Abstract
OBJECTIVE
Depression in patients with type 2 diabetes (T2D) is associated with long-term complications, disability, and early mortality. No studies have systematically examined the length of episodes and remission in adults with major depressive disorder (MDD) and T2D. This study examined the course of depressive disorders in patients with T2D and MDD.
RESEARCH DESIGN AND METHODS
Participants (N = 50) enrolled in a behavioral intervention for adults with T2D and MDD were interviewed using the Structured Clinical Interview for DSM-IV-TR to assess history of depressive disorders at baseline (lifetime history), postintervention, and 3-month follow-up. Onset and remission dates were recorded for all Axis I depressive disorders from birth to final interview.
RESULTS
Average number of MDD episodes was 1.8 with a mean duration of 23.4 months (SD 31.9; range 0.5–231.3). Over the life course, mean exposure to MDD was 43.1 months (SD 46.5; range 0.5–231.3). Kaplan-Meier survival curve analysis indicated median episode duration decreased with subsequent episodes (14 months, first episode; 9 months, second episode; P < 0.002). In patients with multiple depressive episodes, recovery time was shorter with each subsequent episode (P = 0.002). No differences in length of episode or remission were observed based on chronology of T2D diagnosis.
CONCLUSIONS
The overall exposure to depression in this sample of adults with T2D represents a substantial period of time that can contribute to negative medical and psychiatric outcomes. Recurrent episodes decrease in duration as do recovery periods, resulting in a waxing and waning pattern. Findings from this study underscore the need to effectively diagnose and treat depression in patients with T2D to minimize risk of future depressive episodes.
Introduction
Type 2 diabetes (T2D) affects more than 29.1 million Americans and was the seventh leading cause of death in the U.S. in 2010 (1). Additionally, T2D contributes significantly to overall health care expenditures, accounting for $245 billion in total expenses in 2012 alone (1).
Depression is comorbid with T2D. One in four patients with T2D report elevated depressive symptoms, and 11% have been diagnosed with a clinical depressive disorder (2,3). Depressive symptoms and disorders are significantly associated with worsened glycemic control (4), greater severity of diabetes complications (5,6), poorer adherence to diabetes self-care (7–9), decreased quality of life (10), greater functional disability (11), and early mortality (12,13). This literature has been heterogeneous in the measurement and definition of “depression.” Few studies have examined characteristics of depression using standardized psychiatric interview protocols to distinguish depressive disorders from symptoms otherwise attributable to T2D or other medical comorbidities (14–17).
While the course of depression in the general population is well documented (18–21), the progression of major depressive disorder (MDD) in patients with T2D has not been extensively examined. Lustman et al. (16) found that patients with T2D and MDD averaged 4.8 depressive episodes during a 5-year follow-up period. The average duration of the longest episode for each participant was 16.1 months. Lustman et al. found that of the 12 participants who reached full remission during the clinical trial, 58.3% had a subsequent depressive episode within 1 year and 3 more relapsed during the 5-year follow-up period (16).
In a separate study, a combination retrospective and prospective investigation of depression in patients with T2D revealed that 22 of 28 participants with depression evaluated using the Diagnostic Interview Schedule at baseline evaluation met diagnostic criteria for MDD or dysthymia during 5-year follow-up (15). Participants experienced nearly as many depressive episodes (mean 4.2) during the 5-year follow-up period as they had in their lifetime prior to the 5-year follow-up period (mean 6.3 episodes). At baseline, 51% reported that their longest episode lasted 0–12 weeks, 16% reported a longest episode of 13–26 weeks, 5% had a longest episode of 27–52 weeks, and 28% had a longest episode that exceeded 52 weeks. At follow-up, 46% reported a maximum duration of 0–12 weeks, 17% between 13 and 26 weeks, 10% between 27 and 52 weeks, and 17% exceeding 52 weeks (15).
Multiple studies have observed a bidirectional relationship between depression and T2D with a 38–60% increased risk of T2D in adults with a lifetime history of depression (22–25). Findings from these prospective longitudinal trials have suggested that depression may be a predisposing factor for the development of T2D after controlling for obesity, sex, and poverty, which are known risk factors for both disorders. Prior studies using repeated measures cross-sectional and longitudinal designs suggest that depression is persistent in those already diagnosed with T2D (26,27). However, it is not known whether T2D lengthens the duration of depressive episodes, and no comparisons have been made for episode length or burden of depression in patients whose depression has preceded the diagnosis of T2D compared with those in which T2D was diagnosed first.
