Abstract
We examined associations of advanced glycation end products (AGEs) with renal function loss (RFL) and its structural determinants in American Indians with type 2 diabetes. Data were from a 6-year clinical trial that assessed renoprotective efficacy of losartan. Participants remained under observation after the trial concluded. Glomerular filtration rate (GFR) was measured annually. Kidney biopsies were performed at the end of the trial. Five AGEs were measured in serum collected at enrollment and at kidney biopsy. RFL was defined as ≥40% decline of measured GFR from baseline. Of 168 participants (mean baseline age 41 years, HbA1c 9.2%, GFR 164 mL/min, and albumin-to-creatinine ratio 31 mg/g), 104 reached the RFL end point during median follow-up of 8.0 years. After multivariable adjustment, each doubling of carboxyethyl lysine (hazard ratio [HR] 1.60 [95% CI 1.08–2.37]) or methylglyoxal hydroimidazolone (HR 1.30 [95% CI 1.02–1.65]) concentration was associated with RFL. Carboxyethyl lysine, carboxymethyl lysine, and methylglyoxal hydroimidazolone correlated positively with cortical interstitial fractional volume (partial r = 0.23, P = 0.03; partial r = 0.25, P = 0.02; and partial r = 0.31, P = 0.003, respectively). Glyoxyl hydroimidazolone and methylglyoxal hydroimidazolone correlated negatively with total filtration surface per glomerulus (partial r = −0.26, P = 0.01; and partial r = −0.21, P = 0.046, respectively). AGEs improve prediction of RFL and its major structural correlates.
Introduction
Diabetic kidney disease (DKD) is a common complication of type 1 and type 2 diabetes and the leading cause of end-stage renal disease (ESRD) worldwide. Early assessment of DKD is difficult because currently available biomarkers do not identify those at risk with sufficient accuracy. Persistently elevated urinary albumin-to-creatinine ratios (ACR) may return to normal, and structural lesions within the kidneys may be present in the absence of currently available clinical indicators (1–3). Thus, better noninvasive biomarkers are needed that can identify individuals who are at increased risk for DKD even before irreversible structural changes occur.
The complications of diabetes are caused chiefly by persistent hyperglycemia, and intensive glycemic control slows development and progression of the microvascular complications (4,5), including the structural lesions of DKD (6). Hyperglycemia is thought to induce diabetes complications, in part, through nonenzymatic glycation of proteins and the production of advanced glycation end products (AGEs) and related oxidative end products (OPs) (7), with both glycation and oxidation playing roles in the genesis of these adducts. AGEs are produced by nonenzymatic glycation of amino groups on proteins by reducing sugars and dicarbonyl compounds. Glycoxidation adducts are formed by a combination of glycation and oxidation, are more chemically reactive, and are more likely to irreversibly cross-link proteins. AGEs and glycoxidation products are present in higher concentrations among people with diabetes compared with healthy individuals and are thought to increase oxidative stress via interaction with their receptor (RAGE), thereby promoting vascular complications (8).
AGEs are filtered through the glomerulus and reabsorbed by renal proximal tubules. Both their clearance and tubular reabsorption are complex and variable (9,10). AGEs accumulate in glomeruli, where they increase expression of type IV collagen and laminin in the extracellular matricies (11) and induce irreversible cross-linked protein formations (12). In the proximal tubule, they induce premature cell senescence (13). AGEs are signal-transducing ligands for RAGE, a transmembrane receptor. This interaction induces cellular injury through the production of reactive oxygen species and activation of proinflammatory and profibrotic cascades (14,15). Most of the existing RAGE data have used carboxymethyl lysine as the ligand, and it remains to be established which of the dicarbonyl-derived AGEs binds specifically to RAGE.
We reported previously that the highly reactive α-dicarbonyls methylglyoxal and 3-deoxyglucosone are associated with more rapid progression of DKD (16) and that blood levels of AGEs derived from methylglyoxal correlate positively with biopsy documented histological progression of DKD in type 1 diabetes (17). In the current study, we examined the clinical utility of glycation, glycoxidation, and OPs as predictors of renal function loss (RFL) and evaluated their association with the histological lesions of DKD.
Research Design and Methods
Study Subjects and Design
From 1965–2007, Pima Indians from the Gila River Indian Community participated in a longitudinal study of diabetes and its complications. We invited 169 adults with type 2 diabetes from this population to participate in a randomized clinical trial testing the renoprotective efficacy of losartan in early DKD (NCT00340678) (18). Ninety-one participants had normal urinary albumin excretion (ACR <30 mg/g) at baseline, and 78 had persistent microalbuminuria (ACR 30–299 mg/g). Glomerular filtration rate (GFR) was measured annually by the urinary clearance of iothalamate. At the end of the 6-year clinical trial, 111 of the participants underwent percutaneous kidney biopsy (60 with normoalbuminuria and 51 with microalbuminuria at baseline) to determine whether treatment was associated with preservation of kidney structure. Among participants with microalbuminuria at baseline, those who received losartan had, on average, lower mesangial fractional volume than those who received placebo, suggesting that losartan preserved a key aspect of glomerular structure in DKD (19). However, losartan did not significantly affect the primary outcome (i.e., decline in GFR) during the 6-year trial period (18). Upon completion of the clinical trial, participants were returned to the care of their primary physicians and annual research examinations that included measurement of GFR were continued for a median of 7.9 (3.0–9.8) years.
In the current study, we measured AGEs/OPs in serum samples stored at −80°C since collection from the baseline visit of the clinical trial and in up to four subsequent research examinations, each 2 years apart. Samples were collected under standardized conditions and stored at −80°C, undergoing only one freeze-thaw cycle prior to assay. The fourth set of AGE/OP measurements obtained at the 6th year of the clinical trial was only available in subjects with a kidney biopsy. Of the 169 participants in the clinical trial, all but 1 had baseline samples available for measurement of AGEs/OPs. Thus, 168 participants had baseline AGE/OP measurements and were included in the current study, 95 of whom had a kidney biopsy and an AGE/OP measurement at the time of the biopsy (Supplementary Fig. 1).
Vital status and development of ESRD were ascertained independently in all study participants through 31 December 2015. ESRD was defined by the initiation of renal replacement therapy or death from DKD if the participant refused dialysis. Underlying causes of death for the participants who died before reaching the outcomes of interest were determined from death certificates. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Each subject signed an informed consent document.
Clinical and Anthropometric Measures
Blood pressure was measured while the subject was resting in the seated position; mean arterial pressure (MAP) was calculated as (2 × diastolic blood pressure + systolic blood pressure)/3. HbA1c was measured by high-performance liquid chromatography. This method was also used to measure the concentration of nonradioactive iothalamate for GFR determination (11). Urinary albumin concentration was measured by nephelometric immunoassay and urinary creatinine by a modified Jaffé reaction (Siemens, Erlangen, Germany). Urinary albumin concentration below the detection limit of the assay (≤6.8 mg/L) was set to 6.8 mg/L in the analyses.
