Abstract
Rationale and Objectives
Abnormal blood flow with bicuspid aortic valve (BAV) has been characterized with four-dimensional flow magnetic resonance imaging (MRI), but this approach is time consuming and requires technical expertise. We assess the relationship between different leaflets fusion patterns with BAV, eccentric systolic flow, and dilation patterns of the ascending aorta using two-dimensional (2D) phase-contrast (PC) MRI.
Materials and Methods
Fifty-nine patients with BAV who underwent cardiac MRI were identified; 47 had right–left (RL) aortic leaflet fusion and 12 had right-noncoronary (RN) fusion. Flow displacement was calculated, and patients with abnormal displacement (>0.1) were classified as either rightward or leftward. Patterns of aortopathy were determined (0–3), and correlation between leaflet fusion, flow direction, aortopathy type, and other clinical parameters was performed with Pearson correlation, the Fisher exact test and chi-square analysis.
Results
Normal systolic flow was seen in 24% of cases and was significantly correlated with normal aortas (P = .011). Abnormal flow displacement with RL fusion was strongly associated with rightward deviation (36 of 37 cases), whereas RN fusion skewed leftward (seven of eight cases; P < .01). In patients with aortopathy, RL fusion was strongly associated with type 2 aortopathy and RN with type 3 aortopathy (P < .01).
Conclusions
Conventional PC MRI can identify abnormal systolic flow and differences in jet orientation with BAV. RL leaflet fusion is associated with rightward flow deviation and type 2 aortopathy, whereas RN fusion is linked to leftward deviation and type 3 aortopathy. The presence and direction of eccentric flow jets may help risk stratify these patients for valve-related aortic disease.
Keywords: MRI, aorta, valves, BAV, eccentric jets, flow displacement, phase-contrast MRI
Characterization of eccentric systolic blood flow with bicuspid aortic valve (BAV) has been extensively performed using four-dimensional flow (4D Flow) magnetic resonance imaging (MRI) (1,2). Although there is much debate about the significance of these flow patterns, their presence and association with aortic dilation in patients with BAV has been used to support the argument that altered systolic hemodynamics with BAV play a significant role in the aortopathy exhibited by these patients (3,4). However, to date, only a few, small cohorts have suggested the prognostic value of MRI flow data in patients with BAV (5,6). One reason for this is the relatively slow growth rates of the ascending aorta, typically in the range of ≤1 mm/year (7). Another reason is the length and complexity of both the 4D Flow acquisition and calculation of flow-related parameters, which have limited its clinical applicability.
Conventional phase-contrast (PC) MRI is often performed in the ascending aortas of patients with BAV to quantify the degree of aortic regurgitation (8). The calculation is derived from through-plane velocity data for a cross-sectional plane in the tubular ascending aorta, which can be acquired in a single breath-hold. These same data can also be used to assess some of the less-complex findings that have been reported with 4D Flow, such as the presence of eccentric systolic flow and calculation of flow displacement (9). The aim of this study is to use routine PC data (i.e., two-dimensional [2D] PC MRI) to evaluate the interrelationship between systolic flow, aortopathy, and different aortic leaflets fusion patterns in patients with BAV. We hypothesize that this faster and less technically involved MRI approach to assessing aortic hemodynamics with BAV will reveal important abnormalities of flow that are associated with different types of aortopathy and aortic leaflet fusion patterns.
MATERIALS AND METHODS
Diagnostic radiology reports at our institution spanning a period of the past 11 years (2001–2013) were reviewed which identified 59 unique patients with BAVafter application of inclusion/exclusion criteria. Patients of all age were included if they had at least one prior echocardiogram and their MR studies included PC evaluation of the ascending aorta and contrast-enhanced MRA. Exclusion criteria were history of aortic valve or aortic root surgery, complex congenital heart disease, or poor quality study in which dimensions of ascending aortic and/or aortic leaflet fusion pattern could not be determined (total of 29 patients excluded for these reasons). Additionally, two patients were identified with left-noncoronary (LN) fusion patterns but were excluded from analysis because of small group size.
