Abstract
OBJECTIVE
Bariatric surgery may induce remission of type 2 diabetes in obese patients. However, estimates of remission rates reported in the literature range from 25 to 81%, contributing to the uncertainty patients and physicians both face as they assess treatment options. This analysis attempts to reconcile the seemingly disparate rates of diabetes remission reported in studies of Roux-en-Y gastric bypass (RYGB) surgery. It examines variation in the methodologies used to derive the estimates and proposes outcomes that should be reported by all studies.
RESEARCH DESIGN AND METHODS
A literature review yielded 10 large (n > 100), recent (index surgery since 2000) studies of diabetes remission after RYGB. These studies differed in definitions of remission (partial vs. complete), lengths of follow-up (1 year vs. ≥3 years), reported outcomes (cumulative vs. prevalent remission), and risks of attrition bias.
RESULTS
Reported rates of partial remission were 10–30 percentage points higher than rates of complete remission. Study duration explained 69% of the variability in cumulative remission rates, plateauing at 3 years. Adjustment for attrition increased the explained variability to 87%. Attrition-adjusted, 3-year cumulative, complete remission rates ranged from 63 to 65%; however, this does not account for relapse. Attrition-adjusted, 3-year prevalent complete remission rates that accounted for relapse were 23%.
CONCLUSIONS
Variations in reported rates of diabetes remission after RYGB are primarily related to definitions and study duration. Future studies should report both cumulative and prevalent remission to aid decision making and more easily compare studies.
Introduction
Bariatric surgery is commonly regarded as the optimal treatment for severe obesity and as an appropriate treatment for type 2 diabetes (1,2). However, estimates of remission of type 2 diabetes after bariatric surgery are highly variable (3–5), ranging from 25% (6) to 83% (7). This variability can cause concern for patients, physicians, and researchers. Patients faced with such widely disparate results may be uncertain about expected results. Likewise, physicians may find it difficult to endorse a procedure for the treatment of type 2 diabetes that is associated with such widely varying estimates of remission. Researchers, in turn, may question the reliability and validity of results that vary by nearly 60 percentage points. An investigation of the reasons for this variability in outcomes is needed to guide treatment decisions and research interpretation.
Although many surgical approaches have been used in the past decades, two have become dominant in recent years. Roux-en-Y gastric bypass (RYGB) was performed in more than 90% of metabolic operations in 2003 (8) and has been well studied in subsequent years. Sleeve gastrectomy has become increasingly popular because of its low complication rate, being performed in 36% of metabolic operations in 2012 (9). However, because of its recent adoption, few large or long-term studies have studied remission after the procedure. A third surgical approach, laparoscopic adjustable gastric banding, has gained research attention but has not maintained clinical appeal. It accounted for 24% of metabolic operations in 2008, but only 4% in 2012 (9). As a result of the lack of available data for sleeve gastrectomy and the declining interest in laparoscopic adjustable gastric banding operations, we focused our attention on explaining the variability in diabetes remission after RYGB.
This study examined several sources of variability in reported remission rates after RYGB; specifically, variability resulting from 1) different definitions of remission, 2) different lengths of follow-up, 3) different approaches to account for attrition, and 4) different outcomes reported.
Research Design and Methods
A search of MEDLINE and Cochrane databases identified reports in English of outcomes after bariatric surgery. We limited the search to articles published between 1 January 2006 and 1 January 2016. We limited studies to after 2006, because of the move from open surgery to laparoscopic surgery that occurred in 2005 (8). To reduce variability resulting from different surgical procedures, we limited our investigation to RYGB surgery. MeSH search terms included diabetes remission, bariatric surgery, RYGB, and Roux-en-Y. Review articles and original reports were extracted, and studies not identified in the original search were added.
Study Selection Criteria
To support the statistical confidence and clinical relevance of our analysis, we considered only large (initial sample size, n ≥100) studies of diabetes remission after RYGB performed on adults with a BMI of 35 kg/m2 or greater and with a minimum follow-up of 12 months. To limit data to recent surgical techniques, we excluded clinical studies where the index surgery occurred before 2000. A data source diagram is available in Supplementary Fig. 1.
Data Extraction
Data were extracted using a standard template customized to track retention. We identified the initial sample size, the retained sample size, the count (or percentage) of remissions, the definition of remission used, the statistical approach used to calculate the proportion in remission, and the data source. For clinical trials, only the RYGB surgical arm was included for analysis. Incompletely reported data were estimated using overall proportions, as detailed in the Supplementary Table 1. Prior publications defining recruitment procedures, outcomes, and sample sizes were examined as necessary.
