Abstract
OBJECTIVE
The patterns of estimated glomerular filtration rate (eGFR) decline to end-stage renal disease (ESRD) in patients with type 1 diabetes has not been conclusively described. Decline could be linearly progressive to ESRD but with a variable rate. Conversely, decline may be linear but interrupted by periods of plateaus or improvements.
RESEARCH DESIGN AND METHODS
This observational study included 364 patients with type 1 diabetes attending the Joslin Clinic who developed ESRD between 1991 and 2013. We retrieved serum creatinine measurements from clinic visits or research examinations up to 24 years (median 6.7 years) preceding the onset of ESRD. Using serial measurements of serum creatinine to estimate renal function (eGFR), we used regression-based spline methods and a data smoothing approach to characterize individual trajectories of eGFR over time for the 257 patients with five or more data points.
RESULTS
The rate of eGFR decline per year ranged widely, from −72 to −2 mL/min/1.73 m2 (median −8.5). The trajectories, as characterized with linear regression-based spline models, were linear or nearly so for 87% of patients, accelerating for 6%, and decelerating for 7%. Smoothed trajectories evaluated by a Bayesian approach did not significantly depart from a linear fit in 76%.
CONCLUSIONS
The decline of eGFR in type 1 diabetes is predominantly linear. Deviations from linearity are small, with little effect on the expected time of ESRD. A single disease process most likely underlies renal decline from its initiation and continues with the same intensity to ESRD. Linearity of renal decline suggests using slope reduction as the measure of effectiveness of interventions to postpone ESRD.
Introduction
End-stage renal disease (ESRD) and associated morbidity and mortality are major health problems in patients with type 1 diabetes. The lifetime risk of ESRD in type 1 diabetes is 10–15% (1–3). ESRD develops many decades after the disease onset as a result of renal decline (3–5). The decline trajectory and underlying mechanisms are not well understood. Theoretically, the decline may be abrupt, as in acute renal failure (6), episodic with plateaus or periods of improvement (7), or inexorably progressive (8). Each pattern points to a different pathophysiology. Knowledge of the patterns and their frequencies can guide research of the disease mechanisms and help develop new interventions.
Linearity of renal decline in various kidney conditions was already postulated four decades ago (9). Plots of serum creatinine reciprocal were used as a predictive tool for ESRD onset (8,10). Several studies have since examined trajectories of renal function changes, with few patients reaching ESRD. Some studies concluded that trajectories were linear (8,11–13), whereas others found significant variation and nonlinearity (7,14,15). These studies were small (7,8,11–14), included many patients with advanced renal failure, often had short follow-up time, and did not use analytical tools that are now available for evaluating this question.
In our recent large study of patients with type 1 diabetes with normal renal function and proteinuria, estimated glomerular filtration rate (eGFR) decline was present in only one-half. Decline was predominantly linear within an individual, but slopes differed widely among individuals (4). Most of the patients in that study did not reach ESRD, making their trajectories incomplete. Patients who reached ESRD were too few to draw unambiguous conclusions and to rule out these ESRD events being results of acute kidney injury complicating chronic kidney disease.
Different findings were recently reported from the African American Study of Kidney Disease and Hypertension (AASK) (16). That study included 846 patients with at least 3 years of follow-up (median 9 years) and ≥8 GFR estimates. Baseline eGFR was impaired (median 50 mL/min/1.73 m2). The authors reported that more than 41% had nonlinear eGFR trajectories. This finding contrasts sharply with our results in patients with type 1 diabetes and proteinuria (4). Differences in several factors of a biologic nature may have contributed to this discrepancy: 1) mechanisms underlying hypertensive and diabetic kidney diseases, 2) race (17), and 3) characteristics of study subjects and inclusion criteria. For example, volunteers recruited into the AASK study had predominantly slow rates of eGFR decline (18). However, substantially different analytical approaches and definitions of linearity may account for the discrepant conclusions (4,19). We therefore applied both methods in the current study: the method used in our previous publication and the one used for analysis of AASK study data, so the effect of the analytic approach can be assessed. More importantly, in the current study we evaluated, for the first time, complete trajectories of eGFR decline specifically in patients with type 1 diabetes who developed ESRD.
Research Design and Methods
Joslin Clinic Population of Patients With Type 1 Diabetes
A total of 3,500 adult patients with type 1 diabetes diagnosed before age 40 remain under the care of Joslin Clinic, an institution established in 1898 and devoted to the treatment of diabetes (5). Most of these patients come to the clinic within 5 years after the diabetes diagnosis and remain under care for a long period, frequently for life. The current study included the 364 patients who developed ESRD between 1991 and 2013 and were previously enrolled into one or more studies on the natural history of diabetic nephropathy in type 1 diabetes: Joslin Proteinuria Cohort (20), 1st Joslin Study on Natural History of Microalbuminuria (21–24), 2nd Joslin Study on Natural History of Early Renal Decline (25,26), and Joslin Study on Genetics of Diabetic Nephropathy in type 1 diabetes (27,28). Patients with a diagnosis of nondiabetic kidney disease present in their medical records were excluded from enrollment in these studies.
