Abstract
Living cells respond to their environment using networks of signaling molecules that act as sensors, information processors, and actuators. These signaling systems are highly modular at both the molecular and network scales, and much evidence suggests that evolution has harnessed this modularity to rewire and generate new physiological behaviors. Conversely, we are now finding that, following nature’s example, signaling modules can be recombined to form synthetic tools for monitoring, interrogating, and controlling the behavior of cells. Here we highlight recent progress in the modular design of synthetic receptors, optogenetic switches, and phospho-regulated proteins and circuits, and discuss the expanding role of combinatorial design in the engineering of cellular signaling proteins and networks.
Introduction: why design and engineer signaling proteins?
A major goal of modern cell biology is to understand how molecular signaling circuits enable cells to sense their environment and mount an appropriate response. This goal is currently being addressed using two distinct but complementary approaches: research aimed at the dissection, mapping, and analysis of naturally occurring systems, and efforts to engineer new cell signaling pathways. As the traditional analytic approach has revealed the wide diversity of mechanisms and molecular components that underlie cellular communication, a set of common mechanistic themes in signaling have emerged [1,2]. The synthetic approach provides a complementary method for rigorously testing that conceptual framework and for elucidating the core design principles that are used to achieve fundamental classes of response behaviors. By constructing signaling systems, one can precisely alter them in a highly controlled way, and map the landscape of physiological genotype/phenotype relationships. By using orthogonal components, one can ask questions free from the pleiotropic functional entanglement of natural proteins. Thus, these forward engineering approaches may help us better predict how changes wrought by evolution, disease, or therapy will impact cellular behaviors. In addition, the ability to engineer cells with customized signaling responses could also be useful for therapeutic applications. There have been remarkable recent advances in using engineered cells for cancer immunotherapy, treatment of autoimmunity, and regenerative medicine [3], and improving our ability to precisely design therapeutic cells is of growing interest.
Driven by the twin motivations of understanding natural signaling networks and building cells with useful behaviors, researchers are developing methods for engineering diverse cellular signaling molecules and systems [4,5]. Recent efforts in the synthetic biology of signaling are distinct from the transcriptional engineering that dominated early synthetic biology, which largely focused on using gene expression modules to control protein abundance. In cell signaling, protein based receptors and posttranslational protein regulation play a principal role in mediating the cell’s rapid response to changes in its environment. Engineering such fast and spatially coordinated cell signaling behaviors intrinsically focuses on engineering proteins.
Signaling proteins are highly modular in structure, often comprising distinct functional domains — some that catalyze particular information processing reactions (e.g. kinases and phosphatases) and others that mediate regulation or localization. One emerging strategy for engineering posttranslational regulation thus centers on generating novel combinations of modular domains and regulatory elements, which can result in rewiring new connections in the context of a larger cellular circuit. In this review, we will consider three areas of signaling protein design in which this modular approach has been highly successful and has shown recent progress: engineered synthetic cell-surface receptors, optogenetic sensors that allow light control of signaling pathways, and the engineering of synthetic phosphorylation-regulated signaling proteins.
Hierarchical logic of signaling proteins and networks
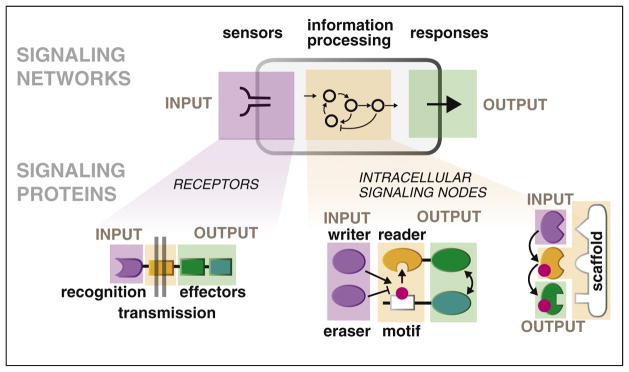
To communicate and respond to its environment, any cell must have at least three components: sensors or receptors that receive input, a downstream layer that processes these inputs, and physiological outputs that change in response to this information (e.g. changes in transcription, cell fate, cell migration or shape, etc.). Remarkably, even if one looks at the scale of individual signaling proteins, one can find the same type of organization. Even within an individual molecule one can find domains responsible for sensing inputs, domains or interactions that mediate decision making, and domains that control output (Figure 1). With this hierarchical architecture, new cellular behaviors — novel physiological INPUT/OUTPUT relationships — do not require the evolution of new systems, but merely new linkages between existing decision-making subsystems.
Figure 1.
Hierarchical organization of signaling systems: cells and individual proteins as input/output nodes. At any scale, a signaling system must have three components — it must have sensors to receive INPUT, an information-processing layer that decides what to make of this information, and an OUTPUT function. These components are found in individual signaling molecules, which detect and effect particular upstream and downstream molecular partners. In receptors that span the cell’s membrane, ligand binding to extracellular domains (INPUT) rapidly regulates the activity of intracellular effector domains (OUTPUT). Similarly, posttranslationally-regulated binding motifs link the activities of upstream enzymes that ‘write’ and ‘erase’ the posttranslational mark (INPUT; e.g. kinases and phosphatases) to recruitment of dedicated ‘reader’ domains (OUTPUT). The same classes of components are found in signaling networks and whole cells, but in this case receptor molecules function as INPUT sensors, networks of intracellular proteins function as the information processing layer, and various cellular response modules control OUTPUT.
