Significance
Hemolytic diseases include a variety of conditions with diverse etiologies in which red blood cells are destroyed and large amounts of hemeproteins are released. Heme has been described as a potent proinflammatory molecule that is able to induce multiple innate immune responses. The mechanisms by which eukaryotic cells respond to the toxic effects induced by heme to maintain homeostasis are not fully understood, however. Here we describe a previously uncharacterized cellular response induced by heme: the formation of p62/SQTM1 aggregates containing ubiquitinated proteins in structures known as aggresome-like induced structures (ALIS). This action is part of a response driven by the transcription factor NRF2 to the excessive generation of reactive oxygen species induced by heme.
Keywords: autophagy, p62/SQSTM1, ALIS, heme, iron
Abstract
Hemolytic diseases include a variety of conditions with diverse etiologies in which red blood cells are destroyed and large amounts of hemeproteins are released. Heme has been described as a potent proinflammatory molecule that is able to induce multiple innate immune responses, such as those triggered by TLR4 and the NLRP3 inflammasome, as well as necroptosis in macrophages. The mechanisms by which eukaryotic cells respond to the toxic effects induced by heme to maintain homeostasis are not fully understood, however. Here we describe a previously uncharacterized cellular response induced by heme: the formation of p62/SQTM1 aggregates containing ubiquitinated proteins in structures known as aggresome-like induced structures (ALIS). This action is part of a response driven by the transcription factor NRF2 to the excessive generation of reactive oxygen species induced by heme that results in the expression of genes involved in antioxidant responses, including p62/SQTM1. Furthermore, we show that heme degradation by HO-1 is required for ALIS formation, and that the free iron released on heme degradation is necessary and sufficient to induce ALIS. Moreover, ferritin, a key protein in iron metabolism, prevents excessive ALIS formation. Finally, in vivo, hemolysis promotes an increase in ALIS formation in target tissues. Our data unravel a poorly understood aspect of the cellular responses induced by heme that can be explored to better understand the effects of free heme and free iron during hemolytic diseases such as sickle cell disease, dengue fever, malaria, and sepsis.
The presence of large amounts of free extracellular heme is the hallmark of several noninfectious and infectious hemolytic diseases such as β-thalassemia, sickle cell disease, ischemia-reperfusion, malaria, and dengue fever (1, 2). Heme is a tetrapyrrole ring containing an atom of iron (Fe) in its center and, in vertebrates, is ubiquitously expressed as the prosthetic group of several key proteins that participate in essential biological processes such as gas transport and electron transfer (3). However, when released into the extracellular milieu, heme can exert several deleterious effects, resulting in damage to lipids (4), proteins (5), and DNA (6). In pathological conditions that result in hemolysis, rhabdomyolysis, or extensive cell damage, large amounts of hemeproteins are released, and under oxidative conditions, the heme moiety is released, further increasing oxidation and cellular stress (7, 8). Recently, it was shown that heme can induce the generation of reactive oxygen species (ROS) through the activation of NADPH oxidase (9) or by the mitochondria (10), and that blocking these pro-oxidant effects protects the host from cell damage and tissue injury.
Along with its oxidative dependent effects, heme also activates innate immune responses and inflammation. In mice, injection of heme results in increased vascular permeability, increased levels of acute-phase proteins, and leukocyte migration and activation through the up-regulation of adhesion molecules, such as intercellular adhesion molecule 1, E-selectin, P-selectin, and von Willebrand factor (11–13). Heme also has been characterized as an agonist for TLR4 and an inducer of proinflammatory cytokines such as TNF and KC (14), as well as the chemoattractant lipid mediator leukotriene B4 (15). In vivo, TLR4 is required for heme-induced acute lung injury (16). Finally, heme also can induce necroptosis in exposed macrophages in a TNF-, ROS-, and RIP1/3-dependent manner (17).
To avoid its potentially deleterious effects, free heme levels are tightly regulated by scavenging proteins, such as haptoglobin (Hp) and hemopexin (Hx), which bind hemoglobin and heme, respectively, and contribute to their clearance from the circulation and to intracellular degradation (18, 19). In vivo studies have demonstrated that Hp- and Hx-deficient mice develop increased renal damage after phenylhydrazine (PHZ)-induced hemolysis compared with wild-type (WT) animals (20). Under strong oxidative stress, the intracellular enzyme heme-oxygenase 1 (HO-1) is rapidly up-regulated to cope with the increased levels of free heme that are generated and internalized (7). HO-1 is an evolutionarily conserved, ubiquitously expressed enzyme (encoded by HMOX genes) that catabolizes heme into equimolar amounts of labile Fe, carbon monoxide (CO), and biliverdin (BV). In contrast to CO and BV, which have cytoprotective effects, labile Fe is able to catabolize the production of free radicals that further increase the oxidative environment. Thus, neutralizing mechanisms are required to avoid these deleterious effects. One such mechanism is mediated by ferritin, an intracellular protein that binds to and oxidizes labile Fe by binding to and oxidizing it, avoiding the generation of more ROS through the engagement of Fe into Fenton reactions (7).
The mechanisms by which heme induces inflammation and tissue damage have been investigated intensely in recent years. Nonetheless, at the cellular level, not much is known about the homeostatic adaptive responses triggered on exposure to heme. Here we show that heme induces a stress response characterized by the formation of protein aggregates known as aggresome-like induced structures (ALIS) in macrophages. Even though these structures are frequently associated with LC3, a key protein in the autophagic machinery, their formation is independent of autophagy. We also show that induction of ALIS formation is part of a cellular response to oxidative stress driven by the transcription factor NRF2 with subsequent up-regulation of p62/SQSTM1 (sequestosome-1) expression levels. Furthermore, the iron released on heme degradation by HO-1 is sufficient and necessary to induce the formation of such aggregates. Notably, cells derived from ferritin-deficient mice of labile Fe display increased levels of p62/SQSTM1 and ALIS formation. Our results support the formation of ALIS as a potential adaptive mechanism for cells to deal with the (oxidative) stress generated by the increased levels of free heme or labile Fe. In the absence of such a mechanism or in situations where its capacity is exceeded, cells could be more susceptible to the toxic effects of heme.