Participants were monitored until 2013 with the goal of obtaining blood and urine specimens at least every 2 years and ascertaining onset of ESRD and deaths. Research specimens were obtained during routine clinic visits or special home visits and stored at −80°C for future use. All clinical data and laboratory determinations for the albumin-to-creatinine ratio (ACR), serum creatinine, and glycated hemoglobin A1c (HbA1c) for all 364 patients included in this study were retrieved from Joslin medical records.
The Joslin Diabetes Center Institutional Review Board approved the study protocols and informed consent procedures for the above studies.
Assessment of Urinary Albumin and HbA1c
In the Joslin Clinic laboratory, albumin concentrations in spot urines were measured by immunonephelometry on a BN100 (Behring, Marburg, Germany) with N Albumin kits. Creatinine measurements in urine were assayed by Jaffe’s modified picrate method. Urinary albumin in milligrams was expressed per gram of urinary creatinine (ACR) (29). HbA1c was measured during routine clinic visits and was standardized to Diabetes Control and Complications Trial units (30).
Laboratory Assessment of Renal Function
Routine measurements of serum creatinine are performed for Joslin patients at Joslin Clinic laboratory at least once a year. In 2011–2014, blood specimens obtained as part of research examinations were sent to the Advanced Research and Diagnostic Laboratory at University of Minnesota to measure serum concentrations of creatinine using the Roche enzymatic assay (prod. no. 11775685) on a Roche/Hitachi Mod P analyzer. This method was calibrated to be traceable to an isotope dilution mass spectrometry reference assay and was verified by measuring National Institutes of Standards and Technology Standard Reference Material No. 967. Whenever a routine visit (n = 1,133) coincided with a research examination, we used duplicate measurements in samples from the same blood draw to calibrate Joslin clinical measurements to the assay method used at the Laboratory at University of Minnesota (4,30). This calibration diminished the possible effect of changes in laboratory assay and equipment over time.
A total of 4,721 calibrated serum creatinine determinations from clinic visits in 364 study patients were used to estimate eGFR with the Chronic Kidney Disease Epidemiology Collaboration formula (31). For our goal of tracing the trajectory of eGFR decline at the beginning of follow-up (baseline) was dated from earliest evaluation of renal function (measurement of serum creatinine) while it was ≥60 mL/min/1.73 m2 and with persistent proteinuria. In patients with eGFR <60 mL/min/1.73 m2 or entering the follow-up without proteinuria, we traced the decline retrospectively from ESRD up to the last observation in chronic kidney disease (CKD) stage 1 to exclude the period of normal and stable renal function before the onset of decline.
Ascertainment of ESRD and Deaths
All patients enrolled into the Joslin Studies described above were queried against rosters of United States Renal Data System (USRDS) and National Death Index (NDI) covering all events up to the end of 2013. ESRD was defined by a match with the USRDS roster (n = 301) or a listing of renal failure among the causes of death on an NDI death certificate (n = 63). Onset of ESRD was given the date of first dialysis or kidney transplantation or date of death for those captured by death certificate. USRDS and NDI data were screened for disease codes indicating nondiabetic causes of ESRD.
Statistical Analysis
Continuous variables are summarized as medians and quartiles, and categorical variables are presented as numbers and percentages. Comparisons between medians were done with the Wilcoxon test, and categorical variables were compared with the χ2 test.
Linear trajectories were estimated with least-squares regression. To characterize eGFR trajectories, as previously reported (4), we used an approach described by Jones and Molitoris (32) in 257 patients with ≥5 eGFR observations placed at least 1 month apart. A spline function was fitted by least squares with position of the knot determined by minimizing the residual sum of the squares. Spline function was tested against the linear model with a partial F test at a significance level of 0.05. Nonlinear trajectories were classified as clinically consequential only if predicted time to ESRD (eGFR = 10 mL/min/1.73 m2) by the simple linear fit missed the observed time to ESRD by ≥1 year; otherwise they were classified as clinically inconsequential. Analysis was performed in SAS 9.3 software (SAS Institute, Inc., Cary, NC).
The Bayesian approach to modeling trajectories in the 257 patients used penalized thin-plate splines, as previously described (16,19). The analysis was performed with R 3.1.0 software (The R Foundation for Statistical Computing, Vienna, Austria) and OpenBUGS 3.2.3 software (University of Helsinki, Helsinki, Finland). Knots were placed 1 year apart in patients with ≥3 years of follow-up and every 0.2 year in patients with <3 years of follow-up. We used noninformative and conjugate priors. Starting values of fixed-effect parameters were estimated with a general additive model using the SemiPar package for R. We performed 700,000 iterations in 4 chains to assess convergence. We discarded the first 200,000 run-in iterations and saved 1 of every 50 from the remaining 500,000 to obtain 10,000 posterior samples. A linear trajectory was then fitted to the smoothed data, and the smoothed trajectory was classified as clinically consequential nonlinearity if any point-wise difference between the smooth and linear trajectories was ≥15 mL/min/1.73 m2. Because the threshold of 15 mL/min/1.73 m2 was an arbitrary choice, we performed a sensitivity analysis using the values of 5 and 10 mL/min/1.73 m2.
