OVERVIEW OF AORTIC IMAGING
Aortic disease is a broad term that encompasses related and sometimes overlapping conditions associated with substantial morbidity and mortality, including aortic aneurysm and dissection.1,2 Imaging has long been used to diagnose and monitor these processes, and along with advances in surgical technique, has markedly improved mortality over the last 50 years.3 Advances in MRI have led to a more sophisticated understanding of intrinsic and valve-related aortic pathology. These developments not only provide insight into underlying pathophysiology, but also may allow imaging to better predict disease progression and inform the timing of interventions.
Hemodynamic imaging with MRI is among the most recent advances in aortic evaluation. The hemodynamic environment of the aorta is unique with extreme pressure variations, high flow rates, and complex flow patterns that exist in both normal and pathologic states. Phase contrast MRI (PC-MRI) has been utilized in routine conventional cardiac MRI for more than 2 decades to accurately measure these flow dynamics. However, the recent optimization of volumetric, time-resolved (cine) and 3-directional PC-MRI (4D Flow), has led to striking visualization of dynamic flow patterns and quantification of associated abnormal hemodynamics.
In this article, we briefly review common clinical applications of 2-directional (2D) PC-MRI, and discuss the emerging applications of 4D Flow for the thoracic aorta. We aim to highlight ongoing research in the field of aortic 4D Flow imaging by focusing on promising quantitative hemodynamic markers of aortic disease.
CURRENT CLINICAL 2-DIRECTIONAL FLOW APPLICATIONS
Aortic blood flow imaging with MRI is conventionally performed in 2 dimensions (2D). This means that single-directional velocity data is captured with PC-MRI through or parallel to a prescribed imaging plane. In contrast, 4D Flow imaging captures 3-directional (3D) velocity data through a volume on interest. Currently, conventional 2D evaluation is routinely used in several clinical scenarios: aortic valve disease, cardiac shunt lesions and aortic coarctation.
Echocardiography is commonly used for assessing aortic valvular disease. However, over the past decade, MRI has become increasingly popular because it provides several distinct advantages, including better evaluation of the ascending aorta, more reproducible measurements of regurgitation fraction, and quantitative measurement left ventricular size, function, and mass.4,5 For evaluation of aortic stenosis, transvalvular gradient is a key parameter for determining disease severity and need for intervention. Transvalvular gradient can be estimated with 2D PC-MRI by imaging parallel the aortic centerline, just beyond the valve, to determine peak jet velocity, which can then be used for estimation of the pressure gradient using the modified Bernoulli equation. However, low temporal resolution and suboptimal imaging plane placement can lead to underestimation of true pressure gradients. For aortic regurgitation, measurement of forward and backward flow volume through the ascending aorta can be used to calculate the regurgitant fraction. Conventional 2D PC-MRI can also be used to quantify cardiac shunt fractions and to assess the hemodynamic impact of aortic coarctation. Similar to aortic regurgitation, through plane flow volumes are measured in the aorta and main pulmonary artery, and the shunt ratio or Qp/Qs can be calculated. In the case of coarctation, acquisition planes are placed across the aorta just distal to the coarctation and at the diaphragm to quantify collateral flow through intercostal vessels, which increases with worsening coarctation severity.6
4-DIRECTIONAL FLOW
Multidimensional MRI (eg, 4D Flow) has become an increasingly popular research tool over the past decade. There are several advantages compared with conventional 2D PC sequences: Free breathing, acquisition of 3D velocity data, no need for prospective 2D plane placement, and powerful flow visualization and quantification software. Currently, the main disadvantages of 4D Flow are the need for labor-intensive data after processing, greater susceptibility to motion artifacts, and longer scan times, although scan times have improved dramatically over the last 5 years.
Data Acquisition, Reconstruction, and Fidelity
4D Flow datasets are acquired over multiple cardiac cycles: Using electrocardiographic gating, data are collected over many hundreds of heartbeats and then compiled to create a cine image of a complete, representative cardiac cycle. Without the use of scan acceleration techniques, datasets can take 1 hour to fully acquire, a prohibitively long time for routine applications. When clinical patients are considered, rather than a population of healthy research volunteers, scan time becomes a dominant concern. A significant amount of research effort has been dedicated to reducing 4D Flow scan times, with promising results reported using spiral (rather than conventional Cartesian) acquisitions and data undersampling techniques for acceleration, including compressed sensing. Many studies report scan times of less than 15 minutes, with some groups acquiring data in as few as 5 minutes.7,8 Data errors with PC-MRI acquisitions have been reported,9 but can be minimized by routine correction of eddy currents, gradient fields, and Maxwell phase effects.