The diagnosis of BAV and specifics of the aortic leaflet fusion was made by MRI and/or echocardiography, and all fusions considered complete for the purposes of analysis. In four cases where there was initial disagreement in fusion pattern between clinical MRI report and echocardiogram report, however, after review of images, consensus was reached in all cases. Aortic stenosis (AS) and aortic insufficiency (AI) severity was based on echocardiography report closest in time to MRI examination. Two blinded reviewers (M.D.H. and N.S.B. with 10 and 6 years experience, respectively, with cardiovascular imaging) measured ascending aortic diameters independently at standard levels through the ascending aorta, and average measurements were used for analysis (10). Aortic segment dilation was determined by aortic size index measurements, as per Roman et al. and Della Cote et al. (11,12), and was then used to classify the aortas into the following groups of aortopathy: type 0 = normal aorta; type 1 = dilated aortic root; type 2 = dilation involving the tubular portion of the ascending aorta; and type 3 = diffuse involvement of the ascending aorta extending to the arch (3,11–13) (Fig 1). In cases where there was initial disagreement on aortopathy type between reviewers (n = 2), independent re-review was performed, and consensus was achieved in all cases. A waiver of informed consent was obtained from our institutional review board for the retrospective data analysis performed for this study, which was compliant with the Health Insurance Portability and Accountability Act.
Figure 1.
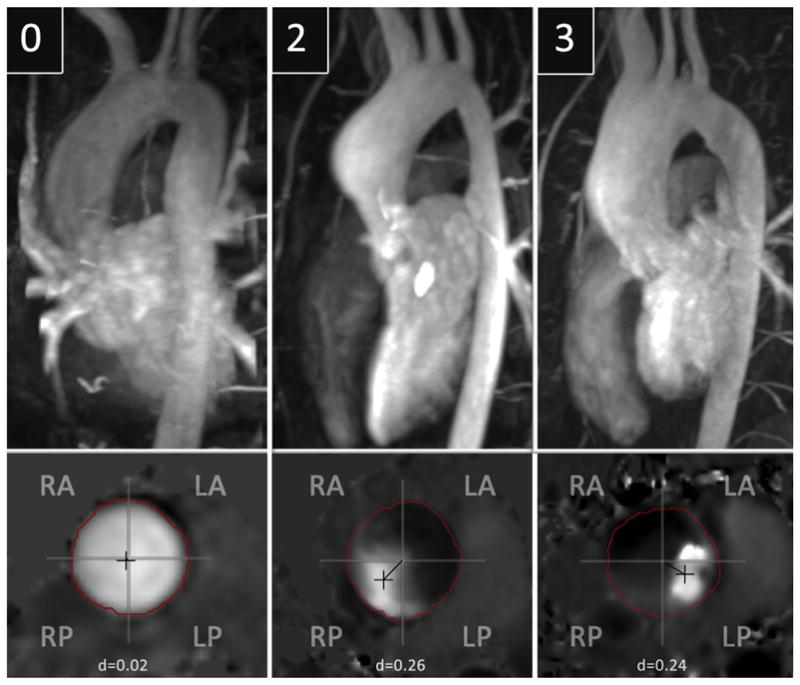
Representative cases of types 0, 2 and 3 aortopathy. Note that type 1 aortopathy was not included because of the limited number of patients with this pattern in our study. Maximum intensity projections of magnetic resonance angiography data demonstrate the pattern of aortopathy, with the associated planar analysis of systolic flow provided for each case. The patient with the normal aorta (type 0) had right–left (RL) aortic leaflet fusion and a flow displacement value of 0.02. The patient with type 2 aortopathy had RL aortic leaflet fusion and a flow displacement value of 0.26 oriented in the right-posterior (RP) quadrant. The patient with type 3 aortopathy had right-noncoronary aortic leaflet fusion and a flow displacement value of 0.24 oriented in the left-posterior (LP) quadrant. LA, left-anterior; RA, right-anterior.