Results
Heme Induces the Accumulation of LC3+ Dot-Like Structures That Are Not Autophagosomes.
The strong pro-oxidant nature of heme generates high levels of oxidative stress, forcing the cell to adapt to cope with redox imbalance and survive. Autophagy is one of the main cellular mechanisms activated in response to different types of stress, particularly oxidative stress (21). Thus, we hypothesized that autophagy would be up-regulated after stimulation of macrophages with heme. To address this question, we first stimulated RAW GFP-LC3 macrophages with increasing concentrations of heme and analyzed the conversion of LC3-I to LC3-II, a hallmark of autophagy, by Western blot analysis. At 100 μM, heme induced a robust conversion of LC3-I to LC3-II (Fig. 1A). We then examined the redistribution of GFP-LC3 in cells treated with either heme for 6 h or rapamycin for 2 h (as a positive control) by fluorescence microscopy. Intriguingly, even though we could observe the formation of LC3+ structures, these were clearly different from the typical autophagosomes induced by rapamycin in both number and shape (Fig. 1B). The LC3+ structures induced by heme were perfectly round dots distributed throughout the cytosol, in contrast to the asymmetrically shaped autophagosomes. Furthermore, in Atg5−/− GFP-LC3 mouse embryonic fibroblasts (MEFs), which do not form autophagosomes, heme did not induce LC3 conversion, as expected. This resulted in increased levels of the autophagy substrate p62/SQSTM1 protein levels (Fig. 1C), although it still induced LC3+ dot-like structures (Fig. 1D). This finding suggests that despite LC3 conversion and cellular redistribution, the LC3+ structures induced by heme are not autophagosomes.
Fig. 1.
Heme-induced ALIS do not depend on autophagy machinery. (A) Immunoblot analysis of LC3-I to LC3-II conversion of RAW GFP-LC3 cells stimulated with heme (30 or 100 μM) for 2 h or with rapamycin (50 μg/mL) for 2 h. Actin served as a loading control. (B) Fluorescence microscopy of RAW GFP-LC3 cells stimulated with heme (100 μM) for 6 h or with rapamycin (50 μg/mL) for 2 h. (Insets) Heme- and rapamycin-induced GFP-LC3+ structures. (C) Immunoblots for LC3-I to LC3-II conversion and p62 from extracts of WT and Atg5−/− MEFs stimulated with heme (100 μM) for 6 h or with rapamycin (50 μg/mL) for 2 h. Actin served as a loading control. (D) Fluorescence microscopy of WT and Atg5−/− MEFs stimulated with heme (100 μM) for 12 h. (E) Quantification of GFP-LC3+ dots in RAW GFP-LC3 cells stimulated with heme (30 or 100 μM) for 6, 12, or 24 h. (F) Quantification of GFP-LC3+ dots in RAW cells pretreated with vehicle or wortmannin (200 nM) and stimulated with heme (100 μM) for 12 or 24 h. (G) Quantification of GFP-LC3+ dots in WT and Atg5−/− MEFs stimulated with heme (100 μM) for 12 or 24 h. (H) Quantification of p62+ dots in WT and Atg5−/− MEFs stimulated with heme (100 μM) for 4, 6, 12, or 24 h. (I) Immunofluorescence images showing cellular distribution of p62 (red) and Ub (green) in BMDMs stimulated with heme (100 μM) for 6 h. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05; ns, not significant.
To further investigate the formation of LC3+ dot-like structures by heme, we performed kinetic studies, in which we observed a maximum accumulation of LC3+ dots at 12 h after stimulation, followed by a reduction in the number of dots per cell at 24 h (Fig. 1E). This transient nature indicates that one or more degradation pathways were engaged to clear the cytosol of heme-treated cells from these structures. As mentioned above, autophagy is one of the main cellular degradation systems and is responsible for the removal of long-lived proteins and damaged organelles from the cytosol. To test whether autophagy, instead of being involved in the formation of LC3+ dot-like structures by heme, is important for the clearance of these structures, we pretreated RAW GFP-LC3 macrophages with wortmannin, a PI3K inhibitor that prevents autophagosome formation, and quantified the number of LC3+ dots after stimulation with heme. As shown in Fig. 1F, impaired autophagy did not alter the number of LC3+ dots at 12 h poststimulation, but significantly reduced the clearance of such structures after 24 h. To confirm these data, we examined heme-induced LC3+ dots in Atg5−/− GFP-LC3 MEFs. In line with our previous result, Atg5−/− MEFs were not able to clear heme-induced LC3+ dots as efficiently as the WT controls (Fig. 1G). Taken together, these results indicate that autophagy is activated at later time points to clear LC3+ structures induced by heme and maintain cellular homeostasis.
Heme Induces ALIS.
Under various stress conditions, misfolded proteins aggregate into ALIS, which are eventually degraded by autophagy (22, 23). These structures are characterized by the presence of p62/SQSTM1 and ubiquitinated proteins (24). p62/SQSTM1 (hereinafter p62) is reported to bind to ubiquitinated proteins through its C-terminal ubiquitin-associated (UBA) domain and to LC3 through its LC3-interacting region, and thus functions as an adaptor to target ubiquitinated substrates to autophagy-dependent degradation (24). In our model, heme induced an increase in p62 protein levels (Fig. 1C), as well as the formation of p62+ puncta in WT and even more so in Atg5−/− MEFs (Fig. 1H).
To test whether p62+ puncta colocalized with LC3+ dots in macrophages after heme stimulation, we immunostained RAW GFP-LC3 macrophages for p62. Whereas in nonstimulated control cells, p62 staining showed a diffuse cellular distribution, cells treated with heme displayed a well-defined punctate staining, very similar to that induced by puromycin (used as a positive control) (Fig. S1A). Furthermore, in stimulated cells, p62 and LC3 staining presented perfect colocalization (Fig. S1A). As mentioned above, p62 also presents a UBA domain that binds to monoubiquitinated and polyubiquitinated proteins. Immunostaining of ubiquitinated proteins in RAW GFP-LC3 macrophages revealed its colocalization with LC3 puncta (Fig. S1B).
Fig. S1.
