Summary
Human pluripotent stem cells (hPSCs) provide a model to study early neural development, model pathological processes, and develop therapeutics. The generation of functionally specialized neural subtypes from hPSCs relies on fundamental developmental principles learned from animal studies. Manipulation of these principles enables production of highly enriched neural types with functional attributes that resemble those in the brain. Further development to promote faster maturation or aging as well as circuit integration will help realize the potential of hPSC-derived neural cells in disease modelling and cell therapy.
Introduction
The human brain is built by neurons and glia that form ordered but intricate networks. Specialized subtypes of neurons and glia dictate the complexity of these neural networks, but the precise number of neural subtypes that are present in the brain is not known. Recent single cell profiling studies point to a diverse array of cell types in the mouse (Usoskin et al., 2015; Zeisel et al., 2015) and human brain (Lake et al., 2016), and how this cellular diversity arises, particularly in the human brain, is also not fully understood. Human pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), offer a model to examine the specification of neural subtypes in human.
Neurons and glia undergo degenerative changes with age. Most neurological diseases preferentially damage specific neural subtypes, at least at an early stage. In Parkinson’s disease (PD), midbrain dopamine neurons, especially those regulating motor functions, are degenerated whereas in Huntington’s disease medium spiny GABA (r-butyric acid) neurons in the striatum are the prime target. In spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS), motor neurons are preferentially affected and degenerated. Among the motor neurons, the fast-twitch fatigable motor neurons undergo the earliest axonal dieback while motor neurons controlling eye and pelvic sphincter are largely preserved (Kanning et al., 2010), highlighting the sensitivity of motor neuron subtypes to pathological insults. Why certain types of neurons are sensitive or resistant to the same insult remains largely unknown. The ability to guide hPSCs to highly enriched or pure neural subtypes may reveal the molecular makeup behind the vulnerability and enable modelling disease processes.
Substantial progress has been made in guiding hPSCs to regionally and functionally specialized neural subtypes. Analysis of these differentiation processes reveals general principles and common strategies for specification of neural subtypes. Manipulation of these principles enables production of highly enriched neural types with functional attributes that resemble those in the brain, setting up the foundation for modelling pathological processes as well as identifying drugs and devising therapeutics for neurological conditions.
Specification of multi-potent neuroepithelia
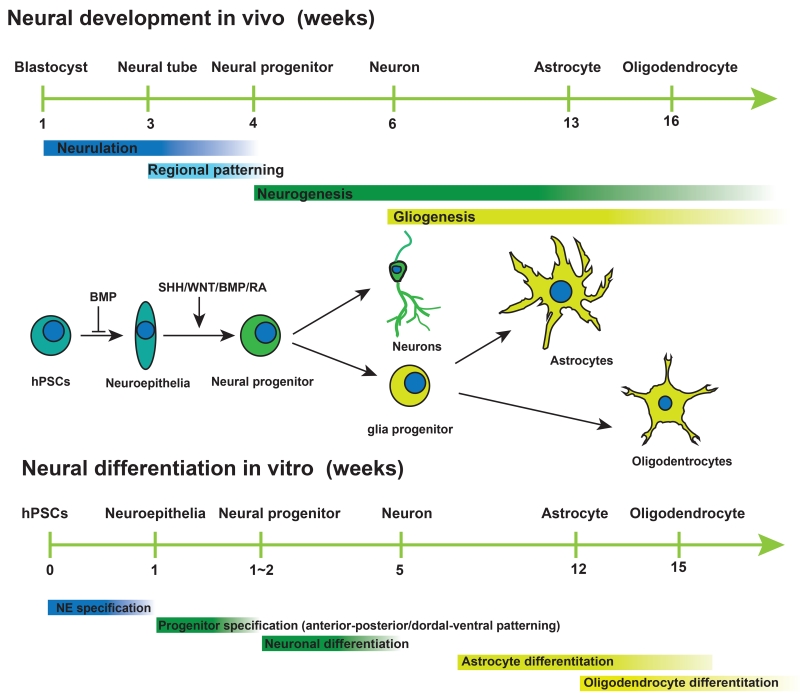
Development of the vertebrate nervous system initiates at the gastrula stage when the ectoderm cells become specialized toward the neural fate, in a process called neural induction. This process is triggered when the “organizer” cells move underneath the ectoderm and release molecules that inhibit the BMP (bone morphogenetic protein) signalling and/or activate the FGF (fibroblast growth factor) pathways (Munoz-Sanjuan and Brivanlou, 2002; Stern, 2005). The first step in neural differentiation essentially follows the neural induction principle (Figure 1). Human PSCs are switched from the self-renewing condition by removing medium components that promote self-renewal. This step is sufficient to trigger differentiation toward all the three embryonic germ layers. Culture of the differentiating cells under the serum-free medium favours neural and limits meso-endoderm cells, thus resulting in the majority of cells becoming neuroepithelia (NE) or neural stem cells (NSC) (Reubinoff et al., 2001; Zhang et al., 2001). Under this condition, exogenous growth factors, such as FGFs or BMP inhibitors, are not necessary, because differentiating cells produce FGFs and BMP inhibitors themselves (LaVaute et al., 2009; Yoo et al., 2011), which perpetuates the neural differentiation process. Historically, neural differentiation involves the formation of PSC aggregates, or embryoid bodies (EBs), which are highly sensitive to variation from experiment to experiment. To overcome this issue, a method was developed to prevent differentiation of extraembryoinc and meso-endoderm tissues using inhibitors of the SMAD-dependent TGFβ (Transforming growth factor beta) and BMP signalling pathways with SB431542 and Noggin (Chambers et al., 2009). This “dual-SMAD inhibition” method efficiently converts hPSCs to NE. This is particularly useful for “monolayer culture” that avoid the formation of EBs in order to reduce culture variability. Nowadays, most laboratories use hybrid methods based on these two protocols.
Figure 1. Parallel between in vitro differentiation in vivo neural development.
Cartoon illustrating the major developmental events in vivo and neural differentiation process in vitro and the morphogens that govern the neural induction, patterning of neural progenitors and neuronal and glial differentiation of the progenitors.
Specification of subtype neural progenitors
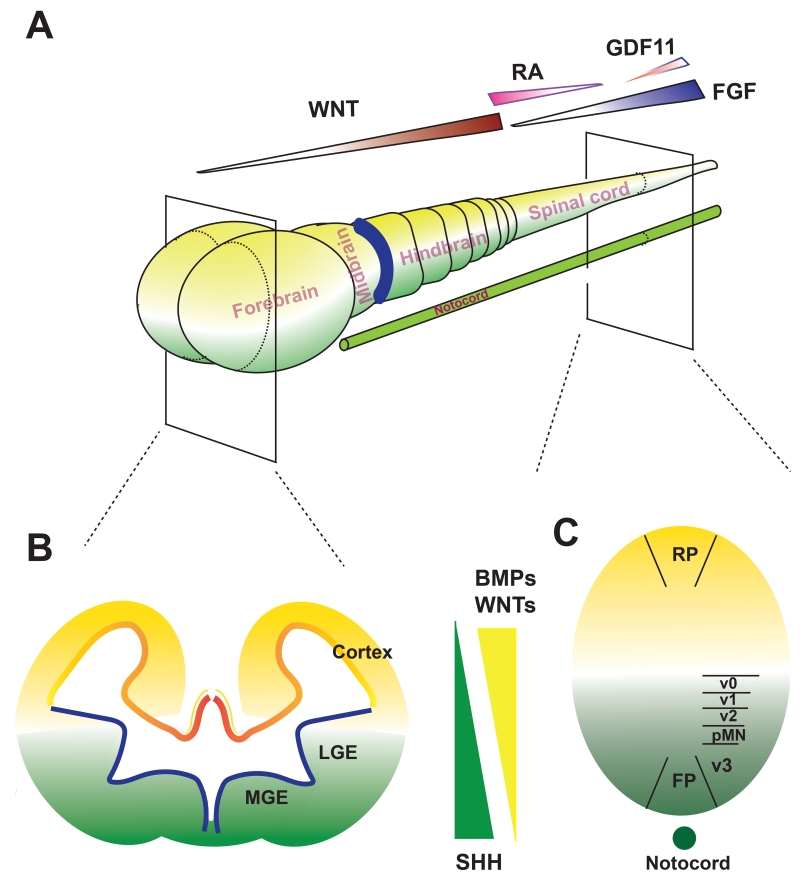
During embryogenesis, the neuroepithelial layer, or neural plate, folds to form the neural tube, from which the brain and spinal cord develop. This morphogenic process, or patterning, is coordinated by temporarily and spatially available morphogen gradients along the anterior-posterior (A-P) and dorsal-ventral (D-V) axes. Morphogens affecting the A-P patterning include FGFs, WNTs, and RA whereas those influencing D-V patterning include WNTs, BMPs, and SHH (sonic hedgehog). The gradient of morphogens, especially those with opposing effects, defines the transcriptional code and hence the identity of the neural progenitors in a particular domain along the A-P and D-V axes (Figure 2). It is this neural patterning principle that forms the basis for specifying the NE to region-specific neural progenitor subtypes.
