Abstract
OBJECTIVE
To compare the effectiveness of three delivery modalities of Decision-making Education for Choices In Diabetes Everyday (DECIDE), a nine-module, literacy-adapted diabetes and cardiovascular disease (CVD) education and problem-solving training, compared with an enhanced usual care (UC), on clinical and behavioral outcomes among urban African Americans with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Eligible participants (n = 182) had a suboptimal CVD risk factor profile (A1C, blood pressure, and/or lipids). Participants were randomized to DECIDE Self-Study (n = 46), DECIDE Individual (n = 45), DECIDE Group (n = 46), or Enhanced UC (n = 45). Intervention duration was 18–20 weeks. Outcomes were A1C, blood pressure, lipids, problem-solving, disease knowledge, and self-care activities, all measured at baseline, 1 week, and 6 months after completion of the intervention.
RESULTS
DECIDE modalities and Enhanced UC did not significantly differ in clinical outcomes at 6 months postintervention. In participants with A1C ≥7.5% (58 mmol/mol) at baseline, A1C declined in each DECIDE modality at 1 week postintervention (P < 0.05) and only in Self-Study at 6 months postintervention (b = −0.24, P < 0.05). There was significant reduction in systolic blood pressure in Self-Study (b = −4.04) and Group (b = −3.59) at 6 months postintervention. Self-Study, Individual, and Enhanced UC had significant declines in LDL and Self-Study had an increase in HDL (b = 1.76, P < 0.05) at 6 months postintervention. Self-Study and Individual had a higher increase in knowledge than Enhanced UC (P < 0.05), and all arms improved in problem-solving (P < 0.01) at 6 months postintervention.
CONCLUSIONS
DECIDE modalities showed benefits after intervention. Self-Study demonstrated robust improvements across clinical and behavioral outcomes, suggesting program suitability for broader dissemination to populations with similar educational and literacy levels.
Introduction
Patient self-management support is recognized as essential for improving and maintaining disease control and quality of life in patients with chronic conditions (1). For patients with type 2 diabetes, self-management support, which seeks to address ongoing patient needs for carrying out and maintaining self-care, is now a recommended standard of care along with diabetes self-management education (2).
Effective self-management support programs for diabetes may be especially important for populations of health disparity, who experience higher disease prevalence and morbidity, and are impacted by health care, societal, and systems barriers to self-care and outcomes (3). In a series of previous studies, we developed the Decision-making Education for Choices In Diabetes Everyday (DECIDE) program, which uses problem-solving training as an evidence-based behavior change skill to facilitate identifying and managing barriers to diabetes self-management (4). The DECIDE intervention was initially tested for usability and effectiveness with people with low literacy and functional impairment, which can impede learning and self-management (5,6). DECIDE intervention acceptability and dose was demonstrated in a clinic-based sample of high-risk African American patients with diabetes within an urban health care system (7).
However, to reach underserved populations in need of such programs, population health management seeks to disseminate interventions across diverse practice and community settings, which necessitates flexibility in the delivery modalities of evidence-based interventions (8). The purpose of this randomized trial was to test the DECIDE intervention in three different delivery formats: 1) a self-study format that can be done in a home or community setting and does not necessitate an interventionist; 2) an individual (one-on-one) in-person format with the patient and an interventionist, which offers directed attention and scheduling to suit the individual patient; and 3) a group format with up to 8–10 patients meeting in person with an interventionist according to a fixed meeting schedule. The trial was designed to compare the effectiveness of the three different DECIDE program delivery modalities with an enhanced usual care condition (UC), which used targeted educational materials suitable for people with low health literacy. In addition to comparing to the Enhanced UC, the focus of the study was to understand the effect of each DECIDE modality on clinical and behavioral outcomes. The study analyzed improvement in A1C as a primary outcome, and blood pressure, lipids, and behavioral skills (problem-solving, disease knowledge, and self-care activities) as secondary outcomes.
Research Design and Methods
Intervention Development
The DECIDE program is a diabetes and cardiovascular disease (CVD) education and problem-solving training that is based on a problem-solving model of chronic disease self-management (4). The intervention addresses patients’ understanding of recommendations for diabetes and CVD self-management and trains patients in problem-solving as a core diabetes self-management skill that facilitates all other self-care behaviors (9). DECIDE uses a social problem-solving framework (10) adapted to diabetes and CVD health goals and everyday barriers to care (11). The program comprises one module of Diabetes and CVD education, which reviews key diabetes self-management behaviors and American Diabetes Association (ADA) recommendations regarding clinical targets (12). The educational module is followed by eight modules of problem-solving training, which focus on recognizing challenges and barriers to everyday self-management, understanding one’s problem-solving orientation, applying effective problem-solving skill training, and learning from past experiences. Two patient workbooks accompany the program: Diabetes and Your Heart: Facts & Information Patient Workbook and Hitting the Targets for Diabetes and Your Heart: Your Problem-Solving Workbook. All DECIDE program materials were previously adapted for low literacy using available consensus criteria (13–16) and were tested for usability with people with low literacy and functional impairment that can impede the learning process (5,6).