Biomarker Analytes
AGEs and OPs were measured in serum samples by liquid chromatography–mass spectrometry using internal stable heavy isotope substituted standards. Analysis was performed in a blinded fashion on the serum filtrate following centrifugation through 10-K cutoff Amicon filters. This fraction contains free AGEs and OPs, as well as peptides of varied sizes, and our analytical method measured the free products. An Agilent model 6490 Triple Quadrupole MS System with a 1290 Rapid Resolution LC System was used (Agilent Technologies). Concurrent quantitative measurement was performed using a single Waters XSelect HSS T3 2.5 μm × 2.1 × 150-mm column (Waters) with a mobile phase of methanol/water gradient with 0.20% heptafluorobutyric acid and a total analysis time of 19 min. Seven biomarkers were measured: five dicarbonyl-derived AGE compounds (carboxymethyl lysine, carboxyethyl lysine, glyoxal hydroimidazolone, methylglyoxal hydroimidazolone, and 3-deoxyglucosone hydroimidazolone) and two OP compounds (methionine sulfoxide and 2-aminoadipic acid). Reproducibility of the biomarker assays was assessed by intraclass correlation of measurements from 50 duplicate samples blinded to the performance laboratory. Intraclass correlation coefficient for carboxymethyl lysine was 0.987, for carboxyethyl lysine was 0.986, for glyoxal hydroimidazolone was 0.944, for methylglyoxal hydroimidazolone was 0.989, for 3-deoxyglycosone hydroimidazolone was 0.986, for methionine sulfoxide was 0.907, and for 2-aminoadipic acid was 0.981, reflecting excellent agreement.
Morphometric Methods
Unbiased random sampling of tissue sections from the kidney biopsy provided digital images for study using quantitative morphometric methods to estimate renal structural parameters (18). Measurements were performed by an investigator (E.J.W.) who was masked to the clinical data. Twelve predefined renal parameters were assessed: glomerular basement membrane (GBM) width, cortical interstitial fractional volume, mesangial fractional volume per glomerulus, glomerular filtration surface density, total filtration surface per glomerulus, number of nonpodocyte cells (endothelial plus mesangial cells) per glomerulus, number of podocytes per glomerulus, podocyte foot process width, percent podocyte detachment, and percentage of normally fenestrated endothelium (18). An equation was used to calculate the percentage of globally sclerotic glomeruli that accounts for the difference in size and therefore in the probability of encountering a sclerotic or nonsclerotic glomerulus in a random cross-section (20). An average ± SD of 14 ± 6 glomeruli were examined in each participant by light microscopy and 3 ± 1 by electron microscopy for the morphometric measurements. Morphometric variables for each individual were calculated as the mean of all measurements for that individual.
Statistical Analysis
Patient characteristics were expressed as mean ± SD, median (interquartile range [IQR]), or n (%). Pearson correlations were used to assess the relationship of biomarkers with each other and with clinical variables. For regression analyses, we log(2)-transformed all biomarker concentrations and morphometric variables with skewed distributions. The primary end point in the longitudinal analyses was RFL as defined by a decline in GFR during follow-up of ≥40% compared with the baseline value. This end point was recently recommended for use in clinical trials of kidney disease (21), and we reported previously that structural lesions strongly predicted this outcome (22). Follow-up continued until the last available research examination; 43 (41%) RFL end points occurred during the clinical trial and 61 (59%) during posttrial follow-up. Five of 26 participants who developed ESRD did not have their GFR drop sufficiently to meet the criteria for RFL by their last research examination, so they were included in the non-RFL group in the analyses. Cox proportional hazards regression was used to assess the effect of each AGE/OP measured at baseline on the risk of RFL. A time-dependent Cox model was also examined to determine if serial measurement of AGEs/OPs (baseline, year 2, and year 4 of the trial) improved the model’s ability to predict RFL. A univariate model and three multivariate models were considered. The first multivariate model (model A) was adjusted for age, sex, treatment assignment during the clinical trial, diabetes duration, HbA1c, and MAP; model B was adjusted for model A plus GFR; model C was adjusted for model B plus ACR. We tested each model for log-linearity, and proportionality assumptions were met by each covariate when using cumulative sums of Martingale residuals. To assess the extent to which AGE/OPs enhanced prediction of RFL, generalized c-statistics were calculated for model C accounting for variable follow-up times (23). Comparisons between nested models that included or excluded the analyte of interest were assessed by likelihood ratio tests (24,25). In addition, the relative integrated discrimination improvement (rIDI) index was calculated to assess the improvement in 10-year RFL risk prediction of each biomarker in addition to traditional DKD risk factors (26); the 10-year risk was selected as it approximates the median follow-up time for the RFL outcome. The 95% CIs for the rIDIs were computed based on 10,000 bootstrap samples. To determine if treatment assignment during the clinical trial modified the relationship between AGEs/OPs and RFL, we tested the interaction of treatment assignment with each AGE/OP.
We performed several sensitivity analyses related to RFL to determine if our findings were robust to competing risk factors, different end point definitions, loss to follow-up, or to the effect of treatment with renin-angiotensin system (RAS) inhibitors, which are known to affect the rate of GFR loss. In the first analysis, we used the competing risk model of Fine and Gray to estimate the subdistribution hazard ratios (HRs) for RFL while accounting for the competing risk of pre-RFL deaths (27). In the second analysis, we computed individual biomarker means and slopes as time-averaged rates of change by simple linear regression on all biomarker measurements in the 143 individuals who had AGE/OP measurements available at all baseline, year 2, and year 4 examinations. We then assessed the effect of the mean and slope for each AGE/OP on RFL. Because the slope was computed from the AGE/OP measurements made at enrollment, year 2, and year 4, the year 4 examination was considered as the “baseline” examination, and these regression analyses were adjusted for age, sex, diabetes duration, MAP, HbA1c, GFR, and ACR measured at this examination. In the third analysis, GFR measured at each research examination at which the participant was treated with a RAS inhibitor was adjusted upward by 3.75% to account for the acute effects of initiating treatment with RAS inhibitors, as previously described (22). In the fourth analysis, we substituted the prespecified primary end point from the clinical trial (decline in GFR to ≤60 mL/min or to half of the baseline value in those who entered the study with a GFR <120 mL/min) for RFL defined by a ≥40% decline in GFR from baseline (18). During the posttrial follow-up, adherence to annual research examinations declined, and five participants progressed to ESRD without documentation of reaching the RFL end point at a research examination. To avoid the bias (informative censoring) that occurs when loss to follow-up is related to the study outcome, we performed the fifth and sixth analyses. In the fifth analysis, the five individuals who developed ESRD without reaching the RFL end point at a research examination were included as cases of RFL, with the last research examination considered as the onset of RFL. In the sixth analysis, we used linear imputation to estimate the date of onset of the study outcomes. To estimate the date of onset of the RFL end point, a linear GFR slope was computed in each participant based on the last two GFR values, with the last GFR value defined as follows:
In participants who did not reach the RFL end point, the GFR measured at their last examination;
In participants who reached the RFL end point at an examination, the GFR value measured at that examination;
In participants who progressed to ESRD without a GFR measurement indicating that they had reached the RFL end point, a GFR of zero was assigned as of the date of onset of renal replacement therapy.