Heme induces LC3+ dots that colocalize with ALIS. (A and B) Immunofluorescence microscopy images of RAW GFP-LC3 cells stimulated with heme (100 μM) for 12 h or with puromycin (5 μg/mL) and stained for p62 (red) (A) or Ub (green) (B). (C and D) Immunofluorescence showing the colocalization of p62 (red) and Ub (green) in RAW cells (C) and in immortalized BMDMs (D) stimulated with heme 100 μM for 12 h. Nuclei were stained with DAPI (blue). (Scale bars: 5 μm.)
Because p62 functions as an adapter linking ubiquitinated substrates to autophagic degradation, we speculated that p62 and ubiquitin (Ub) are localized to the same structure. To test this hypothesis, we stimulated BMDMs with heme, and demonstrated that in fact p62 and Ub colocalize (Fig. 1I). Similar results were obtained in RAW264.7 and immortalized bone marrow-derived macrophages as well (Fig. S1 C and D). Taken together, the foregoing results demonstrate that the cellular stress induced by heme results in protein aggregation in compartments known as ALIS, and that autophagy is essential for their removal from cytosol.
TLR4 and MyD88 Are Not Required for Heme-Induced ALIS Formation.
Activation of macrophages with LPS has been reported to induce the formation of ALIS (22, 25). Heme, as LPS, has been characterized as a TLR4 agonist, because some of its proinflammatory effects are known to be dependent on TLR4 signaling (14). We asked whether TLR4 is involved in heme-induced ALIS formation. To this end, we first confirmed in our settings that LPS induced the formation of LC3+ dots in RAW GFP-LC3 macrophages (Fig. S2A), as well as p62+/Ub+ puncta in BMDMs (Fig. S2 B–D). To determine the role of TLR4 in heme-induced protein aggregation, BMDMs isolated from WT and TLR4−/− mice were stimulated with heme, LPS, or the TLR2 agonist Pam3CSK4. As expected, TLR4 was essential for LPS-induced ALIS formation in these cells, but was dispensable for the formation of ALIS induced by heme and Pam3CSK4 (Fig. S2 F–I), even though it was still required for heme-induced TNF production as described previously (Fig. S2E). Similarly, MyD88, an adaptor protein for TLR4-dependent signaling, was dispensable for heme-induced ALIS formation (Fig. S2 J–L).
Fig. S2.
Indiction of ALIS by heme does not require TLR4 and MYD88. (A) RAW GFP-LC3 cells stimulated with heme 100 μM or LPS (100 ng/mL) for 12 h. (B–D) Immunofluorescence microscopy for p62 (red) and Ub (green) of BMDMs stimulated with heme 100 μM or LPS (100 ng/mL) for 6 h (B) and quantification of p62+ (C) and Ub+ dots (D) of BMDMs stimulated as in B. (E) Secretion of TNF in BMDMs from WT and TLR4−/− mice stimulated with LPS (100 ng/mL), Pam3CSK4 (1 μg/mL), or heme (30, 50 or 100 μM) for 6 h. (F and G) Immunofluorescence images of WT (F) and TLR4−/− (G) BMDMs stimulated with heme (100 μM), LPS (100 ng/mL), or Pam3CSK4 (200 ng/mL) for 6 h and stained for p62 (red) and Ub (green). (H and I) Quantification of p62+ (H) and Ub+ (I) dots in BMDMs from WT and TLR4−/− mice stimulated as in A and B. (J and K) Immunofluorescence microscopy of WT (J) and Myd88−/− (K) immortalized BMDMs stimulated with heme (100 μM), LPS (100 ng/mL), or Pam3CSK4 (200 ng/mL) for 6 h and stained for p62 (red) and Ub (green). (L) Quantification of p62+ (red) dots in WT and MyD88−/− immortalized BMDMs stimulated with heme (100 μM) or LPS (100 ng/mL) for 6 h. (M) Quantification of p62+ dots in BMDMs left untreated or stimulated with 1, 100, or 1,000 ng/mL of LPS for 6 h in the presence or absence of heme (100 μM) for 6 h. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
Given that the release of free heme is often associated with pathological conditions in which microbial products are also present, we tested whether treatment of heme in combination with LPS would enhance ALIS formation. Indeed, BMDMs stimulated with both heme and LPS display an approximate threefold increase in the number of p62+ dots compared with cells treated with heme or LPS alone (Fig. S2M). These data demonstrate that heme induces cellular responses through different pathways, and that ALIS formation is independent of its ability to activate the TLR4 pathway.
MAPKs Signaling and ROS Generation Are Essential for Heme-Induced ALIS Formation.
Given that heme has been reported to induce several signaling pathways in macrophages, including MAPKs (26), we next investigated a possible role for ERK, p38, and JNK in ALIS formation. p38 has been previously implicated in LPS-induced protein aggregation (25). To address this question, BMDMs were pretreated with the specific inhibitors PD98059 (ERK), SB203580 (p38), and SP600125 (JNK) and stimulated with heme for 6 h. As shown in Fig. S3 A and B, the numbers of p62+ and Ub+ dots were dramatically reduced in the cells treated with any one of the inhibitors compared with the untreated control, indicating that MAPKs are involved in heme-induced ALIS formation.
Fig. S3.