Quality Assessment and Risk of Bias
Data collection protocols were designed to focus on three aspects of risk of bias resulting from attrition: retention rate, analytical technique, and data source. Retention rates were recorded as the initial sample size and the retained sample size. The analytical technique was coded for type of estimates (proportions vs. Kaplan-Meier [KM]) and whether the analysis accounted for attrition. Finally, the original data source was coded as population based or clinic based. Population-based data sources were defined as data derived from large electronic medical record systems (EMS) where inclusion in the database was not dependent on follow-up at a specific surgical office or clinic. Clinic-based data sources were defined as data derived from surgical offices or clinics where inclusion or retention in the sample was driven by follow-up at the index site. This specification of data source is critical because attrition in clinical settings can be correlated with treatment failure (10), creating nonignorable missing data. In contrast, patients who leave a population-based health care system might be viewed as missing at random (11,12). We refer to the population-based studies as “less attrition-bias prone” than clinic-based studies.
Another potential source of bias is lack of outcome measurement for data derived from EMS. However, in the case of HbA1c in the population with diabetes, the standard of care is quarterly measurement as well as annual measurement after a treatment such as bariatric surgery. As such, nonmeasurement bias is likely to be small. We made no judgment regarding other risks of bias.
Primary Outcomes
Reported diabetes remission rates were compared with studies using the same definition of remission and approximate length of follow-up. We used the conventional definition of complete remission (HbA1c ≤6.0% [42 mmol/mol] and no antidiabetic medication use) and partial remission (HbA1c ≤6.5% [48 mmol/mol] and no antidiabetic medication use). Studies using alternative definitions rarely had comparable studies (using the same definition of remission) and, thus, were not included. If a study reported both complete and partial remission, each rate was compared with studies that used the same definition. Studies reporting multiple points of follow-up were included for all times where retention was >50%.
Studies using clinic follow-up data reported remission at annual follow-up. Studies using EMS data reported remission as observed from both primary care and surgical follow-up visits.
Other Classifications
Studies were classified as reporting cumulative remission or prevalent remission. Cumulative remission counted any patient who ever achieved remission, and prevalent remission counted only individuals who were in remission at the time of measurement. For studies lasting only 1 year, we assumed that little relapse occurred and denoted the results as cumulative remission. However, these single-year cumulative remission rates were also compared with prevalent remission rates at 1 year.
Studies were also classified as ignoring attrition or as correcting for attrition. Studies that performed available-case analysis and only used subjects who had complete diagnostic data at the time of follow-up (12,13) were classified as ignoring attrition. Studies that used KM estimation corrected for attrition by retaining individuals in the denominator until the time they were lost to follow-up. KM estimation can be biased by nonignorable drop out; but in surgical studies, the KM approach is generally considered less prone to attrition bias.
Statistical Analysis
Proportions and 95% CIs are reported when possible. CIs for the proportion were calculated for studies that reported the number of subjects in remission. For studies reporting KM estimates, we reported the published CIs when available.
Our analysis differed between studies that corrected for attrition and those that ignored attrition. For studies that ignored attrition, we applied extreme-case imputation to the missing observations (13), assuming a worst-case scenario where patients who were lost to follow-up had no remission. Computationally, this worst-case imputation is equivalent to dividing the number of observed remissions at the time of follow-up by the initial sample size rather than dividing by the number of individuals retained. This extreme-case imputation provides a lower bound for the true proportion in remission. In contrast, studies that corrected for attrition using KM estimation were not further corrected for missing data. We compared approaches using extreme-case imputation to KM estimation to investigate the sensitivity of estimates to assumptions regarding attrition.
Results
Cumulative Remission Versus Partial Remission Versus Other Definitions
Table 1 reports the results from the two studies that provided data for complete and partial remission in the same sample. Changing the definition from complete to partial remission increased the cumulative remission rate by 10 to 30 percentage points. The dramatic difference between complete and partial prevalent remission at years 3–5 reported by Brethauer et al. (14) is primarily a result of individuals who initially achieved complete remission but regressed to partial remission. This progression from complete remission to partial remission demonstrates how estimates change over time, not only for prevalent remission but also for reports of cumulative remission. Thus, we examined the length of follow-up when comparing estimates of remission.
Table 1.