Data fidelity is an important consideration because 4D Flow data are subject to multiple artifacts. In addition to the technical issues listed, there are other sources of error related to acquisitions spanning numerous heart beats and the presence of underlying pathologic flow characteristics (eg, turbulence, helicity). Confirmation of 4D Flow data fidelity has largely relied on in vitro, in silico, and animal models.10,11 One in vivo approach for data verification relies on the pathline technique of flow visualization. Pathlines tend to be sensitive to artifact accumulation, because they are calculated by integration over time. Applying conservation-of-mass principles in a specific region of interest, pathlines can be used for data quality verification. Another metric for internal data quality control relies in measurement of aortic (Qs) and pulmonary (Qp) flow rates, which should be equal in the absence of shunts, and can help in the identification of erroneous data. Of note, several important flow parameters are relative to other internal measurements (eg, Qp/Qs, collateral flow, flow displacement) so that the absolute values of the velocity data are less important than relative internal consistency.
Flow Visualization
Several approaches can be used for 4D Flow data visualization, including vector plots and particle traces (ie, streamlines and pathlines), which are typically color coded for velocity data.12 Whereas a vector plot represents the actual velocity data at a given moment in time, streamlines are imaginary lines that smoothly connect together these vectors for a depiction of an instantaneous flow field. They are visually appealing, but do not represent actual blood flow because they only reflect 1 time point. Pathlines, on the other hand, do represent blood flow through time. They are calculated by releasing imaginary particles into the flow field and then using the dataset to determine where particles will travel in an iterative process through the cardiac cycle. Despite the need for time-intensive post processing, these visualization methods have shown promise for several potential clinical applications, including investigation of valve-related abnormal flow patterns,13 blood compartmentalization in the ventricles,14 intimal entry tears and flow patterns in chronic dissection,15 corrected postrepair flow patterns,16 and identification of embolic pathways.17
QUANTITATIVE HEMODYNAMIC MARKERS
Dynamic flow visualization is an obvious appeal of 4D Flow. It affords an intuitive representation of flow and a qualitative assessment of abnormal flow patterns. Quantitative markers, however, can be derived from 4D Flow datasets to more precisely characterize the hemodynamic consequences of pathologic flow disturbances. Although the true clinical utility of aortic 4D Flow remains to be determined, the ability to measure a diverse set of novel hemodynamic markers is likely to be its most clinically applicable feature.
Clinical guidelines for the management of aortic disease use maximal diameter to risk stratify patients and to set thresholds for operative intervention. Aortic dissection and increased mortality, however, are reported with normal-sized and mildly dilated aortas.18 The topic of disease progression and aortic diameter is particularly problematic for patients with bicuspid aortic valves (BAV), with marked variability in management reported in routine surgical practice.19 Markers beyond aortic dimension that reflect the degree of an individual’s risk would be useful to better anticipate disease progression and complication. Herein we review promising new flow-related markers in 3 general contexts: Aortic valve disease, valve-related aortic disease, and aortic wall disease.
