Abstract
Calorie restriction’s (CR) effects on age-associated changes in glycogen-metabolizing enzymes were studied in rat soleus (SOL) and tibialis anterior (TA) muscles. Old (24 months) compared to young (6 months) rats maintained ad libitum on a standard diet had reduced glycogen synthase (GS) activity, lower muscle GS protein levels, increased phosphorylation of GS at site 3a with less activation in SOL. Age-associated impairments in GS protein and activation-phosphorylation were also shown in TA. There was an age-associated reduction in glycogen phosphorylase (GP) activity level in SOL, while brain/muscle isoforms (B/M) of GP protein levels were higher. GP activity and protein levels were preserved, but GP was inactivated in TA with age. Glycogen content was unchanged in both muscles. CR did not alter GS or GP activity/protein levels in young rats. CR hindered age-related decreases in GS activity/protein, unrelated to GS mRNA levels, and GS inactivation-phosphorylation; not on GP. In older rats, CR enhanced glycogen accumulation in SOL. Short-term fasting did not recapitulate CR effects in old rats. Thus, the predominant age-associated impairments on skeletal muscle GS and GP activities occur in the oxidative SOL muscle of rats, and CR can attenuate the loss of GS activity/activation and stimulate glycogen accumulation.
Keywords: Glycogen synthase phosphorylation site 3a (Ser641/0), Gm, RGL, Protein phosphatase 1 regulatory subunit 3A, Protein phosphatase 1 regulatory subunit 3C, Protein phosphatase 1 regulatory subunit 5 (R5)
1. Introduction
Glycogen is a crucial energetic substrate supporting skeletal muscle activity in both humans (Holloszy and Kohrt, 1996; Shulman and Rothman, 2001) and exercising rats (Goldfarb et al., 1989; Ivey and Gaesser, 1987; Richter et al, 1982). A key enzyme of glycogenesis is glycogen synthase (GS) (Kollberg et al., 2007; Manchester et al., 1996; Pederson et al., 2004), while glycogenolysis is controlled by glycogen phosphorylase (GP) activity (Lucia et al., 2008). The predominant isoform of GS in the skeletal muscles of mice (Pederson et al., 2004) and humans (Kollberg et al., 2007) is encoded by the GYS1 gene. The GP muscle isoform, encoded by the PYGM gene, is highly expressed in skeletal muscle in humans (Lucia et al., 2008) and rats (David and Crerar, 1986). Cardiac myocytes, in contrast, contain significant levels of the brain isoform, encoded by the PYGB gene, in both humans (Mair, 1998) and rats (David and Crerar, 1986; Schmid et al., 2008).
Short-term regulation of GS and GP enzymes is mediated allosterically and through phosphorylation. Muscle GS is activated allosterically by glucose-6-phosphate (Nielsen and Wojtaszewski, 2004) and muscle GP by AMP (Buchbinder et al., 2001). Dephosphorylation activates GS and inactivates GP. Mostly protein phosphatase 1 (PP1) mediates dephosphorylation of both enzymes, and its effects are modulated by glycogen-targeting subunits such as GM/RGL-PP1 and PTG/R5-PP1 (Newgard et al., 2000). Several kinases phosphorylate GS at diverse sites although their contribution to the regulation of GS in vivo has not been completely elucidated (Nielsen and Wojtaszewski, 2004). Phosphorylation of muscle GS at site 3a from the COOH terminus has been shown to play a crucial role in the inactivation of the enzyme (Skurat et al., 1994; Skurat and Dietrich, 2004; Wang and Roach, 1993). Muscle GP is phosphorylated by phosphorylase kinase at Ser-14 (Buchbinder et al., 2001).
Muscle glycogen metabolism is susceptible to age-related changes. Indeed, aging is associated with impairment of whole-body glucose disposal in rats during glucose tolerance tests (Park et al., 2006) and under euglycemic–hyperinsulinemic clamping (Escrivá et al., 1997; Nishimura et al., 1988). Muscle glycogenesis has also been shown to be impaired in old rats: under refeeding in vivo in soleus muscle (SOL) (Poland et al., 1982) and following insulin perfusion in isolated fast-twitch gastrocnemius-plantaris muscles (Meynial-Denis et al., 2005). Data on the effects of age on glycogen content are mixed. No difference in muscle glycogen content was found in gastrocnemius-plantaris muscles of aged (22 months) versus young adult (6–8 months) fed rats (Meynial-Denis et al., 2005) or in the SOL or vastus lateralis muscles of aged (24 month or older) versus young (4 months old) fed rats (Poland et al., 1982). However, another study detected progressional reduction of glycogen content in SOL and biceps femoris muscles with age in rats (Dall’Aglio et al., 1987). Data regarding the effects of age on GS and GP are also not conclusive. One study has shown a decrease in GS activity in fast- and slow-twitch muscles in 24-month-old compared with 2-month-old rats (Dall’Aglio et al., 1987). In contrast, other studies found no change in total GS or GP activity in rectus abdominal muscle (Gupta et al., 2000) or in SOL muscle with aging in rats (Narimiya et al., 1984), while in the latter an increase in GS activation and a decrease in GP activation state was observed.
Calorie restriction (CR), undernutrition without malnutrition, is a dietary intervention that attenuates age-related metabolic alterations, extends maximum lifespan and improves glucose tolerance and insulin sensitivity in rodents (Das et al., 2004; Facchini et al., 2000; Masoro, 2005; Park et al., 2006). CR reduces tissue oxidative damage (Hyun et al., 2006) and the age-associated loss of muscle mass and function known as sarcopenia (Marzetti et al., 2008). Earlier studies have shown improvements in whole-body glucose uptake by CR in rats are associated with enhanced muscle glucose utilization and glycogen synthesis. In one study, CR (begun at 4 months of age) improved plasma clearance of 2-deoxyglucose and its uptake in skeletal muscle (only in those in which fast-twitch fibers predominate) while increasing glycogen content in gastrocnemius and SOL muscles in 12-month-old rats (Wetter et al., 1999). In another study, CR stimulated insulin-mediated peripheral glucose uptake and muscle glycogen synthesis and activated GS in rectus abdominal muscle in both 8- and 20-month-old rats (Gupta et al., 2000). Finally, CR has been shown to prevent age-associated alterations in the gene expression profile of metabolic genes in gastrocnemius muscle in mice, including a number of genes involved in glycolysis, the glycerophosphate shunt and regulators of glycogen metabolism, as well as genes involved in mitochondrial function (Lee et al., 1999).
In summary, although CR is known to preserve skeletal muscle glucose metabolism with aging in rats, there are no conclusive data on aging-associated intracellular defects in the regulation of GS and GP gene expression and activity or whether CR could counteract such failings. In this study we examined the mRNA, protein and enzyme activity levels of GS and GP in the slow-twitch oxidative SOL and fast-twitch glycolytic TA muscles of young adult (6-month-old) compared with old (24-month-old) rats. We also assessed whether long-term food restriction or short-term fasting could attenuate any age-related changes.
