Abstract
OBJECTIVE
To compare glycemic control and secondary outcomes of a 4-month telephonic couples behavioral intervention to individual intervention, and to education, for adults with type 2 diabetes.
RESEARCH DESIGN AND METHODS
A randomized trial with the following three arms: couples calls (CC) (n = 104); individual calls (IC) (n = 94); and diabetes education (DE) (n = 82). All arms had self-management education (two calls). CC and IC had 10 additional behavior change calls. CC addressed collaboration and relationships/communication. Participants consisted of 280 couples, among whom one partner had type 2 diabetes and an A1C level ≥7.5%. Blinded assessments occurred at 4, 8, and 12 months. The primary outcome was change in A1C; and secondary outcomes were BMI, waist circumference, blood pressure, depressive symptoms, diabetes self-efficacy, and diabetes distress.
RESULTS
Patients had a mean age of 56.8 years; 61.6% were male, and 30.4% were minorities. The baseline mean A1C level was 9.1%. Intention-to-treat analyses found significant A1C reductions for all (12 months: CC −0.47%, IC −0.52%, DE −0.57%), with no differences between arms. Preplanned within-arm analyses were stratified by baseline A1C tertiles: lowest tertile (7.5–8.2%), no change from baseline; middle tertile (8.3–9.2%), only CC led to significantly lower A1C level; and highest tertile (≥9.3%), significant improvement for all interventions. For BMI, CC showed significant improvement, and CC and DE led to decreased waist circumference. The IC group showed greater blood pressure improvement. Results for secondary psychosocial outcomes favored the CC group.
CONCLUSIONS
In adults with poorly controlled type 2 diabetes, a collaborative couples intervention resulted in significant, lasting improvement in A1C levels, obesity measures, and some psychosocial outcomes. For those with exceedingly high A1C levels, education alone was beneficial, but additional intervention is needed to achieve glycemic targets.
Introduction
For patients with type 2 diabetes, good glycemic control can reduce or forestall complications (1); however, 36–69% do not achieve glycemic targets (2). Poor glycemic control increases the risks of serious complications, poor quality of life, high health care costs, and mortality (3). Although behavioral interventions have led to improved glycemic control, benefits are often short lived, and behavior changes are not sustained (4). Social Ecological Theory (5) suggests that including partners might enhance intervention effects, as the partner might serve as the ongoing reinforcer of behavior change.
There are positive associations between strong marital bonds and better health outcomes (6). A partner’s impact may be strong for patients with type 2 diabetes, whose self-care regimen (e.g., food purchase/preparation) often involves partners (7). Although partner/family member involvement can enhance positive health outcomes (8), the relationship between marital quality and diabetes outcomes is unclear (9,10). Little has been done to intervene at the family level for adults with type 2 diabetes (8,11), with no published reports we are aware of describing interventions with spouses/partners.
We hypothesized that a couples-focused behavior change intervention to enhance self-management would lead to improved glycemic control and improved health and psychosocial outcomes, in the short and longer term, compared with one targeting the individual alone, and that both would be superior to diabetes education (DE) for adults with type 2 diabetes who had poor glycemic control.
Another need is to increase reach to individuals who are unlikely to attend face-to-face interventions (e.g., because they had no transportation or live in rural areas) (12). In couples interventions, two partners must be engaged, a double challenge. Use of the phone may increase reach, although the evidence is inconclusive (13).
We report data from the Diabetes Support Project (DSP), a practical, randomized controlled trial (RCT) of a telephonic couples behavioral diabetes intervention. We present primary (hemoglobin A1C) and secondary (BMI, waist circumference [WC], blood pressure [BP]) health outcomes, and secondary psychosocial outcomes (diabetes distress [DD], depressive symptoms [DS], diabetes self-efficacy [DSE]). Interventions were delivered solely via telephone. This is the first RCT we are aware of that tests the efficacy of a couples intervention for adults with type 2 diabetes. Also, this is an especially strong design because it included an individual intervention comparator.
Research Design and Methods
Trial Design
The DSP, a multicenter, 12-month, randomized clinical trial, involved 280 couples, with one partner having type 2 diabetes with poor glycemic control (2009–2014) (14). Couples were randomized to the following: behavior intervention change couples calls (CC), behavior change intervention individual calls (IC), or individual DE calls. Assessors, who were blind to group assignment, measured outcomes at 4 (i.e., immediately after intervention), 8, and 12 months. Participants were identified through chart review and sent recruitment letters and were recruited by posters and community talks. They were recruited at two sites (upstate New York, northern California), to enhance diversity and generalizability. The trial was approved by the Institutional Review Boards of State University of New York Upstate Medical University and the University of California, San Francisco. Informed participants signed approved consent documents and received compensation for assessments and transportation.
Participants
Couples were eligible if patients, with a willing partner able to speak and read English, met the following criteria: had a diagnosis of type 2 diabetes for >1 year (diagnosis confirmed by medical record and/or A1C level); baseline A1C level of ≥7.5% (58 mmol/mol); ≥21 years of age; able to speak and read English; in a self-defined committed relationship for ≥1 year; no severe medical or psychiatric conditions that might interfere with participation; and telephone access.
Randomization
Randomization was conducted using a computer-generated random assignment scheme by region. We proposed unequal cell sizes; a smaller DE sample was planned to provide more power to compare CC to IC. The biostatistician created a nonuniform random allocation ratio so that participants were assigned to conditions in the proper proportions (15). We stratified by sex and balanced arms for race/ethnicity to ensure comparable representation.