Aortic Valve Disease
Flow assessment has long been central to the clinical evaluation of the aortic valve. Three main flow-derived parameters are currently measured to determine the severity of aortic stenosis and guide surgical intervention: Transvalvular gradient, valve area, and peak velocity. Although these measurements generally perform well as markers of aortic disease severity, they are not always accurate. For example, gradient estimates using the modified Bernoulli equation do not take into account pressure recovery, and severe aortic stenosis with a low ejection fraction can have low gradients (ie, low-flow/low-gradient severe aortic stenosis).20,21 A more accurate composite measurement of the increased overall ventricular workload, “valvuloarterial impedance,” has been developed, and takes into account the degree of valve obstruction, the ventricular response, and the systemic vascular impedance.22 Valvuloarterial impedance may better represent the pathologic burden placed on the left ventricle that leads to overload and failure.20,23
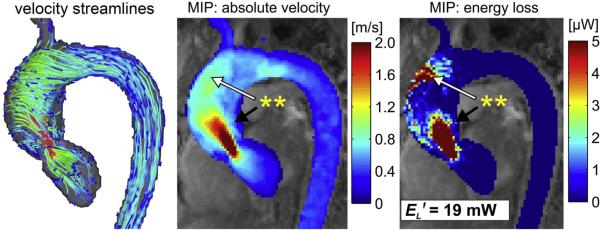
4D Flow affords a complimentary means of calculating the overall ventricular workload experienced with aortic stenosis. Energy loss can be directly assessed with the technique by 2 recently described methods: Estimation of vicious energy loss and turbulent kinetic energy. Viscous dissipation of energy is a normal feature of aortic flow. For normal laminar flow, it is caused by friction between adjacent fluid layers with different velocities. This friction increases with the abnormal flow features that are seen with aortic valve disease, resulting in substantially elevated viscous energy losses (Fig. 1).24 A limitation of measuring viscous energy loss, however, is that it does not take into account turbulence.
Fig. 1.
Systolic velocity streamlines (left), maximum intensity projection (MIP) of the 3-dimensinal (3D) velocity field (middle), and viscous dissipation (right) in the thoracic aorta of a patient with borderline severe stenosis (peak systolic velocity of 4.0 m/s). Regionally high velocity gradients (double asterisk, black arrow) result in elevated energy loss. Flow jet impingement at the aortic wall (double asterisk, white arrow) is co-located with a region of substantial energy loss. EL', cumulative peak systolic energy loss in the ascending aorta. (Courtesy of A. Barker, PhD, and P. van Ooij, PhD, Northwestern University, Chicago, IL.)
Turbulence is a common feature of poststenotic flow and a significant contributor to total irreversible pressure loss owing to the dissipation of mechanical energy into heat. Turbulent kinetic energy (TKE) can be estimated with 4D Flow by measuring the distribution of velocities within each imaging voxel: The greater the standard deviation of velocities, the higher the TKE. Recent studies have shown TKE is significantly increased in aortic stenosis, and that TKE measurements by 4D Flow correlate well with established methods of calculating irreversible pressure loss.25 As a direct imaging measurement of irreversible pressure loss, TKE may best reflect the increased workload placed on the ventricle with aortic stenosis.
Valve-Related Aortic Disease
Valve-related aortic disease has become one of the principal areas of aortic 4D Flow research. Disease of the aortic valve is frequently associated with abnormal dilation of the ascending aorta (i.e., post-stenotic dilation), especially in the case of congenital BAV. Abnormal hemodynamics and intrinsic aortic wall disease both likely play a role in the development of aortic dilation with BAV, with the relative contribution of each factor debated in the literature.26 The asymmetry of ascending aortic dilation that is typically seen with BAV, where there is disproportionate dilation of the aortic convexity, suggests an underlying asymmetric driver of disease.27 4D Flow research has focused on identifying hemodynamic markers that may be responsible for this dilation pattern and be used to predict disease progression. Research efforts have been invigorated by recent data that shows elevated aortic growth rates with restricted cusp opening angle with BAV, which presumably drives abnormal aortic flow.28
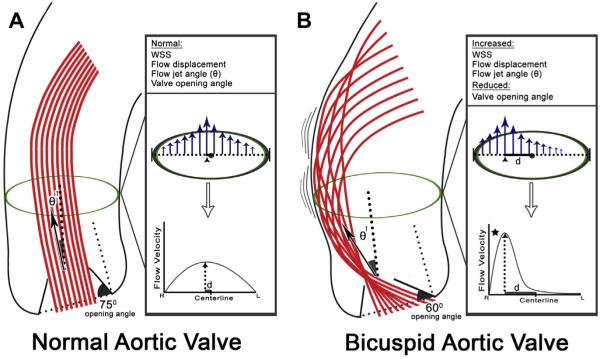
Abnormal systolic aortic flow patterns with BAV were first demonstrated with 4D Flow in 2008.29 Since then, numerous studies have demonstrated similar abnormal flow patterns in the ascending aorta of patients with acquired and congenital aortic valve disease.30–32 Qualitative visualization of disturbed flow has led to the development of qualitative measures of flow abnormality (Fig. 2).
