Abstract
Low-dose antithymocyte globulin (ATG) plus pegylated granulocyte colony-stimulating factor (G-CSF) preserves β-cell function for at least 12 months in type 1 diabetes. Herein, we describe metabolic and immunological parameters 24 months following treatment. Patients with established type 1 diabetes (duration 4–24 months) were randomized to ATG and pegylated G-CSF (ATG+G-CSF) (N = 17) or placebo (N = 8). Primary outcomes included C-peptide area under the curve (AUC) following a mixed-meal tolerance test (MMTT) and flow cytometry. “Responders” (12-month C-peptide ≥ baseline), “super responders” (24-month C-peptide ≥ baseline), and “nonresponders” (12-month C-peptide < baseline) were evaluated for biomarkers of outcome. At 24 months, MMTT-stimulated AUC C-peptide was not significantly different in ATG+G-CSF (0.49 nmol/L/min) versus placebo (0.29 nmol/L/min). Subjects treated with ATG+G-CSF demonstrated reduced CD4+ T cells and CD4+/CD8+ T-cell ratio and increased CD16+CD56hi natural killer cells (NK), CD4+ effector memory T cells (Tem), CD4+PD-1+ central memory T cells (Tcm), Tcm PD-1 expression, and neutrophils. FOXP3+Helios+ regulatory T cells (Treg) were elevated in ATG+G-CSF subjects at 6, 12, and 18 but not 24 months. Immunophenotyping identified differential HLA-DR expression on monocytes and NK and altered CXCR3 and PD-1 expression on T-cell subsets. As such, a group of metabolic and immunological responders was identified. A phase II study of ATG+G-CSF in patients with new-onset type 1 diabetes is ongoing and may support ATG+G-CSF as a prevention strategy in high-risk subjects.
Introduction
Type 1 diabetes results from a period of chronic autoimmunity contributing to pancreatic β-cell death and dysfunction (1), and despite decades of investigation, a cure for the disease remains elusive. The most common clinical research strategies for intervention involve the administration of an immunomodulatory or immunosuppressive agent to potentially slow or halt the autoimmune disease process and preserve remaining β-cell mass (2,3). Though some monotherapies have shown promise, the beneficial effects have generally been limited and short lived.
In recent years, combination therapy has been heralded as a means to conceivably elicit therapeutic synergism and target multiple aspects of the complex pathology (2,4). Indeed, perhaps the most aggressive protocol tested to date utilized autologous nonmyeloablative stem cell transplant involving the administration of cyclophosphamide along with the stem cell mobilizing agent, granulocyte colony-stimulating factor (G-CSF), and high doses of antithymocyte globulin (ATG) for leukocyte depletion. Despite the severe side effects associated with treatment, nearly all patients experienced a period of type 1 diabetes remission and were able to temporarily stop exogenous insulin administration, some for 4 years or longer, representing a clear breakthrough in the field (5–8).
Considering the importance of minimizing therapeutic risks relative to clinical benefit in patients with type 1 diabetes, we have attempted to deconstruct this cocktail and determine whether a safer approach could be defined with comparable efficacy. The Study of Thymoglobulin to Arrest Type 1 Diabetes (START) trial, with a higher dose of ATG alone (6.5 mg/kg), did not achieve the primary end point; however, in post hoc analysis, older subjects maintained stable β-cell function out to 2 years (9,10). Likewise, G-CSF alone (6 mg every 2 weeks × 12 weeks) did not have an effect (11). However, our previous work in NOD mice suggested that lower-dose ATG coupled with G-CSF mediates diabetes reversal via immunomodulatory effects including increased regulatory T cell (Treg) frequency and hematopoietic mobilization. We recently demonstrated that the combination of low-dose ATG (2.5 mg/kg) and pegylated G-CSF (ATG+G-CSF) preserves C-peptide for at least 12 months in subjects with established type 1 diabetes (4–24 months duration) (12). Herein, we report 2-year clinical outcomes and mechanistic data from our study of low-dose ATG and G-CSF in patients with established type 1 diabetes.
Research Design and Methods
Study Patients
For this single-blinded, randomized, placebo-controlled study, screening and enrollment was performed at three study sites (University of California, San Francisco [UCSF], University of Colorado [UC], and University of Florida [UF]) as previously described (12). Briefly, patients aged 12–45 years with type 1 diabetes of duration >4 months to <2 years and minimum peak C-peptide of 0.1 nmol/mL following a 4-h mixed-meal tolerance test (MMTT) were eligible for the study. Patients with an allergy to ATG or G-CSF, prior treatment with ATG, immunodeficiency, or chronic infection (including hepatitis B or C, or HIV) were not eligible.
Study Design
Within 8 weeks of the screening visit, eligible subjects were randomized in a 2:1 ratio to receive ATG (Thymoglobulin; Sanofi) (0.5 mg/kg on day 1 and 2 mg/kg on day 2 via intravenous infusion for total dose of 2.5 mg/kg) and pegylated G-CSF (Neulasta; Amgen) (6 mg via subcutaneous injection administered every 2 weeks for 6 doses) or placebo. Subjects were hospitalized for the first 2 days during the ATG or placebo infusions and the first G-CSF or placebo injection. Prior to and 12 h after ATG infusion, patients were administered methylprednisolone (0.25 mg/kg), and placebo infusions and injections were given on the same schedule to patients randomized to the placebo group. All subjects in both groups received acetaminophen and diphenhydramine prior to ATG or placebo infusion. ATG+G-CSF–treated subjects were given trimethoprim-sulfamethoxazole and acyclovir for the first 3 months of treatment as antimicrobial prophylaxis while placebo-treated subjects received placebo prophylaxis. Throughout the study, all subjects continued intensive diabetes management with their personal diabetes physicians.
Study Assessments and Laboratory Procedures
Physical examination was performed and metabolic and immunological samples obtained at baseline; weeks 1, 2, 4, 6, 8 and 10; and months 3, 6, 9, 12, 18, and 24 from the time of enrollment. The following were performed at each visit. Glycated hemoglobin (HbA1c) was measured via DCA2000 or DCA Vantage Analyzer (Siemens Healthcare Diagnostics, Malvern, PA). MMTT (Boost, 6 mL/kg, maximum 360 mL) was administered, serum C-peptide was measured at the Northwest Lipid Research Laboratories, and 2-h C-peptide area under the curve (AUC) was calculated. Autoantibodies against glutamic acid decarboxylase, insulinoma-associated protein 2, insulin, and zinc transporter 8 were measured via radioimmunoassay at the Barbara Davis Center (13–15). Flow cytometric analysis of peripheral blood immune cell subsets was performed as described below. Adverse events (AEs) were recorded in accordance with Common Terminology Criteria for Adverse Events classifications (version 4.0; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
Flow Cytometry
Fresh whole blood was shipped overnight from UC and UCSF to the UF site, and samples drawn at the UF clinic were rested while rocking for 24 h (rested to match shipped samples). An aliquot of whole blood (24-h rested) was stained using two antibody panels, and the remaining samples were stored as cryopreserved peripheral blood mononuclear cells (PBMCs). The first panel for whole blood included the surface markers CD3, CD4, CD8, and CD19. The second panel included surface markers CD4, CD25, CD45RA, and CD45RO, followed by intracellular staining for the canonical Treg transcription factors FOXP3 and Helios. Whole blood (100 μL) was labeled with surface antibodies at ambient temperature for 20 min. Red blood cells were then lysed with BD FACS lysing solution (BD Biosciences, San Jose, CA) and subsequently washed with 0.9% BSA 1× Dulbecco’s PBS buffer. Following red blood lysis, the first panel was quantified with a BD Accuri C6 flow cytometer (BD Biosciences) while the second panel was fixed, permeabilized, and labeled with intracellular antibodies using a FOXP3 Fix/Perm buffer set (Biolegend, San Diego, CA), and data were quantified using a BD LSRFortessa flow cytometer (BD Biosciences) with data analyzed with FCS Express software (De Novo, Glendale, CA).
