Abstract
Calcific aortic stenosis (AS), the most prevalent heart valve disorder in developed countries,, is characterized by progressive fibro-calcific remodelling and thickening of the aortic valve leaflets that evolve over years to cause severe obstruction to cardiac outflow. In developed countries, AS is the second-most frequent cardiovascular disease after coronary artery disease and systemic arterial hypertension with a prevalence of 0.4% in the general population and 1.7% in the population >65 years old. Congenital abnormality (bicuspid valve) and older age are powerful risk factors for calcific AS. Metabolic syndrome and an elevated plasma level of lipoprotein(a) have also been associated with increased risk of calcific AS. The pathobiology of calcific AS is complex and involves genetic factors, lipoprotein deposition and oxidation, chronic inflammation, osteoblastic transition of cardiac valve interstitial cells and active leaflet calcification Although no pharmacotherapy has proven to be effective in reducing the progression of AS, promising therapeutic targets include lipoprotein(a), the renin-angiotensin system, tumor necrosis factor ligand superfamily member 11 (also called receptor activator of NF-κB ligand (RANKL)) and ectonucleotidases. Currently, aortic valve replacement (AVR) remains the only effective treatment for severe AS. The diagnosis and staging of AS are based on the assessment of stenosis severity and left ventricular systolic function by Doppler echocardiography and the presence of symptoms. The introduction of transcatheter AVR in the past decade has been a transformative innovation for patients at high or extreme-high risk for surgical AVR and this new technology might extend to lower-risk patients in the near future.
Graphical abstract
Calcific aortic stenosis (AS) involves fibro-calcific remodeling of the aortic valve that causes restriction of blood flow. Pibarot and colleagues discuss the mechanisms, diagnosis and management of AS and highlight how the introduction of transcatheter-based valve replacement has transformed patient outcomes.
Introduction
Calcific aortic valve disease is, by far, the most prevalent form of aortic stenosis (AS) worldwide. In the developing world, AS may also be caused by rheumatic heart disease. Calcific aortic valve disease is characterized by slowly progressive fibro-calcific remodelling of the valve leaflets. In the first phase of the disease, termed aortic sclerosis, the valve becomes thickened and mildly calcified but these changes do not cause any obstruction to blood flow. Over the years, the disease evolves to severe valve calcification with impaired leaflet motion and vast blood flow obstruction, which are hallmarks of calcific AS (Table 1)1. In developed countries, AS is one of the third most common cardiovascular diseases after coronary artery disease and systemic arterial hypertension2. Over the past five decades, the management of calcific AS has changed dramatically. Doppler echocardiography has replaced cardiac catheterization as the method of choice for the diagnosis and follow-up of AS, and transcatheter valve therapy has emerged as an alternative to surgery for aortic valve replacement (AVR). However, no pharmacotherapy has proved to reduce either the progression of valve stenosis or the resulting adverse effects on left ventricular (LV) function and patient outcomes. Hence, surgical or transcatheter AVR are the only effective treatment options for severe AS3;4. Overall, this disease is directly responsible for approximately 85,000 AVRs and 15,000 deaths per year in North America2. In this Primer, we discuss the epidemiology, mechanisms, diagnosis and management of calcific AS and highlight how the introduction of transcatheter-based valve replacement has transformed patient outcomes.
Table 1.
Disease progression stages in calcific aortic stenosis
| Disease Stage | Sub-stage | Description | Management |
|---|---|---|---|
| At risk of AS | N/A |
|
|
| Mild or moderate AS | N/A |
|
|
| Severe AS | Asymptomatic severe AS with normal LV systolic function |
|
|
| Asymptomatic severe AS with LV systolic dysfunction |
|
|
|
| Symptomatic severe high-gradient AS |
|
|
|
| Symptomatic low-flow, low-gradient severe AS with preserved LVEF |
|
|
|
| Symptomatic low-flow, low-gradient severe AS with deduced LVEF |
|
|
Indication of AVR: Class I: AVR should be performed; Class IIa: AVR is reasonable; Class IIb: AVR may be considered.
See Table 2 for definitions. AS, aortic stenosis; AVR, aortic valve replacement; LV, left ventricular; LVEF, LV ejection fraction.
Epidemiology
Calcific AS is the consequence of progressive fibro-calcific remodelling occurring on an initially normal (tricuspid) aortic valve or a congenitally abnormal (bicuspid) aortic valve. Although the prevalence of bicuspid aortic valve is only 0.5–1% in children, it accounts for nearly half of aortic valves that are surgically removed due to calcific AS5. During their lifetime, most individuals with a bicuspid aortic valve develop some kind of aortic valve pathology, the most common being AS5–8. Furthermore, patients with bicuspid valve develop calcific AS 1 or 2 decades earlier than those with a tricuspid valve.
Aortic sclerosis, the preclinical phase of calcific aortic valve disease, is defined as focal areas of valve calcification and leaflet thickening without significant cardiac blood flow obstruction (aortic jet velocity of <2.0 m per sec)3. The prevalence of aortic sclerosis increases sharply with age. In developed countries, it is estimated to be 25% in those over 65 years old and almost 50% in those aged over 85 years9–11. According to a recent meta-analysis, the rate of progression to AS in individuals with aortic sclerosis is 1.8–1.9% of patients per year11. Therefore, the prevalence of calcific AS is much lower than that of aortic sclerosis, and has been estimated to be 0.4% in the general population and 1.7% in the population aged over 65 years12 in developed countries. There is a marked increase in the prevalence of calcific AS in those aged > 65, which has been reported by several population-based studies in the United States and Europe (Figure 1)9;13–15. For individuals aged ≥ 75 years, a pooled analysis of available epidemiologic data in developed countries produced an estimated severe AS prevalence of 3.4% (95% confidence interval of 1.1%–5.7%), with 75% of those with severe AS presenting with symptoms16. The incidence of calcific AS has been assessed in a longitudinal Norwegian study and was estimated to be 4.9 per 1000 people per year in a population that had a mean age of 60 years at inclusion13. The geographical distribution of calcific AS is heterogeneous and displays a clustering effect which is probably the consequence of genetic factors17.
Figure 1. The prevalence of aortic stenosis as a function of age.
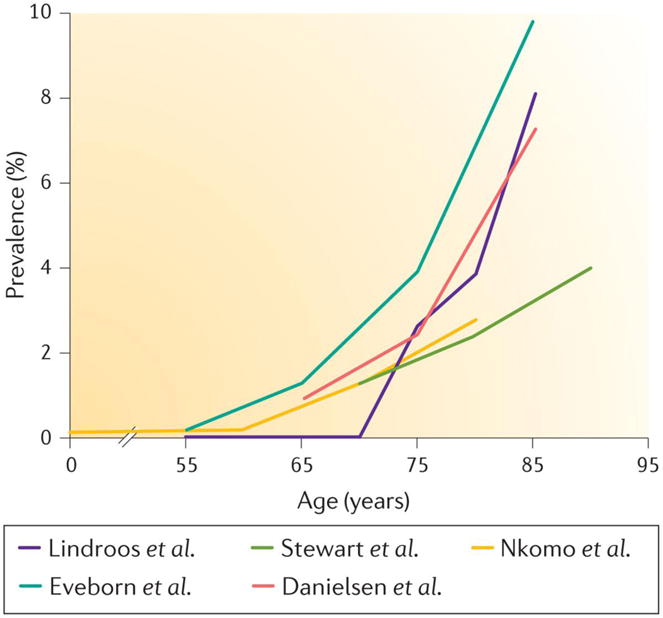
The prevalence of aortic stenosis (AS) according to age in the following population-based series from the USA or Europe: Lindroos et al. (Finland)14, in which AS was defined as an aortic valve area of < 1.2 cm2; Stewart et al. (Cardiovascular Health Study, USA)9, in which AS was defined as a peak aortic jet velocity of > 2.5 m per sec; Nkomo et al. (USA)12, in which AS was defined as an aortic valve area of < 1.5 cm2; Eveborn et al. (Tromsø Study, Norway)13, in which AS defined was as a mean gradient of ≥ 15 mmHg; Danielsen et al. (AGES-Reykjavik Study, Iceland)15, in which AS was defined as an indexed aortic valve area of ≤ 0.6 cm2 per m2.
Although mitral valve regurgitation has a higher prevalence than AS in population-based studies, AS has a more important clinical impact18. In the Euro Heart Survey, AS was more prevalent than mitral valve regurgitation in patients who were referred for in-hospital care and cardiac surgery18. Furthermore, calcific AS accounted for 34% of all native (non-prosthetic) valve diseases, as compared with 25% for mitral regurgitation, and 47% of patients operated for valvular disease, as compared with 14% for mitral regurgitation among patients operated for valvular disease18.
The burden of calcific AS in the community is expected to increase over the next decades owing to population aging and the lack of a prevention strategy aimed at reducing disease progression. Estimates based on current prevalence rates and demographic forecasts predict that the number of patients with calcific AS >70 or >75 years of age will increase twofold to threefold over the next 50 years in developed countries15;16;19.
The epidemiology of AS in developing countries and resource poor settings differs in some respects to that seen in developed countries, in part due to higher rates of rheumatic fever and rheumatic heart disease in poorer communities. Rheumatic heart disease is a chronic condition resulting from acute rheumatic fever, which in turn is caused by an untreated throat infection with group A Streptococcus. Both rheumatic fever and rheumatic heart disease may cause damage to the heart valves and can result in stenosis and regurgitation, in particular of the mitral and aortic valves. Valvular remodelling markedly differs between rheumatic heart disease and calcific AS. Fusion of aortic leaflets at commissures is one hallmark and distinctive feature of rheumatic heart disease. Rheumatic heart disease rarely affects the aortic valve alone (less than 10% in countries where rheumatic fever remains endemic) and most often involves the mitral valve. When the aortic valve is affected, the dysfunction is often mixed: aortic stenosis combined with some degree of aortic regurgitation20;21. The proportion of AS caused by calcific AS is expected to increase in industrially developing countries owing to the decreasing incidence of rheumatic fever. In addition, the overall burden of calcific AS is expected to increase owing to the increasing in life expectancy in these regions.
Mechanisms/pathophysiology
For a long time, calcific aortic valve disease was thought to be a ‘degenerative’ process caused by time-dependent wear-and-tear of the leaflets and passive calcium deposition. Now, there is compelling histopathologic and clinical data suggesting that calcific valve disease is, in fact, an active and multifaceted condition involving lipoprotein deposition, chronic inflammation, osteoblastic transition of valve interstitial cells and active leaflet calcification22,23.
Aortic valve anatomy and remodelling of the aortic valve
The aortic valve is typically composed of three leaflets that are named according to their location with respect to the coronary artery, specifically the left coronary, right coronary and non-coronary leaflets (Figure 2). Each leaflet has a trilaminar structure that determines the biomechanical properties of the aortic valve24. The outermost layers of the leaflet are formed by the fibrosa and ventricularis, which face the aorta and the LV outflow tract, respectively. The spongiosa, which has a high proteoglycan content, is located between the fibrosa and ventricularis (Figure 3). The fibrosa is rich in circumferentially oriented collagen type I and III fibers25, whereas in the ventricularis, radially oriented elastic fibers predominate. The ventricularis composition provides more compliance (the ability to expand under pressure) and allows the apposition of free edge regions of leaflets, thus preventing the backward flow of blood into the LV during diastole. The cellular population of these aortic valve layers includes valve interstitial cells (VICs), smooth muscle cells (SMCs) (<5% of the population) and endothelial cells. The endothelial cells cover the aortic and ventricular surface and therefore provide an interface between the blood and the aortic valve26. VICs is the predominant population of cells in the aortic valve, whereas SMCs reside at the base of the ventricularis27.
Figure 2. Comparison of tricuspid and bicuspid aortic valve structures.
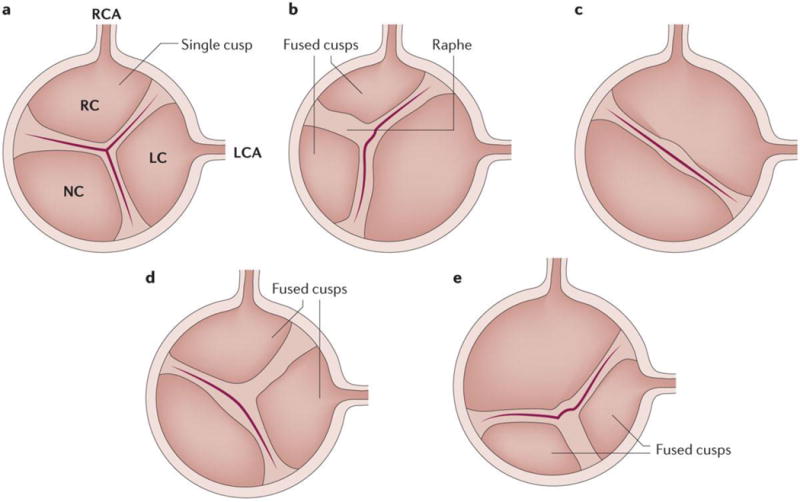
Schematic representation of A) a normal — tricuspid — aortic valve with the 3 cusps, B) a bicuspid valve with right-left coronary cusp fusion and one raphe (the line of union between the fused cups), C) a bicuspid valve with fusion of the right-left coronary cusps and no raphe, D) a bicuspid valve with right-non coronary cusp fusion and one raphe and E) a bicuspid valve with fusion of the left-non coronary cups and one raphe. LC, left coronary; LCA, left coronary artery; NC, non-coronary; RC, right coronary; RCA, right coronary artery.
Figure 3. Macroscopic and histopathologic appearance of normal and abnormal aortic valves.
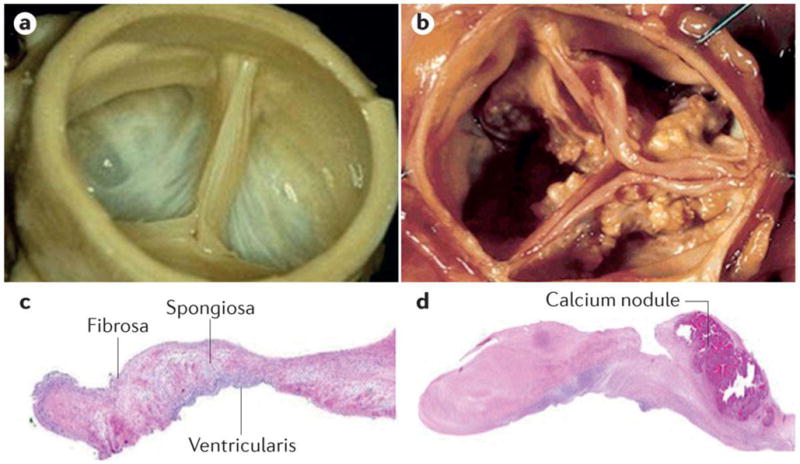
Photographs of A) a normal aortic valve and B) an aortic valve with severe calcific aortic stenosis (AS). C) Histopathologic section of normal aortic valve with hematoxylin staining showing the trilaminar structure of the valve from top to bottom. D) Histopathologic section of a valve with severe calcific AS with hematoxylin staining showing the presence of fibrotic material (pink) and calcified nodule. The tissue is thickened by the excess of fibrotic material and the calcified nodule, located in the fibrosa, contributes to alter the normal architecture of the leaflet.
Inspection of surgically explanted valves with calcific AS reveals two features, fibrosis and calcification (Figure 3), which substantially alter the biomechanical properties of the aortic valve leaflets. A small proportion (10–15%) of calcific AS valves show advanced osteogenic metaplasia with the presence of osteoblast-like cells, chondrocytes and bone marrow28. Calcified valves often contain dense inflammatory infiltrates, which consist mostly of macrophages29;30. Mineralization starts in the fibrosa layer and it is often in the vicinity of lipid deposits. Together, these observations suggest that the fibro-calcific process in the aortic valve is a response to injury, which might be triggered by lipid-derived species and inflammation (Figure 4)31.
Figure 4. Pathogenesis of calcific aortic stenosis.
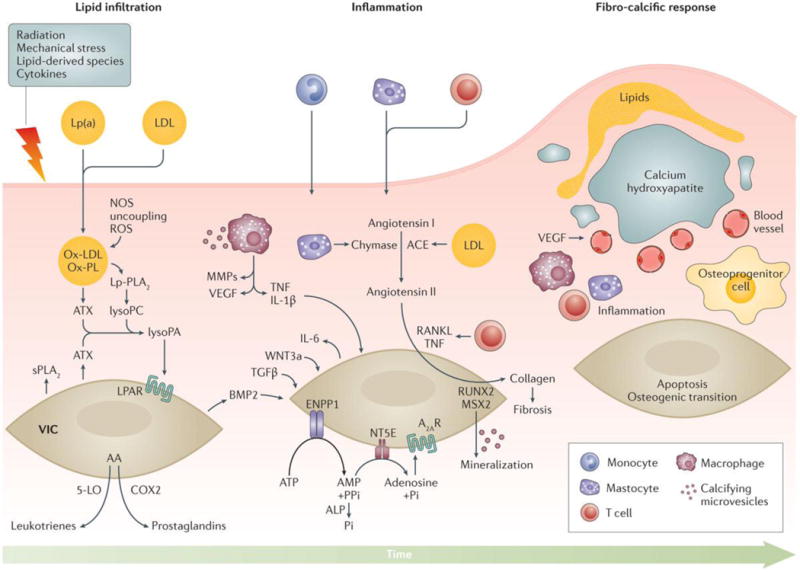
Endothelial damage allows infiltration of lipids, specifically low density lipoprotein (LDL) and lipoprotein(a) (Lp(a)) into the fibrosa and triggers the recruitment of inflammatory cells into the aortic valve. Endothelial injury can be triggered by several factors including lipid-derived species, cytokines, mechanical stress and radiation injury. The production of reactive oxygen species (ROS) is promoted by the uncoupling of nitric oxide synthase (NOS), which increases the oxidation of lipids and further intensifies the secretion of cytokines. Enzymes transported in the aortic valve by lipoproteins (LDL and LP(a)) such as Lp-PLA2 and autotaxin (ATX) produce lysophospholipid derivatives. ATX, which is also secreted by valve interstitial cells (VICs), transforms lysophosphatidylcholine (LysoPC) into lysophosphatidic acid (LysoPA). Several factors including LysoPA, the receptor activator of nuclear factor kappa-B ligand (RANKL) and Wnt3a promote the osteogenic transition of VICs. Arachidonic acid (AA) generated by cytosolic PLA2 promotes the production of eicosanoids (prostaglandins and leukotrienes) through the cyclooxygenase 2 (COX2) and 5-lipoxygenase (5-LO) pathways respectively. In turn, eicosanoids promote inflammation and mineralization. Chymase and angiotensin converting enzyme (ACE) promote the production of angiotensin II, which increases the synthesis and secretion of collagen by VICs. Owing to increased production of matrix metalloproteinases (MMPs) and decreased synthesis of tissue inhibitors of metalloproteinases (TIMPs), disorganized fibrous tissue accumulates within the aortic valve. Microcalcification begins early in the disease, driven by microvesicles secreted by VICs and macrophages. In addition, overexpression of ecto-nucleotidases (NPP1, 5′-NT, ALP) promotes both apoptosis and osteogenic-mediated mineralization. Bone morphogenetic protein 2 (BMP2) entrains osteogenic transdifferentiation, which is associated with the expression of bone-related transcription factors (RUNX2 and MSX2). Osteoblast-like cells subsequently coordinate calcification of the aortic valve as part of a highly regulated process analogous to skeletal bone formation. Deposition of mineralized matrix is accompanied by fibrosis and neovascularization, which is abetted by vascular endothelial growth factor (VEGF). In turn, neovascularization increases the recruitment of inflammatory cells and bone marrow-derived osteoprogenitor cells.