Fig. 2.
Conventional aortic valve anatomy (A) is associated with a normal valve opening angle (~75°), flow jet angle (θ1), wall shear stress (WSS), and flow displacement. Flow displacement measures the displacement of peak systolic flow (arrowhead) from the vessel centerline, and is normalized to aortic size. It is calculated by dividing the distance from centerline of peak systolic flow (d), by the aortic diameter (dotted line with brackets). The abnormal valve anatomy seen with a bicuspid aortic valve (B), here typical of right-left aortic leaflet fusion, leads to a reduced valve opening angle, increased flow jet angle (θ1), and increased flow displacement. The increased near wall velocity gradient (region denoted by star) results in asymmetrically increased systolic WSS at the aortic convexity.
Wall sheer stress (WSS) is an often-reported quantitative marker of abnormal flow. The parameter can be estimated from near-wall velocity gradients with 4D Flow, and represents the frictional force experienced by the endothelium owing to flow viscosity. When deviated from a normal, intermediate range, WSS can adversely affect endothelial activation and signaling. High WSS states have been associated with vascular dilation and remodeling. Using 4D Flow, focally elevated WSS has been demonstrated in patients with BAV at the aortic convexity,30,33,34 a mechanism that may contribute to asymmetric dilation in this region. Although promising, WSS measurements are challenging. Greater differences between subgroups of patients are demonstrated if peak WSS values are reported,33 but these peak values are more subject to noise than the mean values that other groups have reported.34 Furthermore, the accuracy and reliability of WSS estimates derived from 4D Flow data have been called into question, given the difficulty in vessel wall segmentation and limited spatiotemporal resolution.10,35
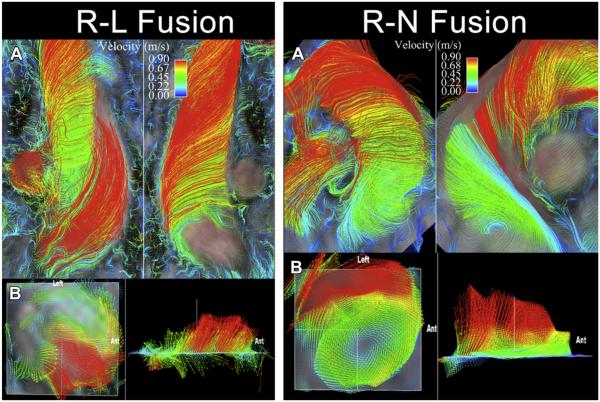
Flow displacement is another quantitative parameter that has shown promise for characterizing BAV-related aortic disease. It measures the displacement of peak systolic flow from the vessel center caused by the presence of a BAV.36 In the more common BAV leaflet fusion pattern (right–left aortic leaflet fusion [RL]), this displacement is toward the aortic convexity, whereas with the other common fusion (right–noncoronary leaflet fusion [RN]), the displacement is typically more posterior. In some cases, differences in the direction of flow displacement result in completely different orientations of helical flow with RL versus RN fusions (Fig. 3). Intriguingly, the differences in flow displacement between RL and RN fusions have recently been associated with often reported differences in patterns of aortopathy; RL is associated with dilation of the tubular portion of the ascending aorta, whereas RN is associated with dilation of the aortic arch.32
Fig. 3.
Right-handed, nested helical flow in a patient with a bicuspid aortic valve involving fusion of the right and left leaflets (R-L Fusion), and normal aortic dimensions. (A) Streamline analysis reveals greater than 180° curvature of peak systolic streamlines in a right-handed twist around slower, central helical flow in the ascending thoracic aorta. (B) Vector analysis reveals a right-anterior eccentric systolic flow jet. Left-handed nested helical systolic flow in a patient with a bicuspid aortic valve involving fusion of the right and noncoronary leaflets (R-N Fusion) and normal aortic dimensions. (A) Streamline analysis reveals greater than 180° curvature of peak systolic streamlines in a left-handed twist around slower, central helical flow in the ascending thoracic aorta. (B) Vector analysis reveals a left-posterior eccentric flow jet. Ant, anterior. (From Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010;255(1):59, 60; with permission.)