2. Material and methods
2.1. Animals
Male Fischer 344 rats born at the Gerontology Research Center (Baltimore, MD) were weaned at 28 days, housed individually and randomly assigned to either AL (standard diet fed ad libitum) or CR (provided with a daily food allotment of 60% of that eaten by the AL rats) and maintained up to 6 months (6-mo) or 24 months (24-mo) of age. All animals were maintained on a 12 h light/dark cycle in a separate vivarium at the Gerontology Research Center under specific pathogen-free conditions, fed with NIH-31 standard rodent chow for AL rats (Harlan Teklad, Indianapolis, IN, USA) and with NIH-31 fortified rodent chow for CR rats (Harlan Teklad). A subgroup of rats within the AL group was fasted for 18 h before sacrifice (ALF); the AL and CR groups were allowed food 18 h before sacrifice. Experiments were performed during the light cycle. Rats were anesthetized with sodium pentobarbital (60mg/kg body weight, i.p). Body weights were assessed and mean values for the 6-mo rats were 353 ± 15, 382 ± 13 and 251±3 g (p < 0.001 versus AL) in the AL, ALF and CR groups respectively and mean body weights in the 24-mo rats were 437 ± 23 (p < 0.01 versus AL young), 444 ± 9 and 255 ± 6 g in the AL, ALF and CR (p < 0.0001 versus AL old) groups respectively. There was an influence of age (F 18.6 p < 0.001) and diet (F 75.5 p < 0.0001) on body weights. SOL and TA tissues from the two legs were excised and immediately frozen in liquid nitrogen prior to storage at −80 °C or placed into RNAlater reagent and stored at 4 °C. All animal procedures for this study were reviewed and approved by the Animal Care and Use Committee (ACUC) at the Gerontology Research Center (NIA/NIH). SOL was taken as a slow-twitch oxidative muscle and TA as a fast-twitch glycolytic muscle (Staron et al., 1999).
2.2. Enzyme activity, western blotting and glycogen quantification
To measure GS and GP activities, extracts were prepared by homogenization of approximately 50 mg muscle samples into 1 ml of homogenization buffer consisting of 10 mM Tris–HCl (pH 7.0), 150 mM NaF, 15 mM EDTA, 600 mM sucrose, 15 mM 2-mercaptoethanol and protease inhibitor cocktail by sonication. The resulting homogenates were used for the determination of enzyme activities. GP activity was determined by the incorporation of [U-14C] glucose 1-phosphate into glycogen in the absence or presence of the allosteric activator AMP (1 mM) (Gilboe et al., 1972). GS activity was measured in the absence or presence of 10 mM glucose 6-P as described (Thomas et al., 1968). Aliquots of the tissue extracts were used for the measurement of protein concentration. For western blotting the tissue extracts prepared as described above were mixed 1:1 with a homogenization buffer consisting of 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 1 mM Na3VO4, 2 μg/μl benzamidine, 2 μg/μl leupeptin, 1% (v/v) Nonidet P40, and 1 mM dithiothreitol. Lysates were then gently rocked for 60 min at 4 °C. All fractions were stored at −80 °C. Protein was resolved in 10% SDS–PAGE and immunoblotting was performed with antibodies against total muscle GS (Cell Signaling, Danvers, MA, USA), phospho-GS (Ser641/0) protein (Cell Signaling), B/M-GP proteins (HyTest, Turku, Finland), skeletal muscle GP (MBL International Corporation, Des Plaines, IL, USA) total glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein (Cell Signaling).
To measure glycogen, extracts were prepared by homogenization of approximately 50 mg of muscle into 0.5 ml of 30% KOH and then boiled for 15 min. Aliquots of the homogenates were used for the measurement of protein concentration. The homogenates were spotted onto Whatman 3MM papers and the glycogen was precipitated by immersing the papers in ice-cold 66% ethanol. Dried papers containing precipitated glycogen were incubated in 0.4 M acetate buffer (pH 4.8) with 25 U/ml of α-amyloglucosidase (Sigma-Aldrich, Madrid, Spain) for 120 min at 37 °C. Glucose released from glycogen was measured enzymatically in a Cobas Fara II autoanalyzer (Biosystems, Barcelona, Spain).
2.3. RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from tissue samples following the instructions provided with the RNeasy Mini Kit (Oiagen, Valencia, CA, USA). Extracts were homogenized using a Polytron homogenizer. An aliquot of 0.5 μg of total RNA was retro-transcribed (RT) with TaqMan reverse transcription reagents from Applied Biosystems (Branchburg, NY, USA) using random hexamers. Real-time PCR was performed using the ABI PRISM 7700 sequence detection system with the TaqMan universal PCR master mix and TaqMan Gene Expression Assays for rat muscle GS (LOC690987, similar to Gys1), muscle GP (Pygm), brain GP (Pygb), PTG/R5 (Ppp1r3c) and GM/RGL (RGD1561312 predicted similar to type 1 protein phosphatase targeting subunit RGL/GM) from Applied Biosystems. The rat 18S rRNA gene was used as the endogenous control to normalize the threshold cycle (Ct) for each probe assay. Relative gene expression was estimated as 2−ΔCt and gene fold change was estimated by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
2.4. Statistical analysis
Data were analyzed using a two-way ANOVA and subsequent multiple pairwise comparisons with Tukey’s post hoc tests. Explanatory variables included in the model were diet, age and the interaction between them. Results were obtained for each muscle type separately. SAS software (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses. Comparisons within muscles were analyzed by the Student’s t-test. Values were considered significant at p < 0.05. Means ± SEM are reported in the figures and tables.
3. Results
3.1. Glycogen synthase and glycogen phosphorylase activity
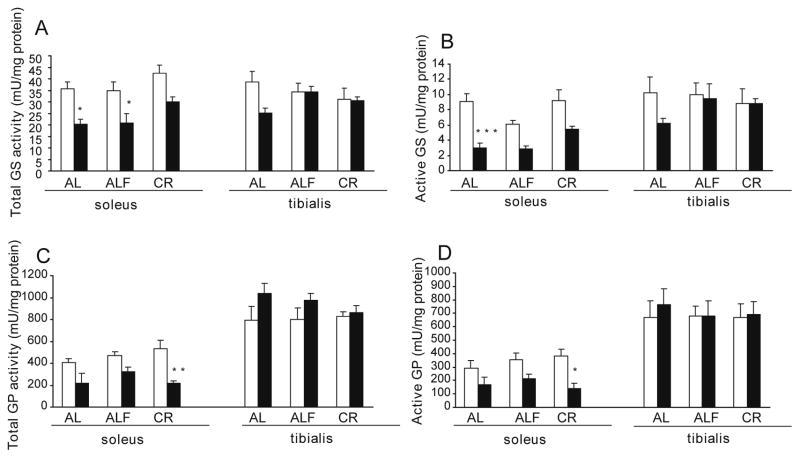
Young (6-mo) rats maintained on a standard diet and euthanized fed (AL) or following an 18 h fast (ALF), or maintained on a CR diet and euthanized fed all showed similar levels of total GS activity (measured with glucose 6-P) in SOL and TA muscles (Fig. 1A). The levels of the active form of GS in young rats (measured without glucose 6-P) (Fig. 1B) were also similar in SOL and TA within the AL and CR groups, whereas in the ALF group a reduction tendency was observed in SOL exclusively, indicating inactivation of the enzyme. Accordingly, the GS (−/+ glucose 6-P) activity ratio was about 33% lower in SOL of the ALF rats compared with the AL group, but equivalent in TA (Table 1). CR did not affect GS activity ratio in young rats.