Summary of published studies comparing complete and partial remission* of diabetes after RYGB
| Time (years) | N | Complete remission,% (95% CI) | Partial remission,% (95% CI) | |
|---|---|---|---|---|
| Reporting cumulative remission | ||||
| Arterburn (17) | 1 | 4,434 | 37 (35.6, 38.7) | 47 (45.6, 48.8) |
| Arterburn (17) | 3 | 4,434 | 63 (61.5, 65.0) | 73 (70.9, 74.1) |
| Arterburn (17) | 5 | 4,434 | 68 (66.4, 70.0) | 77 (75.3, 78.6) |
| Reporting prevalent remission | ||||
| Brethauer (14) | 1–2 | 221† | 52 (45.4, 58.6) | 71 (65.0, 77.0) |
| Brethauer (14) | 3–5 | 221† | 31 (24.9, 37.1) | 61 (54.6, 67.4) |
*Complete remission defined as HbA1c ≤6.0% (42 mmol/mol) and off all antidiabetic medications. Partial remission of diabetes defined as HbA1c ≤6.5% (48 mmol/mol) and off all antidiabetic medications.
†Total sample with diabetes estimated as detailed in Supplementary Table 2.
Cumulative Remission at 1 and 3 Years
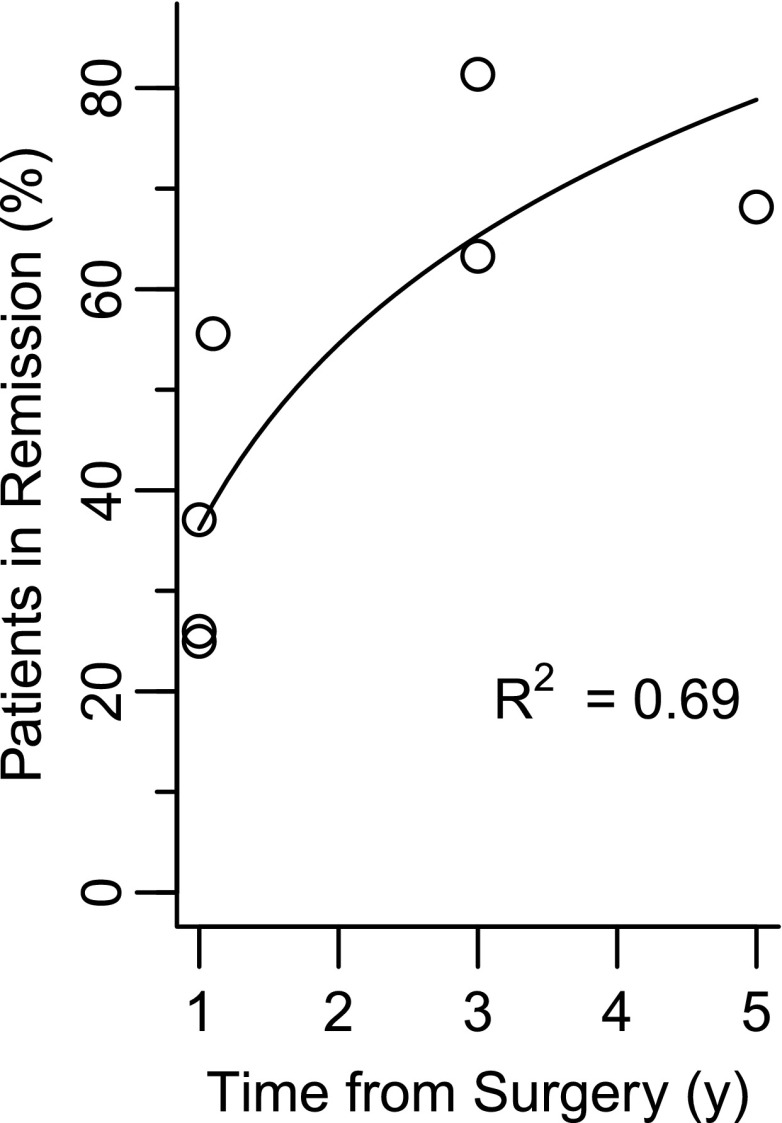
Figure 1 displays the cumulative proportion of individuals in complete remission reported in the literature (details are reported in Table 2). The increasing trend in complete remission over time is a direct result of reporting cumulative remission, because individuals who have a subsequent relapse are not removed from the cumulative count. Also, the plot suggests a plateau at 3 years, and log-scaled regression yields an R2 of 69%, which means study duration explains nearly 70% of the variability in the reported remission rates. However, some of the estimates displayed in Fig. 1 do not consider the effect of attrition, whereas other estimates account for attrition. Some of the estimates displayed in Fig. 1 may thus be inflated because of attrition bias.