MRI Technique
All scans were acquired at 1.5 Twith either an Achieva (Philips Medical Systems, Best, The Netherlands), using a five-channel cardiac coil, or a Signa CV/I (General Electric Medical Systems, Milwaukee, WI), using an eight-channel cardiac coil. PC imaging was performed at a plane perpendicular to the proximal, tubular ascending aorta. The velocity encoding value was adjusted to minimize aliasing of signal; a range of 150–400 cm/s was used. A 16-phase, free-breath sequence was performed on the Philips system in 10 patients. The imaging parameters were as follows: echo time (TE), 3.2–6.6 milliseconds; repetition time (TR), 5.4–10.0 milliseconds; matrix 96 to 256 × 256; field of view (FOV), 240–320 mm; slice thickness, 5–10 mm; and number of excitations, 3. In 33 patients, a 40-phase breath-hold sequence was used with the following imaging parameters: TE, 2.9–3.1 milliseconds; TR, 4.8–5.0 milliseconds; matrix, 128 × 256; FOV, 320 mm; slice thickness, 8 mm; and number of excitations, 1. A 30-phase breath-hold sequence was performed in 16 patients on the GE system with the following imaging parameters: TE, 5.7–5.9 milliseconds; TR, 5.4–10.0 milliseconds; matrix, 128 × 256; FOV, 320 mm; slice thickness, 8 mm; and number of excitations, 1.
Patients underwent three-dimensional (3D) contrast-enhanced angiography in the parasagittal plane after the administration of gadopentetate dimeglumine IV (0.1 mmol/kg body weight, Magnevist; Bayer Schering, Berlin-Wedding, Germany) at a rate of 2.0 mL/s. On the Philips system, imaging parameters were as follows: TE, 1.55 milliseconds; TR, 5.3 milliseconds; matrix, 173 × 400; FOV, 340 × 400; and number of excitations, 1. On the GE system, imaging parameters were as follows: TE, 2.7 milliseconds; TR, 5.5 milliseconds; matrix, 160 × 256; FOV, 320 × 320; and number of excitations, 0.5. The time delay between the start of the contrast injection and image acquisition was determined with bolus tracking. Two breath-hold acquisitions (20–30 seconds) were performed.
Data Collection and Analysis
Segmentation of the aortic lumen was performed at peak systole (ie, the time point of maximum flow). Systolic flow displacement from the vessel center, a parameter developed to quantify flow eccentricity independent of vessel size (9), was calculated from this segmentation using flow analysis software (Medis, Netherlands). This parameter is calculated in the following way: 1) the anatomic center of the aorta for a single cross-sectional imaging plane is defined, 2) the “center of velocity” of the forward flow at peak systole is then defined, 3) the distance between these two points is calculated, and 4) this distance is then normalized by dividing through by the aortic diameter for the analysis plane. The magnitude data from the PC images were used to calculate the aortic diameter and anatomic center; this approach has previously been shown to be reproducible (6). On the basis of prior work with flow displacement, a value of <0.1 was considered normal, “midline” flow (6,9). Additionally, the anatomic quadrant into which the flow displacement was directed (ie, right-anterior, right-posterior, left-anterior, left-posterior) was recorded.
Statistics
Pearson correlation was used to evaluate the interrater reliability of aortic measurements at the four measured locations (sinuses of Valsalva, sinotubular junction, level of main pulmonary artery, and prearch). Frequencies of categorical variables were compared with the chi-square analysis and the Fisher exact test where appropriate. Comparison means for continuous variables was performed with unpaired t tests and ANOVA with Bonferroni correction as appropriate, and a P value of <.05 was considered significant. Pearson correlation was used to measure degree of association. Because of small sample sizes, the variables for severity of AS and AI were collapsed to binary variables where categories of “moderate” or “severe” valve dysfunction were considered significant and “mild” or “none” valve dysfunction categories were considered insignificant. Subgroups of patients with left-anterior and right-anterior quadrant flow displacement were small (n = 7 and 2, respectively), and for purposes of analysis, these groups were merged into “rightward” and “leftward” groups. History of coarctation was defined as a history of prior complete end-to-end resection or patch aortoplasty. All statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, TX).