Depression Course and Duration in the General Population
Lifetime and point prevalence rates of MDD and other depressive disorders (ODD) in the general population have been well characterized by several major prospective trials including the Epidemiologic Catchment Area (ECA) program and the National Institute of Mental Health Collaborative Program on the Psychobiology of Depression trial (18,20). In these studies, participants were assessed longitudinally either in person or via telephone using a structured clinical interview designed to identify psychopathology according to the Research Diagnostic Criteria and the DSM-IV. Participants were followed prospectively and assessed at set intervals such that the duration of their pathology could be plotted over time. In these trials, the lifetime prevalence of MDD was found to range from 10 to 25% in women and 5 to 12% in men, and the point prevalence varied from 5 to 9% for women and 2 to 3% for men (28,29). Additionally, one study found that 85% of participants who recovered from MDD experienced at least one recurrent episode (30). Fifty-eight percent of individuals who remained well for at least 5 years following their baseline episode experienced a subsequent episode (30). Episodes characterized as mild to severe had an average duration of 13.8 to 16.6 weeks, and “very severe” episodes had a mean duration of 23.1 weeks. The overall mean duration was 16 weeks (21). Taken together, data drawn from the general population points to the recurrent nature of clinical depression. However, no studies have examined depression duration in patients with T2D, and there has been no data on the characteristics of first and subsequent episode lengths and periods of depression remission. Understanding the course of depression in people with T2D has implications for treatment approaches and standards of care that should be used to treat this vulnerable population. The current study was designed to describe the lifetime course of MDD and ODD in terms of episode length, remission duration, and progression of episodes in a well-characterized sample of adults with T2D. In addition, we examined episode and remission duration before and after diagnosis of T2D to test whether the order of diagnosis influenced burden of depression.
Research Design and Methods
The current study represents a secondary data analysis of Program ACTIVE (Adults Coming Together to Increase Vital Exercise) (31,32). From 2006 to 2009, a single-arm pilot and feasibility study was conducted to test a combination behavioral approach (10 sessions of cognitive behavioral therapy combined with 12 weeks of community-based exercise) to treating depression in adults with T2D. Participants were recruited from physician practices, community advertisement, and local media from rural Appalachian counties in southeastern Ohio and western West Virginia (31). The study was approved by the Ohio University Institutional Review Board, and informed consent was given by all participants. Eligibility criteria included the following: 18 years of age or older, ambulatory status, receiving care from a primary or specialty care provider, and current MDD (using DSM-IV-TR diagnostic criteria). Participants were medically ineligible if they had been diagnosed with uncontrolled stage 2 hypertension or had a cardiac event in the past year, laser surgery on eyes to correct diabetic retinopathy in the last 6 months, history of stroke, lower limb amputation, asensory neuropathy, severe valvular heart disease, atrial fibrillation, severe chronic obstructive pulmonary disease, class III or IV heart failure, or medical instability. Participants with current suicidal ideation, substance abuse or dependence, a lifetime history of bipolar depression, or psychotic symptoms were excluded. The methods of the parent study are described in detail elsewhere (31).
Participants were assessed at three time points: baseline, postintervention, and 3 months postintervention. A total of 50 participants were enrolled, and 40 participants completed the intervention, the postintervention follow-up assessment, and the 3-month follow-up assessment. At each time point, data were collected on depression, glycemic control, and cardiovascular risk outcomes (31). The current study captured lifetime history and course of depression before, during, and following the 12-week behavioral intervention.
Measures
Demographic Data
Demographic characteristics were obtained via a self-administered questionnaire completed by each participant at baseline. Variables included age, ethnicity, marital status, income, educational status, work status, and health insurance status.
Assessment of Depression: Structured Clinical Interview for DSM-IV-TR
The Structured Clinical Interview for DSM-IV-TR (SCID) is a semistructured psychiatric diagnostic interview protocol that assesses current and lifetime presence of 33 Axis I disorders in adult populations using DSM-IV-TR diagnostic criteria (28,33). Interviewers used a timeline to plot social, occupational, and medical events throughout the period of the interview. Major life events placed on this timeline were used as memory cues for participants to identify the date of onset and offset of depressive symptoms and episodes. Dates of symptom onset and offset were captured at the level of month and year with default values placed on the first day of the month if more specific information was not available from the participant. The time frame for baseline SCID interviews was birth to the date of interview (i.e., lifetime history). In postintervention and 3-month follow-up interviews, participants were queried for life events and symptoms to the date of the last completed interview. The SCID interview uses a “decision tree” format to guide the interviewer through possible diagnoses as the interview proceeds (33).
Interviewers were graduate-level psychology students who had been trained to reliability by the first author using practice interviews to reach consistent levels of accuracy in recognizing symptomology (κ = 0.90–1.00). Case conferences were used to reach group consensus among the study principal investigator and interviewers regarding diagnoses and reduce interviewer drift over the course of the study.
Physical Examination and Medical History
Self-reported medical history data were obtained via interview at baseline, postintervention, and 3-month follow-up by the study research nurse including the following: diabetes type and duration, height, weight, prescribed diabetes treatment regimen, medical contraindications for participation, medical diagnoses, and number and severity of diabetes complications (31). Primary or specialty care providers were contacted to confirm medical eligibility for participation in the study.