Ultimately, reconnecting signaling subsystems requires rewiring individual signaling molecules that lie at the junctions of these higher order subsystems. Links in the cell’s signaling networks are often mediated by protein domains that perform specific functions: protein–protein interaction, subcellular localization, and catalysis. These domains are often found in multi-domain proteins [6] where their combination can yield switch-like enzymes gated by upstream signals, or scaffolds that rewire and guide signaling cascades (Figure 1). In the context of evolution [7,8], development [9], differentiation [10], and disease [11,12], it is clear that new cellular behaviors often arise when existing molecular modules are recombined to generate new receptors, sensors and downstream signaling protein. In this review, we highlight recent advances in the design of synthetic signaling systems made by following the same approach. In other words, by learning how to rewire individual signaling proteins, we are at the same time learning how to rewire whole networks.
Engineering new sensor/receptor molecules
Like a microscopic Argus,3 each cell perceives its environment through an array of molecular sensors and receptors. Synthetic biology now affords us the ability to further expand the cell’s ‘field of view.’ Modularly engineered receptors and sensors can be used readily to link a variety of new inputs to a critical cellular response (as in the case of chimeric antigen receptors), or use a single input (light) to selectively modulate dozens of intracellular signaling systems with precise spatial and temporal control.
CARs: extracellular receptor proteins that detect user-specified antigens
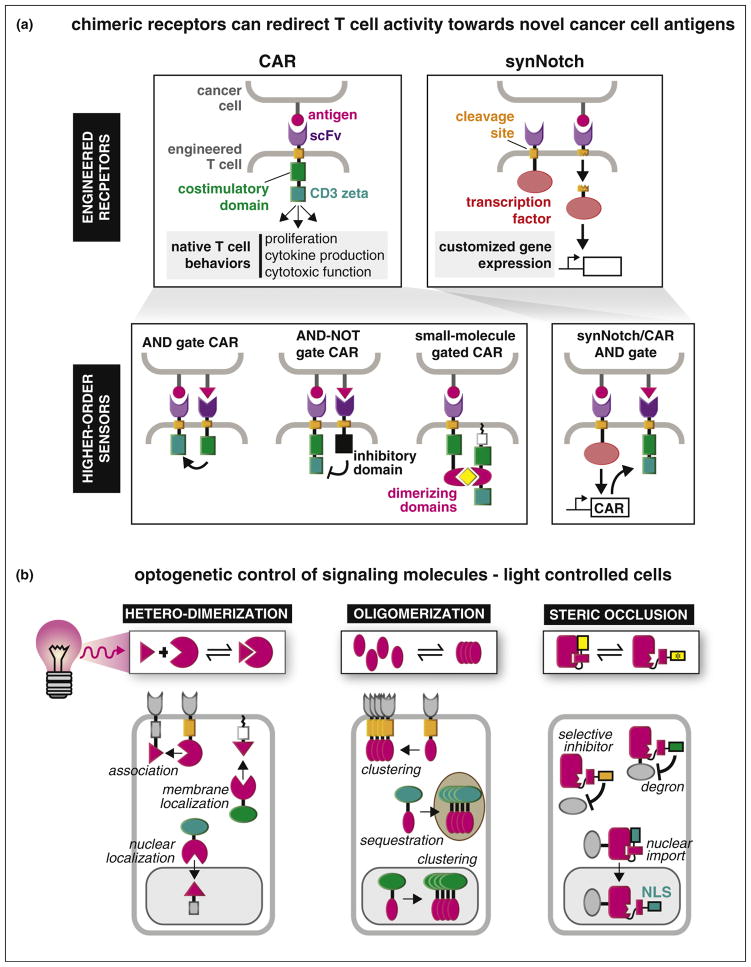
It is hard to believe that simply replacing the extracellular domain of a receptor protein with an unrelated recognition module would allow one to redirect its input sensing, but that is exactly how chimeric antigen receptors (CAR) work. A CAR is a fusion protein combining an extracellular single chain antibody (scFv) with intra-cellular regulatory domains of the T-cell receptor complex. Remarkably, when a CAR is expressed in a T cell, this can be sufficient to reprogram the cell to detect and attack tumor cells bearing the cognate antigen [13,14] (Figure 2a). Initial clinical results with CARs have been highly promising for treatment of B cell cancers (targeting the B cell specific antigen CD19) [15], although over-activation (cytokine storm) and off-target damage are severe problems [16,17]. To address these issues of safety, more precisely regulated CAR variants were recently developed by separating the sensing and intra-cellular signaling domains of the CAR into two separate molecules, and then inducibly reuniting the two components with a modular drug induced heterodimeric interaction. In this way, the split-CAR is essentially a version of the T cell receptor that has been engineered to be controlled by two novel inputs — the disease antigen and the small molecule [18•]. Likewise, CARs gated by the presence [19,20] or absence [21] of secondary antigen on the target cell have been generated using these secondary antigens as a target for co-recruitment of synthetic modulatory receptors that contain intracellular activating or inhibitory domains, respectively.