RESULTS
Average patient age was 29.1 ± 13.6 years, and a majority of patients were men (64%, 38 of 59). Right–left (RL) leaflet fusion (n = 47) was more frequent than right-noncoronary (RN) fusion (n = 12). Roughly half of patients had a history of repaired aortic coarctation (27 of 59), although no patients had persistent, hemodynamically significant coarctation (>20 mm Hg) by gradient analysis. The distribution of aortopathy was the following: type 0, 24 patients; type 1, 2 patients; type 2, 21 patients; and type 3, 12 patients. Average aortic dimensions for the dilated aortic segments by aortopathy type were sinuses of Valsalva, 4.2 ± 0.1 cm for type 1 aortopathy; tubular ascending aorta, 4.2 ± 0.5 cm and 4.5 ± 0.5 cm for types 2 and 3 aortopathies, respectively; and prearch, 3.4 ± 0.3 cm for type 3 aortopathy. A strong correlation was found between reviewers for aortic measurements (r > 0.9). Type of aortopathy showed no statistically significant associations with sex, body surface area, or AS/AI by ANOVA and chi-square analysis.
There was a trend toward younger age in patients with normal aortas (type 0) were versus those with aortopathy, although this was marginally significant (25.3 ± 12.7 vs. 32.3 ± 13.8 years, P = .051). Patients with type 3 aortopathy were less likely to have a history of aortic coarctation compared with other aortic shapes (0 of 12 vs. 27 of 47, P < .001). Less than one-third of patients studied had significant aortic valve disease: seven patients had significant AS, 12 patients had significant AI, and no patients had both significant stenosis and insufficiency. Descriptive statistics of the patient characteristics by valve fusion pattern are presented in Table 1.
TABLE 1.
Characteristics of Study Population by Valve Fusion Pattern
| Characteristic | Right–Left Fusion (n = 47) | Right-Noncoronary Fusion (n = 12) | P Value |
|---|---|---|---|
| Age (years), mean ± standard deviation | 29.8 ± 12.8 | 28.1 ± 17.2 | .7 |
| Male patients, n (%) | 28 (60) | 10 (83) | .18 |
| Coarctation history, n (%) | 24 (51) | 3 (25) | .11 |
| Aortopathy, n (%) | |||
| Type 0 | 20 (43) | 4 (33) | .56 |
| Type 1 | 2 (4) | 0 | .46 |
| Type 2 | 20 (43) | 1 (8) | .023 |
| Type 3 | 5 (10) | 7 (58) | .0002 |
| Systolic flow, n (%) | |||
| Normal | 10 (22) | 4 (33) | .38 |
| Rightward | 36 (76) | 1 (8) | <.0001 |
| Leftward | 1 (2) | 7 (58) | <.0001 |
| Aortic stenosis, n (%)* | 4 (9) | 3 (25) | .12 |
| Aortic insufficiency, n (%)* | 10 (22) | 2 (17) | .72 |
Defined as moderate/severe dysfunction.
Aortic Leaflet Fusion Patterns
There was no significant difference in mean age between patients with RL versus RN aortic leaflet fusion (29.8 ± 12.8 vs. 28.1 ± 17.2 years). Repaired aortic coarctation was more frequent in patients with RL than RN fusion (24/47 vs. 3/12), although differences were nonsignificant (P = .11). Type 2 aortopathy was significantly more frequent in patients with RL fusion versus RN fusion (20 of 47 vs. 1 of 12, P = .023), whereas type 3 aortopathy was significantly more frequent with RN fusion versus RL fusion (7 of 12 vs. 5 of 47, P = .002). Rightward displacement of systolic flow was more frequent with RL fusion compared to RN fusion (36 of 47 vs. 1 of 12, P < .001), whereas leftward displacement was significantly more frequent with RN fusion compared to RL fusion (7 of 12 vs. 1 of 47, P < .001). In patients with aortopathy, RL fusion was strongly associated with type 2 aortopathy (20 of 27 cases) and RN with type 3 aortopathy (7 of 8; P = .002). These results are tabulated in Table 1.