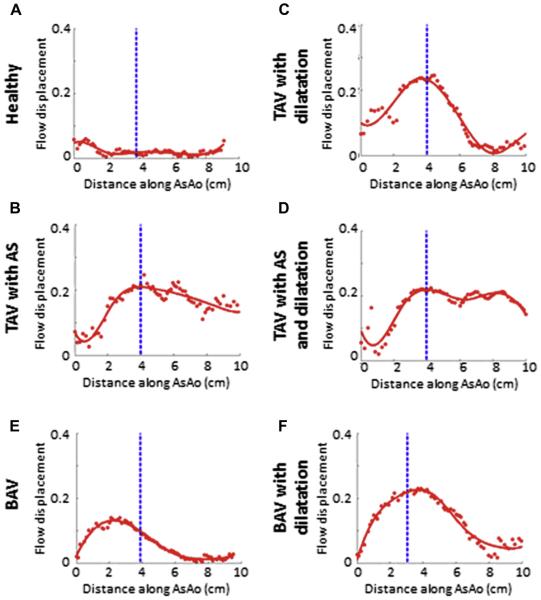
Flow displacement is a very reproducible parameter that can clearly distinguish between aortic valve phenotypes.32,37 It is also the only 4D Flow parameter to date to be correlated with aortic growth in a small cohort study.37 Furthermore, data suggest that using 2D PC-MRI at the typically location for flow analysis in the ascending aorta can identify peak flow displacement values (Fig. 4). Taken together, these findings suggest that the simple parameter of flow displacement could be calculated from conventional 2D PC-MRI for the risk stratification of patients with BAV for aortic disease progression.
Fig. 4.
Representative plots of flow displacement from each of the cross-sections (red dots, red curve-spline fit) as a function of distance along the ascending thoracic aorta (AsAo) for each of the 6 groups labeled. The dotted blue line indicates the location of the 2-dimensional plane, which was placed just distal to the sinotubular junction. No high-flow displacement was observed for the healthy control group (A). Generally, the presence of aortic stenosis resulted in the highest flow displacement values that remained high for longer distances along the AsAo (B-D). Patients with bicuspid aortic valves (BAV) had characteristic 3-dimensional displacement plots with a sharp increase to proximal high peak values, followed by a smooth tapering distally through the ascending aorta (E, F). TAV, tricuspid aortic valve. (Courtesy of M. Sigovan, PhD, University of Lyon, Lyon, France.)
Aortic Wall Disease
Diseases that stiffen the aortic wall can affect aortic flow. Aortic wall stiffening is commonly seen with aging and is an independent predictor of mortality both in the general population and for patients with common chronic illnesses.38 Flow imaging can be used to estimate aortic wall stiffness by determining the speed of the systolic impulse through the aorta, or pulse wave velocity (PWV). Elevated PWV reflects wall stiffness and has been demonstrated using PC-MRI with hypertension and Marfan disease.39,40 For Marfan patients, PWV has long been investigated as means of predicting disease progression, with normal PWV values associated with regional aortic stability.41 4D Flow offers a more comprehensive assessment of aortic PWV than 2D PC-MRI (Fig. 5), and may improve the detection of regional differences that prior studies with Marfan syndrome have reported.41,42 In addition to PWV, 4D Flow is capable of producing accurate, noninvasive measurements of aortic pressure waveforms.43 Relative pressure can then be subdivided into inertial and viscous components, which show unique patterns in different types of aortopathy.44
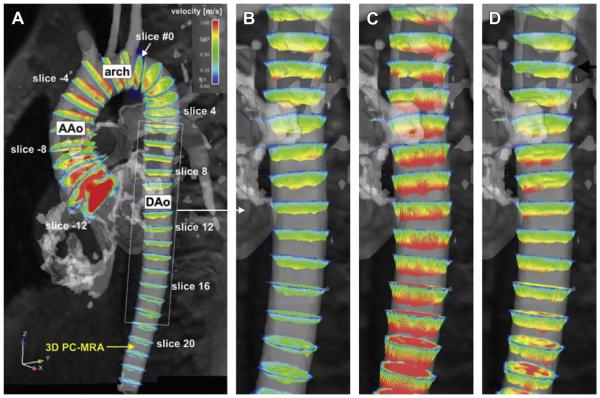
Fig. 5.
