Abstract
Despite the success of potent anti-retroviral drugs in controlling HIV-1 infection, little progress has been made in generating an effective HIV-1 vaccine. Although passive transfer of anti-HIV-1 bNAbs can protect mice or macaques against a single high dose challenge with HIV or SIV/HIV chimeric viruses respectively1-8, the long-term efficacy of a passive antibody transfer approach for HIV-1 has not been examined. Based on the relatively long term protection conferred by Hepatitis A immune globulin, we tested the efficacy of a single injection (20mg/kg) of four anti-HIV-1 neutralizing monoclonal antibodies (MAbs) (VRC01, VRC01-LS, 3BNC117, and 10-10749-12) in blocking repeated weekly low dose virus challenges of the clade B SHIVAD8. Compared to control animals, which required 2 to 6 challenges (median=3 weeks) for infection, a single bNAb infusion prevented virus acquisition for up to 23 weeks. This effect depended on antibody potency and half-life. The highest levels of plasma neutralizing activity and correspondingly, the longest protection, were found in monkeys administered the more potent antibodies, 3BNC117 and 10-1074 (median=13 and 12.5 weeks respectively). VRC01, which showed lower plasma-neutralizing activity, protected for a shorter time (median=8 weeks). The introduction of a mutation that extends antibody half-life into the Fc domain of VRC01 increased median protection from 8 to 14.5 weeks. If administered in to populations at high risk for HIV-1 transmission, such an immunoprophylaxis regimen could have a major impact on virus transmission.
It is now recognized that unlike most other prophylactic vaccines for human viral pathogens, an effective vaccine against HIV-1 will likely need to completely block the establishment of a productive infection within a very short time frame (1 to 3 days of transmission). Such protection has, in fact, been achieved by administering polyclonal and monoclonal anti-HIV-1 neutralizing antibodies (NAbs) to humanized mice or macaques prior to challenge with SIV/HIV chimeric viruses (SHIVs)1-8.
During the past seven years, monoclonal antibodies (MAbs) have been isolated from selected HIV-1 infected individuals, who generate anti-viral NAbs (bNAbs) with broad and potent activity against isolates of diverse genetic and geographic origin13. Several of these bNAbs have been used to suppress ongoing viral infections in humanized mice, macaques, and humans14-18. Pre-exposure immunoprophylaxis with bNAbs has also been evaluated in macaque models. In most of these experiments, a single dose of antibody, typically infused 24 to 48h before a single high dose virus challenge, was sufficient to block infection by a virus challenge, capable of establishing an infection in all untreated animals4,19-21. Humans, however, are usually exposed to much lower doses of virus on several occasions before becoming infected with HIV-122.
It is worth noting that prior to the development of an effective hepatitis A virus vaccine, pre-exposure immunoprophylaxis with Hepatitis A immune globulin was common practice for travelers to endemic regions of the world; protective effects lasted 3 to 5 months23. Prophylactic administration of antibodies against other microbial pathogens has also been used to prevent disease24. Based on this idea, we explored the possibility that a single administration of a potent neutralizing anti-HIV MAb, in the setting of repeated low-dose (RLD) SHIV challenges, might protect for extended periods of time, thereby providing a proof of concept for periodic administration of MAb as an alternative to HIV-1 vaccination.
We initially selected 3 MAbs for the RLD SHIV challenge experiment based on their previously described activity in blocking virus acquisition in a cohort of 60 macaques following a single high dose SHIV challenge21. Two of these antibodies (VRC0112 and 3BNC11711) target the gp120 CD4bs and one (10-107410) is dependent on the presence of HIV-1 gp120 N332 glycan, located immediately downstream of the V3 loop. The challenge virus selected for the present study was SHIVAD8-EO25, an R5-tropic molecular cloned derivative of the clade B SHIVAD826, which possesses multiple properties typical of pathogenic HIV-1 isolates27.
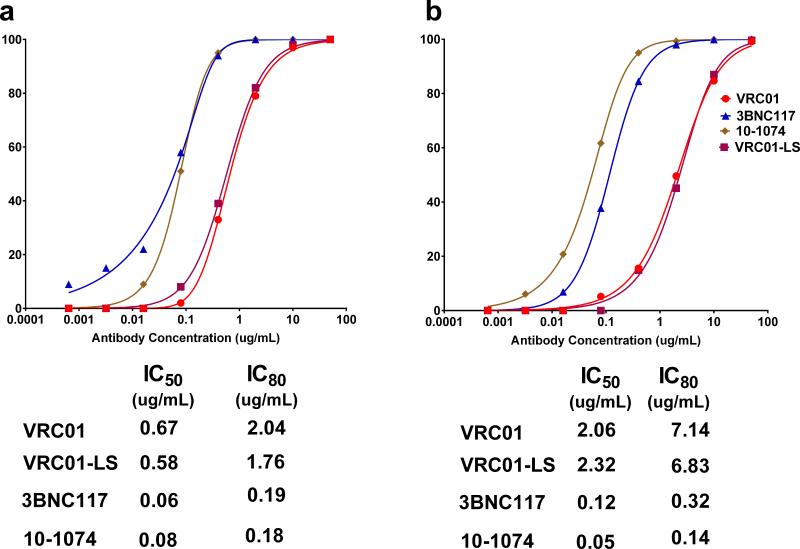
When tested against large HIV-1 pseudovirus panels including multiple clades, 3BNC117 and VRC01 neutralize more than 80% of the viral isolates and 10-1074 neutralizes between 60 and 70%. Against sensitive viruses, 10-1074 is the most potent, followed by 3BNC117 and VRC0128. Consistent with this trend, the IC50s for VRC01, 3BNC117 and 10-1074 against SHIVAD8-EO were 0.67, 0.06 and 0.08 μg/ml, respectively, and the IC80s were 2.04, 0.19 and 0.18 μg/ml, respectively (Extended Data Fig. 1a). Neutralization sensitivities were also measured using the SHIV challenge stock in a single round of infection assay in TZM-bl cells, using replication competent SHIVAD8-EO. The IC50s and IC80s for VRC01, 3BNC117 and 10-1074 in this assay system were 2.06, 0.12, and 0.05 and 7.14, 0.32, and 0.14 μg/ml, respectively (Extended Data Fig. 1b).