Figure 2.
Engineering new sensor/receptor systems. (a) The chimeric antigen receptor (CAR) was engineered to sense a tumor antigen and induce an immunogenic response against tumor cells expressing that antigen. Modular recombination of the CAR domains with new sensor modules has enhanced specificity of the CAR-T response either through logic gates requiring combinations of specific antigens or licensed by small molecule dimerization of critical signaling domains [18•,19–21]. A second type of engineered receptor based on Notch (synNotch) allows both input (target antigen) and output (gene expression) to be fully customized [24]. CAR and synNotch receptors can be combined synergistically, refining the specificity and scope of the T cell response [25]. (b) Modular optogenetic tools for controlling receptors and signaling proteins. Protein domains from plants that undergo light-induced dimerization, oligomerization, or steric regulation have been used to regulate signaling activities throughout the cell in a modular fashion.
A second class of engineered receptors harnesses the regulatory mechanism observed in the native Notch receptor. Notch engagement of an extracellular ligand triggers proteolysis of the receptor, releasing a transcription factor (contained in the receptor’s intracellular fragment) that enters the nucleus and drives downstream gene expression. Following this model, synthetic systems have been constructed in which TEV protease and a membrane tethered transcription factor are co-localized by activated GPCRs (G-protein coupled receptors) and RTKs (receptor tyrosine kinases) [22], or — in a more general form — by any ligand that induces receptor dimerization [23]. It was recently discovered that the proteolytic core of Notch is a modular regulatory element — enabling the generation of synthetic Notch receptors (synNotch) in which both extracellular targeting and induced gene expression are fully customized (Figure 2a) [24]. Importantly, synNotch and CAR receptors are highly complementary. T cells engineered so that synNotch activation drives CAR expression show high specificity for dual-antigen tumors in vivo [25].
Sensors that detect bioorthogonal stimuli such as light and small molecules
Another approach to engineering novel control over cell behavior is to construct signaling proteins that are responsive to flexible user-controlled inputs, such as small molecules and light. Small molecules and light can act within the cell and eliminate the need for transmembrane receptors that are able to transmit signals across the membrane. Inducible signaling proteins help us understand and engineer signaling networks by enabling us to observe network function in response to precisely defined pathway inputs. The first generation of inducible signaling systems relied on chemically induced dimerization (CID) of modular binding domains that homodimerize or heterodimerize upon binding of a small molecule ligand. These have been previously reviewed [26•]. Below, we focus on the next generation of tools that use light as an inducer (broadly termed ‘optogenetics’), providing new strategies for protein control with exquisite spatial and temporal precision. Optogenetic proteins are engineered from natural photoreceptor domains that coordinate with light-sensing cofactors. Absorption of light by these cofactors induces photoisomerization that drives a conformational change in the associated protein domain. This conformational change is then coupled to larger allosteric changes or enhanced protein–protein interactions. A small set of photoreceptor modules that undergo light-induced dimerization, oligomerization, or steric regulation (Figure 2b) have been recurrently used to achieve optogenetic control over diverse signaling proteins and networks.
Light-induced dimerization
Optogenetic protein homo-dimerization and hetero-dimerization has been used in multiple strategies to regulate signaling nodes throughout the cell. Receptor tyrosine kinases (RTKs) EGFR, FGFR, and Ret have been endowed with blue light regulation through fusion of their intracellular tails to a small, blue-light-sensing homo-dimerization domain [27•]. Hetero-dimerization has been used to regulate signaling through protein recruitment to particular cellular compartments. Plasma membrane recruitment of activating guanine nucleotide exchange factors (GEFs) has been an effective strategy for control of small GTPases Rac, Rho, Cdc42, and Ras [28•,29], and, conversely, recruitment of an inhibitory GTPase activating protein (GAP) was reported as a method to inhibit G-protein coupled receptors [30]. Membrane recruitment was also used to regulate signaling through Raf-1 [31] and PI3K [32]. Inducible nuclear recruitment enabled optogenetic control of transcription factor activity [33], and optogenetic recruitment to other cellular compartments was reported as a general strategy for titrating away signaling proteins [34,35]. Still other dimerization-based approaches used homo-association of Dronpa mutants to sterically or allosterically regulate activity of a catalytically active GEF protein [36], and optogenetic dissociation of a constitutive dimer enabled optical control over protein trafficking and secretion [37].
Light-induced oligomerization
Multivalency and higher-order protein assembly play key regulatory roles in many cellular signaling systems [38], and recent work illustrates how protein clustering can be placed under optogenetic control. Blue light-induced oligomerization of the Arabidopsis Cryptochrome2 protein has enabled activation of RTKs, both exogenous [39,40,41] and endogenous [41], as well as the Orai1 calcium channel [42] and the canonical Wnt pathway co-receptor LRP6 [43•]. Within the cytoplasm, clustering has been used to regulate Rho GTPase [43•] and Raf1 kinase [44] activity. Inducible clustering has also been used to regulate DNA damage signaling in the absence of DNA damage through oligomerization of TopBP1 [45].