IL-1β, interleukin-1–β; Lp(a), lipoprotein (a); LDL, low-density lipoprotein; OxPL, oxidized phospholipid; TGF-β transforming growth factor beta; NPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1; 5′-NT, 5′ nucleotidase; ALP, alkaline phosphatase.
In addition, excess production and disorganization of collagen fibers is an important feature of calcific AS. Fibrosis increases the stiffness of the aortic valve and might play a considerable part in promoting mineralization. To this effect, the collagen produced by VICs serves as a scaffold on which the nucleation of calcium and phosphorus can start32. In vitro, serum-induced mineralization of collagen is increased by a population of VICs harbouring a pro-calcifying phenotype with elevated alkaline phosphatase (ALP) expression33;34. In addition, the increased production of several components of the extracellular matrix, including periostin, tenascin (also called tenascin-c) and proteoglycans contributes to the remodelling of the aortic valve during AS35;36. The exact role of non-collagenous proteins in the pathophysiology of AS is still largely unknown, but growing evidence indicates that complex interactions between extracellular matrix proteins and cells provide crucial signals during normal reparative and pathological processes in the aortic valve37.
Lipids
Lipid infiltration and oxidation
Increasing evidence suggests that infiltration of the aortic valve by lipoproteins has a central role in promoting inflammation, which precedes the pathologic mineralization that is characteristic of calcific AS38. Therefore, the retention of lipids promotes a chronic low-grade inflammatory process, which, in turn, might induce an osteogenic program in aortic valves. In this regard, histological studies have demonstrated that several apolipoproteins (apos) such as apoB, apoE, apoA1 and apo(a) are present in surgically removed stenotic aortic valves39.
Oxidative stress has also been implicated in calcific AS. For instance, immunostaining has demonstrated that apoB co-localizes with oxidized low-density lipoproteins (Ox-LDLs) in valves from patients with calcific AS,40;41 and that there is an association between the level of Ox-LDL and the degree of inflammation and fibro-calcific remodelling in surgically removed AS valves40;42. Oxidative stress is increased in AS valves and is related, at least in part, to the uncoupling of the nitric oxide synthase (NOS) pathway43. Also, the expression NAD(P)H oxidase is increased in surgically explanted calcific AS valves and contributes to the production of reactive oxygen species (ROS)44. Therefore, the production of peroxide and superoxide anions, in the vicinity of calcified areas might participate in the production of oxidatively-modified lipid species with osteogenic properties43. Work conducted in vitro has shown that Ox-LDL and several oxidized phospholipid (Ox-PL) species promote the calcification of isolated vascular cells45. In vivo, circulating Ox-PLs are mostly carried by the lipoprotein(a) (Lp(a)) fraction,46 a LDL-like particle in which the apoB protein is linked by a disulfide bridge to apo(a)47. Recent studies that used a Mendelian randomization design showed that the gene encoding apo(a) (LPA) is potentially causally related to calcific aortic valve disease48–50. In addition, Capoulade and colleagues showed that circulating Lp(a) and Ox-PL levels were independently associated with faster progression of calcific AS51. Together, these studies suggest that high circulating levels of Lp(a) might promote the accumulation of Ox-PLs in the aortic valve, which could, in turn, trigger an osteogenic response (Figure 4).
Lipid retention and enzymatically-modified lipid species
Proteoglycans such as biglycan and decorin are overexpressed in aortic valves during calcific AS and might actively participate in lipid retention and modification (Figure 4)52–54. Moreover, transforming growth factor β-1 (TGF-β1), which is activated in calcific AS, has been shown to promote the elongation of glycosaminoglycan (GAG) chains55. In turn, GAG chain elongation increases the interaction between proteoglycans and lipoproteins55. The accumulation and retention of lipoproteins in the aortic valve is a crucial event as lipids might be used by different enzymes to produce bioactive lipid-derived compounds, such as lysophospholipids56.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) levels are increased in stenotic aortic valves and this increase is associated with fibro-calcific remodelling (Figure 4)57;58. Circulating levels of Lp-PLA2 are also positively and independently related to the progression of calcific AS59. Lp-PLA2 is transported by apoB-containing lipoproteins and is enriched in small, dense LDL and Lp(a)60. Lp-PLA2 transforms Ox-PLs into lysophosphatidylcholine (LysoPC), which promotes the loss of mitochondrial membrane potential and apoptosis of VICs57;61. In addition, Bouchareb and colleagues recently showed that ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (also called autotaxin), a lysophospholipase D, is likely transported into the aortic valve by Lp(a) and is also secreted by VICs in response to diverse stimuli including tumor necrosis factor alpha (TNF-α)62. Autotaxin transforms LysoPC into lysophosphatidic acid (lysoPA). Of interest, in vitro knockdown of autotaxin prevented the mineralization of VICs induced by LysoPC, suggesting that LysoPA is probably the mediator that promotes osteogenic programing in VICs. To this effect, in a murine model, the administration of LysoPA increased the deposition of hydroxyapatite (a form of calcium apatite) in the aortic valve and accelerated the development of calcific AS. Therefore, it is possible that autotaxin and lysophosphatidic acid are key factors that explain the link between Lp(a) and AS63.
In addition to lysophospholipids, the arachidonic acid pathway, which produces leukotrienes and prostaglandins, has been shown to also play a considerable part in the mineralization of the aortic valve (Figure 4)64. For instance, the expression of 5-lipoxygenase, which is required for leukotriene synthesis, is increased in aortic valves during calcific AS and leukotriene C4 promotes the expression of bone morphogenetic proteins 2 and 6 (BMP2 and BMP6) as well as the mineralization of VICs in culture64. A recent study has shown that prostaglandin G/H synthase 2 (also called cyclooxygenase 2 (COX2)) is expressed by VICs isolated from AS valves65. In support of a role for COX2 in calcific AS, a loss of function of Cox-2 in Klotho deficient mice, which develop calcification of the aortic valve amongst other features, reduced the mineralization of the aortic valve65. Taken together, these findings suggest that several processes promote the retention of lipids in the aortic valve and produce bioactive lipid species, which promote inflammation and mineralization of aortic valve leaflets.
Inflammation
Tissue remodelling and neovascularization
Fibro-calcific remodelling and inflammation of the aortic valve are intricately linked processes that have several important cross-talks. Inflammatory infiltrate in mineralized aortic valves removed surgically is composed of macrophages, mast cells, CD4+ T cells and CD8+ T cells66. Several oxidized lipid species might activate the innate immune response through toll-like receptors (TLRs) and the nuclear factor-kappa B (NF-κB) pathway. TLRs are also expressed by VICs (TLR2 and TLR4) and may promote an osteogenic phenotype in isolated VICs67;68. On the other hand, the role of adaptive immunity in calcific AS is still largely unknown, but studies have shown that a subset of memory T cells is activated during AS and that clonal expansion of a T cell receptor repertoire is present in surgically removed calcific AS valves69. These data suggest that both innate and adaptive immune responses are likely involved in the pathobiology of calcific AS.
A histopathologic study performed on 285 aortic valves from patients with calcific AS revealed that the presence of dense, chronic inflammatory infiltrates was related to the remodelling score of the leaflets and to the presence of neovascularization29. Although the exact role of neovascularization in driving AS is still largely unknown, it is possible that it is involved in the recruitment of inflammatory and osteoprogenitor cells (Figure 4). In support of this hypothesis, mice deficient of chondromodulin-1 (encoded by Lect1), which is an anti-angiogenic factor, have thickened and mineralized aortic valve leaflets70. Aged Lect1−/− mice develop capillary-like structures in their aortic valve leaflets, which is accompanied by the presence inflammatory cells and lipid deposits70. In human stenotic aortic valves, CD34+ endothelial progenitor cells, which participate in new vessel formation, are observed in clusters in close proximity to SPARC (also called osteonectin) and MMP971. SPARC is a matricellular protein expressed by VICs during calcification that is cleaved by MMPs into peptides with angiogenic activity71. Several MMPs, including MMP2, MMP9 and MMP12, are overexpressed in human calcific AS valve tissue72. As such, angiogenic SPARC peptides might promote neovascularization by CD34+ endothelial progenitor cells and cause inflammation as well as remodelling of the aortic valve. In addition, cathepsins K, V and S, which are proteases that can degrade extracellular matrix proteins, are expressed and activated during AS73, and in ApoE−/− mice, cathepsin S promoted elastolysis and mineralization of the aortic valve74. Therefore, inflammation and neovascularization are linked to remodelling and mineralization of the aortic valve.
Cytokines
TNFα is secreted by monocytes and macrophages and activates TNF receptor superfamily member 1A (TNF-R1). TNF-R1 activation results in activation of NF-κB and its downstream targets including interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) (Figure 4)75–78. These cytokines promote the mineralization of VICs and activate an osteogenic program, which may involve the expression of homeobox protein MSX-2 (MSX-2)75–78. To this effect, treatment of adventitial fibroblasts with TNFα increased the expression of MSX-2 through the production of ROS79. Mice deficient of IL-1 receptor antagonist protein (encoded by IL-1rn) have higher plasma levels of TNFα than wildtype mice and develop a thickening of the aortic valve78. However, double knockout IL-1rn−/− Tnf−/− mice are protected and do not develop a thickening of the aortic valve, suggesting that TNFα plays important part in promoting the remodelling of the aortic valve. In humans, the expression of TNF ligand superfamily member 10 (also called TNF-related apoptosis inducing ligand(TRAIL)), a member of the TNF-related cytokines, is increased in calcific AS valves and promotes the mineralization of VIC cultures through the death receptor 480.
IL-6, another cytokine with pleiotropic activities, has been implicated in calcific AS. IL-6 is increased in human calcified stenotic valves and is secreted in large amounts by cultured human VICs when they are treated with an osteogenic medium81. In addition, knockdown of IL6 substantially reduces the expression of BMP2 and the mineralization of VIC cultures81. Moreover, though not yet investigated in VICs, IL-6 induces the expression of tumor necrosis factor ligand superfamily member 11 (RANKL) in bone cells, which activates its cognate receptor RANK82. Overexpression of RANKL during calcific AS might have an important role in the pathogenesis, as secreted RANKL activates VICs to produce extracellular matrix (Figure 4)83. In support of this role, the administration of osteoprotegerin (OPG), a decoy receptor for/RANKL, to low-density lipoprotein receptor knockout (Ldlr−/−) mice decreased calcification and the expression of osteogenic genes in aortic valves84. Of interest, in bone, RANKL is expressed by osteoblasts and promotes the resorption of mineral by osteoclasts. Therefore, it is possible that a dysregulation of tumor necrosis factor ligand superfamily member 11 (RANKL)/RANK/OPG explains the link between osteoporosis and vascular and valvular calcification66. In this regard, several epidemiological studies have underlined an association between osteoporosis and vascular/valvular calcification66;85–87.
Angiotensin II
Angiotensin converting enzyme (ACE) and chymase are overexpressed in calcific AS valves and are involved in the production of angiotensin II (Figure 4)88;89. Chymase is secreted by mast cells present in calcific AS valve tissues and converts angiotensin I into angiotensin II88. In addition, patients with calcific AS have elevated blood plasma levels of angiotensin II, which correlates with the valvular expression of TNFα and IL-690. Angiotensin II is a potent activator of the NF-κB pathway and promotes a strong fibrotic response in isolated cells. In mice, the administration of angiotensin II promotes fibrosis of the aortic valve91. Moreover, in a rabbit model of hypercholesterolaemia, the administration of olmesartan, an angiotensin receptor blocker (ARB), prevents the thickening of the aortic valve that normally develops in these rabbits92. Retrospective non-randomized studies have reported that administration of ARBs, but not ACE inhibitors, are associated with less fibro-calcific remodelling of aortic valve leaflets and slower progression of valve stenosis93;94. Therefore, it is possible that a substantial amount of angiotensin II is produced by chymase in the aortic valve, the effect of which is blocked downstream by ARBs but not by ACE inhibitors.
Mineralization
Osteogenic differentiation
The endothelium that covers the healthy aortic valve expresses several anti-osteogenic genes in a spatially distributed manner95. The endothelium that covers the aortic side of leaflets shows less expression of anti-osteogenic genes compared with the endothelium on the ventricular side. For instance, aortic side endothelium expressed lower levels of chordin and OPG, respectively negative regulators of BMP2/BMP4 and RANKL. A potential explanation for this difference in expression could be shear stress. Oscillatory shear stress has been shown to modulate the expression of ~1,000 genes and ~30 microRNAs in human primary cultures of aortic valve endothelial cells96. For instance, the expression of miRNA-187, which promotes cell growth and proliferation, was increased when these cultures were exposed to oscillatory shear. Endothelial cells covering the fibrosa (facing the aorta) are exposed to low oscillatory shear stress compared to cells facing the LV. Though the functional relevance of these findings remains to be fully investigated shear stress might explain, at least in part, why the fibro-calcific process predominantly occurs in the fibrosa layer.
In human stenotic aortic valves, several osteogenic genes are overexpressed72, whereas others display altered function that can affect their role in signalling pathways. For instance, Garg and colleagues showed that mutations in NOTCH1 were associated with bicuspid aortic valves, which are prone to developing calcific AS97. The Notch family of receptors are involved in cell fate determination. The activation of NOTCH1 in VICs leads to the formation of the notch intracellular domain (NICD), which associates with the recombining binding protein suppressor of hairless (encoded by RBPJ) in the nucleus where it promotes the expression of the hairy repressors. The hairy repressors prevent the expression of the osteogenic factors in VICs — BMP2 and runt-related transcription factor 2 (RUNX2)98 — suggesting that VICs are driven towards an osteogenic differentiation pathway in calcific AS. To this effect, heterozygous Notch1+/− and Rbpj+/− mice develop mineralization of the aortic valve99. Additionally, the NICD interferes in the nucleus with catenin β-1 (β-catenin), a downstream effector of the Wnt pathway, which is also a key driver of osteogenic differentiation100. A recent study showed in endothelial cells that NOTCH1 regulates the expression of more than a 1,000 genes involved in inflammation and osteogenesis by altering the epigenetic signature at enhancer regions101. Moreover, in human stenotic aortic valves, WNT3a, an agonist of the Wnt pathway, is overexpressed102. The activation of a coreceptor formed by low-density lipoprotein receptor-related protein 5 and G-protein coupled Frizzled receptors, which are expressed by VICs, leads to the stabilization of β-catenin and the osteogenic differentiation (Figure 4)102. In vascular cells, BMP2 promotes the expression of MSX2, a positive regulator of the Wnt pathway103. Several factors, including inflammatory cytokines and oxidized lipid derivatives have been shown to induce the expression of BMP2 in several cell types including VICs104.
Recent studies have also highlighted that the expression of several microRNAs is dysregulated in AS and this might affect the osteogenic programming of VICs. In this regard, miRNA-30b, which is decreased in mineralized aortic valves, is a negative regulator of RUNX2.105 Hence, a dysfunction of Notch and Wnt pathways as well as a dysregulation of microRNAs contribute to increased pro-osteogenic signals in VICs.
Mineral deposition
Osteogenic reprograming of VICs entrains a series of events that promote the deposition of a calcified matrix. The mechanism(s) whereby VICs mineralize the extracellular matrix is still poorly defined but recent observational and experimental work suggests that cells secrete small vesicles rich in ectonucleotidases that promote the nucleation of calcium and phosphorus106;107. A build-up of phosphate in calcifying vesicles, which also contain the annexinV-S100A9 complex that binds calcium, promote the nucleation of mineral108. Classically, secretion of calcifying vesicles has been attributed to cells that transdifferentiate into osteoblast-like cells, in which case calcification proceeds with the deposition of well-organized bone-like mineral matrix (hydroxyapatite).109. However, programmed cell death leads to the production of apoptotic bodies with similar properties to calcifying vesicles. Apoptosis in VICs is promoted by different stimuli including cytokines, ROS and altered purinergic signalling. Apoptotic bodies serve as nidi for dystrophic calcification, a form of mineralization that consists of amorphous deposits of calcium and phosphorus crystals. In human aortic valves, it is likely that both osteogenic and apoptotic processes contribute to the mineralization process and rely, at least in part, on ectonucleotidases110. In support of this involvement, several ectonucleotidases such as ALP, ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (E-NPP 1) and 5′-nucleotidase (5′-NT, also called CD73) are overexpressed in human stenotic aortic valves (Figure 4)110–112. These membrane-bound enzymes use nucleotides and nucleosides secreted by cells as substrates and produce phosphate-derived products that promote mineralization112. For instance, E-NPP 1 hydrolyzes ATP into AMP and pyrophosphate (PPi), a strong inhibitor of mineralization. On the other hand, ALP has a broad spectrum of substrates including the mineralization inhibitor PPi from which it produces phosphate with strong pro-mineralizing activity. Moreover, the over activity of E-NPP 1 and 5′-NT in human stenotic aortic valves depletes extracellular ATP and produces adenosine111. A decrease in the level of extracellular ATP also diminishes purinergic signalling through the P2Y purinoreceptor 2 (P2Y2). In VICs, P2Y2 prevents the mineralization of cells by interfering with apoptosis and also by promoting the activation of carbonic anhydrase 12 (CA12).110;113 CA12 in VICs is normally expressed at the cell membrane following activation of P2Y2 and promotes the acidification of the extracellular space leading to resorption of mineral deposits113. As such, purinergic signalling, which is under the control of ectonucleotidases, plays a central part in controlling the mineralization of the aortic valve.
In summary, studies conducted in the past several years have shown that oxidation and infiltration of the aortic valve by lipids generate several bioactive lipid-species that trigger inflammation of the aortic valve. The activation of several pathways with multiple points of crosstalk disrupts the normal biology of the aortic valve and promotes fibro-calcific remodelling.
Pathophysiology of LV dysfunction
The symptoms in AS are essentially due to an imbalance between the increase in LV haemodynamic load caused by valvular obstruction, on the one hand, and the capacity of the LV to overcome this increase in load both at rest and during exercise, on the other hand. AS results in increased LV systolic pressure that leads to hypertrophy of the cardiomyocytes and interstitial fibrosis (Figure 5). The mechanical signal generated by increased LV systolic pressure initiates a cascade of biological events, including re-expression of immature fetal genes, which lead to coordinated cardiac growth in patients with AS114. This increase in cardiac mass is due to the hypertrophy of existing myocytes rather than hyperplasia, because cardiomyocytes become terminally differentiated soon after birth. The concurrent addition of sarcomeres (force-generating units) causes an increase in myocyte width, which in turn increases wall thickness and therefore contributes to normalize LV wall stress and maintain LV ejection performance despite elevated systolic pressure. To support the increased biomechanical load, the myocyte growth must be accompanied by coordinated increases in the surrounding architecture of connective tissue as well as the capillary and nerve networks114. This ‘reactive’ interstitial fibrosis that results from the increase in collagen synthesis by myofibroblasts in response to pressure overload has a diffuse distribution within the interstitium and might be, at least in part, reversible following AVR115.
Figure 5. Maladaptive remodelling and impaired function of the left ventricle in response to pressure overload from AS.
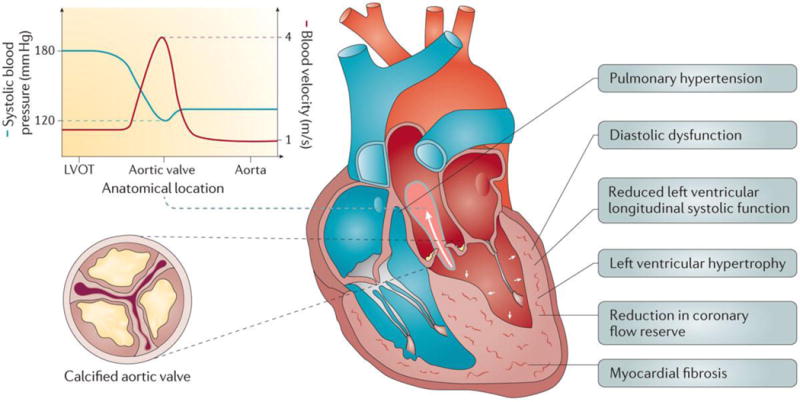
The narrowing of the aortic valve orifice causes an acceleration of the blood flow velocity with a concomitant decrease in systolic blood pressure between the left ventricular (LV) outflow tract (LVOT) and the aorta. The increased LV pressure imposed by AS results in LV hypertrophy (augmentation of the LV myocardial mass), reduced coronary flow reserve, myocardial fibrosis, diastolic dysfunction and decreased longitudinal systolic shortening, although the ejection fraction remains normal in most patients. Left atrial enlargement is common owing to elevated LV filling pressures. The latter often leads to secondary pulmonary hypertension and right ventricular dysfunction in the more advanced stages of the disease.