Fig. 1.
Activity of glycogen-metabolizing enzymes. Rats were maintained from weaning to 6-months (h) or 24-months (j) on a standard, ad libitum (AL) or calorie restricted (CR) diet. AL rats were sacrificed in either a fed state (AL) or after an 18 h fasting period (ALF). All CR rats were sacrificed in the fed state. Enzyme activities were assessed in extracts from SOL and TA muscles: (A and B) GS activity measured (A) with or (B) without glucose 6-P and (C and D) GP activity measured (C) with or (D) without AMP. Means ± SEM from at least 5 rats in each group are shown. Age influenced: total GS activity (F 29.1, p < 0.0001), active GS activity (F 38.6, p < 0.0001), total GP activity (F 25.0, p < 0.0001) and active GP activity (F 17.2, p < 0.001) in SOL. Diet influenced: total GS activity (F 4.52, p < 0.05) and active GS (F 6.10, p < 0.01) in SOL. The significance of the differences is versus 6-month rats comparing equivalent diet and muscle *p < 0.05, **p < 0.01 and ***p < 0.001. The significance of the Student’s t-test for total and active GP activity in TA versus SOL muscle is p < 0.0001.
Table 1.
Activity ratio of GS and GP.
| 6-mo
|
24-mo
|
|||
|---|---|---|---|---|
| Soleus | Tibialis | Soleus | Tibialis | |
| Glycogen synthase activity ratio (−G6P/+G6P) | ||||
| Diet | ||||
| AL | 0.24 ± 0.02 | 0.28 ± 0.02 | 0.12 ± 0.02** | 0.23 ± 0.01 |
| ALF | 0.16 ± 0.01† | 0.32 ± 0.02 | 0.11 ± 0.01 | 0.23 ± 0.03* |
| CR | 0.20 ± 0.03 | 0.27 ± 0.02 | 0.17 ± 0.01 | 0.27 ± 0.01 |
| Glycogen phosphorylase activity ratio (−AMP/+AMP) | ||||
| Diet | ||||
| AL | 0.69 ± 0.08 | 0.84 ± 0.04 | 0.58 ± 0.03 | 0.66 ± 0.09 |
| ALF | 0.74 ± 0.02 | 0.85 ± 0.03 | 0.65 ± 0.07 | 0.65 ± 0.06 |
| CR | 0.71 ± 0.05 | 0.85 ± 0.03 | 0.59 ± 0.06 | 0.79 ± 0.15 |
Enzyme activities were assessed in extracts from SOL and TA muscles and expressed as the activity ratio. Means ± SEM from at least 5 rats in each group are shown. Age influenced: GS activity ratio (F 17.4, p < 0.001) in SOL and (F 8.40, p < 0.01) in TA and GP activity ratio (F 11.3, p < 0.005) in TA. Diet influenced: GS activity ratio (F 5.57, p < 0.01) in SOL. The significance of the differences is: versus AL within same muscle and age †p < 0.05; versus 6-mo rats comparing equivalent diet and muscle *p < 0.05 and **p < 0.001.
There was a significant effect of age on total GS and active GS activities in SOL. In the SOL of old (24-mo) rats in the AL group total GS activity and active GS levels were about 40% lower compared to young (6-mo) rats receiving the same diet (Fig. 1A and B). No significant differences were observed in the CR groups, indicating that the age-associated reduction in total GS activity and the active form of GS was hindered. Fasting had no such effects. No significant effect by age was observed in the TA. While the fed AL group showed a trend towards lower total GS activity and active GS compared with young rats, this was not observed in the CR and ALF groups. Age negatively influenced GS activity ratio in SOL and TA (Table 1), differences reached statistical significance in the AL group in the SOL and in the ALF group in the TA of old compared to young rats. In SOL of young but not old rats, fasting (ALF) caused GS inactivation. No differences between old and young rats on CR were observed in GS activity ratio in SOL or in TA, suggesting partial recovery of the age-associated reduction.
Total GP activity (measured with AMP), as well as the active form of GP (measured without AMP), was not different in the young or old rats as a function of diet (Fig. 1C and D). Total GP activity and active GP levels were higher (1.5- to 3-fold) in the TA than in the SOL muscles in the young and old rats; these data are in accordance with previous results from our lab (Ferrer-Martínez et al., 2006).
There was a significant effect of age on total and active GP activity levels in SOL. In the SOL of old rats, total GP activity (Fig. 1C) was lower in the AL (47%), ALF (33%) and CR (60%) groups compared to young rats receiving the same diets. Similar reductions were observed in the levels of active GP (Fig. 1D) for the AL (42%), ALF (40%) and CR (63%) groups. Statistically significant differences were only reached in the CR groups, suggesting a further negative effect of CR on GP activity. In TA muscle, neither total GP activity nor active GP levels were different between old and young rats, irrespective of diet. GP (−/+ AMP) activity ratio (Table 1) was not changed by diet in either SOL or TA muscles of the younger rats. Age influenced GP activity ratio in TA but not in SOL. GP was less active in the TA of the AL (22%), ALF (24%) and CR (8%) groups of old compared to young rats.
3.2. Glycogen synthase and glycogen phosphorylase protein content
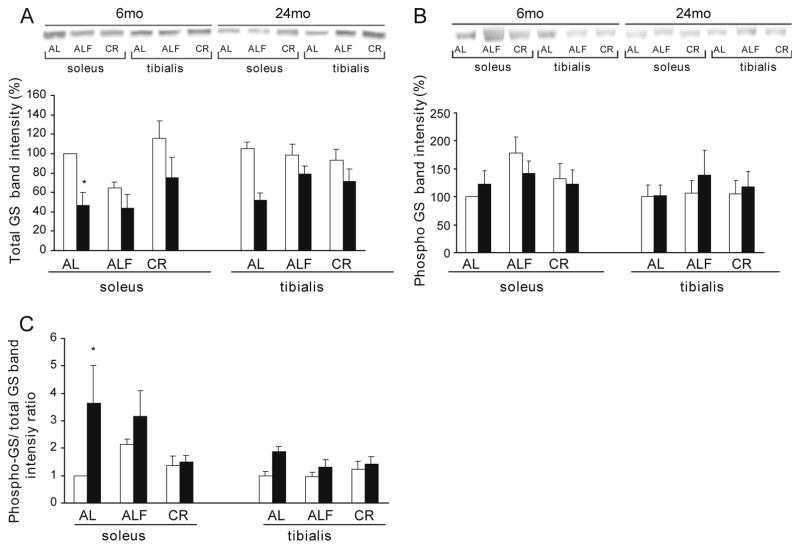
In young rats, the GS protein levels were similar in SOL and TA between the AL and CR groups (Fig. 2A). Fasting, however, caused a reduction tendency in GS protein in the SOL of the ALF rats. We also analyzed the content of phospho-GS (Ser641/0) and the ratio between phospho-GS at site 3a and total GS protein. Only ALF showed a regulatory trend as measured by an increase in the phospho-GS content in SOL compared to the AL group (Fig. 2B) and by an increase in the ratio between phospho-GS at site 3a and total GS protein (Fig. 2C). This corresponded with a significant decrease in GS activity ratio (Table 1). CR did not change the levels of phospho-GS in young rats.