Glycated Hemoglobin
Glycated hemoglobin (HbA1c) was measured using the DCA2000+ Analyzer (Bayer Diagnostics, Wayne, NJ) at each assessment time point. The DCA2000+ Analyzer provides glycated hemoglobin data from whole-blood samples using the measurement of glycated fractions of HbA1c, which reflects the glucose level in blood over a 2- to 3-month time span. The reference range for HbA1c samples was 4.3 to 5.7%.
Data Coding
In order to assess number and duration of psychiatric episodes, data from Program ACTIVE SCID booklets were reviewed and coded for each of the 50 participants enrolled in the study. The following variables were coded for each participant.
Onset Date
The onset date was the date on which the participant met full criteria for the DSM-IV-TR diagnosis of the disorder.
Offset Date
The offset date was the date on which the participant no longer met full criteria for the depressive disorder. For episodes of MDD, this was the date that the participant no longer met criteria for five of the possible nine symptoms required for the diagnosis. For cases in which a depressive episode was ongoing at the time of the last interview, the date of the interview was used as the offset date (i.e., censored). If there was no offset date listed for a current depressive episode but the subsequent SCID booklet did not reflect any continuous symptoms, the date of previous SCID interview was used as the offset date for that episode.
Date of Full Remission
The date on which the participant had been without core mood symptoms (depressed mood or anhedonia) for 2 months was considered the date of full remission. For participants whose MDD episode remitted less than 2 months from the date of the last interview, the depressive episode was coded as MDD in partial remission (28).
ODD
Onset and offset dates were recorded for all ODD including dysthymia, depressive disorder not otherwise specified, adjustment disorder with depressed mood, and simple bereavement. Because these diagnoses do not incorporate a partial remission course specifier per the DSM-IV-TR criteria, the offset date was considered to be the date on which symptoms ended, per participant report.
Remission Periods
Remission periods were calculated as the difference between the date of full remission of the previous episode and the start date of the subsequent episode.
Other Axis I Disorders
Remission status for all other Axis I diagnoses, as defined by the DSM-IV-TR, reported at any of the three assessment time points was recorded. Disorders assessed for remission statuses included bipolar I disorder, alcohol abuse, alcohol dependence, non-alcoholic substance abuse, non-alcoholic substance dependence, panic disorder with/without agoraphobia, agoraphobia without history of panic disorder, social phobia, specific phobia, obsessive-compulsive disorder, somatization disorder, post-traumatic stress disorder, generalized anxiety disorder, anorexia nervosa, bulimia nervosa, and binge eating disorder. Episodes were coded as “current,” “partial remission,” “full remission,” and “diagnosis never present,” consistent with DSM-IV-TR diagnostic criteria.
Statistical Analysis
Data were analyzed using Statistical Analysis Software (SAS) 9.4. Consistent with studies conducted in the general population, depressive episode duration was calculated by subtracting the episode start date from the date of full remission. Descriptive statistics were calculated for episode duration and remission periods. The primary outcome was the time to recurrence of any depressive disorder following the remission of the previous episode. We used Kaplan-Meier survival curves for the first, second, and third recurrences to describe the time to next episode at each observed event time point. Frailty models were calculated to conduct statistical comparisons of two survival curves in order to account the correlation among multiple recurrences within a subject. To assure the proportionality assumption on the hazard ratio, we divided the time into two strata. A similar approach was used to analyze the time to remission and length of episode considering up to three episodes of any depressive disorder within a participant. Finally, Kaplan-Meier curves and log-rank tests were used to determine whether having diabetes before or after the first episode of any depressive disorder resulted in the same remission time or length of episode.
Results
Participant demographic characteristics are presented in Table 1.
Table 1.
Demographic characteristics
| Age (years), mean (SD) |
57.2 (8.8) |
| Sex (female), N (%) |
34 (68.0) |
| Ethnicity (white), N (%) |
50 (100.0) |
| Education, N (%) |
|
| High school diploma/GED or less |
14 (28.0) |
| Trade school/part college |
17 (34.0) |
| 4-year college/postgraduate |
14 (28.0) |
| Other |
5 (10.0) |
| Marital status, N (%) |
|
| Married/living with partner |
37 (74.0) |
| Single |
2 (4.0) |
| Divorced/separated/widowed |
11 (22.0) |
| Income (dollars), N (%) |
|
| 0–10,000 |
3 (6.1) |
| 11,000–20,000 |
5 (10.2) |
| 21,000–40,000 |
14 (28.6) |
| 41,000–60,000 |
13 (27.0) |
| ≥61,000 |
14 (28.1) |
| Health insurance (yes), N (%) |
46 (92.0) |
| Current primary care provider (yes), N (%) |
50 (100.0) |
| Current diabetes specialist (yes), N (%) (missing =1) |
26 (53.1) |
| Age at T2D onset (years), mean (SD) |
46.0 (10.9) |
| Diabetes duration (years), mean (SD) |
10.5 (6.6) |
| Average HbA1c (%), mean (SD) |
|
| Baseline |
7.6 (1.8) |
| Postintervention |
7.0 (1.3) |
| 3-month follow-up | 7.3 (1.2) |
Characteristics and Rate of All Depressive Episodes
Rates of psychiatric disorders and characteristics of depressive episodes across the spectrum of Axis I depressive disorders are presented in Table 2. Participants experienced their first episode of any depressive disorder at a mean (SD) age of 41.7 (15.9) years and had a mean (SD) of 2.3 (1.0) lifetime depressive episodes (range 1.0–5.0). Participants were exposed to a mean (SD) of 71.1 (103.7) months of any depressive disorder (range 0.5–620.5). Mean episode duration of any depression diagnosis was 30.9 months (SD 63.2; range 0.4–536.8).