The estimated date of onset of the RFL end point was then imputed for all participants from the GFR slope, with follow-up continued for each participant for 2 years after the last measured GFR or until the RFL end point, death, or 31 December 2015, whichever came first. This approach permitted us to determine whether a participant who missed scheduled visits and did not reach the RFL end point by their last examination would have done so if they had remained under observation. The 2-year follow-up interval was selected because it represented the median time interval between the last GFR measurement and the onset of ESRD in the study cohort.
Linear regression models were used to assess the relationships between biomarkers measured near the time of the kidney biopsy and renal structural variables. We did not include all previous measures of AGEs/OPs in this analysis, because we considered the measure proximate to the biopsy to be the best measure of lifetime exposure at the time of biopsy. Only the univariate model and models A and B were considered for this analysis; model C was not considered because albuminuria is highly correlated with the underlying structural lesions. We also tested the interaction of treatment assignment with each AGE/OP for the linear regression models. Model fit was assessed for normality and leverage by “studentized” residuals and for multicollinearity using eigenvalues and the condition index (28). Associations between AGEs and morphometric measures were illustrated graphically by partial Pearson correlation coefficients and partial residual regression plots. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC). The P values <0.05 were considered statistically significant.
Results
The 168 participants had a mean age of 41.4 ± 10.6 years, median diabetes duration 8.9 (IQR 6.2–14.9) years, mean HbA1c 9.2 ± 2.3%, mean GFR 164 ± 42 mL/min, and median ACR 31 (IQR 13–76) mg/g. Clinical and biological characteristics of the participants at baseline are summarized in Table 1. AGEs correlated with one another (r = 0.42–0.74; P < 0.001 for all), whereas OPs correlated weakly with themselves (r = 0.13; P = 0.09) and in some cases with AGEs (r = 0.03–0.29; P < 0.001 to 0.70). AGEs correlated negatively with baseline GFR and positively with baseline age and diabetes duration (Table 2). 2-Aminoadipic acid correlated positively with age and BMI, whereas methionine sulfoxide and glyoxal hydroimidazolone correlated negatively with HbA1c. From baseline to year 4, methionine sulfoxide, carboxymethyl lysine, methylglyoxal hydroimidazolone, and 3-deoxyglucosone hydroimidazolone increased significantly in those with measurements at all three time points (P < 0.0001, P = 0.0001, P = 0.004, and P = 0.002, respectively). In the subset of 95 patients who underwent kidney biopsy, all serum AGE/OP concentrations except for 2-aminoadipic acid (P = 0.10) had increased significantly by year 6 (P < 0.001). Losartan treatment assignment did not affect the rise in AGE/OP concentrations.
Table 1.
Clinical characteristics and concentrations of AGEs and OPs at the onset of the clinical trial (n = 168)
| Clinical characteristics | |
| Age (years) | 41.4 ± 10.6 |
| Male | 46 (27) |
| Losartan treatment group | 83 (49) |
| BMI (kg/m2) | 35.7 ± 8.4 |
| Diabetes duration (years) | 8.9 (6.2–14.9) |
| Systolic blood pressure (mmHg) | 118 ± 13 |
| Diastolic blood pressure (mmHg) | 76 ± 8 |
| HbA1c [% (mmol/mol)] | 9.2 ± 2.3 (77.0 ± 25.1) |
| GFR (mL/min) | 164 ± 42 |
| Urinary ACR (mg/g) | 30.6 (13.5–76.3) |
| Biomarker concentrations | |
| Methionine sulfoxide (nmol/L) | 684 (581–803) |
| 2-Aminoadipic acid (nmol/L) | 862 (659–1,098) |
| Carboxymethyl lysine (nmol/L) | 59 (46–74) |
| Glyoxal hydroimidazolone (nmol/L) | 7.3 (6.5–8.6) |
| Carboxyethyl lysine (nmol/L) | 45 (35–58) |
| Methylglyoxal hydroimidazolone (nmol/L) | 71 (47–106) |
| 3-Deoxyglucosone hydroimidazolone (nmol/L) | 194 (160–290) |
Quantitative variables are described by mean ± SD or median (IQR) and qualitative variables by n (%).
Table 2.
Pearson correlations of AGEs and OPs with clinical variables at enrollment in the clinical trial
| Clinical variables | MetSO | 2-AAA | CML | GH1 | CEL | MGH1 | 3DGHI |
|---|---|---|---|---|---|---|---|
| Age | 0.12 (0.13) | 0.21 (0.007) | 0.33 (<0.001) | 0.42 (<0.001) | 0.31 (<0.001) | 0.31 (<0.001) | 0.35 (<0.001) |
| Sex | 0.08 (0.28) | 0.11 (0.16) | 0.05 (0.54) | 0.17 (0.03) | 0.11 (0.16) | 0.09 (0.23) | 0.23 (0.003) |
| Diabetes duration | 0.06 (0.44) | 0.01 (0.94) | 0.22 (0.004) | 0.25 (0.001) | 0.17 (0.02) | 0.22 (0.003) | 0.20 (0.01) |
| BMI | 0.09 (0.24) | 0.21 (0.006) | 0.03 (0.68) | 0.11 (0.15) | 0.01 (0.90) | −0.07 (0.35) | −0.16 (0.04) |
| HbA1c | −0.24 (0.002) | 0.05 (0.53) | −0.02 (0.76) | −0.24 (0.002) | −0.10 (0.21) | −0.03 (0.70) | −0.08 (0.32) |
| MAP | 0.03 (0.73) | −0.02 (0.81) | 0.02 (0.84) | 0.09 (0.25) | 0.01 (0.94) | 0.03 (0.71) | 0.15 (0.05) |
| GFR | −0.04 (0.63) | 0.09 (0.25) | −0.22 (0.004) | −0.28 (<0.001) | −0.29 (<0.001) | −0.19 (0.01) | −0.24 (0.002) |
| Urinary ACR | −0.07 (0.34) | −0.01 (0.95) | 0.003 (0.96) | 0.02 (0.79) | −0.0001 (0.999) | −0.0001 (0.98) | 0.03 (0.65) |
P values <0.05 are shown in boldface.