Results
Cohort Characteristics
The study group comprised 364 Eastern Massachusetts residents, 186 men and 178 women (48.9%) with type 1 diabetes. All were long-term (>11 years) patients of Joslin Clinic (Boston, MA), and 95% were Caucasian (self-reported). Characteristics are summarized in Table 1. Median age at baseline was 33 years, and median diabetes duration was 19 years. Diabetes was diagnosed in most patients during childhood or adolescence. Glycemic control was poor: median HbA1c for the group was 9.5% (80.3 mmol/mol). Median ACR was 1,262 mg/g. Median baseline eGFR was 73 mL/min/1.73 m2. At time of enrollment into Joslin studies, median blood pressure was 134/80 mmHg. There were 66.2% patients treated with ACE inhibitors (ACE-I) or angiotensin receptor blockers (ARB). This proportion reflects gradual entry of renoprotective treatment into clinical practice in 1990s.
Table 1.
Characteristics of the study group according to availability of five observations
| eGFR observations |
||||
|---|---|---|---|---|
| All |
≥5 |
<5 |
P value |
|
| Characteristic |
N = 364 |
N = 257 |
N = 107 |
— |
| Women | 178 (48.9) | 128 (49.8) | 50 (46.7) | 0.59 |
| At the time of earliest eGFR observation | ||||
| Age (years) | 33 (27, 40) | 34 (28, 40) | 32 (26, 40) | 0.42 |
| Diabetes duration (years) | 19 (15, 26) | 19 (16, 26) | 18 (14, 24) | 0.047 |
| Age of diabetes onset (years) | 12 (8, 17) | 12 (7, 17) | 12 (8, 17) | 0.50 |
| ACR (mg/g) | 1,262 (526, 2,500) | 1,299 (559, 2,500) | 966 (471, 2,500) | 0.18 |
| HbA1c (%) | 9.5 (8.4, 10.8) | 9.5 (8.4, 10.8) | 9.6 (8.3, 11.0) | 0.98 |
| HbA1c (mmol/mol) | 80.3 (68.3, 94.5) | 80.3 (68.3, 94.5) | 81.4 (67.2, 96.7) | |
| eGFR (mL/min/1.73 m2) | 73 (57, 97) | 71 (56, 87) | 84 (63, 109) | 0.001 |
| Recorded at the research enrollment examination | ||||
| Systolic blood pressure (mmHg) | 134 (125, 148) | 135 (127, 150) | 134 (120, 144) | 0.21 |
| Diastolic blood pressure (mmHg) | 80 (74, 88) | 80 (74, 86) | 82 (78, 88) | 0.16 |
| Treatment with ACE-I or ARB | 241 (66.2) | 169 (65.8) | 72 (67.3) | 0.78 |
| Smoking | ||||
| Current | 75 (20.6) | 54 (21.0) | 21 (19.6) | 0.77 |
| Past | 111 (30.5) | 74 (28.8) | 37 (34.6) | 0.21 |
| Follow-up | ||||
| Follow-up duration (years) | 6.6 (4.2, 9.9) | 6.7 (4.4, 9.8) | 6.1 (3.7, 10.5) | 0.22 |
| eGFR measurements | 10 (4, 18) | 15 (9, 24) | 3 (2, 4) | |
| eGFR measurements per year | 1.7 (0.6, 3.4) | 2.7 (1.3, 3.9) | 0.5 (0.3, 0.8) | |
| Death at ESRD onset | 63 (17.3) | 48 (18.7) | 15 (14.0) | 0.28 |
| Preemptive kidney transplant | 17 (4.7) | 13 (5.1) | 4 (3.7) | 0.59 |
Data are presented as n (%) or median (1st, 3rd quartile).
P value for comparison between patients with <5 and ≥5 eGFR observations.
Observed time from baseline to the initiation of renal replacement therapy varied widely. Median (1st, 3rd quartiles) interval was 6.6 years (4.2, 9.9). Median number of eGFR observations per patient was 10 with a median density of 1.7 per year. Median (1st, 3rd quartile) individual-specific rate of eGFR decline (estimated with linear regression) was −9.1 mL/min/1.73 m2/year (−5.8, −14.9), whereas the mean per year was −12.2 mL/min/1.73 m2, reflecting that all patients developed ESRD. For most patients, renal replacement therapy began with dialysis, and mortality at ESRD diagnosis was high, at 17.3%. Few patients (4.7%) received preemptive transplants.
The slope of eGFR decline was not associated with age at study entry or diabetes duration (adjusting for baseline eGFR). There was no association with the age at diabetes onset or any significant protective effect of prepubertal diabetes diagnosis. The distributions of slopes were similar in men and women. A steep eGFR slope was associated with higher urinary ACR (P = 0.017) and worse glycemic control (1.2 mL/min/1.73 m2/year steeper per 1% HbA1c increase, P < 0.001,). Higher baseline eGFR was associated with steeper decline (P < 0.001), which reflects the study design, because the patients with slow eGFR decline were likely to be recruited with an already low eGFR. Systolic blood pressure was not associated with eGFR slope, but there was a statistically significant difference between slopes in patients treated and not treated with an ACE-I or ARB (median −7.2 vs. −11.9 mL/min/1.73 m2/year, P < 0.001). No association of eGFR slope was found with tobacco use.