MAPK and ROS are necessary for the formation of heme-induced ALIS. (A) Immunofluorescence microscopy of p62 (red) and Ub (green) in BMDMs pretreated with vehicle or ERK, p38, or JNK inhibitors and stimulated with heme (100 μM) or LPS (100 ng/mL) for 6 h. (B) Quantification of p62+ dots in BMDMs stimulated as in A. (C) Flow cytometry analysis of ROS production in BMDMs pretreated or not with NAC and stimulated with heme (100 μM). (D) Immunofluorescence images of p62+ dot (red) formation in BMDMs pretreated with NAC (10 mM) or mitoTEMPO (500 μM) for 1 h and stimulated with heme (100 μM) for 6 h. (E) Quantification of p62+ dots in BMDMs stimulated as in D. (F–H) Fluorimetric analysis of ROS production in BMDMs stimulated with heme (100 μM) (F), menadione (100 μM) (G), or H2O2 (400 μM) (H). (I–K) Quantification of p62+ dots in BMDMs stimulated as in F, G, and H. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
Compelling evidence from the literature shows that many of the inflammatory and cytotoxic effects exerted by heme are mediated by ROS that can be generated by enzymatic as well as nonenzymatic reactions (9, 17, 27, 28). As an important source of cellular stress, an excess of ROS also has been implicated as a trigger for protein aggregation (29, 30). Thus, we aimed to evaluate the contribution of ROS to ALIS formation in macrophages on stimulation with heme. Flow cytometry analysis using a broad-range cell-permeable free radical probe [5-(and-6)-chloromethyl-20,70-dichlorodihydro-fluorescein diacetate acetyl ester (CM-H2DCFDA)] showed that heme induces a robust production of ROS in BMDMs that is abrogated in the presence of N-acetyl-l-cysteine (NAC), a ROS scavenger through the increase of glutathione intracellular levels (Fig. S3C). Next, BMDMs were pretreated with NAC and then stimulated with heme for quantification of ALIS formation. As shown in Fig. 2 A–C, pretreatment with NAC completely abolished heme-induced ALIS formation. ROS can originate from various enzymatic systems in mammalian cells, including NADPH oxidases, xanthine oxidase, uncoupled NO synthase, and the mitochondrial electron transport chain (31).
Fig. 2.
ROS and NRF2 control heme-induced ALIS formation. (A) Immunofluorescence microscopy showing p62 (red) and Ub (green) localization in BMDMs pretreated or not with NAC (10 mM) for 1 h and stimulated with heme (100 μM) for 6 h. (B and C) Quantification of p62+ (A) and Ub+ (B) BMDMs stimulated as in A. (D) Confocal microscopy images of BMDMs stained for NRF2 (red) and Ub (green) after stimulation with CoPP (100 μM) for 6 h or with heme (100 μM) for 2, 4, and 6 h. (E and F) Western blot analysis of p62 (E) and Nqo1 (F) protein levels in cell extracts of BMDMs stimulated with CoPP (100 μM) for 6 h or with heme (100 μM) for 2, 4, and 6 h. Actin served as a loading control. (G) Western blot analysis of p62 expression levels in BMDMs from WT and Nrf2−/− mice stimulated with heme (100 μM) for 2, 4, and 6 h. Actin served as a loading control. (H and I) Immunofluorescence microscopy of BMDMs from WT (H) and Nrf2−/− (I) mice stimulated with heme (100 μM) or with CoPP (100 μM) for 6 h. (J) Quantification of p62+ dots in BMDMs from WT and Nrf2−/− mice stimulated with heme (100 μM) for 2, 4, and 6 h and with CoPP (100 μM) for 6 h. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
Mitochondria are an important source of ROS (mtROS) induced by heme (10). To evaluate the involvement of mtROS in heme-induced ALIS formation, we pretreated BMDMs with mitoTEMPO, a specific scavenger of mitochondrial superoxide, and again quantified p62+ puncta in these cells. Inhibition of mtROS completely abrogated p62+ protein aggregates (Fig. S3 D and E), underscoring the importance of ROS, particularly mtROS, in the formation of ALIS induced by heme. Further supporting this idea, we observed that inducing oxidative stress with either menadione or hydrogen peroxide was sufficient to induce ALIS formation (Fig. S3 F–K).
ALIS Formation Induced by Heme Is Dependent on NRF2-Driven Transcription.
Western blot analysis revealed that increasing concentrations of heme induced a dose-dependent increase in p62 protein levels (Fig. S4A). Interestingly, preventing oxidative stress by treating BMDMs with NAC significantly reduced the levels of p62 protein (Fig. S4B). Taken together, these results suggest that ALIS formation induced by heme is not the result merely of relocalization of p62, but likely depends on a transcriptional program in response to oxidative stress. To approach the question of whether ALIS formation induced by heme requires new gene expression, we treated BMDMs with actinomycin D to inhibit mRNA synthesis or with cyclohexamide A to inhibit protein synthesis and stimulated cells with heme. Both the increase in p62 protein expression and the assembly of p62+ and Ub+ aggregates induced by heme were completely blocked by either treatment, supporting our hypothesis that transcriptional events are implicated in the formation of ALIS in our experimental model (Fig. S4 C–E).
Fig. S4.
Heme-induced ALIS requires synthesis of p62. (A) Western blot analysis of p62 protein expression in BMDMs stimulated with heme (30 and 100 μM) for 6 h. Actin served as a loading control. (B) Western blot analysis of p62 protein levels from extracts of BMDMs pretreated with NAC and stimulated with heme (100 μM) for 6 h. Actin served as a loading control. (C) Immunofluorescence microscopy for p62 (red) and Ub (green) of BMDMs pretreated with actinomycin D (ACT D; 5 μg/mL) or cyclohexamide (CHX; 5 μg/mL) and stimulated with heme (100 μM) for 6 h. (D) Quantification of p62+ dots of BMDMs stimulated as in C. (E) Western blot analysis of p62 protein levels in extracts from BMDMs stimulated as in A. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
The transcription factor NF-E2–related factor (NRF2) is a master regulator of protective responses in cells under oxidative stress, controlling genes crucial for maintaining the balance in cellular compartments requiring optimal ROS concentrations (32). These cytoprotective genes include those involved in glutathione synthesis, detoxification of xenobiotics, drug transport, and elimination of ROS (32). Thus, we hypothesized that NRF2 could control the transcriptional events required for ALIS formation induced by heme.