Procedures
The study was approved by the Johns Hopkins Medicine Institutional Review Board, and all participants gave written informed consent. All study assessment and intervention visits were conducted at the Johns Hopkins study site. Participants were recruited using mailings, postings on institutional community research volunteer sites, and recruitment at church and community health fairs in downtown and East Baltimore. Interested individuals completed a telephone call with a research assistant, during which they received study information and were screened for study inclusion criteria (age 25 years of age or older, black/African American ethnicity, and diagnosed with diabetes by a physician) and exclusion criteria (pregnant, diagnosed with a life-threatening condition, or unable to attend study visits). People meeting inclusion criteria were scheduled for an in-person visit for anthropometry, phlebotomy, and blood pressure measurement to determine trial eligibility. Eligibility criteria were A1C ≥7.5% (58 mmol/mol) and either suboptimal blood pressure (systolic blood pressure [SBP] >130 mmHg or diastolic blood pressure [DBP] >80 mmHg), based on ADA 2011 Standards of Care (12), or lipids (LDL >100 mg/dL or HDL <50 mg/dL for women and <40 mg/dL for men). In addition, patients with A1C 7.0–7.5% (53–58 mmol/mol) but who met eligibility criteria for both suboptimal blood pressure and lipid values were eligible for participation.
Intervention Delivery
Eligible participants were randomized to one of the four study arms: 1) DECIDE Self-Study; 2) DECIDE Individual; 3) DECIDE Group; and 4) Enhanced UC. Intervention duration for each study arm was 18–20 weeks, allowing for holidays.
Interventionists had bachelor's or master's degrees in health education, psychology, or social work and completed training in the DECIDE program and module content. For quality control, interventionists used an Intervention Manual for module-by-module program delivery. All group and individual sessions were audiotaped. Tapes were randomly selected for full review by the DECIDE intervention supervisor to determine fidelity to the protocol and workbooks.
DECIDE Self-Study
Participants were mailed the nine content modules from the DECIDE workbooks to review and apply in a self-directed format. With the first module, they received a schedule instructing them to complete one content module on a biweekly basis. Subsequent modules were mailed to participants following the biweekly schedule. Participants received one phone call midway through the intervention period to assess their use of the materials, but no training or education was provided by research staff during these calls.
DECIDE Individual
Participants received one-on-one training with an interventionist to complete the nine education and problem-solving training modules. The participants met with their assigned interventionist biweekly and covered one module each session.
DECIDE Group
A group of 8–10 participants attended biweekly group sessions facilitated by an interventionist and a cofacilitator. One module was covered at each group session.
Enhanced UC
In this comparison condition, participants were provided with a set of publicly available educational materials from the ADA that were selected to cover key diabetes and CVD self-management content, and for usability in people with lower literacy (17). This material was also mailed on a biweekly basis in order for participants to review the material on a biweekly schedule, similar to those in the DECIDE Self-Study arm.
Data Collection
During the eligibility screening visit, sociodemographic data, medical history, and clinical data were collected. Participants were asked to bring all of their medications to the assessment visits; medications were recorded by the data collector. The Wide Range Achievement Test (WRAT-3) (18) was used to assess literacy and the Patient Health Questionnaire-2 (PHQ-2) (19) was used to assess symptoms of depression. Blood samples were drawn for determining trial eligibility and for measuring clinical outcomes of the trial. A1C, LDL, and HDL were measured using standard techniques. Blood pressure was assessed using a random-zero sphygmomanometer, and the mean of three blood pressure readings was used.
Data collection visits occurred at baseline and at three follow-up time points. The two primary follow-up time points were at 1 week after the completion of the intervention phase (referred to as postintervention) and at 6 months after completion of the intervention phase (referred to as 6 months postintervention); the intervention phase was for 18–20 weeks for each study arm. The postintervention data collection visit corresponded with 6 months from baseline. The 6-month postintervention corresponded with 1 year from baseline. There was also a data collection visit at 3 months postintervention to be consistent with the primary end point in the pilot study (7). Data from this visit are used in statistical models for the purpose of improving modeling of change in the primary and secondary outcomes.
Health Problem-Solving Scale
The Health Problem-Solving Scale (HPSS) is a 50-item scale designed to assess positive/effective and negative/ineffective health-related problem-solving across three domains (4): 1) problem orientation/motivation, 2) problem-solving skill, and 3) transfer of past experience/learning. Participants respond on a 5-point Likert scale ranging from “not at all true of me” (0) to “extremely true of me” (4). Scoring of the HPSS consists of summing positive/effective items with reverse-scored negative/ineffective items in each domain. Higher scores indicate more effective problem-solving. Psychometric properties of the HPSS have been published elsewhere (20,21). For this current sample, Cronbach α was 0.93.
Diabetes and CVD Knowledge Test
Based on information for diabetes self-management from ADA Standards of Medical Care in Diabetes (12), items assess knowledge of risk for CVD in people with diabetes, awareness of “good” and “bad” cholesterol, clinical targets, and strategies for self-management (5). Total scores range from 0 to 18. Reliability was α = 0.58 for this sample, reflecting differences in knowledge across content items (e.g., accuracy in identifying blood glucose targets vs. accuracy in identifying HDL target or types of foods high in fiber).