Interventions
All groups participated in two telephone sessions (mean length of calls: 75 min) of comprehensive diabetes education. In the DE arm, there was no further intervention. CC and IC interventions had 10 additional calls (mean length: CC 57 min/call, IC 50 min/call). These behavioral interventions, based on social learning theory (16) (which included knowledge development, goal setting, self-monitoring, and behavioral contracting), promoted changes in diet, activity, medication adherence, and blood glucose testing. The CC intervention was also based on Interdependence Theory (17,18); partners were actively involved in calls and homework. Couples were encouraged to provide mutual support for change, using collaborative problem-solving techniques and recognizing their interdependence (i.e., reciprocal effects on one another). Two sessions were relationship focused, as follows: couples practiced the “speaker-listener technique” (partner shares concern, the other restates it until partner feels understood, then they switch roles) and communication/conflict management around a diabetes-related issue. Both techniques are based on a research-supported behavioral approach to relationship enhancement (19). In the IC arm, the intervention was identical, except partners were not involved, and the two CC relationship-focused calls addressed individual problem solving.
Workbooks included precall readings, content for discussion, goal-setting forms, and diet/blood glucose/activity self-monitoring logs. Educators followed a “script,” but tailored interventions to participants’ cultural preferences and cognitive abilities. Calls occurred weekly for 12 weeks.
Educators were dietitians (certified diabetes educators or with significant diabetes experience); were trained for protocol adherence and to promote interaction within couples; and were audiotaped for supervision until deemed competent, with tapes randomly reviewed by an independent team of reviewers for quality assurance. We trained diabetes educators in couples work, not counselors in diabetes-related skills, for two reasons. We believe that diabetes knowledge and patient experience are core educator competencies, which are not easy to teach or to gain experience in. Also, educators are typically more available and, with training, can adapt interventions to couples work, which might increase the likelihood of future replicability and implementation.
Sample Size and Assessments
The minimum sample size necessary, based on A1C data obtained from a 3-month pilot study (20), showed that 80 participants/arm (n = 240) would exceed 80% power to detect significant differences between CC or IC and DE interventions. Because we examined subtle differences between IC and CC interventions, and to include attrition, we conservatively aimed for a larger cohort. Participants were assessed four times (baseline, and 4, 8, and 12 months). Assessors were blind to the treatment group.
Outcomes and Measures
Glycemic control: A1C (21), using the AccuBase A1c Test Kit (Diabetes Technologies, Inc). This mail-in U.S. Food and Drug Administration–approved kit provides highly accurate A1C results. Samples are mailed to a Clinical Laboratory Improvement Amendments–licensed, College of American Pathologist–proficient laboratory. Specimens are screened for abnormal hemoglobin levels, abnormal peaks, and/or red blood cell disturbances.
Obesity: BMI (kg/m2) was calculated with weight (nearest 0.1 kg, using portable digital scale, participants wore street clothes, two readings were averaged) and height (stadiometer); WC, using spring tension stretchless Gulick II tape.
Blood pressure: automated monitor with appropriate cuff sizes. Three seated readings at 1-min intervals; calculated mean of readings 2–3.
Diabetes distress: 17-item Diabetes Distress Scale, to measure the perceived emotional burdens of managing diabetes (22).
Diabetes self-efficacy: 8-item scale developed for the Stanford English Diabetes Self-Management Study, asks how “confident” the individual is in his/her ability to manage the diabetes self-care regimen (i.e., diet, exercise, managing hypoglycemia, and self-assessment) (23).
Depressive symptoms: Patient Health Questionnaire (PHQ-8), a standardized, validated scale, assesses the eight key symptoms of depression (suicidal ideation has been omitted, as is common in research protocols without resources for follow-up) (24).
Patient satisfaction questionnaire: three items, satisfaction with the intervention; two items, attitudes toward phone delivery.
Statistical Analysis
Longitudinal data were analyzed with mixed linear model procedures using SPSS Mixed and SAS Proc Mixed version 9.3. Treatment arm, assessment number (ordinal, 1–4), and treatment × assessment were fixed factors. Random effects were added and retained or discarded based on improvement in model fit, judged by reduction in −2 log likelihoods, the Akaike information criterion and Bayesian information criterion. Autoregressive covariance structures (AR1) provided the best model fit. Preplanned stratified analyses, to analyze the effect of baseline A1C level on change, included the stratifying variable as a fixed effect in the models. Planned contrasts were used to compare baseline measures of the dependent variables with measures at 4, 8, and 12 months. Between-group measures for outcome variables were also compared. All analyses were conducted with an a priori α = 0.05 (two tailed) and a Sidak correction for significance when indicated. Randomization produced treatment arms that did not differ in any participant characteristics except for BP, and no demographic variables predicted change in A1C levels. We statistically controlled for between-arm differences when analyzing BP, but no covariates were used for other outcomes.