Table 2.
Depressive episodes characteristics
| N | Mean or median | SD or SE | Minimum | Maximum | |
|---|---|---|---|---|---|
| Number and exposure of depressive episodes | |||||
| Mean/SD age at first onset of any depressive episode (years) | 50 | 41.7 | 15.9 | 11.4 | 75.1 |
| Mean/SD number of depressive episodes (MDD + ODD) | 50 | 2.3 | 1.0 | 1.0 | 5.0 |
| Mean/SD exposure to all depressive disorders (months) | 50 | 71.1 | 103.7 | 0.5 | 620.5 |
| Median/SE exposure to all depressive disorders (months) | 50 | 38.1 | 16.0–82.0 | 0.5 | 620.5 |
| Mean/SD duration of all depression episodes (months) | 115 | 30.9 | 63.2 | 0.4 | 536.8 |
| Median/SE duration of all depression episodes (months) | 115 | 11.0 | 6.0–30.0 | 0.4 | 536.8 |
| Mean/SD number of MDD episodes | 50 | 1.8 | 0.8 | 1.0 | 4.0 |
| Mean/SD exposure to MDD (months) | 50 | 43.1 | 46.5 | 0.5 | 231.3 |
| Median/SE exposure to MDD (months) | 50 | 24.5 | 13.4–60.1 | 0.5 | 231.3 |
| Mean/SD MDD episode duration (months) | 92 | 23.4 | 31.9 | 0.5 | 231.3 |
| Median/SE MDD episode duration (months) | 92 | 10.6 | 7.0–28.7 | 0.5 | 231.3 |
| Mean/SD number of ODD episodes | 50 | 0.5 | 0.7 | 0.0 | 2.0 |
| Mean/SD exposure to ODD (months) | 18 | 77.8 | 151.6 | 1.0 | 608.8 |
| Median/SE exposure to ODD (months) | 18 | 13.5 | 4.0–60.7 | 1.0 | 608.8 |
| Mean/SD ODD episode duration (months) | 23 | 60.9 | 123.8 | 0.4 | 536.8 |
| Median/SE ODD episode duration (months) |
23 |
12.0 |
2.0–60.0 |
0.4 |
536.8 |
| Episode duration of MDD (onset to full remission) | |||||
| Mean/SD duration of prospective episode (months) | 2 | 2.5 | 0.1 | 2.5 | 2.6 |
| Mean/SD duration of index episode (months) | 50 | 26.3 | 38.7 | 0.5 | 231.3 |
| Mean/SD duration of earliest preindex episode (months) | 30 | 19.8 | 19.2 | 2.2 | 74.0 |
| Mean/SD duration of second earliest preindex episode (months) | 8 | 17.4 | 19.3 | 3.0 | 61.9 |
| Mean/SD duration of third earliest preindex episode (months) | 2 | 50.0 | 50.9 | 14.0 | 86.0 |
Characteristics of MDD
Descriptive characteristics of MDD episodes are presented in Table 2. A total of 92 lifetime MDD episodes were reported. Participants experienced a mean of 1.8 MDD episodes (SD 0.8; range 1.0–4.0) during their lifetime with a mean episode length of 23.4 months (SD 31.9; range 0.5–231.3). Mean lifetime exposure to all MDD episodes combined was 43.1 months (SD 46.5; range 0.5–231.3). Durations of MDD episodes were positively skewed. Sixty-nine percent of episodes were 19.3 months or shorter while the other 31% fell between 25.0 months and 231.3 months with only two episodes above 100 months (107.5 months and 231.3 months).
Censored Episodes
Of the 92 major depressive episodes, 21 were censored and were coded with an artificial full remission date (the date of the participant’s most recent SCID interview) representing a conservative estimate of length for these episodes. Of the 21 censored episodes, 10 were censored because the participant was lost to follow-up and only completed the baseline SCID. A total of 11 episodes were censored because participants did not reach full remission prior to the end of the 3-month follow-up period. Of these 11 episodes, 10 were index episodes reported at baseline and one was a recurrent episode that occurred after recovery from the index episode.