Cryopreserved PBMCs were batch processed and thawed, washed into complete RPMI, and incubated with 100 units DNase (Roche, Indianapolis, IN) at 37°C for 15–30 min. Cells were then labeled with LIVE/DEAD Yellow Viability Dye (Invitrogen, Carlsbad, CA), followed by surface labeling with five antibody panels designed to provide an assessment of both innate and adaptive immune cell subsets. The panels were emulated from Maecker et al. (16) and consist of a B-cell subset panel (CD3, CD19, CD20, CD24, CD27, CD38, and IgD), an innate cell panel (CD3, CD11c, CD14, CD16, CD19, CD20, CD56, CD123, and HLA-DR), a naive and memory T-cell panel (CD3, CD4, CD8, CD38, CD45RA, CD197, and HLA-DR), an effector T-cell subset panel (CD3, CD4, CD38, CD45RO, CD183 [CXCR3], CD196 [CCR6], and HLA-DR), and a Treg panel (CD3, CD4, CD25, CD45RO, CD127, CD194 [CCR4], and HLA-DR). In addition, a T follicular helper cell (Tfh) panel (CD3, CD4, CD45RA, CD183, CD196, CD197 [CCR7], and CD279 [PD-1]) was designed to assess precursor Tfh (17) and memory Tfh (18). Specific antibody clones and suppliers are listed in Supplementary Table 1, and immune cell subset definitions are listed in Supplementary Table 2. Stained cells were analyzed using a BD LSRFortessa flow cytometer and FlowJo data analysis software (Ashland, OR).
End Points
The primary outcome was MMTT 2-h C-peptide AUC change from baseline. Secondary outcomes included change in HbA1c, insulin dose, and AEs, as well as immunological parameters including CD16+CD56hi natural killer cell (NK) frequency, CD4+ effector memory T-cell (Tem) frequency, CD4+PD-1+ central memory T-cell (Tcm) frequency, Tcm PD-1 mean fluorescence intensity (MFI), neutrophil count, CD4+ T-cell frequency, CD4+/CD8+ T-cell ratio, and FOXP3+Helios+ Treg frequency. Markers used to identify immune cell subsets are defined in Supplementary Table 2. Within the ATG+G-CSF treatment group, secondary outcome measures were compared between 12-month responders (previously defined as patients with 12-month MMTT 2-h C-peptide AUC ≥ baseline values [12]) and nonresponders, as well as between 24-month super responders (patients with 24-month MMTT 2-h C-peptide AUC ≥ baseline values) and non–super responders.
Data Collection and Analysis
Data were collected at each study site and consolidated using the Clinical and Translational Research Informatics Program hosted at UF. Statistical analysis was performed using the open source software package R (version 3.1.0) by fitting an ANCOVA model with the outcome being the change of 2-h C-peptide AUC from baseline at 12, 18, and 24 months, and the baseline 2-h AUC is included as an independent variable. Specifically, the analysis used the 2-h AUC, i.e., Yijk for subject j at treatment arm i at measurement time point k (e.g., 12-, 18-, and 24-month) changes from baseline measurement, i.e., Yij0 as the outcomes and fit the following model: E(Yijk) = α0 + α1 Yij0 + α2 I(trtj = 1) where I is an indicator function and trt = 1 represents the drug treatment arm for the subject. Secondary outcomes including metabolic and flow cytometry data were compared using Satterwthaite-corrected t test, unpaired t test, two-way ANOVA with Šidák post hoc correction, or the nonparametric Mann-Whitney test. Spearman correlation was used to investigate the potential relationship between Δ C-peptide AUC and flow cytometry data. Graphs were generated using GraphPad Prism software version 6 (La Jolla, CA). Heat maps were generated in the R programming syntax (version 3.2.4) modeling the baseline and longitudinal changes of the examined parameters (cell frequency percentages and MFI fold change) of the study groups (i.e., 12-month responders, 24-month super responders, and nonresponders). Significance was defined as P < 0.05.
Study Approval
This study was granted an investigational new drug permit (IND 107185) and was conducted according to the guidelines for good clinical practice and the Declaration of Helsinki with the approval of institutional review boards at UF, UCSF, and UC. Written informed consent and in the case of minors, assent, was obtained from each participant prior to study inclusion.
Results
Patients
Patient screening, enrollment, and baseline characteristics were previously reported (12). Briefly, of 37 subjects screened, 25 were eligible and enrolled using 2:1 randomization so that N = 17 subjects were randomly assigned to receive ATG+G-CSF and N = 8 received placebo. Demographic as well as baseline metabolic and immunological measures were comparable between the two groups (12). One subject receiving ATG+G-CSF dropped out of the study following the 6-month visit. For all findings reported below, sex and ethnicity were not found to affect study outcomes.