Results
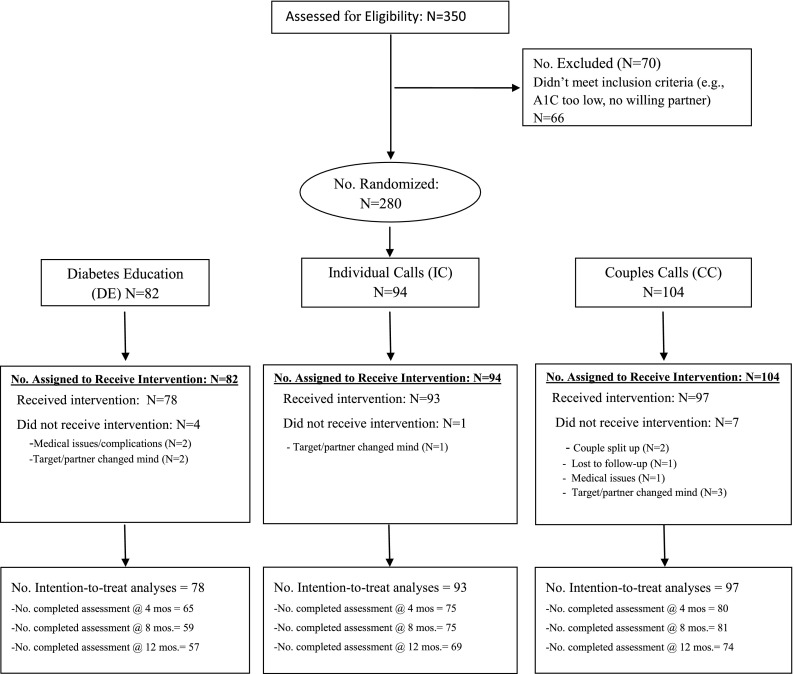
Figure 1 shows the flow of participants through the protocol. Of 350 potential couples who were screened for eligibility, 70 were excluded (20%) for not meeting inclusion criteria. A total of 280 couples completed baseline assessments and were randomized (CC arm = 104, IC arm = 94, DE arm = 82). Of these, 268 (95.7%) participated in at least one intervention call (CC arm = 97, IC arm = 93, DE arm = 78) and were included in intention-to-treat analyses. Others were deemed to have failed inclusion criteria because they were unable/unwilling to participate in procedures. Attrition (i.e., no follow-up A1C level) was 17.9% (4 months), 19.8% (8 months), and 25.4% (12 months), with no significant differences in attrition between arms.
Figure 1.
CONSORT diagram.
Participants
The sample of patient participants (61.6% male, 30.4% self-described minority) had a mean (SD) age of 56.8 years (10.9 years), had a diagnosis of type 2 diabetes for a mean (SD) of 12.4 years (7.9 years), and had been in this committed relationship for a mean (SD) duration of 25.5 years (14.8 years) (Table 1). The mean baseline A1C level was 9.1% (76 mmol/mol) (SD 1.5%). Dropouts (n = 54, no follow-up data) were less likely to be white (53% vs. 74%) and retired (11% vs. 32%), and were more likely to be Asian (18% vs. 7%) and single/widowed/separated/divorced (15% vs. 4%).
Table 1.
Participant characteristics at baseline by intervention arm and overall
| Participant characteristics | Intervention arms |
Total (n = 268) | P value | ||
|---|---|---|---|---|---|
| DE (n = 78) | IC (n = 93) | CC (n = 97) | |||
| Sex (%) | 0.85 | ||||
| Male | 59.0 | 62.4 | 62.9 | 61.6 | |
| Female | 41.0 | 37.6 | 37.1 | 38.4 | |
| Ethnicity (%) | 0.41 | ||||
| Hispanic or Latino | 10.3 | 6.5 | 5.2 | 7.1 | |
| Race1 (%) | 0.37 | ||||
| White | 70.3 | 64.4 | 74.0 | 69.6 | |
| Asian | 12.2 | 12.2 | 4.2 | 9.2 | |
| Black or African American | 13.5 | 20.0 | 17.7 | 17.3 | |
| Other | 4.1 | 3.3 | 4.2 | 3.8 | |
| Relationship status (%) | 0.60 | ||||
| Married | 83.3 | 86.0 | 88.7 | 86.2 | |
| Unmarried/living together | 7.7 | 7.5 | 8.2 | 7.8 | |
| Not living together | 9.0 | 6.5 | 3.1 | 6.0 | |
| Education (%) | 0.95 | ||||
| <High school | 10.3 | 8.6 | 7.2 | 8.6 | |
| High school/GED/tech degrees | 25.6 | 18.3 | 19.6 | 20.9 | |
| Some college or advanced degree | 32.1 | 40.9 | 39.2 | 37.7 | |
| Bachelor's degree | 17.9 | 17.2 | 18.6 | 17.9 | |
| Master's or doctorate degree | 14.1 | 15.1 | 15.5 | 14.9 | |
| Employment status (%) | 0.72 | ||||
| Working full time | 34.6 | 35.5 | 40.2 | 36.9 | |
| Working part time | 11.5 | 10.8 | 7.2 | 9.7 | |
| Retired | 25.6 | 24.7 | 33.0 | 28.0 | |
| On disability | 14.1 | 17.2 | 11.3 | 14.2 | |
| Other | 14.1 | 11.8 | 8.2 | 11.2 | |
| Annual household income (%) | 0.51a | ||||
| <$20,000 | 16.7 | 19.4 | 7.2 | 14.2 | |
| $20,000–30,000 | 5.1 | 6.5 | 7.2 | 6.3 | |
| $30,000–50,000 | 19.2 | 14.0 | 14.4 | 15.7 | |
| $50,000–75,000 | 20.5 | 22.6 | 26.8 | 23.5 | |
| $75,000+ | 28.2 | 28.0 | 28.9 | 28.4 | |
| Missing | 10.3 | 9.7 | 15.5 | 11.9 | |
| Age, mean (SD), years | 56.9 (10.4) | 55.6 (11.4) | 57.8 (10.8) | 56.8 (10.9) | 0.35 |
| Duration of diabetes, mean (SD), years | 12.6 (8.3) | 11.9 (6.9) | 12.8 (8.5) | 12.4 (7.9) | 0.73 |
| Duration of committed relationship, mean (SD), years | 23.9 (14.8) | 25.6 (14.8) | 26.7 (14.9) | 25.5 (14.8) | 0.49 |
| A1C level, mean (SD), % | 9.1 (1.6) | 9.3 (1.7) | 8.9 (1.3) | 9.1 (1.5) | 0.28 |
| SBP, mean (SD), mmHg | 123.5 (13.6) | 126.1 (16.9) | 129.9 (18.9) | 126.7 (17.0) | 0.04 |
| DBP, mean (SD), mmHg | 72.6 (10.5) | 74.6 (10.4) | 75.7 (11.8) | 74.4 (11.0) | 0.18 |
| Weight, mean (SD), kg | 102.1 (22.9) | 102.9 (26.1) | 104.2 (20.8) | 103.1 (23.3) | 0.84 |
| BMI, mean (SD), kg/m2 | 36 (8.1) | 36 (8.2) | 35.7 (6.3) | 35.9 (7.5) | 0.93 |
| WC, mean (SD), cm | 118.3 (18.0) | 117.3 (18.3) | 118.7 (15.2) | 118.1 (17.1) | 0.83 |
| Diabetes Distress Scale, mean (SD) | 2.2 (0.9) | 2.3 (1.1) | 2.4 (0.8) | 2.3 (1.0) | 0.41 |
| Diabetes Self-Efficacy Scale, mean (SD) | 7.0 (1.8) | 6.9 (1.7) | 7.0 (1.7) | 6.9 (1.7) | 0.94 |
| PHQ-8 score, mean (SD) | 5.9 (5.6) | 5.8 (5.1) | 5.8 (5.3) | 5.8 (5.3) | 0.98 |
1n = 74 for DE, n = 90 for IC, n = 96 for CC.