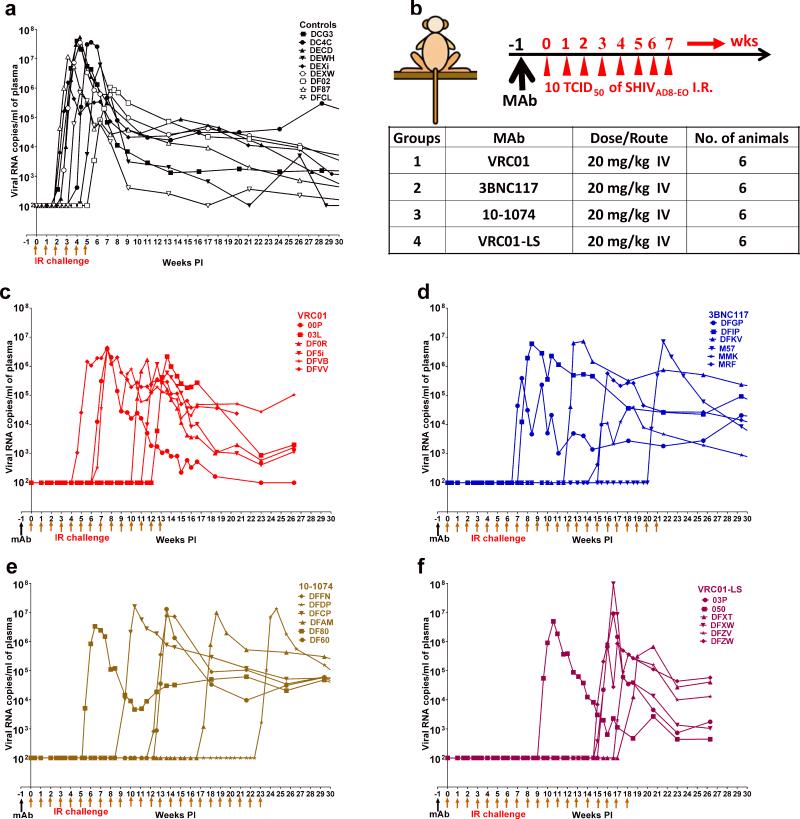
In an initial experiment designed to simulate low dose mucosal transmission in humans, a cohort of 9 monkeys was challenged weekly by the intrarectal (IR) route with 10 TCID50 of SHIVAD8-EO, in the absence of antibody treatment. As shown in Fig. 1a, plasma viremia became detectable following 2 to 6 challenges, with a median of 3.0 weekly virus exposures needed to infect all nine animals. Based on these results, the inoculum size administered to each monkey per challenge was estimated to be 0.27 AID50.
Figure 1. HIV MAbs delay virus acquisition following repeated low-dose intrarectal SHIVAD8-EO challenges.
a, Plasma viral loads in macaques receiving no MAb (controls). b, Diagrammatic representation of the regimen used to assess the protective efficacy of MAbs. Macaques were administered the indicated MAbs at a dose of 20 mg kg−1 and challenged one week later and subsequently, every week thereafter. c, d, e, f, Plasma viral loads in macaques administered VRC01, 3BNC117, 10-1074 and VRC01-LS bNAbs, respectively.
The regimen used to assess the protective efficacy of the three anti-HIV-1 MAbs against a RLD rectal challenge of SHIVAD8-EO is shown in Fig. 1b. Individual MAbs (20 mg/kg) were administered a single time intravenously to three cohorts of 6 animals. Starting one week later, each group was challenged weekly by the IR route with 10 TCID50 of SHIVAD8-EO. Samples of blood, collected at regular intervals, were monitored for levels of viral RNA, concentrations of MAb, and anti-SHIV neutralizing titers. The number of virus challenges required to establish a SHIVAD8-EO infection, indicated by measurable viremia (>100 viral RNA copies/ml plasma), in the recipients of the anti-HIV-1 MAbs was compared to that needed for virus acquisition in the control group.
In all cases, the administration of MAbs delayed virus acquisition. Animals receiving VRC01 required 4-12 challenges; 3BNC117 required 7-20 challenges and 10-1074, 6-23 challenges (Fig. 1c, d, and e). The difference in the number of challenges required for infection, and thus, the median times to virus acquisition compared to control monkeys were 8 weeks for VRC01, 13 weeks for 3BNC117 and 12.5 weeks for 10-1074.
The pharmacokinetic profile of VRC01 was altered by introducing two amino acid mutations (M428L and N434S, referred to as “LS”), into its Fc domain, which increased its half-life in both plasma and tissues9. The neutralization activity of this VRC01 derivative, designated VRC01-LS, was first tested in the TZM-bl assay and, as expected, it exhibited IC50 and IC80 values similar to VRC01 (Extended Data Fig. 1 a and b). When administered to six macaques in the previously described RLD SHIVAD8-EO challenge system, the VRC01-LS treated animals required 9 to 18 challenges (median = 14.5) for all of the monkeys to become infected (Fig. 1f). Thus the modified VRC01-LS Fc domain conferred an estimated 1.8-fold increase in the number of challenges resulting in successful acquisition compared to the parental VRC01 MAb.
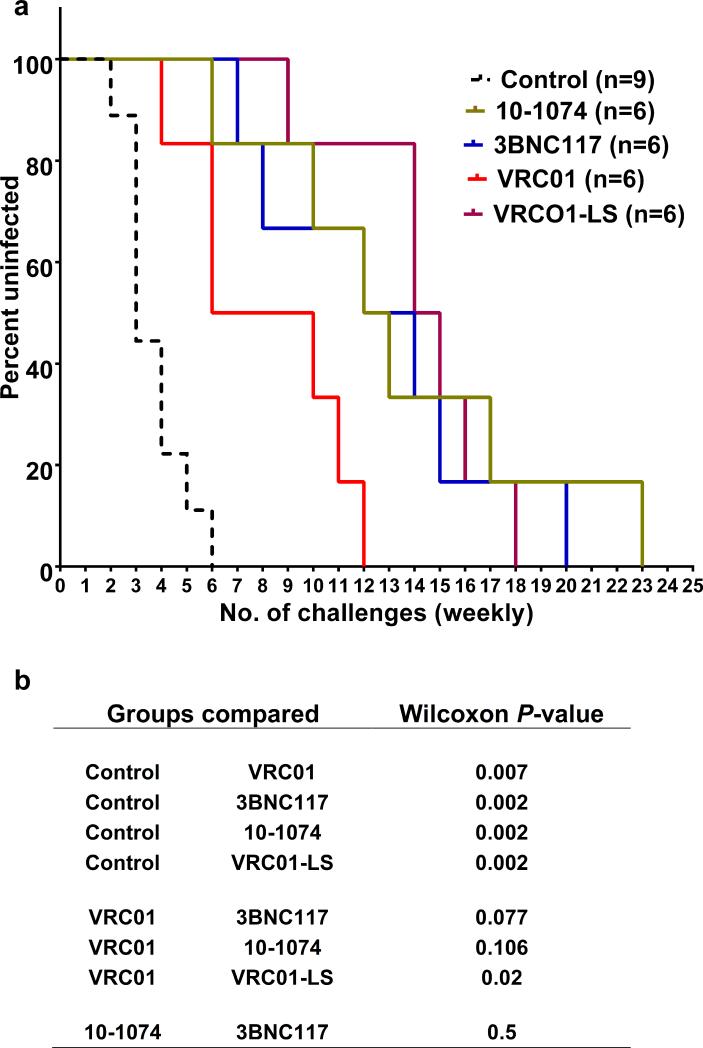
The protective effects of the four anti-HIV-1 MAbs are described by Kaplan-Meier analysis in which the percentage of macaques remaining uninfected is plotted against the number of SHIVAD8EO challenges (Fig. 2a). Significantly increased numbers of challenges were required to establish infections in the recipients of the VRC01, 3BNC117, 10-1074 and VRC01-LS MAbs than in the control animals (P = 0.007, 0.002, 0.002 and 0.002, respectively), using the Wilcoxon Rank Sum test (Fig. 2b). A comparison of the individual pairs of Kaplan-Meier curves revealed that 10-1074, 3BNC117 and VRC01-LS were not significantly different from each other in blocking infection.