Figure 1.
Cumulative complete remission rates after RYGB as a function of time.
Table 2.
Published and attrition-adjusted estimates of the probability of complete remission* of diabetes after RYGB
| Follow-up (years) | N initial | N (%) retained | N remission | Reported,% (method)† | Data source | Attrition-adjusted rate,‡% (95% CI) | |
|---|---|---|---|---|---|---|---|
| Reporting cumulative remission | |||||||
| Iacobellis (28) | 1 | 245 | 206 (84) | 54 | 26 (AC) | Clinic | 22 (16.8, 27.2) |
| Yska (6) | 1 | 280 | NR (54) | 70‖ | 25 (KM) | EMR | 25 (NR) |
| Arterburn (17) | 1 | 4,434 | NR (87) | 1,645‖ | 37 (KM) | EMR | 37 (35.6, 38.7) |
| Blackstone (16) | 14 months | 667 | 505 (76) | 281 | 56 (AC) | Clinic | 42 (38.3, 45.8) |
| Schauer (7) | 3 | 221‖ | 177 (80) | 144 | 81 (AC) | Clinic | 65 (58.7, 71.3) |
| Arterburn (17) | 3 | 4,434 | NR (81) | 2,807‖ | 63 (KM) | EMR | 63 (61.5, 65.0) |
| Arterburn (17) | 5 | 4,434 | NR (76) | 3,024‖ | 68 (KM) | EMR | 68 (66.4, 70.0) |
| Reporting prevalent remission | |||||||
| Brethauer (14) | 1–2 | 221‖ | 162 (73) | 84 | 52 (AC) | Clinic | 38 (31.6, 44.4) |
| Brethauer (14) | 3–5 | 221‖ | 162 (73) | 50 | 31 (AC) | Clinic | 23 (17.5, 28.6) |
AC, available case; EMR, electronic medical record system; NR, not reported.
*Complete remission of type 2 diabetes as defined by HbA1c ≤6.0 (42 mmol/mol) and off all antidiabetic medications.
†Methods for analysis: AC and KM.
‡Extreme-case imputation was used for AC analyses; KM analyses were not further adjusted.
‖Estimated counts. See Supplementary Table 2 for details.
Ignoring Attrition Versus Accounting for Attrition
Higher loss to follow-up among patients with less successful outcomes is well documented (10,13,15). To investigate the effect of this bias, we used worst-case imputation; that is, we assumed that no individuals who were lost to follow-up achieved remission. Table 2 reports these attrition-adjusted proportions of remission for studies that performed available-case analysis. For population-based studies, all of which used KM estimation to account for patient attrition and censoring, we did not make any additional corrections for missing data because the KM estimates are already adjusted for attrition and based on studies prone to less attrition bias.
Table 2 demonstrates the wide variation in reports of cumulative remission for clinic-based studies that did not account for attrition, particularly compared with studies that did account for attrition. When attrition was ignored, the 14-month estimate of Blackstone et al. (16) was closer to population-based 3-year remission rates than it was to 1-year rates. However, after imputing attrition, the percentage in remission was not significantly different from the Arterburn et al. (17) 1-year estimate. Similarly, the 3-year results of Schauer et al. (7), conservatively adjusted for attrition, became consistent with less attrition bias–prone 3-year estimates.