aExcluding missing category.
Glycemic Control
Significant reductions in mean A1C levels were observed at all follow-ups for all interventions with no significant differences between groups at any follow-up (Table 2). In preplanned within-arm analyses, we examined whether baseline A1C level was a factor in outcomes by analyzing by baseline A1C tertiles. In the bottom tertile (7.5–8.2% [58–66 mmol/mol]), no significant differences from baseline were observed in any group. In the middle tertile (8.3–9.2% [67–77 mmol/mol]), the mean A1C was significantly lower at all follow-ups for the CC group only. In the top tertile (≥9.3% [78 mmol/mol]), all three interventions showed significant reductions in A1C levels at all follow-ups. Analyses adjusted for baseline A1C level yielded the same pattern of effects.
Table 2.
Mean (SD) A1C percentages by intervention arm, assessment, and baseline A1C tertile
| Treatment arm | Month | A1C level |
|||
|---|---|---|---|---|---|
| ≤8.20% | 8.21–9.20% | >9.20% | Total | ||
| CC | 0 | 7.8 (1.2) | 8.7 (1.2) | 10.8 (1.2) | 8.9 (1.5) |
| 4 | 7.6 (1.1) | 8.0 (1.1)* | 9.8 (1.1)* | 8.3 (1.4)* | |
| 8 | 7.8 (1.1) | 8.1 (1.1)* | 10.0 (1.1)* | 8.5 (1.5)* | |
| 12 | 7.8 (1.1) | 8.0 (1.1)* | 10.0 (1.1)* | 8.5 (1.5)* | |
| IC | 0 | 7.8 (1.2) | 8.7 (1.2) | 11.0 (1.2) | 9.3 (1.5) |
| 4 | 7.9 (1.1) | 8.4 (1.1) | 9.2 (1.1)* | 8.5 (1.4)* | |
| 8 | 7.8 (1.1) | 8.5 (1.1) | 9.4 (1.1)* | 8.6 (1.4)* | |
| 12 | 8.0 (1.1) | 8.4 (1.1) | 9.7 (1.1)* | 8.8 (1.4)* | |
| DE | 0 | 7.9 (1.2) | 8.6 (1.2) | 10.7 (1.2) | 9.1 (1.5) |
| 4 | 7.6 (1.1) | 8.5 (1.1) | 10.0 (1.1)* | 8.7 (1.5)* | |
| 8 | 7.9 (1.1) | 8.4 (1.1) | 9.7 (1.1)* | 8.7 (1.4)* | |
| 12 | 7.6 (1.1) | 8.5 (1.1) | 9.5 (1.1)* | 8.5 (1.4)* | |
*P < 0.05 for within-treatment comparison with baseline mean. Sidak-adjusted significance levels were used for tertile comparisons.
Secondary Outcomes
BMI
No significant differences in mean BMI were observed between groups at any follow-up (Table 3).
Table 3.
Mean (SD) values for secondary outcomes by treatment arm and assessment
| Treatment arm | Month | BMI (kg/m2) | WC (cm) | Systolic BP1 (mmHg) | DBP1 (mmHg) | DD | DSE | PHQ-8 |
|---|---|---|---|---|---|---|---|---|
| CC | 0 | 35.7 (7.5) | 118.7 (17.5) | 128.1 (12.2) | 74.7 (7.5) | 2.2 (1.0) | 6.8 (1.7) | 5.9 (5.2) |
| 4 | 35.3 (6.8)* | 117.4 (15.8)* | 127.4 (12.0) | 72.7 (7.4)* | 1.6 (1.3)* | 7.8 (2.1)* | 4.3 (5.0)* | |
| 8 | 35.3 (6.8)* | 117.0 (15.9)* | 127.3 (12.1) | 73.6 (7.5) | 1.8(1.2)* | 7.4 (1.9)* | 4.8 (5.0)* | |
| 12 | 35.2 (6.7)* | 116.8 (15.5)* | 128.8 (12.2) | 74.3 (7.5) | 1.7 (1.0)* | 7.5 (1.9)* | 5.3 (4.8) | |
| IC | 0 | 36.0 (7.5) | 117.3 (17.5) | 127.1 (12.3) | 74.5 (7.6) | 2.3 (1.0) | 6.9 (1.7) | 5.8 (5.2)* |
| 4 | 35.8 (6.7) | 116.5 (15.4) | 125.4 (12.3) | 73.1 (7.6) | 1.9 (1.3)* | 7.6 (2.2)* | 4.6 (4.9) | |
| 8 | 35.9 (6.7) | 116.3 (15.6) | 124.9 (12.3) | 72.1 (7.6)* | 2.1 (1.1) | 7.5 (1.9)* | 5.2 (4.9) | |
| 12 | 36.1 (6.7) | 116.9 (15.5) | 125.4 (12.0) | 71.7 (7.4)* | 1.9 (1.0)* | 7.4 (1.9)* | 5.1 (4.9) | |
| DE | 0 | 36.0 (7.5) | 118.3 (17.5) | 126.5 (12.1) | 74.1 (7.5) | 2.4 (1.0) | 6.9 (1.7) | 5.8 (5.2) |
| 4 | 35.8 (6.9) | 117.1 (16.0)* | 127.7 (12.2) | 74.9 (7.5) | 2.0 (1.3)* | 7.1 (2.2) | 5.3 (5.0) | |
| 8 | 35.8 (6.6) | 117.4 (15.3) | 129.8 (11.9) | 74.9 (7.3) | 2.2 (1.1) | 7.1 (1.9) | 5.3 (4.9) | |
| 12 | 35.6 (6.5) | 116.6 (15.2)* | 129.2 (12.0) | 73.7 (7.4) | 2.2 (1.0) | 7.3 (1.9) | 5.5 (4.9) |
1Baseline-adjusted means are presented.