The pattern of the LV adaptive response to pressure overload in AS is highly heterogeneous and includes concentric remodelling, concentric hypertrophy and eccentric hypertrophy (Figure 6) The pattern and magnitude of LV hypertrophic remodelling is influenced not only by AS severity but also by several other factors including age, sex, genetic factors, metabolic factors and the coexistence of coronary artery disease or hypertension116–119. For the same degree of AS, women tend to predominantly develop concentric remodelling/hypertrophy, whereas men are more prone to developing eccentric hypertrophy116. In patients with calcific AS, LV concentric remodelling or hypertrophy has been linked to worse myocardial function and increased risk of cardiac events and mortality compared to patients with normal LV geometry or with LV eccentric hypertrophy120–122. Obesity, metabolic syndrome and diabetes also predispose to the development of more concentric hypertrophy in the presence of AS117;118.
Figure 6. Patterns of left ventricular remodelling.
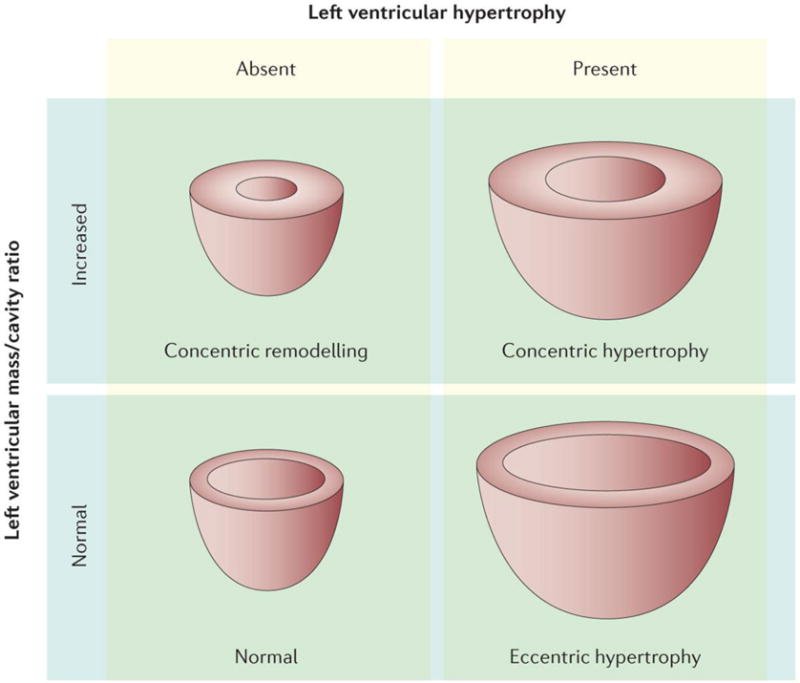
Four left ventricular (LV) remodelling patterns can be defined according to the left ventricular mass and the ratio of the LV mass to the LV cavity size: Normal pattern: both LV mass and mass/cavity ratio are normal; Concentric remodelling: the LV mass is normal but the mass/cavity ratio is increased (thick LV walls with small cavity); Concentric hypertrophy: both LV mass and mass/cavity ratio are increased; Eccentric remodelling: LV mass is increased but the mass/cavity ratio is normal (thickness of LV walls is normal or slightly increased and the LV cavity is enlarged). Reproduced with permission from267
The LV hypertrophy, leading to a reduced density of coronary arteriolar vessels, and increased LV transmural pressures, leading to increased coronary vascular resistance, result in the reduction of coronary flow reserve in patients with AS123;124 The reduction of coronary flow reserve limits the ability of coronary circulation to increase flow to match myocardial oxygen demand, especially during exercise, and it is therefore a key factor in the development of myocardial ischaemia and the occurrence of symptoms. Repetitive myocardial ischaemia related to the exhaustion of coronary flow reserve leads to apoptosis of myocytes and development of ‘replacement’ myocardial fibrosis. This type of fibrosis occurs predominantly in the subendocardial and mid-wall layers of the LV wall and is generally not reversible following relief of LV pressure overload by AVR. The impairment of coronary flow reserve might also explain why patients with severe AS can present with angina symptoms despite having angiographically normal coronary arteries and why these symptoms might regress immediately after AVR125.
LV diastolic dysfunction occurs early in the disease course and worsens with progression of stenosis severity and myocardial fibrosis (Figure 5). In the more advanced stages of the disease, the increased LV filling pressures lead to secondary pulmonary hypertension and dyspnoea symptoms126;127. The global LV systolic function, which is measured using the LV ejection fraction (LVEF), and cardiac output are generally well preserved even in the presence of severe AS because the increase in LV wall thickness allows wall stress to remain relatively normal. Reduced LVEF or cardiac output occurs only in end-stage disease and is usually preceded by clinical symptoms. However, a large proportion of patients with preserved LVEF have subtle LV systolic dysfunction that is characterized by impaired LV longitudinal function with relatively well preserved radial and circumferential function (Box 1) The LV myocardial wall is composed of 3 layers from the inside to the outside of the left ventricle: the subendocardial layer that surrounds the LV cavity, the mid-wall layer, and the subepicardial layer. In pressure overload cardiomyopathies, there is an early and selective alteration of the shortening of myocardial fibers within the subendocardial layer where ischaemia and fibrosis are generally more pronounced (Figure 5)128–130. The fibers in this layer are oriented longitudinally (compared with circumferentially in the mid-wall layer), which explains the selective alteration of the LV longitudinal function in these patients. Hence, a considerable proportion of patients with AS may have subclinical LV systolic dysfunction despite preserved LVEF and the absence of symptoms.
Box 1. Key measurements and tools used for AS assessment.
Aortic valve area (AVA): surface of the aortic valve orifice. It can be measured by Doppler echocardiography, left heart catheterization, or cardiac magnetic resonance.
Aortic valve calcium density: aortic valve calcium score measured by computed tomography divided by the cross-section area of the aortic annulus measured by echocardiography or computed tomography. It is expressed in Agatston unit per cm2.
Carotid upstroke: The pulse pressure of the carotid artery that can be assessed at the level of the neck is characterized by a smooth, relatively rapid upstroke and a smooth, more gradual downstroke. In patients with severe aortic stenosis, the carotid upstroke is delayed.
Circumferential function: circumferential contraction of the LV wall that is in large part driven by the myocytes located in the mid portion of the LV wall.
Class of recommendation for the procedure (aortic valve replacement in the case of AS): Class I: the benefit of the procedure largely outweigh the risk and the procedure should be performed; Class IIa: it is reasonable to perform the procedure; Class IIb: the procedure may be considered; Class III: the procedure is not recommended because it is not useful and may be harmful.
Coronary flow reserve: the maximum increase in blood flow through the coronary arteries above the normal resting flow. The coronary flow reserve can be measured by cardiac catheterization, Doppler-echocardiography or positron emission tomography. The normal coronary flow reserve is 3 to 4. In patients with AS the coronary flow reserve is reduced. When the ratio is 1, the coronary flow reserve is exhausted.
Dobutamine stress echocardiography: echocardiography performed during intravenous infusion of dobutamine, which increases cardiac contractility and flow across the aortic valve.
Mean transvalvular gradient (mean gradient): average value of the pressure loss (or gradient) across the aortic valve. This corresponds to the difference between the pressure in the LV cavity versus that in the aorta. The mean gradient can be measured by Doppler echocardiography of by left heart catheterization.
Left ventricular afterload: pressure in the wall of the left ventricle during ejection
Left ventricular ejection fraction (LVEF): measurement of how much blood is being pumped out of the left ventricle of the heart. It is calculated as the percent decrease in the volume of the LV cavity. It can be measured by echocardiography, angiography, or cardiac magnetic resonance.
Left ventricular longitudinal function: longitudinal (i.e. long-axis direction) contraction of the LV wall that is in large part driven by the myocytes located in the subendocardial layer of the LV wall.
Longitudinal strain: percent shortening of the LV wall in the longitudinal axis during systole. The longitudinal strain is measured by speckle tracking echocardiography.
Peak aortic jet velocity: peak value of the blood flow velocity across the aortic valve. The blood velocity is measured by continuous-wave Doppler.
Radial function: longitudinal (i.e. short-axis direction) contraction of the LV wall that is in large part driven by the myocytes located in the mid-wall layer of the LV wall.
Stress AVA: AVA measured by Doppler echocardiography during dobutamine or exercise stress.
Stress mean gradient: mean gradient measured by Doppler echocardiography during dobutamine or exercise stress.
Stroke volume index: stroke volume (i.e. volume of blood ejected by the heart during systole) indexed to (divided by) the patient’s body surface area.
Transvalvular velocity: blood flow velocity across the aortic valve.
Diagnosis, screening and prevention
Risk factors and prevention
Although some clinical and genetic risk factors have been associated with the onset and progression of calcific AS, no strategy has been yet proven to be efficient for primary or secondary prevention of this disease. Calcific AS shares several risk factors with coronary artery disease but it also presents some important distinctive features.
Clinical risk factors
Congenital leaflet abnormality and older age are both powerful risk factors for developing calcific AS. For instance, the lifetime risk of AVR is around 50% in individuals with a bicuspid valve. Bicuspid aortic valves have two functional leaflets often of unequal size. This abnormality results from incomplete separation of commissures during embryonic development8. Although leaflet orientation varies among patients, the most common form consists of a fusion of the right and left coronary leaflets (~60% of patients) followed by fusion between the right and non-coronary leaflets (~35% of patients) and then fusion between left and non-coronary cusp (~5% of patients) (Figure 2)131. Bicuspid aortic valve is associated with an increased risk of aortopathy, in which genetic, haemodynamic and mechanical factors might participate in the mineralization of aortic valve132. In both individuals with a bicuspid valve and those with a tricuspid valve, age is a powerful risk factor for AS9;133. The other clinical risk factors associated with AS are similar to those associated with atherosclerosis and include male sex, smoking, hypertension, hypercholesterolaemia, obesity, metabolic syndrome, diabetes and elevated Lp(a)9;134,48;135;136.
In patients with AS, the rate of stenosis progression over time varies substantially from one patient to another. The clinical factors associated with faster stenosis progression include older age, female sex, severity of the stenosis and degree of aortic valve calcification at diagnosis, smoking, hypertension, obesity, metabolic syndrome, secondary hyperparathyroidism, renal failure elevated circulating levels of Lp(a), and increased activity of Lp-PLA2 (also called lipoprotein–associated phospholipase A2)51;59;94;137–142. In particular, the presence of elevated plasma Lp(a) (>50 mg per dL; the upper normal limit is <30 mg per dL)) is associated with a twofold faster stenosis progression51.
Additionally, hypertension, and particularly systolic hypertension, is highly prevalent in these patients, affecting 30–70% of those with AS94;143;144. Recent studies suggest that hypertension accelerates the progression of AS, potentially owing to increased mechanical stress on the valve leaflets and activation of renin-angiotensin system (as discussed above)94. Moreover, hypertension further increases the LV afterload (Box 1) that is already elevated in patients with AS and contributes to the risk of developing symptoms and adverse cardiac events94;144.
Genetic risk factors
Several studies suggest that a genetic component is involved in promoting calcific AS associated with bicuspid or tricuspid aortic valves6;17;48;145. However, despite the evidence of a strong inheritance pattern for some cases of bicuspid aortic valve with an incomplete penetrance, the genetic architecture of calcific AS is still poorly understood145. So far, variants of NOTCH1 and GATA binding protein 5 (GATA5) have been associated with bicuspid aortic valves in humans97;146;147. NOTCH1 mutations explain approximately 4% of sporadic cases of AS that occurs in the context of a bicuspid aortic valve148;149. As discussed above, some mutations in NOTCH1 that affect its function might promote aortic valve mineralization. Therefore, it is possible that gene variants that predispose individuals to developing a bicuspid aortic valve also promote valve mineralization later in life, thus further exacerbating the risk of developing calcific AS. Recently, a study has identified in a genome wide association study that variants located in RUNX2 and CACNA1C, which encodes for an osteogenic transcription factor and a voltage-dependent calcium channel subunit respectively, were associated with calcific AS and were found to upregulate their respective mRNA levels.150 Also, studies using a candidate gene approach have linked several gene variants with calcific AS. Although variants of VDR, APOE, APOB, IL10, NOTCH1 and ENPP1 have been found to be significantly associated with AS, these studies suffer from small sample size and require replication in larger series6.
A large study using a Mendelian randomization design identified the single nucleotide polymorphism (SNP) rs10455872 in the LPA gene as the only genome-wide significant SNP associated with the presence of aortic valve calcification and clinical calcific AS48. Subsequent studies have validated these findings and also reported an association between elevated Lp(a) plasma levels with the prevalence of calcific AS and the need for AVR in the general population49;50. The presence of the rs10455872 allele is associated with a 1.5–2.0-fold increase in the risk of incident calcific AS48–50. When considered in the light of the clinical and basic research findings on Lp(a) discussed above, Lp(a) lowering appears to be a promising novel target for the treatment of this disease, particularly to prevent disease progression. However, further studies are needed to evaluate the role of Lp(a) in AS in more detail.
A second study using a Mendelian randomization design reported a strong association between genetic predisposition to elevated LDL-cholesterol, as measured by weighted genetic risk scores, and the presence of aortic valve calcification and incident cases of calcificAS151. However, three randomized clinical trials (RCTs) failed to demonstrate any significant benefit of LDL lowering with statins on the progression of AS152–154. Therefore, it is possible that elevated LDL-cholesterol promotes the initiation of calcific aortic valve disease but has minimal or no effect on AS progression. Moreover, the protective effect of statin therapy in AS might be counterbalanced by its off-target effects including pro-osteogenic properties, worsening of insulin resistance and increased Lp(a) levels51;141. Whether other lipid-lowering strategies (for instance, PCSK9 inhibitors) would prevent or slow AS progression is unknown and this question needs to be addressed. In summary, no pharmacotherapy has proven to be effective in reducing the progression of AS.
Diagnosis
Diagnosis of AS is generally established using an echocardiographic exam, which provides a wealth of information regarding heart valve anatomy and blood flow parameters (Figure 7)155. The same techniques can be used for the diagnosis of calcific AS and rheumatic AS. In the vast majority of patients, the referral to echocardiography is motivated by the auscultation of a systolic murmur and/or the development of symptoms including dyspnoea, angina, syncope and dizziness. In some cases, AS is first recognized on echocardiography requested for other indications. Although most patients are diagnosed long before the onset of symptoms and are followed prospectively on a regular basis until AVR is indicated, a small proportion (5–10%) of patients are not diagnosed with AS until late in the disease course when they present with symptoms of heart failure156. The identification of the presence and stage of AS includes the assessment of the aortic valve anatomy and morphology, the haemodynamic severity of AS, the response of the LV to the pressure overload caused by AS, and the patient’s symptomatic status3;4. On the basis of these assessments, patients can be diagnosed with mild, moderate or severe AS, which can all occur in the presence or absence of symptoms (Table 1). Although Doppler echocardiography is the primary modality to assess the stage of AS, cardiac catheterization, which can measure cardiac blood pressure and flow, may be used to confirm the haemodynamic severity of the stenosis in patients with inconclusive or discordant echocardiography results157. However, this invasive technique is associated with increased risk of bleeding and cerebral embolism158 and should therefore only be considered in patients in whom the reclassification of the stenosis severity by catheterization would change the therapeutic management of the patient (such as AVR versus conservative management). For instance, individuals who might benefit from catheterization assessment include symptomatic patients where there is uncertainty between whether they have moderate or severe AS using echocardiography.
Figure 7. Assessment of aortic stenosis severity by Doppler-echocardiography.
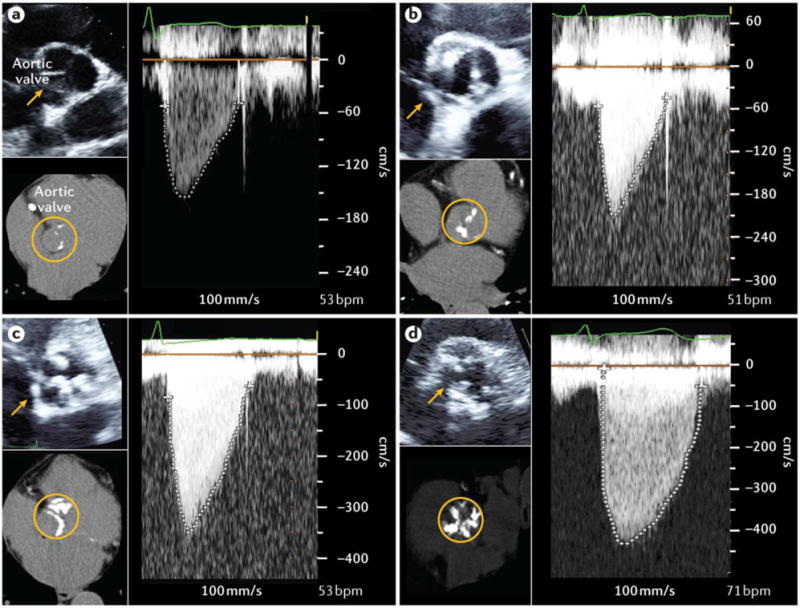
For each degree of disease severity including aortic valve sclerosis (A), mild aortic stenosis (AS) (B), moderate AS (C), and severe AS (D), this figure shows a 2D echocardiographic short-axis view of the aortic valve (top left), the transvalvular velocity by continuous-wave Doppler (right), and the multidetector computed tomography (MDCT) view of aortic valve calcification (bottom left). In the patient with aortic sclerosis (A), there are some small isolated spots of calcification (appears white on the MDCT images) in the aortic valve leaflets but there is no obstruction to blood flow (i.e. no stenosis). The peak aortic jet velocity (1.47 m/s), mean gradient (5 mmHg) and aortic valve area (AVA: 2.87 cm2) are normal. In the patient with mild AS (B), there is mild aortic valve calcification with mild obstruction to blood flow. The peak aortic jet velocity is 2.08 m/s, mean gradient: 9 mmHg, and AVA: 1.62 cm2. In the patient with moderate AS (C), there is more extensive aortic valve calcification with moderate obstruction of blood flow: peak aortic jet velocity: 3.51 m/s, mean gradient: 28 mmHg, and AVA: 1.21 cm2. In the patient with severe AS (D), there is severe aortic valve calcification and severe obstruction to blood flow: peak aortic jet velocity: 4.35 m/s, mean gradient: 48 mmHg, and AVA: 0.75 cm2.
Patients at risk for AS
Individuals with aortic sclerosis and those with a bicuspid valve (irrespective of the presence or absence of sclerosis) are considered to be at risk of developing AS. The identification of bicuspid valve is generally done by echocardiography but might require other imaging modalities such cardiac magnetic resonance (CMR) or CT if the valve is calcified.