Characteristics of ODD
ODD were defined in this study as DSM-IV-TR depressive disorders that did not meet DSM-IV-TR criteria for MDD including dysthymia, depressive disorder not otherwise specified, adjustment disorder with depressed mood, and simple bereavement. Across all participants, there were 23 ODD episodes reported by 18 participants. These episodes lasted a mean of 60.9 months (SD 123.8; range 0.4–536.8) with mean lifetime combined exposure of 77.8 months (SD 151.6; range 1.0–608.8).
Characteristics of Remission Periods
Remission periods between depressive episodes were examined (Table 3). Participants were stratified by the number of depression-free/any depression episodes. The first depression-free period was coded as the period from birth to the first depressive episode.
Table 3.
Well intervals following any depressive episode (years)
| 2 DF periods, N = 13, mean (SD) | 3 DF periods, N = 16, mean (SD) | 4 DF periods, N = 10, mean (SD) | 5 DF periods, N = 1, mean | |
|---|---|---|---|---|
| Between DOB and first depressive episode onset |
53.87 (11.26) |
36.66 (14.59) |
30.37 (11.51) |
26.94 |
| First post-depression well interval |
4.47 (7.62) |
15.15 (10.24) |
12.48 (12.35) |
4.84 |
| Second post-depression well interval |
2.55 (4.49) |
6.01 (4.50) |
9.84 |
|
| Third post-depression well interval |
0.43 (0.41) |
2.58 |
||
| Fourth post-depression well interval | 0.25 |
DF, depression free; DOB, date of birth.
Depressive Episode Recurrence
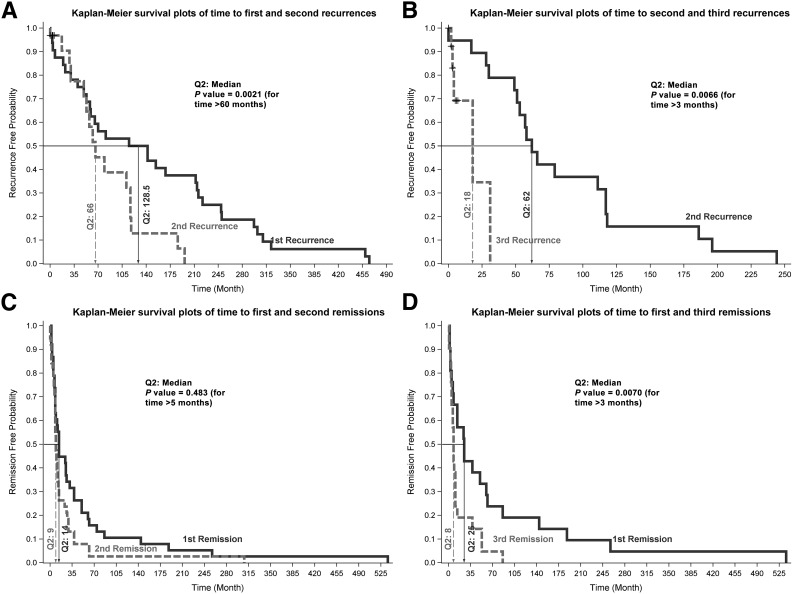
Figure 1A and B shows the survival curves for the time to first, second, and third recurrence of any depressive disorder. The second recurrence occurred significantly sooner (median duration 60 months) than the first recurrence (Fig. 1A) (P = 0.002). The median time to the second recurrence was much shorter than the median time to the first recurrence (66.0 vs. 128.5 months) for those who had at least two recurrences (N = 32). When comparing the time to the third recurrence with the time to first and second recurrences, we considered only those with at least three recurrences (N = 19). The time to the third recurrence was shorter than the time to the first recurrence (18 vs. 70 months), although the difference did not reach statistical significance (P = 0.07 for month >25) because of the small sample size. However, the median time to the third recurrence was significantly shorter than the median time to the second recurrence (Fig. 1B) (18 vs. 62 months; P < 0.007).
Figure 1.
Kaplan-Meier survival curves. A: Time to first and second recurrences. B: Time to second and third recurrences. C: Time to first and second remissions. D: Time to first and third remissions.
Depressive Episode Duration
The Kaplan-Meier survival curves for the length of first, second, and third episodes of any depressive disorder are shown in Fig. 1C and D. Figure 1C shows that the median length of the second episode was significantly shorter than the median length of first episode (9 vs. 14 months; P = 0.048 for time >5 months) for those who had at least two episodes (N = 38). Similarly, Fig. 1D shows that the median length of third episode was significantly shorter than the median length of first episode (8 vs. 25 months; P = 0.007 for time >3 months) for those who had at least three episodes (N = 21). However, the lengths of second and third episodes were not significantly different.