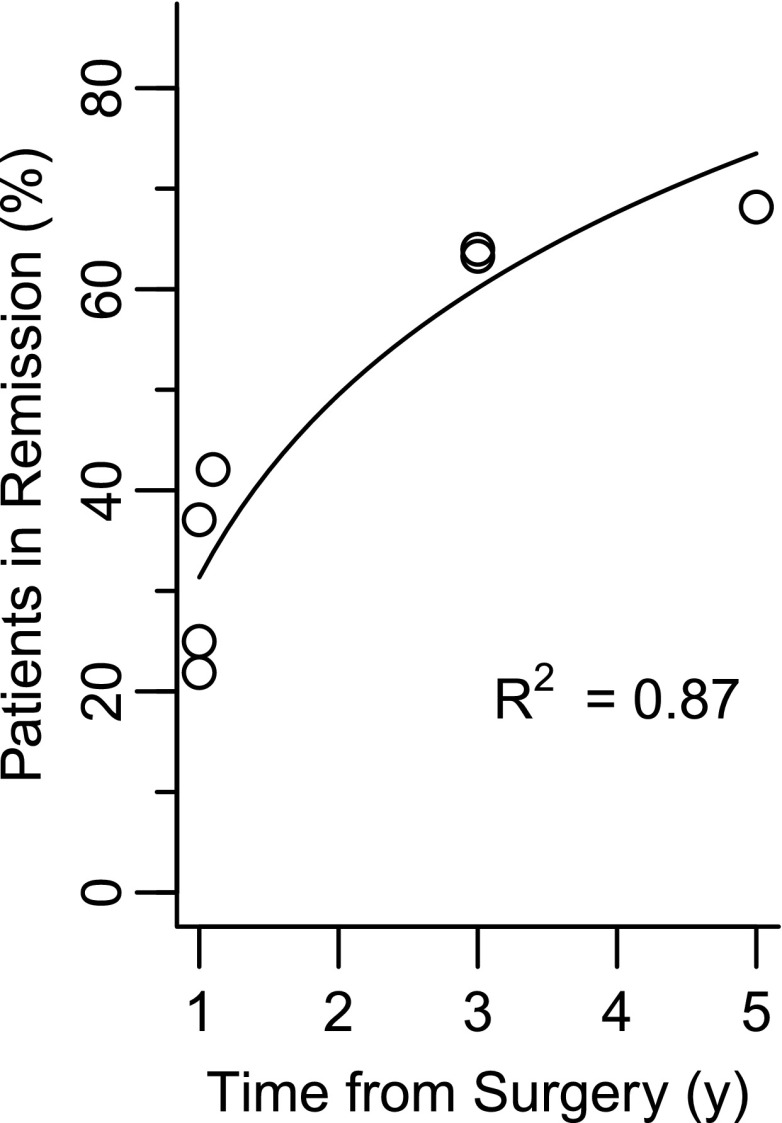
Figure 2 shows the model fit when clinic-based studies are adjusted for attrition bias. The R2 value was 87% compared with 69% before adjusting for attrition. This suggests that study duration explained 87% of the variability in adjusted cumulative remission rates; and worst-case imputation of attrition increased the fit by nearly 20 percentage points. Analogous results for partial remission are available in the Supplementary Table 1 and Supplementary Fig. 3.
Figure 2.
Cumulative complete remission rates after RYGB as a function of time, using estimates that account for attrition by KM analysis or worst-case imputation.
Cumulative Remission Versus Prevalent Remission
The last two rows of Table 2 report period-prevalent remission, extracted from one study. That study′s estimate of remission between years 1 and 2, adjusted for attrition, is consistent with the Arterburn et al. (17) population-based 1-year cumulative estimate. However, the prevalence of complete remission dropped by 21 percentage points after 3 years, emphasizing the difference between cumulative and prevalent remission rates. This decrease in prevalent remission is even clearer in plots of partial remission rates (Supplementary Table 1). The prevalence of partial remission peaks between the first and second year after surgery and then decreases starting in the third year, around the time that cumulative remission rates plateau.
Estimates of prevalent and cumulative remission are not significantly different from each other in the first year after surgery, presumably resulting from a lack of relapse. By 3 years, however, the (attrition-adjusted) cumulative rates are ∼30 percentage points higher than the prevalent rate. This difference is obscured when authors only report cumulative rates.
Conclusions
Our analyses demonstrated two key points. First, we showed that reported rates of diabetes remission after RYGB are related primarily to the definition used and the duration of the study. Second, results differ substantially when cumulative remission and prevalent remission are reported. These results have a number of implications for physicians, patients, and researchers who desire to make decisions based on the literature.
Among the articles we examined, six studies reported complete remission, six reported partial remission, two used both, and three reported other definitions, such as HbA1c ≤5.7% (39 mmol/mol) with no antidiabetic medication (16), HbA1c ≤6.0% (42 mmol/mol) and no antidiabetic medication other than metformin (18), and HbA1c ≤6.0% (42 mmol/mol) with or without antidiabetic medications (19). Only one of these reports used a standard definition so that their results could be compared with other studies. Blackstone et al. (16) demonstrated remission rates ranging from 43 to 60%, depending on which of five definitions of remission was used. For studies that reported complete and partial remission, partial remission was 10 percentage points higher than complete remission. Similarly, when prevalent remission was estimated, partial remission was at least 20 percentage points higher than complete remission. The variability in these estimates emphasizes the importance of anchoring results to a standard definition. In studies that use nonstandard definitions of remission, it is not clear whether differences in the reported rates are a result of the study design, setting, or the nonstandard definition. In addition, the rarity of studies that report more than one definition emphasizes the difficulty physicians, patients, and researchers face when trying to translate published results into contexts that are of interest to themselves. For example, physicians who prescribe metformin as a maintenance drug after metabolic surgery may want to apply research results to their setting but cannot given inconsistencies in its use among the studies.