Aortic valve sclerosis is defined echocardiographically by focal areas of valve calcification and thickening with normal leaflet mobility and normal valvular haemodynamics(Figure 7, Table 2). A systolic outflow murmur may be auscultated on physical examination. Although aortic sclerosis is clinically asymptomatic, its presence is independently associated with a 40% increase in the risk of a coronary event and a 50% increase in the risk of cardiovascular death159. The mechanism of adverse outcomes with aortic sclerosis is not entirely clear but the presence of aortic valve mineralization might be a marker for atherosclerosis and/or for altered phospho-calciummetabolism22;160.
Table 2.
Parameters and criteria for the assessment of aortic stenosis severity
| Technique | Parameter | Aortic Sclerosis | Mild AS | Moderate AS | Severe AS | Very Severe AS | Low Gradient Severe AS |
|---|---|---|---|---|---|---|---|
| Doppler-echocardiography | Peak aortic jet velocity (VPeak) |
< 2 m/s | 2 – 3 m/s | 3 – 4 m/s | ≥ 4 m/s | ≥ 5 m/s | < 4 m/s |
| Mean gradient ΔPMean |
<10 mmHg | 10 – 19 mmHg | 20 – 39 mmHg | ≥ 40 mmHg | ≥ 50 mmHg | <40 mmHg | |
| Aortic Valve Area (AVA=SVLVOT/VTIAo) |
> 2.0 cm2 | 1.6 – 2.0 cm2 | 1.1 – 1.5 cm2 | ≤1.0 cm2 | ≤0.6 cm2 | ≤1.0 cm2 | |
| Indexed AVA (AVAi=AVA/BSA) |
> 1.2 cm2/m2 | 1.0 – 1.2 cm2/m2 | 0.7 – 0.9 cm2/m2 | ≤0.6 cm2/m2 | ≤0.45 cm2/m2 | ≤0.6 cm2/m2 | |
| Dobutamine stress echocardiography | Stress mean gradient | NA | NA | NA | NA | NA | ≥ 40 mmHg |
| Stress AVA | NA | NA | NA | NA | NA | ≤1.0 cm2 | |
| MDCT | Aortic valve calcification score | NA | NA | Men ≥1200 AU Women ≥700 AU |
Men ≥2000 AU Women ≥1200 AU |
NA | Men ≥2000 AU Women ≥1200 AU |
AVA, aortic valve area; AVAi, indexed AVA; BSA, body surface area; ΔPMean, mean transvalvular gradient; MDCT, multidetector computed tomography; SV, stroke volume; VPeak, peak aortic jet velocity; VTIAo, velocity-time integral of the transvalvular flow; NA, not applicable or not available.
Mild or moderate AS
Patients with mild or moderate AS (Figure 7, Tables 1 and 2) are generally asymptomatic unless they have other comorbidities that contribute to the emergence of symptoms. Classic physical findings of AS are a harsh, crescendo-decrescendo systolic murmur, a single second heart sound and a delayed carotid upstroke (Box 1). Using Doppler-echocardiography, the haemodynamic severity of AS can be measured accurately and reliably on the basis of the peak aortic jet velocity, mean transvalvular pressure gradient (mean gradient) and aortic valve area (AVA). With the development of calcific AS, there is a progressive reduction in the AVA that causes an acceleration of the flow (i.e. increase in peak aortic jet velocity) and a loss of pressure (i.e. increase in mean gradient) across the valve (Figure 6, Table 2). AS is confirmed upon the visualization of a thickened aortic valve with a restricted opening and increased peak aortic velocity/mean gradient confirms the diagnosis of AS. Echocardiography is also useful to assess the effects of AS on the geometry and function of cardiac chambers, in particular of the LV (Figures 5 and 6).
Severe AS
Patients with severe AS (typically, those who have a peak aortic jet velocity of ≥ 4m/s, a mean gradient of ≥ 40mmHg and an AVA of ≤1cm2; Tables 1 and 2) may or may not have symptoms and require a closer clinical and Doppler-echocardiographic follow-up than those with mild or moderate forms of the disaese3. Classic symptoms of severe AS include dyspnoea and other symptoms of heart failure, angina and syncope. Patients with severe AS who are apparently asymptomatic according to medical history and physical examination should undergo exercise testing to confirm their asymptomatic status. Indeed, about one-third of patients with severe AS who are a priori asymptomatic in fact have exercise-limiting symptoms detected at an exercise stress test and these patients should be referred for AVR161;162. In addition, a potential marker for risk in AS is a marked increase in mean gradient (absolute increase in gradient >18–20 mmHg) during exercise stress echocardiography, which predicts higher risk of cardiac events in the short-term, independently of symptoms161;162.
Low-gradient AS
The majority of patients with severe AS have a high peak aortic jet velocity and gradient (mean gradient ≥ 40 mmHg). However, a substantial proportion of patients may have a low peak aortic jet velocity and mean gradient despite the presence of a small AVA (<1.0 cm2). The most frequent cause of ‘low gradient’ AS is the presence of low-flow state. There are two main subtypes of low-flow, low-gradient AS (Tables 1 and 2): ‘classical’ low-flow (stroke volume index <35 ml per m2), low-gradient (mean gradient <40 mmHg) AS with reduced LVEF (<50%)163; and ’paradoxical’ low-flow (stroke volume index <35 ml per m2), low-gradient (mean gradient <40 mmHg) AS with preserved LVEF (≥50%)164.
In classical low-flow, low-gradient AS, the decrease in stroke volume and thus in transvalvular flow rate (stroke volume divided by LV ejection time) are predominantly related to LV systolic dysfunction whereas in paradoxical low-flow, low-gradient AS, the low flow state is generally owing to pronounced LV concentric remodelling with impaired LV diastolic filling and reduced LV longitudinal systolic function156. Other conditions, such as mitral regurgitation, mitral stenosis or atrial fibrillation can also contribute to the reduced LV outflow in both classical and paradoxical low-flow, low-gradient AS.
In the presence of low flow, it is thus difficult, using resting Doppler-echocardiography or catheterization, to differentiate truly severe stenosis from pseudo-severe stenosis, that is, a situation wherein the stroke volume is not sufficient to completely open a valve that is only mildly or moderately stenotic. In such low flow conditions, the gradient might underestimate the stenosis severity, whereas the AVA might overestimate the severity. Low-dose dobutamine stress echocardiography should be used for patients with classical (low LVEF) low-flow, low-gradient AS to confirm stenosis severity. Dobutamine is used to mimic the effect of exercise on the heart, thereby increasing cardiac blood flow. Patients with mean gradient ≥40 mmHg (or a peak aortic jet velocity ≥ 4 m per s) and an AVA of <1.0 cm2 on dobutamine stress echocardiography are considered to have truly severe AS (Table 2). In patients who show a limited increase in flow (percent increase in transvalvular flow rate <15%) and persistent discordant grading (small AVA with low mean gradient) during dobutamine stress echocardiography, it is useful to calculate the projected AVA at normal flow rate; a projected AVA of <1.0 cm2 suggests the patient has true severe stenosis165;166. Patients who have no or minimal increase in stroke volume (percent increase <20%) upon dobutamine administration have a high risk of operative mortality with surgical AVR163;167. Low-dose dobutamine stress echocardiography or dobutamine stress cardiac catheterization may also be used in patients with paradoxical low-flow, low-gradient AS168. However, these approaches are often not feasible owing to the presence of restrictive LV physiology or their results are inconclusive owing to limited increases in flow in response to stress.
In patients with classical or paradoxical low-flow, low-gradient AS in whom dobutamine stress echocardiography is not feasible or inconclusive, multidetector computed tomography (MDCT), a high-resolution form of CT, can be used to quantitate aortic valve calcium load and thereby corroborate stenosis severity and indication of AVR (Figure 7 and Table 2). The region of the aortic valve is assessed in contiguous axial slices and the calcium score is measured by the Agatston modified method, in which calcification is defined as 4 adjacent pixels with density >130 Hounsfield units on the MDCT images. Studies have shown that different cut-off values of aortic valve calcium score (AU) should be used in women (>1200 AU) compared with men (>2000 AU) to identify haemodynamically severe stenosis169;170. Furthermore, these studies suggest that aortic valve calcium density (the ratio of calcium load to cross-sectional area of the aortic annulus) might be superior to absolute calcium load to predict hemodynamic severity and clinical outcomes. These studies also demonstrated that different cut-off values should be used in women (>300 AU per cm2) compared with men (500 AU/cm2)169;170. The aortic valve calcium load or density is also a powerful predictor of the risk of fast stenosis progression and of mortality170–172.
Finally, a substantial proportion of patients with AS have a small AVA and low mean gradient but a normal flow (stroke volume index > 35 ml per m2). This category is often referred as to normal-flow, low-gradient AS and might be related to inherent discrepancies in the criteria used to define severe AS (in terms of AVA and mean gradient)173 and/or to markedly reduced aortic compliance169. Patients with normal-flow, low-gradient AS generally have less advanced disease and better outcomes compared with patients who have high gradient or low-flow, low-gradient AS174. However, if the patient is symptomatic, aortic valve calcium scoring using MDCT can be considered to confirm stenosis severity169.
Emerging Biomarkers
Other imaging or blood biomarkers of the severity of AS and its deleterious effects on the LV and other cardiac chambers may also be useful to predict risk of rapid disease progression and adverse events. In particular, these biomarkers may be helpful in identifying patients with asymptomatic severe AS who may benefit from early ‘prophylactic’ AVR.
Biomarkers of aortic valve biology and flow pattern
Positron emission tomography (PET) combined with MDCT (PET-MDCT) is a feasible and reproducible method that combines anatomical imaging from MDCT with the molecular imaging from PET. The valvular uptake of 18F-sodium fluoride (18F-NaF) measured by PET-MDCT is a marker for active mineralization process within the valve (Figure 8)175–177. 18F-NaF uptake correlates well with AS severity and it might provide incremental value beyond aortic valve calcium scoring to predict AS progression over time172. This method might also be useful in assessing the effect of new pharmacotherapies on AS progression. In addition, CMR might be useful to assess valve biology and flow. For instance, data from a previous study suggests that in the future CMR might be able to assess not only the amount of valvular calcification (as can be achieved with MDCT) but also the amount of fibrous-rich and lipid-rich valve tissue178. Moreover, CMR with 4D flow modality might also one day be used to visualize flow patterns in the aorta and therefore to identify patients with AS who are at risk of developing aortic aneurysm and aortic dissection (a breach in the lining of the aorta that causes blood to flow between the layers of the wall of the aorta, forcing layers apart) (Figure 9)179;180.
Figure 8. Assessment of aortic valve mineralization activity by positron emission tomography – computed tomography.
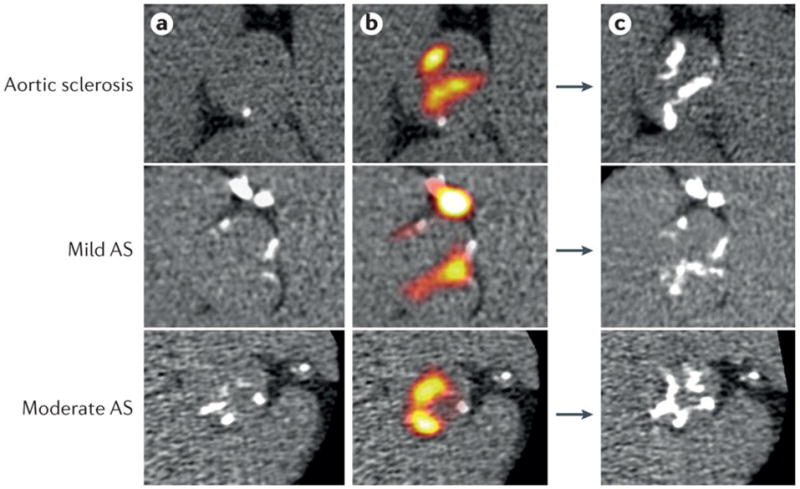
Coaxial short axis views of the aortic valve from one patient with aortic sclerosis, one patient with mild aortic stenosis and one patient with moderate aortic stenosis. Left panels: baseline multi-detector computed tomography (MDCT) images of the aortic valve; regions of macrocalcification appear white. Middle panels: baseline fused MDCT and 18F-sodium fluoride (NaF) positron emission tomography (PET) images showing intense 18F-NaF uptake (red yellow areas) both overlying and adjacent to existing calcium deposits on the MDCT. Right panels: One-year follow-up (without intervention) MDCT images demonstrate increased calcium accumulation in much the same distribution as the baseline PET activity. Reproduced with permission from172.
Figure 9. Assessment of flow patterns in the Aorta by 4D flow cardiac magnetic resonance according to aortic valve phenotype.
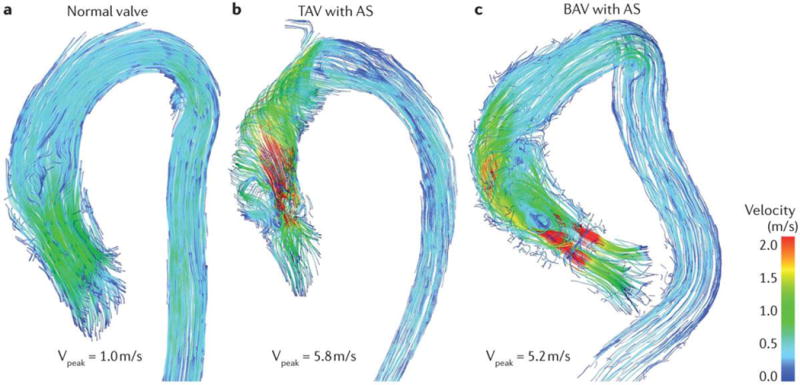
(A) A normal valve systolic flow in a healthy control. (B) A tricuspid aortic valve (TAV) with severe aortic stenosis (AS) and altered systolic flow with helical patterns in the ascending aorta. (C) A bicuspid aortic valve (BAV) with right-left (RL) cusp fusion and severe AS. Altered blood flow with asymmetric helical flow patterns are observed in the proximity of the aortic valve. Courtesy of Julio Garcia, Alex Barker and Michael Markl, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Biomarkers of impact of AS on the left ventricle
Detection of sub-clinical LV dysfunction using biomarkers might prove useful in identifying patients who may need early therapeutic intervention. For example, reduced longitudinal strain is useful to identify subclinical LV dysfunction and predict risk of cardiac events in patients with asymptomatic AS and preserved LVEF181–186. However, further studies are needed to harmonize the different strain analysis platforms between vendors and to propose an optimal cut-off value of longitudinal strain that identifies patients at higher risk of developing LV dysfunction and symptoms in the short-term.
Blood levels of B-type natriuretic peptide (BNP) might also be a useful marker of LV function, as it is secreted from the LV in response to mechanical stress. Although BNP can be used for risk stratification, there is an important inter-study variability in the cut-off serum values of BNP that have been used to identify high-risk patients. A previous study proposed using the BNP ratio (the measured value of BNP divided by the expected value of BNP adjusted for the age and sex of the patient) to overcome this limitation. A BNP ratio of >1 was found to be a powerful independent predictor of mortality in AS, even in patients with asymptomatic AS187. Hence, the BNP ratio as well as its increase during follow-up might be helpful in enhancing risk stratification in AS.
Besides longitudinal strain and BNP, the extent of myocardial fibrosis represents a maladaptive response of the LV to pressure overload from AS. Previous studies188–191 have reported that approximately 20 to 30% of patients undergoing AVR for severe AS have severe myocardial fibrosis documented by CMR or myocardial biopsies. Myocardial fibrosis is often not reversible (or only partially reversible) and is associated with increased risk of cardiovascular events and mortality during follow-up as well as persistence of LV dysfunction and symptoms following AVR188–190;192;193. Therefore, the quantification of myocardial fibrosis by CMR (Figure 10) could potentially be useful to recommend early AVR in patients with asymptomatic severe AS before extensive fibrosis and ensuing irreversible myocardial dysfunction have developed or to improve operative risk stratification and assess potential utility versus futility of AVR in patients with low-flow, low-gradient AS. However, further studies are needed to improve the standardization of the different CMR methods for quantitation of myocardial fibrosis and to establish the thresholds that should be used clinically to identify patients who are at risk for irreversible myocardial dysfunction. The large scale utilization of CMR in the AS population is also limited by its high cost and low availability.
Figure 10. Assessment of myocardial fibrosis by cardiac magnetic resonance in patients with aortic stenosis.
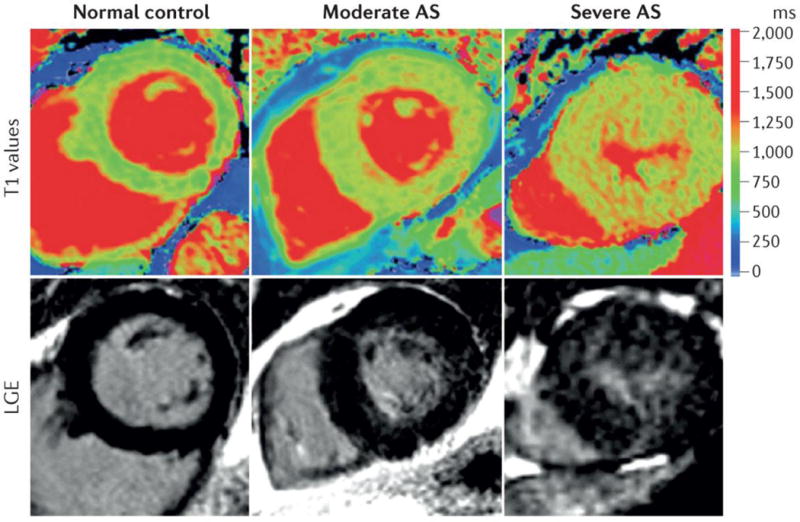
Top panel: colour maps of T1 values using shortened modified Look–Locker inversion in a mid-ventricular short-axis slice; bottom panel: the corresponding slice with late gadolinium enhancement (LGE) imaging. The left panel shows a normal volunteer. The middle panels show moderate aortic stenosis (AS) with moderate left ventricular hypertrophy. The right panel shows severe AS with severe LV hypertrophy. Regions with high T1 values (orange and red) within the LV wall correspond to myocardial fibrosis. Reproduced with permission from Bull et al.268.
Emerging blood biomarkers, such high-sensitivity cardiac troponin194;195, growth/differentiation factor 15, soluble interleukin-1 receptor-like 1 (also called protein ST2) and micro RNAs196–198, might be helpful to detect subclinical and/or irreversible myocardial dysfunction but their incremental value beyond already established clinical, echocardiographic, tomographic and blood biomarkers is yet to be demonstrated.
The main limitation of all aforementioned imaging and blood biomarkers of LV function is that they are non-specific and may be altered by other concomitant diseases, such as hypertension, diabetes mellitus and coronary artery disease. Therefore, these biomarkers should always be interpreted in conjunction with the standard parameters of stenosis severity. Finally, further studies are needed to establish the incremental role of these emerging blood or imaging biomarkers to identify the patients who might benefit from earlier intervention.
Conclusions
In summary, the two main risk factors for calcific AS are older age and bicuspid aortic valve. Other risk factors include metabolic syndrome, diabetes, hypertension, smoking and increased plasma Lp(a). There is currently no preventive or pharmaco-therapeutic approach that has proven effective to prevent the onset or slow the progression of calcific AS The initial screening for this disease is generally based on the auscultation of a systolic murmur by the primary care physician or general cardiologist. Doppler-echocardiography is the method of choice to diagnose AS and assess its severity as well as to follow disease progression over time. Quantitation of aortic valve calcium load by MDCT may be useful to corroborate stenosis severity in patients in whom echocardiography is neither feasible nor conclusive, which is often the case in the setting of low-flow, low-gradient AS. Measurement of circulating BNP levels, assessment of global longitudinal strain by speckle tracking and detection of myocardial fibrosis by CMR are emerging biomarkers that might improve the detection of subclinical LV dysfunction and thus the determination of the optimal timing for AVR.