*P < 0.05 for within-treatment comparison with baseline mean.
Compared with baseline, there were small, significant reductions in BMI only for the CC group at 4 months (−0.354, P = 0.009), 8 months (−0.393, P = 0.027), and 12 months (−0.474, P = 0.021). Stratifying by baseline tertiles, no differences by treatments were observed for the bottom and middle tertiles. For the top tertile, the BMI of the CC group was significantly lower than that of the DE group at all assessments, and was lower than that of the IC group at 12 months. Also, mean BMI was significantly lower than baseline for the CC group at 12 months, and for the IC group only at 4 months.
Waist Circumference
No significant differences in mean WC were observed between groups at any follow-up. Compared with baseline, there were significant WC reductions for the CC arm at all follow-ups (P < 0.001), and for the DE arm at 4 and 12 months (Table 3). We stratified by baseline tertiles and found that, for the bottom tertile, means were significantly lower at all follow-ups for the CC group only; and for the top tertile, only means for the CC group were significantly lower at 8 and 12 months.
Systolic and Diastolic BP
Systolic BP
The IC group mean was significantly lower than the DE group mean at 8 months (P = 0.021). No significant differences from baseline were observed for any intervention at any follow-up (Table 3). Stratifying by tertiles, there was a significant increase in systolic BP (SBP) for the bottom tertile, and no differences for the middle tertile for any arm. For the top tertile, the IC arm showed significant declines at all follow-ups, whereas the CC arm showed significant declines at 4 months only.
Diastolic BP
The IC group mean was significantly lower than the DE group mean at 8 months (P = 0.032) and was lower than the CC group mean at 12 months. Compared with baseline, IC group mean diastolic BP (DBP) was significantly lower at 8 and 12 months; for the CC arm, only the 4-month DBP was significantly lower, and the DE group showed no differences (Table 3). Stratifying into baseline DBP tertiles, the IC group mean was significantly lower than the CC group mean at 12 months in the top tertile.
Diabetes Distress
The CC group mean was significantly lower than the DE group mean at 12 months (P = 0.009) and was marginally lower at 8 months (P = 0.057). CC group mean was significantly lower than baseline at 4 months (P < 0.001), 8 months (P = 0.003), and 12 months (P < 0.001); this was also true for the IC group at 4 months (P = 0.006) and 12 months (P = 0.003). The DE group mean was lower only at 4 months (P = 0.014) (Table 3).
Diabetes Self-Efficacy
The CC group mean was marginally greater than the DE group mean at 4 months (P = 0.058), and no other group differences emerged. Compared with baseline, both the IC and CC groups improved, with baseline-adjusted means higher at all follow-ups (all P values <0.002). The DE group showed no improvement (all P values > 0.081) (Table 3).
Depressive Symptoms
There were no differences between group means. Compared with baseline, the CC group had lower PHQ-8 scores at 4 months (P = 0.001) and 8 months (P = 0.014). The IC group improved only at 4 months (P = 0.009). The DE group showed no improvement (Table 3).
Participant Satisfaction
Interventions are only effective if participants value them. We examined several indices of satisfaction. The mean numbers of sessions completed (of 12) were 10.43 (CC group) and 9.83 (IC group) (1.94, of 2, in the DE group). This very high attendance is strong evidence for participant engagement. On the participant satisfaction questionnaire, participants were asked about “satisfaction with amount of help received”; 83.5% (CC group), 70.3% (IC group), and 41.3% (DE group) were “very satisfied,” the CC arm reported higher satisfaction than the IC arm (P = 0.05), and the percentages for both the CC and IC group were greater than those for the DE group (P < 0.001). Only 1.3% of CC group participants and 0% of IC group participants were “mostly” or “very dissatisfied” versus 22.2% of DE group participants. Asked to what extent the DSP helped them manage diabetes more effectively, 82% (CC group), 66% (IC group), and 38% (DE group) responded “a great deal,” with CC group percentages greater than those for the IC group (P = 0.02) and the DE group (P < 0.001), and percentages for the IC group greater than those for the DE group (P < 0.001). Asked whether they would recommend the DSP to a friend/family member, 85% (CC group), 78% (IC group), and 44% (DE group) said “yes, enthusiastically,” with both the CC and IC groups greater than the DE group (P < 0.001). Including those replying simply “yes” finds near total acceptance for both the CC and IC interventions. If offered face-to-face interventions, 24% said they were “somewhat” or “very unlikely” to participate.