Figure 2. Kaplan-Meier analysis and magnitude of protection by HIV MAbs in repeated low-dose challenge.
a, The Kaplan-Meier survival curves for recipients of the four bNAbs and the cohort of control animals. The percentage of macaques remaining uninfected is plotted against the number of 10 TCID50 SHIVAD8-EO intrarectal challenges required to establish infections. b, Statistical differences are represented as P-values (Wilcoxon Rank sum test) by comparing the number of challenges resulting in infection between control animals and an individual MAb recipient group or between different MAb treated groups.
Ultrasensitive nested qRT-PCR and qPCR assays for plasma viral RNA and cell-associated viral RNA and DNA29 were performed on plasma and PBMC samples, collected from recipients of the different neutralizing MAbs, prior to SHIVAD8-EO breakthrough infections, as assessed by plasma viremia measured in our standard assay. In all cases, the levels of viral RNA and DNA measured with the ultrasensitive assays were below detectable limits (Extended Data Table 1).
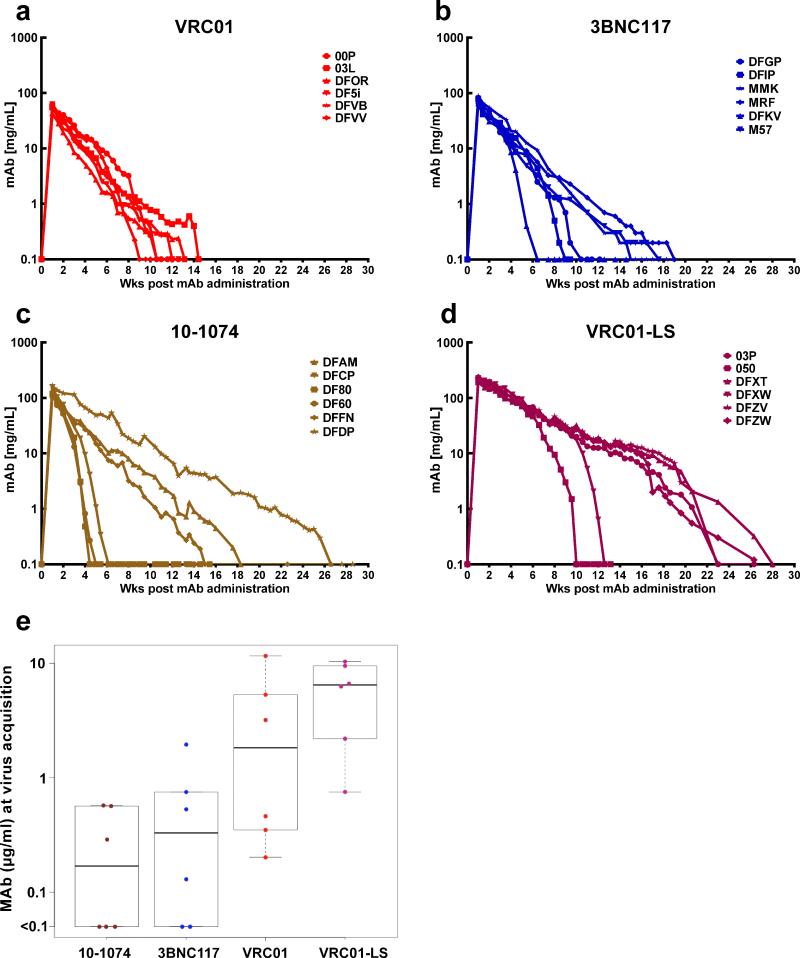
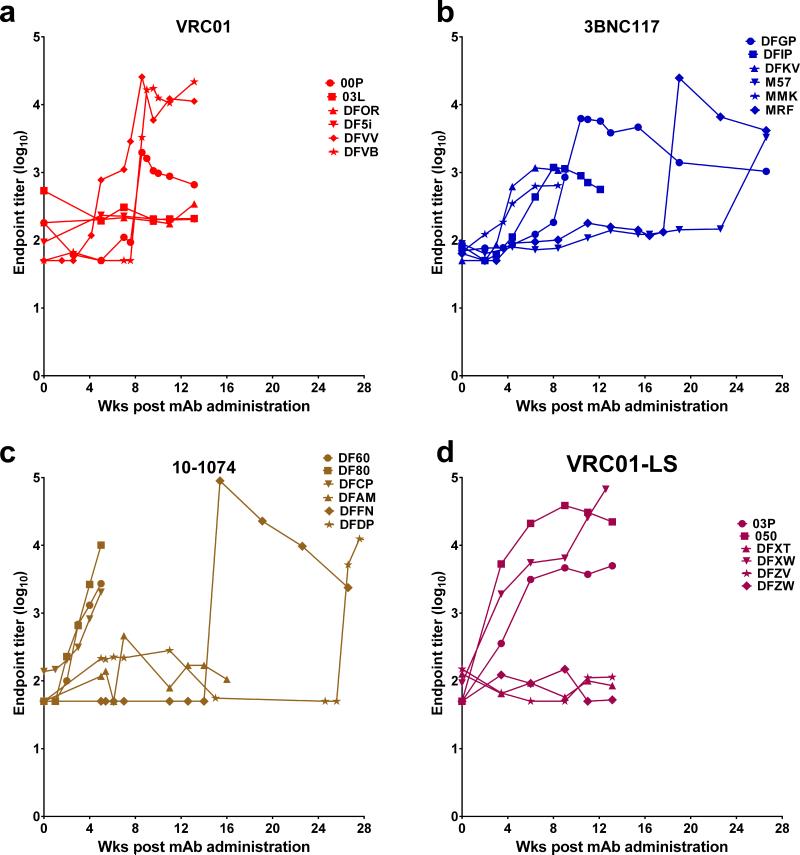
The plasma concentrations of the infused MAbs were measured longitudinally in individual animals beginning 1 week following infusion. (Fig. 3; Extended data Tables 2 and 3). The median plasma concentrations at the times of virus breakthrough for the 10-1074 and 3BNC117 recipient cohorts were 0.169 and 0.330 μg/ml, respectively (Fig. 3e). These values are comparable to the IC80 values determined in vivo, using TZM-bl assays (Extended data, Fig. 1b). The median plasma concentrations at the times of virus acquisition for VRC01 and VRC01-LS were 10 to 20-fold higher (1.825 and 6.446 μg/ml) and were also in the same range as the IC80 values determined in vitro. It is worth noting that three of the six recipients of the 10-1074 MAb experienced rapid decay of plasma antibody, which fell to background levels between weeks 4 to 6 following administration (Fig. 3c). A similar pattern occurred for three of the 3BNC117 MAb and VRC01-LS recipients, although the decline of antibody in plasma was delayed in these two groups animals (Fig. 3b). This rapid clearance of plasma MAbs in the subgroups of 10-1074 and 3BNC117 recipients tracked with the emergence of anti-antibody responses to the infused anti-HIV-1 human MAbs (Extended Data Fig. 2). For monkeys infused with the 10-1074 MAb, the median number of challenges for successful infection in the three-animal subgroup not experiencing the rapid anti-antibody induced decay, was 17.0 weeks compared to 12.5 for the entire 10-1074 recipient cohort.