Management
The only treatment available to treat patients with symptomatic severe AS is to implant a prosthetic heart valve either surgically or percutaneously (through a catheter). The therapeutic management is similar for calcific versus rheumatic AS. As discussed above, there is no pharmacotherapy specifically targeting AS to prevent progressive leaflet calcification or to delay time to valve replacement3;199. Although there was hope that statins would fill that void, several randomized trials showed no effect of statins on haemodynamic progression or AS-related clinical events152–154. However, the combination of simvastatin (drug that lowers plasma LDL cholesterol levels) and ezetimibe (drug that decreases cholesterol absorption in the small intestine) did reduce ischaemic cardiovascular events in patients with mild to moderate AS153. Therefore, as valve stenosis progresses into the moderate to severe range, greater vigilance is required regarding assessment for symptoms associated with significant AS to decide when to perform AVR.
Management decisions regarding AVR are often straightforward (Figure 11). However, in the current era of transcatheter AVR (TAVR), there are more options to consider when intervention is contemplated than in previous decades (Figure 12). In addition, older (> 80 years) and sicker patients who previously were not candidates for definitive therapy are being treated200;201. Increasingly, clinicians must integrate complex information about the severity of AS, ambiguous symptoms, LV remodelling and function, comorbidities, frailty and disabilities to make decisions about whether, when, and how to perform AVR3;199;202. This complex information ought to be discussed and debated in the context of a heart valve team — a multidisciplinary group comprised of cardiac surgeons, interventionalists, cardiac imaging experts, and often nurses, geriatricians and anesthesiologists203–205. In addition, it is important for management decisions to be patient-centered and not myopically focused on AS severity alone3. First, a decision should be made whether valve replacement is indicated. Subsequently, consideration can be given to how the valve should be replaced (surgical versus transcatheter) (Table 3 and Figure 12). Finally, at any stage of AS, associated medical conditions such as atrial fibrillation, coronary disease, hypertension and heart failure should be treated according to guideline recommendations3;4;199.
Figure 11. Algorithm for the management of aortic stenosis.
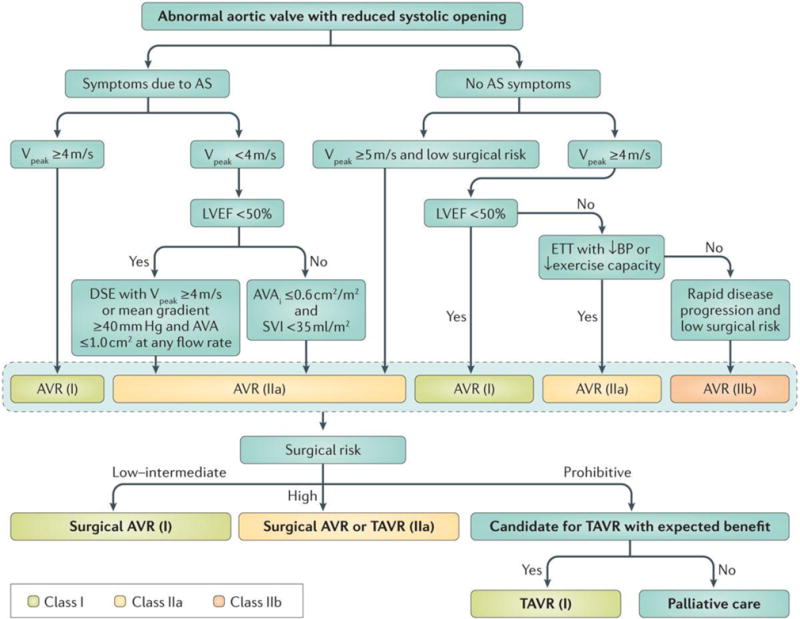
This figure presents the algorithm recommended by the 2014 ACC/AHA guidelines for the management of aortic stenosis3. AS:, aortic stenosis; AVA, aortic valve area; AVAi, AVA indexed for body surface area; BP, blood pressure; AVR, aortic valve replacement; ETT, exercise treadmill test; LVEF, LV ejection fraction; SVi, stroke volume index; TAVR, tr anscatheter AVR; VPeak, peak aortic jet velocity.
Figure 12. Different types of surgical aortic valve replacement.
(A) Surgical aortic valve replacement with a bileaflet mechanical valve. (B) Surgical aortic valve replacement with a bioprosthetic valve.
Table 3.
Key management decisions when selecting a technique and prosthetic valve for aortic valve replacement.
| AVR technique or valve type | Indication | Contra-indication | Advantages | Limitations |
|---|---|---|---|---|
| Surgical AVR | Indication of AVR Low to high surgical risk |
Prohibitive surgical risk Life expectancy < 1 year |
Standard therapy with well-established record of safety, efficacy and durability | Invasive |
| Surgical AVR with biological valve | Patient preference Achievement of good anticoagulation unlikely Age > 65 years |
Life expectancy < 1 year | Does not require anticoagulation | Limited long-term durability |
| Surgical AVR with mechanical valve | Patient preference Patients already on anticoagulation |
Life expectancy < 1 year | Long-term durability | Requires life-time anticoagulation (increased risk of bleeding) |
| Transcatheter AVR* | Indication of AVR High or prohibitive surgical risk |
Life expectancy < 1 year | Less invasive than surgical AVR | Long-term durability unknown Higher risk of paravalvular AR |
With balloon-expandable or self-expanding valves.
AR, aortic regurgitation; AVR, aortic valve replacement
Indications for aortic valve replacement
Symptomatic severe AS
Severe high-gradient AS accompanied by symptoms related to AS is the most common and straightforward indication for AVR, and those with severe AS who present with symptoms and/or LV systolic dysfunction (defined as a LVEF of <50%) have a firm (Class I, Box 1) indication for AVR (Figure 11, Table 1)3;4. Low-flow, low gradient AS presents somewhat of a challenge as the combination of a small AVA with a low gradient raises uncertainty about the severity of the stenosis and thus the indication of AVR. Symptomatic patients with classical low-flow, low-gradient and reduced LVEF (<50%) are reasonable candidates for AVR (Class IIa indication, Box 1) provided there is anatomic evidence (MDCT calcium score) or haemodynamic evidence (peak aortic jet velocity ≥4 m per sec or mean gradient ≥40 mmHg with dobutamine stress echocardiography) that the AS is truly severe3;4;170. AVR may be considered in patients with classical low-flow, low-gradient AS having no flow reserve at dobutamine stress echocardiography, but the operative risk is higher4;163;167;206. It is also reasonable to perform AVR in symptomatic patients with paradoxical low-flow, a low-gradient and preserved LVEF (≥50%) (Class IIa indication) provided there is clinical, haemodynamic and anatomic evidence that the obstruction is severe and the most likely cause of symptoms3;4;168. Although there has been some debate about the outcome and therapeutic management of patients with paradoxical low-flow, low-gradient AS, a recent meta-analysis confirms that these patients have worse outcomes compared to moderate or high-gradient severe AS and that their survival is markedly improved by AVR174.
Asymptomatic severe AS
Patients with severe AS who are asymptomatic by history but who have a reduced LVEF (<50%) (Table 1) or are undergoing another cardiac surgical procedure should have their valve replaced (Class I indication) (Figure 11)3;4. It also is reasonable to perform AVR (Class IIa indication) in asymptomatic patients with severe AS and decreased exercise tolerance or a drop in blood pressure with exercise, and in those at low surgical risk with very severe AS (peak aortic jet velocity >5 m/sec or 5.5 m/s, depending on the guidelines,), or findings suggestive of rapid progression (severe valve calcification or increase in peak aortic jet velocity of ≥0.3 m per sec per year)3;4.
Surgical aortic valve replacement
The first successful surgical AVR was performed in 1960207. Over the past half century, tremendous advances in operative management, techniques and valve design have transformed the outlook for patients with AS. Despite increasing age and comorbidities, the mortality associated with AVR has decreased dramatically during the past two decades208;209. For an isolated AVR, the overall 30-day mortality rate is currently under 3% as reported in the Society of Thoracic Surgeons (STS) database and German Aortic Valve Registry (GARY)209;210. Table 3 presents the advantages and limitations of the different types of AVR. There has been a shift away from mechanical valves toward greater use of bioprosthetic valves, particularly in patients >65 years of age (Figure 12)209. Increasingly, younger patients or those with an active lifestyle opt for a bioprosthetic valve to avoid anticoagulation despite its shorter durability compared to a mechanical valve. The most frequently used bioprosthetic valves are the stented bioprostheses, which are composed of three biologic leaflets made from porcine aortic valve or bovine pericardium and mounted on a metal or polymeric stented ring. Bioprosthetic valves also include stentless bioprostheses that are manufactured from intact porcine aortic valves or from bovine pericardium. These valves have better hemodynamics compared to stented valves but their implantation is more complex and thus requires longer cardiopulmonary bypass time. Sutureless stent-mounted bioprosthetic valves have also been developed to allow easier and faster implantation of the valve without sutures.
Additional alternatives for AVR in younger patients include the implantation of an aortic homograft (aortic valve harvested from a donor) or the Ross procedure, which involves the replacement of the diseased aortic valve with the patient’s pulmonary valve followed by pulmonary valve replacement using a donor pulmonary valve211–213. These options are however more controversial and less frequently used. A recent propensity analysis showed no difference in mortality or stroke among patients 50–69 years of age treated with a bioprosthetic versus mechanical valve, although a bioprosthetic valve was associated with a higher incidence of reoperation and a mechanical valve was associated with a higher incidence of major bleeding during the 15-year follow-up214. A mini-sternotomy, which is a minimally invasive way of performing cardiac surgery, is a viable option for isolated AVR and is associated with similar mortality, but decreased morbidity and resource utilization, compared to a full sternotomy215.
Operative mortality for AVR varies according to the skill and experience of the surgical team as well as hospital volume216. Increasing age and comorbidities substantially increase both operative and long-term mortality after AVR217;218. A number of risk scores, including the EuroSCORE (http://www.euroscore.org) and the STS risk calculator (http://riskcalc.sts.org), incorporate these factors to estimate operative risk. These risk scores are imperfect and iteratively being refined. They often do not include important factors such as frailty, chest wall radiation, porcelain aorta, pulmonary hypertension and liver cirrhosis. Owing to age, LV dysfunction, multiple comorbidities and other factors, approximately one-third of patients with indications for AVR are not treated200;219.
Transcatheter aortic valve replacement
TAVR is a minimally invasive procedure that involves insertion of a bioprosthetic aortic valve within the orifice of the native stenotic valve using a catheter. For patients at high or prohibitive risk of operative mortality, with surgical AVR, TAVR has been a transformative innovation, providing a life-saving treatment for patients who were previously not candidates for AVR (Table 3)201;220–224. In the PARTNER Trial, there was a 20% absolute reduction in 1-year mortality (HR 0.55; 95% CI 0.40 to 0.74) with TAVR compared to standard therapy (30.7% versus 50.7%)201. This survival benefit was accompanied by relief of symptoms and improvement in functional capacity in many patients201;225. Randomized trials of balloon-expandable and self-expanding valves have also demonstrated that TAVR is a viable alternative to surgery in patients at high risk for AVR (Table 4)220;221.
Table 4.
Completed, ongoing and future clinical trials on different AS of therapeutic procedures and strategies
| Trial name | Patient population and surgical risk | Number of patients | Design | Intervention(s) | Endpoints and results | Status |
|---|---|---|---|---|---|---|
| PARTNER-IB201;222;269 |
Symptomatic Severe AS Inoperable |
358 | Randomized | TAVR (SAPIEN) vs. conservative management | 1-year mortality: 30.7% TAVR vs. 49.7% conservative* 1-year mortality of Major Stroke: 33% TAVR vs. 50.3% conservative* 5-year mortality: 71.8% TAVR vs. 93.6% conservative* |
Completed |
| PARTNER-IA220;223;270 | Symptomatic Severe AS High risk |
699 | Randomized | TAVR (SAPIEN) vs. SAVR | 30-day mortality: 3.4% TAVR vs. 6.5% SAVR 1-year mortality: 24.2% TAVR vs. 26.8% SAVR 1-year mortality or major stroke: 26.5% TAVR vs. 28% SAVR 5-year mortality: 67.8% TAVR vs. 62.4% SAVR |
Completed |
| PARTNER-IIB271 | Symptomatic Severe AS Inoperable |
560 | Randomized | TAVR (SAPIEN) vs. TAVR (SAPIEN-XT) | 30-day mortality: 5.1% SAPIEN vs. 3.5 % SAPIEN-XT 1-year mortality: 23.3% SAPIEN vs. 22.3% SAPIEN-XT 1-year major stroke: 5.5% SAPIEN vs. 4.8% SAPIEN-XT |
Completed |
| SAPIEN 3-HR272;273 | Symptomatic Severe AS High risk/inoperable |
583 | Non-Randomized | TAVR (SAPIEN 3) | 30-day mortality: 2.2% 30-day major stroke: 0.86% 1-year mortality: 14.4% 1-year major stroke: 2.4% |
Ongoing |
| SAPIEN 3-IR272 | Symptomatic Severe AS Intermediate risk |
1076 | Non-Randomized | TAVR (SAPIEN 3) | 30-day mortality: 1.1% 30-day major stroke: 1.02% |
Ongoing |
| CoreValve ER224 | Symptomatic Severe AS Inoperable |
509 | Non-Randomized | TAVR (SAPIEN 3) | 1-year mortality: 26 % 1-year major stroke: 2.3 % |
Completed |
| CoreValve IR221;274 | Symptomatic Severe AS Intermediate risk |
750 | Randomized | TAVR (CoreValve) vs. SAVR | 1-year mortality: 19.1 % TAVR vs. 14.2 % SAVR* 1-year major stroke: 22.2 % TAVR vs. 28.6 % SAVR* 2-year mortality: 22.2 % TAVR vs. 28.6 % SAVR* 2-year major stroke: 22.2 % TAVR vs. 28.6 % SAVR* |
Completed |
| CHOICE275 | Symptomatic Severe AS Low risk |
241 | Randomized | TAVR (SAPIEN-XT) vs. SAVR (CoreValve) | 30-day mortality: 4.1% (SAPIEN-X) vs. 4.3% (CoreValve) 30-day stroke: 5.8% (SAPIEN-XT) vs. 2.6% (CoreValve) |
Completed |
| NOTION276 | Symptomatic Severe AS Low risk |
280 | Randomized | TAVR vs. SAVR | 1-year mortality: 4.9% TAVR vs. 7.5% SAVR 1-year all stoke: 2.9% TAVR vs. 4.6% SAVR |
Completed |
| PARTNER-IIA277 | Symptomatic Severe AS Intermediate risk |
2000 | Randomized | TAVR (SAPIEN-XT) vs. SAVR | Primary end-point: 2-year mortality or major stroke | Ongoing |
| SURTAVI277 | Symptomatic Severe AS Intermediate risk |
2500 | Randomized | TAVR (CoreValve) vs. SAVR | Primary end-point: 2-year mortality or major stroke | Ongoing |
| TAVR-UNLOAD277 | Moderate AS Low LVEF HF symptoms |
600 | Randomized | HF therapy alone vs. HF therapy plus TAVR | Primary end-point: hierarchical composite of 1-year death, stroke, HF hospitalization and quality of life | Future |
| AVATAR RECOVERY EARLY-TAVR277 | Asymptomatic Severe AS | 144–800 | Randomized | Early AVR (SAVR and/or TAVR) vs. watchful waiting | Primary end-point: composite of death, stroke, myocardial infarction, HF hospitalization, LV systolic dysfunction and quality of life | Future |
Difference between groups is statistically significant.
AS, aortic stenosis; AVR, aortic valve replacement; HF, heart failure; LVEF, Left ventricular ejection fraction; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement; HF, heart failure.
TAVR may be performed by several different approaches; the most common access routes include transfemoral, transapical and transaortic (Figure 12, Table 5). Approximately two-thirds (56–75%) of TAVR procedures are performed via a transfemoral approach226–229. As catheter sheath sizes decrease, the balance is anticipated to shift even more toward a transfemoral approach. A transfemoral approach is associated with lower mortality and quicker recovery compared to alternative access approaches227–229. Other approaches include via the subclavian, axillary or carotid arteries. There have even been recent reports of transcaval approaches230.
Table 5.
Comparison of TAVR access routes
| Approach | Indication | Contra-indication | Advantages | Limitations |
|---|---|---|---|---|
| Transfemoral | Default approach for TAVR | Small, tortuous or calcified femoro-iliac arteries | Least invasive approach | Requires a minimal femoral and iliac artery diameter of 6–6.5 mm |
| Transapical (via the chest between the ribs) | Femoral and other vascular access not possible | LV aneurysm or thrombus Severe pulmonary disease* Severe LV systolic dysfunction* |
Better control of the positioning of the valve | More invasive More myocardial injury More respiratory complications |
| Transaortic (via mini-sternotomy) | Femoral and other vascular access not possible Viable alternative to transapical |
Complete porcelain aorta (rare) | Avoids manipulation and suturing of LV apex with potential resulting apical dysfunction | Requires a non-calcified area on the aorta for access and purse suture |
| Other approaches# | Alternative access routes in the context of severe peripheral artery disease | Unsuitable anatomy or size of the alternative artery | Provides an option to transfemoral that is potentially less invasive that transapical or transaortic | Depends on route (carotid approach may increase stroke risk; transcaval approach may have bleeding or damage to the aorta that is difficult to control) |
Relative contra-indication
Left subclavian or axillary artery, carotid artery or transcaval route. LV, left ventricle; TAVR, transcatheter aortic valve replacement
Balloon-expandable and self-expanding transcatheter valves have been the most rigorously studied to date, specifically the CoreValve (Medtronic, Dublin, Ireland) and SAPIEN (Edwards, Irvine CA, USA) valves (Figure 11, Table 5)201;220;221;224;226;231;232. This clinical arena is a very active area of development including iterative improvements on existing valves and novel designs233. Although TAVR has been a successful therapy in many ways, several complications and challenges have been encountered233. The most notable has been paravalvular aortic regurgitation234–236. The association between moderate or severe paravalvular aortic regurgitation and increased mortality has been clearly established, with some studies even suggesting that this adverse association extends to mild regurgitation235;237;238. Other complications of TAVR have included major vascular injury, heart block requiring a permanent pacemaker and acute kidney injury; more rare complications include stroke, aortic rupture and coronary obstruction233.
The TAVR field is rapidly evolving. Clinical trials comparing TAVR to surgery in intermediate risk populations are ongoing with results expected soon (Table 4). Surgical AVR has excellent results with low mortality in low risk populations209. For TAVR to make inroads into lower risk populations, device improvements are needed (principally to reduce paravalvular regurgitation and heart block, which is an arrhythmia that occurs when electrical impulses in the heart are blocked or delayed), vascular and stroke complications must be minimized and valve durability needs to be demonstrated. There is a growing movement away from general anesthesia to conscious sedation that might decrease the morbidity of the procedure239. Finally, valve-in-valve procedures for failed bioprostheses are becoming more common as an alternative to re-doing surgical AVR240.
Choice of surgical versus transcatheter aortic valve replacement
The choice of how to perform AVR should occur only after a decision that AVR is indicated (Table 1)3. Currently, surgical AVR is indicated for patients with low to moderate surgical risk and TAVR is indicated for patients at prohibitive risk for surgery (Figure 11, Table 3)3;4. Patients may be at prohibitive risk for surgery owing to technical factors (such as porcelain aorta) or for clinical reasons (such as multiple comorbidities or frailty)3;241. Presently, intermediate risk patients may be treated with surgical AVR or enrolled in a clinical trial for TAVR. High-risk patients who are candidates for either surgical AVR or TAVR should have their therapy determined by careful consideration by the heart valve team3;4. Factors to weigh in this decision include anatomic considerations, concomitant coronary disease and associated mitral or tricuspid valve disease. In patients with considerable associated mitral or tricuspid regurgitation, it is unclear whether concomitant surgical repair of the mitral or tricuspid valve at the time of AVR would improve clinical outcomes242;243.