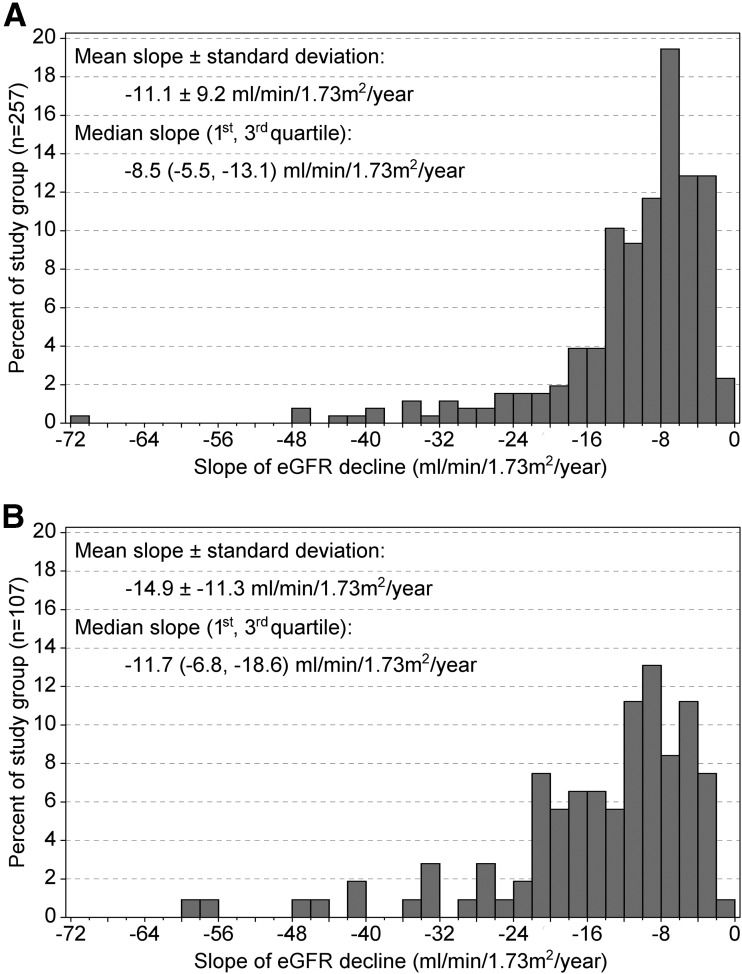
Because 5 observations are required for tests of nonlinearity, patients with fewer eGFR observations could not be included in the analysis of trajectories. Clinical characteristics of the 257 patients with ≥5 observations and the 107 with <5 were generally similar (Table 1). Exceptions were steeper slopes of eGFR decline in those with <5 observations (median −11.7 vs. −8.5 mL/min/1.73 m2/year, P < 0.001 (Fig. 1A and B), slightly shorter diabetes duration (P = 0.047 (Table 1), and higher baseline eGFR (84 vs. 71 mL/min/1.73 m2, P = 0.001) (Table 1). Paucity of observations in these patients was perhaps caused by rapid rates of eGFR decline.
Figure 1.
Distribution of eGFR decline slopes with ≥5 (A) and <5 (B) eGFR observations.
For the 257 patients with ≥5 observations, the median interval to ESRD was 6.7 years, with a median of 15 eGFR observations for a median density of 2.7 observations per year. At the first measurement of serum creatinine, distribution according to CKD stage was 55 at stage 1, 127 at stage 2, and 70 at stage 3.
Patterns of eGFR Decline
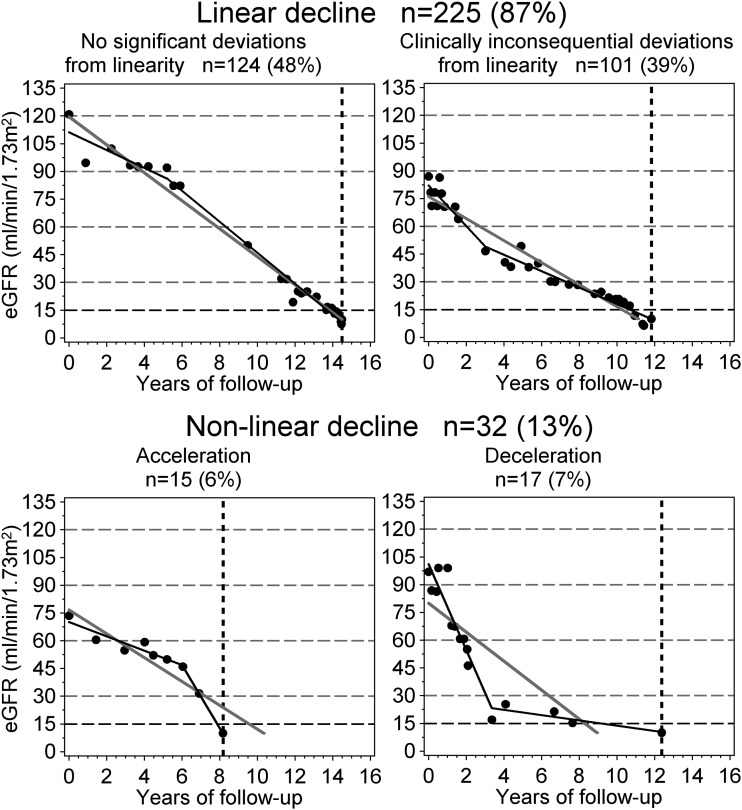
Criteria for classifying trajectories as nonlinear are summarized in Table 2. We used an approach described by Jones and Molitoris (32) for fitting a nonlinear spline model to an individual’s eGFR data. Nonlinearity was subsequently classified as clinically consequential if predicted time to ESRD (eGFR = 10 mL/min/1.73 m2) by simple linear fit missed the observed time to ESRD by 1 year or more. Clinically consequential nonlinear trajectories were further characterized as decelerating or accelerating (Table 2).