β-Cell Function
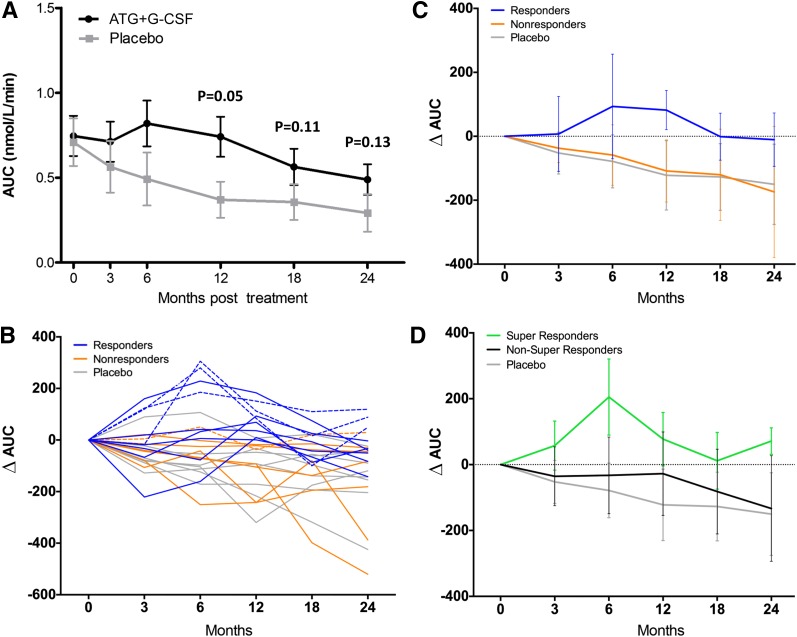
MMTT-stimulated 2-h C-peptide peak and AUC were comparable at baseline for ATG+G-CSF and placebo treatment groups. We previously demonstrated significantly greater C-peptide response in ATG+G-CSF–treated versus placebo-treated subjects for both 2-h AUC at 12 months following therapy and the change in 2-h AUC from baseline to 12 months (12); although differences still persisted at 18 and 24 months, they were no longer statistically significant likely because of the small numbers in each group (Fig. 1A and B and Table 1). Interestingly, when subjects were further segregated by diabetes duration (<1 year vs. ≥1 year) at study enrollment, there was a trend toward higher peak C-peptide in ATG+G-CSF–treated versus placebo-treated subjects with disease duration >1 year (P = 0.09) (Table 2). We found no significant differences in change in insulin dose or HbA1c levels (baseline to 24 months or 12 to 24 months) in ATG+G-CSF–treated compared with placebo-treated subjects (Table 1).
Figure 1.
ATG+G-CSF combination therapy preserves β-cell function for at least 12 months in a subset of responders and for at least 24 months in a small group of super responders. A: C-peptide AUC for ATG+G-CSF–treated (black circles; N = 16) vs. placebo-treated (gray squares; N = 8) subjects over time (mean ± SD). C-peptide AUC was significantly higher in ATG+G-CSF subjects at 12 months (P = 0.05) (12) but not at 18 or 24 months (P = 0.11 and P = 0.13, respectively, for ∆ AUC). B: Within the ATG+G-CSF treatment group, subjects were classified according to 12-month MMTT 2-h C-peptide AUC as responders (blue; N = 9; AUC ≥ baseline at 12 months) and nonresponders (orange; N = 7; AUC < baseline at 12 months). Individual MMTT 2-h C-peptide AUC values are shown for responders, nonresponders, and placebo subjects (gray). Subjects with AUC ≥ baseline at 24 months were classified as super responders (dashed lines; N = 4; 3/4 were 12-month responders and 1/4 was a 12-month nonresponder). C: Responders (blue) demonstrated a trend toward greater ∆ C-peptide AUC at 24 months after time of enrollment while nonresponders (orange) resembled placebo control subjects (gray; P = 0.09; mean ± SD). D: Super responders (green; N = 4) demonstrated a trend toward greater ∆ C-peptide AUC compared with non–super responders (gray; N = 12; P = 0.29; mean ± SD) who were comparable to placebo-treated subjects (black) at 24 months.
Table 1.
Changes (Δ) in metabolic outcomes reported as mean (SD) between the baseline and 24 months (Δ0–24), as well as 12- and 24-month (Δ12–24) evaluations.
| ATG+G-CSF (N = 16) | Placebo (N = 8) | P value* | ATG+G-CSF |
P value** | ||
|---|---|---|---|---|---|---|
| Responders (N = 9) | Nonresponders (N = 7) | |||||
| Δ Insulin requirement (units/kg/day) | ||||||
| (Δ24–0) | 0.110 (0.499) | 0.207 (0.212) | 0.68 | 0.127 (0.158) | 0.0867 (0.783) | 0.89 |
| (Δ24–12) | 0.084 (0.276) | 0.102 (0.115) | 0.19 | 0.182 (0.239) | −0.074 (0.278) | 0.11 |
| Δ HbA1c (%) | ||||||
| (Δ24–0) | 1.471 (3.105) | 1.243 (1.385) | 0.86 | −0.025 (0.883) | 3.467 (3.953) | <0.05 |
| (Δ24–12) | 0.750 (2.329) | 0.371 (0.720) | 0.38 | 0.275 (0.857) | 1.383 (3.498) | 0.40 |
| Δ C-peptide AUC (nmol/mL/120 min) | ||||||
| (Δ24–0) | −82.03 (166.0) | −150.3 (125.5) | 0.13 | −10.46 (83.45) | −174.1 (205.0) | <0.05 |
| (Δ24–12) | −80.75 (121.3) | −28.12 (117.6) | 0.67 | −92.53 (65.80) | −65.61 (174.7) | 0.68 |
| Δ C-peptide peak (nmol/mL) | ||||||
| (Δ24–0) | −0.996 (1.740) | −1.599 (1.358) | 0.40 | −0.281 (0.977) | −1.916 (2.131) | 0.06 |
| (Δ24–12) | −1.016 (1.273) | −0.333 (0.891) | 0.19 | −1.048 (0.936) | −0.976 (1.696) | 0.92 |
P values are calculated via Satterthwaite corrected t test for
*Placebo vs. ATG+G-CSF total and
**ATG+G-CSF responders vs. ATG+G-CSF nonresponders.
Table 2.