Fig. 2.
Immunoblot analysis of GS protein content. Western blot analyses were performed on total extracts (20 μg protein) from SOL and TA muscle samples and membranes hybridized with antibodies against (A) GS, (B) phospho-GS (Ser641/0). (C) phospho-GS/GS ratio was calculated. For each age group, muscle and dietary treatment a representative image is shown and bands were quantified with a LAS-3000 (FujiFilm). Means ± SEM from at least 5 rats for each group are shown. Data were compared using percentages with the mean value of the young AL group in the SOL Age influenced: total GS in SOL (F 15.8, p < 0.001) and in TA (F 11.3, p < 0.005); phospho-GS/GS ratio (F 5.16, p < 0.05) in SOL and in TA (F 6.63, p < 0.05). Diet influenced: total GS (F 5.36, p < 0.05) in SOL The significance of the differences is versus young rats comparing equivalent diet and muscle *p < 0.05.
Age influenced GS protein content in SOL and TA. In the SOL of AL old rats, there was a reduction in the total GS protein content compared to AL young rats (more than 50%) (Fig. 2A). No significant decrease was observed in the CR group. In the TA of old rats there was a reduction tendency in GS protein compared to young rats in the AL group. This reduction was not observed within the ALF or CR groups. All these changes corresponded with those of total GS activity. There were no changes in the level of phospho-GS (Ser641/0) by age in either SOL or TA (Fig. 2B). Age altered the ratio between phospho-GS at site 3a and total GS protein in SOL and TA causing an increase. A greater difference was observed in the AL group of old compared with young rats in SOL (Fig. 2C) and this corresponded with GS inactivation (Table 1). No difference was observed in the CR group, suggesting that CR hindered the age-associated increase in the phosphorylation ratio of GS at site 3a, and in correlation with its effect on GS activation state (Table 1).
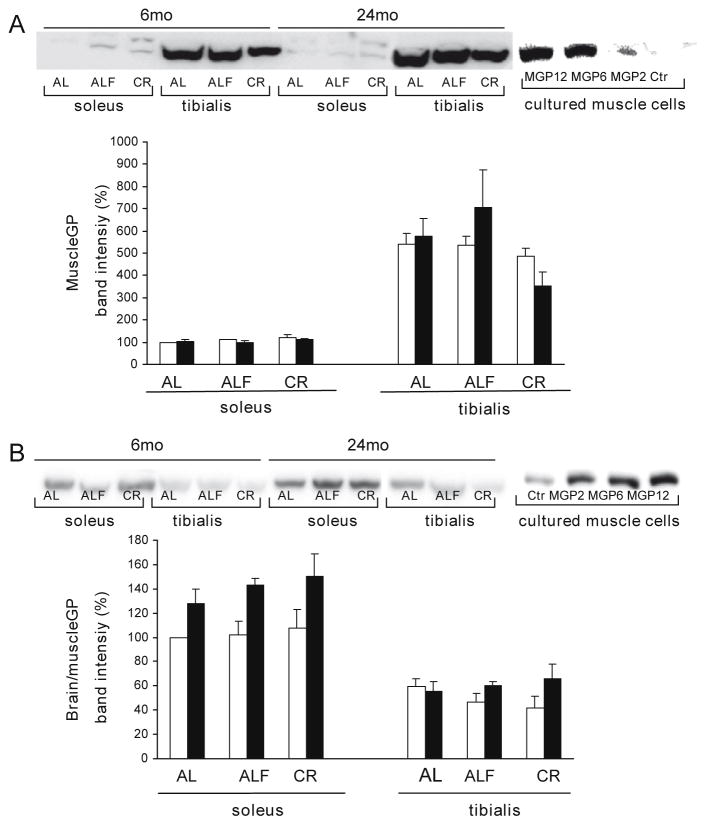
Regarding GP, immunoblot analysis showed higher values of muscle GP protein (between 3.5- and 5-fold) in TA than in SOL in all the studied conditions (Fig. 3A) in correspondence with values of total GP activity. The muscle GP protein pattern of expression was similar to that of the glycolytic protein glyceraldehyde-3-phosphate dehydrogenase whose content in young AL rats was higher (183 ± 38%, p < 0.01) in TA compared to that in SOL. Data were the opposite for immunoblotted B/M-GP protein content: values were about double in SOL compared to TA muscle, irrespective of diet or age (Fig. 3B), although the content was about 3-fold lower than that in rat heart and 20-fold lower than that in rat brain (data not shown). These data suggest that the SOL muscle contains higher brain GP protein than the TA. Both the muscle GP-specific and the B/M-GP antibodies could detect rabbit muscle GP protein in extracts of genetically engineered muscle cells (Baqué et al., 1996) (Fig. 3A and B).
Fig. 3.
Immunoblot analysis of muscle and brain/muscle GP protein content. Western blot analyses were performed on total extracts from SOL and TA muscle samples (20 μg of protein) and on extracts from cultured human muscles cells transfected with an adenovirus encoding GFP (Ctr) or an adenovirus encoding rabbit muscle GP cDNA (MGP) at increasing multiplicity of infection (10×) (20 μg (A) or 5 μg (B) of protein). Membranes hybridized with antibodies against (A) muscle GP, (B) brain/muscle GP. For each age group, muscle and dietary treatment a representative image is shown and bands were quantified with a LAS-3000 (FujiFilm). Means ± SEM from at least 5 rats for each group are shown. Data were compared using percentages with the mean value of the young AL group in the SOL Age influenced the brain/muscle GP protein in SOL (F 14.8, p < 0.001). The significance of Student’s t-test for muscle and brain/muscle GP protein in TA versus SOL muscle is p < 0.0001.
No significant diet or age effects were observed in muscle GP protein content (Fig. 3A) in SOL or TA. There were age effects on B/M-GP protein content in SOL (Fig. 3B), in which an increase in B/M-GP protein content compared to young rats was shown in each diet group: AL (27%), ALF (40%) and CR (40%). Of note, the increase in GP protein was opposite to the decrease in total GP enzyme activity (Fig. 1C and D). In the TA no effects of age were observed. None of the tested diets modified the content of the B/M-GP protein in either SOL or TA.
3.3. Glycogen synthase, glycogen phosphorylase and glycogen-targeting subunits of PP1 gene expression
We assessed mRNA levels relative to the 18S rRNA control gene in the SOL and TA muscles of young AL rats. The mRNA levels of muscle GS were about 2-fold higher (p < 0.05) in SOL (−554 ± 140) than in TA (−1073 ± 166), unlike the protein levels that were similar. The mRNA levels of muscle GP were 4-fold lower (p < 0.005) in SOL (−234 ± 74) than in TA (−41 ± 3), as has been previously shown (Ferrer-Martínez et al., 2006) and is in accordance with our results for muscle GP protein content. The mRNA levels of brain GP were low and equivalent in SOL (−230473 ± 78076) and TA (−216603 ± 81340), despite higher B/M protein content in SOL. We also analyzed the mRNA levels of the glycogen-targeting subunits of PP1 expressed in rat skeletal muscle: GM/RGL-PP1 (Lannér et al., 2001) and PTG/R5-PP1 (Printen et al., 1997). The PTG and GM genes were similarly expressed in both muscles (−215 ± 36 in SOL and −205 ± 36 in TA for PTG and −3369 ± 720 in SOL and −2261 ± 315 in TA for GM).