Light-induced steric regulation
The blue light-induced conformational change of the LOV2 domain from A. sativa phototropin has been successfully used to sterically regulate small peptides controlling multiple cellular functions in a modular fashion. These functions include protein interaction [46,47], protein degradation [48,49], and nuclear translocation [50,51]. Steric occlusion of the appropriate peptides has also yielded optogenetic inhibition of specific kinases [52•] and activation of calcium channels [53].
Engineering phosphorylation control: intracellular posttranslational circuitry
Once a cellular sensor is activated, the signal is often relayed through a posttranslational regulatory network that processes that information and directs the cell to execute an appropriate response. Phosphorylation is the most common posttranslational modification [54], and efforts to engineer phospho-signaling proteins have shed light on how posttranslational networks function and how they can be rewired.
Engineered scaffolds for phospho-signaling
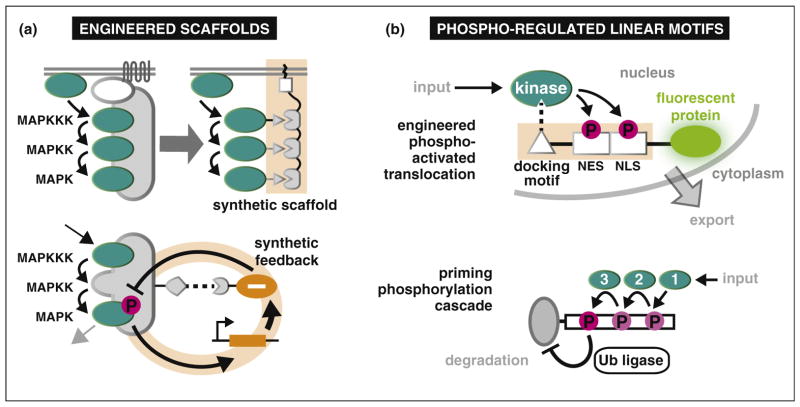
Proteins with multiple interaction domains can serve as molecular scaffolds, organizing multiple proteins in a signaling pathway into a complex (Figure 1). In yeast, the scaffold protein Ste5 orchestrates the mating pheromone MAP kinase (MAPK) pathway. Ste5 co-localizes a kinase cascade (MAPKKK → MAPKK → MAPK) and serves as a platform for the spatial and temporal control of these enzymes. To investigate how modular interactions mediate kinase cascades, Ryu and Park designed synthetic scaffolds using strings of repeated peptide binding domains (PDZ) and fused complementary PDZ ligands to each of the Ste5-associated kinases: Ste11 (MAPKKK), Ste7 (MAPKK), and Fus3 (MAPK). Synthetic scaffolds that co-localized two or more kinases (MAPKKK AND [MAPKK OR MAPK]) at the cell membrane were sufficient to functionally replace Ste5 [55] (Figure 3a). Moreover, these minimal scaffolds demonstrated logic gate properties and could be tuned by the co-recruitment of negative regulatory phosphatases. This result matches findings with endogenous MAPK pathways, where recruitment of regulators to engineered scaffolds reshapes the amplitude and timing of pathway behavior [56]. This approach was recently extended by the use of bacterial effectors [57]. The utility of this approach is exemplified by OspF, a toxic protein that irreversibly inactivates MAPKs by catalyzing a beta-elimination reaction that removes the hydroxyl group of the key phosphothreonine side chain, thereby preventing MAPK phosphorylation and consequent activation. This mechanism enables Shigella flexneri to disable human epithelial and dendritic cells. Repurposed as a tool for synthetic biology, this pathogenic inhibitor has been used to engineer a negative feedback loop that reshapes the dynamic response of the yeast osmolarity pathway (Figure 3a) and a T-cell ‘pause switch’ for adoptive immunotherapy [57].
Figure 3.
Post-translational signaling: rewiring phosphorylation devices. Novel linkages between signaling modules enable new functionality in signaling proteins and networks. (a) Synthetic scaffolding of the MAPK pathway was sufficient to induce downstream signaling [55] (top left), while co-scaffolding with negative regulatory effectors can tune the pathway response [57]. (b) Combining kinase docking motifs with phospho-regulated nuclear import and export sequences is a successful strategy to create dynamic fluorescent reporters of specific kinase activity [62••] (top right). Mutation of a phospho-site in a 3-pronged AND-gate for protein degradation generated a 2-pronged degradation AND-gate [69•].
