Introduction
Complex environmental, social, behavioral, and emotional factors, known as psychosocial factors, influence living with diabetes, both type 1 and type 2, and achieving satisfactory medical outcomes and psychological well-being. Thus, individuals with diabetes and their families are challenged with complex, multifaceted issues when integrating diabetes care into daily life. To promote optimal medical outcomes and psychological well-being, patient-centered care is essential, defined as “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions” (1). Practicing personalized, patient-centered psychosocial care requires that communications and interactions, problem identification, psychosocial screening, diagnostic evaluation, and intervention services take into account the context of the person with diabetes (PWD) and the values and preferences of the PWD.
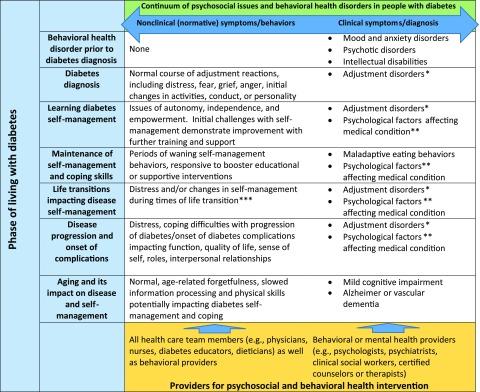
This article provides diabetes care providers with evidence-based guidelines for psychosocial assessment and care of PWD and their families. Recommendations are based on commonly used clinical models, expert consensus, and tested interventions, taking into account available resources, practice patterns, and practitioner burden. Consideration of life span and disease course factors (Fig. 1) is critical in the psychosocial care of PWD. This Position Statement focuses on the most common psychological factors affecting PWD, including diabetes distress and psychological comorbidities, while also considering the needs of special populations and the context of care.
Figure 1.
Psychosocial care for PWD: life and disease course perspectives. *With depressed mood, anxiety, or emotion and conduct disturbance. **Personality traits, coping style, maladaptive health behaviors, or stress-related physiological response. ***Examples include changing schools, moving, job/occupational changes, marriage or divorce, or experiencing loss.
GENERAL CONSIDERATIONS IN PSYCHOSOCIAL CARE
Recommendations
Psychosocial care should be integrated with collaborative, patient-centered medical care and provided to all people with diabetes, with the goals of optimizing health outcomes and health-related quality of life. A
Providers should consider an assessment of symptoms of diabetes distress, depression, anxiety, and disordered eating and of cognitive capacities using patient-appropriate standardized/validated tools at the initial visit, at periodic intervals, and when there is a change in disease, treatment, or life circumstance. Including caregivers and family members in this assessment is recommended. B
Consider monitoring patient performance of self-management behaviors as well as psychosocial factors impacting the person’s self-management. E
Consider assessment of life circumstances that can affect physical and psychological health outcomes and their incorporation into intervention strategies. E
Addressing psychosocial problems upon identification is recommended. If an intervention cannot be initiated during the visit when the problem is identified, a follow-up visit or referral to a qualified behavioral health care provider may be scheduled during that visit. E
Practitioners should identify behavioral/mental health providers, ideally those who are knowledgeable about diabetes treatment and the psychosocial aspects of diabetes, with whom they can form alliances and use for referrals (Table 1) in the psychosocial care of PWD. Ideally, psychosocial care providers should be embedded in diabetes care settings. Shared resources such as electronic health records, management data, and patient-reported information regarding adjustment to illness and life course issues facilitate providers’ capacity to identify and remediate psychosocial issues that impede regimen implementation and improve diabetes management and well-being. Care models that take into account cultural influences, as well as personal, family, and community resources, and tailor care to the core values and lifestyle of the individual are more likely to be successful (2). Regardless of how the diabetes care team is constituted, the PWD is central to the care process. If a PWD cannot act on behalf of him/herself in the care process, a support person needs to be identified to participate in treatment decisions and facilitate disease management. It is also important that providers enlist members of the patient’s social support network to aid in the identification, prevention, and resolution of psychosocial problems.
Table 1.
Situations that warrant referral of a person with diabetes to a mental health provider for evaluation and treatment
| • If self-care remains impaired in a person with diabetes distress after tailored diabetes education |
| • If a person has a positive screen on a validated screening tool for depressive symptoms |
| • In the presence of symptoms or suspicions of disordered eating behavior, an eating disorder, or disrupted patterns of eating |
| • If intentional omission of insulin or oral medication to cause weight loss is identified |
| • If a person has a positive screen for anxiety or FoH |
| • If a serious mental illness is suspected |
| • In youth and families with behavioral self-care difficulties, repeated hospitalizations for diabetic ketoacidosis, or significant distress |
| • If a person screens positive for cognitive impairment |
| • Declining or impaired ability to perform diabetes self-care behaviors |
| • Before undergoing bariatric surgery and after if assessment reveals an ongoing need for adjustment support |
Medical management of diabetes requires patient implementation of a treatment regimen. Thus, psychosocial factors impacting self-care such as diabetes distress (burdens of diabetes and its treatment, worries about adverse consequences), lack of social and economic resources, and other psychological states (e.g., depression, anxiety, eating disorders, cognitive impairment) (3), as well as health literacy and numeracy, should be monitored. To detect problems early and prevent health deterioration, all PWD should be evaluated at the initial visit and on a periodic basis going forward even if there is no patient specific indication (4). In addition, evaluation is indicated during major disease and life transitions, including the onset of complications and significant changes in treatment (i.e., initiation of insulin pump or other forms of intensification) or life circumstances (i.e., living arrangements, job, and significant social relationships), with prospective monitoring for 6 months (a period of increased risk) (5).
All care providers should include queries about well-being in routine care. Standardized and validated tools (Table 2) for psychosocial monitoring, assessment, and diagnosis can be used by providers in a stepped sequence with positive findings leading to further evaluation, starting with informal verbal inquiries for monitoring followed by questionnaires for assessment (e.g., PHQ-9) and finally by structured interviews for diagnosis (e.g., Structured Clinical Interview for the DSM-V). For example, the diabetes care provider can ask whether there have been changes in mood during the past 2 weeks or since their last visit. Further, providers should consider asking whether there are new or different barriers to treatment and self-management, such as feeling overwhelmed or stressed by diabetes or other life stressors. Positive responses can be probed with additional questions and/or use of standardized measures to inform assessment and guide the selection of appropriate interventions.
Table 2.