Table 2.
Criteria for classifying trajectories of eGFR decline according to analytic approach
| Classification of trajectory if criterion met | Criterion | Classification of trajectory if criterion not met |
|---|---|---|
| Spline regression method | ||
| Nonlinear | Linear model is rejected in favor of spline model at P < 0.05 with partial F test. | Linear |
| Clinically consequential nonlinearity | Linear model rejected and linear projection of time to reach eGFR = 10 mL/min/1.73 m2 misses the observed time of ESRD by 1 year or more. | Clinically inconsequential nonlinearity |
| Acceleration | Clinically consequential nonlinear trajectory and the slope of the second spline segment is steeper than the first. | |
| Deceleration | Clinically consequential nonlinear trajectory and the slope of the first spline segment is steeper than the second. | |
| Bayesian method | ||
| Nonlinear | Any eGFR difference between the smoothed trajectory and the line fitted to it is ≥15 mL/min/1.73 m2. | Linear |
| Bayesian method with criteria of linearity proposed in AASK study | ||
| Nonlinear | Mean slope for the half of the follow-up months with faster decline differs from the mean slope for the half with slower yearly decline >3 mL/min/1.73 m2. | Linear |
The test of nonlinearity failed to reject the null hypothesis of linearity for 124 trajectories. Furthermore, 101 nonlinear trajectories failed to meet the criterion of clinical consequence. Thus, 225 trajectories (87%) were satisfactorily characterized by a constant slope, and representation of eGFR decline as a single slope, as shown in Fig. 1A, is valid for the vast majority of patients. The remaining 32 trajectories were equally divided between 15 significant accelerations (6%) and 17 significant decelerations (7%). Examples are presented in Fig. 2.
Figure 2.
Examples and frequency of linear and nonlinear of trajectories of eGFR decline classified with the linear spline approach. Trajectories were classified by comparing the linear model (solid gray line) with spline regression (solid black line). The dots represent eGFR observations, the horizontal gridlines correspond to boundaries of CKD stages, and the vertical dashed line indicates the onset of ESRD.
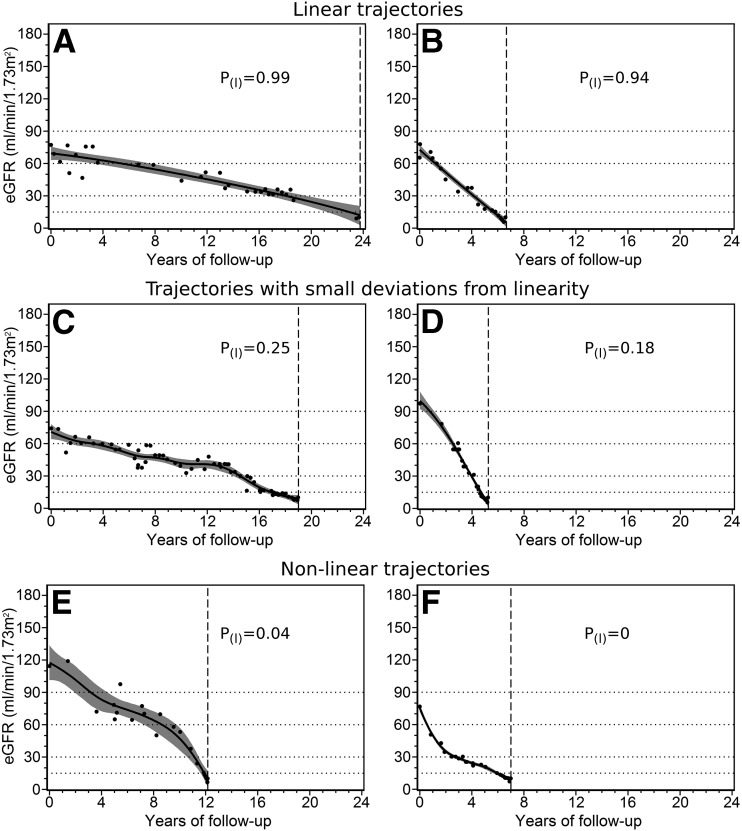
Apart from the previously applied method of testing linearity with the spline model, we analyzed data with a Bayesian technique previously used in the analysis of data from the AASK (16,19). The same criteria of linearity applied to our ESRD cohort (Table 2) resulted in rejection of the linear model in most patients. A careful inspection of eGFR decline trajectories of patients declared nonlinear identified a large number of trajectories, for which the criterion suggested in the AASK study (16) was clearly too stringent, as shown in the examples presented in Fig. 3. In the examples in Fig. 3C and D, the trajectory is apparently linear, but only a minority of the Bayesian trajectories conform to AASK study criteria. As an alternative, we based the test of nonlinearity on the magnitude of the largest departure of the smoothed trajectory from a simple regression line fitted to it (Table 2). Provisionally, we chose a departure ≥15 mL/min/1.73 m2 as the criterion for nonlinearity and then performed a sensitivity analysis using values of 5 and 10 mL/min/1.73 m2. According to the 15 mL/min/1.73 m2 criterion, overall prevalence of linear trajectories in posterior Bayesian samples was 76.0%, and 5 and 10 mL/min/1.73 m2 departures yielded 42.2% and 62.7% of linear trajectories, respectively.