Phospho-regulated linear motifs
Signaling proteins are enriched in unstructured regions (linear motifs) where the ‘writers’, ‘readers’, and ‘erasers’ of phosphorylation (kinases, phospho-binding domains, and phosphatases, respectively) collectively regulate protein binding, concentration, and localization [58,59] (Figure 1). Phospho-regulated linear motifs have been used to create dynamic reporters of intracellular signaling. FRET reporters for specific kinases link a phospho-regulated intramolecular binding event (phosphorylated substrate peptide + phospho-binding domain) to fluorescent protein co-localization [60]. More recently, a synthetic, phospho-stabilized, version of the destabilizing PEST domain was fused to a fluorescent protein to construct a live cell reporter of Erk activity [61]. In a third example of phospho-engineering, Regot et al. built a reporter that translocates in response to c-Jun N-terminal kinase (JNK) activation by combining a nuclear export signal (NES) activated by JNK phosphorylation, a phospho-inhibited nuclear import signal (NLS), and a fluorescent protein [62••] (Figure 3b). Importantly, this design can be readily adapted to multiple types of kinases by either substituting the docking site for JNK with that of another MAP kinase (MAPK), or mutating a kinase’s naturally occurring substrate to introduce the NLS and NES modules. This work illustrates the potential for modular engineering with linear motifs, and complements recent advances in the computational design of posttranslational regulation [63].
Scaling up phospho-circuit design
In proteins that contain multiple phosphorylation sites, phospho-regulated linear motifs can collectively form information processors that integrate inputs, set response thresholds [64], tune binding affinities [65], amplify weak signals, and serve as ‘conduits’ for sequential signal transduction [66]. Analogous synthetic ‘devices’, built from combinations of phospho-regulatory modules, may ultimately make it possible to endow engineered signaling proteins with complex information processing behaviors.
One of the best-studied examples of multisite phosphorylation is Sic1, a critical cell cycle regulator whose phospho-induced degradation triggers S phase in budding yeast [64,67]. Cyclin dependent kinase 1 (Cdk1), in complex with a cyclin and the phospho-adaptor Cks1, phosphorylates Sic1 in an ordered sequence at multiple sites, culminating in the activation of phosphodegrons within Sic1 that recruit the SCFCdc4 ubiquitin ligase complex, thereby targeting Sic1 for rapid degradation. Using synthetic Cdk1 substrates, Loog and colleagues found that the rate at which Cdk1 phosphorylation propagates is determined by how well the fixed spatial orientation of the three docking sites on the cyclin-Cdk1–Cks1 complex fits with the linear pattern of phosphorylation sites along the length of substrates like Sic1 [68•]. These results suggest that a simple set of rules might define the overall pattern and rates of phosphorylation in a multisite cluster.
Eco1, a regulator of sister chromatid cohesion, is also degraded after multisite phosphorylation. The timing of Eco1 function is usually restricted to S phase by the collective action of three different kinases (Cdk1, Cdc7, Mck1). In healthy cells, sequential phosphorylation by these kinases forms an SCFCdc4 phosphodegron, triggering Eco1 destruction. However, inhibition of one kinase (Cdc7) by the DNA damage response prevents Eco1 destruction, allowing establishment of cohesion after S phase. Lyons et al. characterized this system and generated a mutant version of Eco1 in which Cdk1 directly primes Mck1 phosphorylation — bypassing Cdc7’s phospho-regulation. This study revealed that a single point deletion converts the naturally occurring 3-pronged AND gate into a synthetic 2-pronged gate with altered cell cycle sensitivity [69•] (Figure 3b).
Overall, these findings suggest that fairly simple rules governing linear phospho-motifs are used by nature to achieve quite sophisticated information processing. As we learn to better manipulate these motifs, we may be able to test and harness these emerging rules.
Forward evolution of signaling networks
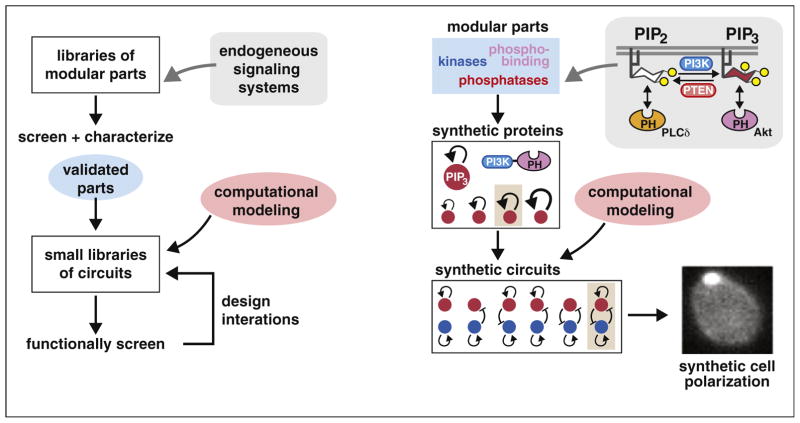
Ultimately, to engineer cellular behavior, we want to engineer cellular networks. But as described above, to engineer cellular networks we need to learn how to engineer individual signaling proteins and their connectivity. We postulate that much of the functional innovation in cellular signaling networks has evolved through repeated duplication and recombination of modular domains [7]. Researchers interested in posttranslational engineering can now use bioinformatics tools [70,71,72] to mine these naturally occurring circuits for domains that are, in essence, ‘pre-validated’ for a high degree of modularity (successfully functioning in many fusion contexts) and minimal crosstalk with other cellular components. These parts complement the growing toolbox of regulatory domains that have been validated (in an analogous manner) through repeated use in synthetic circuits [5,73]. With these enriched building blocks, generating new signaling proteins could potentially be a straightforward matter of screening domain combinations (combinatorial libraries) and optimizing protein expression [18•,28•,56]. The same approach can be iterated to generate posttranslational circuits of increasing complexity. One successful example has been the construction of a synthetic circuit for inducing artificial cell polarization in yeast. Modular binding domains that recognize phospho-inositide species were combined with modular catalytic domains that modify these species, yielding a set of proteins that form spatially localized positive and negative feedback loops. Together, this system of synthetic proteins generates a self-organizing asymmetric pole of the signaling molecule PIP3 [74••] (Figure 4). It is likely that in the coming years, we will see more examples of the construction of more complex synthetic signaling systems, enabled by a better understanding of modular domains, but also by advances in computational design and experimental combinatorial screening of libraries of modular synthetic circuits. As the field of synthetic signaling systems matures, a semi-predictive approach that combines computational design with combinatorial screening offers a pragmatic strategy for learning nature’s design principles while tailoring cellular responses to applications in medicine and biotechnology.