Selected measures for the evaluation of psychosocial constructs in the clinical setting
| Topic area | Measure title | Citations | Description | Validated population |
|---|---|---|---|---|
| Diabetes-related distress | Problem Areas in Diabetes (PAID) | Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 | 20-item measure of diabetes-specific distress measuring emotional distress and burden associated with diabetes | Adults with type 1 and type 2 diabetes |
| Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas in Diabetes (PAID) questionnaire. Diabet Med 2003;20:69–72 | ||||
| Diabetes Distress Scale (DDS) | Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care 2005;28:626–631 | 17-item questionnaire measuring diabetes-specific distress in four domains: emotional burden, diabetes interpersonal distress, physician-related distress, and regimen-related distress | Adults with type 1 and type 2 diabetes | |
| Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–64 (39) | ||||
| PAID–Pediatric Version (PAID-Peds) | Markowitz JT, Volkening LK, Butler DA, Laffel LM. Youth-perceived burden of type 1 diabetes: Problem Areas in Diabetes Survey-Pediatric Version (PAID-Peds). J Diabetes Sci Technol 2015;9:1080–1085 | 20-item measure of diabetes burden | Youth (ages 8–17 years) with type 1 diabetes | |
| PAID–Teen Version | Weissberg-Benchell J, Antisdel-Lomaglio, J. Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes 2011;12:341–344 | 26-item questionnaire measuring perceived burden of diabetes | Adolescents (ages 11–19 years) with diabetes | |
| PAID–Parent Revised version (PAID-PR) | Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio JH, Anderson BJ, Laffel LM. Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey–Parent Revised version (PAID-PR). Diabet Med 2012;29:526–530 | 18-item questionnaire assessing perceived parental burden of diabetes | Parents of children and adolescents (ages 8–18 years) with type 1 diabetes | |
| Depression | Patient Health Questionnaire (PHQ-9) | Spitzer RL, Williams JB, Kroenke K, et al. Utility of new procedure for diagnosis mental-disorders in primary-care: the PRIME-MD 1000 Study. JAMA 1994;272:1749–1756 | 9-item measure of depressive symptoms (corresponding to criteria for major depressive disorder) | Adults |
| Beck Depression Inventory–II (BDI-II) | Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II, 2nd ed. San Antonio, TX, Harcourt, Brace & Company, 1996 | 21-item questionnaire evaluating somatic and cognitive symptoms of depression | Adults | |
| Child Depression Inventory (CDI) (current edition is CDI-2) | Kovacs, M. The Children’s Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY, Multi-Health Systems, 2003 | 27-item measure assessing depressive symptoms using child and parent report | Youth (ages 7–17 years) | |
| Geriatric Depression Scale (GDS) | Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist 1986;5:165–172 | 15-item measure was developed to assess depression in older adults | Adults (ages 55–85 years) | |
| Eating disorders | Eating Disorders Inventory–3 (EDI-3) | Garner DM. Eating Disorder Inventory-3: Professional Manual. Odessa, FL, Psychological Assessment Resources, 2004 | 2 interview and self-report surveys aimed at the measurement of psychological traits or symptom clusters relevant to the development and maintenance of eating disorders | Females (ages 13–53 years) |
| Diabetes Eating Problems Survey (DEPS-R) | Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care 2010;33:495–500 | 16-item self-report measure designed to assess diabetes-specific eating issues | Youth (ages 13–19 years) with type 1 diabetes | |
| Diabetes Treatment and Satiety Scale (DTSS-20) | Young-Hyman D, Davis C, Grigsby C, Looney S, Peterson C. Development of the Diabetes Treatment and Satiety Scale: DTSS-20 (Abstract). Diabetes 2011;60(Suppl. 1):A218 | 20-item self-report measure that assesses perception of satiety and fullness in the context of food intake, physical activity, medication dosing, and glycemic levels | Youth (ages 10–17 years) with type 1 diabetes | |
| Health literacy and numeracy | General Health Numeracy Test (GHNT) | Osborn CY, Wallston KA, Shpigel A, Cavanaugh K, Kripalani S, Rothman RL. Development and validation of the General Health Numeracy Test (GHNT). Patient Educ Couns 2013;91:350–356 | 21-item self-report questionnaire designed to assess patient level of understanding of the use of numbers in medications and health | Adults |
| Diabetes Numeracy Test (DNT) | Huizinga MM, Elasy TA, Wallston KA, et al. Development and validation of the Diabetes Numeracy Test (DNT). BMC Health Ser Res 2008;1:96 | 5-, 15-, and 43-item word problem–based test to assess understanding of tables, graphs, and figures specific to the management of diabetes | Adults (ages 18–80 years) | |
| Brief Health Literacy Scale (BHLS) | Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric properties of the Brief Health Literacy Screen in clinical practice. J Gen Intern Med 2014;29:119–126 | 3-item measure read aloud to patients in an outpatient and emergency department setting to assess understanding of health concepts | Adults | |
| Self-care efficacy | Diabetes self-efficacy | Ritter PL, Lorig K, Laurent D. Characteristics of the Spanish- and English-language self-efficacy to manage diabetes scales. Diabetes Educ 2016;42:167–177 | 8-item self-report scale designed to assess confidence in performing diabetes self-care activities | Adults |
| Self-efficacy for diabetes management | Iannotti RJ, Schneider S, Nansel TR, et al. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. J Dev Behav Pediatr 2006;27:98–105 (26) | 10-item self-report self-efficacy scale | Adolescents (ages 10–16 years) with type 1 diabetes | |
| Anxiety | State-Trait Anxiety Inventory for Children (STAIC) | Spielberger CD, Edwards CD, Lushene R, Monturi J, Plotzek D. State-Trait Anxiety Inventory for Children Professional Manual. Menlo Park, CA, Mind Garden, Inc., 1973 | 40 items on two dimensions—trait and state anxiety | Youth with and without type 1 diabetes |
| Beck Anxiety Inventory (BAI) | Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX, The Psychological Corporation, 1993 | 21 items assessing self-reported anxiety | Adults | |
| Hypoglycemia Fear Survey-II (HFS-II) | Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 (63) | 33 items assessing behavioral and worry dimensions of hypoglycemia in adults | Adults with type 1 diabetes | |
| Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care 2011;34:801–806 (71) | ||||
| Children’s Hypoglycemia Index (CHI) | Kamps JL, Roberts MC, Varela RE. Development of a new fear of hypoglycemia scale: preliminary results. J Pediatr Psychol 2005;30:287–291 | Designed to assess FoH (25 items) | Youth (ages 8–16 years) with type 1 diabetes | |
| Cognitive screening in older adults | Mini-Mental State Examination (MMSE) | Folstein MF, Folstein SE, McHugh PR. “Mini-mental” state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 | 11-item (30-point) screen for cognitive impairment in adults | Adults (ages 18 – 100 years) |
| Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–2391 | ||||
| Telephone Interview for Cognitive Status (TICS) | Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol 1988;1:111–117 | 11-item measure assessing cognitive status by telephone | Adults (ages 60–98 years) | |
| Brandt J, Folstein MF. Telephone Interview for Cognitive Status (TICS) Professional Manual. Lutz, FL, Psychological Assessment Resources, 2003 | ||||
| Cognitive assessment toolkit | Cordell CB, Borson S, Boustani M, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement 2013;9:141–150 | Designed for use during a medical office visit to screen for cognitive impairment in older adults (includes informant interviews also) | Adults | |
| Chronic pain | Short-form McGill Pain Questionnaire (SF-MPQ-2) | Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009;144:35–42 | 22-item questionnaire designed to assess pain | Adults |
| Adherence to self-care | Summary of Diabetes Self-Care Activities (SDSCA) | Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 | 11-item and expanded 25-item measure of diabetes self-care behaviors | Adults with type 1 and type 2 diabetes |
| Adherence to Refills and Medications Scale (ARMS-D) | Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health 2009;12:118–123 | 11-item self-report questionnaire designed to assess the extent to which patients take and refill their diabetes-related medications | Adults | |
| Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D outperforms the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract 2013;102:96–104 | ||||
| Barriers to diabetes adherence | Mulvaney SA, Hood KK, Schlundt DG, et al. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract 2011;94:77–83 | 21-item self-report questionnaire designed to assess barriers to diabetes self-care behaviors | Adolescents (ages 12–17 years) with diabetes |
When referral is warranted (Table 1), formal diagnostic assessments and interviews should be conducted by a qualified behavioral health provider familiar with the care of PWD. Standardized, age- and literacy-appropriate assessment and diagnostic tools should be used (Table 2). These established measures were selected from a wider literature on the basis of the scientific rigor used in their development and the availability of norms for clinical use. The recommendation of specific measures for clinical use is beyond the scope of this statement. Care providers should implement interventions to address the day-to-day problems of living with diabetes, particularly diabetes-related distress related to self-management behaviors, as well as diabetes-related family conflict (6–8). Support from a behavioral health provider may be effective when difficulties are persistent. However, as soon as there is indication of a diagnosable psychological condition, consultation and/or referral should be sought with a provider having the appropriate mental health expertise. Standardized/validated intervention strategies specific to PWD should be utilized whenever possible.