Figure 3. Plasma concentrations of the infused MAbs in macaques correlate with long-term protection from SHIV infection.
a, b, c, d, Plasma antibody concentrations in macaques administered VRC01, 3BNC117, 10-1074, and VRC01-LS decay over time. e, The median plasma concentrations at the times of virus breakthrough in bNAb recipients were 0.169 (10-1074), 0.330 (3BNC117), 1.825 (VRC01), and 6.446 (VRC01-LS), respectively.
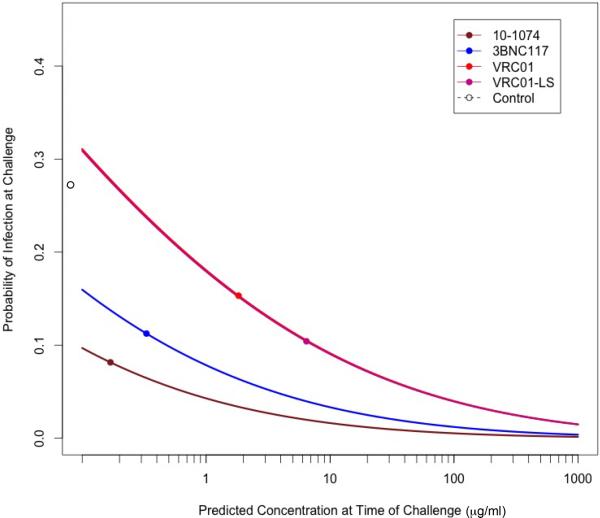
Probit analysis was also used to estimate the probability of infection as a function of the imputed plasma MAb concentration at the time of each challenge. The probability of infection per infection for the control monkeys was 0.27, estimated by pooling all of the SHIVAD8-EO challenges to this group of animals; this is indicated by the single open circle along the ordinate of Extended Data Fig. 3. Not unexpectedly, the curves relating antibody concentration and virus acquisition for VRC01 and VRC01-LS were superimposed on one another even though VRC01-LS had a longer half-life in vivo. In this same analysis, the curves for 10-1074 and 3BNC117, which conferred lower probabilities for infection at each plasma MAb concentration, reflected their greater neutralization potency against the challenge virus, relative to the VRC01 antibodies. At a 10-1074 MAb plasma concentration of 1 μg/ml, the model predicts a probability of infection, for a single challenge, of 0.044, approximately 6-fold less than that estimated for animals receiving no antibodies.
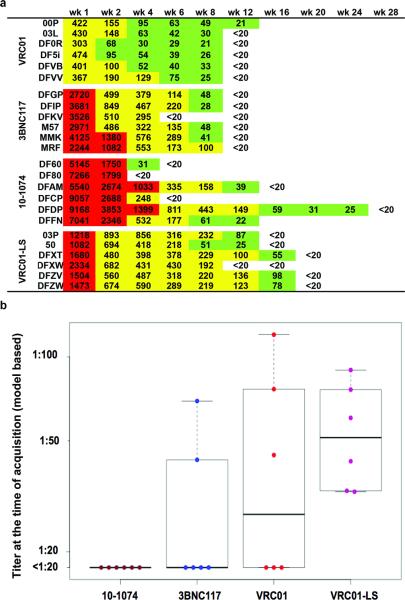
The plasma neutralization titer was also determined for each of the MAb recipients at multiple times post infusion (Figure 4a). The median plasma neutralizing titers for the four groups of macaques at the time of SHIVAD8-EO acquisition were low: <1:20 (below the level of detection) for 10-1074 and 3BNC117 recipients; 1:27 for the VRC01 group; and 1:51 for the VRC01-LS cohort (Fig. 4b). As noted earlier for plasma MAb concentrations, the levels of detectable neutralizing activity in members of each cohort inversely correlated with the emergence of anti-antibodies (Compare Fig. 4a and Extended Data Fig. 2).
Figure 4. The decline of neutralizing antibody titers in plasma over time in macaques corresponds to the time of virus acquisition.
a, Plasma IC50 titers of the indicated MAbs were determined longitudinally using the TZM-bl cell assay. b, Plasma neutralizing titers at the time of virus acquisition for the four groups of MAb recipients. Boxes represent the 25th and 75th percentiles and the heavier line represents the median value for each group.
In conclusion a single administration of potent anti-HIV-1 neutralizing MAbs to naïve macaques was protective against repeated low dose SHIV infection for several months. The duration of protection was directly related to antibody potency and half-life. When considered in the context of a potential exposure to HIV-1 in regions of the world where the HIV-1 is endemic, the barrier to infection when antibody concentrations remain above protective levels in infused individuals, could have a profound impact on virus transmission. As noted earlier, anti-antibodies directed against some of the administered MAbs emerged quite rapidly in some macaques, and diminished their prophylactic efficacy. However, this is not likely to occur in humans as reported in a recent study of VRC0130. Based on the results obtained with VRC01 and VRC01-LS, it is also anticipated that the creation and use of 3BN117 and/or 10-1074 derivatives with the LS mutation should exhibit increased durability in vivo, resulting in protection of up to 6 months against SHIVAD8-EO infected macaques. The administration of a multivalent cocktail of these anti-viral bNAbs could augment their efficacy by increasing overall breadth and their capacity to block the transmission of resistant HIV-1 strains.