Episode Duration and Order of Depression Versus Diabetes Onset
Finally, in order to assess whether the duration of any depressive disorder differed in the presence of T2D, we conducted Kaplan-Meier survival curves stratified by order of depression versus diabetes onset (i.e., those whose first diagnosis was depression vs. those whose first diagnosis was T2D). We did not observe any differences in the survival curves for the time to first remission or length of first episode between the two groups: those with diagnosis of diabetes before the first episode of any depressive disorder (median 11 months) versus those with diagnoses of diabetes after having the first episode of any depressive disorder as well as after the remission time (median 14 months; P = NS).
Conclusions
This study characterizes the course of depressive disorders in a well-defined cohort of adults with T2D drawn from a rural Appalachian geographic region. This is the first study to carefully document episode and remission duration in a sample of participants exclusively with T2D. This is also the first study to examine length of depressive episodes vis-à-vis the onset of T2D.
We found an average duration of MDD episodes of 23.4 months, which is an order of magnitude longer than the average duration of MDD observed in the general population (22 weeks) (34). Although the design of this study did not include a control group without diabetes, this finding is consistent with prior intervention trials (15,16) as well as cross-sectional repeated measures studies (26,27) that have suggested that depression is more persistent among T2D patients than the general population. The mean episode duration of major depression in our study was 7 months longer than the average of the longest episodes reported by Lustman et al. (16). We also observed that MDD and ODD were recurrent in participants with T2D with mean total exposure to all depressive episodes lasting nearly 6 years (i.e., 71 months) over the life course.
We observed that the first episode of depression had the longest duration (median 14–25 months) with subsequent episodes lasting a median of 8–9 months. Prior studies of depressive episode duration in the general population have had mixed results; some studies (34) have shown a comparable episode duration across recurrences while others (35,36) have documented a shorter duration with each subsequent episode. These studies prospectively assessed for psychopathology using structured clinical interviews other than the SCID. Solomon et al. (34) conducted assessments every 6 months, and Spijker et al. (36) conducted assessments every 3 months. Eaton et al. (35) conducted a single follow-up interview 13–15 years after the initial assessment using a visual interactive life chart to provide memory cues for each of the years under assessment. In our sample, the time between episodes (interepisode recovery) became shorter in those with two or more depressive episodes. These findings are consistent with the pattern of decreasing interepisode depression-free periods seen in the general population (37).
We observed a considerable variation in the duration of MDD episodes across our sample. Sixty-nine percent of MDD episodes were 19.3 months or shorter in duration, and the other 31% ranged from 25.0 to 231.3 months. The variance in episode length captured here could reflect the varying nature with which MDD persists in patients with T2D. Further study is needed to determine if the dissimilarity among episodes observed here is generalizable to all individuals with T2D and MDD.
There are a number of factors that may contribute to this overall pattern. Insufficient detection and/or treatment of depressive episodes may result in incomplete recovery from the primary episode for individuals with T2D. Evaluation of the course and duration of depressive episodes in the general population has indicated that partial remission increases the likelihood of depression relapse. One study (38) reported a 67.6% relapse rate for those in partial remission from MDD and a 15.2% relapse rate for those in full remission from MDD at the beginning of a 2-year follow-up period. Another investigation (39) observed that participants with residual symptoms during recovery from an MDD episode relapsed three times faster (median 23 vs. 68 weeks) than patients who achieved asymptomatic recovery. Patients with ODD relapsed five times faster (median 33 vs. 184 weeks) than patients who reached asymptomatic recovery. In addition, another study (39) found that individuals with residual, subthreshold symptoms had shorter well intervals (median 22.0 weeks) between any depressive episode than their asymptomatic counterparts (median 154.0 weeks). This study also found that participants with subthreshold symptoms had more depressive episodes (mean 2.5 episodes) than participants who reached asymptomatic recovery (mean 1.5 episodes). They reported that those with subthreshold symptoms spent a lower percentage of weeks without depressive symptoms than those who reached asymptomatic recovery. Patients with residual symptoms spent a significantly higher percentage of time with subthreshold depressive symptoms, minor depression, or dysthymia (40).
If these observations from the general population hold true for patients with T2D, these findings in combination with the current data point to the importance of depression detection and well-monitored progressive treatment to fully resolve depressive episodes. The longer the exposure to depression, the greater the risk of adverse diabetes outcomes (3). Such exposure can be significantly reduced when depression is effectively treated using a range of behavioral and medication strategies (3,32).
We did not observe a significant difference in the length of depressive episodes for people whose first diagnosis was depression compared with those whose first diagnosis was T2D. Prior prospective longitudinal trials (23–25) have documented that a lifetime history of depression confers a 38–60% increased risk for the onset of T2D after controlling for other common factors including obesity, sex, and poverty, suggesting a biological predisposing factor. While a lifetime history of depression may increase the risk for the development of T2D, the length of depressive episodes was consistently prolonged whether depression was diagnosed before or after T2D. This suggests that stressors or other epigenetic factors may set the stage for greater burden of depression regardless of the timing of the onset of T2D (41).