Metabolic outcomes reported as mean (SD) at the baseline (month 0) and 12- and 24-month evaluations for ATG+G-CSF–treated vs. placebo-treated subjects with type 1 diabetes duration ≥1 year and <1 year at the time of study enrollment
| Time point (months) | ATG+G-CSF |
Placebo |
P value | ATG+G-CSF |
Placebo |
P value | ATG+G-CSF |
Placebo |
P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 16) | Total (N = 8) | Duration ≥1 year (N = 10) | Duration ≥1 year (N = 3) | Duration <1 year (N = 6) | Duration <1 year (N = 5) | |||||
| Insulin requirement (units/kg/day) | 0 | 0.44 (0.49) | 0.45 (0.30) | 0.99 | 0.40 (0.24) | 0.75 (0.23) | 0.09 | 0.51 (0.78) | 0.27 (0.17) | 0.84 |
| 12 | 0.48 (0.40) | 0.54 (0.29) | 0.98 | 0.46 (0.29) | 0.82 (0.03) | 0.09 | 0.50 (0.60) | 0.38 (0.24) | 0.97 | |
| 24 | 0.56 (0.40) | 0.53 (0.39) | 0.99 | 0.44 (0.21) | 0.94 (NA) | NA | 0.72 (0.55) | 0.43 (0.37) | 0.78 | |
| HbA1c (%) | 0 | 6.7 (1.1) | 6.0 (1.0) | 0.78 | 6.8 (0.9) | 6.1 (1.3) | 0.86 | 6.6 (1.4) | 6.0 (0.9) | 0.96 |
| 12 | 7.2 (2.1) | 7.0 (1.0) | 0.99 | 6.8 (1.1) | 6.5 (0.7) | 0.99 | 8.0 (3.1) | 7.2 (1.2) | 0.94 | |
| 24 | 8.1 (3.2) | 7.1 (1.1) | 0.61 | 7.7 (2.6) | 6.7 (0.8) | 0.70 | 8.6 (4.1) | 7.4 (1.4) | 0.84 | |
| C-peptide AUC (nmol/mL/120 min) | 0 | 2.24 (1.42) | 2.13 (1.19) | 0.99 | 2.39 (1.45) | 1.07 (0.35) | 0.33 | 1.99 (1.47) | 2.76 (1.04) | 0.61 |
| 12 | 2.23 (1.42) | 1.11 (0.90) | 0.11 | 2.30 (1.54) | 0.56 (0.46) | 0.13 | 2.10 (1.31) | 1.73 (0.94) | 0.73 | |
| 24 | 1.56 (1.07) | 0.88 (0.94) | 0.50 | 1.73 (1.19) | 0.28 (0.18) | 0.25 | 1.27 (0.86) | 1.24 (1.05) | 0.99 | |
| C-peptide peak (nmol/mL) | 0 | 2.96 (1.81) | 2.84 (1.44) | 0.99 | 3.14 (1.83) | 1.55 (0.25) | 0.36 | 2.66 (1.90) | 3.61 (1.28) | 0.66 |
| 12 | 2.98 (1.82) | 1.57 (1.12) | 0.12 | 3.11 (1.96) | 0.75 (0.51) | 0.09 | 2.76 (1.71) | 2.06 (1.11) | 0.83 | |
| 24 | 1.96 (1.32) | 1.24 (1.29) | 0.63 | 2.12 (1.42) | 0.44 (0.32) | 0.31 | 1.69 (1.20) | 1.72 (1.44) | 0.99 |
Multiplicity adjusted P values were calculated via two-way ANOVA with Šidák post hoc correction. Insulin requirement data at the 24-month time point were not available (NA) for two subjects in the placebo group with ≤1 year duration.
Responders Versus Nonresponders
Within the ATG+G-CSF treatment group, we had previously defined responders as those with MMTT-stimulated 2-h C-peptide AUC at the 12 month time point equal to or above baseline values. Nine (56.3%) were responders, and seven (43.8%) were not (compared with 1 of 8 [12.5%] in the placebo-treated group) (12). Using this definition, we found that compared with nonresponders, responders still exhibited a trend toward greater capacity for C-peptide secretion at 24 months, although the difference was no longer statistically significant (mean difference 0.28 nmol/L/min, P = 0.088) (Fig. 1B and C and Table 1). Four ATG+G-CSF–treated subjects maintained MMTT-stimulated C-peptide AUC equal to or above mean baseline values even at the 24-month time point (super responders) while all remaining subjects (both ATG+G-CSF–treated and control subjects) had fallen below baseline C-peptide AUC levels (Fig. 1B and D). One of these four was considered a nonresponder at the 12-month time point but improved over months 12–24 (Fig. 1B, dashed lines).
Clinical Characteristics of Super Responders