PSYCHOSOCIAL ISSUES IMPACTING DIABETES SELF-MANAGEMENT
Recommendations
People with diabetes should be evaluated and receive training until they attain competence in diabetes self-care skills and the use of technologies at the time of diagnosis, annually, if/when complications arise, and if/when transitions in care occur. The diabetes care team is encouraged to directly and regularly assess these self-management behaviors. B
Providers should consider the burden of treatment and patient levels of confidence/self-efficacy for management behaviors as well as level of social and family support when making treatment recommendations. E
While following treatment regimens consistently improves A1C (9–12), the impact is modest. Multiple factors other than patient behavior affect diabetes treatment outcomes, including adequacy of medical management, duration of diabetes, weight gain, and other health-related (e.g., comorbid illness and concomitant medication) and social-structural factors (e.g., poverty, access to care, health insurance coverage) (13–16). Therefore, it is not appropriate to automatically attribute suboptimal A1C and adverse events such as hypoglycemia (17) solely to self-management behaviors without their direct assessment.
Provider communications with patients/families should acknowledge that multiple factors impact glycemic management but also emphasize that following collaboratively developed treatment regimens and recommended lifestyle changes can significantly improve disease outcomes and well-being (14,18–20). Thus, the goal of provider–patient communication should be to empower the PWD without blaming them for “noncompliance” when the outcomes of self-management are not optimal.
The familiar term, noncompliance, denotes a passive, obedient role for PWD in “following doctor’s orders” that is at odds with the active role PWD are asked to take in directing the day-to-day planning, monitoring, evaluation, and problem-solving involved in diabetes self-management (21). Patient perceptions about their own ability, or self-efficacy, to self-manage diabetes are one important psychosocial factor related to improved diabetes self-management and treatment outcomes in diabetes (22–26) and should be a target of ongoing assessment and treatment planning.
Suboptimal self-management may be due to functional limitations (e.g., blindness, problems with dexterity, low health literacy and numeracy), lack of appropriate diabetes education, forgetting and disruption in routines, or psychosocial barriers, such as inadequate family and/or social support, misinformation or inaccurate beliefs about illness and treatment, emotional distress/depressive symptoms, or deficits in problem-solving or coping skills (23,27–30). Therefore, individual needs should be evaluated so that interventions can be tailored to the problem (31–35). Self-report measures are available and can be used in most practice settings (see Table 2). Using a nonjudgmental approach that normalizes periodic lapses in self-management may help minimize patients’ resistance to reporting problems with self-management.
Making healthy food choices on a daily basis is among the most difficult aspects of diabetes self-care (36). Current medical nutrition therapy guidelines promote flexible and healthy eating patterns personalized to the individual rather than defining a wide range of behaviors as dietary “nonadherence” (37). Self-monitoring of food intake may help the individual with diabetes become more aware of their own eating patterns while providing information that helps the registered dietitian nutritionist assist with meal planning and develop personalized dietary recommendations. Through monitoring, it is important to assess for disordered eating behaviors (see disordered eating behavior: clinical and subclinical).
DIABETES DISTRESS
Recommendation
Routinely monitor people with diabetes for diabetes distress particularly when treatment targets are not met and/or at the onset of diabetes complications. B
Diabetes distress is very common and is distinct from a psychological disorder (38–40). The constant behavioral demands (medication dosing, frequency, and titration; monitoring blood glucose, food intake and eating patterns, and physical activity) of diabetes self-management and the potential or actuality of disease progression are directly associated with reports of diabetes distress (39). Its prevalence is reported to be 18–45% with an incidence of 38–48% over 18 months (41). High levels of diabetes distress significantly impact medication-taking behaviors and are linked to higher A1C, lower self-efficacy, and poorer dietary and exercise behaviors (39,41,42). It may be helpful to provide counseling regarding expected diabetes-related versus generalized psychological distress at diagnosis and when disease state or treatment changes (43).
About one-third of adolescents with diabetes develop diabetes distress, which may be associated with declines in self-management behaviors and suboptimal blood glucose levels (44). Parents of children with type 1 diabetes are also prone to diabetes distress (45), which could impact their ability to provide psychological and diabetes management support for their child.
Diabetes distress should be routinely monitored (46) using patient-appropriate validated measures (Table 2). If diabetes distress is identified, the person should be referred for diabetes education to address areas of diabetes self-care that are most relevant to the patient and have the most impact on diabetes outcomes. People whose self-care remains impaired after tailored diabetes education should be referred by their care team to a behavioral health provider for evaluation and treatment.
PSYCHOLOGICAL COMORBIDITIES
Prevalence of clinically significant psychopathology in PWD ranges across diagnostic categories, and some diagnoses are considerably more common in PWD than in those without the disease (47–52). Symptoms, both clinical and subclinical, that interfere with the person’s ability to carry out diabetes self-management must be addressed.
Depression
Recommendations
Providers should consider annually screening all patients with diabetes and/or a self-reported history of depression for depressive symptoms with age-appropriate depression screening measures, recognizing that further evaluation will be necessary for individuals who have a positive screen. B
Beginning at diagnosis of complications or when there are significant changes in medical status, consider assessment for depression. B
Referrals for treatment of depression should be made to mental health providers with experience using cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches in conjunction with collaborative care with the patient’s diabetes treatment team. A
History of depression, current depression, and antidepressant medication use are risk factors for the development of type 2 diabetes, especially if the individual has other risk factors such as obesity and family history of type 2 diabetes (53–55).
Elevated depressive symptoms and depressive disorders affect one in four patients with type 1 or type 2 diabetes (47). Thus, routine screening for depressive symptoms is indicated in this high-risk population including people with prediabetes (particularly those who are overweight), type 1 and/or type 2 diabetes, gestational diabetes mellitus, and postpartum diabetes. Regardless of diabetes type, women have significantly higher rates of depression than men (56).
Routine monitoring with patient-appropriate validated measures (Table 2) can help to identify whether referral is warranted. Remission of depressive symptoms or disorder in adult patients suggests the need for ongoing monitoring of depression recurrence within the context of routine care (53).
Integrating mental and physical health care can improve outcomes. The mental health provider should be incorporated into the diabetes treatment team when a patient is in psychological therapy (talk therapy) (57). Incorporation of a physical activity regimen into routine self-management has also been shown to improve the health and mental well-being of PWD (58,59). Please refer to the Position Statement of the American Diabetes Association (ADA) on physical activity/exercise and diabetes (60) for additional information.
Anxiety Disorders
Recommendations
Consider screening for anxiety in people exhibiting anxiety or worries regarding diabetes complications, insulin injections or infusion, taking medications, and/or hypoglycemia that interfere with self-management behaviors and in those who express fear, dread, or irrational thoughts and/or show anxiety symptoms such as avoidance behaviors, excessive repetitive behaviors, or social withdrawal. Refer for treatment if anxiety is present. B
People with hypoglycemia unawareness, which can co-occur with fear of hypoglycemia, should be treated using Blood Glucose Awareness Training (or other evidence-based similar intervention) to help re-establish awareness of hypoglycemia and reduce fear of hypoglycemia. A
Anxiety symptoms and diagnosable disorders (e.g., generalized anxiety disorder [GAD], body dysmorphic disorder, obsessive compulsive disorder [OCD], specific phobias, and posttraumatic stress disorder [PTSD]) are common in PWD (61); the Behavioral Risk Factor Surveillance System estimated the lifetime prevalence of GAD to be 19.5% in people with either type 1 or type 2 diabetes (62). Common diabetes-specific concerns include fears related to hyperglycemia (63,64), not meeting blood glucose targets (61), and insulin injections or infusion (65). General anxiety is a predictor of injection-related anxiety and associated with fear of hypoglycemia (FoH) (64,66).
Preoccupation with an imagined defect in appearance associated with having diabetes that interferes with social, occupational, or other important areas of function may reflect body dysmorphic disorder (51). When ideas and symptoms (e.g., perceived deficits in strength, attractiveness, and sexual function) do not reach the level of clinical diagnosis, identification of these beliefs provides a context for provider education about disease processes, reframing disease processes as distinct from the emotional response to having diabetes and questioning the inevitability of health decline. Onset of complications presents another critical point where these thoughts/beliefs can occur and may require re-education and disease-based counseling (67).