2-AAA, 2-aminoadipic acid; CEL, carboxyethyl lysine; CML, carboxymethyl lysine; 3DGHI, 3-deoxyglucosone hydroimidazolone; GH1, glyoxal hydroimidazolone; MetSO, methionine sulfoxide; MGH1, methylglyoxal hydroimidazolone.
AGEs and RFL
During a median follow-up of 8.0 (IQR 4.9–13.1) years, the primary end point of RFL (≥40% decline in GFR) occurred in 104 (62%) of the participants. In addition, 24 participants died before reaching the RFL end point: 5 from malignancy, 4 from cardiovascular disease, 4 from alcoholic liver disease, 5 from other natural causes, and 3 from external causes; death certificates were not yet available for the 3 remaining deaths). In univariate Cox models, a higher concentration of methylglyoxal hydroimidazolone at baseline was associated with a higher risk of RFL (Table 3). After multivariable adjustment (model C), carboxyethyl lysine (HR per doubling 1.60 [95% CI 1.08–2.37]) and methylglyoxal hydroimidazolone (HR 1.30 [95% CI 1.02–1.65]) each predicted RFL. Adjustment for both GFR and ACR strengthened the association between these compounds and RFL, in comparison with models A and B. The c-statistic from model C for predicting the primary end point was 0.672, when neither an AGE nor OP was included in the model. The addition of methylglyoxal hydroimidazolone to the model C covariates alone increased the c-statistic to 0.680 (difference in c-statistic, 0.008; P = 0.02; rIDI 14.9% [95% CI 1.7–51.8], P = 0.04) and the addition of carboxyethyl lysine increased the c-statistic to 0.682 (difference in c-statistic, 0.010; P = 0.02; rIDI 13.4% [95% CI −1.1 to 47.8], P = 0.10). There was no interaction between losartan treatment assignment and AGEs/OPs in any of the models. None of the AGEs/OPs predicted RFL in the time-dependent Cox models that took all available measurements of AGEs/OPs into account.
Table 3.
Cox proportional hazards model for the risk of >40% decline in GFR from baseline associated with a doubling of the serum concentration of AGEs and OPs
| Variable | Univariate | Model A | Model B | Model C |
|---|---|---|---|---|
| Cox models | ||||
| MetSO | 0.77 (0.46–1.31) | 0.98 (0.57–1.70) | 0.93 (0.54–1.62) | 0.97 (0.56–1.69) |
| P = 0.34 | P = 0.94 | P = 0.80 | P = 0.88 | |
| 2-AAA | 0.95 (0.66–1.39) | 0.96 (0.63–1.46) | 0.82 (0.55–1.24) | 0.82 (0.55–1.22) |
| P = 0.80 | P = 0.83 | P = 0.35 | P = 0.38 | |
| CML | 1.35 (0.99–1.85) | 1.30 (0.91–1.85) | 1.38 (0.97–1.96) | 1.39 (0.97–1.98) |
| P = 0.06 | P = 0.15 | P = 0.07 | P = 0.06 | |
| GH1 | 0.96 (0.59–1.58) | 1.00 (0.54–1.85) | 1.27 (0.69–2.32) | 1.29 (0.70–2.38) |
| P = 0.88 | P = 0.998 | P = 0.45 | P = 0.40 | |
| CEL | 1.29 (0.93–1.80) | 1.35 (0.92–1.97) | 1.59 (1.07–2.35) | 1.60 (1.08–2.37) |
| P = 0.12 | P = 0.13 | P = 0.02 | P = 0.01 | |
| MGH1 | 1.26 (1.03–1.56) | 1.27 (1.00–1.61) | 1.28 (1.01–1.63) | 1.30 (1.02–1.65) |
| P = 0.03 | P = 0.046 | P = 0.04 | P = 0.03 | |
| 3DGHI | 1.21 (0.93–1.58) | 1.16 (0.86–1.57) | 1.20 (0.88–1.64) | 1.21 (0.88–1.64) |
| P = 0.15 | P = 0.32 | P = 0.25 | P = 0.21 | |
| Fine and Gray competing risk models* | ||||
| MetSO | 0.61 (0.37–1.01) | 0.82 (0.50–1.33) | 0.78 (0.48–1.28) | 0.78 (0.48–1.29) |
| P = 0.05 | P = 0.41 | P = 0.33 | P = 0.33 | |
| 2-AAA | 0.99 (0.68–1.43) | 1.03 (0.70–1.52) | 0.96 (0.64–1.44) | 0.96 (0.64–1.43) |
| P = 0.94 | P = 0.89 | P = 0.84 | P = 0.83 | |
| CML | 1.33 (0.99–1.79) | 1.37 (0.97–1.92) | 1.44 (1.02–2.03) | 1.47 (1.05–2.06) |
| P = 0.06 | P = 0.07 | P = 0.04 | P = 0.03 | |
| GH1 | 0.92 (0.57–1.49) | 1.10 (0.59–2.04) | 1.33 (0.73–2.41) | 1.34 (0.73–2.43) |
| P = 0.73 | P = 0.76 | P = 0.35 | P = 0.34 | |
| CEL | 1.24 (0.8–1.73) | 1.30 (0.88–1.92) | 1.40 (0.95–2.07) P = 0.09 | 1.40 (0.95–2.08) |
| P = 0.22 | P = 0.18 | P = 0.09 | ||
| MGH1 | 1.29 (1.05–1.58) | 1.34 (1.08–1.67) | 1.36 (1.10–1.69) | 1.38 (1.11–1.71) |
| P = 0.01 | P = 0.009 | P = 0.004 | P = 0.004 | |
| 3DGHI | 1.13 (0.89–1.43) | 1.18 (0.93–1.49) | 1.24 (0.96–1.60) | 1.23 (0.95–1.64) |
| P = 0.31 | P = 0.17 | P = 0.11 | P = 0.11 | |
Data are presented as HR (95% CI). HRs are given per doubling of the AGE variable. P values <0.05 are shown in boldface.
Model A was adjusted for age, sex, treatment assignment, diabetes duration, HbA1c, and MAP. Model B was adjusted for model A covariates plus GFR. Model C was adjusted for model B covariates plus urinary ACR.
2-AAA, 2-aminoadipic acid; CEL, carboxyethyl lysine; CML, carboxymethyl lysine; 3DGHI, 3-deoxyglucosone hydroimidazolone; GH1, glyoxal hydroimidazolone; MetSO, methionine sulfoxide; MGH1, methylglyoxal hydroimidazolone. *Competing risk is all-cause death.