Summary of Diabetes Self-Care Activities Scale
The Summary of Diabetes Self-Care Activities Scale (SDSCA) was used to assess frequency of diabetes self-care behaviors with subscale items for diet, exercise, glucose testing, foot care, smoking, and medication taking (22). Scoring instructions as provided by Toobert et al. (22) were followed to obtain the mean number of days following a general diet, exercising, following a specific diet, and blood glucose testing. Cronbach α for the SDSCA subscales were as follows: general diet (0.85), specific diet (0.37), exercise (0.73), and blood glucose testing (0.90). The lower reliability coefficient for specific diet is deemed reflective of the observed differences individuals exhibit between behavior frequencies for very specific aspects of diet (e.g., high-fat food consumption vs. fruit/vegetable frequency or spacing carbohydrates).
Statistical Analyses
Descriptive statistics were used to compare the means and SDs for continuous variables and frequency distributions for categorical variables for the total study sample and by intervention arm. Differences between intervention arms on these variables were assessed using χ2 test for categorical variables and general linear model for continuous variables.
Power for the trial was calculated using A1C as a primary outcome. A sample size of 240 (60 people per arm) was targeted for randomization, with a minimum sample size of 180 (45 people per arm) required to provide 80–100% power to detect differences in a clinically meaningful change in A1C (0.5% or greater) and moderate effect sizes in the other outcomes while adjusting for multiple comparisons. Secondary outcomes were SBP, DBP, HDL, LDL, health-related problem-solving, diabetes and CVD knowledge, and subscales from the SDSCA.
Linear mixed-effects models were used to examine the effect of intervention on change in the primary and secondary outcomes at postintervention and at 6 months postintervention according to the intent-to-treat principle. Random effects were estimated for intercept only when modeling change from baseline to postintervention; models included the baseline and postintervention time points of the outcome. When modeling change from baseline to 6 months postintervention, models included all time points of the outcome (i.e., baseline, postintervention, 3 months postintervention, and 6 months postintervention). An unstructured covariance model was assumed. Random effects were estimated for intercept and slope, which allowed adjustment for any individual variability around the intercept and the slope of the outcome. The treatment arm was dummy coded with Enhanced UC as the reference for all analyses. Models were adjusted by age, years of education, and baseline PHQ-2 score. Missing data were handled by applying full information maximum likelihood. In addition, because of the intervention design, piecewise linear mixed-effects models were used. This allowed examination of change in the outcomes during the intervention period (baseline to postintervention) and maintenance of that change in the primary and secondary outcomes once there were no more intervention contacts (postintervention to 6 months postintervention). For each participant, a piecewise linear function was specified with an intercept at baseline and two slopes (i.e., baseline to postintervention and postintervention to 6 months postintervention). Thus, all analyses included baseline and follow-up values of the outcome. Analyses were conducted using SAS version 9.3 and Hierarchical Linear Modeling software version 6.08.
Results
Participant Characteristics
The CONSORT diagram is available as Supplementary Fig. 1. The study eligibility screening visit was completed by 382 people. Among those screened, the eligibility rate was 48%, indicating that almost half of the community sample had A1C, blood pressure, and lipid values within recommended clinical ranges. A resulting 182 people comprised the trial sample, with balanced allocation to each treatment arm. The retention rate for this study was 87.4%.
Table 1 displays the baseline characteristics for the total sample, and by treatment arm. The Self-Study arm participants were older than Enhanced UC participants, but there were no other differences between treatment arms at baseline. The sample was 70% female, with a mean age of 57 years. Although mean years of education was 13, 19% of participants had less than a fifth grade reading level. One-third of the sample reported an annual household income of less than $10,830. Forty-one (23%) participants screened positive for depression (PHQ-2 score ≥3). Participant self-reported complications and comorbidities were as follows: 39 (22%) retinopathy; 53 (29%) peripheral vascular disease; 99 (54%) peripheral neuropathy; 9 (5%) ulcers or gangrene; 6 (3%) amputations; 19 (10%) kidney problems; 5 (3%) liver problems or cirrhosis; 54 (30%) asthma, emphysema, or chronic bronchitis; 97 (54%) arthritis; 20 (11%) digestive problems; 39 (22%) heart trouble; 25 (14%) stroke; 14 (8%) weakness or paralysis due to stroke; and 22 (30%) mental health diagnosis.
Table 1.