For 24-month super responders, the change in HbA1c from the 12 to 24 month time points was −0.2% ± 0.6 (mean ± SD) vs. 1.0% ± 2.6 in non–super responders, although the difference was not statistically significant (P = 0.18, two-tailed Mann-Whitney test). The change in daily insulin requirements from 12 to 24 months was comparable between super responders (0.09 ± 0.12 units/kg/day) and non–super responders (0.08 ± 0.31 units/kg/day, P = 0.94). The majority of subjects (7/9 [78%] 12-month responders, 6/7 [86%] 12-month nonresponders) administered insulin via injection rather than pump, and few used a continuous glucose monitor (CGM) (1/9 [11%] responders, 0/7 nonresponders), as documented at the 12-month visit. Of the four 24-month super responders, three used a pump to administer insulin and one used injections; one super responder also used a CGM. None of these four subjects altered the mode of insulin delivery (pump) or the use of a CGM between the 12- and 24-month visits. Long-term preservation of β-cell function in super responders does not appear to be attributable solely to more rigorous blood glucose management; however, because of the small sample size (N = 4 super responders), we cannot definitively determine the relationship between C-peptide secretory capacity and glycemic control.
Complete Blood Count and Flow Cytometry
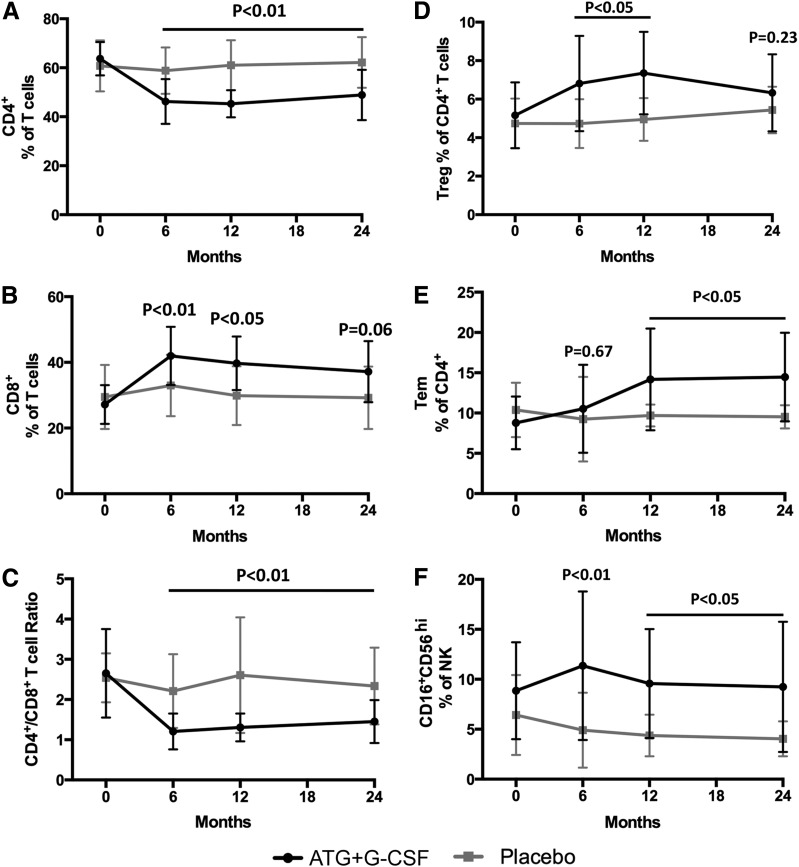
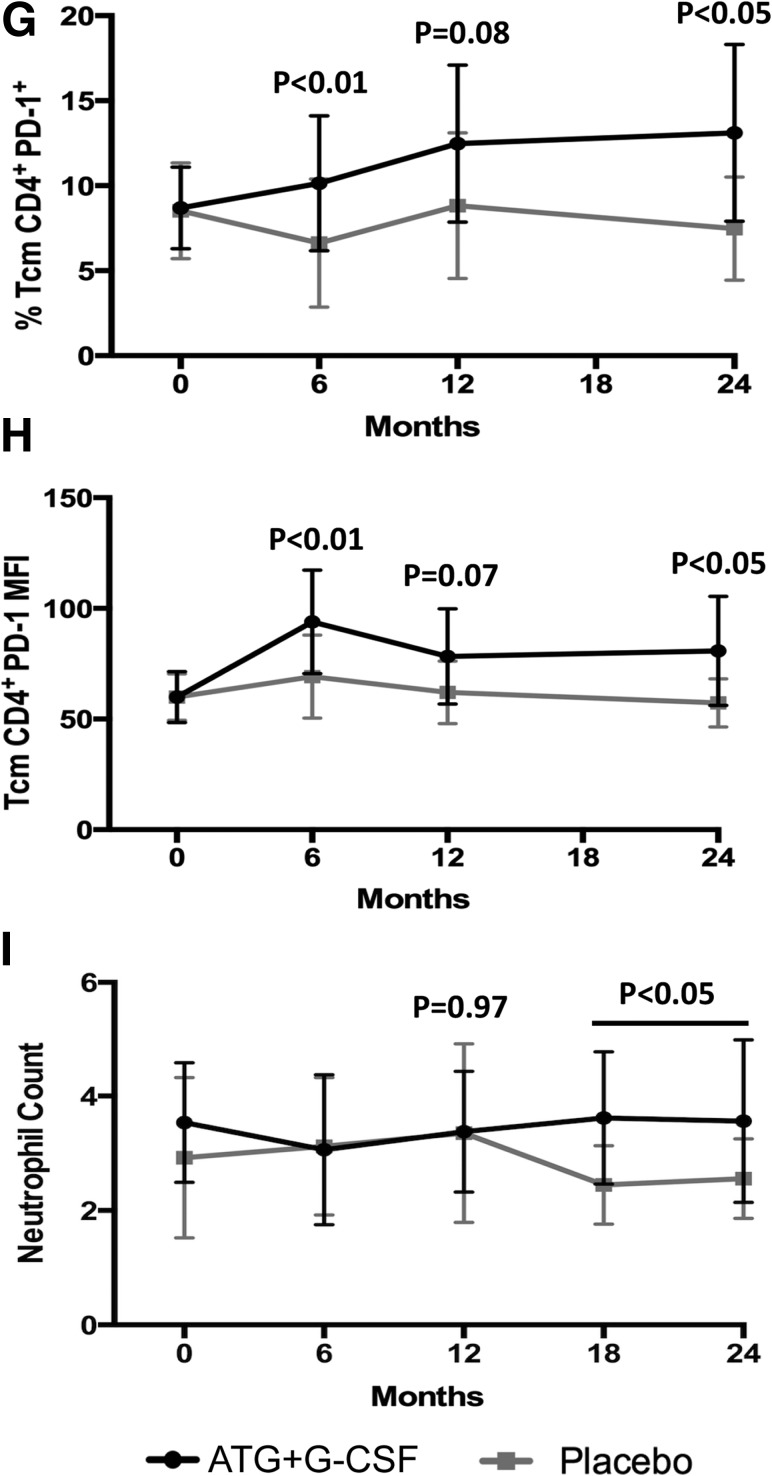
When compared with control subjects, subjects treated with ATG+G-CSF had significantly reduced CD4+ T-cell frequencies (P < 0.01) (Supplementary Fig. 1 and Fig. 2A) and a trend toward increased CD8+ T-cell frequencies (P = 0.06) (Supplementary Fig. 1 and Fig. 2B), leading to significantly reduced CD4+/CD8+ T-cell ratios (P < 0.01) (Fig. 2C). These observations remained consistent over time, even at 24 months following therapy. FOXP3+Helios+ Treg frequency was elevated in ATG+G-CSF subjects at 6, 12, and 18 months (P < 0.05), but the differences were no longer statistically significant 24 months from the time of enrollment (P = 0.23) (Supplementary Fig. 2 and Fig. 2D). Additionally, CD4+ Tem (Fig. 2E), CD16+CD56hi NK (Fig. 2F), and CD4+PD-1+ Tcm frequencies (Fig. 2G), as well as Tcm PD-1 expression levels (Fig. 2H) and neutrophil counts (Fig. 2I), were significantly elevated in ATG+G-CSF–treated subjects compared with those that received placebo, even 24 months from the time of enrollment. Together these data suggest that many of the immunomodulatory effects of combination treatment with ATG+G-CSF are persistent well beyond the therapeutic window, suggesting long-term immunoregulation contributes toward the preservation of residual β-cell function.
Figure 2.
Immunomodulatory effects of ATG+G-CSF combination therapy remain detectable at least 24 months following treatment. PBMCs were characterized by flow cytometric analysis, and complete blood count was performed. Compared with placebo (gray squares; N = 8), subjects treated with ATG+G-CSF (black circles; N = 16) exhibited reduced CD4+ T-cell frequency (A), increased CD8+ T-cell frequency (B), and reduced CD4+/CD8+ T-cell ratio (C). D: CD4+CD25+FOXP3+Helios+ Tregs were significantly elevated in ATG+G-CSF–treated subjects at 6 and 12 months, but the difference was not significant at 24 months. CD4+ Tem frequency (E), CD16+CD56hi NK percent (F), CD4+PD-1+ Tcm frequency (G), PD-1 MFI on CD4+ Tcm (H), and neutrophil counts (I) were significantly greater in ATG+G-CSF–treated subjects compared with control subjects. All P values shown are for ATG+G-CSF vs. placebo at the indicated time points.