Sensitivity Analyses for AGEs and RFL
After accounting for the competing risk of mortality, the subdistribution HRs for baseline methylglyoxal hydroimidazolone and carboxymethyl lysine were strengthened and for carboxyethyl lysine was attenuated (Table 3). In the subset of 143 participants with complete data for AGE/OP measurements at enrollment, year 2, and year 4 examinations, 93 (65%) developed RFL. When the mean value of all three AGE/OP measurements and the slope of the three measurements were included in model C, mean methylglyoxal hydroimidazolone was significantly associated with the risk of RFL (HR 1.55 [95% CI 1.01–2.39]) after accounting for the slope of methylglyoxal hydroimidazolone. Adjustment for the acute effects of RAS-inhibitor use on GFR had no effects on the results.
Supplementary Table 1 shows the results of the remaining sensitivity analyses. When the five participants who developed ESRD but did not meet the GFR criteria for RFL were included as cases of RFL, carboxymethyl lysine, in addition to carboxyethyl lysine and methylglyoxal hydroimidazolone, each predicted RFL in model C (HR 1.44 [95% CI 1.02–2.04]; 1.68 [1.14–2.46]; and 1.32 [1.05–1.67], respectively). When RFL was ascertained by linear imputation, 117 participants reached the RFL end point, and the findings were equivalent to the primary analyses. When we reanalyzed the data using the prespecified primary GFR outcome from the clinical trial (decline in GFR to ≤60 mL/min or to half of the baseline value in those who entered the study with a GFR <120 mL/min), 37 (22%) participants reached this end point, and 3-deoxyglucosone hydroimidazolone was predictive in model C in addition to carboxyethyl lysine and methylglyoxal hydroimidazolone, which predicted the RFL outcome defined by a ≥40% decline in GFR.
AGEs and Renal Structure
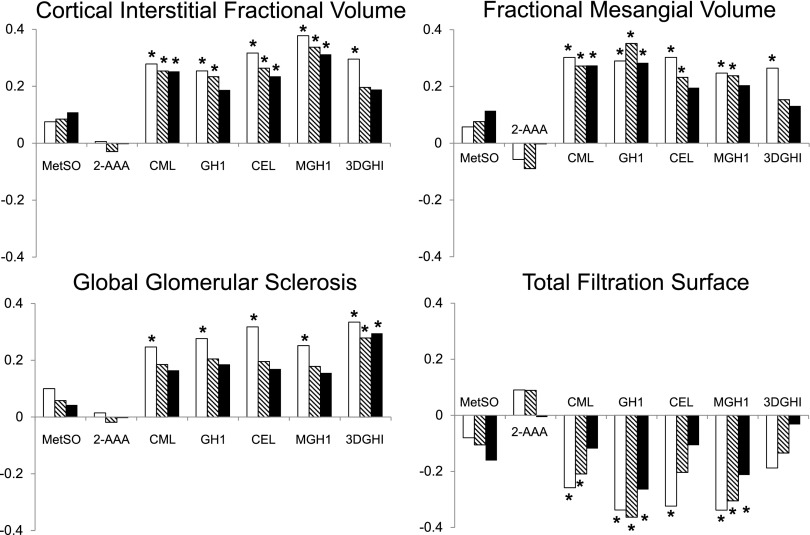
Characteristics of the 95 participants who underwent a kidney biopsy and for whom AGE/OP concentrations were available near the time of biopsy are presented in Table 4. Forty-nine (52%) of those who underwent kidney biopsy had ACR <30 mg/g, and 46 (48%) had an ACR of 30–299 mg/g at enrollment into the clinical trial; 54 (57%) were assigned to receive losartan (Supplementary Fig. 1). AGE/OP concentrations were measured in serum samples obtained a median of 85 (IQR 43–87) days from the kidney biopsy. In univariate models (Supplementary Table 2), all dicarbonyl-derived compounds correlated significantly and positively with the proportion of globally sclerotic glomeruli (r = 0.25–0.33; P < 0.001–0.02), mesangial fractional volume per glomerulus (r = 0.25–0.30; P < 0.001–0.01), and cortical interstitial fractional volume (r = 0.25–0.38; P = 0.003–0.02). All AGEs, with the exception of 3-deoxyglucosone hydroimidazolone, correlated negatively with total filtration surface per glomerulus (r = −0.34 to −0.26; P < 0.001–0.01). Glomerular volume correlated negatively with carboxyethyl lysine and methylglyoxal hydroimidazolone (r = −0.22, P = 0.03; and r = −0.21, P = 0.04, respectively). Filtration surface density correlated negatively with glyoxal hydroimidazolone (r = −0.24; P = 0.02). Fenestrated endothelium correlated negatively with carboxymethyl lysine (r = −0.25; P = 0.02). Podocyte foot process width correlated positively with glyoxal hydroimidazolone (r = 0.21; P = 0.04). One participant had a foot process width 6.5 SDs above the mean; removal of this outlier strengthened the relationship with glyoxal hydroimidazolone (r = 0.29; P = 0.004). Neither of the OPs was associated with any structural measures.
Table 4.
Clinical characteristics and concentrations of AGEs and OPs at the time of biopsy (morphometric characteristics from the biopsy are also shown) (n = 95)
| Clinical characteristics | |
| Age (years) | 46.1 ± 9.9 |
| Male | 26 (27) |
| Losartan treatment group | 54 (57) |
| BMI (kg/m2) | 36.1 ± 8.2 |
| Diabetes duration (years) | 14.2 (11.3–19.9) |
| Systolic blood pressure (mmHg) | 124 ± 17 |
| Diastolic blood pressure (mmHg) | 78 ± 10 |
| HbA1c [% (mmol/mol)] | 9.3 ± 2.0 (78.0 ± 21.9) |
| GFR (mL/min) | 144 ± 60 |
| Urinary ACR (mg/g) | 36.1 (12.1–110.7) |
| Biomarker concentrations | |
| Methionine sulfoxide (nmol/L) | 947 (812–1,075) |
| 2-Aminoadipic acid (nmol/L) | 776 (646–1,018) |
| Carboxymethyl lysine (nmol/L) | 74 (59–100) |
| Glyoxal hydroimidazolone (nmol/L) | 8.2 (6.5–9.9) |
| Carboxyethyl lysine (nmol/L) | 54 (43–76) |
| Methylglyoxal hydroimidazolone (nmol/L) | 113 (65–190) |
| 3-Deoxyglucosone hydroimidazolone (nmol/L) | 300 (209–430) |
| Morphometric characteristics | |
| Mean glomerular volume (× 106 µm3) | 5.7 (4.7–6.9) |
| Global glomerular sclerosis (%) | 5.6 (0.0–18.7) |
| Glomerular filtration surface density (µm2/µm3) | 0.08 (0.06–0.09) |
| Total filtration surface per glomerulus (× 105 µm2) | 4.0 (3.2–5.5) |
| GBM width (nm) | 500 (413–584) |
| Mesangial fractional volume (%) | 18.0 (13.3–23.4) |
| Cortical interstitial fractional volume (%) | 30.0 (24.4–33.4) |
| Podocyte number per glomerulus | 620 (462–766) |
| Foot process width (nm) | 458 (402–524) |
| Podocyte detachment (%) | 0.4 (0.0–1.5) |
| Fenestrated endothelium (%) | 27.4 (22.4–33.0) |
Quantitative variables are described by mean ± SD or median (IQR) and qualitative variables by n (%).