Flow Displacement
Midline systolic flow (displacement <0.10) was seen in 14 patients: 10 with RL fusion and 4 with RN fusion. Midline flow was significantly associated with normal aortas (type 0) compared to those patients with eccentric flow (10/14 vs. 14/45, P = .007), although age did not significantly differ between patients with midline and eccentric flow (23.9 ± 12.2 vs. 31.2 ± 13.8, P = .08). Patients with any type of aortopathy showed a higher flow displacement than those with normal aortas (0.21 ± 0.06 vs. 0.15 ± 0.09, P = .002). In patients with eccentric systolic flow (flow displacement >0.10), rightward flow displacement was more frequent than leftward displacement (37 vs. 8). No significant difference in flow displacement values was found between rightward and leftward displacement orientations (0.21 ± 0.04 vs. 0.26 ± 0.09, P = .09). No significant association was found between displacement and age (r = 0.17, P = .18). Mean displacement values did not differ by the presence of significant AS (0.2 ± 0.01 vs. 0.19 ± 0.08, P = .68) or significant AI (0.17 ± 0.08 vs. 0.19 ± 0.08, P = .33).
Rightward flow displacement was associated with type 2 aortopathy compared to leftward flow displacement (19 of 21 vs. 2 of 21, P = .001), whereas leftward flow displacement was associated with type 3 aortopathy compared to rightward flow (8 of 12 vs. 4 of 12, P = .46). When only eccentric flow was considered, a strong association was found between rightward flow displacement and RL fusion (36 of 37 patients), whereas a similarly strong association was found between leftward flow displacement and RN fusion (7 of 8 patients; P < .001). These results are tabulated in Table 2 with representative cases exhibited in Figure 1. No differences in magnitude of flow displacement were found between studies performed on Phillips or GE scanners (0.19 ± 0.8 vs. 0.19 ± 0.9, P = .9) or between 16-, 30-, or 40-phase techniques by ANOVA (F = 0.19, P = .82).
TABLE 2.
Associations by Flow Subgroup
| Characteristic | Normal Flow (n = 14) | Rightward Flow (n = 37) | Leftward Flow (n = 8) |
|---|---|---|---|
| Age (years) | 23.8 ± 12.6 | 32.2 ± 12.5 | 26.6 ± 19.0 |
| Flow displacement | 0.06 ± 0.02 | 0.21 ± 0.04* | 0.26 ± 0.09* |
| Aortic shape | |||
| Type 0 | 10 (72)† | 11 (30) | 3 (37) |
| Type 1 | 1 (7) | 1 (3) | 0 |
| Type 2 | 1 (7) | 19 (51)† | 1 (13) |
| Type 3 | 2 (14) | 6 (16) | 4 (50)‡ |
| Fusion pattern | |||
| RL | 10 (71) | 36 (97)† | 1 (13) |
| RN | 4 (29) | 1 (3) | 7 (87)† |
The values are presented as mean ± standard deviation or n (%).
Statistically significant compared to normal flow (P < .01).
Statistically significant (P < .01) by chi-square.
Statistically significant (P < .05) by chi-square.