(A): Visualization of pulse wave propagation within the thoracic aorta, based on flow-sensitive 3-directional phase contrast MRI (4D) data. Equidistant analysis planes with an interanalysis plane gap of 10 mm were positioned upstream (negative analysis plane numbers) and downstream (positive analysis plane numbers) along the thoracic aorta, starting with analysis plane #0 (outlet of the left subclavian artery). (B–D) Spatially varying flow profiles from the proximal to the descending thoracic aorta (DAo) in analysis planes can be appreciated in successive systolic time frames: during early systole (B), profiles in the proximal DAo are already fully developed, whereas velocities are continuously lower further downstream. During peak systole (C), flow profiles reach their maxima in the entire DAo, whereas during late systole (D), flow profiles in the proximal DAo are already reduced compared with flow further downstream. AAo, ascending thoracic aorta. (From Markl M, Wallis W, Brendecke S, et al. Estimation of global aortic pulse wave velocity by flow-sensitive 4D MRI. Magn Reson Med 2010;63(6):1579; with permission.)
Beside aortic wall stiffening, altered aortic flow patterns have been linked to the development of atherosclerosis. Low and oscillatory WSS have been shown induce endothelial changes that lead to plaque formation.45,46 Using 4D Flow estimates of WSS, further evidence has been gathered showing the interrelationship of altered aortic flow and atherosclerotic plaque formation.47,48 This suggests that 4D Flow could be used for identification of abnormal flow markers to predict an atherogenic-prone region, and thus guide risk stratification and medical management.
SUMMARY
Aortic disease is routinely monitored with anatomic imaging, but until the recent advent of 4D Flow imaging, associated blood flow abnormalities have gone largely undetected. 4D Flow is a rapidly evolving technique that is currently able to measure a range of aortic hemodynamic markers in less than 15 minutes. Initial qualitative flow visualization has spurred the investigation of new quantitative flow markers. For example, eccentric systolic flow with BAV has led to the application of WSS and flow displacement for assessment of related ascending aortic disease. Many promising 4D Flow markers of aortic disease have been proposed, although larger prospective studies are needed to validate their clinical relevance. Within the next decade, 4D sequences may be commonly acquired during routine clinical cardiac MR studies, and provide valuable information to guide the medical and surgical management of patients with aortic disease.
KEY POINTS.
Three-directional phase contrast MRI (4D flow MRI) is able to visualize and quantify abnormal flow related to a wide variety of aortic pathologies.
Limitations of the technique, including scan time and artifacts, have been greatly reduced making 4D flow a clinically feasible technique.
Novel quantitative hemodynamic markers have been developed to characterize abnormal flow, and to investigate underlying mechanisms of disease.
Markers beyond aortic diameter could improve risk stratification of patients with aortic disease and better determine the timing of intervention.
Larger, prospective studies are needed to validate the clinical relevance of 4D flow.
Acknowledgments
Funding: Covidien/Radiological Society of North America Research Scholar Grant RSCH1215 (M.D. Hope).
Footnotes
Disclosures: No conflicts to disclose.