To address this question, we first analyzed the subcellular localization of NRF2 during stimulation of BMDMs with heme. Under normal conditions, NRF2 is found in the cytosol, where it interacts with Kelch-like ECH-associated protein 1 (KEAP1). KEAP1 associates with Cullin 3 to form a Ub-E3 ligase complex that mediates the polyubiquitination of NRF2, resulting in constitutive degradation of NRF2 by the proteasome. In the presence of ROS or electrophilic reagents, thiols from reactive cysteines in KEAP1 are modified, impairing KEAP1-Cullin3-E3 ligase complex activity and thus reducing NRF2 degradation, which can then accumulate in the nucleus (32). Confocal microscopy showed that stimulation of BMDMs with heme induced NRF2 translocation to the nucleus after 6 h, similar to cobalt protoporphyrin (CoPP), a known activator of NRF2 (33) (Fig. 2D). Furthermore, Ub staining demonstrated that this coincides with the formation of Ub+ protein aggregates (Fig. 2D).
p62 has been identified as a target gene for NRF2 because it harbors an NRF2-antioxidant response element that drives its transcription. We observed that on stimulation with heme, the levels of p62 protein expression increased with the same kinetics as that of NQO1, a known NRF2-dependent gene (34) (Fig. 2 E and F). This prompted us to investigate whether p62 expression, which is required for ALIS formation induced by heme, is dependent on NRF2. To this end, we examined p62 protein expression in WT and Nrf2−/− BMDMs at different time points after stimulation with heme. As shown in Fig. 2G, p62 expression on stimulation with heme was severely reduced in Nrf2−/− macrophages compared with WT controls in all time points tested. As a consequence, ALIS formation was also severely reduced in Nrf2−/− cells after stimulation with heme or CoPP compared with WT controls (Fig. 2 H–J). Taken together, this set of results shows that heme induces an ROS- and NRF2-dependent transcriptional program that drives p62 expression, which is essential for the formation of ALIS.
Heme Must Be Degraded to Induce ALIS Formation.
Once released from hemeproteins, heme is potentially cytotoxic, and thus adaptive cellular responses are initiated to maintain tolerable levels of free heme (1). Heme catabolism is achieved by the enzymatic activity of heme-oxygenase (HO)-1 and HO-2, the products of HMOX1 and HMOX2, respectively. HO-2 is expressed constitutively in homeostatic conditions, whereas HO-1 is induced by a variety of stimuli. HO-1 is the rate-limiting enzyme for heme degradation, and cells from Hmox1−/− mice are unable to cope with increased levels of free heme and display exacerbated cytotoxicity (7). As shown in Fig. S5 A and B, heme induced a robust time- and dose-dependent increase in HO-1 and p62 protein expression concomitantly.
Fig. S5.
HO-1 enzymatic activity and heme-derived iron are necessary for ALIS formation. (A and B) Western blot analysis from extracts of BMDMs stimulated by heme (100 μM) for 2, 4, and 6 h (A) or by heme (30, 50, and 100 μM) for 6 h (B). Actin served as a loading control. (C and D) Quantification of p62+ (C; red) and Ub+ (D; green) dots in BMDMs pretreated or not with SnPP (100 μM) and stimulated with heme (100 μM) for 6 h. (E) Quantification of p62+ dots in BMDMs stimulated with heme (100 μM) in the presence or absence of DFO (2 mM 1 h before). (F) Immunofluorescence images showing the colocalization of p62+ (red) and Ub+ (green) dots in BMDMs stimulated as in E. (G) Schematic representation of heme (Top) and PPIX (Bottom). Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
To investigate whether these events are related, we analyzed the levels of p62 protein expression in Hmox1+/+, Hmox1+/−, and Hmox1−/− BMDMs after 4 h and 6 h of stimulation with heme. The increase in p62 protein expression observed in Hmox1+/+ and Hmox1+/− macrophages treated with heme was not observed in Hmox−/− BMDMs (Fig. 3A), which explains the dramatically reduced number of ALIS in these cells (Fig. 3 B–D). Under an oxidative environment, HO-1 has been reported to localize to the nucleus, where it binds to and stabilizes NRF2, suggesting an additional role independent of its enzymatic activity (35). Our data indicate that both HO-1 and NRF2 are essential for heme-induced ALIS.
Fig. 3.
HO-1 and iron released from heme are essential for ALIS formation induced by heme. (A) Western blot analysis of HO-1 and p62 expression in cell extracts from Hmox1+/+, Hmox1+/−, and Hmox1−/− BMDMs stimulated with heme (100 μM) for 4 and 6 h. Actin served as a loading control. (B) Quantification of p62+ dots in from Hmox1+/+, Hmox1+/−, and Hmox1−/− BMDMs stimulated as in A. (C and D) Immunofluorescence microscopy for p62 (red) and Ub (green) in Hmox1+/+ (C) and Hmox1−/− (D) BMDMs. (E) Immunofluorescence microscopy images of BMDMs stained for p62 (red) and Ub (green) after stimulation with heme (100 μM), heme plus deferoxamine (H+DFO), or PPIX (100 μM) for 6 h. (F) Quantification of p62+ dots in BMDMs stimulated as described in E. (G and H) Western blot analysis of HO-1 (G) and p62 (H) of cell extracts from BMDMs stimulated with heme (100 μM), H+DFO, PPIX (100 μM), FeCl3 (Fe3+; 100 μM), or FeSO4 (Fe2+; 100 μM) for 6 h. Actin served as a loading control. (I) Immunofluorescence images of the colocalization of (red) and Ub (green) in BMDMs stimulated with FeCl3 (Fe3+; 100 μM) or FeSO4 (Fe2+; 100 μM) for 6 h. (J) Quantification of p62+ dots in BMDMs stimulated as in I. (K) Quantification of p62+ dots in WT and Nrf2−/− BMDMs stimulated with FeCl3 (Fe3+; 100 μM) or FeSO4 (Fe2+; 100 μM). (L and M) Quantification of p62+ dots in BMDMs stimulated with FeCl3 (Fe3+; 100 μM) or FeSO4 (Fe2+; 100 μM) with or without succinylacetone (SA; 1 μM 1 h before) for 6 h (L) and 24 h (M). DAPI was used to stain nuclei (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
To address whether the impact of HO-1 is related to its enzymatic activity or to its role as a NRF2 regulator, we pretreated BMDMs with tin protoporphyrin (SnPP), an inhibitor of HO-1 enzymatic activity that does not affect its expression (31), before stimulation with heme. This resulted in a significant reduction in the number of p62+ and Ub+ puncta in treated cells compared with the untreated controls (Fig. S5 C and D), indicating that the enzymatic activity of HO-1 and the generation of heme degradation products are required for heme-induced formation of ALIS.
Labile Fe Released on Heme Degradation Is Sufficient to Induce ALIS.