Figure 3.
Examples of Bayesian trajectories of eGFR decline. The solid black line represents the mean estimated trajectory, the gray zone indicates the 95% point-wise Bayesian credible interval, the black dots represent eGFR observations, the horizontal gridlines represent CKD stages boundaries, and the vertical gridline indicates the time of ESRD onset. A and B: Patients have varying slopes of eGFR decline, but all of their trajectories are linear. C and D: Patients have visibly linear trajectories but present some fluctuations of eGFR. E and F: Patients have clearly nonlinear trajectories. Probability of linearity [P(I)] according to AASK study criteria is provided.
The linear spline approach classified 13% of the trajectories as sufficiently nonlinear to have a clinically consequential effect on the anticipated time to ESRD. The Bayesian approach classified 24% of the posterior samples as nonlinear because they departed by at least 15 mL/min/1.73 m2 from a linear trajectory. To determine concordance of classifications by the two approaches, we calculated the frequency of Bayesian nonlinear posterior samples according to class of trajectory determined by the linear spline approach. The frequency of Bayesian nonlinearity was 10.8% in 124 patients whose trajectories were classified as linear by the spline method, 31.6% in 104 patients classified as inconsequentially nonlinear, and 51.1% among 32 patients whose decline was concluded consequentially nonlinear with the spline method.
We tested patients’ characteristics, such as sex, age, duration of diabetes, age at diabetes onset, ACR, HbA1c, eGFR at baseline, blood pressure, ACE-I or ARB treatment, and smoking status for their association with deviations from linearity and with accelerations or decelerations. None were statistically significant.
Conclusions
We characterized trajectories of eGFR decline that preceded onset of ESRD in a large group of patients with type 1 diabetes. For more than 70%, serial measurements of serum creatinine began while their eGFR was >60 mL/min/1.73 m2. The eGFR declined at a very rapid rate in most patients. In half of the group, the yearly rate of eGFR loss was steeper than 9 mL/min/1.73 m2 and up to 72 mL/min/1.73 m2. In the other half, the yearly rate of eGFR loss was between 2 and 9 mL/min/1.73 m2.
In most of the patients studied, trajectories of eGFR decline were linear on the arithmetic scale and not constant on the percentage scale, as it is erroneously assumed and used in outcome definitions recommended for clinical trials (33–35). Furthermore, acceleration or deceleration was observed in a small minority of trajectories. There was no evidence that acute kidney injury or series of it, as suggested by others (6,7), leads to onset of ESRD in a significant proportion of patients with type 1 diabetes.
There was a statistically significant association of glycemic control, urinary ACR, and renoprotective/antihypertensive treatment with eGFR decline. No sex differences in the slopes were present in our study group, and the protective effect of prepubertal diabetes onset was absent (3,36,37). We were not able to identify predictors of deviations from linearity in our study group. This may be partly explained by the lack of statistical power and the inclusion, by design, of only those patients who developed ESRD.
Infrequent presence of accelerations and decelerations, regardless of the initial slope, suggests that eGFR decline is determined by constitutive factors, possibly genetic susceptibilities. A disease process that initiates the onset of eGFR decline occurs when patients have normal eGFR, as found in this study and in our previous report (26). In the Joslin Proteinuria Cohort, baseline HbA1c and blood pressure were higher in those who reached ESRD (20). An intriguing question is, “Is persistence of a trigger necessary to sustain the disease process that underlies progressive eGFR decline once it is initiated?” Our recent reports suggest that persistence of triggers is important. Improvement in glycemic control sustained for several years in patients with previously poor control had salutary effects on the incidence rate of ESRD (30) and on the influence of markers of constitutive susceptibility (38).
We previously (4) traced eGFR decline trajectories in 244 patients with type 1 diabetes and persistent proteinuria who had normal baseline renal function. Renal function was stable in approximately half of the patients. Significant nonlinear decline was rare, and decelerations were more frequent than accelerations. Most patients did not reach ESRD, making their trajectories incomplete. Patients in that cohort who reached ESRD were too few to draw unambiguous conclusions and to rule out that these ESRD events resulted from acute kidney injury complicating the chronic condition. Our current results, for the first time, describe complete trajectories in a large number of patients and indicate that eGFR decline is linear during the early stage of the process (4) and just before ESRD. The same disease process seems to be responsible for the early (eGFR ≥60 mL/min/1.73 m2) and late (eGFR <60 mL/min/1.73 m2) decline in eGFR.