Figure 4.
Combinatorial design for engineering signaling proteins and networks. We present here a conceptual workflow for engineering signaling networks with desired properties. Small libraries of candidate circuits can be semi-rationally designed using a combination of validated signaling and regulatory components together with computational models. These circuits can then be screened and optimized for the proper function. The design of a network for cell polarization [74••] is provided as an example of this approach.
Acknowledgments
The authors sincerely thank Dr. Nicholas Frankel, Dr. Ricardo Almeida, Dr. Scott Coyle, Dr. Kole Roybal and Dr. Kyle Daniels for comments on the manuscript, as well as other members of the Lim lab for helpful discussions. We apologize to authors whose relevant work was not cited in this review due to space constraints. The authors are supported by the National Institutes of Health (P50 GM081879, PN2 EY016546, R01 GM055040, R01 GM096164, R01 CA196277), NSF Synthetic Biology and Engineering Research Center, and the Howard Hughes Medical Institute.
Footnotes
Watchman in Greek mythology whose body was covered with 100 eyes.
Conflict of interest statement
Wendell Lim is a Founder of Cell Design Labs and a member of its scientific advisory board.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Alon U. An introduction to systems biology: design principles of biological circuits. CRC Press; 2006. p. 324. [Google Scholar]
- 2.Lim W, Mayer B, Pawson T. Cell signaling: principles and mechanisms. Taylor & Francis; 2014. [Google Scholar]
- 3.Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11:393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brophy JAN, Voigt CA. Principles of genetic circuit design. Nat Methods. 2014;11:508–520. doi: 10.1038/nmeth.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slusarczyk AL, Lin A, Weiss R. Foundations for the design and implementation of synthetic genetic circuits. Nat Rev Genet. 2012;13:406–420. doi: 10.1038/nrg3227. [DOI] [PubMed] [Google Scholar]
- 6.Tordai H, Nagy A, Farkas K, Banyai L, Patthy L. Modules multidomain proteins organismic complexity. FEBS J. 2005;272:5064–5078. doi: 10.1111/j.1742-4658.2005.04917.x. [DOI] [PubMed] [Google Scholar]
- 7.Di Roberto RB, Peisajovich SG. The role of domain shuffling in the evolution of signaling networks. J Exp Zool B Mol Dev Evol. 2014;322:65–72. doi: 10.1002/jez.b.22551. [DOI] [PubMed] [Google Scholar]
- 8.Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell. 2010;142:661–667. doi: 10.1016/j.cell.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buljan M, Chalancon G, Eustermann S, Wagner GP, Fuxreiter M, Bateman A, et al. Tissue-specific splicing of disordered segments that embed binding motifs rewires protein interaction networks. Mol Cell. 2012;46:871–883. doi: 10.1016/j.molcel.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 11.Aman P. Fusion oncogenes in tumor development. Semin Cancer Biol. 2005;15:236–243. doi: 10.1016/j.semcancer.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Davey NE, Travé G, Gibson TJ. How viruses hijack cell regulation. Trends Biochem Sci. 2011;36:159–169. doi: 10.1016/j.tibs.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011:3. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther J Am Soc Gene Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Wu C-Y, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. Illustrates systematic combinatorial design – assembly and screening of a small library of domain combinations yielded a split version of the CAR whose function is conditional upon drug-induced hetero-dimerization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, et al. Chimeric antigen receptor T cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorov VD, Themeli M, Sadelain M. PD-1-based and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, et al. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daringer NM, Dudek RM, Schwarz KA, Leonard JN. Modular extracellular sensor architecture for engineering mammalian cell-based devices. ACS Synth Biol. 2014;3:892–902. doi: 10.1021/sb400128g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164:780–791. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Voß S, Klewer L, Wu Y-W. Chemically induced dimerization: reversible and spatiotemporal control of protein function in cells. Curr Opin Chem Biol. 2015;28:194–201. doi: 10.1016/j.cbpa.2015.09.003. A recent review of chemically induced dimerization, which has been widely used for small-molecule induction of signaling proteins. [DOI] [PubMed] [Google Scholar]