On heme degradation by HO-1, the protoporphyrin IX (PPIX) ring is cleaved, releasing biliverdin and equimolar amounts of CO and Fe. The labile Fe can catalyze the generation of ROS via a Fenton reaction (7). Given our results showing that the formation of ALIS induced by heme is dependent on its degradation and ROS, we next examined whether Fe alone could account for the capacity of heme to induce ALIS. To this end, we stimulated BMDMs with heme in the presence of the iron chelator deferoxamine (DFO). As shown in Fig. S5 E and F, treatment with DFO completely abrogated the formation of p62+ and Ub+ aggregates. We then stimulated BMDMs with PPIX (Fig. S5G), a direct heme precursor that lacks the Fe atom, and did not detect ALIS formation (Fig. 3 E and F). Taken together, these results support the idea that Fe is the minimal molecular requirement for the formation of ALIS induced by heme.
To further explore this hypothesis, we stimulated RAW GFP-LC3 macrophages with FeSO4 (Fe2+) and FeCl3 (Fe3+) and observed that, similar to heme, both Fe2+ and Fe3+ induced a time- and dose-dependent increase in GFP-LC3+ dot-like structures that are cleared from the cytosol through autophagy (Fig. S6 A–C). Importantly, these structures were characterized as ALIS because they were also p62+ and Ub+ (Fig. 3 I and J). Furthermore, both Fe2+ and Fe3+ induced strong ROS production (Fig. S6 D and E), as well as increased HO-1 and p62 protein levels in BMDMs (Fig. 3 G and H). Finally, Fe2+ and Fe3+ failed to induce ALIS in Nrf2−/− macrophages (Fig. 3K), which is consistent with our previous results showing that NRF2 is essential for heme-induced ALIS formation. To exclude the possibility that the ALIS formation observed in cells treated with labile Fe was a result of increased heme synthesis, we pretreated BMDMs with succinylacetone (an inhibitor of heme synthesis) and stimulated the cells with Fe2+ or Fe3+ for 6 and 24 h. As observed in Fig. 3 L and M, ALIS formation is not likely related to increased heme synthesis on labile Fe stimulation. These data demonstrate that labile Fe recapitulates important features related to the formation of ALIS induced by heme, and thus can be considered the minimal heme-derived motif required for the formation of ALIS.
Fig. S6.
Fe2+ and Fe3+ induce ALIS. (A and B) Quantification of p62+ dots in BMDMs stimulated with Fe2+ (A) or Fe3+ (B) (25, 50, or 100 μM) for 2, 4, 6, 12, or 24 h. (C) Quantification of GFP-LC3+ dots in RAW cells pretreated with vehicle or wortmannin (200 nM) and stimulated with Fe2+ (100 μM) for 12 or 24 h. (D and E) Flow cytometry analysis for ROS production in BMDMs stimulated with Fe2+ (100 μM) (D) or Fe3+ (100 μM) (E). Results are representative of one of at least three independent experiments. For all quantification experiments, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
Ferritin Protects Against Iron-Induced ALIS Formation.
Following heme degradation, the deleterious effects of labile Fe can be neutralized by a myriad of metabolic pathways that are triggered by the presence of Fe itself. These pathways include induction of Fe efflux pumps and increased expression of the ferritin H chain (FtH). FtH associates with the ferritin L chain (FtL) to form a multimeric complex that is able to bind to and oxidize up to 4,500 Fe atoms (7). Because our results show that labile Fe induces ALIS formation, we speculated that FtH could counteract this effect. Indeed, heme, Fe2+, and Fe3+, but not PPIX, induced a significant increase in ferritin protein levels in BMDMs (Fig. 4A). FtH−/− BMDMs displayed dramatically higher p62 protein levels compared with WT controls when stimulated with Fe2+ (Fig. 4B). This was accompanied by a remarkable increase in the number of p62+ and Ub+ aggregates induced by Fe2+ in FtH−/− macrophages compared with WT controls (Fig. 4 C–E). Collectively, these data indicate that the ability of ferritin to limit the levels of intracellular labile Fe prevents the formation of ALIS.
Fig. 4.
Ferritin is essential to avoid iron-induced ALIS. (A) Western blot analysis of cell extracts from BMDMs stimulated with heme (100 μM), H+DFO (2 mM 1 h before), PPIX (100 μM), FeCl3 (Fe3+; 100 μM), or FeSO4 (Fe2+; 100 μM) for 6 h. Actin served as a loading control. (B) Western blot analysis of p62 protein expression in BMDMs from WT and FtH−/− BMDMs stimulated with Fe2+ or Fe3+ (100 μM) for 6 h. Actin served as a loading control. (C and D) Immunofluorescence images showing the colocalization of p62 (red) and Ub (green) in WT (C) and FtH−/− (D) BMDMs stimulated with Fe2+ (100 μM) for 6 h. (E) Quantification of p62+ dots in an experiment performed as in C and D. Nuclei were stained with DAPI (blue). (Scale bars: 20 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least 300 cells were counted. Data are mean ± SD. *P < 0.05.
Hemolysis Induces the Formation of ALIS in Vivo.
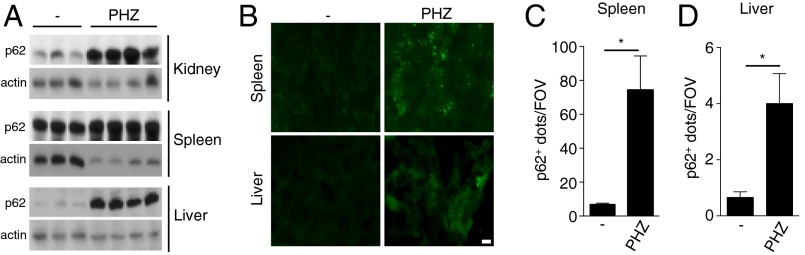
Independent studies have demonstrated that during hemolytic diseases, high amounts of hemoglobin and, subsequently, free heme promote tissue damage and inflammation. To test the hypothesis that such an increase in liberated free heme would induce ALIS formation in the damaged tissues in vivo, we mimic what happens during hemolytic diseases by injecting PHZ intraperitoneally in mice for 12 h. We initially observed a dramatic increase in p62 protein levels in kidneys, spleens, and livers of PHZ-injected mice compared with saline-injected mice, suggesting that ALIS formation in response to high levels of free heme does occur in vivo (Fig. 5A). To confirm this, we quantified the number of p62+ dots in tissue sections of spleens and livers from PHZ- and saline-injected mice. Our results showed a robust increase in the number of p62+ dots in the tissues obtained from PHZ-treated mice, especially in the spleen, compared with mice injected with vehicle (Fig. 5 B–D). In conclusion, these data reinforce our hypothesis that ALIS formation is indeed an integral part of cell response to high levels of free heme and, as such, likely has an important role in homeostasis maintenance.