Participant characteristics at baseline
| Total (n = 182) | Enhanced UC (n = 45) | DECIDE Self-Study (n = 46) | DECIDE Individual (n = 45) | DECIDE Group (n = 46) | |
|---|---|---|---|---|---|
| Age (years)* | 57.18 (10.55) | 54.51 (10.34) | 60.57 (10.27)* | 54.82 (9.31) | 58.72 (11.21) |
| Female, n (%) | 127 (70%) | 32 (71%) | 33 (72%) | 30 (67%) | 32 (70%) |
| Education (years) | 13.21 (2.30) | 13.51 (2.27) | 12.76 (2.55) | 13.38 (1.83) | 13.22 (2.49) |
| Literacy (WRAT-3) | 8.79 (3.59) | 9.48 (2.90) | 8.33 (3.80) | 8.62 (3.88) | 8.74 (3.72) |
| >12th grade, n (%) | 48 (27%) | 9 (20%) | 12 (27%) | 14 (31%) | 13 (29%) |
| 9–12th grade, n (%) | 46 (26%) | 17 (38%) | 8 (18%) | 9 (20%) | 12 (27%) |
| 5–8th grade, n (%) | 51 (28%) | 14 (31%) | 16 (36%) | 13 (29%) | 8 (18%) |
| <5th grade, n (%) | 35 (19%) | 5 (11%) | 9 (20%) | 9 (20%) | 12 (27%) |
| Annual income <$10,830, n (%) | 55 (30%) | 9 (20%) | 13 (28%) | 18 (40%) | 16 (35%) |
| A1C (%), mmol/mol | 9.03 (1.70), 75 | 9.24 (1.69), 77 | 8.78 (1.65), 72 | 9.18 (1.68), 77 | 8.93 (1.81), 74 |
| SBP (mmHg) | 137.58 (23.22) | 134.34 (20.69) | 143.64 (24.46) | 136.01 (22.84) | 136.22 (24.29) |
| DBP (mmHg) | 95.09 (14.38) | 93.50 (13.71) | 98.11 (14.82) | 94.80 (15.04) | 93.92 (13.94) |
| HDL (mg/dL) | 53.69 (17.03) | 51.20 (13.88) | 54.41 (20.77) | 51.98 (15.04) | 57.04 (17.42) |
| LDL (mg/dL) | 105.54 (39.36) | 115.11 (54.19) | 97.83 (32.26) | 100.91 (28.56) | 108.33 (36.37) |
| Total Health Problem-Solving Scale | 19.99 (3.91) | 19.83 (3.24) | 19.97 (4.12) | 19.95 (4.22) | 20.21 (4.10) |
| Diabetes and CVD knowledge | 12.56 (2.47) | 12.89 (2.52) | 12.16 (2.47) | 12.42 (2.77) | 12.76 (2.08) |
| General diet (no. days) | 4.03 (2.02) | 3.99 (2.03) | 4.20 (1.77) | 4.19 (1.92) | 3.75 (2.34) |
| Specific diet (no. days) | 3.79 (1.84) | 3.50 (1.73) | 4.16 (1.93) | 4.00 (1.61) | 3.52 (2.02) |
| Exercise (no. days) | 3.38 (2.34) | 3.00 (2.50) | 3.03 (2.16) | 3.77 (2.37) | 3.67 (2.28) |
| Blood glucose testing (no. days) | 4.42 (2.70) | 4.61 (2.71) | 5.08 (2.34) | 3.91 (2.63) | 4.08 (2.99) |
| PHQ-2 | 1.44 (1.64) | 1.84 (1.81) | 1.22 (1.68) | 1.38 (1.47) | 1.30 (1.56) |
| PHQ-2 positive screen, n (%) | 41 (23%) | 14 (8%) | 7 (4%) | 10 (6%) | 10 (6%) |
| Insulin shot, n (%) | 111 (61%) | 29 (64%) | 28 (61%) | 27 (60%) | 27 (59%) |
| Diabetes pills, n (%) | 132 (73%) | 31 (69%) | 33 (72%) | 30 (67%) | 38 (83%) |
Data are mean (SD), except where indicated.
*Significantly different mean between Enhanced UC and Self-Study conditions at P < 0.05.
Intervention Participation Rates
Intervention participation rates are reported for the two intervention arms that necessitated in-person visits. For the DECIDE Group arm, 82% of participants attended more than five out of nine sessions and were thus deemed to have had adequate program exposure. Sixty-five percent attended seven or more sessions, and 30% attended all nine of the group sessions. In the DECIDE Individual arm, 66% attended more than five of the nine sessions, 54% attended seven or more sessions, and 23% of participants attended all nine sessions. There was no significant association between total number of sessions attended and change in A1C, problem-solving, or health knowledge for both the Individual and Group arms (data not shown).
Change in A1C
Change in A1C was analyzed for all participants (n = 182) and for participants with suboptimal baseline A1C (≥7.5% [58 mmol/mol], n = 142). There was no significant relationship between age, years of education, or baseline PHQ-2 score and change in A1C. Among all participants, A1C declined by 0.57% (P < 0.05) in the Group arm between baseline and postintervention, a greater reduction than Enhanced UC (b = −0.68, P < 0.05). There was no significant change in A1C for any other treatment arm at follow-up time points (data not shown). Among participants with A1C ≥7.5% (58 mmol/mol) at baseline, the DECIDE Self-Study, Individual, and Group arms had significant declines in A1C at postintervention (Table 2). Group had a greater reduction in A1C than Enhanced UC (b = −0.84, P < 0.05). DECIDE Self-Study also had a significant decline at the 6-month postintervention time point, and A1C did not increase significantly at 6 months in the Individual and Group arms compared with each group’s baseline. A1C did not improve significantly in Enhanced UC at either time point.
Table 2.