Age influenced muscle GS, muscle GP and GM gene expression in SOL only (Table 2), causing their down-regulation. Diet influenced muscle GS and GM gene expression in SOL and the interaction of diet and age also exerted a significant effect on muscle GP and GM genes in SOL. CR reduced in SOL of older rats the expression of muscle GS and GM genes and a negative tendency was observed for the muscle GP gene. Fasting significantly reduced the expression in SOL of muscle GP and GM and a negative tendency was observed for muscle GS. No effects of age or diet were observed on brain GP in either muscle.
Table 2.
Differences in the mRNA levels of GS, GP, GM and PTG in SOL and TA muscles of young and old rats maintained on different diets.
| mRNA | Fold of change (1/2−ΔΔCt)
|
|||||
|---|---|---|---|---|---|---|
| Soleus
|
Tibialis
|
|||||
| AL | ALF | CR | AL | ALF | CR | |
| MGS | −1.31 | −1.74 | −2.30** | +1.01 | +1.37 | +1.34 |
| MGP | −1.05 | −2.12* | −2.01 | +1.10 | −1.00 | −1.01 |
| BGP | +1.16 | −1.74 | −1.09 | +1.68 | +1.26 | −1.10 |
| GM | −1.04 | −2.47** | −1.96* | +1.44 | −1.21 | +1.30 |
| PTG | −1.85 | −2.72 | +4.04* | −1.91 | −1.23 | +1.62 |
The relative mRNA levels of muscle GS (MGS), muscle GP (MGP), brain GP (BGP), GM and PTG were analyzed in muscles from at least 5 rats per group by RT-PCR. The Ct values for the reference probe (18S rRNA gene) were subtracted from each probe Ct (ΔCt). To determine changes in gene expression, the ΔCt values of 6-mo rats were subtracted from the ΔCt values of 24-mo rats for each muscle type and dietary condition. Data are expressed as mean values of 2−ΔΔCt for upregulated genes and l/2−ΔΔCt for downregulated ones. The former are shown by + and the latter by −. Age influenced the mRNA levels of: MGS (F 20.4, p < 0.0001), MGP (F12.5 p < 0.005), GM (F 19.0, p< 0.001) in SOL. Diet influenced the mRNA levels of: MGS (F 4.82, p < 0.05) and GM (F 13.8, p < 0.0001) in SOL The interaction of diet and age was also significative for MGP (F 3.92, p<0.05), GM (F 4.37, p<0.05) and PTG (F 8.80, p < 0.001) in SOL and PTG (F 4.38, p < 0.05) in TA. The significance of the differences between 24-mo and 6-mo in the equivalent diet and muscle is *p < 0.05 and **p<0.01.
Regarding PTG mRNA, a marked interaction between age and diet was observed in SOL and TA (Table 2). The CR diet increased by 4.6-fold (p < 0.01) PTG gene expression in SOL of old but not young rats; a positive tendency was also observed in TA. The changes in PTG mRNA in old rats correspond with the recovery of GS activation- site 3a phosphorylation in both muscle types. However, the content of PTG protein could not be established with the commercially available antibody as previously described (Jurczak et al., 2007).
3.4. Glycogen content
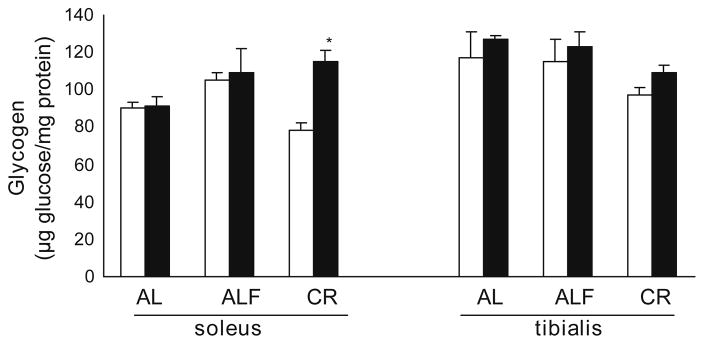
There was a significant interaction between the effects of age and diet in the glycogen content in SOL. A reduction tendency was observed in the glycogen content in SOL of young rats on CR (15% of reduction) compared to AL (Fig. 4) and a smaller reduction trend was observed in TA. Neither fasting nor age caused a significant effect on glycogen content in either muscle. CR increased glycogen content in SOL of old rats by 46% over the values in CR young rats and by 25% over the values in AL young or old rats, which correlates with a positive effect on activation of GS by CR in this muscle.
Fig. 4.
Glycogen content. Glycogen content was measured in extracts from SOL and TA muscles. Means ± SEM from at least 5 rats for each group are shown. There was an influence of diet (F 4.0, p < 0.05). The interaction of diet and age was also significative (F 4.4, p < 0.05) in SOL The significance of differences is versus young rats comparing equivalent diet and muscle *p < 0.05.
4. Discussion
In this study we show that the activity levels of GS are decreased in the SOL muscle of old (24-mo-old) compared to young adult (6-mo-old) rats maintained on a standard diet and fed ad libitum. The reduction in GS activity corresponds with reduced GS protein content as detected by immunoblotting. This is in contrast to GS gene expression, where mRNA levels of muscle GS gene are unchanged. In TA there is only an age-related reduction in GS protein, with no change in muscle GS mRNA CR hinders the age-associated reduction in GS activity and protein levels in SOL, but is not linked to the regulation of muscle GS mRNA levels. Short-term fasting does not mimic CR effects in the SOL.
The activity ratio of GS (−/+ glucose 6-P) is negatively affected by age in SOL and TA muscles. No decreases are observed in the CR group. Fasting is not equivalent to CR in this regard; in our old rats GS is still inactivated and in the young rats fasting inactivates GS in SOL. The activation state of muscle GS may be regulated by phosphorylation/dephosphorylation at multiple sites. Phosphorylation at site 3a (Ser640 in rabbit muscle GS) in particular has a strong inhibitory effect on the enzyme (Skurat et al., 1994; Skurat and Dietrich, 2004; Wang and Roach, 1993). We show that the age-associated inactivation of GS in rats maintained on a standard diet corresponds with increased phosphorylation at site 3a in both SOL and TA while CR-mediated maintenance of GS activity ratio corresponds with normalization of phosphorylation at this site.
Our observation of decreased GS activity in aged rats agrees with previous data in SOL and fast-twitch biceps femoris of 24-versus 2-month-old rats (Dall’Aglio et al.1987). This is in contrast to the observations made in SOL of 12-compared to 1.5-month-old rats (Narimiya et al., 1984), in which GS activity ratio was slightly increased. No differences in GS activity were either observed in rectus abdominal muscle among 2-, 8- and 20-month-old rats after an hyperinsulinemic-euglycemic clamp (Gupta et al., 2000), what may be interpreted as that high-insulin has an effect. In the latter study, CR was shown to activate GS in 8- and 20-month-old rats (Gupta et al., 2000) after the insulin infusion. We observe a positive effect on the activation state of GS by CR, but only in the aged (24-mo) rats.