Limitations of the current study include a relatively small sample size that may contribute to limited power to detect effects. Further, the lack of diversity among participants may hinder generalizability of this predominantly white, rural, and insured sample to other populations with T2D. In addition, this study did not include a control group without diabetes, which would have allowed for a direct comparison of the course of MDD in people with and without T2D. Because the parent study (31,32) was designed as a pilot trial of a behavioral intervention for people with T2D, people without diabetes were excluded. However, the assessment conducted to diagnose MDD and measure episode duration was consistent with the methods used by prior studies in the general population. While the specific tools may differ, structured diagnostic interviews allow for a level of symptom identification and differentiation that reliably achieve the same level of diagnostic validity.
Further, individuals with suicidal ideation were also excluded from the current study. Because suicidal ideation often marks a more severe level of MDD, it is possible that there is a selection bias in the sample. If reported durations are taken as conservative estimates, it remains notable that episode lengths are an order of magnitude longer than those previously reported for the general population.
We used a combined retrospective and prospective design following the introduction of a combination behavioral intervention designed to treat depression. Prospective study designs reduce recall error in participants and permit more granular estimates of episode duration. Use of retrospective data are consistent with previous work (15). In order to maximize accuracy of participant recall, we used the timeline method throughout the SCID interview to minimize the effects of this limitation. Similar to prior studies (16), estimates of depression episode durations may be artificially limited by the effectiveness of the intervention. However, our data suggest that episode duration is significantly longer in participants with T2D even if this is a conservative estimate attributable to the effectiveness of the cognitive behavioral therapy and exercise intervention.
Another limitation is the lack of availability of medical record data to verify prescribed use of antidepressant medications or referral for behavioral therapies over the life course. While antidepressant medication use was collected prospectively in the current trial, it could not be accurately estimated for past episodes. It is not possible to estimate the effects of these medications on episode duration in our data set. However, the presence of recurrent depressive episodes in this sample suggests that even when antidepressant medication was present, dosing, timing, and monitoring were insufficient to effectively treat depression in these participants.
Further, the absence of medical record data prevents evaluation of the effects of treatment on subsequent MDD or ODD episode timing or duration. It is possible that a known history of diagnosis and/or treatment for MDD could result in further screening and effective treatment. Episode number and duration data from this study suggest, however, that patients did not receive adequate screening or treatment to successfully prevent subsequent depressive episodes from their providers. Future studies that record detailed histories of psychiatric diagnoses and the timing of medication and/or behavioral therapies are needed to better understand the impact of treatment on depression relapse.
In sum, clinical depression poses a significant burden on adults and families with T2D. Data from the first study to document depression episode and remission duration over the life course has shown that depression is a recurrent disorder that persists for prolonged periods and imposes a significant burden on patients with T2D. Whether depression or T2D is diagnosed first, depression in these patients requires consistent and progressive treatment to reduce and prevent the host of adverse consequences associated with prolonged exposure to these comorbid conditions.
Article Information
Acknowledgments. The authors thank the following individuals and organizations for their contributions to Program ACTIVE: the Athens Community Center; Al Miller, Debbie Piatt, and Christine Roush at the Marietta Family YMCA; Joe Leaman at HealthSouth Sports Medicine and Outpatient Rehabilitation, Inc.; and Jean Andrews, Mark McGlynn, Mike Knutson, Todd Doyle, Jennifer Averyt, Dustin Hammers, Zina Trost, Candace Patterson, Jessica Turchik, Diane Turcotte, Andrea Waltje, Travis Lovejoy, Petya Demireva, Troy Robison, and Jamie Huckins, all of the Program ACTIVE team at Ohio University. The authors thank Frank Schwartz and Michael Kushnick of Ohio University for their collaboration in the parent study. The authors also thank all of the study participants of Program ACTIVE whose collaboration made the study possible.