- 27•.Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, et al. Spatiotemporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 2014;33:1713–1726. doi: 10.15252/embj.201387695. The authors use a small homodimerizing domain to regulate association and signaling of receptor tyrosine kinase activation using blue light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell. 2013;155:1422–1434. doi: 10.1016/j.cell.2013.11.004. The authors leverage rapid optogenetic switching to quantify temporal filtering and frequency response of signal transduction through the Ras/ Erk pathway. This demonstrates the power of optogenetics for a probing signaling network function within living cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill PR, Gautam N. Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Mol Biol Cell. 2014;25:2305–2314. doi: 10.1091/mbc.E14-04-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang K, Duan L, Ong Q, Lin Z, Varman PM, Sung K, et al. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. PLOS ONE. 2014;9:e92917. doi: 10.1371/journal.pone.0092917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toettcher JE, Gong D, Lim WA, Weiner OD. Light-based feedback for controlling intracellular signaling dynamics. Nat Methods. 2011;8:837–839. doi: 10.1038/nmeth.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beyer HM, Juillot S, Herbst K, Samodelov SL, Müller K, Schamel WW, et al. Red light-regulated reversible nuclear localization of proteins in mammalian cells and zebrafish. ACS Synth Biol. 2015;4:951–958. doi: 10.1021/acssynbio.5b00004. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Park H, Kyung T, Kim NY, Kim S, Kim J, et al. Reversible protein inactivation by optogenetic trapping in cells. Nat Methods. 2014;11:633–636. doi: 10.1038/nmeth.2940. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Jost AP-T, Weiner OD, Tang C. A light-inducible organelle-targeting system for dynamically activating and inactivating signaling in budding yeast. Mol Biol Cell. 2013;24:2419–2430. doi: 10.1091/mbc.E13-03-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou XX, Chung HK, Lam AJ, Lin MZ. Optical control of protein activity by fluorescent protein domains. Science. 2012;338:810–814. doi: 10.1126/science.1226854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D, Gibson ES, Kennedy MJ. A light-triggered protein secretion system. J Cell Biol. 2013;201:631–640. doi: 10.1083/jcb.201210119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mammen M, Choi S-K, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Chang K-Y, Woo D, Jung H, Lee S, Kim S, Won J, et al. Light-inducible receptor tyrosine kinases that regulate neurotrophin signalling. Nat Commun. 2014;5:4057. doi: 10.1038/ncomms5057. [DOI] [PubMed] [Google Scholar]
- 40.Kim N, Kim JM, Lee M, Kim CY, Chang K-Y, Heo WD. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem Biol. 2014;21:903–912. doi: 10.1016/j.chembiol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Bugaj LJ, Spelke DP, Mesuda CK, Varedi M, Kane RS, Schaffer DV. Regulation of endogenous transmembrane receptors through optogenetic Cry2 clustering. Nat Commun. 2015;6:6898. doi: 10.1038/ncomms7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyung T, Lee S, Kim JE, Cho T, Park H, Jeong Y-M, et al. Optogenetic control of endogenous Ca(2+) channels in vivo. Nat Biotechnol. 2015;33:1092–1096. doi: 10.1038/nbt.3350. [DOI] [PubMed] [Google Scholar]
- 43•.Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods. 2013;10:249–252. doi: 10.1038/nmeth.2360. The authors describe the natural light-induced oligomerizaton of Arabidopsis Cryptochrome 2 and demonstrate that this activity may be used in a modular fashion to aggregate and regulate multiple signaling effectors. [DOI] [PubMed] [Google Scholar]
- 44.Wend S, Wagner HJ, Müller K, Zurbriggen MD, Weber W, Radziwill G. Optogenetic control of protein kinase activity in mammalian cells. ACS Synth Biol. 2014;3:280–285. doi: 10.1021/sb400090s. [DOI] [PubMed] [Google Scholar]
- 45.Ozkan-Dagliyan I, Chiou Y-Y, Ye R, Hassan BH, Ozturk N, Sancar A. Formation of Arabidopsis cryptochrome 2 photobodies in mammalian nuclei: application as an optogenetic DNA damage checkpoint switch. J Biol Chem. 2013;288:23244–23251. doi: 10.1074/jbc.M113.493361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat Methods. 2012;9:379–384. doi: 10.1038/nmeth.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, et al. Engineering an improved light-induced dimer (iLID) for controlling the localization activity of signaling proteins. Proc Natl Acad Sci U S A. 2015;112:112–117. doi: 10.1073/pnas.1417910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonger KM, Rakhit R, Payumo AY, Chen JK, Wandless TJ. General method for regulating protein stability with light. ACS Chem Biol. 2014;9:111–115. doi: 10.1021/cb400755b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renicke C, Schuster D, Usherenko S, Essen L-O, Taxis C. A LOV2 domain-based optogenetic tool to control protein degradation and cellular function. Chem Biol. 2013;20:619–626. doi: 10.1016/j.chembiol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Yumerefendi H, Dickinson DJ, Wang H, Zimmerman SP, Bear JE, Goldstein B, et al. Control of protein activity and cell fate specification via light-mediated nuclear translocation. PLOS ONE. 2015;10:e0128443. doi: 10.1371/journal.pone.0128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niopek D, Benzinger D, Roensch J, Draebing T, Wehler P, Eils R, et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat Commun. 