Changes in suboptimal clinical measures at the postintervention and 6-month post-intervention follow-up visits
| Enhanced UC |
DECIDE Self-Study |
DECIDE Individual |
DECIDE Group |
|||||
|---|---|---|---|---|---|---|---|---|
| Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | |
| A1C ≥7.5%, 58 mmol/mol | 0.06 (0.26), P = 0.81 | −0.03 (0.11), P = 0.75 | −0.57 (0.27), P < 0.05 | −0.24 (0.11), P < 0.05 | −0.55 (0.27), P < 0.05 | −0.10 (0.11), P = 0.37 | −0.78 (0.27), P < 0.01 | −0.09 (0.12), P = 0.42 |
| SBP >130 mmHg | −9.43 (3.77), P < 0.05 | −2.48 (1.30), P = 0.06 | −9.69 (3.47), P < 0.01 | −4.04 (1.20), P = 0.001 | −8.25 (3.56), P < 0.05 | −1.59 (1.31), P = 0.23 | −15.13 (3.83), P < 0.001 | −3.59 (1.34), P < 0.01 |
| DBP >80 mmHg | −4.66 (1.95), P < 0.05 | −1.47 (0.67), P < 0.05 | −2.86 (1.82), P = 0.12 | −1.37 (0.62), P < 0.05 | −3.61 (1.94), P = 0.07 | −1.22 (0.68), P = 0.08 | −8.17 (1.98), P < 0.001 | −2.20 (0.67), P < 0.01 |
| LDL >100 | −18.88 (7.08), P < 0.01 | −9.04 (2.28), P < 0.001 | −12.38 (7.49), P = 0.10 | −6.02 (2.59), P < 0.05 | −22.17 (7.71), P < 0.01 | −6.54 (2.52), P < 0.05 | 16.91(6.87), P < 0.05 | −4.23 (2.19), P = 0.06 |
| HDL <40 (men) or <50 (women) | 3.23 (1.94), P = 0.10 | 0.42 (0.74), P = 0.58 | 1.60 (1.81), P = 0.38 | 1.76 (0.74), P < 0.05 | −1.12 (2.10), P = 0.60 | −0.78 (0.84), P = 0.36 | 0.38 (2.48), P = 0.88 | −0.32 (1.20), P = 0.79 |
Results from linear mixed-effects models (baseline to postintervention and baseline to 6 months postintervention) adjusted for age, education, and PHQ-2 score. Data are presented as β coefficient (SE), P value.
Change in Blood Pressure and Lipids
Changes in blood pressure and lipids, among participants with suboptimal values at baseline, are also shown in Table 2. There were no significant differences between each DECIDE modality and Enhanced UC for change in SBP or DBP. Among participants with SBP >130 mmHg at baseline (n = 117), there was a significant reduction in SBP in the Self-Study and Group arms from baseline to 6 months postintervention. Similarly, among participants with DBP >80 mmHg (n = 157), there was a significant reduction in DBP at 6 months postintervention in the Self-Study, Group, and Enhanced UC arms.
There were no significant differences in lipids between DECIDE modalities and Enhanced UC at postintervention follow-ups. Among participants with LDL >100 mg (n = 94), Self-Study, Individual, and Enhanced UC arms had significant declines in LDL at 6 months postintervention. In patients with suboptimal HDL (n = 62) at baseline (<40 mg for men and <50 mg for women), the Self-Study arm showed significant increases in HDL from baseline to 6 months postintervention (b = 1.76, P < 0.05).
Change in Behavioral Outcomes
Table 3 shows change in health-related problem-solving, diabetes and CVD knowledge, and diabetes self-care activities. There was a significant increase in problem-solving across all treatment arms from baseline to 6 months postintervention. The PHQ-2 score moderated the relationship between treatment (for Self-Study vs. Enhanced UC) and change in health-related problem-solving (b = 0.88, P < 0.05). Specifically, at higher depression scores (above the mean) at baseline, Self-Study was more effective than Enhanced UC in increasing participants’ problem-solving from baseline to postintervention. This interaction did not significantly predict change in problem-solving from baseline to 6 months postintervention. Knowledge increased in all treatment arms from baseline to 6 months postintervention. The Self-Study (b = 0.33, P = 0.05) and Individual (b = 0.33, P = 0.05) arms increased in knowledge significantly more than Enhanced UC. With regard to number of days per week participants engaged in self-care behaviors, at postintervention and/or 6 months postintervention, diet behaviors (general diet and/or specific diet) increased in all treatment arms. Days per week engaging in at least 30 min of physical activity increased from baseline to 6 months postintervention only in the Self-Study arm. Participants in the Group arm and in the Individual arm showed an increase in the number of days per week they tested their blood glucose, at the postintervention and 6-month postintervention follow-up time points, respectively. Effect sizes (Cohen d) (7) for changes in behavioral variables in each treatment arm are presented in Supplementary Table 1.
Table 3.