Funding. Funding for this study was provided by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (R34-DK-71545 and R18-DK-092765), the Ohio Department of Health, the Office of Healthy Ohio, the Bureau of Health Promotion and Risk Reduction, the Ohio Diabetes Prevention and Control Program, and the Ohio University Diabetes Research Initiative.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.d.G. wrote the manuscript. K.A.C. wrote the manuscript and analyzed data. M.L. analyzed data. C.S. analyzed data. J.H.S. reviewed/edited the manuscript. M.d.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster presentation at the 37th Annual Scientific Sessions of the Society of Behavioral Medicine, Washington, DC, 30 March–2 April 2016 and at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
References
- 1.Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 2.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 3.Holt RI, de Groot M, Lucki I, Hunter CM, Sartorius N, Golden SH. NIDDK international conference report on diabetes and depression: current understanding and future directions. Diabetes Care 2014;37:2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–942 [DOI] [PubMed] [Google Scholar]
- 5.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–630 [DOI] [PubMed] [Google Scholar]
- 6.Clouse RE, Lustman PJ, Freedland KE, Griffith LS, McGill JB, Carney RM. Depression and coronary heart disease in women with diabetes. Psychosom Med 2003;65:376–383 [DOI] [PubMed] [Google Scholar]
- 7.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278–3285 [DOI] [PubMed] [Google Scholar]
- 8.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry 2003;25:246–252 [DOI] [PubMed] [Google Scholar]
- 9.Wagner JA, Tennen H, Osborn CY. Lifetime depression and diabetes self-management in women with type 2 diabetes: a case-control study. Diabet Med 2010;27:713–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson AM, de Groot M, Samson JA. The effects of psychiatric disorders and symptoms on quality of life in patients with type I and type II diabetes mellitus. Qual Life Res 1997;6:11–20 [DOI] [PubMed] [Google Scholar]
- 11.Von Korff M, Katon W, Lin EH, et al. Potentially modifiable factors associated with disability among people with diabetes. Psychosom Med 2005;67:233–240 [DOI] [PubMed] [Google Scholar]
- 12.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005;28:2668–2672 [DOI] [PubMed] [Google Scholar]
- 13.Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med 2009;7:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustman PJ, Griffith LS, Clouse RE, Cryer PE. Psychiatric illness in diabetes mellitus. Relationship to symptoms and glucose control. J Nerv Ment Dis 1986;174:736–742 [DOI] [PubMed] [Google Scholar]
- 15.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes. Results of 5-yr follow-up study. Diabetes Care 1988;11:605–612 [DOI] [PubMed] [Google Scholar]
- 16.Lustman PJ, Griffith LS, Freedland KE, Clouse RE. The course of major depression in diabetes. Gen Hosp Psychiatry 1997;19:138–143 [DOI] [PubMed] [Google Scholar]
- 17.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psychiatric Disorders in America: the Epidemiologic Catchment Area Study. Robins L, Regier D, Eds. New York, The Free Press, 1991 [Google Scholar]
- 19.Unützer J, Katon W, Callahan CM, et al.; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment . Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836–2845 [DOI] [PubMed] [Google Scholar]
- 20.Shapiro RW, Keller MB. Initial 6-month follow-up of patients with major depressive disorder. A preliminary report from the NIMH collaborative study of the psychobiology of depression. J Affect Disord 1981;3:205–220 [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, Berglund P, Demler O, et al.; National Comorbidity Survey Replication . The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–3105 [DOI] [PubMed] [Google Scholar]
- 22.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care 1996;19:1097–1102 [DOI] [PubMed] [Google Scholar]
- 24.Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 1999;22:1071–1076 [DOI] [PubMed] [Google Scholar]
- 25.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peyrot M, Rubin RR. Persistence of depressive symptoms in diabetic adults. Diabetes Care 1999;22:448–452 [DOI] [PubMed] [Google Scholar]
- 27.de Groot M, Doyle T, Averyt J, Risaliti C, Shubroo J. Depressive symptoms and type 2 diabetes mellitus in rural appalachia: an 18-month follow-up study. Int J Psychiatry Med 2015;48:263–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition- Text Revision. Washington, DC, American Psychological Association 2000. [Google Scholar]
- 29.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 2012;21:169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller TI, Leon AC, Keller MB, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry 1999;156:1000–1006 [DOI] [PubMed] [Google Scholar]
- 31.de Groot M, Kushnick M, Doyle T, et al. A model of community-based behavioral intervention for depression in diabetes: Program ACTIVE. Diabetes Spectr 2010;23:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Groot M, Doyle T, Kushnick M, et al. Can lifestyle interventions do more than reduce diabetes risk? Treating depression in adults with type 2 diabetes with exercise and cognitive behavioral therapy. Curr Diab Rep 2012;12:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry 1992;49:624–629 [DOI] [PubMed] [Google Scholar]
- 34.Solomon DA, Keller MB, Leon AC, et al. Recovery from major depression. A 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry 1997;54:1001–1006 [DOI] [PubMed] [Google Scholar]
- 35.Eaton WW, Anthony JC, Gallo J, et al. Natural history of Diagnostic Interview Schedule/DSM-IV major depression. The Baltimore Epidemiologic Catchment Area follow-up. Arch Gen Psychiatry 1997;54:993–999 [DOI] [PubMed] [Google Scholar]
- 36.Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Br J Psychiatry 2002;181:208–213 [DOI] [PubMed] [Google Scholar]
- 37.Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry 2000;157:229–233 [DOI] [PubMed] [Google Scholar]
- 38.Pintor L, Gastó C, Navarro V, Torres X, Fañanas L. Relapse of major depression after complete and partial remission during a 2-year follow-up. J Affect Disord 2003;73:237–244 [DOI] [PubMed] [Google Scholar]
- 39.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord 1998;50:97–108 [DOI] [PubMed] [Google Scholar]
- 40.Judd LL, Paulus MJ, Schettler PJ, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry 2000;157:1501–1504 [DOI] [PubMed] [Google Scholar]
- 41.Post RM. Heading off depressive illness evolution and progression to treatment resistance. Dialogues Clin Neurosci 2015;17:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]