The activity levels of GP are reduced in the SOL but not TA by age, irrespective of diet, although the reduction is stronger in the CR group. Compared with TA SOL has lower GP activity and increased B/M-GP immunoblotted protein. This suggests a higher content of the brain GP isoform, and as such may reflect the aerobic phenotype of the SOL muscle and its similarity to cardiac muscle, which also has a high content of the brain GP isoform (David and Crerar, 1986; Mair, 1998; Schmid et al., 2008). The lower total GP activity in SOL compared to TA may be due in part to differences between the brain and muscle isoenzyme kinetics. Although these two isoforms are highly activated by AMP, unlike the liver isoform, (Buchbinder et al., 2001), the brain nonphosphorylated b form is less responsive to activation by phosphorylation than the muscle isozyme and requires AMP for full activation (Crerar et al., 1995). Unexpectedly, the age-associated reduction in SOL GP activity occurs despite preservation of muscle GP protein and increased content of the B/M-GP protein. Brain GP mRNA levels are unchanged by age in the SOL and TA. Our data indicates that the reduction in GP specific activity in SOL by age is not prevented by fasting and further impaired by CR.
The activity ratio of GP (−/+ AMP) is decreased in the TA muscle by age suggesting regulation of the phosphorylation state. However, age does not affect the GP activity ratio in the SOL. Therefore, post-translational mechanisms other than phosphorylation might be responsible for the age-associated changes in GP kinetics. Changes in GP kinetics have been noted in GP-b purified from skeletal muscle of aged rats, where a decrease in the specific activity was correlated with the accumulation of 3-nitrotyrosine (Sharov et al., 2006). Impairment of GP enzyme activity by exposure of purified muscle GP-b, or C2C12 myotubes, to peroxynitrite has also been demonstrated and this corresponds with increased nitration of Tyr613, a key amino acid in the allosteric inhibitor site (Dairou et al., 2007). More recently, however it has been shown that enzymatic activity of muscle GP-b does not directly correlate with protein nitration levels (Sharov et al., 2008). In his context, we show decreased GP specific activity not in the TA muscle, where the muscle GP isoform predominates, but in the oxidative SOL muscle that displays higher B/M-GP protein.
Previous studies have shown no differences in total GP activity in the fast-twitch rectus abdominal muscle of 20- versus 2-month-old rats after an hyperinsulinemic-euglycemic clamp (Gupta et al., 2000), similar to our data in TA. In SOL of 12-compared to 1.5-month-old rats no differences were found (Narimiya et al., 1984) unlike in our study, although GP was more inactive as in our study.
We assessed the effects of aging and CR on the gene expression of GS, GP and key glycogen-targeting subunits of PP1 in skeletal muscle such as GM and PTG. Both GM (Lerin et al., 2003) and PTG (Lerin et al., 2000) promote GS activation in cultured muscle cells and GM inactivates GP (Lerin et al., 2003). We found that age, fasting and CR can negatively affect, muscle GS, muscle GP and GM mRNA levels in SOL, while having no effect in TA In contrast, the PTG mRNA levels are positively regulated by CR, with a marked upregulation in SOL. Short-term fasting does not mimic this effect on PTG mRNA levels. Therefore, upregulation by CR of PTG gene expression corresponds with recovery of GS activity/phosphorylation in both muscle types. The importance of PTG in the regulation of skeletal muscle glycogen metabolism has been revealed by heterozygous deletion of the PTG gene in mice; young mice (1–2 months) exhibited a small reduction of glycogen stores in white fiber epitrochlearis muscle corresponding with GS inactivation and no change in mixed fiber gastrocnemius, while older mice (18 months) showed inhibition of the IRS-1/Akt insulin signaling pathway involved in glucose transport and glycogen synthesis (Crosson et al, 2003).
Glycogen content is not changed in the SOL or TA by age despite considerably decreased GS and GP activities in SOL, suggesting than an impairment of total GS activity of more than 40% is required before defects in glycogen synthesis are seen. Similar to our data, mice heterozygous for the disruption of GYS1, the gene encoding the muscle isoform of GS, and allowed food ad libitum showed about 50% reduction in GS activity with only a tendency toward lower glycogen levels (Pederson et al., 2004); whereas homozygous mice lacked muscle glycogen. In addition, patients with glycogenosis type V have mutations in both alleles of the PYGM gene that result in a lack of functional mature protein (Lucia et al., 2008). Maintenance of skeletal muscle glycogen content in aged rats is a consistent finding. No change in glycogen content was also observed in the gastrocnemius-plantaris muscles of aged (22 months) versus young adult (6–8 months) fed rats (Meynial-Denis et al., 2005) or in SOL and vastus lateralis muscle of aged (24 month or older) versus 4-month-old fed rats (Poland et al., 1982). Although, these are in contrast with the progressive fell in glycogen levels found in SOL and biceps femoris muscles with aging in rats (Dall’Aglio et al., 1987). Furthermore, no differences in glycogen content, phosphate content of glycogen and branching have been noted between 9- to 12-month-old and 3-month-old mice, but an increased average diameter of glycogen particles (Tag-liabracci et al. (2008)).
In the current study we show a significant interaction between the effects of diet and age on muscle glycogen content. In young rats, CR causes a reduction tendency in glycogen content in SOL and TA Short-term fasting does not exert the same effect as CR. In a previous rat study (Holness and Sugden, 1991), 6 h of fasting slightly reduced TA glycogen and did not change SOL glycogen content while fasting for 24 h led to markedly reduced glycogen content in both muscle types. Other studies have also found that fasting, whether for 24 h (Jensen et al., 2006) or 5 days (Meynial-Denis et al., 2005), depletes muscle glycogen content in young rats. Therefore, according to our data in young rats CR resembles long-term fasting in that muscle glycogen is reduced and the CR effect is not related to regulation of GS or GP activity ratio or total activity levels. We also demonstrate that in SOL of older rats CR does not cause glycogen depletion; indeed the levels attained are even higher than in rats fed a standard diet. A previous study in accordance with our data (Wetter et al., 1999) showed increased glycogen concentration in muscle from 12-month-old rats subjected to CR (from 4 months of age) compared to those fed ad libitum, and this effect was greater in the SOL than in gastrocnemius. Likewise, 5 days of food deprivation in aged (22 months) rats did not reduce gastrocnemius-plantaris muscles glycogen content as it did in young rats (Meynial-Denis et al., 2005). Overall these data suggest a differential response of skeletal muscle glycogen to food restriction or deprivation in aged and young rats. In our study, enhancement of glycogen content by CR in older rats corresponds with a positive effect on the activation of GS, the effects being more significant in SOL than in TA Besides increased glycogen synthesis, it is however also possible that glycogen is spared in older rats in favor of other substrates. In this regard a marked rise in triglyceride content in skeletal muscle of aged rats, particularly in SOL, has been shown (Poland et al., 1982).