Figure 2. Neural patterning principle for neural progenitor subtype specification.
A. A-P patterning is under the regulation of several morphogens during development. The gradient of WNTs dictates the regionalization of the forebrain, mid-hindbrain, and anterior spinal cord whereas gradients of RA and FGFs govern the spinal cord segmentation. B and C, D-V patterning in the forebrain (B) and spinal cord (C) is set by the dorsally derived morphogens WNTs and BMPs (yellow color) and the (notocord) ventrally derived SHH (green color).
Specification of neural progenitors along the A-P axis
During neural induction, NE are specified first in the head region and extend caudally. Correspondingly, the NE differentiated from hPSCs, whether by the EB method or dual-SMAD inhibition approach, carry an anterior identity by expressing PAX6 (Paired box 6) and OTX2 (Orthodenticle homeobox 2) but not caudal markers like EN1 (Engrailed Homeobox 1), GBX2 (Gastrulation brain homeobox 2), or HOX (Homeobox) genes (Chambers et al., 2009; Zhang et al., 2001). However, this anterior phenotype is transient. Depending upon the presence of morphogens, the NE will take on a definitive regional identity in the next 1-2 weeks. In the absence of morphogens or presence of FGFs or inhibitors of WNTs, the neural progenitors become committed to the forebrain fate (Figure 2).
The main caudalizing morphogens are Wnts, Fgfs, and RA. Wnt1 and Fgf8 are produced by cells in the midbrain-hindbrain boundary (MHB) and are required for defining the midbrain and hindbrain identity through regulation of Otx2 (define the forebrain and midbrain) and Gbx2 (define the hindbrain). For human NE, FGF8, in a wide range of concentrations, has little effect in patterning cells to the mid-hindbrain fate (Perrier et al., 2004; Yan et al., 2005). Activation of the WNT pathway by small molecules, including CHIR99021 (CHIR), exerts precise dose-dependent effect in patterning the NE to forebrain, midbrain, hindbrain, and anterior spinal cord identities (Kirkeby et al., 2012). At a low concentration of CHIR, the NE cells become progenitors with posterior forebrain identity. With increasing concentrations, the NE cells are fated to progenitors with midbrain, hindbrain, and anterior spinal cord identities (Figure 2). This is also revealed by single-cell RNAseq analysis, showing that neural progenitors with mid/hindbrain identities begin to segregate as early as 12 days of hESC differentiation in response to morphogens that activate the canonical WNT pathway (Yao et al., 2016). The identification of the CHIR role in patterning NE cells was the tipping point that led to the efficient generation of midbrain DA neurons from hPSCs almost independently by different laboratories (Kirkeby et al., 2012; Kriks et al., 2011; Xi et al., 2012). Fine-tuning of CHIR concentrations enables specification of progenitors in two closely related regions, the ventral midbrain and subthalamic nucleus, thus enriching the midbrain DA neuron population (Kee et al., 2016). It also leads to the efficient production of hindbrain serotonin neurons from hPSCs (Lu et al., 2016).
The patterning of the spinal cord cells along the A-P axis is more complicated. It is usually thought that the spinal cord cells are originated from the neural tube. However, recent studies have revealed that both spinal cord and paraxial mesoderm cells are derived from neuromesodermal progenitors (NMPs) (reviewed in (Gouti et al., 2015; Henrique et al., 2015)). Generation of spinal cord cells have also been achieved following both principles either by manipulating the combination of WNT, RA and FGFs on NE (Maury et al., 2015) or differentiating from NMPs (Gouti et al., 2014). It will be interesting to determine whether the hPSC-derived cells with the spinal cord identity, generated in these two approaches, are molecularly and functionally similar.
Specification of neural progenitors along the D-V axis
The neural progenitor identity is not only defined by its A-P location but also D-V position. The D-V patterning is governed by SHH for ventralization and the antagonizing activity of WNT canonical pathway and BMP pathway for dorsalization (Kiecker and Lumsden, 2012; Le Dreau and Marti, 2012). SHH, secreted from the notochord and later from the floor plate, patterns cells at the ventral part of the neural tube. With exposure to increasing concentrations of SHH cells are fated to more ventral identities (Ribes and Briscoe, 2009). In contrast, WNTs and BMPs derived from the roof plate, mediate the dorsal patterning (Figure 2B, C). Following this principle, it is now possible to effectively specify NE to progenitors with particular identities along the D-V axis. The vast majority of the anterior NE become progenitors of the cerebral cortical identity with few cells acquiring the ventral identity in the absence of SHH (Espuny-Camacho et al., 2013; Li et al., 2009). This “default” cerebral cortical identity is partly due to the expression of numerous WNT ligands in the differentiating NE (Li et al., 2009). In the presence of low concentrations of SHH, the NE are patterned to cells equivalent to the lateral ganglionic eminence (LGE) by expressing GSX2 (GS Homeobox 2), CTIP2 (B-cell CLL/lymphoma 11B), and MEIS2 (Meis homeobox 2). These progenitors will mainly differentiate to the medium spiny GABA neurons in the striatum (Arber et al., 2015; Ma et al., 2012). With increasing concentration of SHH, the NE are patterned to the most ventral part of the forebrain, equivalent to the medial ganglionic eminence (MGE) by expressing NKX2.1 (NK2 homeobox 1) and give rise to GABA interneurons and basal forebrain cholinergic neurons (Kim et al., 2014; Liu et al., 2013b; Maroof et al., 2013; Nicholas et al., 2013). Thus, by regulating the SHH concentrations and/or balance between SHH and WNTs, a whole host of forebrain cell types are specified along the D-V axis. The same principle has been employed to specify ventral neuronal types of other brain regions, including midbrain DA neurons (Kirkeby et al., 2012; Kriks et al., 2011; Xi et al., 2012), hindbrain serotonin neurons (Lu et al., 2016), and spinal motor neurons (Li et al., 2005; Maury et al., 2015).
Enrichment of subtype neural progenitors by coordinating morphogens with opposing effects
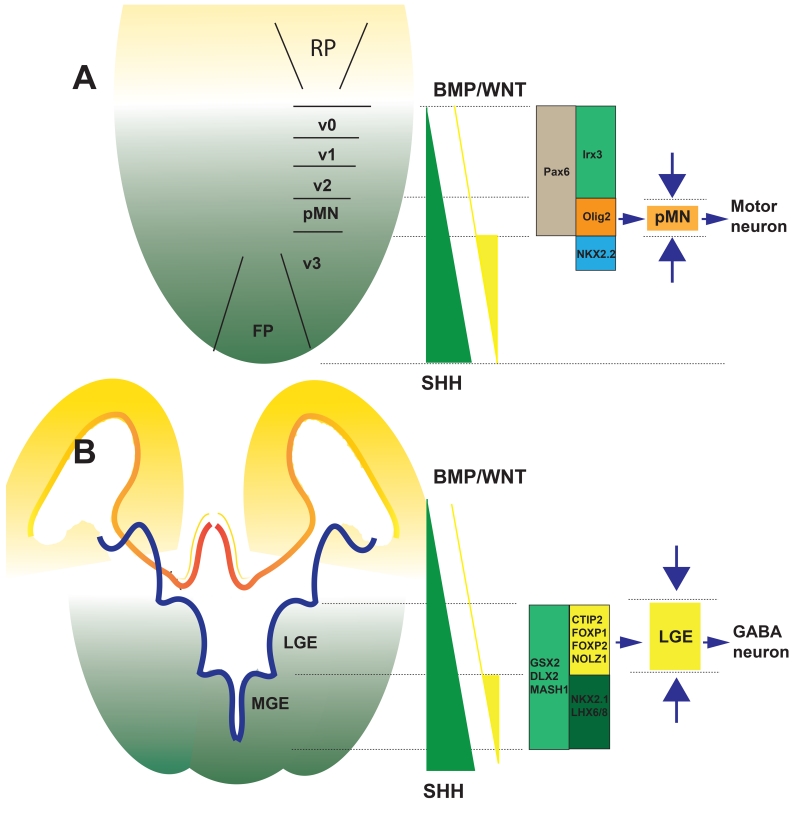
Patterning NEs by morphogens along both the A-P axis and D-V axis is used to generate the target progenitor population with varying degrees of purity. It is generally easier to specify progenitors at the most dorsal or ventral and most anterior or posterior domains. For example, nearly pure populations of MGE progenitors can be generated from the anterior NEs with a high concentration of SHH whereas almost pure cortical (dorsal) progenitors are produced without the presence of morphogens. When it comes to progenitors located in between the ends, a single concentration of a morphogen often results in a mix of target progenitors and those residing in the neighbouring domains. In order to specify motor neuron progenitors, SHH, at a concentration slightly lower than the maximal level for the most ventral cells, is used. That level of SHH certainly results in specification of OLIG2 (Oligodendrocyte transcription factor 2)-expressing motor neuron progenitors, but it also yields a substantial population of NKX2.2 (NK2 homeobox 2)-expressing interneuron progenitors that are ventral to the motor neuron progenitor domain and some IRX3-expressing interneuron progenitors that are dorsal to the motor neuron progenitor domain (Figure 3A). If the neighboring progenitors are limited, the target progenitors will be enriched. By this principle, combination of SHH (to induce the NKX2.2- and OLIG2-expressing progenitors) and CHIR (to antagonise the induction of NKX2.2-expressing cells by SHH) enriches the OLIG2-expressing progenitors, thus producing motor neurons at over 90% purity (Du et al., 2015) (Figure 3A).