DISCUSSION
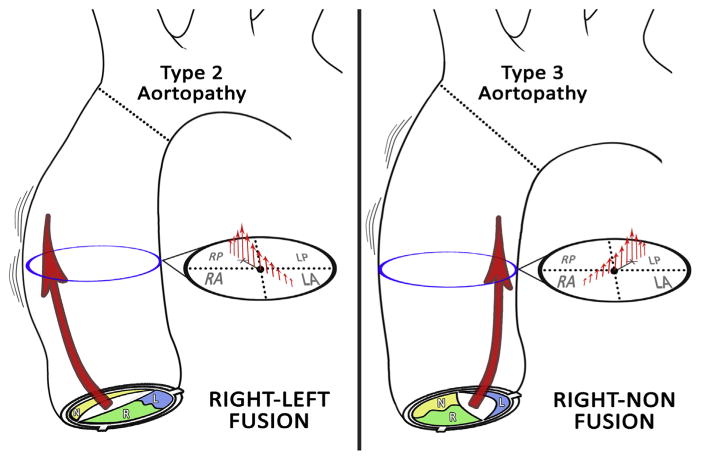
Conventional 2D PC MRI using a standard cross-sectional plane in the tubular ascending aorta can characterize key differences in systolic blood flow with BAV. Many, although not all, patients studied (45 of 59) demonstrated abnormal systolic flow. Fusion of the right and left (RL) aortic leaflets was associated with rightward flow displacement and type 2 aortopathy, whereas fusion of the RN aortic leaflets was associated to leftward flow displacement and type 3 aortopathy (Fig 2). Patients with BAV and normal flow were significantly more likely to have normal aortas despite being comparable in age.
Figure 2.
Characteristic associations between leaflet fusion pattern, flow, and aortopathy. Right–left aortic leaflet fusion is associated with rightward displacement of systolic flow and type 2 aortopathy (ie, dilation involving the tubular ascending aorta), whereas right-noncoronary aortic leaflet fusion is linked to leftward displacement of systolic flow and type 3 aortopathy (ie, diffuse dilation extending to the transverse arch).
BAV is common (1%–2% of population) and can lead to significant aortic morbidity in young, relatively healthy patients (14,15). The aortopathy seen with BAV is favored to represent intrinsic, structural aortic disease in many reports (16). However, visualization of abnormal systolic flow with 4D Flow MRI has suggested a contributory role for hemodynamics in the development of BAVaortopathy (17). Eccentric systolic jets oriented toward the aortic convexity with the more common RL aortic leaflet fusion pattern correlate with the site of asymmetric aortic dilation often observed with BAV, whereas the left-posterior jets that have been reported with the less-common RN fusion may explain the increased aortic arch dimensions in this subgroup (1,2,18–22).
The association between aortopathy and aortic leaflet fusion with BAV, as well as its clinical significance, has been further explored in recent publications (3,12,13). Kang et al. show that type 3 aortopathy (ie, dilation extending to the transverse arch) is linked to RN fusion. Mahadevia et al. show RL fusion is linked to type 2 aortopathy (ie, dilation involving the tubular portion), whereas RN fusion is associated with non–type 2 aortopathy. They also demonstrate clear differences in flow jet orientations between RL and RN fusion, with right-anterior jets seen with RL fusion and right-posterior jets with RN fusion.
Our study confirms these findings using routine 2D PC, a more clinically available technique. Among patients with aortopathy, RL fusion is linked to type 2 aortopathy, whereas RN fusion is linked to type 3 aortopathy (P < .01). Underlying jet orientations are clearly different between RL and RN fusions, with RL linked with rightward flow and RN linked to leftward flow; these associations grow even stronger when only patients with abnormal flow are included (36 of 37 RL cases have rightward flow, whereas 7 of 8 RN cases have leftward flow, P < .001). Differences in the terminology for describing jet orientation between our study and that of Mahadevia et al. (ie, right-anterior and right-posterior vs. rightward and leftward) may reflect differences in the axes of 4D Flow acquisitions and/or the different levels at which flow was assessed (more proximal for Mahadevia et al.) and the evolution of eccentric flow (ie, helicity) along the ascending aorta.