Conclusions
Value of a Couples Intervention
For the primary outcome of glycemic control, although there were no between-group differences, subgroup within-arm analyses found that the CC intervention was efficacious in lowering A1C levels for individuals with a high A1C level (i.e., 8.3–9.2% [67–77 mmol/mol]), although a comparable individual intervention, and DE alone, were not. And, it is highly significant that these benefits were sustained for a full 8 months after the intervention concluded. In contrast, all three interventions were efficacious for those with very high A1C level (i.e., ≥9.3% [78 mmol/mol]).
Although other behavioral interventions have led to improved glycemic control, analyses examining the data by baseline A1C level often show that it is only the group with the highest A1C levels (i.e., very poor glycemic control) that improves. A three-session intervention for patients plus family member versus usual care reported a 0.4% difference in A1C level (P = 0.04) (25). However, stratification by baseline A1C level showed no significant differences within groups with moderately high A1C levels (8.0–8.4% or 8.5–9.4%). Those with baseline A1C levels ≥9.5% drove the positive results, with an A1C decrease of 1.2%. Similarly, in a trial of telephonic education versus education with print materials, positive results (0.4% difference between groups) were driven by significant change only for those with baseline A1C levels >9% (26). It is significant that in the DSP, the couples intervention resulted in a significant decrease in A1C for the high—but not exceedingly high—A1C group, the group that is most commonly seen in clinical practice. Compared with baseline, changes in BMI and WC also favored the CC intervention; however, although statistically significant, changes were clinically small. Changes in BP were more variable, but favored the individual intervention.
We also assessed psychosocial outcomes because partner engagement might have a negative effect on the patient, including erosion of their sense of self-efficacy and increased distress (27). We found that both individual and couples interventions led to improved DSE, with a somewhat stronger short-term effect of the CC intervention. The CC intervention led to longer-lasting decreases in depressive symptoms. Although both the IC and CC interventions resulted in less DD, again, the CC intervention showed a somewhat stronger effect compared with DE. Again, though statistically significant, some of these changes were small and may not be clinically meaningful, but they do help to allay concerns that partner involvement leads to increased DD or decreased DSE. Finally, the CC intervention yielded the highest levels of participant satisfaction and perceived value.
How do we understand the positive effects of a couples intervention in this clinical trial? This may reflect the benefits of social support, and of having a partner “coach” to reinforce healthy behaviors. This is consistent with the many studies indicating that social support facilitates coping with chronic illness, and that greater partner provision of health-related support and better marital functioning relate to better health outcomes (28). It may also reflect a direct effect of decreased relationship stress on health outcomes (29). Although the underlying mechanisms remain elusive, there have been calls for a “family-focused” approach to disease and diabetes management (8,30). Yet, the couples intervention literature is sparse. Martire et al. (31) performed a meta-analysis of couples interventions for varied diseases and found positive impacts, but only on pain, depression, and relationship quality. A meta-analysis (32) of couples versus individual weight loss interventions found a significant, but small and short-lived, benefit of interventions that included partners. Couples interventions with fibromyalgia patients (33) and for smoking cessation (34) reported no benefit.
For adults with type 2 diabetes, the few family intervention studies are often limited by selection bias, limited follow-up, and lack of RCT standards. A systematic review of family interventions for adults with diabetes found only 10 studies, 6 with randomization and 6 that targeted type 2 diabetes. Of the four studies that measured A1C 6 months after intervention, only one (25) reported significant intervention effects (final n = 12–15/group), and only in those with an A1C level ≥9.5%. An RCT (n = 28/group) comparing a family partnership intervention to usual care reported absolute improvements in both groups, with no significant differences between groups in A1C level or BMI (35).
The limited efficacy of reported couples interventions may reflect the limits of underlying models, which typically define a couples intervention simply as one that includes partners. We adopted a “dyad-level” model, reflecting the “interdependence” of partners (36). Interdependence theory (17,18), our theoretical base, suggested an intervention to promote communal coping, effective communication, and shared problem-solving. We may have avoided the trap of partner involvement being experienced as a form of social control, and thus eliciting behavioral resistance and emotional distress (27). Our data support the hypothesis that the active engagement of partners, and promoting their collaborative coping, in diabetes behavior change interventions may result in significant and lasting improvements in glycemic control for individuals with poor glycemic control, and modest improvements in weight and some psychosocial outcomes. It will be important to identify the underlying mechanisms to further refine couples interventions and build on these positive effects.
Value of DE
For glycemic control, there was a benefit of the DE intervention alone, resulting in no significant differences between group means. However, this finding reflected the benefit of DE only for those with very high A1C levels. The meta-analysis of 31 RCTs of education versus usual care by Norris et al. (4) found that there was an initial benefit (−0.76% at post-test) that declined with time (−0.26% at ≥4 months), and contact time was the only predictor of improved A1C level. In a systematic review (37) of behavioral interventions for adults with type 2 diabetes, effect sizes for “minimally intensive” (<10 h) interventions were not “clinically significant” (i.e., <0.04% change in A1C level); thus, longer interventions were recommended. Our two-session DE group showed a 1-year decrease in A1C of 0.57%; but again, the data driving this was the decrease of 1.19% for those in the highest tertile (A1C ≥9.3%). Thus, our data suggest that even brief DE can be helpful for those with very high A1C levels, but these patients require additional interventions to approach glycemic targets (e.g., intensification of medical therapy). The DE group showed a benefit in WC, though not in DSE, DD, or depressive symptoms.