REFERENCES
- 1.Olsson C, Thelin S, Stahle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114(24):2611–8. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 2.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306(10):1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 3.Clouse WD, Hallett JW, Jr, Schaff HV, et al. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA. 1998;280(22):1926–9. doi: 10.1001/jama.280.22.1926. [DOI] [PubMed] [Google Scholar]
- 4.Tsai SF, Trivedi M, Daniels CJ. Comparing imaging modalities for screening aortic complications in patients with bicuspid aortic valve. Congenit Heart Dis. 2012;7(4):372–7. doi: 10.1111/j.1747-0803.2012.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/ AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48(3):e1–148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Steffens JC, Bourne MW, Sakuma H, et al. Quantification of collateral blood flow in coarctation of the aorta by velocity encoded cine magnetic resonance imaging. Circulation. 1994;90(2):937–43. doi: 10.1161/01.cir.90.2.937. [DOI] [PubMed] [Google Scholar]
- 7.Schnell S, Markl M, Entezari P, et al. k-t GRAPPA accelerated four-dimensional flow MRI in the aorta: effect on scan time, image quality, and quantification of flow and wall shear stress. Magn Reson Med. 2014;72(2):522–33. doi: 10.1002/mrm.24925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao A, Lustig M, Alley MT, et al. Rapid pediatric cardiac assessment of flow and ventricular volume with compressed sensing parallel imaging volumetric cine phase-contrast MRI. AJR Am J Roentgenol. 2012;198(3):W250–9. doi: 10.2214/AJR.11.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatehouse PD, Rolf MP, Graves MJ, et al. Flow measurement by cardiovascular magnetic resonance: a multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J Cardiovasc Magn Reson. 2010;12:5. doi: 10.1186/1532-429X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersson S, Dyverfeldt P, Ebbers T. Assessment of the accuracy of MRI wall shear stress estimation using numerical simulations. J Magn Reson Imaging. 2012;36(1):128–38. doi: 10.1002/jmri.23610. [DOI] [PubMed] [Google Scholar]
- 11.Boussel L, Rayz V, Martin A, et al. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: comparison with computational fluid dynamics. Magn Reson Med. 2009;61(2):409–17. doi: 10.1002/mrm.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta PP, Pedrizzetti G, Kilner PJ, et al. Emerging trends in CV flow visualization. JACC Cardiovasc Imaging. 2012;5(3):305–16. doi: 10.1016/j.jcmg.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Hope MD, Wrenn SJ, Sigovan M, et al. Imaging Biomarkers of Aortic Disease: increased growth rates with eccentric systolic flow. J Am Coll Cardiol. 2012;60(4):356–7. doi: 10.1016/j.jacc.2012.01.072. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson J, Carlhall CJ, Dyverfeldt P, et al. Semiautomatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson. 2010;12:9. doi: 10.1186/1532-429X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clough RE, Waltham M, Giese D, et al. A new imaging method for assessment of aortic dissection using four-dimensional phase contrast magnetic resonance imaging. J Vasc Surg. 2012;55(4):914–23. doi: 10.1016/j.jvs.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Allen BD, Barker AJ, Kansal P, et al. Impact of aneurysm repair on thoracic aorta hemodynamics. Circulation. 2013;128(17):e341–3. doi: 10.1161/CIRCULATIONAHA.112.000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harloff A, Strecker C, Frydrychowicz AP, et al. Plaques in the descending aorta: a new risk factor for stroke? Visualization of potential embolization pathways by 4D MRI. J Magn Reson Imaging. 2007;26(6):1651–5. doi: 10.1002/jmri.21126. [DOI] [PubMed] [Google Scholar]
- 18.Chau KH, Elefteriades JA. Natural history of thoracic aortic aneurysms: size matters, plus moving beyond size. Prog Cardiovasc Dis. 2013;56(1):74–80. doi: 10.1016/j.pcad.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Yanagawa B, Kalra S, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: a survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg. 2013;146(5):1033–40. doi: 10.1016/j.jtcvs.2013.06.037. e4. [DOI] [PubMed] [Google Scholar]
- 20.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47(11):2141–51. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P, Magne J. Valvuloarterial impedance in aortic stenosis: look at the load, but do not forget the flow. Eur J Echocardiogr. 2011;12(5):354–7. doi: 10.1093/ejechocard/jer044. [DOI] [PubMed] [Google Scholar]