Figure 3. Using opposing mitogens to enrich the neural progenitor population.
A. To enrich the neural progenitors from the pMN domain in the spinal cord, a high concentration of SHH or Purmorphamine (SHH signalling agonist) is employed to induce the OLIG2+ and the more ventral NKX2.2+ progenitors. Since the more ventral NKX2.2+ cells give rise to interneurons rather than motor neurons, CHIR (activate the WNT pathway) is used to antagonize the effect of SHH in induction of NKX2.2 but not OLIG2, leading to the enrichment of the OLIG2+ progenitors. Such a strategy enables generation of highly enriched population of spinal motor neurons. B. To enrich LGE progenitors and striatal GABA neurons, forebrain NE are ventralized to the LGE and MGE domains. At the same time, the most ventral MGE domain is antagonized by activin (on the BMP pathway). Together, the opposing morphogens restrict the cells to the LGE domain, thus producing enriched striatal GABA neurons.
Similarly, in order to generate LGE progenitors and then striatal GABA neurons, Arber et al first differentiated hPSCs to anterior NEs with the dual-SMAD inhibition method. NE cells generated by this condition contain a mixed population of both the dorsal and ventral origins. Activin (activating the BMP signalling) was then applied to block the NKX2.1-expressing MGE neural progenitors induced by SHH (Figure 3B). This strategy leads to an enriched cell population of LGE progenitors that express CTIP2, FOXP2 (Forkhead box protein P2), DLX2 (Distal-less homeobox 2), GSX2, MASH1 (Achaete-scute family bHLH transcription factor 1), NOLZ1 (Zinc finger protein 503) and EBF1 (Early B-cell factor 1) (Arber et al., 2015).
The strategy of using morphogens with opposing effects to sharpen/narrow the cell domain along the A-P and D-V axes, illustrated in the above examples, should apply to the differentiation of most neural subtypes. Technically, it involves the titration of the opposing morphogens so that the target progenitor population is maximized and the unwanted population is minimized. With increasing application of such a strategy, we expect that most of the neural subtypes may be differentiated from hPSCs to the level of near purity.
Enrichment of subtype neural progenitors by exploiting the intrinsic programs
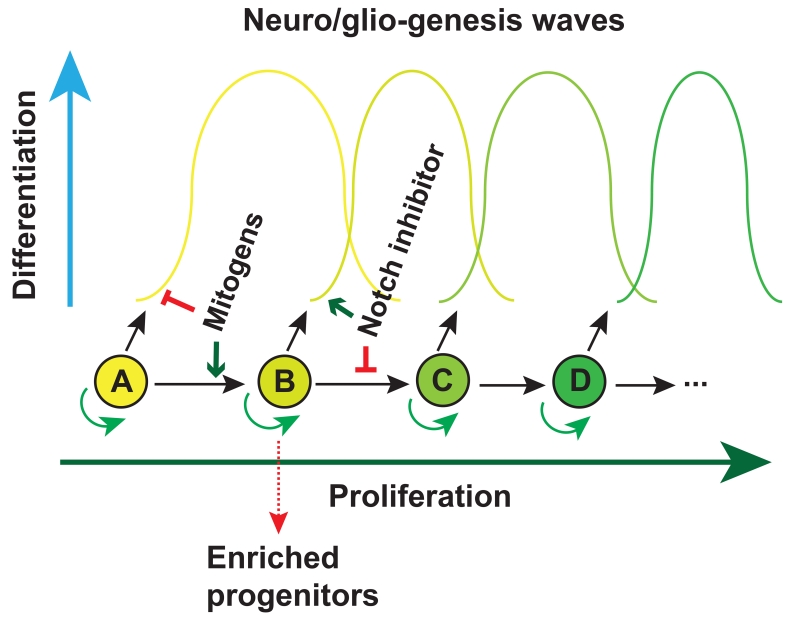
In addition to secreted factors for patterning region-specific neural progenitors, intrinsic properties of neural progenitors can also be exploited for generation and enrichment of sub-type neural progenitors. During development, neural progenitors give rise to different types of neurons and then glial cells according to the intrinsic time course (Figure 1). During cerebral cortex development, radial glia (NSCs) generate neurons in the deep layer (layer VI) and the most superficial layer (layer I) first. The differentiating radial glia will then sequentially generate neurons that reside in layer V, IV, III, and II. Correspondingly, mouse ESC-derived NE cells with the cortical identity first give rise to progenitors that express FOXP2 and CTIP2, corresponding to those in layer V-VI, followed by those expressing POU3F3 (POU class 3 homeobox 3), CUX1/2 (Cut like homeobox 1/2) and LHX2 (LIM homeobox protein 2) (layer IV), LMO3 (LIM domain only 3) and TLE3 (Transducin like enhancer of split 3) (layer III), and PLXND1 (Plexin D1) (layer II) (Gaspard et al., 2008). Similarly, hPSC-derived retinal progenitor cells generate ganglion cells, horizontal cells, cone, amacrine, rod, bipolar and Müller glia in order (Kaewkhaw et al., 2015; Zhong et al., 2014). By taking advantage of the intrinsic time course in the production of progenitor subtypes, one may enrich the subtype neural progenitors at a particular temporal window when the progenitors emerge (Figure 4).
Figure 4. Enriching neural progenitor subtypes according to their intrinsic temporal course.
Neural progenitors such as radial glia or retinal progenitors give rise to multiple progenies (waves) over development. If the progenitor cell is kept in cell cycle (to prevent differentiation) it shifts to the next progenitor pool. If the progenitor is forced to exit cell cycle (e.g., by γ-secretase inhibitors (also known as Notch inhibitors)), no subsequent progenitor pools are available. Together, a particular neuronal subtype is enriched.
This approach works well with the first wave of progenitors. It may not be straightforward with subsequent waves of progenitors as the early-born progenitors and their differentiated progenies will be present, yielding a mixed population. One way to overcome this hurdle is to prevent the differentiation of the prior waves of progenitors by maintaining the cells in proliferation until the time when the target progenitors are born (Figure 4). This wave of progenitors is thus enriched by forcing them to exit cycle (Figure 4), e.g., by γ-secretase inhibitors, to yield post-mitotic cells (Borghese et al., 2010). This strategy has so far been applied for differentiating glial cells (Krencik et al., 2011). The key is to find a mitogen that keeps cells in cycle without altering the progenitor identity (see below). Together, manipulation of NE with morphogens along the intrinsic program will maximize the production of the intended progenitor subtypes.
Neuronal differentiation and functional maturation
Regionally patterned neural progenitors acquire a specific transmitter phenotype after exiting from the cell cycle. The progenitors with the dorsal forebrain identity become exclusively glutamate neurons whereas the vast majority of ventrally patterned forebrain progenitors differentiate to GABA neurons with some of the most ventral progenitors becoming cholinergic neurons. Similarly, the progenitors patterned to the spinal pMN domain differentiate to motor neurons with acetylcholine as a transmitter (Figure 5). Under most circumstances, regional progenitors become neurons with a particular transmitter phenotype based on their intrinsic properties. This phenomenon is in line with the proposal that the transmitter phenotype may be determined by the transcriptional coregulatory mechanism (Flames and Hobert, 2011). In some cases, additional signals may enhance the acquisition of the transmitter phenotype. Ventrally patterned midbrain progenitors, even though they express FOXA2 (forkhead box protein A2), EN1 (Homeobox protein engrailed-1), OTX2, and LMX1A (LIM Homeobox Transcription Factor 1 Alpha), do not readily become DA-producing neurons (Xi et al., 2012). Interestingly, a recent study aiming at identifying markers to predict the yield of DA neurons in transplant showed that the commonly used DA neuron markers (FOXA2, LMX1A and CORIN) poorly correlate with DA neuron production in vivo whereas the markers of caudal ventral midbrain (FGF8, PAX5, EN2 and CNPY1 (Canopy FGF signaling regulator 1)) are associated with higher DA neuron yield. Addition of FGF8 later than day 9, but not earlier (before day 9), maintains the FOXA2/LMX1A/B expression in progenitors and increases the generation of DA neurons (Kirkeby et al., 2016). This result suggests that FGF8 promotes DA neuron program by patterning the progenitors to the caudal fate at a late stage. It is also possible that FGF8 promotes differentiation given the fact that the midbrain identity of the progenitors has been determined by day-9. Indeed, FGF8, even added from day-13, substantially boosts the expression of NURR1 (Nuclear receptor related 1) and tyrosine hydroxylase (Xi et al., 2012), which is necessary for the synthesis of DA. Similarly, although the ventral hindbrain progenitors have the potential to become serotonin neurons, they do not do so unless FGF4 is applied to stimulate the expression of tryptophan hydroxylase and suppress the expression of Phox2B (Paired-like homeobox 2b) in the appropriately patterned progenitors (Lu et al., 2016). Thus, while regional progenitors take on a transmitter identity during neuronal differentiation based on their intrinsic properties, extracellular signals may facilitate the acquisition of the transmitter phenotype either through late-stage patterning and/or coordination with the transcriptional code of the progenitors.