We did not include a no-active-intervention usual care arm; thus, the DE group data may reflect usual care outcomes. However, given the strong evidence for the positive effects of diabetes self-management education (DSME) on glycemic control (38), we feel reasonably confident that the changes in the very high A1C group were due to the DE. The American Diabetes Association takes the formal position that all diabetes patients should receive DSME at diagnosis and as needed, and diabetes self-management support (DSMS) thereafter to ensure sustained change (39). Thus, DSME/DSMS is now the standard for usual care. Because our intervention did not meet specific DSME/DSMS standards (40), the added benefit of our DE intervention after a more comprehensive DSME program would be interesting to assess.
Value of Phone
Analyses report that in-person programs are more effective than those using technology, including telephones, and in-person intervention is recommended (4,37). However, there is a very low rate of participation in DSME. In one study (41), 4% of Medicare patients participated in DSME; in another study (42), 6.8% did so in the year after diagnosis. This is the first study we are aware of to provide a couples intervention by telephone. The very high level of engagement and satisfaction clearly support the feasibility and acceptability of a couples intervention. And, 24% said it was unlikely they would have participated if the intervention were only offered face to face. The high proportion of males enrolled (61.6%) may mean that males are more open to a phone intervention.
Strengths/Limitations
Our study is unique in its targeting of a committed partner relationship. It contains the key elements of valid couples intervention trials (i.e., is theoretically grounded and includes an individual intervention comparison group) (43). Without an individual intervention comparator, one cannot conclude that partner involvement has an effect, even if differences emerge, just that the intervention was efficacious compared with usual care. Other strengths include an RCT design, blinded assessments, a high proportion of male (61.6%) and minority (30.4%) participants, and a 1-year follow-up. The main limitation was that couples had been in this relationship for many years (mean 25.5 ± 14.8 years, range 1–67 years); thus, the results are not generalizable to relationships of shorter duration. Also, because both partners had to be willing to participate, understanding that a couples intervention was one arm, it is possible that couples were recruited who were specifically interested in a couples intervention, and the results may not generalize to those not interested in one. The attrition rate, and the lack of significant between-group differences for some outcomes, are also concerns in terms of the strength of the conclusions we can draw. Finally, because we did not track changes in treatment over the course of the trial, it is possible that treatment changes were instituted differently across arms.
Conclusions
For adults with poorly controlled type 2 diabetes, engaging their committed partner in a collaborative couples intervention may be needed to yield significant and lasting improvement in glycemic control. For those with exceedingly high A1C levels, diabetes education alone can achieve improvement, but they require additional intervention to approach glycemic targets. A couples intervention appears to benefit participants in other ways, too. This approach shows promise for enhancing the potential positive impact of partners of type 2 diabetes patients in poor glycemic control.
Article Information
Acknowledgments. The authors thank the patients and partners who participated in the study. The authors also thank the excellent educators Pam Blackmer, Mary Griffin, Carina Lagua, Juliann Mellen, Jacqueline Pallas, Nancy Rindfuss, Christina Bellino Sullivan, and Jennifer Vallone, who delivered the interventions. In addition, the authors thank Dr. Roberto Izquierdo, at the State University of New York Upstate Medical University, who served as the Data and Safety Monitoring Official; and Dr. Kasandra Scales, who supported all activities during the first year of the study. The authors also thank Ifeoma Okwu, Valerie Pintado, and Tatum Toner, the research staff at the University of California, San Francisco, who performed assessments and recruitment activities. Finally, the authors thank Roche, Inc., who provided some material support.
Funding. This study was supported by National Institutes of Health (NIH) grant 1R18-DK-080867-01A2. The first year of the study was funded by an NIH Diversity Fellowship Supplement.
Duality of Interest. L.F. reported that he serves as a consultant or member of an advisory board for Roche Diagnostics, Elli Lilly, Novo Nordisk, Sanofi, and Abbott. R.S.W. reported that she receives research grant funding from Medtronic, Mylan GmbH Inc., Calibra Medical Inc., Sanofi, Novo Nordisk, and Intarcia. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.M.T. contributed to every aspect of this article. L.F., J.S., and R.S.W. contributed to the study design, researching of the data, discussion, and editing of the manuscript. D.A.C., J.D., D.M.H., and P.F. contributed to researching of the data, discussion, and review and editing of the manuscript. P.M.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral poster form at the 75th Scientific Sessions of the American Diabetes Association, Boston MA, 5–9 June 2015.