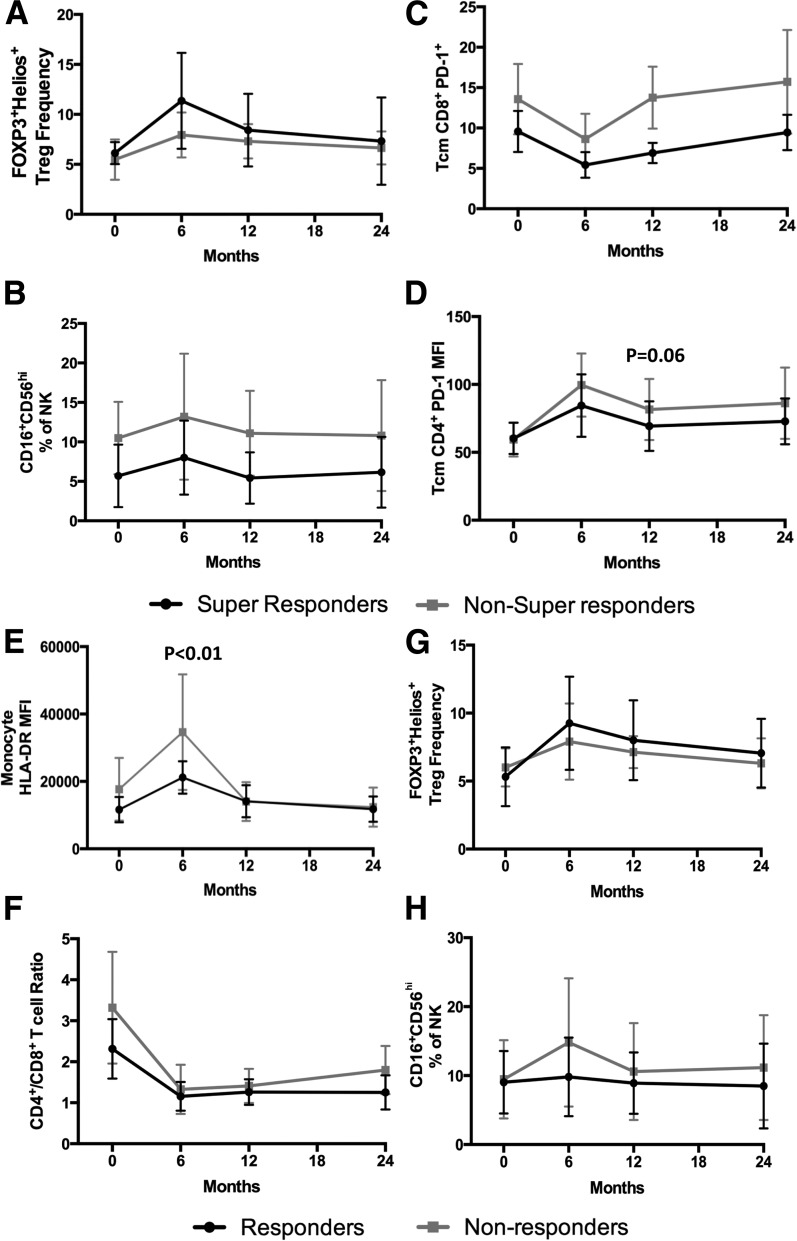
We further assessed the flow cytometric data for indications of immunological responders versus nonresponders. Our data indicated super responders had a transient increase in FOXP3+Helios+ Treg frequency at the 6-month time point (Fig. 3A); however, our secondary mechanistic outcomes were not powered for immune subset analysis. Nevertheless, several innate and adaptive subsets showed intriguing trends in 12-month responders and 24-month super responders (Supplementary Figs. 1 and 2 and Fig. 3). Interestingly, the immune profile appeared to differ even prior to study enrollment in the four super responders versus non–super responders (Supplementary Fig. 1D and Fig. 3A–D). Elevated monocyte HLA-DR MFI at the 6-month time point may identify ATG+G-CSF 12-month nonresponders (mean fold change = 1.6 relative to responders, P < 0.01, two-way ANOVA with Šidák post hoc analysis) (Supplementary Fig. 1A and Fig. 3E). Low HLA-DR MFI among monocytes, high CD279 (PD-1) MFI among CD8+ effector memory RA (TEMRA) T cells, low naive CD4+ T-cell frequency, and low naive CD8+ T-cell frequency may help identify ATG+G-CSF responders prior to treatment (Supplementary Fig. 1C); however, these expression patterns and cellular subsets will need to be confirmed in larger clinical cohorts.
Figure 3.
Peripheral blood immune cell populations were characterized by flow cytometric analysis in ATG+G-CSF 24-month super responders (black circles; N = 4) vs. non–super responders (gray squares; N = 12). There were no significant differences in Treg (A), CD16+CD56hi NK (B), or CD8+PD-1+ Tcm (C) frequency change from baseline, but there was a trend toward reduced CD4+ Tcm PD-1 MFI change (D) from baseline in super responders at the 12-month time point. E: HLA-DR MFI on monocytes was significantly elevated in ATG+G-CSF 12-month nonresponders (gray squares; N = 7) compared with responders (black circles; N = 9) at the 6-month time point. In 12-month responders vs. nonresponders, there were no significant differences in CD4+/CD8+ T-cell ratio (F), FOXP3+Helios+ Treg frequency (G), or CD16+CD56hi NK percent (H) at all time points evaluated.
Association Between Immunomodulation and Preservation of β-Cell Function
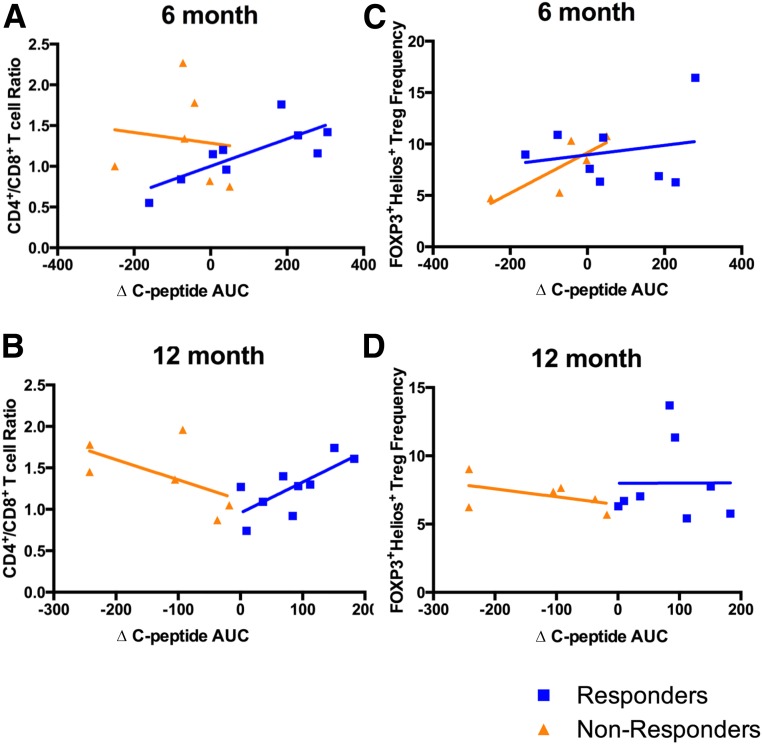
We next asked whether persistent immunomodulatory effects of ATG+G-CSF combination therapy correlated to greater preservation of β-cell function. Indeed, we found that among the 12-month responders, there was a significant correlation between Δ C-peptide AUC and CD4+/CD8+ T-cell ratios at the 6-month (Fig. 4A) (P < 0.05, R2 = 0.60) and 12-month (Fig. 4B) (P < 0.05, R2 = 0.53) time points. Interestingly, nonresponders appeared to exhibit trends toward inverse correlation between Δ C-peptide AUC and CD4+/CD8+ T-cell ratio, although these were not statistically significant (Fig. 4A and B), which suggests that CD4+/CD8+ T-cell ratios alone are likely not sufficient to discriminate responders from nonresponders. Trends were observed suggesting a positive association between FOXP3+Helios+ Treg frequency and Δ C-peptide AUC at 6 months after the initiation of treatment (Fig. 4C), although the correlation was most apparent among nonresponders and not statistically significant (responders: P = 0.84, R2 = 0.04; nonresponders: P = 0.08, R2 = 0.64); this trend did not persist to the 12-month time point (Fig. 4D). Indeed, this may suggest that the long-term (i.e., 6- to 12-month) immunomodulatory effects of ATG+G-CSF therapy are associated with greater β-cell secretory capacity among responders, but given the small sample sizes, confirmation with a larger cohort is necessary.