If the PWD exhibits excessive diabetes self-management behaviors well beyond what is prescribed or needed to achieve glycemic targets, reports repetitive negative thoughts about inability to prevent poor health outcomes, and/or has related thoughts and behaviors that interfere with other functions of daily living, the PWD may be experiencing symptoms of OCD (68). OCD symptoms can represent generalized anxiety or be diabetes specific, and referral to a mental health professional (such as a psychiatrist) familiar with OCD treatment should be considered, especially if diabetes re-education does not prove effective in reducing obsessive thoughts, behaviors, or feelings of general anxiety. Caution should be exercised in diagnosing OCD-like symptoms, as regimen behaviors contain similar characteristics, such as repetition, and are aimed at achieving control over a perceived threat.
FoH and hypoglycemia unawareness often co-occur, and interventions aimed at treating one often benefit both (69). FoH may explain avoidance of behaviors associated with lowering glucose such as increasing insulin doses or frequency of monitoring. If FoH is identified and a person does not have symptoms of hypoglycemia, a structured program, Blood Glucose Awareness Training, delivered in routine clinical practice can improve A1C, reduce the rate of severe hypoglycemia, and restore hypoglycemia awareness (70,71). Such improvements in disease state have been shown to reduce diabetes distress and improve psychological well-being (69,72,73).
Occurrence of severe hypoglycemia has been shown to be associated with PTSD and PTSD-like and panic disorder symptoms (74,75). The potential for increased prevalence of PTSD and panic disorder in this population, though not well studied, is intuitive given the potentially life-threatening nature of the disease, particularly for those who use exogenous insulin. Given that potential stimuli for PTSD-like symptoms are recurrent for PWD, PTSD should be considered among other anxiety disorders.
Disordered Eating Behavior: Clinical and Subclinical
Recommendations
Providers should consider re-evaluating the treatment regimen of people with diabetes who present with symptoms of disordered eating behavior, an eating disorder, or disrupted patterns of eating. B
Consider screening for disordered or disrupted eating using validated screening measures when hyperglycemia and weight loss are unexplained by self-reported behaviors related to medication dosing, meal plan, and physical activity. In addition, a review of the medical regimen is recommended to identify potential treatment-related effects on hunger/caloric intake. B
Estimated prevalence of disordered eating behaviors and diagnosable eating disorders in PWD varies (51,76,77). PWD with diagnosable eating disorders have high rates of comorbid psychiatric disorders (78). People with type 1 diabetes and eating disorders have high rates of diabetes distress and FoH (79). For people with type 1 diabetes, insulin omission causing glycosuria in order to lose weight is the most commonly reported disordered eating behavior (80,81), and in people with type 2 diabetes, bingeing (excessive food intake with an accompanying sense of loss of control) is most commonly reported. For people with type 2 diabetes treated with insulin, intentional omission is also frequently reported (82). Binge eating disorder has been found to be more likely in PWD than in the nondiabetes population, though studies of prevalence in specific diabetes samples show varying rates (77,83). Other diagnostic categories of eating disorders have a very low prevalence in PWD (77).
Potential confounders to the identification of symptoms of disordered eating are behaviors that are prescribed as part of treatment (e.g., carbohydrate counting and calorie restriction), behaviors or effects that are part of the disease (e.g., loss of control over satiety regulation secondary to disease processes), and adverse effects of treatment such as excessive hunger secondary to hypoglycemia. When evaluating symptoms of disordered or disrupted eating in PWD, etiology and motivation for the behavior should be considered (51,84). For example, missed insulin injections as a result of suboptimal self-management differ significantly from intentional medication omission to produce weight loss. Assessment and screening of disordered and disrupted eating requires methods that account for treatment prescription, regimen behaviors, and diabetes-specific eating problems attributable to disease processes (see Table 2). If night eating syndrome, which is recurrent eating at night either after awakening from sleep or excessive eating after dinner, is diagnosed, changes to the medication regimen are required until maladaptive eating patterns can be modified (85). Adjunctive medication such as glucagon-like peptide 1 receptor agonists (86) may not only help individuals meet glycemic targets but also help regulate hunger and food intake, thus having the potential to reduce uncontrollable hunger and bulimic symptoms.
Serious Mental Illness
Recommendations
Annually screen people who are prescribed atypical antipsychotic medications for prediabetes/diabetes. B
Incorporate monitoring of diabetes self-care activities into treatment goals in people with diabetes and serious mental illness. B
Studies of people with serious mental illness, particularly schizophrenia and other thought disorders, show significantly increased rates of type 2 diabetes (87). People with schizophrenia should be monitored for type 2 diabetes because of the known comorbidity. Disordered thinking and judgment can be expected to make it difficult to engage in behaviors that reduce risk factors for type 2 diabetes, such as restrained eating for weight management. Individuals with major psychiatric disorders may need consistent monitoring and oversight in their diabetes management, even if thought disorders remit. Coordinated management of diabetes or prediabetes and serious mental illness is recommended to achieve diabetes treatment targets. In addition, those taking olanzapine require greater monitoring because of an increase in risk of type 2 diabetes associated with this medication (88). Further study is needed to examine the association of other antipsychotic medications with the onset of diabetes and glycemic management (48,89).
LIFE COURSE CONSIDERATIONS
PWD are diagnosed earlier (e.g., type 2 diabetes in childhood) (90) and living longer (48). At each point in the life course, providers should consider which resources and accommodations are needed to maximize disease outcomes and well-being. In particular, identification of psychosocial factors influencing self-management are recommended (e.g., culture, environment, social determinants, life roles and responsibilities, and interpersonal dynamics, as well as person-based characteristics such as sex, race/ethnicity, age, language, and socioeconomic status) (91).
Youth and Emerging Adults
Recommendations
At diagnosis and during routine follow-up care, consider assessing psychosocial issues and family stresses that could impact diabetes management and provide appropriate referrals to trained mental health professionals, preferably experienced in childhood diabetes. E
Providers should consider monitoring youth and their parents about social adjustment (peer relationships) and school performance to determine whether further evaluation is needed. B
Consider assessing youth with diabetes for generic and diabetes-related distress starting at about 7–8 years of age. B
Providers should encourage developmentally appropriate family involvement in diabetes management tasks for children and adolescents, recognizing that premature transfer of diabetes care to the child can result in poor self-management behaviors and deterioration in glycemic management. A
Consider the inclusion of children in consent processes as early as cognitive development indicates understanding of health consequences of behavior. E
Adolescents may have time by themselves with their care provider(s) starting at age 12 years. E
Providers should consider initiating discussions about care transition to an adolescent medicine/transition clinic/adult provider no later than 1 year prior to starting the transfer but preferably during early adolescence (∼14 years of age). E
Consider monitoring support from parents/caretakers of emerging adults with diabetes and encouraging instrumental support (e.g., ordering supplies) and collaborative decision making among caregivers. E
Starting at puberty, preconception counseling should be incorporated into routine diabetes clinic visits for all females of childbearing potential. A
Consider counseling males, starting at puberty, regarding adoption of a healthy lifestyle to reduce risk for sexual dysfunction. E
Given the rapid and dynamic nature of cognitive, developmental, and emotional changes in youth, early detection of depression, anxiety disorders, eating issues, and learning disabilities enhance the range and effectiveness of potential treatment options and may help to minimize adverse effects on diabetes management and disease outcomes.
Because youth are dependent on social support systems (family and care providers) and must eventually transition to independent diabetes self-management, their families and related social networks need to be included in psychosocial assessment and treatment (92–94). Parents of children with type 1 diabetes are prone to high rates of depression, especially around the time of diagnosis (95,45). Persistence of parental depression is associated with poorer child adjustment and diabetes management, especially in younger children (96). Teaching family members effective problem-solving and conflict-resolution skills can improve diabetes management and facilitate better glycemic management, with the potential to reduce diabetes distress (92).