Changes in behavioral outcomes at the postintervention and 6-month postintervention follow-up visits
| Enhanced UC |
DECIDE Self-Study |
DECIDE Individual |
DECIDE Group |
|||||
|---|---|---|---|---|---|---|---|---|
| Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | Postintervention | 6 months postintervention | |
| Health Problem-Solving Scale | 0.97 (0.45), P < 0.05 | 0.41 (0.14), P < 0.01 | 1.38 (0.45), P < 0.01 | 0.59 (0.14), P < 0.001 | 1.67 (0.46), P < 0.001 | 0.55 (0.14), P < 0.001 | 2.03 (0.45), P < 0.001 | 0.39 (0.14), P < 0.01 |
| Diabetes and CVD knowledge | 1.07 (0.36), P < 0.01 | 0.28 (0.12), P < 0.05 | 1.02 (0.36), P < 0.01 | 0.61 (0.11), P < 0.001 | 1.67 (0.37), P < 0.001 | 0.61 (0.12), P < 0.001 | 0.94 (0.36), P < 0.01 | 0.40 (0.12), P < 0.001 |
| General diet* | 1.02 (0.29), P < 0.001 | 0.20 (0.09), P < 0.05 | 0.57 (0.28), P < 0.05 | 0.15 (0.08), P = 0.07 | 0.54 (0.29), P = 0.07 | 0.22 (0.09), P < 0.05 | 1.44 (0.29), P < 0.001 | 0.32 (0.09), P < 0.001 |
| Specific diet* | 0.85 (0.29), P < 0.01 | 0.28 (0.09), P < 0.01 | 0.17 (0.29), P = 0.55 | 0.20 (0.09), P < 0.05 | 0.90 (0.30), P < 0.01 | 0.16 (0.10), P = 0.09 | 0.93 (0.29), P < 0.01 | 0.32 (0.09), P < 0.001 |
| Exercise* | 0.41 (0.38), P = 0.28 | 0.19 (0.12), P = 0.11 | 0.98 (0.38), P < 0.05 | 0.31 (0.12), P < 0.01 | −0.14 (0.39), P = 0.73 | 0.04 (0.12), P = 0.73 | 0.42 (0.38), P = 0.28 | −0.09 (0.12), P = 0.44 |
| Blood glucose testing* | 0.69 (0.35), P < 0.05 | 0.16 (0.12), P = 0.17 | 0.40 (0.34), P = 0.24 | 0.03 (0.12), P = 0.79 | 0.56 (0.35), P = 0.11 | 0.41 (0.32), P < 0.01 | 1.41 (0.35), P < 0.001 | 0.12 (0.12), P = 0.32 |
Results from linear mixed-effects models (baseline to postintervention and baseline to 6 months postintervention) adjusted for age, education, and PHQ-2 score. Data are presented as β coefficient (SE), P value.
*Subscales from the Summary of Diabetes Self-Care Activities Scale.
Change and Maintenance of Treatment Effects Based on Piecewise Linear Mixed-Effects Models
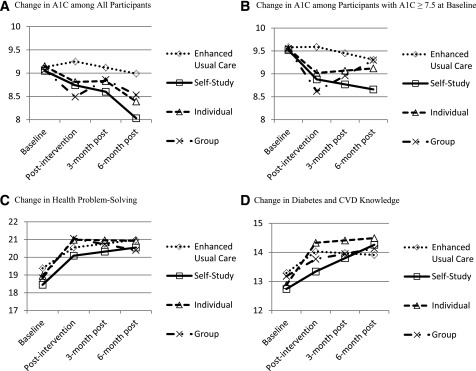
Figure 1 displays results from the piecewise linear mixed-effects models for A1C, health problem-solving, and diabetes and CVD knowledge. Change in A1C is presented for all participants and for participants with A1C ≥7.5% (58 mmol/mol). Participants in the Group arm showed a significant reduction in A1C at postintervention but a significant increase between postintervention and 6 months postintervention, irrespective of baseline A1C (Fig. 1A). Although A1C for the Self-Study arm is declining over time, this change was not significant (Fig. 1A). Among participants with A1C ≥7.5% (58 mmol/mol) at baseline (Fig. 1B), A1C for the Self-Study arm significantly declined from baseline to postintervention and continued to decline from postintervention to 6 months postintervention, but not statistically significantly. Both Individual and Group participants had a significant decline in A1C from baseline to postintervention, but a nonsignificant increase in A1C from postintervention to 6 months postintervention (Fig. 1B).
Figure 1.
A–D: Change in clinical and behavioral outcomes based on piecewise mixed-effects models. Each line is derived from a piecewise linear mixed-effects model with treatment arm dummy coded as a predictor and adjusting for age, years of education, and PHQ-2 score. The model consists of an intercept and two slopes (baseline to postintervention and postintervention to 6 months postintervention).
There was a significant increase in health problem-solving across all treatment arms from baseline to postintervention, which was sustained from postintervention to 6 months postintervention (Fig. 1C). Similarly, diabetes and CVD knowledge increased for each treatment arm initially and improvement was maintained. Knowledge continued to increase significantly for the Self-Study arm from postintervention to 6 months postintervention (Fig. 1D).
Conclusions
DECIDE was designed as a structured, behavioral diabetes self-management support program that uses problem-solving skills training to identify and address barriers to diabetes self-care in the context of everyday life. Findings reveal that the DECIDE training, in each delivery modality (Self-Study, Individual, and Group), increased participants’ health-related problem-solving ability and diabetes and CVD knowledge after intervention, and these skill improvements were sustained at long-term follow-up.
A1C improved in participants with suboptimal A1C (≥7.5%, 58 mmol/mol), between baseline and postintervention. Between postintervention and 6 months postintervention, with the exception of Group, there was no significant increase in A1C. The programs showed greater utility in people with poorer glycemic control. Improvements were found across DECIDE intervention arms in participants with suboptimal SBP and DBP. HDL improved in the Self-Study arm.
Of the DECIDE delivery modalities, the Self-Study modality demonstrated robust results across primary (A1C) and secondary (problem-solving, knowledge, SBP, DBP, and HDL) outcomes. This finding may indicate materials suitability for the educational and literacy levels in this community sample. The Individual modality appeared least robust of the DECIDE delivery modalities, both with regard to adoption (attendance rates were less than in the Group arm) and impact on primary and secondary outcomes. Prior literature has determined individual and group modalities of type 2 diabetes education to be comparable for knowledge and quality of life outcomes, with individual generally equal to usual care in A1C outcomes (23). However, the body of literature remains small, and there is not as yet a body of evidence comparing modalities for psychosocial-oriented interventions or outcomes in diabetes. To our knowledge, there are no prior studies examining comparisons with a self-directed treatment modality.