Several authors have argued for changing the standard definitions for remission after metabolic surgery to allow the use of metformin (18,20,21). Metformin therapy is now considered a standard of care for the prevention of diabetes (22) and, thus, could be considered consistent with complete remission. One study reported prevalent remission rates as HbA1c <6.0% (42 mmol/mol), including patients using metformin, and found 59%, 64%, and 51% at 1, 2, and 5 years, respectively (18). These rates are up to 30 percentage points higher than reports of prevalent partial remission for comparable years, but because the authors did not provide standard definitions of remission rates, it is not clear whether this increase in remission was a result of differences in the underlying population, the effect of metformin, or the nonstandard definition used. Thus, their results are less informative than they could be. Another study reported remission rates with and without metformin and found a remission rate that was 15 percentage points higher when metformin was allowed (23). However, this study used a definition of HbA1c <7.0% (53 mmol/mol), so that their results do not reflect current research standards. Opening a discussion of the role of metformin in diabetes remission would be a valuable contribution to the literature; however, this discussion requires a bridge between the standard definitions and new conceptualizations of remission.
For clinicians and patients making decisions about treatment, what constitutes remission (i.e., how remission is defined) and the long-term benefits of remission (complete vs. partial) are both important to identify and understand. Little is known about the long-term outcomes after remission, particularly because reported outcomes are often based on competing definitions of remission. Simulation models have suggested that delaying the onset of diabetes delays the onset of complications and comorbidities and reduces their cumulative incidence, partly because of competing mortality (24,25). A recent study indicates that even temporary partial remission reduces the risk of incident microvascular disease (26). However, further research is needed to measure the magnitude of the effect on other outcomes such as complications, comorbidities, and survival. Furthermore, understanding the legacy effect of partial remission versus complete remission, with and without the use of metformin, would provide a better understanding of the benefits of transient remission.
In addition to the variability explained by the definition of remission, methodological choices inform clinical decisions and research interpretation. We examined the effect of three methodological choices: imputation methods, time of follow-up, and statistical technique. Our analysis demonstrated that worst-case imputation of missing data caused attrition-unadjusted estimates to become consistent with estimates from less attrition bias–prone studies. This congruence between worst-case imputation and less attrition bias–prone estimates suggests that there is a strong effect from selective patient attrition. One study has shown that patients with less weight loss are less likely to follow-up with their surgical clinic (10). Given that individuals who lose less weight after surgery are less likely to achieve remission and more likely to relapse (14), selective attrition will inflate surgical success rates unless authors account for those missing observations.
Population-based EMS data are less likely to face attrition bias but have the potential for a different form of bias. If research outcomes of interest are not regularly measured in the clinical setting, then outcomes may be missed or their ascertainment delayed, thus underestimating the rate of occurrence. However, in the case of patients with diabetes, the standard of care is quarterly measurement of HbA1c. Thus, any measurement delay will be small, and the attrition bias is likely to be a larger source of variability in reports of remission of diabetes.
Our analysis highlighted two approaches to handling these missing data. The first, worst-case imputation, is computationally simple, provides a lower bound to the estimated rates, and can be calculated in the margin of an article using a rudimentary calculator. In contrast, KM estimation generally requires individual-level data and statistical software. Yet, both techniques yielded similar results in the case of diabetes remission after RYGB. Estimates in the clinical setting are thus consistent with estimates in the population when every patient who did not return is assumed to have had a relapse. This is surprising. We expected the population-based estimates to be higher than the lower bound; instead, the population-based estimates were consistent with the extreme-case scenario. More research is necessary to verify this phenomenon. Moreover, this consistency between extreme-case imputation and less attrition bias–prone estimates may not be the case in other applications. Congruence between these two approaches should be investigated whenever possible, and in the context of metabolic surgery, we recommend extreme-case imputation as the standard for reporting point estimates in the same way that intent-to-treat analyses have become the standard in pharmaceutical studies
Even accounting for attrition bias, published estimates of remission rates after RYGB range from 25 to 65%. However, we demonstrated that differences in the follow-up time among studies could explain much of this variability. Remission after bariatric surgery is clearly a function of time (17,27), but so is the variability in the estimates. Cumulative remission rates among population-based studies varied significantly in the first year; but given the rapid change in weight and other metabolic indicators during the first year (6,14,18,28), the variability in remission rates is not surprising. At 3 years, however, the observed cumulative proportion plateaus around the rate commonly reported by other authors (5,29). This third-year plateau suggests that few new remissions are observed beyond that time. Moreover, the total increase in cumulative remission beyond 3 years was less than 4%, which equals a typical remission rate in the control group of a population-based cohort (27). Thus, it is difficult to argue that any remission beyond 3 years is due to the effect of RYGB.