The adolescent years are known for disruption in diabetes care and communication between family members, youth, and providers. Hallmarks of normal adolescence are increased independence in decision making and turning to the peer group for validation of self-concept and self-worth. Wishing to “fit in” may contribute to youth hiding or minimizing diabetes care behaviors, thereby compromising management in the school setting (97). Cognitive development and medical decision-making skills will impact a wide variety of risk-taking behaviors and acceptance of self-management behaviors into daily life (98,99). Suboptimal glycemic management should not automatically be attributed to adolescent rebellion or lack of concern for health. A thorough age-appropriate psychosocial evaluation and review of the medical regimen will suggest targets for modification to facilitate self-management and well-being. If the adolescent is resistant to accepting support from clinicians, family, and friends, the possibility of a more serious psychological issue must be considered and evaluated.
Although legal and ethical issues of youth accepting or refusing treatment components (e.g., an insulin pump) has not been extensively studied, these issues will undoubtedly surface in the process of treatment decisions. Thus, the issue of treatment consent must be considered when making regimen choices. Although cognitive abilities vary, the ethical position often adopted is the “mature minor rule,” whereby children after age 12 or 13 years who appear to be “mature” ought to have the right to consent or to withhold consent to general medical treatment, except in cases in which refusal would significantly endanger health (99). Emerging technologies, such as phone and computer transmission of management data, can be useful in maintaining communication of information through nonconfrontational channels and may provide a means for youth to communicate directly with care providers as they transition to more independent self-management (100).
Adolescents should have time by themselves with their care provider(s) starting at age 12 years. Care should be taken to respect a teen/young adult’s privacy, as lack of confidentiality is known to negatively affect adolescents’ health behavior, especially regarding what are considered taboo or risky behaviors (101). Discussions with adolescents should include questions about well-being in general, diabetes distress, and risk behaviors (e.g., substance use and sexual activity) (102,103).
Preconception counseling for females during reproductive years increases knowledge about diabetes-related risk, delays age of sexual initiation, decreases unprotected sex, and improves preconception care and health (104,105). Less research is available regarding sexual health for males, particularly in youth; however, males with diabetes have a threefold increased risk of erectile dysfunction compared with men who do not have diabetes (106,107). Open and factual discussions of these topics facilitate future comfort in disclosing any concerns regarding sexual function. As less frequent attendance to diabetes care visits is typical in the 18- to 30-year-old age-group, screening regarding risk behaviors may be necessary at each visit.
Adults
Recommendations
In the care of adults with childbearing potential, include a discussion of life choices that could be impacted by diabetes self-management, such as pregnancy and sexual functioning. B
Providers should consider assessing for the presence of social support providers (e.g., family, peer support, lay diabetes educators/caretakers) who may facilitate self-management behaviors, reduce burden of illness, and improve diabetes and general quality of life. B
As people enter adulthood, establishment of a work role, intimate partnering, childbearing, and parenting are typical life tasks (7). Living with and self-managing diabetes can be expected to impact all life-course decisions for PWD and their partners. PWD may question whether intimate partnering and biological parenthood are viable in the context of their health status (108). High-quality relationships with and diabetes management support from intimate partners improve diabetes-specific and general quality of life, self-management behaviors, and metabolic outcomes (7). Partner roles may change if functional ability is impacted by poor health outcomes (109). Sexual dysfunction is often associated with depression and is routinely reported in clinical encounters (see depression). In one study of individuals with type 1 diabetes, sexual dysfunction was reported in as many as 50% of male patients (107). It is beyond the scope of these guidelines to discuss psychosocial issues related to pregnancy and gestational diabetes mellitus (see ref. 110).
Older Adults
Recommendations
Annual screening for early detection of mild cognitive impairment or dementia is indicated for adults aged 65 years or older. B
Assessment of neuropsychological function and dementia using available standards for conducting evaluations of dementia and age-related cognitive changes is recommended. E
Within the primary care setting, a collaborative care model, incorporating structured nurse care management intervention, is recommended for treatment of comorbid depression in older adults with diabetes. A
Older adults with diabetes may be functional and cognitively intact and have significant life expectancy, and they may not require psychosocial care beyond that of younger adults. However, older adults may have issues particular to their age, such as advanced disease, cognitive dysfunction, complex treatment regimens, comorbid health conditions, functional impairment, limited social and financial resources, and depression (111). Meeting glycemic targets may be impacted by unique nutritional requirements, physical limitations (such as reduced sensation), memory loss, and low literacy and numeracy skills. As older adults with diabetes may receive care support from family members and staff at assisted living facilities, during hospitalizations, and in long-term care facilities, the treatment regimen must consider context and caregiver capacities. Support people (e.g., adult children, caretakers) who provide instrumental, social, or emotional support for older adults with diabetes should be included in diabetes management discussions and shared decision making.
Psychosocial targets for intervention include self-management support, access to health care, and financial and emotional support, as well as day-to-day facilitation of physical and mental well-being. Within the primary care setting, older adults with diabetes and comorbid depression are likely to benefit from a collaborative care intervention approach, which uses a nurse care manager supervised by a primary care physician and psychiatrist (58,112,113).
Compared with older adults without diabetes, those with the disease are at an increased risk of mild cognitive impairment (114). A meta-analysis of prospective and observational studies in PWD showed a 73% increased risk of all types of dementia, a 56% increased risk of Alzheimer dementia, and a 127% increased risk of vascular dementia compared with individuals without diabetes (115). For detection of cognitive dysfunction, people >65 years of age should receive cognitive screening annually within routine health care, using recommended procedures and resources for practitioners (Table 2) (116–118). Medical providers should address reversible contributors to cognitive dysfunction including but not limited to depression, combinations of medications, thyroid disease, and delirium (116).
PWD in Need of Special Considerations
People With Diabetes Complications and Functional Limitations
Recommendation
Care providers should consider routinely monitoring for chronic pain associated with diabetes complications and its impact on quality of life. Appropriate pain management interventions, including referral to a behavioral health provider for pain management strategies, should be provided. B
Diabetes complications, including peripheral neuropathy, foot ulcers, limb amputation, diabetic kidney disease, vision impairment, stroke, and heart attack, are associated with depression, anxiety, reduced autonomy, role impairment, and reduced overall physical function and quality of life (119–122). Fear of complications is a major component of diabetes distress, and depression associated with complications increases mortality (123,124). Care should be taken when discussing rates, causes, and probability of diabetes complications. Providers should acknowledge that discussing complications can be uncomfortable and distressing and should encourage dialogue over multiple visits.
Chronic pain from neuropathy is associated with prevalent psychosocial distress, depression, and sleep disturbance (125,126). Care providers should routinely monitor for chronic pain associated with diabetes complications and its impact on quality of life. Appropriate pain management therapies, including referral to a mental health provider for pain self-management strategies, should be provided.
Onset of diabetes complications threatens independence, self-image, and quality of life. To identify the level of self-care independence and necessary adjunctive supports, providers should evaluate whether individuals have a cognitive impairment impacting the ability to do a task (e.g., poor memory or information processing), a functional limitation that interferes with task performance (e.g., poor motor control or impaired vision), a disability that impacts doing the task without assistance or accommodation (e.g., paralysis or amputation); or a combination of the above (127). Unless limitations are profound and/or formal evaluation clearly determines decreased capabilities, providers should not assume a patient is unable to self-manage. Reassessment of self-management efficacy, abilities, and need for adaptations or assistance is indicated with the onset or worsening of functional limitations or disabilities including vision, hearing, or physical impairment. For example, people with visual disability may benefit from materials that meet low-vision guidelines (128).
Bariatric Surgery
Recommendations
People presenting for bariatric surgery should receive a comprehensive mental health assessment by a professional familiar with weight-loss interventions and postbariatric surgery behavioral requirements. B
If psychopathology is evident, particularly suicidal ideation and/or significant depression, postponement of surgery should be considered so that patient suffering can be addressed before adding the burden of recovery and lifestyle/psychosocial adjustment. E
For people who undergo bariatric surgery, consider assessment for need of ongoing mental health services to help them adjust to medical and psychosocial changes postsurgery. C
Bariatric surgery supports weight loss in people with severe obesity, often with adjunctive remission of type 2 diabetes (129,130). People presenting for bariatric surgery have increased rates of depression and other major psychiatric disorders compared with healthy people and are prone to clinically significant body image disorders, sexual dysfunction, and suicidal behavior (131). Psychosocial well-being and depression, anxiety, and self-care behaviors should be an essential component of the pre- and postsurgical evaluation and monitored during the year after surgery (132).