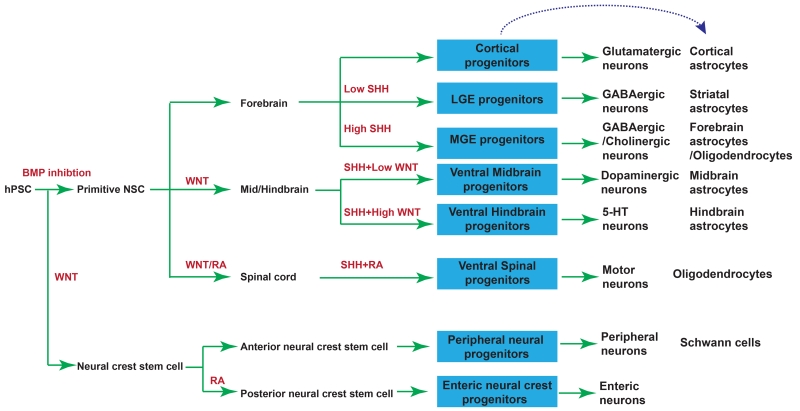
Figure 5. Differentiation of neuronal and glial subtypes from hPSCs.
Neuronal and glial subtypes that have been successfully differentiated from hPSCs through NE induction, regional patterning, and neural differentiation. Red indicates morphogens applied in each step. Blue boxes indicate subtype progenitors.
It takes quite a while for the postmitotic neurons to become functionally mature in terms of electrophysiological properties and synaptic capabilities. The maturation process is species dependent. In the adult non-human primate brain, new neurons need at least 6 months to mature (Kohler et al., 2011). Indeed, hPSC-derived neurons mature gradually over a period of several weeks based on the membrane characteristics and their ability to transmit signals through synapses. Under standard neuronal culture conditions in the presence of neurotrophic factors such as BDNF, GDNF, and NT3, hPSC-derived neurons with cerebral cortical identities gradually decrease their membrane potentials, gain characteristic Na+ and K+ currents, and fire mature action potentials over a 7-week period. In subsequent weeks, the neurons begin to fire repetitive action potentials and exhibit synaptic currents, showing further maturation (Johnson et al., 2007). Modification of the concentration of inorganic salts, amino acids, D-glucose and vitamins, termed as BrainPhys media, appears to support the basic synaptic functions and activity of human neurons (Bardy et al., 2015).
The in vivo maturation of in vitro produced human neurons appears to follow the intrinsic maturation program of the human cells. It usually takes 3-4 months for grafted human neural cells to project and connect with the target cells (Espuny-Camacho et al., 2013; Ma et al., 2012). The time required for the innervation and electrophysiologically functional maturation is also largely dependent on the types of neurons. Following transplantation into the mouse brain, hPSC-derived CTIP2 positive neurons (layer V) target the midbrain, hindbrain, and spinal cord whereas the TBR1 (T-box, brain 1) positive neurons (layer VI) project mainly to the thalamus. The grafted human neurons synapse with host cells and exhibit electrophysiological properties 6 months after transplantation (Espuny-Camacho et al., 2013). Similarly, granule neurons derived from hPSCs integrate into the mouse dentate gyrus 6 weeks after transplantation and exhibited transient sodium inward current and sustained potassium outward currents after 6 months (Yu et al., 2014). These findings suggest that the intrinsic property of the human cells dictates the neuron projection and functional maturation.
Functional circuits formed by human neurons
Neurons are organized into circuits to process and transmit information. When the hPSC-derived neurons are mature, they form networks and express synaptic proteins, including synapsin and PSD95 (postsynaptic density protein 95). Electrophysiological recording reveals spontaneous synaptic currents, indicating the formation of functional neural networks. To determine if the in vitro produced human neurons can functionally integrate into an existing network, Weick et al co-cultured the mCherry-labelled human neurons with GFP-labelled primary mouse cortical neurons. The primary mouse cortical neurons often exhibit rhythmic firing patterns in culture whereas the human neurons do not. However, after 4 weeks of co-culture, the human neurons also display the rhythmic activity at the same frequency, suggesting that the human neurons can integrate into the existing networks post-synaptically. To determine if the human neurons can also integrate into a network presynaptically, channelrhodopsin (ChR2)-expressing human neurons were co-cultured with the mouse neurons. Stimulation of the human neurons with light led to a post-synaptic response in mouse neurons (Weick et al., 2011). Similarly, by co-culturing the ChR2-expressing human motor neurons with muscle cells to form neuromuscular junctions, Steinbeck et al demonstrate that muscle contractions can be precisely controlled by the light-regulated motor neurons (Steinbeck et al., 2016), demonstrating the functional connection between the human motor neurons and the muscles.
Not only can the hPSC-derived neurons participate in a neural network in vitro but also can they integrate into a neural circuit in vivo. When the ChR2-expressing hPSC-derived DA neurons are transplanted into the striatum of the PD model mouse, light stimulation of the grafted human neurons regulates the glutamate inputs into the striatal GABA neurons. Consequently, the motor behaviours of the PD mouse are modulated (Steinbeck et al., 2015). By using both excitatory and inhibitory DREADDs (designer receptor exclusively activated by designer drugs) to enable remote control of the transplanted human DA neurons in the brain, Chen et al demonstrated that the transmission from glutamate neurons to striatal GABA neurons can be positively and negatively regulated, respectively. Correspondingly, the behaviours of the PD mice are modulated effectively (Chen et al., 2016). All these findings indicate that the in vitro generated human neurons can functionally integrate into a neural circuit, signifying the therapeutic potential of the hPSC-derived neurons.
Formation of the neural circuits in vitro by human neurons derived from hPSCs will help decipher the network abnormalities underlie disease conditions. This is particularly useful for modelling psychiatric disorders in which abnormal neural transmission/plasticity rather than structural defect is likely the main underlying pathology. Building such a model will also facilitate drug development for mental disorders. Indeed, neurons derived from schizophrenia patient iPSCs exhibit diminished neuronal connectivity in conjunction with decreased neurite numbers (Brennand et al., 2011). Similarly, the generation of cortical glutamate neurons and GABA neurons from Down’s syndrome patient iPSCs is not altered when compared to their isogenic controls. However, the synaptic transmission, measured by postsynaptic currents, is reduced in Down syndrome neurons (Weick et al., 2013). Neurons derived from hPSCs carrying the mutant NRXN1 (Neurexin 1) gene, which is associated with autism and schizophrenia, selectively impaired neurotransmitter release without altering neuronal differentiation or synapse formation (Pak et al., 2015). In GABAergic neurons and glutamatergic neurons that are derived from epilepsy patient iPSCs, the frequency and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) but not spontaneous excitatory postsynaptic currents (sEPSC) are significantly lower than those of control and genetically corrected neurons (Liu et al., 2016). These studies indeed suggest defects in transmission in neurons that are produced from patients with mental disorders.
The frequency or amplitude in spontaneous synaptic currents is influenced by many factors, including technical issues. If such changes are persistent and responsive to activities, or exhibiting synaptic plasticity, these synaptic changes may better reflect the pathological alterations in psychiatric diseases. Synaptic plasticity, typically measured by long-term potentiation (LTP) and long-term depression (LTD) in brain slices, is regarded as the basis of learning and memory. To begin to develop techniques that measure neuronal plasticity, Odawara et al cultured hiPSC-derived cortical neurons on multi-electrode array (MEA) and measured neuronal activity by calcium waves (Odawara et al., 2016). The authors observed changes in evoked responses in neurons that are grown for over 100 days under high-frequency electrical stimulation. This result suggests that the iPSC-derived neurons exhibit LTP-like neuronal activity. It is presently not validated by classical electrophysiological recording. The MEA based LTP/LTD assay could provide a way to compare plasticity of neural circuits from the healthy control and patients. However, long-term culture (over 100 days) on the chip makes it technically challenging and interpretation difficult. A shorter culture system that shows direct link between simple stimulation (activity) and robust synaptic adaptation (plasticity) will likely substantially change the way we peek into the pathological changes in mental illness.