Fig. 5.
Hemolysis triggers ALIS formation in vivo. (A) Western blot analysis of p62 expression levels in the kidneys (Top), spleen (Middle), and liver (Bottom) from control (−) and PHZ-injected mice. Actin served as a loading control. (B and C) Immunofluorescence of spleen (B) and liver (C) tissue sections from control and PHZ-injected mice stained for p62 (green). (D and E) Quantification of p62+ dots per field of view (FOV) in spleen and liver tissue sections of control (−) and PHZ-injected mice. (Scale bars: 10 μm.) Results are representative of one of at least three independent experiments. For all quantification experiments using microscopy, at least five fields were counted. Data are mean ± SD. *P < 0.05.
Discussion
Heme is ubiquitously distributed and exists essentially as a prosthetic group of hemeproteins with various biological functions such as the transport of diatomic gases, chemical catalysis and electron transfer (3). When released from hemeproteins, however, free heme becomes highly cytotoxic, owing mainly to its pro-oxidant properties, and participates in the pathogenesis of inflammatory/hemolytic diseases such as malaria and sepsis (1, 2). This in turn induces an equally potent cellular response to cope with the stress, minimize redox imbalance, and maintain homeostasis (33). We show here that the stress response triggered by heme includes the transient assembly of p62+/Ub+ protein aggregates known as ALIS. This response is mediated by ROS generated in response to heme and/or its degradation product, labile Fe, and operates through activation of MAPKs and the transcriptional factor NRF2, resulting in increased levels of p62 expression. Importantly, in severe hemolytic conditions in vivo, the up-regulation of p62 and the formation of ALIS were observed in target tissues, especially in the spleen, indicating that this mechanism might be important for the cells to cope with the toxic effects of heme.
ALIS are stress-induced p62+/Ub+ aggregates, distinct from aggresomes or stress granules formed in various cell types under a wide range of stress conditions, indicating that they are part of a general stress response (23). During stress situations in which protein synthesis exceeds degradation rates, the rapid accumulation of protein aggregates could be either toxic to the cell or necessary to adjust synthesis and degradation rates. Even though the precise role of ALIS in the stress response remains unclear, its transient nature owing to continuous removal through autophagy suggests that its accumulation might be toxic to the cell. Indeed, accumulation of protein aggregates is a feature observed in various degenerative diseases, such as Alzheimer’s and Parkinson’s (36).
During oxidative conditions, heme is released from hemeproteins and quickly catabolized by HO-1 (7). The expression of HO-1 is induced ubiquitously in response to oxidative stress. Most, if not all, forms of oxidative stress are associated with a rapid increase in the rate of cellular heme catabolism through the induction of HMOX1 transcription and HO-1 expression (7). Here we observed that heme induced a robust time- and dose-dependent increase of HO-1 concomitant with p62. Interestingly, the increased level of p62 expression induced by heme was abolished in macrophages from Hmox1−/− mice. Consistent with this previous result, the number of ALIS induced by heme in Hmox1−/− macrophages was significantly lower than that in WT macrophages. Furthermore, functional inhibition of HO-1 with SnPP also resulted in decreased formation of ALIS induced by heme, indicating that the enzymatic activity of HO-1 and the release of free iron that results from heme degradation are required for the formation of ALIS.
It may seem counterintuitive that a detoxifying mechanism, such as those mediated by HO-1, would result in the release of large amounts of free Fe, which can act as a Fenton reagent to catalyze the production of free radicals in an unfettered manner (1). Indeed, in several experimental models, the cytotoxicity attributed to heme is likely mediated by the free Fe released from its PPIX ring. In our model, we show that stimulation with free Fe recapitulates most of the features observed on heme stimulation, i.e., ROS production, increases in HO-1 and p62 protein levels, and ALIS formation. On the other hand, various Fe metabolic pathways induced by Fe itself represent yet another layer of protection against the cytotoxicity induced by heme degradation products. These include the induction of Fe efflux pump, as well as the induction of FtH expression, both of which contribute to the neutralization of the potential toxic effects of free intracellular Fe (7). FtH is also a stress-responsive gene and regulates Fe metabolism. Some of the proinflammatory effects of heme are not affected in FtH−/− cells. For example, in WT and FtH−/− macrophages, IL-1β processing is similar in response to heme stimulation. In contrast, we show here that FtH−/− cells displayed dramatically higher levels of p62 protein, as well as a greater number of ALIS, compared with WT cells. The ability of ferritin is due in part to the ferroxidase activity of FtH, which converts reactive iron (Fe2+) into inert and nucleates iron (Fe3+), no longer available to catalyze the production of free radicals via a Fenton reaction (7, 8). Consistently, the absence of FtH resulted in higher levels of p62 expression on treatment with Fe2+, but not on treatment with Fe3+.
Our results highlight a previously uncharacterized cellular response (ALIS formation) induced by heme or one of its degradation products, free Fe. Usually, the formation of protein aggregates inside the cell is associated with deleterious events; however, the fact that the cytoprotective enzyme HO-1 is involved in ALIS formation may suggest a function for ALIS as a mechanism that helps cells under stress to maintain or return to homeostatic conditions. Alternatively, accumulation of such aggregates may turn out to be toxic to the cell, resulting in loss of viability. Most likely, both hypotheses will hold true in different cellular contexts, depending on the intensity and duration of stress conditions, as well as on the efficiency of the cell in dealing with these conditions.
Experimental Procedures
Reagents and Materials.