People with preoperative psychopathology should be assessed at regular intervals following surgery to optimize control over psychiatric conditions and to ensure that psychiatric symptoms do not interfere with weight loss and/or lifestyle change. History of eating patterns, disordered eating behaviors, and clinically significant eating disorders, including night eating syndrome, should be evaluated and monitored pre- and postsurgically at regularly scheduled medical management visits. Bariatric surgery in and of itself does not alleviate psychiatric symptoms, but it may result in remission of food addiction among those who were extremely obese (133).
CONCLUSIONS: PSYCHOSOCIAL CARE IN CONTEXT
PWD must master many complex tasks and behaviors to successfully incorporate diabetes care into daily life. Disease management cannot be successful unless the lifestyle and emotional status of the individual is taken into consideration. As detailed in this Position Statement, routine monitoring and screening for diabetes distress, depression, anxiety, eating issues, and appropriate levels of social and family support, as well as contextual factors that impede implementation of care, are clearly indicated. Effectiveness of regimen and care provision will be enhanced by the inclusion of behavioral health services into the diabetes treatment team. Collaborative care shows the most promise for supporting physical and behavioral health outcomes.
The integration of screening into clinical settings, with appropriate referrals to qualified mental health professionals for reasons noted in Table 1, can improve outcomes. Challenges to accomplishing this standard of care are considerable, including too few qualified mental health professionals who understand living with diabetes and medical care models that are not conducive to team care. Those in most need, the disadvantaged lower socioeconomic level families, have the poorest access to diabetes services (134). The psychosocial services recommended are reimbursable for mental health providers in routine medical care under Centers for Medicare & Medicaid Services (CMS). In addition, new CMS reimbursement is planned for the Collaborative Care Model in routine care. With changing laws mandating minimum standards and payment for diabetes care services (135) and the availability of low-cost insurance that also reimburses preventive services, this balance is changing, allowing the practitioner to incorporate previously unsupported services into routine practice. Knowing how to evaluate and treat common psychosocial issues that impact PWD can inform routine care. The integration of psychosocial care and ensuring access to services will benefit the PWD and the care team.
Article Information
Acknowledgments. The process leading to a Position Statement regarding guidelines for the psychosocial care of PWD originated during the tenure of Dr. Richard Rubin as President, Health Care & Education, ADA, 2006–2007. His leadership in the field of behavioral diabetes helped establish the importance of psychosocial care for success in promoting the health and well-being of those affected by diabetes. The authors also thank Dr. David Marrero, President, Health Care & Education, ADA, 2013–2015, for his support of this effort.
The authors acknowledge the following contributors to the book Psychosocial Care for People with Diabetes, which provided the foundation material for this article: Linda Gonder-Frederick, Daniel Cox, Harsimran Singh, Jaclyn Shepard, Clarissa Holmes, Christopher Ryan, Garry Welch, Sofija Zagarins, Paula Trief, Lori Laffel, Judith Wylie-Rosett, Brook Bailer, Thomas Wadden, Lucy Faulconbridge, David Sarwar, Richard Rubin, Tim Wysocki, Barbara Anderson, David Marrero, Jill Weissberg-Benchell, Suzanne Bennett Johnson, and Linda Delahanty.
Funding. M.d.G. was funded in part by the National Institute of Diabetes and Digestive and Kidney Diseases (R18-DK-092765). J.S.G. is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01-DK-104845, R18-DK-098742, and P30-DK-111022).
The content and views represent those of the authors and do not represent the position of the National Institutes of Health.
Duality of Interest. F.H.-B. is a member of the ADA Board of Directors. K.H. has served as a consultant to Bigfoot Biomedical and Johnson & Johnson Diabetes Institute and has received research support from Dexcom. M.P. has received research grants from Bristol-Meyers Squibb, Genentech, and Novo Nordisk; has received consulting fees from AstraZeneca, Calibra, Genentech, Eli Lilly, and Novo Nordisk; has received speaking honoraria from Novo Nordisk; and has participated in advisory panels for GlaxoSmithKline, Eli Lilly, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This position statement was reviewed and approved by the American Diabetes Association Professional Practice Committee in September 2016 and ratified by the American Diabetes Association Board of Directors in October 2016.
References
- 1.Institute of Medicine Crossing the Quality Chasm: A New Health System for the 21st Century [Internet]. Washington, DC, National Academies Press, 2001. Available from http://www.nap.edu/catalog/10027. Accessed 8 September 2016 [PubMed]
- 2.Kahn R, Anderson JE. Improving diabetes care: the model for health care reform. Diabetes Care 2009;32:1115–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA 2014;312:691–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones A, Vallis M, Pouwer F. If it does not significantly change HbA1c levels why should we waste time on it? A plea for the prioritization of psychological well-being in people with diabetes. Diabet Med 2015;32:155–163 [DOI] [PubMed] [Google Scholar]
- 5.Young-Hyman D, Peyrot M. Psychosocial Care for People with Diabetes. 1st ed. Alexandria, VA, American Diabetes Association, 2012, p. 240 [Google Scholar]
- 6.Anderson BJ. Involving family members in diabetes treatment. In Practical Psychology for Diabetes Clinicians. 2nd ed. Anderson BJ, Rubin RR, Eds. Alexandria, VA, American Diabetes Association, 2002, p. 199–207 [Google Scholar]
- 7.Trief PM. Lifespan development issues for adults. In Psychosocial Care for People with Diabetes. 1st ed. Young-Hyman D, Peyrot M, Eds. Alexandria, VA, American Diabetes Association, 2012, p. 251–271 [Google Scholar]
- 8.Wysocki T, Anderson BJ. Special Issues in children and adolescents. In Psychosocial Care for People with Diabetes. 1st ed. Young-Hyman D, Peyrot M, Eds. Alexandria, VA, American Diabetes Association, 2012, p. 229–250 [Google Scholar]
- 9.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011;33:74–109 [DOI] [PubMed] [Google Scholar]
- 10.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics 2009;124:e1171–e1179 [DOI] [PubMed] [Google Scholar]
- 11.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care 2002;25:1015–1021 [DOI] [PubMed] [Google Scholar]
- 12.Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. . Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One 2014;9:e108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant RW, Buse JB, Meigs JB; University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team . Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care 2005;28:337–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 15.Vigersky RA. An overview of management issues in adult patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace TM, Matthews DR. Poor glycaemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM 2000;93:369–374 [DOI] [PubMed] [Google Scholar]
- 17.Lopez JM, Annunziata K, Bailey RA, Rupnow MF, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer Adherence 2014;8:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 19.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 20.White NH, Cleary PA, Dahms W, Goldstein D, Malone J, Tamborlane WV; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–812 [DOI] [PubMed] [Google Scholar]
- 21.Anderson RM, Funnell MM. Compliance and adherence are dysfunctional concepts in diabetes care. Diabetes Educ 2000;26:597–604 [DOI] [PubMed] [Google Scholar]
- 22.Sarkar U, Fisher L, Schillinger D. Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care 2006;29:823–829 [DOI] [PubMed] [Google Scholar]
- 23.King DK, Glasgow RE, Toobert DJ, et al. . Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care 2010;33:751–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouwen A, Urquhart Law G, Hussain S, McGovern S, Napier H. Comparison of the role of self-efficacy and illness representations in relation to dietary self-care and diabetes distress in adolescents with type 1 diabetes. Psychol Health 2009;24:1071–1084 [DOI] [PubMed] [Google Scholar]
- 25.Beckerle CM, Lavin MA. Association of self-efficacy and self-care with glycemic control in diabetes. Diabetes Spectr 2013;26:172–178 [Google Scholar]