Although, in general, TAVR is associated with a survival advantage compared to conservative (no AVR) management, there is a sizable sub-group that dies soon after TAVR or does not experience an improvement in quality of life, suggesting potential futility of TAVR in some patients201;202;220;244;245. For instance, among inoperable patients treated with TAVR in the PARTNER I Cohort B trial (Table 4), at 1 year after the procedure approximately 31% were dead and 18 % had less than a moderate improvement in their quality of life or New York Heart Association functional class201;244 Among patients treated in the high-risk Cohort A of the PARTNER I trial with TAVR or surgical AVR (Table 4), death from non-cardiovascular causes was more common than death from cardiovascular causes48. Moreover, when cause of death was difficult to categorize, it often occurred in frail patients who were failing to thrive246. Therefore, when lifespan or quality of life is profoundly limited by frailty, noncardiac disease, mental or physical disability, the potential benefit of AVR may be low11. These cases highlight the importance of a heart valve team in the management decisions of these complex patients3;4. In some of these patients, the most appropriate approach is palliative care, taking the values and preferences of the patient and family into consideration in the decision making process202.
Management of coronary artery disease in patients with AS
The prevalence of considerable coronary disease in the setting of severe AS increases with age and was as high as 75% in recent trials comprised mostly of very elderly patients201;220. Decisions regarding revascularization at the time of valve replacement used to be somewhat simpler when surgical valve replacement was the only option. If significant coronary artery stenosis was present at preoperative coronary angiogram, coronary artery bypass graft was performed at the time of valve replacement surgery. With the emergence of TAVR, decisions regarding the treatment of coronary disease have become more complex, including which coronary lesions to treat versus leave alone, how to treat them (percutaneous versus bypass) and when to treat them (before, during, or after valve replacement)247. These decisions are influenced by numerous factors including lesion location and complexity, overall burden of coronary disease, the presence or absence of angina, LV function, bleeding risk on dual antiplatelet therapy and other factors. How these decisions affect clinical outcomes requires further investigation as many questions remain247;248. The way in which coronary disease should influence decisions between valve replacement with TAVR versus surgical valve replacement is also unclear in some scenarios. A detailed discussion of these complex decisions is beyond the scope of this Primer, but has been recently reviewed elsewhere247;249.
Balloon aortic valvuloplasty
Balloon aortic valvuloplasty (BAV), which uses the pressure of an inflated balloon to widen the opening of the stenotic valve, is not a definitive therapy for AS3. The changes produced by BAV in valve area and transvalvular pressure gradient are usually modest and short-lived (weeks to months)250;251. In particularly ill patients, BAV may be used as a ‘bridge’ to stabilize the patient prior to definitive therapy with valve replacement3. When there is uncertainty whether a patient will benefit clinically from valve replacement owing to markedly depressed LV function or concomitant oxygen dependent lung disease or other factors, a BAV may have diagnostic utility to determine whether valve replacement is appropriate202. In patients with severe AS undergoing non-cardiac surgery, a BAV is generally not warranted unless the patient is symptomatic or hemodynamically unstable and needs to undergo non-cardiac surgery before aortic valve replacement can be performed3. In some circumstances, a BAV may be utilized for palliative care as there is some evidence that it might provide a short-term benefit in terms of improved survival, functional capacity and quality of life, but these benefits are not sustained251.
Quality of life
Severe AS primarily impairs quality of life by causing heart failure symptoms including shortness of breath, fatigue and diminished functional capacity199;252. However, because patients who develop severe AS are usually older adults, these symptoms may also result, in part, from normal aging, numerous comorbidities or frailty202. In older patients at high or extreme surgical risk undergoing TAVR, disease-specific and generic health status are often extremely poor221;224;244;245. Given the high prevalence of frailty and disability in this patient population, the relationship between valvular stenosis and overall quality of life is also complex and variable.202
AVR is indicated in patients with severe symptomatic AS both to increase life expectancy and improve symptoms and quality of life3;4;199;202;252;253. For a patient with severe AS and heart failure symptoms, who is at low surgical risk, surgical AVR is associated with a fairly predictable improvement in shortness of breath and functional capacity. For patients who are at high-risk for surgical interventions were previously not treated with AVR, TAVR has been a transformative innovation that has improved survival and quality of life200;201. Compared with inoperable patients treated with conservative management, patients treated with TAVR had less severe heart failure symptoms and better disease-specific and generic health status over the year after randomization201;244.
To determine the anticipated benefit of valve replacement in terms of quality of life, it is important to consider how much of the patient’s symptoms and impaired health status are due to the valvular obstruction and heart failure versus other comorbidities and geriatric conditions253. This can be challenging to determine. When a patient’s diminished quality of life is clearly related to heart failure symptoms from severe AS, valve replacement conveys a predictable and noticeable improvement in quality of life and extends life expectancy. Notably, however, some patients have residual heart failure symptoms (albeit not as severe) after valve replacement owing to persistent diastolic dysfunction; this may manifest similar to the common syndrome of heart failure with preserved LVEF. When poor health status is principally due to comorbidities and geriatric conditions, valve replacement might lead to an unsatisfactory result both in terms decreased survival and a decline or lack of improvement in quality of life253–256. Elucidating which factors contribute to worse quality of life after TAVR and identifying how those factors might be targeted with adjunctive interventions to improve outcomes require further study. It is likely that systemic, non-cardiac factors play an important role.
Outlook
Valve biology
Although long considered to be a passive and degenerative process, it is now clear that calcific AS results from an active biology that promotes fibrosis and calcification of the valve leaflets1. The pathobiology of AS is complex and likely involves genetic factors, multiple signalling pathways, ageing, sex hormones, haemodynamic factors and shear stress, and the systemic milieu. Disease initiation and progression are influenced by different factors. Several laboratories worldwide are working to elucidate the pathobiology of aortic sclerosis and stenosis, which will likely yield novel insights into potential therapeutic targets to prevent or reverse calcific aortic valve disease.
Pilot trials to slow disease progression
Several intervention studies have been performed to test the hypothesis that lipid lowering with statin medications would slow the progression of AS, however the results were generally disappointing152–154. Equipped with new insights into valve biology, there will likely be a new wave of clinical trials testing interventions that target diverse pathways to slow the progression of (or even reverse) calcific AS. Specific interventions might target the initiation of disease or the progression of disease. Promising targets on the horizon include Lp(a), the renin-angiotensin system, RANKL and ectonucleotidases. Novel composite endpoints are likely to be developed for these trials based on the mechanism of action of the intervention and the phase of disease targeted.
AS as a disease of the ventricle
The LV response to chronic pressure overload from AS is characterized by hypertrophic remodelling (myocyte hypertrophy and fibrosis) and diastolic and systolic dysfunction. In many ways, this LV response considerably influences the morbidity and mortality of the disease199;257–260. Future research will likely clarify the mechanisms driving the formation of fibrosis in the pressure overloaded heart and elucidate the abnormal diastolic properties (such as stiffness versus relaxation) involved in AS. In asymptomatic patients, targeting the adverse remodelling sequelae of the valvular stenosis with a therapeutic medical intervention might delay the onset of symptoms and allow us to put new valves into healthier hearts, thereby potentially improving long-term cardiac performance and functional capacity.
TAVR will move into lower risk populations
With iterative improvements in transcatheter valves and lower procedural complications (less paravalvular leak, permanent pacemakers, stroke and vascular injury), TAVR will likely move into lower risk populations (Table 4). However, questions about valve durability will need to be addressed. Although TAVR might become a viable option in low-risk patients with isolated AS, there will likely continue to be a group of patients for whom surgical AVR is preferable because it allows for more optimal treatment of concomitant pathology such as left main coronary disease or severe mitral or tricuspid valve disease. The currently available option of a transcatheter valve-in-valve procedure might lead cardiac surgeons to implant bioprosthetic valves (rather than mechanical valves) in younger patients, with the understanding that a new bioprosthetic valve can be subsequently implanted using TAVR.
Improved accuracy of risk prediction for TAVR
Although the STS score and EuroSCORE have reasonable accuracy in predicting morbidity and mortality after TAVR, they were developed in younger patient cohorts with fewer comorbidities undergoing cardiac surgery261. With multiple clinical trials and registries collecting detailed data on patients undergoing TAVR, there will be several risk prediction models developed specifically in and for TAVR patients that will improve upon existing ones. These scores will incorporate geriatric factors (for example, frailty, disability and cognitive impairment) and will be developed to predict quality of life outcomes, not just mortality.
Increased use of biomarkers
Biomarkers have not been widely utilized in the management of patients with AS. Natriuretic peptides, such as BNP, are somewhat of an exception, but their role in management decisions has not been clearly defined3;4. In the coming years, there will be more specific cut-offs of natriuretic peptide levels to guide management decisions187. High sensitivity cardiac troponin will be more routinely integrated into our evaluation of patients with AS194. Increasingly, as in non-AS heart failure populations, a multimarker approach will be taken to measure diverse biological pathways in a more integrated manner to gain insight into ventricular health and systemic factors that might affect clinical outcomes and influence management strategies regarding valve replacement and adjunctive therapies198.
Tailored management strategies for AVR
Treatment decisions will become more personalized regarding when, whether and how to perform valve replacement. Previously, management decisions were largely conceptualized in terms of the severity of AS and the presence or absence of symptoms. Phenotyping and risk stratification has and will become more sophisticated, allowing for more nuanced management decisions. The LV response to a given degree of pressure overload, systemic factors, biomarkers, patient symptoms and operative risk will be integrated alongside an assessment of AS severity to influence management strategies regarding valve replacement.
In the near future, the realization of randomized trials might pave the way for new indications for AVR. The trials that should be considered a priority by the cardiology community include: early ‘prophylactic’ AVR versus a watchful waiting strategy in asymptomatic patients with severe AS; and TAVR combined with heart failure therapy versus heart failure therapy alone in patients with moderate AS, low LVEF and heart failure symptoms (Table 4). Also, the data from the ongoing and future trials will help to better individualize the type of AVR according to the baseline risk profile of patients. Results from some recent studies suggest that TAVR might be preferable to surgical AVR in patients with diabetes, chronic obstructive pulmonary disease, pulmonary hypertension, small aortic annulus and low-flow, low-gradient AS262–266.
Interventions after AVR to improve clinical outcomes
Given that AS is conceptualized as a mechanical problem (valve obstruction) in need of a mechanical solution (valve replacement), it is common to view the problem or disease of AS as ’fixed or solved’ after the valve is replaced, with little attention directed toward strategies and interventions that might improve clinical outcomes in the post-valve replacement period. We anticipate that there will be a growing recognition of factors that impair an optimal clinical outcome in patients with AS after valve replacement, with interventions identified that might improve these outcomes. These might include interventions such as adjunctive medical therapies (for example, anti-fibrotic and anti-hypertrophic agents) to improve LV reverse remodelling and function or lifestyle interventions targeting frail patients undergoing TAVR.
Figure 13. Different types of transcatheter aortic valve replacement.
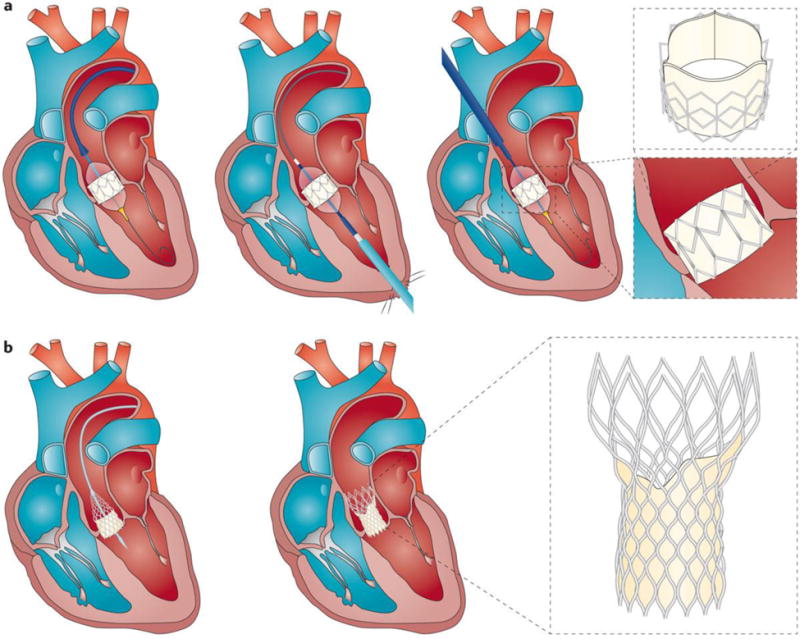
(A) Transcatheter aortic valve replacement with a balloon expandable valve via the transfemoral, transapical or transaortic approach. (B) Transcatheter aortic valve replacement with a self-expanding valve via the transfemoral approach.
Acknowledgments
B.R.L. is supported by NIH K23 HL116660 and was a Gilead Sciences Research Scholars Program in Cardiovascular Disease Award Recipient. P.P. holds the Canada Research Chair in Valvular Heart Disease and his research program is funded by the Canadian Institutes of Health Research (Grant numbers FDN-143225, MOP 126072, MOP 114997 and MOP 102737) (Ottawa, Ontario, Canada). P.M. is a research scholar from the Fonds de Recherche du Québec-Santé (FRQS) and his research programme is supported by Canadian Institutes of Health Research (MOP114893, MOP245048, MOP341860), the Heart and Stroke Foundation of Canada and the Fonds nature et Technologies-Québec.
Footnotes
Competing interests
B.R.L. has received research support from and served on the scientific advisory board for Roche Diagnostics. B.I. has received consultant fees from Abbott and Boehringer Ingelheim and speaker’s fees from Edwards Lifesciences. P.P. has Core Lab contracts with Edwards Lifesciences, for which he receives no direct compensation, and is a speaker for St. Jude Medical. The other authors have no disclosure.
Author contributions
Introduction (P.P.); Epidemiology (B.I. and P.P.); Mechanisms/pathophysiology (P.M. and P.P.); Diagnosis, screening and prevention (B.R.L., M.-A.C., P.L., P.M. and P.P.); Management (B.R.L., C.M.O., P.L., P.M. and P.P.); Quality of life (B.R.L., C.M.O. and P.P); Outlook (B.L. and P.P.); Overview of Primer (P.P)
References
- 1.Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group * executive summary: calcific aortic valve disease – 2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. A good review of the state of knowledge (in 2011) and future research directions for calcific aortic valve disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. Most recent version of the clinical guidelines for the management of valvular heart disease, including aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 5.Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- 6.Bosse Y, Mathieu P, Pibarot P. Genomics: the next step to elucidate the etiology of calcific aortic valve stenosis. J Am Coll Cardiol. 2008;51:1327–1336. doi: 10.1016/j.jacc.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 8.Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (BAVCon) Circulation. 2014;129:2691–2704. doi: 10.1161/CIRCULATIONAHA.113.007851. A review of the diagnosis, complications, and management of bicuspid aortic valve syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 10.Cosmi JE, Kort S, Tunick PA, et al. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. doi: 10.1001/archinte.162.20.2345. [DOI] [PubMed] [Google Scholar]
- 11.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2852–2861. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. An epidemiology study reporting the prevalence of valvular heart disease, including calcific aortic stenosis, in the general population. [DOI] [PubMed] [Google Scholar]
- 13.Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromso Study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 14.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 15.Danielsen R, Aspelund T, Harris TB, Gudnason V. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: the AGES-Reykjavik study. Int J Cardiol. 2014;176:916–922. doi: 10.1016/j.ijcard.2014.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Probst V, Le SS, Legendre A, et al. Familial aggregation of calcific aortic valve stenosis in the western part of France. Circulation. 2006;113:856–860. doi: 10.1161/CIRCULATIONAHA.105.569467. [DOI] [PubMed] [Google Scholar]
- 18.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe : The Euro Heart Survey on Valvular heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. A large European survey describing the clinical management of valvular heart diseaseand adherence to guidelines in the real-life clinical practice. [DOI] [PubMed] [Google Scholar]
- 19.Iung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart. 2012;98(Suppl 4):iv7–13. doi: 10.1136/heartjnl-2012-302395. [DOI] [PubMed] [Google Scholar]
- 20.Demirbag R, Sade LE, Aydin M, Bozkurt A, Acarturk E. The Turkish registry of heart valve disease. Turk Kardiyol Dern Ars. 2013;41:1–10. doi: 10.5543/tkda.2013.71430. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Carrington M, Mayosi BM, Zigiriadis E, Mvungi R, Stewart S. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the heart of Soweto study. Eur Heart J. 2010;31:719–727. doi: 10.1093/eurheartj/ehp530. [DOI] [PubMed] [Google Scholar]
- 22.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 23.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4:254–262. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 24.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 25.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 26.Taylor PM, Allen SP, Yacoub MH. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J Heart Valve Dis. 2000;9:150–158. [PubMed] [Google Scholar]
- 27.Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur Heart J. 2015;36:1335–1345. doi: 10.1093/eurheartj/eht547. [DOI] [PubMed] [Google Scholar]
- 28.Steiner I, Kasparova P, Kohout A, Dominik J. Bone formation in cardiac valves: a histopathological study of 128 cases. Virchows Arch. 2007;450:653–657. doi: 10.1007/s00428-007-0430-7. [DOI] [PubMed] [Google Scholar]
- 29.Cote N, Mahmut A, Bosse Y, et al. Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation. 2013;36:573–581. doi: 10.1007/s10753-012-9579-6. [DOI] [PubMed] [Google Scholar]
- 30.Helske S, Syvaranta S, Kupari M, et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27:1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu P, Boulanger MC. Basic Mechanisms of Calcific Aortic Valve Disease. Can J Cardiol. 2014;30:982–993. doi: 10.1016/j.cjca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Price PA, Toroian D, Chan WS. Tissue-nonspecific alkaline phosphatase is required for the calcification of collagen in serum: a possible mechanism for biomineralization. J Biol Chem. 2009;284:4594–4604. doi: 10.1074/jbc.M803205200. [DOI] [PubMed] [Google Scholar]
- 33.Price J, Lapierre H, Ressler L, Lam BK, Mesana TG, Ruel M. Prosthesis-patient mismatch is less frequent and more clinically indolent in patients operated for aortic insufficiency. J Thorac Cardiovasc Surg. 2009;138:639–645. doi: 10.1016/j.jtcvs.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Rattazzi M, Bertacco E, Iop L, et al. Extracellular pyrophosphate is reduced in aortic interstitial valve cells acquiring a calcifying profile: implications for aortic valve calcification. Atherosclerosis. 2014;237:568–576. doi: 10.1016/j.atherosclerosis.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Hinton RB, Jr, Lincoln J, Deutsch GH, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 36.Satta J, Melkko J, Pollanen R, et al. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. 2002;39:96–101. doi: 10.1016/s0735-1097(01)01705-3. [DOI] [PubMed] [Google Scholar]
- 37.Pawade TA, Newby DE, Dweck MR. Calcification in Aortic Stenosis: The Skeleton Key. J Am Coll Cardiol. 2015;66:561–577. doi: 10.1016/j.jacc.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 38.Abdelbaky A, Corsini E, Figueroa AL, et al. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis. 2015;238:165–172. doi: 10.1016/j.atherosclerosis.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 40.Mohty D, Pibarot P, Despres JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 2008;28:187–193. doi: 10.1161/ATVBAHA.107.154989. [DOI] [PubMed] [Google Scholar]
- 41.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 42.Cote C, Pibarot P, Despres JP, et al. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94:1175–1180. doi: 10.1136/hrt.2007.125740. [DOI] [PubMed] [Google Scholar]