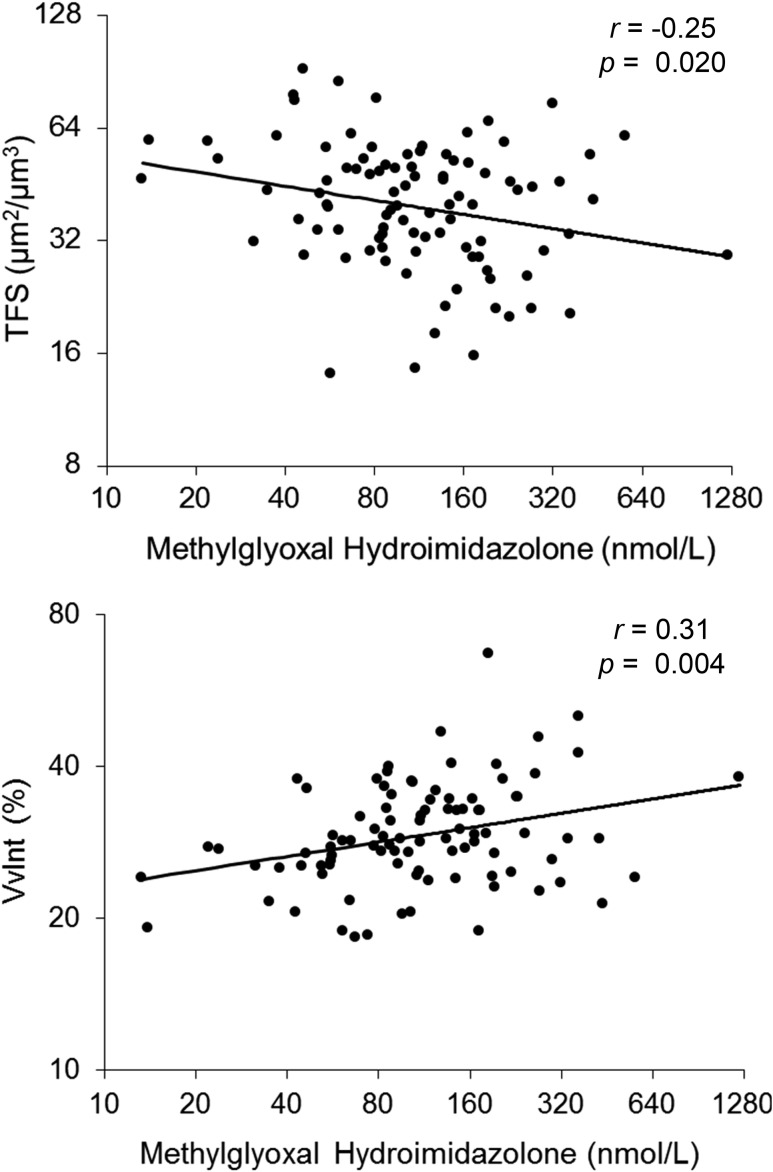
Although multivariate adjustment (models A and B) attenuated many of the observed relationships, all dicarbonyl-derived AGEs remained associated with at least one morphometric parameter after full adjustment. In model B, carboxyethyl lysine and methylglyoxal hydroimidazolone correlated positively with cortical interstitial fractional volume (partial r = 0.27, P = 0.01; and partial r = 0.28, P = 0.008, respectively) and carboxymethyl lysine, carboxyethyl lysine, and methylglyoxal hydroimidazolone with mesangial fractional volume (partial r = 0.25, P = 0.02; partial r = 0.23, P = 0.03; and partial r = 0.31, P = 0.003, respectively). Glyoxal hydroimidazolone and methylglyoxal hydroimidazolone correlated negatively with total filtration surface per glomerulus (partial r = −0.26, P = 0.01; and partial r = −0.21, P = 0.05, respectively). 3-Deoxyglucosone hydroimidazolone correlated positively with percentage of globally sclerotic glomeruli (partial r = 0.30; P = 0.005). Finally, carboxymethyl lysine correlated negatively with the percentage of fenestrated endothelium. The partial correlations of each AGE/OP with relevant structural variables are shown in Fig. 1. For illustration, partial residual regression plots of methylglyoxal hydroimidazolone with total filtration surface per glomerulus and cortical interstitial fractional volume are shown in Fig. 2. We found no interactions between losartan treatment assignment and any of the AGEs/OPs, indicating that the relationship between these biomarkers and the morphometric variables was not modified by treatment.
Figure 1.
Partial correlations of AGEs and OPs with renal structural variables. Partial Pearson correlation coefficients are shown on the y-axis for three different models: unadjusted (open bars), model A (hatched bars), and model B (closed bars). Model A was adjusted for age, sex, losartan treatment assignment, diabetes duration, HbA1c, and MAP. Model B was adjusted for model A covariates plus GFR. *P < 0.05. 2-AAA, 2-aminoadipic acid; CEL, carboxyethyl lysine; CML, carboxymethyl lysine; 3DGHI, 3-deoxyglucosone hydroimidazolone; GH1, glyoxal hydroimidazolone; MetSO, methionine sulfoxide; MGH1, methylglyoxal hydroimidazolone.
Figure 2.
Partial regression residual plots of methylglyoxal hydroimidazolone concentration and renal structural variables. The residuals were computed from regressing each of these variables on age, sex, diabetes duration, HbA1c, treatment assignment, MAP, and GFR. Methylglyoxal hydroimidazolone, total filtration surface, and cortical fractional interstitial volume are shown on a log base 2 scale. Pearson partial r and the corresponding P value are shown. TFS, total filtration surface; VvInt, cortical interstitial fractional volume.