Generation of glial subtypes
The most abundant cells in the brain are glial cells, which take up about 90% of total cells. They are critical for normal brain function and are involved in a wide range of neurological and psychiatric disorders such as multiple sclerosis (MS), ALS, spinocerebellar ataxia, PD, Huntington’s disease and brain ischemia (Phatnani and Maniatis, 2015; Yates, 2015). Among the glial types, astrocytes and oligodendrocytes are originated from NE after neurogenesis.
Generation of astrocytes from hPSCs
During development, astrocytes are generated from radial glia or NSCs at the subventricular zone. The molecular signals that specify NSCs to the astrocyte fate are not clear. Hence, the strategy for differentiating hPSCs to astrocytes usually takes the following three steps. First, hPSCs are specified to NE through either the EB or dual-SMAD inhibition method. Since the NE produce primarily neurons upon differentiation and since there is currently no effective non-genetic means to block neurogenesis and/or promote gliogenesis, the second step is to expand the NE until the onset of gliogenesis (Figure 1; Figure 4). The appearance of glial progenitors, defined by expression of NF1A, S100β, CD44 but downregulation of neurogenic marker PAX6, occurs at the 3rd month of hPSC differentiation (Krencik et al., 2011). The third step is to differentiate the glial progenitors to astrocytes under the condition in which BMPs and CNTF (ciliary neurotrophic factor) are present. BMPs and CNTF promote astrocyte differentiation by activating the STAT3 pathway (Rajan and McKay, 1998). An interesting observation is that although the glial progenitors are already present by the end of the third month, generation of a high proportion of functional astrocytes, defined by their expression of GFAP, display of rectifying inward potassium currents, propagation of calcium waves across cells, and uptake of glutamate, takes an additional 3 months (Krencik et al., 2011). This delayed differentiation to astrocytes is also observed following transplantation of the progenitors into the mouse brain in which the differentiated astrocytes exhibit characteristic human astrocyte phenotypes with a substantially larger cell body and many more and longer processes than the neighbouring endogenous mouse astrocytes. This differentiation process, to a large degree, corresponds to in vivo astrocyte development in human. Nevertheless, it is a time consuming process.
Efforts to shorten the process of astrocyte generation from hPSCs, ranging from 2 months to 4 months (Emdad et al., 2012; Gupta et al., 2012; Juopperi et al., 2012; Lafaille et al., 2012; Roybon et al., 2013; Serio et al., 2013; Shaltouki et al., 2013) have been attempted, using similar strategies (summarized in Table 1). The question then is why astrocytes are differentiated from hPSCs in a substantially shorter time in some reports. One potential issue is how to define the astrocytes, which is a major issue for the field. If expression of GFAP and S100β is the main criterion, then many neural progenitors, like radial glia, may be regarded as astrocytes. Hence, it is important to include functional characterization of the differentiated cells. It is also clear that identification of a means to activate the neurogenesis-to-gliogenesis switch will accelerate the astrocyte differentiation.
Table 1.
Comparison of astrocyte differentiation from hPSCs
| Differentiation study | Duration (days) |
Efficiency (GFAP positive) |
Astrocyte markers |
Method (Neuralization) |
Additions in Medium (expansion phase) |
Maturation (astrocyte differentiation) |
Function test |
|---|---|---|---|---|---|---|---|
| Krencik et al., 2011 | 180 | >90 % | GFAP S100β CD44 NF1A |
EB | EGF FGF2 (Suspension) |
CNTF, LIF or 10% FBS |
Astrocyte-neuron co-culture Glutamate uptake Propagation of calcium |
| Emdad et al., 2012 | 35 | 50-80% | GFAP A2B5 S100β |
EB | N2 FGF EGF CNTF | N2 CNTF | Transwell migration |
| Gupta et al., 2012 | 70 | >90% | GFAP S100β EAAT1 EAAT2 |
EB | EGF FGF2 Heparin (Suspension) |
BMP2,BMP4, LIF |
Glutamate uptake |
| Juopperi et al., 2012 | 120 | - | GFAP S100β | EB | Astrocyte medium contain FBS (Monolayer) |
FBS | - |
| Lafaille et al., 2012 | 90 | - | GFAP | Dual-smad inhibition |
EGF FGF2 | 5% FBS | - |
| Roybon et al., 2013 | >80 | >90% | CD44 S100β, CX43 Vimentin NF1A aldolase C |
Dual-smad Inhibition |
B27 NTFs FBS (Monolayer) |
FGF1 or FBS | Glutamate uptake |
| Serio et al., 2013 | >70 | >90% | Vimentin, NF1A GFAP S100β EAAT1 |
EB with dual- smad inhibition |
EGF LIF->EGF FGF2 (Suspension) |
CNTF | Glutamate uptake Astrocyte-neuron co-culture Calcium Wave |
| Shaltouki et al., 2013 | >70 | 60-80% | CD44 GFAP NF1A S100β EAAT1 |
EB | Actinvin A Heregulin 1 β IGFI FGF2 (Monolayer) |
CNTF BMP2 | Glutamate uptake Astrocyte-neuron co-culture |
Like neurons, there are many subtypes of astrocytes depending on their location, morphology, molecular and physiological functions. As discussed above, the identity of neuronal subtypes is largely endowed during the patterning of neural progenitors. Since neural progenitors first give rise to neurons and then glia (Figure 1), it is reasonable to expect that part of the astrocyte heterogeneity comes from regional patterning of the progenitors. To test this hypothesis, Krencik et al first generated neural progenitors with dorsal forebrain, ventral forebrain, dorsal spinal cord, and ventral spinal cord identities. These regional progenitors, after long-term expansion and become gliogenic, retain the regional identities by expressing respective homeodomain transcription factors. Such regional identities are even retained after the progenitors are transplanted into the mouse brain and become astrocytes (Krencik et al., 2011). Thus, part of the astrocyte heterogeneity comes from the regional identity of their progenitors endowed during development. Of course, further complexity may come from their interaction with neighboring neurons (Farmer et al., 2016). Future work is needed to determine if the regional astrocytes are functionally distinct and how the functional properties are evolved.
Generation of Oligodendrocytes from hPSCs
Similar to astrocytes, oligodendrocytes appear late during development, providing support and insulation by forming myelin sheath around the axons in the central nervous system. Oligodendrocytes have several origins, including ventral neural tube (MGE, LGE and pMN), dorsal neural tube, and SVZ during development (Gallo and Deneen, 2014; Goldman and Kuypers, 2015). The best-characterized origin is at the ventral part of telencephalon and spinal cord where the oligodendrocyte progenitor cells (OPCs) are specified from NE in response to SHH signal by expressing the Olig1/2 gene (Lu et al., 2002; Zhou and Anderson, 2002). This is essentially the guiding principle for oligodendrocyte differentiation from hPSCs to date (Douvaras et al., 2014; Gorris et al., 2015; Izrael et al., 2007; Nistor et al., 2005; Piao et al., 2015; Stacpoole et al., 2013; Wang et al., 2013) (summarized in table 2).
Table 2.