- 26.Iannotti RJ, Schneider S, Nansel TR, et al. . Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. J Dev Behav Pediatr 2006;27:98–105 [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez JS, Peyrot M, McCarl LA, et al. . Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherman A, Schnur J, Montgomery G, Sassu R, Veresiu I, David D. How are adherent people more likely to think? A meta-analysis of health beliefs and diabetes self-care. Diabetes Educ 2011;37:392–408 [DOI] [PubMed] [Google Scholar]
- 29.Duangdao KM, Roesch SC. Coping with diabetes in adulthood: a meta-analysis. J Behav Med 2008;31:291–300 [DOI] [PubMed] [Google Scholar]
- 30.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract 2011;93:1–9 [DOI] [PubMed] [Google Scholar]
- 31.Nieuwlaat R, Wilczynski N, Navarro T, et al. . Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014. (11):CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev 2013. (2):CD008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umpierre D, Ribeiro PA, Kramer CK, et al. . Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 34.Norris SL, Engelgau MM, Narayan KMV. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]
- 35.Driscoll KA, Young-Hyman D. Use of technology when assessing adherence to diabetes self-management behaviors. Curr Diab Rep 2014;14:521. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Foundations of Care and Comprehensive Medical Evaluation. Sec. 3. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S23–S35 [DOI] [PubMed] [Google Scholar]
- 37.Evert AB, Boucher JL, Cypress M, et al. . Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolucci A, Kovacs Burns K, Holt RIG, et al.; DAWN2 Study Group . Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabet Med 2013;30:767–777 [DOI] [PubMed] [Google Scholar]
- 39.Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful? Establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 2012;35:2472–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher L, Hessler D, Glasgow RE, et al. . REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36:2551–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher L, Skaff MM, Mullan JT, et al. . Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 44.Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016;16:9. [DOI] [PubMed] [Google Scholar]
- 45.Hessler D, Fisher L, Polonsky W, Johnson N. Understanding the areas and correlates of diabetes-related distress in parents of teens with type 1 diabetes. J Pediatr Psychol 2016;41:750–758 [DOI] [PubMed] [Google Scholar]
- 46.Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes Endocrinol 2015;3:450–460 [DOI] [PubMed] [Google Scholar]
- 47.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 48.McIntyre RS, Konarski JZ, Misener VL, Kennedy SH. Bipolar disorder and diabetes mellitus: epidemiology, etiology, and treatment implications. Ann Clin Psychiatry 2005;17:83–93 [DOI] [PubMed] [Google Scholar]
- 49.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson RJ, Grigsby AB, Freedland KE, et al. . Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med 2002;32:235–247 [DOI] [PubMed] [Google Scholar]
- 51.Young-Hyman DL, Davis CL. Disordered eating behavior in individuals with diabetes: importance of context, evaluation, and classification. Diabetes Care 2010;33:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrence JM, Standiford DA, Loots B, et al.; SEARCH for Diabetes in Youth Study . Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 2006;117:1348–1358 [DOI] [PubMed] [Google Scholar]
- 53.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes. Results of 5-yr follow-up study. Diabetes Care 1988;11:605–612 [DOI] [PubMed] [Google Scholar]
- 54.de Groot M, Crick KA, Long M, Saha C, Shubrook JH. Lifetime duration of depressive disorders in patients with type 2 diabetes. Diabetes Care 2016;39:2174–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin RR, Ma Y, Marrero DG, et al.; Diabetes Prevention Program Research Group . Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008;31:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clouse RE, Lustman PJ, Freedland KE, Griffith LS, McGill JB, Carney RM. Depression and coronary heart disease in women with diabetes. Psychosom Med 2003;65:376–383 [DOI] [PubMed] [Google Scholar]
- 57.Katon WJ, Lin EH, Von Korff M, et al. . Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piette JD, Richardson C, Himle J, et al. . A randomized trial of telephonic counseling plus walking for depressed diabetes patients. Med Care 2011;49:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umpierre D, Ribeiro PA, Kramer CK, et al. . Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 60.Colberg SR, Sigal RJ, Yardley JE, et al. . Physical activity/exercise and diabetes: a Position Statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith KJ, Béland M, Clyde M, et al. . Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res 2013;74:89–99 [DOI] [PubMed] [Google Scholar]
- 62.Li C, Barker L, Ford ES, Zhang X, Strine TW, Mokdad AH. Diabetes and anxiety in US adults: findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet Med 2008;25:878–881 [DOI] [PubMed] [Google Scholar]
- 63.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 64.Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15 [DOI] [PubMed] [Google Scholar]
- 65.Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract 1999;46:239–246 [DOI] [PubMed] [Google Scholar]
- 66.Mitsonis C, Dimopoulos N, Psarra V. Clinical implication of anxiety in diabetes: a critical review of the evidence base. Eur Psychiatry 2009;24:S526 [Google Scholar]
- 67.Rubin RR, Peyrot M. Psychosocial adjustment to diabetes and critical periods of psychological risk. In Psychosocial Care for People with Diabetes. Young-Hyman D, Peyrot M, Eds. Alexandria, VA, American Diabetes Association, 2012, p. 219–228 [Google Scholar]
- 68.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA, American Psychiatric Publishing, 2013
- 69.Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA. Interventions that restore awareness of hypoglycemia in adults with type 1 diabetes: a systematic review and meta-analysis. Diabetes Care 2015;38:1592–1609 [DOI] [PubMed] [Google Scholar]
- 70.Cox DJ, Gonder-Frederick L, Polonsky W, Schlundt D, Kovatchev B, Clarke W. Blood Glucose Awareness Training (BGAT-2). Diabetes Care 2001;24:637–642 [DOI] [PubMed] [Google Scholar]
- 71.Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. . Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care 2011;34:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walz L, Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wandell P. Impact of symptomatic hyoglycemia on medication adherence, patients satisfaction with treatment, and glycemic control in patients with type 2 diabetes. Patient Prefer Adherence 2014;8:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez JM, Annunziata K, Bailey RA, Rupnow MF, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer Adherence 2014;8:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Egede LE, Dismuke CE. Serious psychological distress and diabetes: a review of the literature. Curr Psychiatry Rep 2012;14:15–22 [DOI] [PubMed] [Google Scholar]
- 75.Trief PM, Ouimette P, Wade M, Shanahan P, Weinstock RS. Post-traumatic stress disorder and diabetes: co-morbidity and outcomes in a male veterans sample. J Behav Med 2006;29:411–418 [DOI] [PubMed] [Google Scholar]
- 76.Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: Challenges in diagnosis and treatment. World J Diabetes 2015;6:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papelbaum M, Appolinário JC, Moreira RdeO, Ellinger VC, Kupfer R, Coutinho WF. Prevalence of eating disorders and psychiatric comorbidity in a clinical sample of type 2 diabetes mellitus patients. Rev Bras Psiquiatr 2005;27:135–138 [DOI] [PubMed] [Google Scholar]
- 78.Hudson JI, Hiripi E, Pope HG Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007;61:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol 2014;51:683–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinhas-Hamiel O, Hamiel U, Greenfield Y, et al. . Detecting intentional insulin omission for weight loss in girls with type 1 diabetes mellitus. Int J Eat Disord 2013;46:819–825 [DOI] [PubMed] [Google Scholar]
- 81.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 2008;31:415–419 [DOI] [PubMed] [Google Scholar]
- 82.Weinger K, Beverly EA. Barriers to achieving glycemic targets: who omits insulin and why? Diabetes Care 2010;33:450–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crow S, Kendall D, Praus B, Thuras P. Binge eating and other psychopathology in patients with type II diabetes mellitus. Int J Eat Disord 2001;30:222–226 [DOI] [PubMed] [Google Scholar]