Discussion
In Pima Indians with type 2 diabetes and early-stage DKD, serum AGEs predicted RFL and correlated with the severity of DKD lesions associated with RFL. Both carboxyethyl lysine and methylglyoxal hydroimidazolone predicted RFL, and methylglyoxal hydroimidazolone significantly improved the accuracy of RFL prediction when considered in addition to traditional renal risk factors. These results were consistent across several definitions of renal function decline. In addition, several AGEs were associated with the severity of DKD lesions, including increased cortical interstitial fractional volume, mesangial fractional volume, decreased total filtration surface area per glomerulus, podocyte foot process width, and the percentage of endothelial cell surface with fenestrations. We reported previously that the early decline of GFR in type 1 diabetes is primarily related to classical DKD glomerular lesions (29,30). Tubulointerstitial lesions are critically important in the progression of DKD from moderately reduced GFR to ESRD (29,31). Hence, increased concentrations of the AGEs appear to be associated with both the initiation and progression of DKD. Measuring AGEs in renal tissue may more accurately reflect production and accumulation in the kidneys. Nevertheless, the serum measures used in the current study predicted RFL and its structural determinants and may provide a noninvasive means to more precisely determine risk of progressive DKD than traditional risk factors alone.
These results expand previous work showing a cross-sectional relationship between serum AGEs and renal function in dialyzed or renal-transplanted patients with diabetes by demonstrating a relationship with RFL in early DKD (32). They also accord with prior studies showing higher AGE concentrations associated with worsening renal structural lesions in patients with type 1 diabetes and either normal (17) or elevated urinary albumin excretion (16). We previously showed that in a group of 45 Pima Indians, elevated methylglyoxal, the precursor of carboxyethyl lysine and methylglyoxal hydroimidazolone, was associated with increased GBM width and reduced podocyte number per glomerulus (16). In the same study, we found that oxidative compounds were not associated with worsening of renal structural lesions, which is consistent with the current study, in which neither methionine sulfoxide nor 2-aminioadipic acid was associated with RFL or any morphometric variables. Although the dicarbonyl-derived AGE species we measured were individually associated with different structural variables, all AGEs were consistently associated with signs of greater structural damage commonly attributed to diabetes (33). The morphometric parameters associated with AGEs have previously been related to the level of renal function in type 1 (34) and type 2 diabetes (22,35).
All AGE concentrations increased during follow-up. This increase was expected, as AGEs accumulate with aging (36) and increased diabetes duration (37), and AGE clearance is reduced by renal impairment (38). In the current study, all AGEs correlated positively with age and diabetes duration and negatively with GFR. We believe that declining renal function with attendant decreases in the renal clearance of these AGEs (39) were predominantly responsible for their increasing concentrations seen during follow-up and are responsible for the time-dependent model not enhancing the prediction of RFL. The rising concentrations of AGEs/OPs attributable to decreased renal clearance may confound the biological processes that link AGEs with DKD in persons with normal renal function. Nevertheless, the progressive retention of AGEs that occurs with declining renal function may create a vicious cycle of kidney damage that accelerates the decline in renal function in the later stages of DKD. Negative correlations of methionine sulfoxide and glyoxal hydroimidazolone with HbA1c are consistent with the observation that AGE/OP formation is partly genetically determined and not entirely dependent on high ambient glycemia (40,41). Multiple glycation pathways in diabetes could produce discrepant levels of end products, and adaptive mechanisms that alter processing of AGEs/OPs could also play a role (42).
Some drugs may affect AGE production or clearance. Among the angiotensin II receptor blockers, olmesartan (43) and valsartan (44,45) decrease AGE concentrations, whereas irbesartan does not (46,47). Despite losartan’s known effect on AGE concentrations in an animal model (48), in the current study, losartan treatment did not affect the relationship between AGE/OP concentrations and RFL or renal structural lesions, suggesting the absence of a class effect. Biguanides, especially metformin, may also decrease AGE concentrations via an indirect effect by lowering glucose and reacting directly with dicarbonyl adducts (49). Seventy-five percent of our study participants reported taking metformin, but 93% of those had only intermittent exposure during the study period.
The strengths of this study include an extended follow-up period, multiple measurements of AGE/OP concentrations over time, a detailed renal phenotype based on serial measures of GFR by the urinary clearance of iothalamate, and measures of kidney structure based on standardized unbiased random sampling morphometric methods. Samples were collected under standardized conditions and stored at −80°C, undergoing only one prior freeze-thaw cycle. We previously showed that AGE/OP concentrations were stable when stored under these conditions (17), and the assay used to measure these samples was robust and highly reproducible (50). In addition, a large proportion of the studied population reached the functional study end point, giving us good statistical power to examine the effects of AGEs/OPs on RFL. The conclusions of the study were largely unchanged when different definitions of GFR decline were used in the analysis. Increased loss to follow-up as kidney disease progressed in the study participants was a potential limitation. This could lead to differential misclassification of the GFR outcome if participants with RFL were more likely to miss research examinations at which the GFR was measured. Sensitivity analyses suggest that bias attributable to this factor had little effect on the findings of the study. Another limitation is the single kidney biopsy at the end of the clinical trial, which did not allow us to assess structural changes over time. We recently completed a second kidney biopsy on a subset of the study participants and plan to explore the effects of AGEs/OPs on the structural changes of DKD when these data become available.
In conclusion, a number of serum AGEs in American Indians with type 2 diabetes and early DKD are associated with specific renal lesions of DKD and the loss of renal function that occurs in the presence of those lesions. These findings persisted after adjusting for traditional risk factors. Additionally, methylglyoxal hydroimidazolone improved the accuracy of predicting RFL over traditional renal risk factors, although the magnitude of improvement was modest. These results suggest that some serum AGEs may be useful biomarkers for progressive DKD and may play an active pathophysiological role in its development.
Article Information
Acknowledgments. The authors thank the participants of the losartan clinical trial and the doctors, nurses, and support staff for the role in collecting and processing the data. The authors also thank Dr. R. Hanson (National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ) for statistical help and critical comments.
Funding. This research was supported by the Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases, the American Diabetes Association (Clinical Science Award 1-08-CR-42), and Small Business Innovation Research grant 4R44-DK-101226-02 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. S.H. is employed by PreventAGE Health Care, where the assays of AGEs and OPs were performed. P.J.B. has a financial interest in PreventAGE Health Care. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.-J.S., R.G.N., and P.J.B. researched data and wrote the manuscript. K.M.W., S.H., E.J.W., S.K.T., and B.Y. researched data and reviewed and edited the manuscript. W.C.K., K.V.L., and M.M. researched data, reviewed and edited the manuscript, and contributed to the discussion. R.G.N. and P.J.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Society of Nephrology’s Kidney Week, San Diego, CA, 3–8 November 2015, and at the 12th International Symposium on the Maillard Reaction, Tokyo, Japan, 1–4 September 2015.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0310/-/DC1.