Participants in this study were healthier than our previous urban, African American study populations. Based on our previous trials (7,24), we anticipated a 65% eligibility rate among those screened for suboptimal disease control. However, of those screened for the current study, we observed a 48% eligibility rate, indicating better disease control in this community sample. In addition, the community sample had higher education and literacy than our prior, clinic-based sample. This could reflect the difference between community samples (seeking health information/intervention) and clinic patients identified from an administrative database. The Self-Study delivery modality, with DECIDE program adaptations for usability and understandability, proved viable in this community sample.
Although it has been recommended that all patients, irrespective of disease control status, may benefit from receipt of self-management support services and programs over the course of their chronic disease (1), the DECIDE program was more effective with patients in suboptimal glycemic and blood pressure control, demonstrating better utility with patients identified as being at higher risk for adverse diabetes outcomes. Longer-term studies are needed to detect whether improvements in the behavioral skills confer clinical benefit over longer duration in patients with controlled blood glucose and blood pressure at the time of the DECIDE self-management training.
In people with type 2 diabetes, the prevalence of comorbid depression is ∼18%, with a 24% prevalence in women with type 2 diabetes (25). Comorbid depression has been shown to be associated with poorer self-management behaviors and quality of life in type 2 diabetes (26). We used the PHQ-2 as a depression screening instrument and found that 23% of the study sample screened positive. Problem-solving intervention has a long history as an evidence-based therapeutic approach for depression and other mental health conditions (27,28). In the current study, higher depressive symptom scores at baseline in Self-Study were in fact associated with greater improvement in problem-solving after intervention, consistent with known benefits of this approach. With the exception of this finding, baseline PHQ-2 had no other impact on intervention outcomes.
The comparator condition, Enhanced UC, showed improvement in blood pressure over the study period. This is consistent with trends seen in other clinical trials with hypertensive African American participants. For example, Pavlik et al. (29) reported that, based on three National Institutes of Health (NIH)–funded cluster randomized trials of behavioral interventions for uncontrolled hypertensive African Americans (30–32), control groups are improving and can be expected to improve over the duration of a trial, due to health care initiatives in the current era of health care reform. Improvements in control conditions, as widespread, systematic interventions take place on chronic conditions, including hypertension and diabetes, have implications for researchers’ ability to detect the effectiveness of experimental study arms on outcomes that are targeted metrics in care delivery systems and surrounding communities. Interestingly, although A1C is also a targeted metric in current health care improvement initiatives, in the current study, the Enhanced UC condition did not demonstrate improvements in A1C in people in suboptimal glycemic control, whereas the DECIDE intervention arms did. Enhanced UC did show improvements in disease knowledge and problem-solving, which may be attributable to careful selection of publicly available diabetes and CVD educational materials that met many criteria for low literacy and patient engagement. The increased knowledge and problem-solving, however, were not accompanied by improved self-care behaviors or A1C.
One limitation of the study is the sample size. Although the achieved sample size of 180 provided adequate power for our primary outcome, a larger sample size would have conferred greater power for our secondary outcomes. While this study furthers available evidence of the DECIDE approach in an urban sample, there is also evidence of generalizability of this problem-solving intervention within populations including rural and underserved Hispanic, non-Hispanic white, and Native American people at risk for CVD (33).
A strength of this study is that it is among the first to compare various delivery modalities of a psychosocial intervention for diabetes self-management support. Moreover, the Self-Study modality, which emerged as the most effective of the treatment arms, is the lowest-resource delivery modality, requiring no intervention visits, allowing participants to complete the program in the home or community setting and in a self-directed manner. Ultimately, for dissemination of evidence-based interventions across clinical and community settings and populations, flexibility in efficacious modalities is optimal. It is recommended that future implementation work examine patterns of modality effectiveness across populations and settings in need of evidence-based, structured, approaches to patient self-management support.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants in this study and research personnel Angela Doswell, Kristina Schumann, Mary Stainback, Kristen Starks, Arianne Jennings, Dawna McGlynn, Nina Jackson-Goode, and Haseeb Majid.
Funding. This work was supported by National Heart, Lung, and Blood Institute grant R01HL089751 (to F.H-B.), National Institute of Diabetes and Digestive and Kidney Diseases Diabetes Research Center grant P30 DK079637, and NIH National Center for Research Resources grant M01RR000052 to the Johns Hopkins General Clinical Research Center.
Duality of Interest. F.H.-B. is a member of the ADA Board of Directors. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.L.F. researched data, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. S.H.G. and F.H.-B. researched data, contributed to discussion, and reviewed and edited the manuscript. K.S., J.S., S.D., T.B., N.-Y.W., and L.A.C. researched data and reviewed and edited the manuscript. J.A. contributed to discussion and reviewed and edited the manuscript. F.H.-B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of this research were presented orally at the 75th Scientific Sessions of the ADA, Boston, MA, 5–9 June 2015.