Comparison of the oligodendrocyte differentiation from hPSCs
| Differentiation study |
Durati on (days) |
Efficien cy (OPC) |
markers | Method (Neuralization) |
Generation of pre- OPC |
Transition from Pre-OPC to OPC |
OPC to oligodendrocyte |
|---|---|---|---|---|---|---|---|
| Nistor et al., 2005 | 42 | >80% | OLIG1 SOX10 A2B5 NG2, PDGFRα GalC RIP O4 |
EB | FGF EGF RA | EGF | |
| Izrael et al., 2007 | >45 | >80% | OLIG1/2 SOX10 NKX2.2 PDGFRα O4 |
EB | EGF RA Noggin | EGF FGF Noggin | Noggin |
| Hu et al., 2009 | 112 | >80 % | OLIG2 NKX2.2 PDGFRα O4 |
EB | SHH B27 RA FGF2 | PDGF IGFI NT3 T3 | PDGF IGFI NT3 |
| Stacpoole et al., 2013 | 100 | 80% | OLIG2 NKX2.2 NG2 PDGFRα O4 |
EB with low oxygen |
SAG FGF RA (pMN) SAG FGF (forebrain) |
PDGF T3 purmorphamine/SAG RA (pMN) PDGF FGF purmorphamine/SAG T3 (forebrain) |
SAG PDGF NT3 IGFI T3 cAMP |
| Wang et al., 2013 | 140 | 70- 90% |
OLIG2 SOX10 NKX2.2 MBP PDGFRα O4 |
EB | RA B27 FGF Purmorphamine |
PDGF IGFI T3 NT3 Purmorphamine |
PDGF IGFI NT3 B27 BDNF |
| Douvaras et al., 2014 | 95 | 70% | OLIG2 SOX10 NKX2.2 MBP O4 |
Dual-SMAD inhibition (Monolayer) |
RA SAG | PDGF HGF IGFI NT3 Insulin T3 Biotin cAMP |
Insulin T3 Biotin cAMP AA |
| Gorris et al., 2015 | >90 | 80% | OLIG1/2 NKX6.2 NKX2.2 NG2 MBP SOX10 |
EB | PDGF EGF forskolin |
PDGF T3 AA Noggin | T3 AA laminin |
| Piao et al., 2015 | 70 | 90% | OLIG2 NKX2.2 SOX10 O1 MBP O4 |
Dual-SMAD and WNT inhibition |
Purmorphamine AA BDNF FGF8 |
PDGF IGFI T3 cAMP | BDNF AA T3 cAMP |
AA: Ascorbic acid RA: retinoic acid EB: Embryoid body
The process of oligodendrocyte differentiation from hPSCs begins with neural induction, followed by specification of OPCs by SHH and oligodendrocyte differentiation. Neural specification in the first step is the same as that for neuron and astrocyte differentiation. In the second phase, the NE are patterned to the pMN domain of the spinal cord, which express OLIG2, or ventral forebrain progenitors that express NKX2.1. The OLIG2-expressing spinal progenitors normally give rise to motor neurons. In order to maintain the progenitor identity (by preventing differentiation to motor neurons) and promote transition to the gliogenic progenitors by acquiring the co-expression of OLIG2 and NKX2.2, the spinal progenitors are cultured in the continued presence of SHH or its agonist purmorphamine. At the same time, the cells are maintained in the presence of mitogens like FGF2 and EGF (Figure 4). Under such a condition, the progenitors express both OLIG2 and NKX2.2 in about a week. The OLIG2/NKX2.2-expressing ventral spinal progenitors in mice usually become oligodendrocytes shortly. However, the human progenitors do not become oligodendrocytes in another two months. Examination of OLIG2/NKX2.2-expressing progenitors revealed that they do not express SOX10 and PDGFRα (platelet derived growth factor receptor α) which, together with OLIG2 and NKX2.2, are required for the progenitors to become oligodendrocytes. The OLIG2/NKX2.2-expressing progenitors are thus termed pre-OPCs (Hu et al., 2009). Therefore, a critical step is to transition the pre-OPCs to OPCs. Ideally, a simple way is to expand the pre-OPCs with a mitogen like FGF2 until they reach the stage of OPCs (Figure 4). However, FGF2 inhibits the generation of OPCs by interfering with the SHH signalling thus disrupting the co-expression of OLIG2 and NKX2.2 (Hu et al., 2009). Hence, most protocols use growth factors other than FGF2, such as PDGF and insulin like growth factor-1 (IGF1), to promote the transition from pre-OPCs to OPCs (Table 2). The downside of such an approach is the weak proliferation of the pre-OPCs over a 2-month period, not yielding large quantities of OPCs. The OPCs can indeed differentiate to O4-expressing immature oligodendrocytes and MBP (myelin basic protein)-expressing mature oligodendrocytes in vitro over a long period. When transplanted into the mouse brain or spinal cord, they mature and produce myelin sheaths, usually over 3-4 months (Erceg et al., 2010; Hu et al., 2009; Kerr et al., 2010; Kim et al., 2012; Piao et al., 2015; Sharp et al., 2010; Wang et al., 2013; Yasuda et al., 2011). To date, almost all the protocols consistently show that the oligodendrocyte differentiation is a long process, taking about 3 months to differentiate hPSCs to OPCs and 3 more months to myelin-producing oligodendrocytes.
The long oligodendrocyte differentiation process, to a large degree, is delayed by the transition from pre-OPC to OPC, which takes up to 2/3 of the whole differentiation. It should be possible to shorten the process by promoting the proliferation of the pre-OPCs while maintaining the co-expression of OLIG2 and NKX2.2. In other words, coordinating the effects of mitogens and morphogens (Figure 4) would be possible to facilitate the transition from pre-OPCs to OPCs. Addition of SHH or its agonists may help the process although the use of SHH or its agonists in a long term is either costly or toxic. A simplest solution is to find a mitogen that does not alter the identity of the progenitors.
Challenges and future directions
Generating additional neural cell types
The neural cell types specified from hPSCs, described above, are targets of major neurological diseases, including Alzheimer’s disease, Huntington disease, PD, ALS, and multiple sclerosis, etc. These cells can be successfully generated from hPSCs because of the relatively clear molecular pathways that govern the development of these neural subtypes (Figure 5). For those whose developmental pathway is not known or less clear, the differentiation is less directed and the efficiency is low. The patterning of cerebellum or hypothalamus is reasonably delineated but the specification of major cell types in the cerebellum or hypothalamus is much less clear. Consequently, differentiation of cerebellar Purkinje cells (Muguruma et al., 2015; Wang et al., 2015) or hypothalamic hormone-producing neurons (Merkle et al., 2015) is less efficient, even though the proportion of Purkinje cells may be increased through cell sorting (Muguruma et al., 2015; Wang et al., 2015). For some brain structures, such as the hippocampus and amygdala, their developmental processes are quite complicated. Consequently, differentiation of hippocampal neurons (Sakaguchi et al., 2015; Yu et al., 2014) or amygdala neurons from hPSCs is not straightforward.
Because of the involvement of these neural cell types in a wide range of neurological and psychiatric disorders, efforts are being made to generate them from hPSCs. One simple way is to enrich the desired cell type through cell sorting, exemplified by cerebellar Purkinje cells (Muguruma et al., 2015; Wang et al., 2015). An alternative is to force stem or progenitors to adopt the target cell identity by expressing a cell type-specific transcription factor. This approach was initially used by Zhang et al to drive hPSCs to become glutamate neurons by transiently overexpressing NGN2 (Zhang et al., 2013). Such a strategy to transiently or inducibly express a cell-type specific transcription factor in the developing progenitors can be a way to generate the neurons of interest from hPSCs. This strategy requires transgene expression in PSCs or progenitors but expressing a transgene in hPSCs is technically simple. In addition, the transcriptional codes for specific neural subtypes are increasingly available, especially those learned from studies with direct neural conversion. At the present, it is not clear if the NGN2-induced glutamate neurons resemble the cortical glutamate neurons. If so, such a strategy may help unravel the molecular pathways governing the generation of the target cell type. It is possible that the glutamate neurons, generated by forced expression of NGN2 at the PSC stage, lose their regional identity. If so, additional factors, or expression of the cell type determinant in the regionally specified progenitors may be necessary to generate regionally and functionally specialized neural subtypes.
Speeding up the differentiation of interneurons and glia
The process of neural differentiation from hPSCs mirrors in vivo development (Figure 1). It thus offers a good model to look at aspects of early human neural development. It also places challenges to the differentiation of neural cell types that are normally generated late in embryonic development, including cortical GABA interneurons and glia. There are many types of cortical GABA interneurons. They are originated from MGE in the ventral developing brain. Interestingly, the NKX2.1-expressing MGE progenitors can be differentiated from hPSCs in 2-3 weeks (Liu et al., 2013b). However, the generation of subclasses of GABA interneurons, especially the parvalbumin-expressing cells, takes many additional weeks or even months (Liu et al., 2013a; Nicholas et al., 2013). Even so, the differentiation efficiency is low. The main reason is the lack of understanding what the existing markers for defining the GABA neuronal subtypes mean and how they are related to the specification of these neuronal types (DeFelipe et al., 2013). Consequently, few solutions are available to tackle the issue but following their intrinsic o’clock. One potential shortcut is expression of GABAergic neuron related transcription factors to force the stem cells or progenitors to take on the GABAergic fate, which hopefully will aid in our understanding of the GABAergic fate determination and future development of non-genetic strategies for faster generation of cortical GABA neuronal subtypes.
Similarly, the differentiation of astrocytes and oligodendrocytes takes a protracted length of time. The field is anxiously waiting for a fast and robust method of glial differentiation from hPSCs. The rate-limiting step is the switch of neural progenitors from neurogenesis to gliogenesis. The neurogenesis-to-gliogenesis switch is tightly regulated by both extrinsic and intrinsic factors, including Notch pathway, bHLH transcription factors, and epigenetic remodelling (Hirabayashi and Gotoh, 2010). Strategies to shorten the time for neural-glial switch or to jump NE directly to gliogenesis will speed up the glial differentiation. Potential approaches include regulation of the Notch pathway or transient expression of gliogenic transcription factors such as NF1A (astrocytes) or SOX10 (oligodendrocytes) along neural differentiation.