In summary, age exerts negative robust effects on GS and GP activities in SOL muscle. The loss of GS activity is due to a reduction in protein content and the decrease in GP activity to a reduction in its specific activity. GS is inactivated-phosphorylated at site 3a by effect of age in both muscles SOL and TA Glycogen accumulation is well preserved in older rats on a standard diet despite reduced GS activity. CR tends to decrease muscle glycogen content in muscle of young rats without affecting GS or GP activity/protein levels, suggesting an effect of limited nutrient availability. In old rats, however, CR hinders age-associated reduction in GS activity/protein and activity ratio and decreases the phosphorylation ratio at site 3a, mainly in SOL and this corresponds with an increase in glycogen content, suggesting an important role for GS activity in these conditions. To conclude, a failing in GS activity and activation occurs in aged rats in skeletal muscle mostly in the slow-twitch oxidative SOL rather than in the fast-twitch glycolytic TA. CR, which is known to exert beneficial effects on age-associated tissue oxidative damage, hinders such changes, but not short-term fasting. Glycogen storage is enhanced in old rats on the CR diet despite restricted nutrient availability. The positive effects of the CR diet on skeletal muscle glycogen metabolism in aged rats may contribute to improve glucose disposal and sustain exercise performance.
Acknowledgments
We thank Anna Orozco (Bioquímica i Biologia Molecular, Universitat de Barcelona) and Roy Cutler (Laboratory of Neuroscience, NIA/NIH) for technical assistance. We thank Oliver Valero (Servei d’Estadística UAB, Barcelona, Spain) for assistance in the statistical analysis of data.
This work was supported by Grants SAF2006-07228 from the Ministerio de Ciencia y Tecnología (MCyT) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas which is an ISCIII project, Spain, and in part by the Intramural Research Program of the National Institute on Aging, USA. CGM was the recipient of a Ramon y Cajal fellowship from the Ministerio de Educación y Ciencia, Spain.
References
- Baqué S, Guinovart JJ, Gómez-Foix AM. Overexpression of muscle glycogen phosphorylase in cultured human muscle fibers causes increased glucose consumption and nonoxidative disposal. J Biol Chem. 1996;271:2594–2598. doi: 10.1074/jbc.271.5.2594. [DOI] [PubMed] [Google Scholar]
- Buchbinder JL, Rath VL, Fletterick RJ. Structural relationships among regulated and unregulated phosphorylases. Annu Rev Biophys Biomol Struct. 2001;30:191–209. doi: 10.1146/annurev.biophys.30.1.191. [DOI] [PubMed] [Google Scholar]
- Crerar MM, Karlsson O, Fletterick RJ, Hwang PK. Chimeric muscle and brain glycogen phosphorylases define protein domains governing isozyme- specific responses to allosteric activation. J Biol Chem. 1995;270:13748–13756. doi: 10.1074/jbc.270.23.13748. [DOI] [PubMed] [Google Scholar]
- Crosson SM, Khan A, Printen J, Pessin JE, Saltiel AR. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J Clin Invest. 2003;111:1423–1432. doi: 10.1172/JCI17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Aglio E, Chang H, Reaven GM, Azhar S. Age-related changes in rat muscle glycogen synthase activity. J Gerontol. 1987;42:168–172. doi: 10.1093/geronj/42.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairou J, Pluvinage B, Noiran J, Petit E, Vinh J, Haddad I, Mary J, Dupret JM, Rodrigues-Lima F. Nitration of a critical tyrosine residue in the allosteric inhibitor site of muscle glycogen phosphorylase impairs its catalytic activity. J Mol Biol. 2007;372:1009–1021. doi: 10.1016/j.jmb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13–19. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- David ES, Crerar MM. Quantitation of muscle glycogen phosphorylase mRNA and enzyme amounts in adult rat tissues. Biochim Biophys Acta. 1986;880:78–90. doi: 10.1016/0304-4165(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Escrivá F, Agote M, Rubio E, Molero JC, Pascual-Leone AM, Andrés A, Satrústegui J, Carrascosa JM. In vivo insulin-dependent glucose uptake of specific tissues is decreased during aging of mature Wistar rats. Endocrinology. 1997;138:49–54. doi: 10.1210/endo.138.1.4862. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua NW, Reaven GM, Stoohs RA. Hyperinsulinemia: the missing link among oxidative stress and age-related diseases? Free Radic Biol Med. 2000;29:1302–1306. doi: 10.1016/s0891-5849(00)00438-x. [DOI] [PubMed] [Google Scholar]
- Ferrer-Martinez A, Marotta M, Turini M, Macé K, Gémez-Foix AM. Effect of sucrose and saturated-fat diets on mRNA levels of genes limiting muscle fatty acid and glucose supply in rats. Lipids. 2006;41:55–62. doi: 10.1007/s11745-006-5070-1. [DOI] [PubMed] [Google Scholar]
- Gilboe DP, Larson KL, Nuttall FQ. Radioactive method for the assay of glycogen phosphorylases. Anal Biochem. 1972;47:20–27. doi: 10.1016/0003-2697(72)90274-6. [DOI] [PubMed] [Google Scholar]
- Goldfarb AH, Bruno JF, Buckenmeyer PJ. Intensity and duration of exercise effects on skeletal muscle cAMP, phosphorylase, and glycogen. J Appl Physiol. 1989;66:190–194. doi: 10.1152/jappl.1989.66.1.190. [DOI] [PubMed] [Google Scholar]
- Gupta G, She L, Ma XH, Yang XM, Hu M, Cases JA, Vuguin P, Rossetti L, Barzilai N. Aging does not contribute to the decline in insulin action on storage of muscle glycogen in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R111–R117. doi: 10.1152/ajpregu.2000.278.1.R111. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM. Regulation of carbohydrate and fat metabolism during and after exercise. Annu Rev Nutr. 1996;16:121–138. doi: 10.1146/annurev.nu.16.070196.001005. [DOI] [PubMed] [Google Scholar]
- Holness MJ, Sugden MC. Glucose disposal by skeletal muscle in response to re-feeding after progressive starvation. Biochem J. 1991;277:429–433. doi: 10.1042/bj2770429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Hernandez JO, Mattson MP, de Cabo R. The plasma membrane redox system in aging. Ageing Res Rev. 2006;5:209–220. doi: 10.1016/j.arr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Ivey PA, Gaesser GA. Postexercise muscle and liver glycogen metabolism in male and female rats. J Appl Physiol. 1987;62:1250–1254. doi: 10.1152/jappl.1987.62.3.1250. [DOI] [PubMed] [Google Scholar]
- Jensen J, Jebens E, Brennesvik EO, Ruzzin J, Soos MA, Engebretsen EM, O’Rahilly S, Whitehead JP. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab. 2006;290:E154–E162. doi: 10.1152/ajpendo.00330.2005. [DOI] [PubMed] [Google Scholar]