Our study is different from that of Mahadevia et al. in three important ways. First, we have made our observations regarding flow with BAV and aortopathy using conventional 2D PC MRI, rather than with 4D Flow. The advantage of our approach is that any imager can use a routine and short MRI sequence to collect these data. Second, we focus on the parameter of flow displacement, which can be easily calculated with commercially available software (Medis, Netherlands), rather than on more complex parameters such as wall shear stress (WSS) that require lengthy postprocessing and propriety analysis. Flow displacement was developed as a straightforward way to measure flow eccentricity that could be used as a surrogate for WSS (9). Compared to WSS, it is both easier to calculate and more reproducible (6). Third, we looked at a series of unselected patients with BAV and a typical distribution of RL and RN fusions (23,24), rather than a selected cohort of patients. In addition to being more representative of patients with BAV in general, our patients were younger and more likely to have normal aortas (24 of 59 in our study vs. 0 of 30 in Mahadevia et al). As a substantial number of patients with BAV do not develop aortopathy (14), these patients with normal aortas are key to understanding the range and significance of flow abnormalities seen with BAV. Importantly, we have shown that normal systolic flow is linked to normal aortas (P = .011), a finding which others have also demonstrated (2). This finding suggests that the presence or absence of flow abnormality could be used to risk stratify younger patients for the likelihood of developing aortopathy and potentially that the degree of flow abnormality could be used to fine-tune risk assessment, as recent reports looking at the prognostic significance of eccentric flow with BAV suggest (25).
Our study has several limitations. First, we do not have longitudinal data to evaluate the prognostic significance of our findings. This was beyond the aim of our study, which was to demonstrate that key associations between aortic leaflet fusion, aortopathy, and flow can be made with conventional 2D PC MRI. However, in a recent longitudinal study of patients with BAV using MRI, increased flow displacement was associated with elevated aortic growth rates, suggesting the prognostic value of our findings (25). Second, we have a relatively small number of patients with RN fusion (n = 12). Despite this relatively small number, however, we were able to draw clear and statistically significant conclusions about this BAV subgroup. Third, as our medical center is a tertiary/quaternary care facility, more advanced disease may be expected than in the general population. Yet, less than a third of our patients had significant AS or regurgitation, and the inclusion of more patients with limited disease would presumably strengthen the correlation we have found between normal flow and the absence of aortopathy. Additionally because of referral biases at our center, we noted a relatively high incidence of concomitant aortic coarctation (51%), potentially limiting generalization of our conclusions. However, other studies of different patient populations with BAV have reached similar conclusions to those we present (3,13). Fourth, we do not show an association between AI and root dilation (ie, type 1 aortopathy) as others have reported (26). This is likely due to both the relatively small number of patients with AI and type 1 aortopathy in our cohort. Finally, the flow data we used were acquired from clinical scans where there was technician-dependent variation in the placement of imaging planes. Plane placement was based on anatomic landmarks (ie, perpendicular to the proximal tubular ascending aorta) without regard to 3D flow features through the ascending aorta. Nonetheless, we were able to confirm previously reported associations between aortic leaflet fusion, flow, and aortopathy.
In conclusion, routine 2D PC MRI can identify abnormal systolic flow and differences in jet orientation in the ascending aortas of patients with BAV. The presence and direction of eccentric flow jets are clearly associated with different aortic shapes and may help risk stratify these patients for valve-related aortic disease. The wide availability of this MRI sequence makes the analysis we have performed feasible at centers without advanced technical and computational support.
Abbreviations
- BAV
bicuspid aortic valve
- TAV
tricuspid aortic valve
- MRI
magnetic resonance imaging
- 2D
two-dimensional
- 3D
three-dimensional
- PC MRI
phase-contrast MRI
- 4D Flow
3D, time-resolved phase-contrast MRI
- WSS
wall shear stress
- AS
aortic stenosis
- AI
aortic insufficiency
Footnotes
Conflicts of Interest: M.D.H. received Radiological Society of North America Research Scholars Grant 2012–2014 and N.S.B. received National Institutes of Health T32 Training Grant RR0814.