2014;5:4404. doi: 10.1038/ncomms5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Yi JJ, Wang H, Vilela M, Danuser G, Hahn KM. Manipulation of endogenous kinase activity in living cells using photoswitchable inhibitory peptides. ACS Synth Biol. 2014;3:788–795. doi: 10.1021/sb5001356. The authors describe how the light-induced conformational change of LOV2 might be engineered to sterically regulate inhibitors of endogenous proteins. This study both provides general guidelines for engineering the LOV2 photoswitch and provides methods for optogenetic signal control without overexpression of the enzymes under control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pham E, Mills E, Truong K. A synthetic photoactivated protein to generate local or global Ca(2+) signals. Chem Biol. 2011;18:880–890. doi: 10.1016/j.chembiol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the Swiss-Prot database. Sci Rep. 2011;13:1. doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryu J, Park S-H. Simple synthetic protein scaffolds can create adjustable artificial MAPK circuits in yeast and mammalian cells. Sci Signal. 2015;8:ra66. doi: 10.1126/scisignal.aab3397. [DOI] [PubMed] [Google Scholar]
- 56.Bashor CJ, Helman NC, Yan S, Lim WA. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science. 2008;319:1539–1543. doi: 10.1126/science.1151153. [DOI] [PubMed] [Google Scholar]
- 57.Wei P, Wong WW, Park JS, Corcoran EE, Peisajovich SG, Onuffer JJ, et al. Bacterial virulence proteins as tools to rewire kinase pathways in yeast and immune cells. Nature. 2012;488:384–388. doi: 10.1038/nature11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gsponer J, Babu MM. The rules of disorder or why disorder rules. Prog Biophys Mol Biol. 2009;99:94–103. doi: 10.1016/j.pbiomolbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Van Roey K, Uyar B, Weatheritt RJ, Dinkel H, Seiler M, Budd A, et al. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem Rev. 2014;114:6733–6778. doi: 10.1021/cr400585q. [DOI] [PubMed] [Google Scholar]
- 60.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, et al. Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22:4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Regot S, Hughey JJ, Bajar BT, Carrasco S, Covert MW. High-sensitivity measurements of multiple kinase activities in live single cells. Cell. 2014;157:1724–1734. doi: 10.1016/j.cell.2014.04.039. Concretely demonstrates how desired signaling behaviors can be created by combining or overlaying phospho-regulated linear motifs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strumillo M, Beltrao P. Towards the computational design of protein post-translational regulation. Bioorg Med Chem. 2015;23:2877–2882. doi: 10.1016/j.bmc.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, et al. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 65.Lee CW, Ferreon JC, Ferreon ACM, Arai M, Wright PE. Graded enhancement of p53 binding to CREB-binding protein (CBP) by multisite phosphorylation. Proc Natl Acad Sci U S A. 2010;107:19290–19295. doi: 10.1073/pnas.1013078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galea CA, Wang Y, Sivakolundu SG, Kriwacki RW. Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits. Biochemistry (Mosc) 2008;47:7598–7609. doi: 10.1021/bi8006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2007;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 68•.Kõivomägi M, Ord M, Iofik A, Valk E, Venta R, Faustova I, et al. Multisite phosphorylation networks as signal processors for Cdk1. Nat Struct Mol Biol. 2013;20:1415–1424. doi: 10.1038/nsmb.2706. These articles shed light on the structural basis for ordered multisite phosphorylation, suggesting a basis for rationale design of phospho-regulated systems that rapidly perform multistep computations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Lyons NA, Fonslow BR, Diedrich JK, Yates JR, Morgan DO. Sequential primed kinases create a damage-responsive phosphodegron on Eco1. Nat Struct Mol Biol. 2013;20:194–201. doi: 10.1038/nsmb.2478. These articles shed light on the structural basis for ordered multisite phosphorylation, suggesting a basis for rationale design of phospho-regulated systems that rapidly perform multistep computations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43(Database issue):D257–D260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dinkel H, Van Roey K, Michael S, Davey NE, Weatheritt RJ, Born D, et al. The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res. 2014;42(Database issue):D259–D266. doi: 10.1093/nar/gkt1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Roey K, Dinkel H, Weatheritt RJ, Gibson TJ, Davey NE. The switches. ELM resource: a compendium of conditional regulatory interaction interfaces. Sci Signal. 2013;6:rs7. doi: 10.1126/scisignal.2003345. [DOI] [PubMed] [Google Scholar]
- 73.Lienert F, Lohmueller JJ, Garg A, Silver PA. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat Rev Mol Cell Biol. 2014;15:95–107. doi: 10.1038/nrm3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Chau AH, Walter JM, Gerardin J, Tang C, Lim WA. Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell. 2012;151:320–332. doi: 10.1016/j.cell.2012.08.040. This article illustrates how modules derived from naturally occurring posttranslational networks can be extracted and used to build cells with customized signaling behaviors of increasing complexity. [DOI] [PMC free article] [PubMed] [Google Scholar]