- 43.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberman M, Bassi E, Martinatti MK, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 45.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 46.Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008;19:369–377. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 47.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–140. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 48.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. First large genetic study to demonstrate an association between the LPA gene with incident aortic stenosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 50.Arsenault BJ, Boekholdt SM, Dubé MP, et al. Lipoprotein(a) levels, genotype and incident aortic valve stenosis: A prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7:304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 51.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a) and progression of aortic valve stenosis. J Am Coll Cardiol. 2015;66:1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 52.Derbali H, Bossé Y, Côté N, et al. Increased biglycan in aortic valve stenosis leads to the overexpression of phospholipid transfer protein via toll-like receptor 2. Am J Pathol. 2010;176:2638–2645. doi: 10.2353/ajpath.2010.090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song R, Zeng Q, Ao L, et al. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler Thromb Vasc Biol. 2012;32:2711–2720. doi: 10.1161/ATVBAHA.112.300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahmut A, Boulanger MC, Fournier D, et al. Lipoprotein lipase in aortic valve stenosis is associated with lipid retention and remodelling. Eur J Clin Invest. 2013;43:570–578. doi: 10.1111/eci.12081. [DOI] [PubMed] [Google Scholar]
- 55.Osman N, Grande-Allen KJ, Ballinger ML, et al. Smad2-dependent glycosaminoglycan elongation in aortic valve interstitial cells enhances binding of LDL to proteoglycans. Cardiovasc Pathol. 2013;22:146–155. doi: 10.1016/j.carpath.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hung MY, Witztum JL, Tsimikas S. New therapeutic targets for calcific aortic valve stenosis: The lipoprotein(a)-lipoprotein-associated phospholipase A2-oxidized phospholipid axis. J Am Coll Cardiol. 2014;63:478–480. doi: 10.1016/j.jacc.2013.08.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmut A, Boulanger MC, El Husseini D, et al. Elevated expression of Lp-PLA2 in calcific aortic valve disease: Implication for valve mineralization. J Am Coll Cardiol. 2014;63:460–469. doi: 10.1016/j.jacc.2013.05.105. [DOI] [PubMed] [Google Scholar]
- 58.Mahmut A, Mahjoub H, Boulanger MC, et al. Lp-PLA2 is associated with structural valve degeneration of bioprostheses. Eur J Clin Invest. 2014;44:136–145. doi: 10.1111/eci.12199. [DOI] [PubMed] [Google Scholar]
- 59.Capoulade R, Mahmut A, Tastet L, et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis. J Am Coll Cardiol Img. 2015;8:26–33. doi: 10.1016/j.jcmg.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009;1791:327–338. doi: 10.1016/j.bbalip.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Lehti S, Kakela R, Horkko S, et al. Modified lipoprotein-derived lipid particles accumulate in human stenotic aortic valves. PLoS One. 2013;8:e65810. doi: 10.1371/journal.pone.0065810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouchareb R, Mahmut A, Nsaibia MJ, et al. Autotaxin Derived From Lipoprotein(a) and Valve Interstitial Cells Promotes Inflammation and Mineralization of the Aortic Valve. Circulation. 2015;132:677–690. doi: 10.1161/CIRCULATIONAHA.115.016757. [DOI] [PubMed] [Google Scholar]
- 63.Rogers MA, Aikawa E. A Not-So-Little Role for Lipoprotein(a) in the Development of Calcific Aortic Valve Disease. Circulation. 2015;132:621–623. doi: 10.1161/CIRCULATIONAHA.115.018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagy E, Andersson DC, Caidahl K, et al. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- 65.Wirrig EE, Gomez MV, Hinton RB, Yutzey KE. COX2 inhibition reduces aortic valve calcification in vivo. Arterioscler Thromb Vasc Biol. 2015;35:938–947. doi: 10.1161/ATVBAHA.114.305159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathieu P, Bouchareb R, Boulanger MC. Innate and Adaptive Immunity in Calcific Aortic Valve Disease. J Immunol Res. 2015;2015:851945. doi: 10.1155/2015/851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venardos N, Nadlonek NA, Zhan Q, et al. Aortic valve calcification is mediated by a differential response of aortic valve interstitial cells to inflammation. J Surg Res. 2014;190:1–8. doi: 10.1016/j.jss.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West XZ, Malinin NL, Merkulova AA, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winchester R, Wiesendanger M, O’Brien W, et al. Circulating activated and effector memory T cells are associated with calcification and clonal expansions in bicuspid and tricuspid valves of calcific aortic stenosis. J Immunol. 2011;187:1006–1014. doi: 10.4049/jimmunol.1003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshioka M, Yuasa S, Matsumura K, et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151–1159. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 71.Charest A, Pépin A, Shetty R, et al. Distribution of SPARC during the neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–1849. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosse Y, Miqdad A, Fournier D, Pepin A, Pibarot P, Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet. 2009;2:489–498. doi: 10.1161/CIRCGENETICS.108.820795. [DOI] [PubMed] [Google Scholar]
- 73.Helske S, Syvaranta S, Lindstedt KA, et al. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006;26:1791–1798. doi: 10.1161/01.ATV.0000228824.01604.63. [DOI] [PubMed] [Google Scholar]
- 74.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu YC, Boston JR, Simaan MA, Antak JF. Minimally invasive estimation of systemic vascular parameters. Ann Biomed Eng. 2001;29:595–606. doi: 10.1114/1.1380420. [DOI] [PubMed] [Google Scholar]
- 76.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 77.Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 2010;391:1087–1092. doi: 10.1016/j.bbrc.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 78.Isoda K, Matsuki T, Kondo H, Iwakura Y, Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol. 2010;30:708–715. doi: 10.1161/ATVBAHA.109.201749. [DOI] [PubMed] [Google Scholar]
- 79.Lai CF, Shao JS, Behrmann A, Krchma K, Cheng SL, Towler DA. TNFR1-activated reactive oxidative species signals up-regulate osteogenic Msx2 programs in aortic myofibroblasts. Endocrinology. 2012;153:3897–3910. doi: 10.1210/en.2012-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galeone A, Brunetti G, Oranger A, et al. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int J Cardiol. 2013;169:296–304. doi: 10.1016/j.ijcard.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 81.El HD, Boulanger MC, Mahmut A, et al. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: implication for calcific aortic valve disease. J Mol Cell Cardiol. 2014;72:146–156. doi: 10.1016/j.yjmcc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 82.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Kaden JJ, Bickelhaupt S, Grobholz R, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Weiss RM, Lund DD, Chu Y, et al. Osteoprotegerin inhibits aortic valve calcification and preserves valve function in hypercholesterolemic mice. PLoS One. 2013;8:e65201. doi: 10.1371/journal.pone.0065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Demer LL. Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int J Epidemiol. 2002;31:737–741. doi: 10.1093/ije/31.4.737. [DOI] [PubMed] [Google Scholar]
- 86.Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol. 2009;104:122–124. doi: 10.1016/j.amjcard.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 87.Osako MK, Nakagami H, Koibuchi N, et al. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 88.Helske S, Lindstedt KA, Laine M, et al. Induction of local angiotensin II-producing systems in stenotic aortic valves. J Am Coll Cardiol. 2004;44:1859–1866. doi: 10.1016/j.jacc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 89.O’Brien KD, Shavelle DM, Caulfield MT, et al. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 90.Cote N, Pibarot P, Pepin A, et al. Oxidized low-density lipoprotein, angiotensin II and increased waist cirumference are associated with valve inflammation in prehypertensive patients with aortic stenosis. Int J Cardiol. 2010;145:444–449. doi: 10.1016/j.ijcard.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 91.Fujisaka T, Hoshiga M, Hotchi J, et al. Angiotensin II promotes aortic valve thickening independent of elevated blood pressure in apolipoprotein-E deficient mice. Atherosclerosis. 2013;226:82–87. doi: 10.1016/j.atherosclerosis.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 92.Arishiro K, Hoshiga M, Negoro N, et al. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol. 2007;49:1482–1489. doi: 10.1016/j.jacc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 93.Cote N, Couture C, Pibarot P, Despres JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest. 2011;41:1172–1179. doi: 10.1111/j.1365-2362.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 94.Capoulade R, Clavel MA, Mathieu P, et al. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur J Clin Invest. 2013;43:1262–1272. doi: 10.1111/eci.12169. [DOI] [PubMed] [Google Scholar]
- 95.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holliday CJ, Ankeny RF, Jo H, Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H856–H867. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. First study to report that mutations in NOTCH1 cause both development of bicuspid aortic valve and calcification of aortic valve. [DOI] [PubMed] [Google Scholar]
- 98.Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009;47:828–834. doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nus M, MacGrogan D, Martinez-Poveda B, et al. Diet-induced aortic valve disease in mice haploinsufficient for the Notch pathway effector RBPJK/CSL. Arterioscler Thromb Vasc Biol. 2011;31:1580–1588. doi: 10.1161/ATVBAHA.111.227561. [DOI] [PubMed] [Google Scholar]
- 100.Deregowski V, Gazzerro E, Priest L, Rydziel S, Canalis E. Notch 1 overexpression inhibits osteoblastogenesis by suppressing Wnt/beta-catenin but not bone morphogenetic protein signaling. J Biol Chem. 2006;281:6203–6210. doi: 10.1074/jbc.M508370200. [DOI] [PubMed] [Google Scholar]
- 101.Theodoris CV, Li M, White MP, et al. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell. 2015;160:1072–1086. doi: 10.1016/j.cell.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun. 2004;320:424–427. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]
- 105.Zhang M, Liu X, Zhang X, et al. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2014;147:1073–1080. doi: 10.1016/j.jtcvs.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 106.Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12:576–583. doi: 10.1038/nmat3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bouchareb R, Boulanger MC, Fournier D, Pibarot P, Messaddeq Y, Mathieu P. Mechanical strain induces the production of spheroid mineralized microparticles in the aortic valve through a RhoA/ROCK-dependent mechanism. J Mol Cell Cardiol. 2014;67:49–59. doi: 10.1016/j.yjmcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 108.New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hjortnaes J, New SE, Aikawa E. Visualizing novel concepts of cardiovascular calcification. Trends Cardiovasc Med. 2013;23:71–79. doi: 10.1016/j.tcm.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cote N, El HD, Pepin A, et al. ATP acts as a survival signal and prevents the mineralization of aortic valve. J Mol Cell Cardiol. 2012;52:1191–1202. doi: 10.1016/j.yjmcc.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Mahmut A, Boulanger MC, Bouchareb R, Hadji F, Mathieu P. Adenosine derived from ecto-nucleotidases in calcific aortic valve disease promotes mineralization through A2a adenosine receptor. Cardiovasc Res. 2015;106:109–120. doi: 10.1093/cvr/cvv027. [DOI] [PubMed] [Google Scholar]
- 112.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis. 2005;14:353–357. [PubMed] [Google Scholar]
- 113.Bouchareb R, Cote N, Marie CB, et al. Carbonic anhydrase XII in valve interstitial cells promotes the regression of calcific aortic valve stenosis. J Mol Cell Cardiol. 2015;82:104–115. doi: 10.1016/j.yjmcc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 115.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–1107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 117.Pagé A, Dumesnil JG, Clavel MA, et al. Metabolic syndrome is associated with more pronounced impairment of LV geometry and function in patients with calcific aortic stenosis: A substudy of the ASTRONOMER trial. (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 118.Lund BP, Gohlke-Barwolf C, Cramariuc D, Rossebo AB, Rieck AE, Gerdts E. Effect of obesity on left ventricular mass and systolic function in patients with asymptomatic aortic stenosis (a Simvastatin Ezetimibe in Aortic Stenosis [SEAS] substudy) Am J Cardiol. 2010;105:1456–1460. doi: 10.1016/j.amjcard.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 119.Lindman BR, Arnold SV, Madrazo JA, et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–292. doi: 10.1161/CIRCHEARTFAILURE.110.960039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cramariuc D, Cioffi G, Rieck AE, et al. Low-flow aortic stenosis in asymptomatic patients: Valvular arterial impedance and systolic function from the SEAS substudy. J Am Coll Cardiol Img. 2009;2:390–399. doi: 10.1016/j.jcmg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 121.Cioffi G, Faggiano P, Vizzardi E, et al. Prognostic value of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 122.Duncan AI, Lowe BS, Garcia MJ, et al. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg. 2008;85:2030–2039. doi: 10.1016/j.athoracsur.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 123.Rajappan K, Rimoldi OE, Dutka DP, et al. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105:470–476. doi: 10.1161/hc0402.102931. [DOI] [PubMed] [Google Scholar]
- 124.Rajappan K, Rimoldi OE, Camici PG, Bellenger NG, Pennell DJ, Sheridan DJ. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation. 2003;107:3170–3175. doi: 10.1161/01.CIR.0000074211.28917.31. [DOI] [PubMed] [Google Scholar]
- 125.Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation. 1997;95:892–898. doi: 10.1161/01.cir.95.4.892. [DOI] [PubMed] [Google Scholar]
- 126.Mutlak D, Aronson D, Carasso S, Lessick J, Reisner SA, Agmon Y. Frequency, determinants and outcome of pulmonary hypertension in patients with aortic valve stenosis. Am J Med Sci. 2012;343:397–401. doi: 10.1097/MAJ.0b013e3182309431. [DOI] [PubMed] [Google Scholar]
- 127.Lancellotti P, Magne J, Donal E, et al. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. 2012;126:851–859. doi: 10.1161/CIRCULATIONAHA.111.088427. [DOI] [PubMed] [Google Scholar]
- 128.Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–1034. doi: 10.1161/01.cir.59.5.1024. [DOI] [PubMed] [Google Scholar]
- 129.Lancellotti P, Donal E, Magne J, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–1371. doi: 10.1136/hrt.2009.190942. Prospective study that shows the prognostic value of valve stenosis severity, valvulo-arterial impedance, left ventricular longitudinal function, and left atrial dilation in patients with calcific aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 130.Kusunose K, Goodman A, Parikh R, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7:938–945. doi: 10.1161/CIRCIMAGING.114.002041. [DOI] [PubMed] [Google Scholar]
- 131.Fernandez B, Duran AC, Fernandez-Gallego T, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54:2312–2318. doi: 10.1016/j.jacc.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 132.Mathieu P, Bossé Y, Huggins GS, et al. The pathology and pathobiology of bicuspid aortic valves: State of the art and novel research perspective. J Path: Clin Res. 2015;1:195–206. doi: 10.1002/cjp2.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mohty D, Pibarot P, Despres JP, et al. Age-related differences in the pathogenesis of calcific aortic stenosis: The potential role of resistin. Int J Cardiol. 2010;142:126–132. doi: 10.1016/j.ijcard.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 134.Aronow WS, Schwartz KS, Koenigsberg M. Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol. 1987;59:998–999. doi: 10.1016/0002-9149(87)91144-1. [DOI] [PubMed] [Google Scholar]
- 135.Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 136.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors–a causal relationship? A clinical morphologic study. Clin Cardiol. 1991;14:995–999. doi: 10.1002/clc.4960141210. [DOI] [PubMed] [Google Scholar]
- 137.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–70. doi: 10.1161/01.cir.95.9.2262. One of the first prospective studies to describe the clinical and echocardiographic predictors of the progression and outcomes of calcific aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 138.Livanainen AM, Lindroos M, Tilvis R, Heikkila J, Kupari M. Natural history of aortic valve stenosis of varying severity in the elderly. Am J Cardiol. 1996;78:97–101. doi: 10.1016/s0002-9149(96)00235-4. [DOI] [PubMed] [Google Scholar]
- 139.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101:2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- 140.Ngo MV, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ. Smoking and obesity are associated with the progression of aortic stenosis. Am J Geriatr Cardiol. 2001;10:86–90. doi: 10.1111/j.1076-7460.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 141.Capoulade R, Clavel MA, Dumesnil JG, et al. Impact of metabolic syndrome on progression of aortic stenosis: Influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. doi: 10.1016/j.jacc.2012.03.052. Post-hoc analysis of the ASTRONOMER study showing that metabolic syndrome is associated with faster progression of aortic stenosis and that statins may accelerate the stenosis progression in these patients. [DOI] [PubMed] [Google Scholar]
- 142.Hekimian G, Boutten A, Flamant M, et al. Progression of aortic valve stenosis is associated with bone remodelling and secondary hyperparathyroidism in elderly patients–the COFRASA study. Eur Heart J. 2013;34:1915–1922. doi: 10.1093/eurheartj/ehs450. [DOI] [PubMed] [Google Scholar]
- 143.Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 144.Rieck AE, Cramariuc D, Boman K, et al. Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension. 2012;60:90–97. doi: 10.1161/HYPERTENSIONAHA.112.194878. [DOI] [PubMed] [Google Scholar]
- 145.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 146.Laforest B, Andelfinger G, Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. J Clin Invest. 2011;121:2876–2887. doi: 10.1172/JCI44555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shi LM, Tao JW, Qiu XB, et al. GATA5 loss-of-function mutations associated with congenital bicuspid aortic valve. Int J Mol Med. 2014;33:1219–1226. doi: 10.3892/ijmm.2014.1700. [DOI] [PubMed] [Google Scholar]
- 148.Foffa I, Ait AL, Panesi P, et al. Sequencing of NOTCH1, GATA5, TGFBR1 and TGFBR2 genes in familial cases of bicuspid aortic valve. BMC Med Genet. 2013;14:44. doi: 10.1186/1471-2350-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ducharme V, Guauque-Olarte S, Pibarot P, Mathieu P, Bossé Y. NOTCH1 genetic variants in patients with tricuspid calcific aortic valve stenosis. J Heart Valve Dis. 2013;22:142–149. [PubMed] [Google Scholar]
- 150.Guauque-Olarte S, Messika-Zeitoun D, Droit A, et al. Calcium signalings pathway genes RUNX2 and CACNA1C are associated with calcific aortic valve disease. Circ Cardiovasc Genet. 2015 doi: 10.1161/CIRCGENETICS.115.001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith JG, Luk K, Schulz CA, et al. Association of Low-Density Lipoprotein Cholesterol-Related Genetic Variants With Aortic Valve Calcium and Incident Aortic Stenosis. JAMA. 2014;312:1764–1771. doi: 10.1001/jama.2014.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 153.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. Randomized clinical trial that reported no effect of statins on the progression and outcomes of calcific aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 154.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis. Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 155.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 156.Pibarot P, Dumesnil JG. Low-flow, low-gradient aortic stenosis with normal and depressed left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1845–1853. doi: 10.1016/j.jacc.2012.06.051. Review on the diagnosis and management of low-flow, low-gradient aortic stenosis, which is one of the most challenging entities in patients with valvular heart diseases. [DOI] [PubMed] [Google Scholar]
- 157.Nishimura RA, Carabello BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation. 2012;125:2138–2150. doi: 10.1161/CIRCULATIONAHA.111.060319. [DOI] [PubMed] [Google Scholar]
- 158.Omran H, Schmidt H, Hackenbroch M, et al. Silent and apparent cerebral embolism after retrograde catheterisation of the aortic valve in valvular stenosis: a prospective, randomised study. Lancet. 2003;361:1241–1246. doi: 10.1016/S0140-6736(03)12978-9. [DOI] [PubMed] [Google Scholar]
- 159.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 160.Owens DS, Budoff MJ, Katz R, et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. J Am Coll Cardiol. 2012;5:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–I382. doi: 10.1161/CIRCULATIONAHA.104.523274. [DOI] [PubMed] [Google Scholar]
- 162.Maréchaux S, Hachicha Z, Bellouin A, et al. Usefulness of exercise stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–1397. doi: 10.1093/eurheartj/ehq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Monin JL, Quere JP, Monchi M, et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–324. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- 164.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 165.Blais C, Burwash IG, Mundigler G, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low flow, low-gradient aortic stenosis: The multicenter TOPAS (Truly or Pseudo Severe Aortic Stenosis) study. Circulation. 2006;113:711–721. doi: 10.1161/CIRCULATIONAHA.105.557678. [DOI] [PubMed] [Google Scholar]