- 22.Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46(2):291–8. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 23.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54(11):1003–11. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 24.Barker AJ, van Ooij P, Bandi K, et al. Viscous energy loss in the presence of abnormal aortic flow. Magn Reson Med. 2014;72(3):620–8. doi: 10.1002/mrm.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyverfeldt P, Hope M, Tseng EE, et al. Noninvasive magnetic resonance measurement of turbulent kinetic energy for the estimation of irreversible pressure loss in aortic stenosis. JACC Cardiovasc Imaging. 2013;6(1):64–71. doi: 10.1016/j.jcmg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girdauskas E, Borger MA, Secknus MA, et al. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39(6):809–14. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Lu MT, Thadani SR, Hope MD. Quantitative assessment of asymmetric aortic dilation with valve-related aortic disease. Acad Radiol. 2013;20(1):10–5. doi: 10.1016/j.acra.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Della Corte A, Bancone C, Conti CA, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: a new risk marker for aortopathy. J Thorac Cardiovasc Surg. 2012;144(2):360–9. doi: 10.1016/j.jtcvs.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Hope MD, Meadows AK, Hope TA, et al. Images in cardiovascular medicine. Evaluation of bicuspid aortic valve and aortic coarctation with 4D flow magnetic resonance imaging. Circulation. 2008;117(21):2818–9. doi: 10.1161/CIRCULATIONAHA.107.760124. [DOI] [PubMed] [Google Scholar]
- 30.Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6(4):499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255(1):53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 32.Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic 3D outflow patterns, wall shear stress and expression of aortopathy. Circulation. 2014;129(6):673–82. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hope MD, Hope TA, Crook SE, et al. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc Imaging. 2011;4(7):781–7. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Barker AJ, Markl M, Burk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5(4):457–66. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 35.Stalder AF, Russe MF, Frydrychowicz A, et al. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60(5):1218–31. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 36.Sigovan M, Hope MD, Dyverfeldt P, et al. Comparison of four-dimensional flow parameters for quantification of flow eccentricity in the ascending aorta. J Magn Reson Imaging. 2011;34(5):1226–30. doi: 10.1002/jmri.22800. [DOI] [PubMed] [Google Scholar]
- 37.Hope MD, Sigovan M, Wrenn SJ, et al. MRI hemodynamic markers of progressive bicuspid aortic valverelated aortic disease. J Magn Reson Imaging. 2014;40(1):140–5. doi: 10.1002/jmri.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavalcante JL, Lima JA, Redheuil A, et al. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–22. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Westenberg JJ, Scholte AJ, Vaskova Z, et al. Age-related and regional changes of aortic stiffness in the Marfan syndrome: assessment with velocity-encoded MRI. J Magn Reson Imaging. 2011;34(3):526–31. doi: 10.1002/jmri.22646. [DOI] [PubMed] [Google Scholar]
- 40.Brandts A, Westenberg JJ, van Elderen SG, et al. Site-specific coupling between vascular wall thickness and function: an observational MRI study of vessel wall thickening and stiffening in hypertension. Invest Radiol. 2013;48(2):86–91. doi: 10.1097/RLI.0b013e31827f6410. [DOI] [PubMed] [Google Scholar]
- 41.Kroner ES, Scholte AJ, de Koning PJ, et al. MRI-assessed regional pulse wave velocity for predicting absence of regional aorta luminal growth in Marfan syndrome. Int J Cardiol. 2013;167(6):2977–82. doi: 10.1016/j.ijcard.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 42.Markl M, Wallis W, Brendecke S, et al. Estimation of global aortic pulse wave velocity by flow-sensitive 4D MRI. Magn Reson Med. 2010;63(6):1575–82. doi: 10.1002/mrm.22353. [DOI] [PubMed] [Google Scholar]
- 43.Delles M, Rengier F, Jeong YJ, et al. Estimation of aortic pressure waveforms from 4D phase-contrast MRI. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:731–4. doi: 10.1109/EMBC.2013.6609604. [DOI] [PubMed] [Google Scholar]
- 44.Lamata P, Pitcher A, Krittian S, et al. Aortic relative pressure components derived from four-dimensional flow cardiovascular magnetic resonance. Magn Reson Med. 2014;72(4):1162–9. doi: 10.1002/mrm.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113(23):2744–53. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 46.Slager CJ, Wentzel JJ, Gijsen FJ, et al. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat Clin Pract Cardiovasc Med. 2005;2(8):401–7. doi: 10.1038/ncpcardio0274. [DOI] [PubMed] [Google Scholar]
- 47.Markl M, Brendecke SM, Simon J, et al. Co-registration of the distribution of wall shear stress and 140 complex plaques of the aorta. Magn Reson Imaging. 2013;31(7):1156–62. doi: 10.1016/j.mri.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Frydrychowicz A, Stalder AF, Russe MF, et al. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. J Magn Reson Imaging. 2009;30(1):77–84. doi: 10.1002/jmri.21790. [DOI] [PubMed] [Google Scholar]