METHODS
Animal experiments
Thirty-three male and female rhesus macaques (Macaca mulatta) of Indian genetic origin from 2 to 4 years of age were housed and cared for in accordance with Guide for Care and Use of Laboratory Animals Report no. NIH 82-53 (Department of Health and Human Services, Bethesda, Maryland, 1985) in a biosafety level 2 NIAID facility. All animal procedures and experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of NIAID, NIH. Animals were not randomized and the data collected was not blinded. Phlebotomies, euthanasia and sample collection were performed as previously described 31. All of the macaques used in this study were negative for the MHC class I Mamu-A*01, Mamu-B*08 and Mamu-B*17 alleles. No animals were excluded from the analysis.
Antibodies
The VRC01, 3BNC117, 10-1074 and VRC01-LS anti-HIV-1 monoclonal NAbs were isolated and produced as described elsewhere 9-12. 10-1074 was produced by transient transfection of IgH and IgL expression plasmids into the human embryonic kidney (HEK) cells whereas VRC01, VRC01-LS, and 3BNC117 were produced from Chinese hamster ovary (CHO) cells. All of the MAbs were IgG1. All of the monoclonal antibodies were purified by chromatography and sterile filtration and were endotoxin free. A single dose (20 mg/kg) of each MAb was administered intravenously to individual animals in four cohorts of monkeys.
Virus challenge
The origin and preparation of the tissue-culture-derived SHIVAD8-EO stock have been previously described25. One week following MAb infusion, animals were challenged intrarectally with 10 TCID50 of SHIVAD8-EO, and every week thereafter, until a virus infection was established. A pediatric nasal speculum was used to gently open the rectum and a 1 ml suspension of virus was slowly infused into rectal cavity using a plastic tuberculin syringe. An IR challenge SHIVAD8-EO inoculum size of 10 TCID50 was chosen for RLD experiments based on previous results indicating that: 1) 1000 TCID50 of SHIVAD8-EO administered by the IR route resulted in the establishment of infections of 30 of 30 rhesus monkeys and 2) an IR virus titration suggested that 1000 TCID50 of SHIVAD8-EO was equivalent to approximately 10 animal infectious doses (AID)5021.
Quantification of viral nucleic acids
Viral RNA levels in plasma were determined by real-time reverse transcription-PCR (ABI Prism 7900HT sequence detection system; Applied Biosystems) as previously described31. Ultrasensitive measurement of plasma SIV gag RNA was performed as described, and cell-associated levels of SIV RNA and DNA were determined by a nested, hybrid real-time/digital PCR assay, essentially as reported previously29.
Antibody concentrations in plasma
Plasma antibody levels were quantified by ELISA using purified MAbs as a standard and anti-antibody responses in plasma were also evaluated as reported earlier9. These assays were performed twice.
Neutralization assays
The titers of each MAb against SHIVAD8-EO was assessed by two types of in vitro neutralization assays: (1) TZM-bl entry assay with pseudotype challenge virus25,27 and (2) a single-round TZM-bl infectivity assay with replication competent challenge virus32. Antibody concentrations required to inhibit infection by 50% or 80% are reported as 50% or 80% inhibitory concentrations (IC50 or IC80), respectively. TZM-bl cells were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH from Dr. John C. Kappes, Dr. Xiaoyun Wu and Tranzyme Inc 33. These cells were not authenticated for this study. The neutralization activity present in plasma samples collected from rhesus macaques was assessed by TZM-bl entry assay with pseudotype challenge virus. The IC50 titer was calculated as the plasma dilution causing 50% reduction in RLUs compared with virus controls. The neutralization assays were repeated two times.
Statistical analyses
The Wilcoxon rank-sum test was used to compare number of challenges until infection between each MAb groups and control; these comparisons were considered primary and were compared to a Bonferroni-adjusted alpha of .05/4=.0125 to determine significance. Comparisons between antibodies were considered secondary and are not adjusted for multiple comparisons. Finally, probit models were used to model the probability of infection at each challenge as a function of concurrent antibody concentration. Since these values were not always measured at the precise time of challenge, antibody concentration were modeled separately for each animal over time, and these models were used to impute the concentration at the exact time of each challenge for the probit model.
Extended Data
Extended Data Figure 1. Neutralization sensitivity of SHIVAD8-EO to four broadly acting neutralizing anti-HIV-1 MAbs.
a, Neutralizing activity of the indicated bNAbs was determined against SHIVAD8-EO pseudovirions using TZM-bl target cells. The calculated IC50 and IC80 values are shown at the bottom. b, Neutralizing activity of the indicated bNAbs was determined against replication competent SHIVAD8-EO in a single round TZM-bl infectivity assay. The calculated IC50 and IC80 values are shown at the bottom. The assay was carried out in the presence of indinavir. Both experiments were performed twice.
Extended Data Figure 2. Development of anti-MAb immune responses in recipients of anti-HIV-1 bNAbs.
a, b, c, d, Longitudinal analysis of anti-VRC01, anti-3BNC117, anti-10-1074 and anti-VRC01-LS antibody responses respectively, following a single intravenous infusion of indicated MAbs. This assay was performed twice.
Extended Data Figure 3. Predicted probability of infection as a function of antibody levels.
The per-challenge probability of infection was modeled as a function of antibody concentration at the time of each challenge using a probit regression model. The fitted probabilities from the models are plotted separately for each MAb group, with the estimated probability of infection for the control animals (0.27) indicated by the open circle adjacent to each ordinate. The VRC01 and VRC01-LS curves are superimposed. The points on each curve represent the median concentration at the time of breakthrough infection for each group of monkeys.
Extended Data Table 1.