Figure 4.
Immmunomodulatory effects of ATG+G-CSF combination therapy were associated with MMTT-stimulated C-peptide AUC change from baseline in 12-month responders (blue; 12-month C-peptide AUC > baseline) and nonresponders (orange; 12-month C-peptide AUC < baseline). For responders, ∆ C-peptide AUC significantly correlated with CD4+/CD8+ T-cell ratios at 6 months (P < 0.05, R2 = 0.60) (A) and 12 months (P < 0.05, R2 = 0.53) (B) following treatment, and nonresponders demonstrated trends toward inverse correlation (A: P = 0.24, R2 = 0.01; B: P = 0.30, R2 = 0.33). Nonresponders demonstrated a trend toward positive correlation between FOXP3+Helios+ Treg frequency and Δ C-peptide AUC at 6 months (nonresponders: P = 0.08, R2 = 0.64; responders: P = 0.84, R2 = 0.04) (C), but no associations were observed between FOXP3+Helios+ Treg frequency and Δ C-peptide AUC at 12 months (nonresponders: P = 0.50, R2 = 0.23; responders: P = 0.79, R2 < 0.01) (D).
Autoantibody Titers
We previously reported that treatment with ATG+G-CSF did not significantly change autoantibody titers over the course of the first year (12). We evaluated the change in glutamic acid decarboxylase, insulinoma-associated protein 2, insulin, and zinc transporter 8 antibody titers from 12 to 24 months and again found no significant differences within or between ATG+G-CSF or placebo treatment groups over time (Supplementary Table 3).
AEs
AEs during the first 12 months were reported previously (12). Importantly, in the second year following treatment, few minor AEs were observed, and these occurred with similar frequency and severity in both the ATG+G-CSF– and placebo-treated groups. It was determined that these AEs were unlikely to be attributable to therapy except for one patient in the ATG+G-CSF treatment arm who presented transiently with reduced CD4+ T-cell counts possibly related to treatment (Supplementary Table 4). Of note, we saw no greater risk for infection or trouble clearing infection in the ATG+G-CSF–treated group compared with those assigned to the placebo arm. While potential effects of antimicrobial treatment during the first 3 months of ATG+G-CSF therapy have not been directly examined, we do not consider persisting immunomodulation or preservation of C-peptide to be attributable to antimicrobial agents.
Conclusions
Efforts to reverse type 1 diabetes in human subjects have varied widely regarding immunological or metabolic targets (reviewed in refs. 2,19), and it is clear that a combination approach will be necessary to halt autoimmunity and maintain β-cell mass. This notion is perhaps best supported by the efforts of Voltarelli et al., Li et al., and Snarski et al., in which an aggressive approach involving autologous nonmyeloablative stem cell transplant along with cyclophosphamide, G-CSF, and high-dose ATG provided a period of insulin independence in most patients (5–8). More recently, similar results were reported in a small cohort of patients with type 1 diabetes who, in an outpatient setting, underwent autologous stem cell transplantation with G-CSF, cyclophosphamide, and the chemotherapeutic agent fludarabine in place of ATG (20). Ultimately, the associated risks and observed adverse effects preclude implementation of these treatment protocols as standard of care, especially in children and adolescents. Nevertheless, these results represent progress in the field and a platform for our recent studies. Efforts to utilize a single agent from this combination therapy, either ATG alone or G-CSF alone, have not met with success. However, in the START trial, the older ATG-treated subjects appeared to fare far better with slower rate of β-cell loss than adults in the placebo group or ATG-treated adolescents (9,10). No immunological study accounted for these apparent treatment differences.
We administered low-dose ATG plus pegylated G-CSF to patients with established type 1 diabetes, and although subjects did not experience clinical remission, we observed significant preservation of β-cell function for 12 months following treatment (12). At 24 months, β-cell function appears to remain greater in ATG+G-CSF–treated patients compared with those that received placebo, but in this small cohort, the difference was no longer statistically significant. Mechanistically, a number of immunological parameters were altered as result of ATG+G-CSF treatment—CD4+ T-cell frequency was reduced while CD8+ T-cell frequencies increased, resulting in reduced CD4+/CD8+ T-cell ratios. Additionally, FOXP3+Helios+ Treg, CD4+ Tem, CD16+CD56hi NK, and CD4+PD-1+ Tcm frequencies, as well as Tcm PD-1 expression levels and neutrophil counts, were significantly elevated in ATG+G-CSF–treated subjects compared with those that received placebo. Importantly, these therapeutic effects were sustained even 24 months from the time of treatment with the exception of increased Treg frequency in treated patients, which was only detectable through the first 18 months. These data suggest that ATG+G-CSF combination treatment imparts prolonged immunomodulatory effects, although with small sample sizes reported here, these results are exploratory and need to be confirmed.
We previously reported that within the ATG+G-CSF treatment group, nine responders maintained or even improved their capacity for insulin production over the first 12 months following treatment while MMTT-stimulated C-peptide AUC fell below baseline values in the remaining seven nonresponders (12). At 24 months, these responders still demonstrated a trend toward higher C-peptide AUC compared with nonresponders. With age as the only demographic factor differentiating these two groups (responders were older than nonresponders by a mean difference of 9.95 years, P < 0.05) (12), there is a need for further studies including larger cohorts to investigate potential biomarkers or genetic haplotypes that might help us to better predict patients as strong candidates for ATG+G-CSF combination therapy. Interestingly, both ATG+G-CSF combination treatment tested here as well as ATG monotherapy are more efficacious in older patients in situations of both new-onset and established disease (9,12). A subgroup of four super responders with C-peptide AUC greater than baseline at the 24-month time point were identified, but the small sample size precluded statistical analysis of immune markers or potential age-related effects. Collectively, these data suggest that immune profiles may identify candidates for such therapy, albeit validation in a larger cohort is clearly necessary. Further studies are needed to accurately determine if T cells are moving to a more anergic/senescent phenotype following ATG+G-CSF therapy, as has been observed with other T cell–targeting agents that have demonstrated clinical responses (21,22).
The notion of responders and nonresponders implies that some patients may be refractory to certain therapies, likely representing differences in genetics, physiology, and/or disease pathogenesis. Alternatively, patients may be effective immunological responders without a recovery of endogenous β-cell mass or function. This is an important phenomenon not only from the perspective of clinical practice but also in clinical trials where, on first analysis, nonresponders may mask statistically and biologically significant effects of a given therapy in the responder group. Moving forward, further investigation is needed to identify biomarkers differentiating these patient populations so that we can better predict those who will benefit from treatment. While not adequately powered for subset analysis in this initial trial, our immune profiling of responders demonstrated a trend toward low monocyte expression of HLA-DR (an antigen-presenting cell activation marker) at baseline, as well as high expression of the negative regulator PD-1 on CD8+ effector memory RA T cells as potential immune biomarkers of response. Importantly, significant positive correlation between CD4+/CD8+ T-cell ratios and Δ C-peptide AUC was observed among responders at 6 and 12 months after beginning treatment, suggesting that long-term immunoregulation is associated with greater preservation or recovery of β-cell secretory capacity. However, from these data we cannot definitively ascertain if this represents a causal relationship.