Making human neurons mature faster or age
One of the major applications of hPSCs is to model neurological or psychiatric diseases and to use them as a drug discovery platform. Most neurological and psychiatric diseases occur in adults or at least in mature neurons. The hPSC-differentiated neurons, however, resemble those in the fetal brain, at least based on the similarity in gene expression profiles between in vitro generated neurons and those from fetal brain tissues (Ho et al., 2016). And the maturation process often takes weeks, if not months. Hence, it is critical to mature the hPSC-derived neurons faster to enable presentation of disease-relevant phenotypes. It is even more critical for building high throughput screening platforms based on hPSC-derived neurons as it is technically prohibitive to grow hPSC-derived neurons in a 1536-well plate for longer than 3-7 days that requires periodic medium changes. As discussed, blockade of the Notch signalling by compound E or DAPT eliminates the dividing progenitors, thus synchronizing the maturation process (Borghese et al., 2010). In theory, it is not promoting neuronal maturation per se. Interestingly, however, treatment with γ-secretase inhibitors (also known as Notch inhibitors) often results in electrophysiologically active neurons in just 2-4 weeks (Borghese et al., 2010; Du et al., 2015). This is possibly due to the elimination of neural stem/progenitor cells, resulting in homogeneous post-mitotic neurons (Chen et al., 2014; Du et al., 2015; Lu et al., 2016), which interact each other to promote maturation. This phenomenon suggests the possibility of speeding up the maturation process by regulating signalling pathways that are not known for enhancing maturation, including those forcing cell cycle exit, enhancing neuronal survival, and stimulating neuronal activities. Some of the molecules are included in the BrainPhys media (Bardy et al., 2015).
Age is a common factor associated with many degenerative diseases. Capturing the age signature in the in vitro produced neural cells will likely facilitate the presentation of the pathological processes. One way to accelerate the age process is to overexpress progerin in neurons derived from hPSCs (Miller et al., 2013). Progerin, an aberrantly spliced and processed form of nuclear-envelope protein lamin A, causes Hutchinson-Gilford progeria syndrome (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003). Progerin is produced at very low rate in healthy cells and accumulates in senescent cells. Overexpression of progerin in neurons results in decrease in overall telomeres length and an increase in the percentage of short telomeres, which are typical features of aging (Miller et al., 2013). As progerin overexpression by itself causes cellular pathology, attentions should be paid to separating the non-age consequences caused by progerin overexpression from the pathology inherent to the target disease. Ideally, non-genetic approaches to induce cellular aging would overcome such a drawback. These non-transgenic means may include cellular stresses caused by toxins or reactive oxygen species (reviewed in (Studer et al., 2015)). Another approach is to convert the aged somatic cells directly to desired neurons. Mertens et al. recently showed that directly reprogrammed human neurons retain the aging–associated transcriptomic signatures (Mertens et al., 2015). However, the number of neurons derived from direct reprogramming is often limited, hindering its application in disease modelling and drug testing.
Producing large quantities of neurons with consistent quality
Large quantities of homogenous quality neural cells are required for high throughput screening (HTS) or for potential cell therapy. Billions or hundreds of millions of cells are usually needed for a large library by HTS. Such quantity would also allow therapeutic application in dozens or hundreds of patients. Typically, a hPSC can generate 10 to 100 neural cells during a month-long differentiation process. Hence, a large number of starting stem cells or multiple times (lots) of differentiation will be necessary to achieve the number for application. Either method introduces variations, making it difficult for quality control and HTS. One potential solution is to guide the hPSCs to a population of committed subtype-specific progenitors and then expand this pool of progenitors to the desired quantity. Unfortunately, the general way to expand neural stem/progenitor cells by EGF and/or FGF, while producing large numbers, almost always changes the cell fate. Hence, a strategy to expand the progenitor cells without altering the fate identity is needed. Li et al has attempted to expand hPSC-derived NE by a growth factor cocktail that consists of LIF, CHIR and SB431542 (Li et al., 2011). After a long-term expansion (27 passages), the resultant progenitor cells can still generate tyrosine hydroxylase positive DA neurons and spinal motor neurons although it is not examined if the progenitors can still generate neurons with forebrain identities (Li et al., 2011). Because of the presence of CHIR, which activates the WNT pathway, the expanded progenitors will gradually acquire a more caudal fate. Thus, blocking the patterning effect of the mitogens and/or maintaining the regional identity is necessary. This is particularly true for committed neural progenitors, such as midbrain DA neural progenitors or spinal motor neuron progenitors. Taking these factors into consideration, Du et al expanded the specified human spinal motor neuron progenitors in a similar culture medium but with additional patterning molecules such as SHH and DMH1 to maintain the progenitor identity. Such a strategy enabled expansion up to 5 passages without losing the motor neuron identity, resulting in over 100-fold increase in cell number and thus producing sufficient number of cells for HTS (Du et al., 2015). The limited capacity of expansion also suggests a need of further technological development in that direction. The ability to renew the lineage-committed neural progenitors will dramatically enhance basic research, disease modelling, drug screening and cell therapy.
Choosing between 2D and 3D culture system
From the self-organized neuroepithelial rosettes derived through EBs (Zhang et al., 2001) to the self-assembled cortical tissues (Eiraku et al., 2008) from hPSCs, the 3D floating culture for neural differentiation has made remarkable progress during the past decade. The initial serum-free embryoid body (SFEB) culture (Watanabe et al., 2007) was developed by optimizing the EB system in the “U” shape low cell-adhesion 96-well plate to generate uniform cell aggregates in suspension, leading to self-organizing brain tissues such as cortical tissues (Eiraku et al., 2008). Modification of the culture system by using spinning bioreactor (Martin et al., 2004) enables long-term culture and growth of larger tissues or organoids. Addition of inductive signals pattern the differentiating tissues to different regional identities (Qian et al., 2016), very much like the way that has been used for 2D cultures. These technical improvements yield organoids that resemble the cerebral cortex (Lancaster et al., 2013), midbrain (Jo et al., 2016) and many other brain regions (Qian et al., 2016).
The 3D organoid culture system allows the formation of brain tissues through either self-assembly or active induction. While these tissues resemble those in the brain, they are not the same. The cerebral organoids, for instance, exhibit a layered structure but they generally lack the GABA interneurons that are originated from the ventral neural tube. The ability to differentiate region-specific progenitors may enable assembly of brain tissues closer to those in the human brain. Because of the 3D structure, organoids are useful for modelling brain development and developmental disorders. When it comes to application in building a screening platform, a substantial technical improvement is needed to produce homogenous organoids in a consistent manner. At the present, the neural cell types generated using the 2D culture system described above are marching to industrial application. For cell therapy, the cell types generated by the 2D system are relatively easy for GMP manufacture and quality control. Hence, we need to find a balance between system complexity and cell type homogeneity (Kelava and Lancaster, 2016). The ability to produce highly enriched neural progenitor subtypes, described herein, makes it feasible to print brain tissues with necessary complexities yet in a reliable and consistent manner.