References
- 1.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994;43:1358–1364 [DOI] [PubMed] [Google Scholar]
- 2.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 2003;52:1036–1040 [DOI] [PubMed] [Google Scholar]
- 3.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 5.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 6.Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM. Effect of glycemic control on early diabetic renal lesions. A 5-year randomized controlled clinical trial of insulin-dependent diabetic kidney transplant recipients. JAMA 1994;272:600–606 [PubMed] [Google Scholar]
- 7.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 2010;3:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Babaei-Jadidi R, Howell SK, Beisswenger PJ, Thornalley PJ. Degradation products of proteins damaged by glycation, oxidation and nitration in clinical type 1 diabetes. Diabetologia 2005;48:1590–1603 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed N, Babaei-Jadidi R, Howell SK, Thornalley PJ, Beisswenger PJ. Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes. Diabetes Care 2005;28:2465–2471 [DOI] [PubMed] [Google Scholar]
- 11.Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest 1997;100:2995–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternberg M, Urios P, Grigorova-Borsos AM. Effects of glycation process on the macromolecular structure of the glomerular basement membranes and on the glomerular functions in aging and diabetes mellitus. C R Seances Soc Biol Fil 1995;189:967–985 [in French] [PubMed] [Google Scholar]
- 13.Liu J, Huang K, Cai GY, et al. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 2014;26:110–121 [DOI] [PubMed] [Google Scholar]
- 14.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem 2001;81:102–113 [DOI] [PubMed] [Google Scholar]
- 15.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol 2004;165:2033–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 2005;54:3274–3281 [DOI] [PubMed] [Google Scholar]
- 17.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 2013;36:3234–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weil EJ, Fufaa G, Jones LI, et al. Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes. Diabetes 2013;62:3224–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Squarer A, Lemley KV, Ambalavanan S, et al. Mechanisms of progressive glomerular injury in membranous nephropathy. J Am Soc Nephrol 1998;9:1389–1398 [DOI] [PubMed] [Google Scholar]
- 21.Coresh J, Turin TC, Matsushita K, et al.; CKD Prognosis Consortium . Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014;311:2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fufaa GD, Weil EJ, Lemley KV, et al. Structural predictors of loss of renal function in American Indians with type 2 diabetes. Clin J Am Soc Nephrol 2016;11:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–2123 [DOI] [PubMed] [Google Scholar]
- 24.Demler OV, Pencina MJ, D’Agostino RB Sr. Misuse of DeLong test to compare AUCs for nested models. Stat Med 2012;31:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepe MS, Kerr KF, Longton G, Wang ZY. Testing for improvement in prediction model performance. Stat Med 2013;32:1467–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 28.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Observations and Sources of Collinearity. Hoboken, NJ, John Wiley and Sons, 1980 [Google Scholar]
- 29.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M; International Diabetic Nephopathy Study Group . The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 2005;54:2164–2171 [DOI] [PubMed] [Google Scholar]
- 30.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 2013;24:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond K, Mauer M; International Diabetic Nephropathy Study Group . The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002;51:1580–1587 [DOI] [PubMed] [Google Scholar]
- 32.Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991;325:836–842 [DOI] [PubMed] [Google Scholar]
- 33.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol 2007;27:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauer M, Caramori ML, Fioretto P, Najafian B. Glomerular structural-functional relationship models of diabetic nephropathy are robust in type 1 diabetic patients. Nephrol Dial Transplant 2015;30:918–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 2000;11:1667–1673 [DOI] [PubMed] [Google Scholar]
- 36.Dyer DG, Dunn JA, Thorpe SR, et al. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 1993;91:2463–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino T, Horii Y, Shiiki H, et al. Immunohistochemical detection of advanced glycosylation end products within the vascular lesions and glomeruli in diabetic nephropathy. Hum Pathol 1995;26:308–313 [DOI] [PubMed] [Google Scholar]
- 38.Yamada K, Miyahara Y, Hamaguchi K, et al. Immunohistochemical study of human advanced glycosylation end-products (AGE) in chronic renal failure. Clin Nephrol 1994;42:354–361 [PubMed] [Google Scholar]
- 39.Stinghen AE, Massy ZA, Vlassara H, Striker GE, Boullier A. Uremic Toxicity of Advanced Glycation End Products in CKD. J Am Soc Nephrol 2016;27:354–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie RD, Beyan H, Sawtell P, Boehm BO, Spector TD, Snieder H. Level of an advanced glycated end product is genetically determined: a study of normal twins. Diabetes 2003;52:2441–2444 [DOI] [PubMed] [Google Scholar]
- 41.Adams JN, Raffield LM, Martelle SE, et al. Genetic analysis of advanced glycation end products in the DHS MIND study. Gene 2016;584:173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care 2011;34:968–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyata T, van Ypersele de Strihou C, Ueda Y, et al. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol 2002;13:2478–2487 [DOI] [PubMed] [Google Scholar]
- 44.Saisho Y, Komiya N, Hirose H. Effect of valsartan, an angiotensin II receptor blocker, on markers of oxidation and glycation in Japanese type 2 diabetic subjects: blood pressure-independent effect of valsartan. Diabetes Res Clin Pract 2006;74:201–203 [DOI] [PubMed] [Google Scholar]
- 45.Monacelli F, Poggi A, Storace D, et al. Effects of valsartan therapy on protein glycoxidation. Metabolism 2006;55:1619–1624 [DOI] [PubMed] [Google Scholar]
- 46.Busch M, Franke S, Wolf G, Rohde RD, Stein G; Collaborative Study Group . Serum levels of the advanced glycation end products Nepsilon-carboxymethyllysine and pentosidine are not influenced by treatment with the angiotensin receptor II type 1 blocker irbesartan in patients with type 2 diabetic nephropathy and hypertension. Nephron Clin Pract 2008;108:c291–c297 [DOI] [PubMed] [Google Scholar]
- 47.Persson F, Rossing P, Hovind P, et al. Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes 2006;55:3550–3555 [DOI] [PubMed] [Google Scholar]
- 48.Sebeková K, Schinzel R, Münch G, Krivosíková Z, Dzúrik R, Heidland A. Advanced glycation end-product levels in subtotally nephrectomized rats: beneficial effects of angiotensin II receptor 1 antagonist losartan. Miner Electrolyte Metab 1999;25:380–383 [DOI] [PubMed] [Google Scholar]
- 49.Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999;48:198–202 [DOI] [PubMed] [Google Scholar]
- 50.Thornalley PJ. Measurement of protein glycation, glycated peptides, and glycation free adducts. Perit Dial Int 2005;25:522–533 [PubMed] [Google Scholar]