Our findings have important implications for the design and evaluation of clinical trials of interventions to reduce risk of ESRD in type 1 diabetes. First, the predominance of linear trajectories is fortunate because the slope is easy to interpret and makes possible simple predictions of a patient’s near and more distant future. Its stability over time allows it to be used as a dependent variable in studies of determinants of eGFR decline and ESRD risk biomarkers (M. Yamanouchi, A.S.K., unpublished observations). Linearity of eGFR decline implies that an association with the slope observed during 3–6 years of follow-up translates strongly into an association with the risk of ESRD. An early slope of renal decline may serve as a surrogate end point as long as it is estimated from sufficiently dense data (we postulate measurements for research purposes every 3–6 months).
From data presented in Fig. 1 we may estimate that >80% of patients with type 1 diabetes who will develop ESRD in the fourth to sixth decade of life will have a yearly eGFR loss ≥5 mL/min/1.73 m2. Once prognostic algorithms are developed, these high-risk patients should be screened to identify them while they have still normal renal function. Effective interventions at this early stage might decelerate eGFR slopes and postpone onset of ESRD by years rather than by several months, as is expected when treatment is implemented in CKD stage 3 or 4. Our recent study suggests that improvement in HbA1c can decrease the rate of eGFR decline and postpone ESRD onset (30); however, such improvement had to be sustained for several years. Interventions aimed at glycemic control, if initiated too late, will likely fail.
Patients described in this study represent a group in which available preventive measures failed. In our previous study, where only a minority of patients with type 1 diabetes with proteinuria developed ESRD, there were more eGFR trajectories with clinically significant deceleration (4). Such decelerations may represent responses to effective therapeutic interventions (30). Change in slope of eGFR may be considered for future use as the primary end point in clinical trials, possibly tested using a quadratic model of decline (30). Under such a model, an expected linear decline in the placebo group will be compared with a change of slope (deceleration) in the treated group. Use of a quantitative outcome may provide more power than current threshold-based end points.
Emphasizing the strength of our study we would like to point out the large size and unbiased selection of our study group, the reliable measurements of serum creatinine (most eGFR observations in the range <90 mL/min/1.73 m2, where less noise is observed), and two alternative analytical methods to analyze eGFR trajectories that provided similar results. The Bayesian approach is appealing because the fit to the data is not constrained to just two or three linear segments (20,27) and it provides estimates of uncertainty about the character of a trajectory. However, the approach based on fitting linear spline segments provides results that are easier to summarize numerically in clinical trials and epidemiologic studies. Once the predominance of the linear pattern is established, the preferred summary measure of eGFR decline should be a single slope. This metric is also suitable as an outcome measure for evaluating clinical interventions. We would like to emphasize that the conclusion about linearity is highly sensitive to a chosen definition of what constitutes a significant departure from it. Current definitions are arbitrary and data driven; therefore, we postulate adopting uniform consensus criteria for the future research.
Although our study has many strengths, we acknowledge its limitations. First, its generalizability would benefit from replication, especially in type 2 diabetes and non-Caucasian individuals. Second, we were unable to test trajectories of all ESRD patients at Joslin Clinic because too few eGFR measurements were available, often because of the very rapid decline of renal function. Also, eGFR measurements were not uniformly spaced, a weakness that favors detection of too many rather than too few nonlinear trajectories as they become unstable in areas with sparse observations. Finally, we acknowledge that direct GFR measurements were not available in our patients; however, a recent study found that measured GFR compared with eGFR did not show an enhanced association with risk of ESRD, cardiovascular events, or death (39).