Plasma viral RNA and cell-associated viral RNA/DNA in rhesus macaques prior to breakthrough of infection.
| Animal | Wks post mAb treatment | Plasma Viral RNA (copies/ml) | SIV Gag RNA copies per 106 cell eq | SIV Gag DNA copies per 106 cell eq |
|---|---|---|---|---|
| DF60 | 7.4 | <2 | <1 | <1 |
| 11.4 | <2 | <1 | <1 | |
| DF80 | 3.6 | <2 | <1 | <1 |
| 5.4 | <2 | <1 | <1 | |
| DFAM | 11.4 | <2 | <1 | <1 |
| 15.4 | <2 | <1 | <1 | |
| DFCP | 5.4 | <2 | <1 | <1 |
| 7.4 | <2 | <1 | <1 | |
| DFDP | 13.6 | <2 | <1 | <1 |
| 26.6* | 3,600,000 | 140000 | 610 | |
| DFFN | 7.4 | <2 | <1 | <1 |
| 11.4 | <2 | <1 | <1 | |
| DFGP | 3.6 | <2 | <1 | <1 |
| 5.4 | <2 | <1 | <1 | |
| DFIP | 5.4 | <2 | <1 | <1 |
| 6.4 | <2 | <1 | <1 | |
| DFKV | 7.4 | <2 | <1 | <1 |
| 11.4* | 10 | <1 | <1 | |
| M57 | 11.4 | <2 | <1 | <1 |
| 17.6 | <2 | <1 | <1 | |
| MMK | 11.4 | <2 | <1 | <1 |
| 15.4 | <2 | <1 | <1 | |
| MRF | 9.4 | <2 | <1 | <1 |
| 13.6 | <2 | <1 | <1 | |
| OOP | 4.6 | <2 | <1 | <1 |
| 6.6* | 270 | 3.2 | 4.2 | |
| 03L | 8.6 | <2 | <1 | <1 |
| 12.6* | 25,000 | 5100 | 25 | |
| DFOR | 6.6 | <2 | <1 | <1 |
| 10.6* | 73,000 | 16000 | 22 | |
| DF5i | 6.6 | <2 | <1 | <1 |
| 10.6 | <2 | <1 | <1 | |
| DFVB | 4.6 | <2 | <1 | <1 |
| 6.6* | 1,300 | <1 | <1 | |
| DFVV | 2.6 | <2 | <1 | <1 |
| 4.6* | 930 | <1 | <1 | |
| 03P | 10.6 | <2 | <1 | <1 |
| 14.6 | <2 | <1 | <1 | |
| O50 | 4.6 | <2 | <1 | <1 |
| 8.6 | <2 | <1 | <1 | |
| DFXT | 10.6 | <2 | <1 | <1 |
| 14.6 | <2 | <1 | <1 | |
| DFXW | 10.6 | <2 | <1 | <1 |
| 14.6 | <2 | <1 | <1 | |
| DFZV | 10.6 | <2 | <1 | <1 |
| 14.6 | <2 | <1 | <1 | |
| DFZW | 10.6 | <2 | <1 | <1 |
| 14.6* | 10 | <1 | <1 |
Time point collected post breakthrough of infection. Ultrasensitive measurements of plasma SIV RNA or cell-associated SIV RNA and SIV DNA in PBMC were determined by a nested, hybrid real-time/digital PCR assay.
Extended Data Table 2.
VRC01 and 3BNC117 antibody concentrations in the plasma of macaques after a single administration of the indicated MAbs.
| VRC01 conc (μg/ml) | 3BNC117 conc (μg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wks | OOP | 03L | DFOR | DF5i | DFVB | DFW | Wks | DFGP | DFIP | MMK | MRF | DFKV | M57 |
| 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1.0 | 63 | 55.4 | 43.1 | 62.41 | 40.07 | 50.86 | 1.0 | 72.6 | 78.1 | 88.7 | 64.3 | 65.5 | 63 |
| 1.6 | 43.23 | 35.25 | 28.4 | 42.53 | 31.03 | 39.16 | 1.4 | 57 | 41.4 | 65.7 | 49 | 44.2 | 41.9 |
| 2.0 | 40.16 | 30.21 | 19.51 | 34.13 | 22.88 | 32.85 | 2.0 | 47.4 | 39 | 54.6 | 35.1 | 30.8 | 37.9 |
| 2.6 | 32.65 | 22.64 | 12.98 | 22.54 | 19.93 | 30.92 | 3.0 | 19.6 | 29.2 | 38.8 | 29.1 | 23.2 | 23.4 |
| 3.0 | 25.98 | 16.11 | 8.49 | 14 | 13.46 | 23.38 | 3.6 | 14.4 | 20.9 | 32.9 | 17.2 | 14.7 | 12.7 |
| 3.4 | 18.19 | 12.74 | 7.17 | 10.93 | 11.03 | 16.62 | 4.0 | 12.2 | 16.8 | 20.7 | 14.7 | 8.6 | 11 |
| 4.1 | 16.16 | 9.53 | 4.87 | 8.12 | 8.41 | 13.97 | 4.4 | 8.6 | 15.5 | 20.3 | 11.3 | 4.1 | 8.8 |
| 4.6 | 14.43 | 8.15 | 3.77 | 6.47 | 7.53 | 14.69 | 5.4 | 7.8 | 8 | 12.6 | 9.1 | 0.4 | 4.9 |
| 5.0 | 12.23 | 6.65 | 2.75 | 4.78 | 7 | 10.77 | 6.4 | 2.5 | 4.4 | 9.3 | 5.7 | 0.1 | 3.3 |
| 5.6 | 9.28 | 3.78 | 1.66 | 2.5 | 3.81 | 6.87 | 7.4 | 1.5 | 1.4 | 4.3 | 3.3 | 0.1 | 2 |
| 6.0 | 8.08 | 3.55 | 1.61 | 2.26 | 3 | 4.45 | 8.0 | 1.3 | 0.5 | 0.1 | |||
| 6.6 | 6.03 | 2.51 | 1.39 | 1.52 | 2.45 | 2.41 | 8.4 | 1.2 | 0.2 | 2.7 | 3 | 0.1 | 1.3 |
| 7.0 | 4.38 | 1.85 | 0.7 | 0.96 | 2.11 | 1.61 | 9.0 | 0.7 | 0.1 | ||||
| 7.6 | 3.46 | 1.53 | 0.67 | 0.95 | 1.55 | 0.66 | 9.4 | 0.2 | 0.1 | 1.6 | 2.3 | 0.1 | 1.2 |
| 8.0 | 3.21 | 1.35 | 0.55 | 0.92 | 1.38 | 10.4 | 0.1 | ||||||
| 8.6 | 1.06 | 1.32 | 0.5 | 0.78 | 0.8 | 0.22 | 11.0 | 0.1 | 0.7 | 1.3 | 0.1 | 0.7 | |
| 9.0 | 0.83 | 1.12 | 0.39 | 0.51 | 0.52 | 0.1 | 11.4 | 0.1 | |||||
| 9.6 | 0.33 | 0.89 | 0.32 | 0.48 | 0.5 | 0.1 | 12.1 | 0.1 | |||||
| 10.0 | 0.27 | 0.78 | 0.31 | 0.45 | 0.37 | 0.1 | 12.6 | 0.3 | 0.7 | 0.1 | 0.4 | ||
| 10.6 | 0.1 | 0.73 | 0.3 | 0.1 | 0.1 | 13.6 | 0.3 | 0.6 | 0.1 | 0.3 | |||
| 11.0 | 0.1 | 0.58 | 0.29 | 0.27 | 0.1 | 0.1 | 14.0 | 0.2 | 0.5 | 0.3 | |||
| 11.6 | 0.1 | 0.46 | 0.29 | 0.26 | 0.1 | 0.1 | 14.6 | 0.2 | 0.4 | 0.1 | 0.2 | ||
| 12.0 | 0.1 | 0.43 | 0.23 | 0.1 | 0.1 | 0.1 | 15.0 | 0.1 | 0.4 | 0.2 | |||
| 12.6 | 0.1 | 0.48 | 0.24 | 0.1 | 0.1 | 0.1 | 15.4 | 0.1 | 0.3 | 0.2 | |||
| 13.1 | 0.1 | 0.42 | 0.1 | 0.1 | 0.1 | 0.1 | 16.0 | 0.1 | 0.3 | 0.2 | |||
| 13.6 | 0.60 | 16.4 | 0.1 | 0.2 | 0.2 | ||||||||
| 14.0 | 0.40 | 17.0 | 0.1 | 0.2 | |||||||||
| 14.4 | 0.10 | 17.6 | 0.1 | 0.2 | 0.1 | ||||||||
| 18.3 | 0.1 | 0.2 | |||||||||||
| 19.0 | 0.1 | 0.1 | |||||||||||
The plasma concentrations of the infused VRC01 and 3BNC117 were measured longitudinally in the indicated animals.