References
- 1.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255(1):53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 2.Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6(4):499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129(6):673–682. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uretsky S, Gillam LD. Nature versus nurture in bicuspid aortic valve aortopathy: more evidence that altered hemodynamics may play a role. Circulation. 2014;129(6):622–624. doi: 10.1161/CIRCULATIONAHA.113.007282. [DOI] [PubMed] [Google Scholar]
- 5.Della Corte A, Bancone C, Conti CA, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: a new risk marker for aortopathy. J Thorac Cardiovasc Surg. 2012;144(2):360–369. doi: 10.1016/j.jtcvs.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Hope M, Sigovan M, Wrenn J, et al. MRI hemodynamic markers of progressive bicuspid aortic valve-related aortic disease. J Magn Reson Imaging. 2014;40(1):140–145. doi: 10.1002/jmri.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanassoulis G, Yip JW, Filion K, et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med. 2008;5(12):821–828. doi: 10.1038/ncpcardio1369. [DOI] [PubMed] [Google Scholar]
- 8.Muzzarelli S, Monney P, O’Brien K, et al. Quantification of aortic flow by phase-contrast magnetic resonance in patients with bicuspid aortic valve. Eur Heart J Cardiovasc Imaging. 2014;15(1):77–84. doi: 10.1093/ehjci/jet129. [DOI] [PubMed] [Google Scholar]
- 9.Sigovan M, Hope MD, Dyverfeldt P, et al. Comparison of four-dimensional flow parameters for quantification of flow eccentricity in the ascending aorta. J Magn Reson Imaging. 2011;34(5):1226–1230. doi: 10.1002/jmri.22800. [DOI] [PubMed] [Google Scholar]
- 10.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Devereux RB, Kramer-Fox R, et al. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64(8):507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 12.Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6(12):1301–1310. doi: 10.1016/j.jcmg.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC Cardiovasc Imaging. 2013;6(2):150–161. doi: 10.1016/j.jcmg.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83(1):81–85. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 16.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55(25):2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 17.Girdauskas E, Borger MA, Secknus MA, et al. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39(6):809–814. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Bauer M, Gliech V, Siniawski H, et al. Configuration of the ascending aorta in patients with bicuspid and tricuspid aortic valve disease undergoing aortic valve replacement with or without reduction aortoplasty. J Heart Valve Dis. 2006;15(5):594–600. [PubMed] [Google Scholar]
- 19.Cotrufo M, Della Corte A. The association of bicuspid aortic valve disease with asymmetric dilatation of the tubular ascending aorta: identification of a definite syndrome. J Cardiovasc Med (Hagerstown) 2009;10(4):291–297. doi: 10.2459/JCM.0b013e3283217e29. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer BM, Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94(12):1634–1638. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 21.Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg. 2008;135(4):901–907. 907.e1–2. doi: 10.1016/j.jtcvs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920–1929. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 23.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133(5):1226–1233. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Sabet HY, Edwards WD, Tazelaar HD, et al. Congenitally bicuspid aortic valves: a surgical pathology study of 542 cases (1991 through 1996) and a literature review of 2,715 additional cases. Mayo Clin Proc. 1999;74(1):14–26. doi: 10.4065/74.1.14. [DOI] [PubMed] [Google Scholar]
- 25.Burris NS, Sigovan M, Knauer HA, et al. Systolic flow displacement correlates with future ascending aortic growth in patients with bicuspid aortic valves undergoing magnetic resonance surveillance. Investigative radiology. 2014;49(10):635–639. doi: 10.1097/RLI.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 26.Girdauskas E, Borger MA. Bicuspid aortic valve and associated aortopathy: an update. Semin Thorac Cardiovasc Surg. 2014;25(4):310–316. doi: 10.1053/j.semtcvs.2014.01.004. [DOI] [PubMed] [Google Scholar]