- Jurczak MJ, Danos AM, Rehrmann VR, Allison MB, Greenberg CC, Brady MJ. Transgenic overexpression of protein targeting to glycogen markedly increases adipocytic glycogen storage in mice. Am J Physiol Endocrinol Metab. 2007;292:E952–E963. doi: 10.1152/ajpendo.00559.2006. [DOI] [PubMed] [Google Scholar]
- Kollberg G, Tulinius M, Gilljam T, Ostman-Smith I, Forsander G, Jotorp P, Oldfors A, Holme E. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. New Eng J Med. 2007;357:1507–1514. doi: 10.1056/NEJMoa066691. [DOI] [PubMed] [Google Scholar]
- Lannér C, Suzuki Y, Bi C, Zhang H, Cooper LD, Bowker-Kinley MM, DePaoli-Roach AA. Gene structure and expression of the targeting subunit, RGL, of the muscle-specific glycogen-associated type 1 protein phosphatase, PP1G. Arch Biochem Biophys. 2001;388:135–145. doi: 10.1006/abbi.2001.2283. [DOI] [PubMed] [Google Scholar]
- Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Lerin C, Montell E, Berman HK, Newgard CB, Gómez-Foix AM. Overexpression of protein targeting to glycogen in cultured human muscle cells stimulates glycogen synthesis independent of glycogen and glucose 6-phosphate levels. J Biol Chem. 2000;275:39991–39995. doi: 10.1074/jbc.M006251200. [DOI] [PubMed] [Google Scholar]
- Lerin C, Montell E, Nolasco T, Clark C, Brady MJ, Newgard CB, Gómez-Foix AM. Regulation and function of the muscle glycogen-targeting subunit of protein phosphatase 1 (GM) in human muscle cells depends on the COOH-terminal region and glycogen content. Diabetes. 2003;52:2221–2226. doi: 10.2337/diabetes.52.9.2221. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucia A, Nogales-Gadea G, Pérez M, Martín MA, Andreu AL, Arenas J. McArdle disease: what do neurologists need to know? Nat Clin Pract Neurol. 2008;4:568–577. doi: 10.1038/ncpneuro0913. [DOI] [PubMed] [Google Scholar]
- Mair J. Glycogen phosphorylase isoenzyme BB to diagnose ischaemic myocardial damage. Clin Chem Acta. 1998;272:79–86. doi: 10.1016/s0009-8981(97)00254-4. [DOI] [PubMed] [Google Scholar]
- Manchester J, Skurat AV, Roach P, Hauschka SD, Lawrence JC., Jr Increased glycogen accumulation in transgenic mice overexpressing glycogen synthase in skeletal muscle. Proc Natl Acad Sci USA. 1996;93:10707–10711. doi: 10.1073/pnas.93.20.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Meynial-Denis D, Miri A, Bielicki G, Mignon M, Renou JP, Grizard J. Insulin-dependent glycogen synthesis is delayed in onset in the skeletal muscle of food-deprived aged rats. J Nutr Biochem. 2005;16:150–154. doi: 10.1016/j.jnutbio.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Narimiya M, Azhar S, Dolkas CB, Mondon CE, Sims C, Wright DW, Reaven GM. Insulin resistance in older rats. Am J Physiol Endocrinol Metab. 1984;246:E397–E404. doi: 10.1152/ajpendo.1984.246.5.E397. [DOI] [PubMed] [Google Scholar]
- Newgard CB, Brady MJ, O’Doherry RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49:1967–1977. doi: 10.2337/diabetes.49.12.1967. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Wojtaszewski JF. Regulation of glycogen synthase activity and phosphorylation by exercise. Proc Nutr Soc. 2004;63:233–237. doi: 10.1079/PNS2004348. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Kuzuya H, Okamoto M, Yoshimasa Y, Yamada K, Ida T, Kakehi T, Imura H. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol Endocrinol Metab. 1988;254:E92–E98. doi: 10.1152/ajpendo.1988.254.1.E92. [DOI] [PubMed] [Google Scholar]
- Park S, Komatsu T, Hayashi H, Yamaza H, Chiba T, Higami Y, Kuramoto K, Shimokawa I. Calorie restriction initiated at middle age improved glucose tolerance without affecting age-related impairments of insulin signaling in rat skeletal muscle. Exp Gerontol. 2006;41:837–845. doi: 10.1016/j.exger.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Pederson BA, Chen H, Schroeder JM, Shou W, DePaoli-Roach AA, Roach PJ. Abnormal cardiac development in the absence of heart glycogen. Mol Cell Biol. 2004;24:7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland JL, Poland JW, Honey RN. Substrate changes during fasting and refeeding contrasted in old and young rats. Gerontology. 1982;28:99–103. doi: 10.1159/000212517. [DOI] [PubMed] [Google Scholar]
- Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid H, Pfeiffer-Guglielmi B, Dolderer B, Thiess U, Verleysdonk S, Hamprecht B. Expression of the brain and muscle isoforms of glycogen phosphorylase in rat heart. Neurochem Res. 2008 doi: 10.1007/s11064-008-9825-3. [DOI] [PubMed] [Google Scholar]
- Sharov VS, Galeva NA, Kanski J, Williams TD, Schöneich C. Age-associated tyrosine nitration of rat skeletal muscle glycogen phosphorylase b: characterization by HPLC-nanoelectrospray-tandem mass spectrometry. Exp Gerontol. 2006;41:407–416. doi: 10.1016/j.exger.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Sharov VS, Galeva NA, Dremina ES, Williams TD, Schoneich C. Inactivation of rabbit muscle glycogen phosphorylase b by peroxynitrite revisited: Does the nitration of Tyr(613) in the allosteric inhibition site control enzymatic function? Arch Biochem Biophys. 2008 doi: 10.1016/j.abb.2008.12.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL. The “glycogen shunt” in exercising muscle: a role for glycogen in muscle energetics and fatigue. Proc Natl Acad Sci USA. 2001;98:457–461. doi: 10.1073/pnas.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurat AV, Dietrich AD. Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem. 2004;279:2490–2498. doi: 10.1074/jbc.M301769200. [DOI] [PubMed] [Google Scholar]
- Skurat AV, Wang Y, Roach PJ. Rabbit skeletal muscle glycogen synthase expressed in COS cells. Identification of regulatory phosphorylation sites. J Biol Chem. 1994;269:25534–25542. [PubMed] [Google Scholar]
- Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GE. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol. 1999;111:117–123. doi: 10.1007/s004180050341. [DOI] [PubMed] [Google Scholar]
- Tagliabracci VS, Girard JM, Segvich D, Meyer C, Turnbull J, Zhao X, Minassian BA, Depaoli-Roach AA, Roach PJ. Abnormal metabolism of glycogen phosphate as a cause for Lafora disease. J Biol Chem. 2008;283:33816–33825. doi: 10.1074/jbc.M807428200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JA, Schlender KK, Larner J. A rapid filter paper assay for UDP-glucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968;25:486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Roach PJ. Inactivation of rabbit muscle glycogen synthase by glycogen synthase kinase-3. Dominant role of the phosphorylation of Ser-640 (site-3a) J Biol Chem. 1993;268:23876–23880. [PubMed] [Google Scholar]
- Wetter TJ, Gazdag AC, Dean DJ, Cartee GD. Effect of calorie restriction on in vivo glucose metabolism by individual tissues in rats. Am J Physiol Endocrinol Metab. 1999;276:E728–E738. doi: 10.1152/ajpendo.1999.276.4.E728. [DOI] [PubMed] [Google Scholar]