Extended Data Table 3.
10-1074 and VRC01-LS antibody concentrations in the plasma of macaques after a single administration of the indicated MAbs.
| 10-1074 conc (μg/ml) | VRC01-LS conc (μg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wks | DFAM | DFCP | DF80 | DF60 | DFFN | DFDP | Wks | 03P | 050 | DFXT | DFXW | DFZV | DFZW |
| 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 1.0 | 112.2 | 157.1 | 118.4 | 123.2 | 105 | 165.2 | 1.0 | 225.6 | 192.3 | 191.8 | 226.3 | 206 | 234 |
| 1.4 | 85.79 | 102.9 | 83.04 | 73.83 | 100.2 | 137.1 | 1.6 | 169.9 | 181.9 | 153.7 | 205.5 | 185.8 | 169.8 |
| 2.0 | 65.71 | 75.66 | 54.04 | 51.46 | 70.47 | 121.3 | 2.0 | 147.1 | 183.8 | 144.9 | 192.1 | 196.5 | 180.9 |
| 3.0 | 40.26 | 30.14 | 19.31 | 13.14 | 34.16 | 115.3 | 2.6 | 161.1 | 158.4 | 134.1 | 175.7 | 143.3 | 160 |
| 3.6 | 38.54 | 18.54 | 3.09 | 2.92 | 33.9 | 76.13 | 3.0 | 139.6 | 121 | 114.2 | 146.9 | 122.4 | 126.1 |
| 4.0 | 29.05 | 9.41 | 0.48 | 0.82 | 26.9 | 66.49 | 3.4 | 100.8 | 123.2 | 97.5 | 149.2 | 99.62 | 109.9 |
| 4.4 | 23.98 | 4.13 | 0.1 | 0.27 | 23.48 | 58.02 | 4.1 | 85.8 | 99.2 | 85.39 | 115.4 | 83.86 | 110.5 |
| 5.0 | 19.22 | 1.07 | 0.1 | 0.1 | 14.98 | 48.57 | 4.6 | 80.75 | 81.47 | 84.05 | 96.66 | 90.35 | 89.88 |
| 5.4 | 15.36 | 0.34 | 0.1 | 0.1 | 11.37 | 50.83 | 5.0 | 74.15 | 70.61 | 81.56 | 94 | 76.03 | 78.98 |
| 6.1 | 10.25 | 0.1 | 0.1 | 0.1 | 7.47 | 43.68 | 5.6 | 66.4 | 50.01 | 62.09 | 66.09 | 64.61 | 57.82 |
| 6.4 | 11.31 | 0.1 | 0.1 | 0.1 | 7.15 | 53.86 | 6.0 | 68.3 | 46.59 | 68.85 | 59.62 | 64.89 | 55.32 |
| 7.0 | 11.17 | 0.1 | 0.1 | 0.1 | 5.95 | 34.49 | 6.6 | 48.25 | 32.37 | 48.55 | 49.35 | 59.46 | 52.37 |
| 7.4 | 10.01 | 0.1 | 0.1 | 0.1 | 6.78 | 22.26 | 7.0 | 41.47 | 19.19 | 42.24 | 42.01 | 45.4 | 43.91 |
| 8.0 | 8.04 | 0.1 | 0.1 | 0.1 | 3.19 | 18.74 | 7.6 | 35.11 | 12.16 | 35.04 | 35.43 | 37.21 | 33.66 |
| 8.4 | 6.29 | 0.1 | 0.1 | 0.1 | 2.74 | 16.32 | 8.0 | 35.83 | 9.45 | 44.51 | 32.74 | 46.14 | 40.3 |
| 9.0 | 5.69 | 0.1 | 0.1 | 0.1 | 2.43 | 14.52 | 8.6 | 28.34 | 5.09 | 33.09 | 28.2 | 37.1 | 34.81 |
| 9.4 | 4.52 | 0.1 | 0.1 | 0.1 | 1.65 | 20.28 | 9.0 | 27.42 | 3.01 | 29.52 | 25.81 | 31.81 | 29.42 |
| 10.4 | 3.78 | 0.1 | 0.1 | 0.1 | 1.05 | 13.05 | 9.6 | 20.82 | 1.5 | 27.67 | 20.34 | 26.1 | 21.27 |
| 11.0 | 2.51 | 0.1 | 0.1 | 0.1 | 0.71 | 9.75 | 10.0 | 19.85 | 0.2 | 32.19 | 17.53 | 27.91 | 27.6 |
| 11.4 | 2.21 | 0.1 | 0.1 | 0.1 | 0.84 | 8.53 | 10.6 | 15.94 | 0.2 | 20.39 | 9.9 | 24.64 | 21.42 |
| 12.1 | 1.71 | 0.1 | 0.1 | 0.1 | 0.65 | 6.48 | 11.0 | 13.36 | 0.2 | 19.64 | 5.28 | 20.15 | 19.54 |
| 12.6 | 0.85 | 0.1 | 0.1 | 0.1 | 0.37 | 3.94 | 11.6 | 12.62 | 0.2 | 17.85 | 2.06 | 17.7 | 16.72 |
| 13.3 | 0.72 | 0.1 | 0.1 | 0.27 | 5.44 | 12.0 | 12.52 | 0.2 | 16.9 | 0.64 | 18.82 | 18.1 | |
| 13.6 | 1.3 | 0.1 | 0.1 | 0.34 | 4.5 | 12.6 | 12.4 | 0.2 | 15.67 | 0.2 | 15.06 | 15.68 | |
| 14.0 | 0.91 | 0.1 | 0.24 | 4.42 | 13.1 | 9.66 | 0.2 | 14.02 | 0.2 | 13.15 | 13.11 | ||
| 14.6 | 0.72 | 0.1 | 0.1 | 0.1 | 0.19 | 4.19 | 13.6 | 10.40 | 15.20 | 16.80 | 14.10 | ||
| 15.0 | 0.61 | 0.1 | 4.21 | 14.0 | 9.70 | 14.10 | 16.20 | 13.60 | |||||
| 15.4 | 0.58 | 0.1 | 0.1 | 0.1 | 0.1 | 3.5 | 14.4 | 8.10 | 12.70 | 13.50 | 11.90 | ||
| 16.0 | 0.45 | 3.72 | 15.0 | 8.00 | 12.00 | 14.40 | 11.80 | ||||||
| 17.6 | 1.87 | 15.6 | 7.00 | 11.00 | 12.80 | 10.00 | |||||||
| 18.3 | 1.95 | 16.0 | 6.00 | 10.40 | 12.70 | 9.20 | |||||||
| 19.0 | 0.1 | 1.8 | 16.6 | 4.40 | 9.00 | 11.10 | 6.30 | ||||||
| 19.6 | 1.6 | 17.0 | 4.60 | 9.30 | 10.90 | 2.00 | |||||||
| 20.0 | 1.09 | 17.6 | 4.10 | 7.50 | 10.00 | 2.40 | |||||||
| 20.6 | 1.03 | 18.1 | 2.40 | 6.60 | 8.20 | 1.70 | |||||||
| 21.0 | 0.93 | 18.6 | 1.95 | 5.76 | 7.52 | 1.29 | |||||||
| 22.0 | 0.78 | 19.0 | 5.64 | 6.57 | |||||||||
| 22.6 | 0.1 | 0.73 | 19.6 | 1.80 | 4.70 | 2.98 | 0.84 | ||||||
| 23.0 | 0.62 | 20.0 | 3.29 | ||||||||||
| 23.4 | 0.59 | 20.6 | 1.07 | 2.06 | 2.10 | 0.55 | |||||||
| 24.1 | 0.46 | 23.0 | 0.10 | 1.33 | 0.10 | 0.30 | |||||||
| 24.6 | 0.41 | 26.3 | 0.10 | 0.32 | 0.10 | 0.12 | |||||||
| 25.0 | 0.43 | ||||||||||||
| 25.6 | 0.3 | ||||||||||||
| 26.6 | 0.1 | 0.1 | |||||||||||
The plasma concentrations of the infused 10-1074 and VRC01-LS were measured longitudinally in the indicated animals.
ACKNOWLEDGEMENTS
We thank R. Plishka, A. Peach and T. Lewis for determining plasma viral RNA loads and K. Rice, R. Engel, R. Petros and S. Fong for diligently assisting in the maintenance of animals and assisting with procedures. We also thank R. Schwartz, Vaccine Production Program Laboratory, Vaccine Research Center, NIAID, for clinical grade VRC01 and VRC01-LS; and X. Chen, Vaccine Research Center, NIAID, for protein reagents for ELISA. We thank NIH AIDS Research and Reference Reagent Program for TZM-bl cells. Finally, we thank R. Fast, Frederick National Laboratory for Cancer Research, for ultrasensitive plasma SIV RNA assays and W. Bosche and M Hull for ultrasensitive PBMC SIV RNA/DNA assays. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) and, in part, with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E (J.D.L.). The research was also funded in part by the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery Grants OPP1033115 and OPP1092074 (to M.C.Nu.), by the NIH under award numbers, AI-100148, UM1 AI100663-01. M.C. Nu. is supported by the Robertson Foundation and HHMI.