Article Information
Funding. This project was supported through JDRF fellowship grant 3-2009-397 (J.S.) and research grant 17-2013-8 (A.S.K.) and National Institutes of Health grant DK41526 (A.S.K.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.S. performed statistical analysis. J.S., J.H.W., R.C.S., and A.S.K. drafted the manuscript. J.S., J.H.W., and A.S.K. contributed to the study concept and design. All authors were involved in data analysis and interpretation. J.S. and A.S.K. and obtained the funding. J.H.W., R.C.S., and A.S.K. supervised the study. A.M.S. acquired the data and was responsible for the administrative, technical, and material support. R.C.S. and A.S.K. critically revised the manuscript for important intellectual content. J.S. and A.S.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Laing SP, Swerdlow AJ, Slater SD, et al. . Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R, Dorman JS, Bosnyak Z, Tajima N, Becker DJ, Orchard TJ; Diabetes Epidemiology Research International Mortality Study; Allegheny County Registry . Incidence of ESRD and survival after renal replacement therapy in patients with type 1 diabetes: a report from the Allegheny County Registry. Am J Kidney Dis 2003;42:117–124 [DOI] [PubMed] [Google Scholar]
- 3.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 4.Skupien J, Warram JH, Smiles AM, et al. . The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 2012;82:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015;38:954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onuigbo MA, Agbasi N. Diabetic nephropathy and CKD-analysis of individual patient serum creatinine trajectories: a forgotten diagnostic methodology for diabetic CKD prognostication and prediction. J Clin Med 2015;4:1348–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ, Dominguez JH. Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol 2010;32:469–475 [DOI] [PubMed] [Google Scholar]
- 8.Jones RH, Hayakawa H, Mackay JD, Parsons V, Watkins PJ. Progression of diabetic nephropathy. Lancet 1979;1:1105–1106 [DOI] [PubMed] [Google Scholar]
- 9.Mitch WE, Walser M, Buffington GA, Lemann J Jr. A simple method of estimating progression of chronic renal failure. Lancet 1976;2:1326–1328 [DOI] [PubMed] [Google Scholar]
- 10.Bleyer AJ. A reciprocal graph to plot the reciprocal serum creatinine over time. Am J Kidney Dis 1999;34:576–578 [DOI] [PubMed] [Google Scholar]
- 11.Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest 1976;36:383–388 [DOI] [PubMed] [Google Scholar]
- 12.Parving HH, Smidt UM, Friisberg B, Bonnevie-Nielsen V, Andersen AR. A prospective study of glomerular filtration rate and arterial blood pressure in insulin-dependent diabetics with diabetic nephropathy. Diabetologia 1981;20:457–461 [DOI] [PubMed] [Google Scholar]
- 13.Viberti GC, Bilous RW, Mackintosh D, Keen H. Monitoring glomerular function in diabetic nephropathy. A prospective study. Am J Med 1983;74:256–264 [DOI] [PubMed] [Google Scholar]
- 14.Shah BV, Levey AS. Spontaneous changes in the rate of decline in reciprocal serum creatinine: errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol 1992;2:1186–1191 [DOI] [PubMed] [Google Scholar]
- 15.Szeto CC, Leung CB, Wong TY, et al. . Extrapolation of reciprocal creatinine plot is not reliable in predicting the onset of dialysis in patients with progressive renal insufficiency. J Intern Med 2003;253:335–342 [DOI] [PubMed] [Google Scholar]
- 16.Li L, Astor BC, Lewis J, et al. . Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012;59:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese G, Friedman DJ, Ross MD, et al. . Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329:841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JT Jr, Bakris G, Greene T, et al.; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–2431 [DOI] [PubMed] [Google Scholar]
- 19.Crainiceanu CM, Ruppert D, Wand MP. Bayesian analysis for penalized spline regression using WinBUGS. J Stat Softw 2005;14:1–24 [Google Scholar]
- 20.Rosolowsky ET, Skupien J, Smiles AM, et al. . Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol 2011;22:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krolewski AS, Laffel LM, Krolewski M, Quinn M, Warram JH. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med 1995;332:1251–1255 [DOI] [PubMed] [Google Scholar]
- 22.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 23.Ficociello LH, Perkins BA, Roshan B, et al. . Renal hyperfiltration and the development of microalbuminuria in type 1 diabetes. Diabetes Care 2009;32:889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkins BA, Ficociello LH, Ostrander BE, et al. . Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 25.Nowak N, Skupien J, Niewczas MA, et al. . Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 2016;89:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krolewski AS, Niewczas MA, Skupien J, et al. . Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014;37:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller PW, Rogus JJ, Cleary PA, et al. . Genetics of Kidneys in Diabetes (GoKinD) study: a genetics collection available for identifying genetic susceptibility factors for diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 2006;17:1782–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al.; DCCT/EDIC Research Group . Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 2009;58:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warram JH, Gearin G, Laffel L, Krolewski AS. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 1996;7:930–937 [DOI] [PubMed] [Google Scholar]
- 30.Skupien J, Warram JH, Smiles A, Galecki A, Stanton RC, Krolewski AS. Improved glycemic control and risk of ESRD in patients with type 1 diabetes and proteinuria. J Am Soc Nephrol 2014;25:2916–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RH, Molitoris BA. A statistical method for determining the breakpoint of two lines. Anal Biochem 1984;141:287–290 [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Inker LA, Matsushita K, et al. . GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014;64:821–835 [DOI] [PubMed] [Google Scholar]
- 34.Coresh J, Turin TC, Matsushita K, et al.; CKD Prognosis Consortium . Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inker LA, Lambers Heerspink HJ, Mondal H, et al. . GFR decline as an alternative endpoint for kidney failure: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis 2014;64:848–859 [DOI] [PubMed] [Google Scholar]
- 36.Harvey JN. The influence of sex and puberty on the progression of diabetic nephropathy and retinopathy. Diabetologia 2011;54:1943–1945 [DOI] [PubMed] [Google Scholar]
- 37.Donaghue KC, Fairchild JM, Craig ME, et al. . Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care 2003;26:1224–1229 [DOI] [PubMed] [Google Scholar]
- 38.Skupien J, Warram JH, Niewczas MA, et al. . Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5-18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabetes Care 2014;37:2601–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku E, Xie D, Shlipak M, et al.; CRIC Study Investigators . Change in measured GFR versus eGFR and CKD outcomes. J Am Soc Nephrol 2016;27:2196–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]