Footnotes
References
- 1.Pearson ML, Mattke S, Shaw R, Ridgely MS, Wiseman SH. Patient self-management support programs: an evaluation. Agency for Healthcare Research and Quality Web site. http://www.ahrq.gov/research/findings/final-reports/ptmgmt/index.html. Accessed 1 March 2016
- 2.Powers MA, Bardsley J, Cypress M, et al. . Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. J Acad Nutr Diet 2015;115:1323–1334 [DOI] [PubMed] [Google Scholar]
- 3.Golden SH, Brown A, Cauley JA, et al. . Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill-Briggs F. Problem solving in diabetes self-management: a model of chronic illness self-management behavior. Ann Behav Med 2003;25:182–193 [DOI] [PubMed] [Google Scholar]
- 5.Hill-Briggs F, Lazo M, Renosky R, Ewing C. Usability of a diabetes and cardiovascular disease education module in an African American, diabetic sample with physical, visual, and cognitive impairment. Rehabil Psychol 2008;53:1–8 [Google Scholar]
- 6.Hill-Briggs F, Renosky R, Lazo M, et al. . Development and pilot evaluation of literacy-adapted diabetes and CVD education in urban, diabetic African Americans. J Gen Intern Med 2008;23:1491–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill-Briggs F, Lazo M, Peyrot M, et al. . Effect of problem-solving-based diabetes self-management training on diabetes control in a low income patient sample. J Gen Intern Med 2011;26:972–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barr VJ, Robinson S, Marin-Link B, et al. . The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q 2003;7:73–82 [DOI] [PubMed] [Google Scholar]
- 9.Boren SA. AADE7TM self-care behaviors: Systematic reviews. Diabetes Educ 2007;33:866, 871 [DOI] [PubMed]
- 10.D’Zurilla T, Nezu A. Problem-Solving Therapy: A Positive Approach to Clinical Intervention. 3rd ed. New York, Springer Publishing Company, 2007 [Google Scholar]
- 11.Schumann K, Sutherland JA, Majid HM, Hill-Briggs F. Evidence-based behavioral treatments for diabetes: problem-solving therapy. Diabetes Spectr 2011;24:64–69 [Google Scholar]
- 12.American Diabetes Association Standards of Medical Care in Diabetes--2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill-Briggs F, Schumann KP, Dike O. Five-step methodology for evaluation and adaptation of print patient health information to meet the < 5th grade readability criterion. Med Care 2012;50:294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Scientific and Technical Information Simply Put. 2nd ed. Atlanta, GA, Office of Communication, Centers for Disease Control and Prevention, 1999 [Google Scholar]
- 15.Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA, J.B. Lippincott Company, 1996 [Google Scholar]
- 16.National Cancer Institute Clear and Simple: Developing Effective Print Materials for Low-Literate Readers. Bethesda, MD, U.S. Department of Health and Human Services, 1994 [Google Scholar]
- 17.Hill-Briggs F, Smith AS. Evaluation of diabetes and cardiovascular disease print patient education materials for use with low-health literate populations. Diabetes Care 2008;31:667–671 [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson GS. The Wide Range Achievement Test: Manual. 3rd ed. Wilmington, DE, Wide Range, 1993 [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284–1292 [DOI] [PubMed] [Google Scholar]
- 20.Hill-Briggs F, Gemmell L, Kulkarni B, Klick B, Brancati FL. Associations of patient health-related problem solving with disease control, emergency department visits, and hospitalizations in HIV and diabetes clinic samples. J Gen Intern Med 2007;22:649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick SL, Hill-Briggs F. Measuring health-related problem solving among African Americans with multiple chronic conditions: application of Rasch analysis. J Behav Med 2015;38:787–797 [DOI] [PubMed] [Google Scholar]
- 22.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 23.Duke S-AS, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database of Syst Rev 2009;Jan 21:CD005268 [DOI] [PMC free article] [PubMed]
- 24.Gary TL, Batts-Turner M, Yeh HC, et al. . The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2009;169:1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165–1173 [DOI] [PubMed] [Google Scholar]
- 26.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry 2003;25:246–252 [DOI] [PubMed] [Google Scholar]
- 27.Bell AC, D’Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Rev 2009;29:348–353 [DOI] [PubMed] [Google Scholar]
- 28.Malouff JM, Thorsteinsson EB, Schutte NS. The efficacy of problem solving therapy in reducing mental and physical health problems: a meta-analysis. Clin Psychol Rev 2007;27:46–57 [DOI] [PubMed] [Google Scholar]
- 29.Pavlik VN, Chan W, Hyman DJ, et al. . Designing and evaluating health systems level hypertension control interventions for African-Americans: lessons from a pooled analysis of three cluster randomized trials. Curr Hypertens Rev 2015;11:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pezzin LE, Feldman PH, Mongoven JM, McDonald MV, Gerber LM, Peng TR. Improving blood pressure control: results of home-based post-acute care interventions. J Gen Intern Med 2011;26:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyman DJ, Pavlik VN, Greisinger AJ, et al. . Effect of a physician uncertainty reduction intervention on blood pressure in uncontrolled hypertensives--a cluster randomized trial. J Gen Intern Med 2012;27:413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogedegbe G, Tobin JN, Fernandez S, et al. . Counseling African Americans to control hypertension: cluster-randomized clinical trial main effects. Circulation 2014;129:2044–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lilly CL, Bryant LL, Leary JM, et al.; MSHA . Evaluation of the effectiveness of a problem-solving intervention addressing barriers to cardiovascular disease prevention behaviors in 3 underserved populations: Colorado, North Carolina, West Virginia, 2009. Prev Chronic Dis 2014;11:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.