Footnotes
AUTHOR CONTRIBUTIONS
R.G., Y.N., M.A.M., M.C.Nu., and J.R.M. designed experiments; R.G., Y.N., A.P., F.K, A.G., J.G., A.B.W., R.S., K.W., Z.M., and S.D.S. performed experiments; R.G., Y.N., M.C.Na., M.A.M., M.C.Nu., J.R.M., and J.D.L. analyzed data; R.G., Y.N., M.A.M., M.C.Nu., J.R.M. and J.D.L. wrote the manuscript.
Online Content
Methods, along with any additional Extended Data display items and source data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
References
- 1.Baba TW, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. doi:10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. doi:10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. doi:10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 4.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. doi:10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura Y, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietzsch J, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. doi:10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. doi:10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 9.Ko SY, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. doi:10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouquet H, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–3277. doi: 10.1073/pnas.1217207109. doi:10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. doi:10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou T, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. doi:10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol. 2015;16:571–576. doi: 10.1038/ni.3158. doi:10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. doi:10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. doi:10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492:118–122. doi: 10.1038/nature11604. doi:10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. doi:10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch RM, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7:319ra206. doi: 10.1126/scitranslmed.aad5752. doi:10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 19.Rudicell RS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88:12669–12682. doi: 10.1128/JVI.02213-14. doi:10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders KO, et al. Sustained Delivery of a Broadly Neutralizing Antibody in Nonhuman Primates Confers Long-Term Protection against Simian/Human Immunodeficiency Virus Infection. J Virol. 2015;89:5895–5903. doi: 10.1128/JVI.00210-15. doi:10.1128/JVI.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shingai M, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. doi:10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel P, et al. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. doi:10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Advisory Committee on Immunization, P. Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2006;55:1–23. [PubMed] [Google Scholar]
- 24.Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS. 2015;10:129–134. doi: 10.1097/COH.0000000000000154. doi:10.1097/COH.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shingai M, et al. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A. 2012;109:19769–19774. doi: 10.1073/pnas.1217443109. doi:10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura Y, et al. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J Virol. 2010;84:4769–4781. doi: 10.1128/JVI.02279-09. doi:10.1128/JVI.02279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautam R, et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J Virol. 2012;86:8516–8526. doi: 10.1128/JVI.00644-12. doi:10.1128/JVI.00644-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West AP, Jr., et al. Computational analysis of anti-HIV-1 antibody neutralization panel data to identify potential functional epitope residues. Proc Natl Acad Sci U S A. 2013;110:10598–10603. doi: 10.1073/pnas.1309215110. doi:10.1073/pnas.1309215110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. doi:10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledgerwood JE, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182:289–301. doi: 10.1111/cei.12692. doi:10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo Y, et al. Short-and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J Virol. 2000;74:6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]