- 84.Peterson CM, Fischer S, Young-Hyman D. Topical review: a comprehensive risk model for disordered eating in youth with type 1 diabetes. J Pediatr Psychol 2015;40:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morse SA, Ciechanowski PS, Katon WJ, Hirsch IB. Isn’t this just bedtime snacking? The potential adverse effects of night-eating symptoms on treatment adherence and outcomes in patients with diabetes. Diabetes Care 2006;29:1800–1804 [DOI] [PubMed] [Google Scholar]
- 86.Garber AJ. Novel GLP-1 receptor agonists for diabetes. Expert Opin Investig Drugs 2012;21:45–57 [DOI] [PubMed] [Google Scholar]
- 87.Suvisaari J, Perälä J, Saarni SI, et al. . Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. Eur Arch Psychiatry Clin Neurosci 2008;258:129–136 [DOI] [PubMed] [Google Scholar]
- 88.Koro CE, Fedder DO, L’Italien GJ, et al. . Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002;325:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schulte PF, Bocxe JT, Doodeman HJ, van Haelst IM, Cohen D. Risk of new-onset diabetes after long-term treatment with clozapine in comparison to other antipsychotics in patients with schizophrenia. J Clin Psychopharmacol 2016;36:115–119 [DOI] [PubMed] [Google Scholar]
- 90.Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Golden SH, Brown A, Cauley JA, et al. . Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wysocki T, Harris MA, Buckloh LM, et al. . Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol 2006;31:928–938 [DOI] [PubMed] [Google Scholar]
- 93.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr 1997;130:257–265 [DOI] [PubMed] [Google Scholar]
- 94.Gruhn MA, Lord JH, Jaser SS. Collaborative and overinvolved parenting differentially predict outcomes in adolescents with type 1 diabetes. Health Psychol. 21 March 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Streisand R, Mackey ER, Elliot BM, et al. . Parental anxiety and depression associated with caring for a child newly diagnosed with type 1 diabetes: opportunities for education and counseling. Patient Educ Couns 2008;73:333–338 [DOI] [PubMed] [Google Scholar]
- 96.Ducat L, Rubenstein A, Philipson LH, Anderson BJ. A review of the mental health issues of diabetes conference. Diabetes Care 2015;38:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Silverstein J, Klingensmith G, Copeland K, et al.; American Diabetes Association . Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]
- 98.Kuther TL. Medical decision-making and minors: issues of consent and assent. Adolescence 2003;38:343–358 [PubMed] [Google Scholar]
- 99.Coleman DL, Rosoff PM. The legal authority of mature minors to consent to general medical treatment. Pediatrics 2013;131:786–793 [DOI] [PubMed] [Google Scholar]
- 100.Driscoll KA, Young-Hyman D. Use of technology when assessing adherence to diabetes self-management behaviors. Curr Diab Rep 2014;14:521. [DOI] [PubMed] [Google Scholar]
- 101.Peters A, Laffel L; American Diabetes Association Transitions Working Group . Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care 2011;34:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hanna KM, Stupiansky NW, Weaver MT, Slaven JE, Stump TE. Alcohol use trajectories after high school graduation among emerging adults with type 1 diabetes. J Adolesc Health 2014;55:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaser SS, Yates H, Dumser S, Whittemore R. Risky business: risk behaviors in adolescents with type 1 diabetes. Diabetes Educ. 2011;37:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sereika S, Schmitt P, Powell A, et al. . Long-term effects of preconception counseling (PC) during adolescence on family planning vigilance (FPV) in adult women with type 1 diabetes (TID): 15 year follow-up. Diabetes 2014;63(Suppl. 1):A198 [Google Scholar]
- 105.Charron-Prochownik D, Sereika SM, Becker D, et al. . Long-term effects of the booster-enhanced READY-Girls preconception counseling program on intentions and behaviors for family planning in teens with diabetes. Diabetes Care 2013;36:3870–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes 2014;7:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hatzimouratidis K, Hatzichristou D. How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep 2014;14:545. [DOI] [PubMed] [Google Scholar]
- 108.Sereika SM, Becker D, Schmitt P, et al. . Operationalizing and examining family planning vigilance in adult women with type 1 diabetes. Diabetes Care 2016;39:2197–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beverly E, Wray LA, Miller CK. Practice implications of what couples tell us about type 2 diabetes management. Diabetes Spectr 2008;21:39–45 [Google Scholar]
- 110.Wouters MGAJ, Snoek FJ. Psychological issues in the management of diabetes and pregnancy. In Psychology in Diabetes Care. 2nd ed Snoek FJ, Skinner TC, Eds. Chichester, U.K., Wiley, 2005
- 111.Kirkman MS, Briscoe VJ, Clark N, et al. . Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Unützer J, Katon W, Callahan CM, et al.; IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment . Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836–2845 [DOI] [PubMed] [Google Scholar]
- 113.Katon WJ, Von Korff M, Lin EHB, et al. . The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042–1049 [DOI] [PubMed] [Google Scholar]
- 114.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575 [DOI] [PubMed] [Google Scholar]
- 115.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J Diabetes Investig 2013;4:640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Institute of Medicine (IOM) Health care response to cognitive aging. In IOM, Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington, DC, The National Academies Press, 2015, p. 209–226 [PubMed] [Google Scholar]
- 117.American Psychological Association Guidelines for the evaluation of dementia and age-related cognitive change. Am Psychol 2012;67:1–9 [DOI] [PubMed] [Google Scholar]
- 118.Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc 2014;20:278–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vileikyte L, Peyrot M, Gonzalez JS, et al. . Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia 2009;52:1265–1273 [DOI] [PubMed] [Google Scholar]
- 120.Carrington AL, Mawdsley SK, Morley M, Kincey J, Boulton AJ. Psychological status of diabetic people with or without lower limb disability. Diabetes Res Clin Pract 1996;32:19–25 [DOI] [PubMed] [Google Scholar]
- 121.Palmer S, Vecchio M, Craig JC, et al. . Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 2013;84:179–191 [DOI] [PubMed] [Google Scholar]
- 122.Cukor D, Coplan J, Brown C, et al. . Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis 2008;52:128–136 [DOI] [PubMed] [Google Scholar]
- 123.Winkley K, Sallis H, Kariyawasam D, et al. . Five-year follow-up of a cohort of people with their first diabetic foot ulcer: the persistent effect of depression on mortality. Diabetologia 2012;55:303–310 [DOI] [PubMed] [Google Scholar]
- 124.Palmer SC, Vecchio M, Craig JC, et al. . Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis 2013;62:493–505 [DOI] [PubMed] [Google Scholar]
- 125.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia 2000;43:957–973 [DOI] [PubMed] [Google Scholar]
- 126.Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006;81(Suppl.):S3–S11 [DOI] [PubMed] [Google Scholar]
- 127.Hill-Briggs F. Diabetes-related functional impairment and disability. In Psychosocial Care for People with Diabetes. Young-Hyman D, Peyrot M, Eds. American Diabetes Association, Alexandria, VA, 2012, p. 273–289 [Google Scholar]
- 128.American Association of Diabetes Educators (AADE) Diabetes education for people with disabilities. Diabetes Educ 2002;28:916–921 [DOI] [PubMed] [Google Scholar]
- 129.Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks. Diabetes Care 2011;34(Suppl. 2):S361–S266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kalarchian MA, Marcus MD, Levine MD, et al. . Psychiatric disorders among bariatric surgery candidates: relationship to obesity and functional health status. Am J Psychiatry 2007;164:328–334; quiz 374 [DOI] [PubMed] [Google Scholar]
- 132.Greenberg I, Sogg S, M Perna F. Behavioral and psychological care in weight loss surgery: best practice update. Obesity (Silver Spring) 2009;17:880–884 [DOI] [PubMed] [Google Scholar]
- 133.Pepino MY, Stein RI, Eagon JC, Klein S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obesity (Silver Spring) 2014;22:1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hill J, Nielsen M, Fox MH. Understanding the social factors that contribute to diabetes: a means to informing health care and social policies for the chronically ill. Perm J 2013;17:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burge MR, Schade DS. Diabetes and the Affordable Care Act. Diabetes Technol Ther 2014;16:399–413 [DOI] [PMC free article] [PubMed] [Google Scholar]