ACKNOWLEDGEMENTS
We thank Dr. A. Bhattacharyya for helpful comments on the manuscript. Studies in the Zhang laboratories have been supported in part by the NIH-NINDS (NS076352, NS086604), NIH-NIMH (MH099587, MH100031), the Bleser Family Foundation, the Busta Foundation, and the NICHD (HD076892, U54 HD090256).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Arber C, Precious SV, Cambray S, Risner-Janiczek JR, Kelly C, Noakes Z, Fjodorova M, Heuer A, Ungless MA, Rodriguez TA, et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C, van den Hurk M, Eames T, Marchand C, Hernandez RV, Kellogg M, Gorris M, Galet B, Palomares V, Brown J, et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2725–2734. doi: 10.1073/pnas.1504393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brustle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature biotechnology. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Qian K, Du Z, Cao J, Petersen A, Liu H, Blackbourn L.W.t., Huang CL, Errigo A, Yin Y, et al. Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell stem cell. 2014;14:796–809. doi: 10.1016/j.stem.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, Zhou W, Zhang SC. Chemical Control of Grafted Human PSC-Derived Neurons in a Mouse Model of Parkinson’s Disease. Cell stem cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature reviews Neuroscience. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem cell reports. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, Zhong X, Fan F, Zhang SC. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nature communications. 2015;6:6626. doi: 10.1038/ncomms7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell stem cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem cells and development. 2012;21:404–410. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, Lopez MG, Radojevic I, Moreno-Manzano V, Rodriguez-Jimenez FJ, Bhattacharya SS, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux JF, Peng J, et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science. 2016;351:849–854. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- Flames N, Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annual review of neuroscience. 2011;34:153–184. doi: 10.1146/annurev-neuro-061010-113824. [DOI] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development. 2015;142:3983–3995. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorris R, Fischer J, Erwes KL, Kesavan J, Peterson DA, Alexander M, Nothen MM, Peitz M, Quandel T, Karus M, et al. Pluripotent stem cell-derived radial glia-like cells as stable intermediate for efficient generation of human oligodendrocytes. Glia. 2015;63:2152–2167. doi: 10.1002/glia.22882. [DOI] [PubMed] [Google Scholar]
- Gouti M, Metzis V, Briscoe J. The route to spinal cord cell types: a tale of signals and switches. Trends in genetics: TIG. 2015;31:282–289. doi: 10.1016/j.tig.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS biology. 2014;12:e1001937. doi: 10.1371/journal.pbio.1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Patani R, Baxter P, Serio A, Story D, Tsujita T, Hayes JD, Pedersen RA, Hardingham GE, Chandran S. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell death and differentiation. 2012;19:779–787. doi: 10.1038/cdd.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. 2015;142:2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Gotoh Y. Epigenetic control of neural precursor cell fate during development. Nature reviews Neuroscience. 2010;11:377–388. doi: 10.1038/nrn2810. [DOI] [PubMed] [Google Scholar]
- Ho R, Sances S, Gowing G, Amoroso MW, O’Rourke JG, Sahabian A, Wichterle H, Baloh RH, Sareen D, Svendsen CN. ALS disrupts spinal motor neuron maturation and aging pathways within gene co-expression networks. Nature neuroscience. 2016;19:1256–1267. doi: 10.1038/nn.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Molecular and cellular neurosciences. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Goke J, Tan ZY, Saw TY, Tan CP, Lokman H, et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell stem cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA, Ming GL, Song H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Molecular brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewkhaw R, Kaya KD, Brooks M, Homma K, Zou J, Chaitankar V, Rao M, Swaroop A. Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks. Stem Cells. 2015;33:3504–3518. doi: 10.1002/stem.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annual review of neuroscience. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Kee N, Volakakis N, Kirkeby A, Dahl L, Storvall H, Nolbrant S, Lahti L, Björklund ÅK, Gillberg L, Joodmardi E, et al. Single-cell Analysis Reveals A Close Relationship Between Differentiating Dopamine and Subthalamic Nucleus Neuronal Lineages. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.10.003. 10.1016/j.stem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA. Stem Cell Models of Human Brain Development. Cell stem cell. 2016;18:736–748. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Kerr CL, Letzen BS, Hill CM, Agrawal G, Thakor NV, Sterneckert JL, Gearhart JD, All AH. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. The International journal of neuroscience. 2010;120:305–313. doi: 10.3109/00207450903585290. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. The role of organizers in patterning the nervous system. Annual review of neuroscience. 2012;35:347–367. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- Kim H, Walczak P, Kerr C, Galpoththawela C, Gilad AA, Muja N, Bulte JW. Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis. Stem Cells. 2012;30:2820–2829. doi: 10.1002/stem.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TG, Yao R, Monnell T, Cho JH, Vasudevan A, Koh A, Peeyush KT, Moon M, Datta D, Bolshakov VY, et al. Efficient specification of interneurons from human pluripotent stem cells by dorsoventral and rostrocaudal modulation. Stem Cells. 2014;32:1789–1804. doi: 10.1002/stem.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell reports. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, Ottosson DR, Lelos MJ, Rifes P, Dunnett SB, et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.09.004. 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nature biotechnology. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature. 2012;491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake BB, Ai R, Kaeser GE, Salathia NS, Yung YC, Liu R, Wildberg A, Gao D, Fung HL, Chen S, et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science. 2016;352:1586–1590. doi: 10.1126/science.aaf1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVaute TM, Yoo YD, Pankratz MT, Weick JP, Gerstner JR, Zhang SC. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dreau G, Marti E. Dorsal-ventral patterning of the neural tube: a tale of three signals. Developmental neurobiology. 2012;72:1471–1481. doi: 10.1002/dneu.22015. [DOI] [PubMed] [Google Scholar]
- Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nature biotechnology. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao C, Chen W, Ma W, Li X, Shi Y, Zhang H, Zhang L, Long Y, Xu H, et al. CRISPR/Cas9 facilitates investigation of neural circuit disease using human iPSCs: mechanism of epilepsy caused by an SCN1A loss-of-function mutation. Translational psychiatry. 2016;6:e703. doi: 10.1038/tp.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nature protocols. 2013a;8:1670–1679. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nature biotechnology. 2013b;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhong X, Liu H, Hao L, Huang CT, Sherafat MA, Jones J, Ayala M, Li L, Zhang SC. Generation of serotonin neurons from human pluripotent stem cells. Nature biotechnology. 2016;34:89–94. doi: 10.1038/nbt.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Ma L, Hu B, Liu Y, Vermilyea SC, Liu H, Gao L, Sun Y, Zhang X, Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell stem cell. 2012;10:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, et al. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell stem cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends in biotechnology. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Maury Y, Come J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nature biotechnology. 2015;33:89–96. doi: 10.1038/nbt.3049. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Maroof A, Wataya T, Sasai Y, Studer L, Eggan K, Schier AF. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development. 2015;142:633–643. doi: 10.1242/dev.117978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Paquola AC, Ku M, Hatch E, Bohnke L, Ladjevardi S, McGrath S, Campbell B, Lee H, Herdy JR, et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell stem cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, Tu EY, Mandal PK, Vera E, Shim JW, Kriks S, et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell stem cell. 2013;13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell reports. 2015;10:537–550. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nature reviews Neuroscience. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell stem cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Odawara A, Katoh H, Matsuda N, Suzuki I. Induction of long-term potentiation and depression phenomena in human induced pluripotent stem cell-derived cortical neurons. Biochemical and biophysical research communications. 2016;469:856–862. doi: 10.1016/j.bbrc.2015.12.087. [DOI] [PubMed] [Google Scholar]
- Pak C, Danko T, Zhang Y, Aoto J, Anderson G, Maxeiner S, Yi F, Wernig M, Sudhof TC. Human Neuropsychiatric Disease Modeling using Conditional Deletion Reveals Synaptic Transmission Defects Caused by Heterozygous Mutations in NRXN1. Cell stem cell. 2015;17:316–328. doi: 10.1016/j.stem.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harbor perspectives in biology. 2015;7 doi: 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell stem cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nature biotechnology. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Ribes V, Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harbor perspectives in biology. 2009;1:a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia-Diaz A, Yang EJ, Sattler R, Jackson-Lewis V, Kim YA, Kachel CA, Rothstein JD, Przedborski S, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell reports. 2013;4:1035–1048. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nature communications. 2015;6:8896. doi: 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltouki A, Peng J, Liu Q, Rao MS, Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31:941–952. doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole SR, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, Franklin RJ. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem cell reports. 2013;1:437–450. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, Mosharov EV, Studer L. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nature biotechnology. 2015;33:204–209. doi: 10.1038/nbt.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck JA, Jaiswal MK, Calder EL, Kishinevsky S, Weishaupt A, Toyka KV, Goldstein PA, Studer L. Functional Connectivity under Optogenetic Control Allows Modeling of Human Neuromuscular Disease. Cell stem cell. 2016;18:134–143. doi: 10.1016/j.stem.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Studer L, Vera E, Cornacchia D. Programming and Reprogramming Cellular Age in the Era of Induced Pluripotency. Cell stem cell. 2015;16:591–600. doi: 10.1016/j.stem.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell stem cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang B, Pan N, Fu L, Wang C, Song G, An J, Liu Z, Zhu W, Guan Y, et al. Differentiation of human induced pluripotent stem cells to mature functional Purkinje neurons. Scientific reports. 2015;5:9232. doi: 10.1038/srep09232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature biotechnology. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Weick JP, Held DL, Bonadurer GF, 3rd, Doers ME, Liu Y, Maguire C, Clark A, Knackert JA, Molinarolo K, Musser M, et al. Deficits in human trisomy 21 iPSCs and neurons. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9962–9967. doi: 10.1073/pnas.1216575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick JP, Liu Y, Zhang SC. Human embryonic stem cell-derived neurons adopt and regulate the activity of an established neural network. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20189–20194. doi: 10.1073/pnas.1108487108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells. 2012;30:1655–1663. doi: 10.1002/stem.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Mich JK, Ku S, Menon V, Krostag A-R, Refugio, Martinez A, Furchtgott L, Mulholland H, Bort S, et al. A single cell roadmap of lineage bifurcation in human ESC models of embryonic brain development. Cell stem cell. 2016 doi: 10.1016/j.stem.2016.09.011. 10.1016/j.stem.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda A, Tsuji O, Shibata S, Nori S, Takano M, Kobayashi Y, Takahashi Y, Fujiyoshi K, Hara CM, Miyawaki A, et al. Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells. 2011;29:1983–1994. doi: 10.1002/stem.767. [DOI] [PubMed] [Google Scholar]
- Yates D. Neurodegenerative disease: Factoring in astrocytes. Nature reviews Neuroscience. 2015;16:67. doi: 10.1038/nrn3908. [DOI] [PubMed] [Google Scholar]
- Yoo YD, Huang CT, Zhang X, Lavaute TM, Zhang SC. Fibroblast growth factor regulates human neuroectoderm specification through ERK1/2-PARP-1 pathway. Stem Cells. 2011;29:1975–1982. doi: 10.1002/stem.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, Marchetto MC, Brennand K, Wright R, Mei A, McHenry L, Lisuk D, Grasmick JM, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem cell reports. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature biotechnology. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature communications. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]