Footnotes
References
- 1.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Dall TM, Halder P, Gallo P, Kowal SL, Hogan PF; American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]
- 5.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot 1996;10:282–298 [DOI] [PubMed] [Google Scholar]
- 6.Lyons RF, Sullivan MJL, Rivo PG. Relationships in Chronic Illness and Disability. Thousand Oaks, CA, Sage, 1995 [Google Scholar]
- 7.Gonder-Frederick LA, Cox DJ, Ritterband LM. Diabetes and behavioral medicine: the second decade. J Consult Clin Psychol 2002;70:611–625 [DOI] [PubMed] [Google Scholar]
- 8.Fisher L, Weihs KL. Can addressing family relationships improve outcomes in chronic disease? Report of the National Working Group on Family-Based Interventions in Chronic Disease. J Fam Pract 2000;49:561–566 [PubMed] [Google Scholar]
- 9.Trief PM, Himes CL, Orendorff R, Weinstock RS. The marital relationship and psychosocial adaptation and glycemic control of individuals with diabetes. Diabetes Care 2001;24:1384–1389 [DOI] [PubMed] [Google Scholar]
- 10.Trief PM, Morin PC, Izquierdo R, et al. . Marital quality and diabetes outcomes: the IDEATel Project. Fam Syst Health 2006;24:318–331 [Google Scholar]
- 11.Torenholt R, Schwennesen N, Willaing I. Lost in translation--the role of family in interventions among adults with diabetes: a systematic review. Diabet Med 2014;31:15–23 [DOI] [PubMed] [Google Scholar]
- 12.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med 2007;32:419–434 [DOI] [PubMed] [Google Scholar]
- 14.Trief PM. Challenges and lessons learned in the development and implementation of a couples-focused telephone intervention for adults with type 2 diabetes: the Diabetes Support Project. Transl Behav Med 2011;1:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piantadosi S. Clinical trials: A Methodologic Perspective. New York, Wiley, 1997 [Google Scholar]
- 16.Bandura A, Walters RH. Social Learning Theory and Personality Development. New York, Wiley, 1978 [Google Scholar]
- 17.Kelley HH, Thibaut TW. Interpersonal Relations: A Theory of Interdependence. New York, Wiley, 1978 [Google Scholar]
- 18.Rusbult CE, Van Lange PA. Interdependence, interaction, and relationships. Annu Rev Psychol 2003;54:351–375 [DOI] [PubMed] [Google Scholar]
- 19.Laurenceau JP, Stanley SM, Olmos-Gallo A, Baucom B, Markman HJ. Community-based prevention of marital dysfunction: multilevel modeling of a randomized effectiveness study. J Consult Clin Psychol 2004;72:933–943 [DOI] [PubMed] [Google Scholar]
- 20.Trief P, Sandberg JG, Ploutz-Snyder R, et al. . Promoting couples collaboration in type 2 diabetes: the Diabetes Support Project pilot data. Fam Syst Health 2011;29:253–261 [DOI] [PubMed] [Google Scholar]
- 21.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984;310:341–346 [DOI] [PubMed] [Google Scholar]
- 22.Polonsky WH, Fisher L, Earles J, et al. . Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 23.Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: a randomized trial. Diabetes Educ 2009;35:641–651 [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173 [DOI] [PubMed] [Google Scholar]
- 25.Keogh KM, Smith SM, White P, et al. . Psychological family intervention for poorly controlled type 2 diabetes. Am J Manag Care 2011;17:105–113 [PubMed] [Google Scholar]
- 26.Chamany S, Walker EA, Schechter CB, et al. . Telephone intervention to improve diabetes control: a randomized trial in the New York City A1c Registry. Am J Prev Med 2015;49:832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook KS, August KJ, Stephens MAP, Franks MM. When does spousal social control provoke negative reactions in the context of chronic illness? The pivotal role of patients’ expectations. J Soc Pers Relat 2011;28:772–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martire LM, Franks MM. The role of social networks in adult health: introduction to the special issue. Health Psychol 2014;33:501–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull 2001;127:472–503 [DOI] [PubMed] [Google Scholar]
- 30.Marrero DG, Ard J, Delamater AM, et al. . Twenty-first century behavioral medicine: a context for empowering clinicians and patients with diabetes: a consensus report. Diabetes Care 2013;36:463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is it beneficial to involve a family member? A meta-analysis of psychosocial interventions for chronic illness. Health Psychol 2004;23:599–611 [DOI] [PubMed] [Google Scholar]
- 32.Black DR, Gleser LJ, Kooyers KJ. A meta-analytic evaluation of couples weight-loss programs. Health Psychol 1990;9:330–347 [DOI] [PubMed] [Google Scholar]
- 33.deVoogd JN, Knipping AA, deBlecourt ACE, vanRijswijk MH. Treatment of fibromyalgia syndrome with psychomotor therapy and marital counseling. J Musculoskelet Pain 1993;1:273–281 [Google Scholar]
- 34.Palmer CA, Baucom DH, McBride CM. Couple approaches to smoking cessation. In The Psychology of Couples and Illness. Schmaling T, Sher TG, Eds. Washington, DC, American Psychological Association, 2000, p. 311–336 [Google Scholar]
- 35.Kang CM, Chang SC, Chen PL, et al. . Comparison of family partnership intervention care vs. conventional care in adult patients with poorly controlled type 2 diabetes in a community hospital: a randomized controlled trial. Int J Nurs Stud 2010;47:1363–1373 [DOI] [PubMed] [Google Scholar]
- 36.Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc Sci Med 2006;62:1369–1380 [DOI] [PubMed] [Google Scholar]
- 37.Agency for Healthcare Research and Quality. Behavioral programs for diabetes mellitus [article online], 2014. Available from www.ahrq.gov/research/findings/evidence-based-reports/er221-abstract.html. Accessed 19 June 2015
- 38.Steinsbekk A, Rygg LØ, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers MA, Bardsley J, Cypress M, et al. . Diabetes self management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care 2015;38:1372–1382 [DOI] [PubMed] [Google Scholar]
- 40.Haas L, Maryniuk M, Beck J, et al.; 2012 Standards Revision Task Force . National standards for diabetes self-management education and support. Diabetes Care 2014;37(Suppl. 1):S144–S153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duncan I, Birkmeyer C, Coughlin S, Li QE, Sherr D, Boren S. Assessing the value of diabetes education. Diabetes Educ 2009;35:752–760 [DOI] [PubMed] [Google Scholar]
- 42.Li R, Shrestha SS, Lipman R, Burrows NR, Kolb LE, Rutledge S; Centers for Disease Control and Prevention (CDC) . Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes–United States, 2011-2012. MMWR Morb Mortal Wkly Rep 2014;63:1045–1049 [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell TA. Physical disorder and effectiveness research in marriage and family therapy. In Effectiveness Research in Marriage and Family Therapy. Sprenkle D, Ed. Alexandria, VA, American Association for Marriage and Family Therapy, 2002, p. 311–337 [Google Scholar]