Not only does incidence of remission decrease after 3 years, but relapses also begin to accumulate. Yet, it is remarkable how many studies report cumulative remission without reporting relapse (6,7,29–31). Other studies estimate that up to 40% of individuals in remission experience a relapse at 5 years, with the median time to relapse ranging between 5 and 8 years for RYGB (17,32) in contrast to a reported 80% relapse rate at 5 years after sleeve gastrectomy (33). Even in the most optimistic cases, analysis of prevalent remission rates over 3–5 years after RYGB emphasize that relapse begins within 3 years of surgery (14,27). Even more telling, Brethauer et al. (14) observed that 73% of individuals in remission at 5 years had fluctuated in, out, and back into remission at some point during that period. These results reemphasize the importance of reporting both cumulative and prevalent remission rates to guide treatment decisions on a population level.
For individual treatment decisions, it is important to recognize that this report focuses on the average rates for large samples or populations. Individual characteristics significantly increase or decrease the likelihood of remission (7,29,30,34). For example, duration of diabetes of less than 5 years is associated with an increased chance of remission (14,34). Likewise, younger age and diet-controlled diabetes are commonly associated with higher odds of remission (7,17,29). Thus, although our synthesis explained nearly 90% of the variability in overall proportions, individual risk can still vary greatly from the overall mean, depending on patient characteristics. Clinicians and patients seeking to assess individual risks and benefits can use our estimates as an anchor from which to adjust their estimates of individual odds of remission.
One limitation of our study is that our numerical results are specific to RYGB. Other surgical procedures are known to have different outcomes (17,34). However, our conceptual approach to synthesizing postsurgical remission rates can be applied to other surgical procedures as more data become available. Individuals and consensus panels seeking to evaluate these alternative treatments can observe the time of follow-up, differentiate between cumulative and prevalent remission rates, and apply a simple adjustment for attrition bias. Using these steps, clinicians, policy makers, and patients can interpret the literature to answer questions of interest.
Researchers can also use our methods to guide future analyses. For example, they can make their results more useful and relevant by anchoring their results to standard definitions of remission. They can make their results more credible by accounting for attrition. They can report and discuss how the study duration affects their observed results, and they can report statistics that provide a sophisticated depiction of their data.
Unfortunately, a single statistic does not provide complete information. Prevalent remission rates adjust for relapse, are easily interpreted, and are amenable to clinic-based data sets with annual follow-up; however, they do not automatically account for attrition bias. KM estimates naturally account for attrition and are amenable to continual follow-up but ignore relapse. Whenever possible, attrition-adjusted prevalent remission rates and cumulative remission and cumulative relapse rates should both be reported. Using these methods, researchers can provide useful information for patients and clinicians.
In conclusion, the commonly held perception that there is a 60% remission rate after RYGB is accurate only for less attrition bias–prone, 3-year cumulative rates that ignore relapse. For clinical practice, less emphasis should be placed on cumulative rates and more emphasis on the entire postsurgical experience, including remission, relapse, and attrition. More research is needed to evaluate the effects of temporary remission on long-term complications, comorbidities, and survival to better understand the risks and benefits of bariatric surgery as a treatment for type 2 diabetes.
Supplementary Material
Article Information
Funding. The project described was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant P30-DK-020572 (Michigan Diabetes Research Center) and grant P30-DK-092926 (Michigan Center for Diabetes Translational Research) and used core services supported by grant P30-DK-089503 (Michigan Nutrition Obesity Research Center).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.J.M.I. contributed to the analysis design, conducted the literature searches and data analysis, and wrote the manuscript. A.E.R. interpreted data and contributed to manuscript development. W.H.H. contributed to the study design, interpreted data, and supervised the study and manuscript development. All authors reviewed, edited, and approved the final manuscript. D.J.M.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0954/-/DC1.