It remains to be determined whether earlier intervention with ATG+G-CSF in subjects with recent-onset type 1 diabetes (duration <4 months) may offer improved efficacy (23). This concept is currently being explored in the National Institutes of Health (NIH)-funded Type 1 Diabetes TrialNet utilizing a three-arm trial of 1) low-dose ATG alone, 2) low-dose ATG+G-CSF, and 3) placebo in patients with new-onset disease (ClinicalTrials.gov identifier NCT02215200). Repeat dosing of ATG+G-CSF or G-CSF, potentially in combination with autoantigen vaccination, is an additional concept that might promote continued immune regulation for prolonged elevated Treg frequency and extended preservation of endogenous β-cell function. In addition to ATG+G-CSF, combinatorial agents promoting β-cell regeneration or islet transplant may be necessary to provide recovery of functional β-cell mass in subjects with established disease. Indeed, we previously reported a four-drug combination treatment that was able to reverse not only new-onset diabetes but even established disease in the NOD model (24) using low-dose ATG+G-CSF plus dipeptidyl peptidase 4 inhibitor and proton pump inhibitor. These two additional agents have together afforded efficacy in preclinical type 1 diabetes studies (25), are already approved by the U.S. Food and Drug Administration for use in treating type 2 diabetes and gastroesophageal reflux disease, and are commonly prescribed in the clinic with low associated risks. Keeping in mind the importance of equipoise, we expect that a similar approach using the immunomodulatory combination of ATG+G-CSF along with these or other methods for β-cell replacement and/or regeneration may provide a means to improve metabolic control and slow autoimmune β-cell destruction in human subjects with type 1 diabetes.
Article Information
Acknowledgments. The authors would like to acknowledge Miriam Cintron, Kieran McGrail, Sean McGrail, Joshua Peterson, Theresa Sumrall, Robert T. Davis, Richard Williams, Robert Camacho, Peter Hong, and Zachary Webster (all from UF) for assistance, as well as the extended team of research coordinators and nurses. Most importantly, the authors thank the patients who participated in the study.
Funding. This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust (2014PG-T1D010 and 2015PG-T1D032). Additional research support was provided by NIH P01 Grant AI42288, JDRF Career Development Award (2-2012-280 to T.M.B.) and postdoctoral fellowship award (2-PDF-2016-207-A-N to D.J.P.), and from the McJunkin Family Charitable Foundation. Research reported in this publication was supported by the UF Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1-TR-001427, and by NIH National Center for Research Resources UCSF-CTSI Grant Number UL1-TR-000004. This work was also supported by the Mentor-based Post-Doctoral Fellowship from the American Diabetes Association (ADA 7-12-MN-030) awarded to M.A.A. (mentor) and M.A.H. (mentee).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Duality of Interest. M.A.A. is a co-inventor on a patent for the use of ATG+G-CSF for type 1 diabetes. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J.H. conceived of the study, researched the data, and wrote the manuscript. S.E.G., P.A.G., and A.W.M. researched the data and reviewed/edited the manuscript. D.J.P., A.R.S., M.A.H., and T.M.B. contributed to discussion, analyzed the data, and reviewed/edited the manuscript. J.J.S. and B.Z. analyzed the data and reviewed/edited the manuscript. C.H.W., C.E.M., and M.A.A. contributed to discussion and reviewed/edited the manuscript. A.L.P. wrote the manuscript and analyzed the data. D.A.S. conceived of the study and reviewed/edited the manuscript. M.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01106157, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db16-0823/-/DC1.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes 2013;62:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol 2013;9:92–103 [DOI] [PubMed] [Google Scholar]
- 4.Ludvigsson J. Combination therapy for preservation of beta cell function in type 1 diabetes: new attitudes and strategies are needed! Immunol Lett 2014;159:30–35 [DOI] [PubMed] [Google Scholar]
- 5.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–1576 [DOI] [PubMed] [Google Scholar]
- 6.Snarski E, Milczarczyk A, Torosian T, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 2011;46:562–566 [DOI] [PubMed] [Google Scholar]
- 7.Li L, Shen S, Ouyang J, et al. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab 2012;97:1729–1736 [DOI] [PubMed] [Google Scholar]
- 8.D’Addio F, Valderrama Vasquez A, Ben Nasr M, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes 2014;63:3041–3046 [DOI] [PubMed] [Google Scholar]
- 9.Gitelman SE, Gottlieb PA, Felner EI, et al.; ITN START Study Team . Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia 2016;59:1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitelman SE, Gottlieb PA, Rigby MR, et al.; START Study Team . Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol 2013;1:306–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haller MJ, Atkinson MA, Wasserfall CH, et al. Mobilization without immune depletion fails to restore immunological tolerance or preserve beta cell function in recent onset type 1 diabetes. Clin Exp Immunol 2016;183:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller MJ, Gitelman SE, Gottlieb PA, et al. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest 2015;125:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlosser M, Mueller PW, Törn C, Bonifacio E, Bingley PJ; Participating Laboratories . Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620 [DOI] [PubMed] [Google Scholar]
- 14.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories . Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 15.Lampasona V, Schlosser M, Mueller PW, et al. Diabetes antibody standardization program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem 2011;57:1693–1702 [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 2012;12:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5⁺ CD4⁺ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013;39:770–781 [DOI] [PubMed] [Google Scholar]
- 18.Locci M, Havenar-Daughton C, Landais E, et al.; International AIDS Vaccine Initiative Protocol C Principal Investigators . Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013;39:758–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes--considerations for attempts to prevent and reverse the disease. Diabetes Care 2015;38:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantú-Rodríguez OG, Lavalle-González F, Herrera-Rojas MA, et al. Long-term insulin independence in type 1 diabetes mellitus using a simplified autologous stem cell transplant. J Clin Endocrinol Metab 2016;101:2141–2148 [DOI] [PubMed] [Google Scholar]
- 21.Ehlers MR. Immune interventions to preserve β cell function in type 1 diabetes. J Investig Med 2016;64:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckner JH, Nepom GT. Obstacles and opportunities for targeting the effector T cell response in type 1 diabetes. J Autoimmun 2016;71:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed]
- 24.Xue S, Posgai A, Wasserfall C, et al. Combination therapy reverses hyperglycemia in NOD mice with established type 1 diabetes. Diabetes 2015;64:3873–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarez-Pinzon WL, Cembrowski GS, Rabinovitch A. Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia 2009;52:1680–1682 [DOI] [PubMed] [Google Scholar]