- 166.Clavel MA, Burwash IG, Mundigler G, et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: the multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr. 2010;23:380–386. doi: 10.1016/j.echo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 167.Tribouilloy C, Levy F, Rusinaru D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–1873. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 168.Clavel MA, Ennezat PV, Maréchaux S, et al. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. J Am Coll Cardiol Img. 2013;6:175–183. doi: 10.1016/j.jcmg.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 169.Clavel MA, Messika-Zeitoun D, Pibarot P, et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–2338. doi: 10.1016/j.jacc.2013.08.1621. Multicenter study demonstrating the usefulness of aortic valve calcium scoring by multi-detector computed tomography to corroborate the stenosis severity in patients with discordant findings at echocardiography. [DOI] [PubMed] [Google Scholar]
- 170.Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–1213. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 172.Jenkins WS, Vesey AT, Shah AS, et al. Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol. 2015;66:1200–1201. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]
- 173.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96:1463–1468. doi: 10.1136/hrt.2009.181982. [DOI] [PubMed] [Google Scholar]
- 174.Dayan V, Vignolo G, Magne J, Clavel MA, Mohty D, Pibarot P. Outcome and Impact of Aortic Valve Replacement in Patients with Preserved LV Ejection Fraction and Low Gradient Aortic Stenosis: A Meta-analysis. J Am Coll Cardiol. 2015;66:2594–603. doi: 10.1016/j.jacc.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 175.Hyafil F, Messika-Zeitoun D, Burg S, et al. Detection of (18)fluoride sodium accumulation by positron emission tomography in calcified stenotic aortic valves. Am J Cardiol. 2012;109:1194–1196. doi: 10.1016/j.amjcard.2011.11.060. [DOI] [PubMed] [Google Scholar]
- 176.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 177.Dweck MR, Jenkins WS, Vesey AT, et al. 18F-NaF uptake Is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. Study that demonstrates the utility of sodium fluoride positron emission tomography to detect the mineralization activity in the aortic valve and predict the rapidity of aortic stenosis progression. [DOI] [PubMed] [Google Scholar]
- 178.Le Ven F, Tizon-Marcos H, Fuchs C, Mathieu P, Pibarot P, Larose E. Valve tissue characterization by magnetic resonance imaging in calcific aortic valve disease. Can J Cardiol. 2014;30:1676–1683. doi: 10.1016/j.cjca.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 179.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 180.Hope MD, Hope TA, Crook SES, et al. 4D flow CMR in assessment of valve-related ascending aortic disease. J Am Coll Cardiol. 2011;4:781–787. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 181.Bartko PE, Heinze G, Graf S, et al. Two-dimensional strain for the assessment of left ventricular function in low flow-low gradient aortic stenosis, relationship to hemodynamics and outcome : A substudy of the multicenter TOPAS study. Circ Cardiovasc Imaging. 2012;6:268–276. doi: 10.1161/CIRCIMAGING.112.980201. [DOI] [PubMed] [Google Scholar]
- 182.Dahou A, Bartko PE, Capoulade R, et al. Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low-gradient aortic stenosis: results from the multicenter true or pseudo-severe aortic stenosis study. Circ Cardiovasc Imaging. 2015;8:e002117. doi: 10.1161/CIRCIMAGING.114.002117. [DOI] [PubMed] [Google Scholar]
- 183.Lancellotti P, Donal E, Magne J, et al. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr. 2010;11:537–543. doi: 10.1093/ejechocard/jeq014. [DOI] [PubMed] [Google Scholar]
- 184.Dahl JS, Eleid MF, Michelena HI, et al. Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002917. [DOI] [PubMed] [Google Scholar]
- 185.Lancellotti P, Moonen M, Magne J, et al. Prognostic effect of long-axis left ventricular dysfunction and B-type natriuretic peptide levels in asymptomatic aortic stenosis. Am J Cardiol. 2010;105:383–388. doi: 10.1016/j.amjcard.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 186.Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol. 2011;8:494–501. doi: 10.1038/nrcardio.2011.80. [DOI] [PubMed] [Google Scholar]
- 187.Clavel MA, Malouf J, Michelena HI, et al. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J Am Coll Cardiol. 2014;63:2016–2025. doi: 10.1016/j.jacc.2014.02.581. Study that demonstrates the prognostic value of plasma B-type natriuretic peptide (BNP) in patients with calcific aortic stenosis. This study shows the importance of normalizing the measured level of BNP for the patient’s age and sex normal refrence value. [DOI] [PubMed] [Google Scholar]
- 188.Weidemann F, Herrmann S, Stork S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 189.Azevedo CF, Nigri M, Higuchi ML, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. One of the first studies to demonstrate the prognostic value of myocardial fibrosis measured by cardiac magnetic resonance in patients with calcific aortic stenosis. [DOI] [PubMed] [Google Scholar]
- 190.Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 191.Milano AD, Faggian G, Dodonov M, et al. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg. 2012;144:830–837. doi: 10.1016/j.jtcvs.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 192.Herrmann S, Stork S, Niemann M, et al. Low-gradient aortic valve stenosis: Myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 193.Nazarian S. Is ventricular arrhythmia a possible mediator of the association between aortic stenosis-related midwall fibrosis and mortality? J Am Coll Cardiol. 2011;58:1280–1282. doi: 10.1016/j.jacc.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 194.Chin CW, Shah AS, McAllister DA, et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rosjo H, Andreassen J, Edvardsen T, Omland T. Prognostic usefulness of circulating high-sensitivity troponin T in aortic stenosis and relation to echocardiographic indexes of cardiac function and anatomy. Am J Cardiol. 2011;108:88–91. doi: 10.1016/j.amjcard.2011.02.346. [DOI] [PubMed] [Google Scholar]
- 196.Chen Z, Li C, Xu Y, Li Y, Yang H, Rao L. Circulating level of miR-378 predicts left ventricular hypertrophy in patients with aortic stenosis. PLoS One. 2014;9:e105702. doi: 10.1371/journal.pone.0105702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rosjo H, Dahl MB, Bye A, et al. Prognostic value of circulating microRNA-210 levels in patients with moderate to severe aortic stenosis. PLoS One. 2014;9:e91812. doi: 10.1371/journal.pone.0091812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lindman BR, Breyley JG, Schilling JD, et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart. 2015 doi: 10.1136/heartjnl-2015-307742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lindman BR, Bonow RO, Otto CM. Current management of calcific aortic stenosis. Circ Res. 2013;113:223–237. doi: 10.1161/CIRCRESAHA.111.300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 201.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Eng J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. Randomized clinical trial that demonstrates the major superiority of transcatheter aortic valve replacement compared to conservative management in patients who can not undergo surgical aortic valve replacement. [DOI] [PubMed] [Google Scholar]
- 202.Lindman BR, Alexander KP, O’Gara PT, Afilalo J. Futility, Benefit, and Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv. 2014;7:707–716. doi: 10.1016/j.jcin.2014.01.167. Review that describes the challenges and strategies for decision making in patients with severe calcific aortic stenosis who are potential candidates for transcatheter aortic valve replacement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Holmes DR, Jr, Rich JB, Zoghbi WA, Mack MJ. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61:903–907. doi: 10.1016/j.jacc.2012.08.1034. [DOI] [PubMed] [Google Scholar]
- 204.Holmes DR, Jr, Mohr F, Hamm CW, Mack MJ. Venn diagrams in cardiovascular disease: the Heart Team concept. Eur J Cardiothorac Surg. 2013;43:255–257. doi: 10.1093/ejcts/ezs656. [DOI] [PubMed] [Google Scholar]
- 205.Holmes DR, Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 206.Levy F, Laurent M, Monin JL, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis: Operative risk stratification and long-term outcome: A European multicenter study. Journal of the American College of Cardiology. 2008;51:1466–1472. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 207.Harken DE, Soroff HS, Taylor WJ, Lefemin AA, Gupta SK, Lunzer S. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–762. [PubMed] [Google Scholar]
- 208.Lee R, Li S, Rankin JS, et al. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg. 2011;91:677–684. doi: 10.1016/j.athoracsur.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 209.Brown JM, O’Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 210.Hamm CW, Mollmann H, Holzhey D, et al. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J. 2014;35:1588–1598. doi: 10.1093/eurheartj/eht381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.David TE, Woo A, Armstrong S, Maganti M. When is the Ross operation a good option to treat aortic valve disease? J Thorac Cardiovasc Surg. 2010;139:68–73. doi: 10.1016/j.jtcvs.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 212.Stulak JM, Burkhart HM, Sundt TM, III, et al. Spectrum and outcome of reoperations after the Ross procedure. Circulation. 2010;122:1153–1158. doi: 10.1161/CIRCULATIONAHA.109.897538. [DOI] [PubMed] [Google Scholar]
- 213.David TE. Reoperations after the Ross procedure. Circulation. 2010;122:1139–1140. doi: 10.1161/CIRCULATIONAHA.110.977991. [DOI] [PubMed] [Google Scholar]
- 214.Chiang YP, Chikwe J, Moskowitz AJ, Itagaki S, Adams DH, Egorova NN. Survival and long-term outcomes following bioprosthetic vs mechanical aortic valve replacement in patients aged 50 to 69 years. JAMA. 2014;312:1323–1329. doi: 10.1001/jama.2014.12679. [DOI] [PubMed] [Google Scholar]
- 215.Brown ML, McKellar SH, Sundt TM, Schaff HV. Ministernotomy versus conventional sternotomy for aortic valve replacement: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2009;137:670–679. doi: 10.1016/j.jtcvs.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 216.Astor BC, Kaczmarek RG, Hefflin B, Daley WR. Mortality after aortic valve replacement: results from a nationally representative database. Ann Thorac Surg. 2000;70:1939–1945. doi: 10.1016/s0003-4975(00)01670-2. [DOI] [PubMed] [Google Scholar]
- 217.Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112:224–231. doi: 10.1161/CIRCULATIONAHA.104.515049. [DOI] [PubMed] [Google Scholar]
- 218.Ashikhmina EA, Schaff HV, Dearani JA, et al. Aortic valve replacement in the elderly: determinants of late outcome. Circulation. 2011;124:1070–1078. doi: 10.1161/CIRCULATIONAHA.110.987560. [DOI] [PubMed] [Google Scholar]
- 219.Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr, Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2:533–539. doi: 10.1161/CIRCOUTCOMES.109.848259. [DOI] [PubMed] [Google Scholar]
- 220.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 221.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. Randomized clinical trial that demonstrates a superiority of transcatheter aortic valve replacement compared to surgical aortic valve replacementin patients with high surgical risk. [DOI] [PubMed] [Google Scholar]
- 222.Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–2491. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 223.Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 224.Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 225.Biere L, Launay M, Pinaud F, et al. Influence of sex on mortality and perioperative outcomes in patients undergoing TAVR: insights from the FRANCE 2 registry. J Am Coll Cardiol. 2015;65:755–757. doi: 10.1016/j.jacc.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 226.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Eng J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 227.Ludman PF, Moat N, de Belder MA, et al. Transcatheter Aortic Valve Implantation in the United Kingdom: Temporal Trends, Predictors of Outcome, and 6-Year Follow-Up: A Report From the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation. 2015;131:1181–1190. doi: 10.1161/CIRCULATIONAHA.114.013947. [DOI] [PubMed] [Google Scholar]
- 228.Holmes DR, Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 229.Blackstone EH, Suri RM, Rajeswaran J, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral TAVR: A PARTNER-I Trial Substudy. Circulation. 2015;131:1989–2000. doi: 10.1161/CIRCULATIONAHA.114.012525. [DOI] [PubMed] [Google Scholar]
- 230.Martinez-Clark PO, Singh V, Cadena JA, et al. Transcaval retrograde transcatheter aortic valve replacement for patients with no other access: first-in-man experience with CoreValve. JACC Cardiovasc Interv. 2014;7:1075–1077. doi: 10.1016/j.jcin.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 231.Moat NE, Ludman P, Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 232.Walther T, Hamm CW, Schuler G, et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol. 2015;65:2173–2180. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 233.Rodes-Cabau J. Transcatheter aortic valve implantation: current and future approaches. Nat Rev Cardiol. 2012;9:15–29. doi: 10.1038/nrcardio.2011.164. [DOI] [PubMed] [Google Scholar]
- 234.Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61:1585–1595. doi: 10.1016/j.jacc.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 235.Genereux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol. 2013;61:1125–1136. doi: 10.1016/j.jacc.2012.08.1039. [DOI] [PubMed] [Google Scholar]
- 236.Pibarot P, Hahn RT, Weissman NJ, Monaghan MJ. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8:340–360. doi: 10.1016/j.jcmg.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 237.Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36:449–456. doi: 10.1093/eurheartj/ehu384. [DOI] [PubMed] [Google Scholar]
- 238.Van Belle E, Juthier F, Susen S, et al. Postprocedural aortic regurgitation in balloon-expandable and self-expandable TAVR procedures: Analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation. 2014;129:1415–1427. doi: 10.1161/CIRCULATIONAHA.113.002677. [DOI] [PubMed] [Google Scholar]
- 239.Babaliaros V, Devireddy C, Lerakis S, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv. 2014;7:898–904. doi: 10.1016/j.jcin.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 240.Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. doi: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 241.Makkar RR, Jilaihawi H, Mack M, et al. Stratification of outcomes after transcatheter aortic valve replacement according to surgical inoperability for technical versus clinical reasons. J Am Coll Cardiol. 2014;63:901–911. doi: 10.1016/j.jacc.2013.08.1641. [DOI] [PubMed] [Google Scholar]
- 242.Toggweiler S, Boone RH, Rodes-Cabau J, et al. Transcatheter aortic valve replacement: outcomes of patients with moderate or severe mitral regurgitation. J Am Coll Cardiol. 2012;59:2068–2074. doi: 10.1016/j.jacc.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 243.Lindman BR, Maniar HS, Jaber WA, et al. Effect of Tricuspid Regurgitation and the Right Heart on Survival After Transcatheter Aortic Valve Replacement: Insights From the Placement of Aortic Transcatheter Valves II Inoperable Cohort. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 245.Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 246.Svensson LG, Blackstone EH, Rajeswaran J, et al. Comprehensive analysis of mortality among patients undergoing TAVR: Results of the PARTNER trial. J Am Coll Cardiol. 2014;64:158–168. doi: 10.1016/j.jacc.2013.08.1666. [DOI] [PubMed] [Google Scholar]
- 247.Goel SS, Ige M, Tuzcu EM, et al. Severe aortic stenosis and coronary artery disease – implications for management in the transcatheter aortic valve replacement era: A comprehensive review. J Am Coll Cardiol. 2013;62:1–10. doi: 10.1016/j.jacc.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 248.Stefanini GG, Stortecky S, Cao D, et al. Coronary artery disease severity and aortic stenosis: clinical outcomes according to SYNTAX score in patients undergoing transcatheter aortic valve implantation. Eur Heart J. 2014;35:2530–2540. doi: 10.1093/eurheartj/ehu074. [DOI] [PubMed] [Google Scholar]
- 249.Poulin A, Rodes CJ, Paradis JM. Management of coronary disease in the era of transcatheter aortic valve replacement: comprehensive review of the literature. In: Kodali SK, Price MJ, editors. Transcatheter aortic valve replacement. 1. 2015. pp. 13–21. [DOI] [PubMed] [Google Scholar]
- 250.Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation. 1994;89:642–650. doi: 10.1161/01.cir.89.2.642. [DOI] [PubMed] [Google Scholar]
- 251.Kapadia S, Stewart WJ, Anderson WN, et al. Outcomes of inoperable symptomatic aortic stenosis patients not undergoing aortic valve replacement: insight into the impact of balloon aortic valvuloplasty from the PARTNER trial (Placement of AoRtic TraNscathetER Valve trial) JACC Cardiovasc Interv. 2015;8:324–333. doi: 10.1016/j.jcin.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 252.Otto CM, Prendergast B. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 253.Schwarz F, Baumann P, Manthey J, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 254.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–981. doi: 10.1016/j.jcin.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI) Eur Heart J. 2013;34:692. doi: 10.1093/eurheartj/ehs304. [DOI] [PubMed] [Google Scholar]
- 256.Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489–496. doi: 10.1016/j.jcin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 257.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–1278. doi: 10.1016/j.jtcvs.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 258.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 259.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: A disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 260.Biner S, Rafique AM, Goykhman P, Morrissey RP, Naghi J, Siegel RJ. Prognostic value of E/E′ ratio in patients with unoperated severe aortic stenosis. JACC Cardiovasc Imaging. 2010;3:899–907. doi: 10.1016/j.jcmg.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 261.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 262.Lindman BR, Pibarot P, Arnold SV, et al. Transcatheter versus surgical aortic valve replacement in patients with diabetes and severe aortic stenosis at high risk for surgery: An analysis of the PARTNER Trial (Placement of Aortic Transcatheter Valve) J Am Coll Cardiol. 2014;63:1090–1099. doi: 10.1016/j.jacc.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Herrmann HC, Pibarot P, Hueter I, et al. Predictors of mortality and outcomes of therapy in low flow severe aortic stenosis: A PARTNER trial analysis. Circulation. 2013;127:2316–2326. doi: 10.1161/CIRCULATIONAHA.112.001290. [DOI] [PubMed] [Google Scholar]
- 264.Dvir D, Waksman R, Barbash IM, et al. Outcomes of patients with chronic lung disease and severe aortic stenosis treated with transcatheter versus surgical aortic valve replacement or standard therapy: Insights for the PARTNER Trial. J Am Coll Cardiol. 2014;63:269–279. doi: 10.1016/j.jacc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 265.Mok M, Nombela-Franco L, Dumont E, et al. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. 2013;6:1072–1084. doi: 10.1016/j.jcin.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 266.Rodes-Cabau J, Pibarot P, Suri RM, et al. Impact of aortic annulus size on valve hemodynamics and clinical outcomes after transcatheter and surgical aortic valve replacement: insights from the PARTNER Trial. Circ Cardiovasc Interv. 2014;7:701–711. doi: 10.1161/CIRCINTERVENTIONS.114.001681. [DOI] [PubMed] [Google Scholar]
- 267.Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level–risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8:673–685. doi: 10.1038/nrcardio.2011.154. [DOI] [PubMed] [Google Scholar]
- 268.Bull S, White SK, Piechnik SK, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 270.Kodali SK, Williams MR, Smith CR, et al. Two-Year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. Article that reports the 2-year outcomes of the first randomized trial comparing transcatheter versus surgical aortic valve replacement. This study shows that paravalvular regurgitation following transcatheter aortic valve replacement is associated with increased risk of mortality. [DOI] [PubMed] [Google Scholar]
- 271.Webb JG, Doshi D, Mack M, et al. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv. 2015;8:1797–806. doi: 10.1016/j.jcin.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 272.Kodali S, the PARTNER 3 TAVR investigators Clinical and echocardiographic outcomes at 30 days with the SAPIEN 3 TAVR system in inoperable, high-risk and intermediate-risk AS patients. [abstract]Kodali S, and the PARTNER 3 TAVR investigators. J Am Coll Cardiol. 2015 [Google Scholar]
- 273.Herrmann HC. Evaluation of a balloon-expandable transcatheter aortic valve in high-risk and inoperable patients with aortic stenosis – One year outcomes [abstract]Herrmann HC. J Am Coll Cardiol. 2015 [Google Scholar]
- 274.Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113–121. doi: 10.1016/j.jacc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 275.Abdel-Wahab M, Mehilli J, Frerker C, et al. Comparison of Balloon-Expandable vs Self-expandable Valves in Patients Undergoing Transcatheter Aortic Valve Replacement: The CHOICE Randomized Clinical Trial. JAMA. 2014;311:1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 276.Thyregod HG, Steinbruchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: one-year results from the all-comers nordic aortic valve intervention (NOTION) randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. doi: 10.1016/j.jacc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 277.Clinical Trials Registry. National Insitutes of Health. https://clinicaltrials.gov/