References
- 1.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–2425 [DOI] [PMC free article] [PubMed]
- 2.Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care 2016;39:861–877 [DOI] [PubMed] [Google Scholar]
- 3.Capoccia D, Coccia F, Guida A, et al. Is type 2 diabetes really resolved after laparoscopic sleeve gastrectomy? Glucose variability studied by continuous glucose monitoring. J Diabetes Res 2015;2015:674268 [DOI] [PMC free article] [PubMed]
- 4.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed]
- 5.Yu J, Zhou X, Li L, et al. The long-term effects of bariatric surgery for type 2 diabetes: systematic review and meta-analysis of randomized and non-randomized evidence. Obes Surg 2015;25:143–158 [DOI] [PubMed]
- 6.Yska JP, van Roon EN, de Boer A, et al. Remission of type 2 diabetes mellitus in patients after different types of bariatric surgery: a population-based cohort study in the United Kingdom. JAMA Surg 2015;150:1126–1133 [DOI] [PubMed]
- 7.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:465–467 [DOI] [PMC free article] [PubMed]
- 8.Nguyen NT, Masoomi H, Magno CP, Nguyen XMT, Laugenour K, Lane J. Trends in use of bariatric surgery, 2003-2008. J Am Coll Surg 2011;213:261–266 [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NT, Nguyen B, Gebhart A, Hohmann S. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg 2013;216:252–257 [DOI] [PubMed] [Google Scholar]
- 10.Harper J, Madan AK, Ternovits CA, Tichansky DS. What happens to patients who do not follow-up after bariatric surgery? Am Surg 2007;73:181–184 [PubMed]
- 11.Little RJ, Cohen ML, Dickersin K, et al. The design and conduct of clinical trials to limit missing data. Stat Med 2012;31:3433–3443 [DOI] [PMC free article] [PubMed]
- 12.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012;367:1355–1360 [DOI] [PMC free article] [PubMed]
- 13.Hollis S. A graphical sensitivity analysis for clinical trials with non-ignorable missing binary outcome. Stat Med 2002;21:3823–3834 [DOI] [PubMed]
- 14.Brethauer SA, Aminian A, Romero-Talamas H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:627–628 [DOI] [PMC free article] [PubMed]
- 15.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311:74–86 [DOI] [PMC free article] [PubMed]
- 16.Blackstone R, Bunt JC, Cortes MC, Sugerman HJ. Type 2 diabetes after gastric bypass: remission in five models using HbA1c, fasting blood glucose, and medication status. Surg Obes Relat Dis 2012;8:548–555 [DOI] [PubMed]
- 17.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed]
- 18.Dicker D, Yahalom R, Comaneshter DS, Vinker S. Long-term outcomes of three types of bariatric surgery on obesity and type 2 diabetes control and remission. Obes Surg 2016;26:1814–1820 [DOI] [PubMed]
- 19.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed]
- 20.Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016;39:954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern B, Cercato C, Mancini MC. Diabetes remission off medications is not a suitable endpoint for comparing bariatric/metabolic surgery with pharmacotherapy. Diabetologia 2016;59:2040–2041 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S52–S5926696682 [Google Scholar]
- 23.Ikramuddin S, Korner J, Lee W-J, et al. . Durability of addition of Roux-en-Y gastric bypass to lifestyle intervention and medical management in achieving primary treatment goals for uncontrolled type 2 diabetes in mild to moderate obesity: a randomized control trial. Diabetes Care 2016;39:1510–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed]
- 25.Herman WH, Hoerger TJ, Brandle M, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed]
- 26.Coleman KJ, Haneuse S, Johnson E, et al. . Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care 2016;39:1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulliford MC, Booth HP, Reddy M, et al. Effect of contemporary bariatric surgical procedures on type 2 diabetes remission. A population-based matched cohort study. Obes Surg 2016;26:2308–2315 [DOI] [PMC free article] [PubMed]
- 28.Iacobellis G, Xu C, Campo RE, De La Cruz-Munoz NF. Predictors of short-term diabetes remission after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2015;25:782–787 [DOI] [PubMed]
- 29.Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38–45 [DOI] [PMC free article] [PubMed]
- 30.Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:254–259 [DOI] [PubMed]
- 31.Kim S, Richards WO. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann Surg 2010;251:1049–1055 [DOI] [PubMed]
- 32.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed]
- 33.Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg 2015;150:1051–1057 [DOI] [PubMed]
- 34.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg 2012;16:42–45 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.