Heme, PPIX, SnPPIX (tin protoporphyrin), and CoPPIX (cobalt protoporphyrin) were purchased from Frontier Scientific. These reagents were dissolved in NaOH (0.1 M), diluted in RPMI-1640, and filtered (0.22 μM pore size) just before use. Stock solutions of porphyrins were prepared in the dark to avoid free radical generation. LPS 0111:B4 from Escherichia coli and synthetic Pam3CSK4 were purchased from InvivoGen. NAC, DFO, FeSO4, FeCl3, actinomycin D, cyclohexamide A, puromycin ERK (PD98059), p38 (SB203580), JNK (SP600125) inhibitors, and succinylacetone were obtained from Sigma-Aldrich. Antibodies for mouse p62/SQSTM1 and GFP were obtained from Abcam. Antibody for NRF2 and Ub were from Sigma-Aldrich and Enzo Lifesciences, respectively. Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 568 goat anti-rabbit IgG were obtained from Molecular Probes. ELISA for TNF measurements was from Peprotech. MitoTEMPO was from Enzo Lifesciences. RPMI-1640, DMEM, penicillin-streptomycin, FCS, and l-glutamine were obtained from Lonza.
Cells.
BMDMs were cultured in RPMI-1640 medium supplemented with 10% (vol/vol) FCS, 1% penicillin-streptomycin (vol/vol), and 30% (vol/vol) L929 supernatant. RAW 264.7 and immortalized BMDMs were cultured in RPMI-1640 medium supplemented with 10% (vol/vol) FCS and 1% penicillin-streptomycin (vol/vol). MEFs were cultured in DMEM supplemented with 10% (vol/vol) FCS, 1% penicillin-streptomycin (vol/vol), and 1% l-glutamine (vol/vol). Time and concentration of stimuli and inhibitors are described in the figure legends. Stimulation of macrophages and MEFs with heme was performed in RPMI-1640 medium supplemented with 1% (vol/vol) FCS and 1% penicillin-streptomycin (vol/vol). All experiments were performed in accordance with guidelines of the institutional Ethical Committee and were approved by the Commission for the Ethical Use of Animals of the Federal University of Rio de Janeiro (approval CEUA/CCS/UFRJ/IMPPG 011).
Lentiviral Transduction.
Recombinant viral particles were obtained by cotranfection of HEK293T cells with the lentiviral vector (pHR-SIN-CSGWΔNotI-GFP-LC3, from C. Münz, University of Zurich) with the helper plasmids pCMVΔR8.91 and pMDG. Lentiviral supernatant was added onto the target cells in the presence of polybrene (8 mg/mL) and centrifuged at 2,061 × g for 90 min. After an overnight incubation at 37 °C in 5% CO2, fresh medium was added to the cells.
Immunofluorescence Microscopy.
After stimulation, cells were fixed with 4% (wt/vol) paraformaldehyde, blocked with PBS-BSA (1% wt/vol), made permeable with Triton X-100 (Sigma-Aldrich), and incubated with primary antibodies. Cells were then washed three times with PBS and stained with the appropriate secondary antibodies and DAPI (Sigma-Aldrich).
In Vivo Hemolysis Experiments.
Hemolysis was induced by intraperitoneal injection of PHZ. The PHZ was freshly prepared by dissolving it in sterile PBS and adjusting the pH to 7.2 with NaOH. Mice were injected with 0.1 mg/g and then reinjected with 0.05 mg/g 16 h later. Mice were killed at 12 h after the second injection, and the kidneys, spleens, and livers were collected for detection of p62 by Western blot analysis. Spleens and livers from PHZ- and saline-treated mice were harvested, embedded in OCT, and immediately frozen. Tissue sections (5 µm thick, at least three sections per sample) were cut and transferred to microscope slides. Sections were allowed to dry at room temperature and then stored at −20 °C until use. Tissue sections were permeabilized in PBS containing 0.25% Triton X-100 for 5 min at room temperature. Slides were washed with PBS, and unspecific binding sites were blocked with PBS supplemented with 0.1% Triton X-100, 2% BSA, and 5% horse serum for 60 min at room temperature. Slides were then washed and incubated overnight at 4 °C with rabbit anti-p62 antibody (diluted 1:250 in PBS containing 0.1% Triton X-100 and 0.5% BSA). Slides were washed extensively in PBS and then incubated for 2 h at room temperature with Alexa Fluor 488-conjugated anti-rabbit IgG (1:500 in PBS, 0.1% Triton X-100, and 0.5% BSA). Slides were finally washed in PBS, fixed for 20 min in PBS and 1% PFA, and mounted with ProLong Gold with DAPI (Molecular Probes).
Image Analysis.
GFP-LC3+, p62+, and Ub+ dots were counted from images acquired with a Leica epifluorescence microscope. The number of dots per cell was quantified using ImageJ software (37), and the results were representative of five random fields analyzed per experimental condition (300 cells at least). For analysis of the in vivo experiments, images were acquired with a Leica microscope using a 100× immersion objective. At least five fields were acquired for each sample. The number of p62 dots per field of view was quantified using ImageJ.
Immunoblot Analysis.
After stimulation, cells were lysed in ice-cold RIPA buffer, and extracts were separated by 10% (wt/vol) SDS/PAGE, transferred to nitrocellulose membranes, and subjected to immunodetection by standard procedures.
ROS Measurements.
Cells were incubated in prewarmed PBS-BSA 1% containing 10 mM H2DCFDA (Molecular Probes) for 20 min at 37 °C, washed in PBS, and analyzed by FACS. For fluorimetric ROS measurements, cells were washed in serum-free culture medium, incubated with H2DCFDA for 30 min, and then washed and kept in PBS until read.
Statistical Analysis.
Results are expressed as mean ± SD of data obtained in independent experiments. Statistical differences between groups were determined using Student’s t test or one-way ANOVA followed by the Mann–Whitney U test. Statistical significance was set at P < 0.05.
Acknowledgments
We thank N. Mizushima (Tokyo Medical and Dental Center) for the Atg5−/− MEFs; C. Münz (University of Zurich) for pHR-SIN-CSGWNotI-GFP-LC3; M. Soares (Instituto Gulbenkian de Ciência) for femurs from Nrf2−/−, FtH−/−, and Hmox1−/− mice; and Clarissa Damaso (Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro) for anti-p62. L.R.V. and J.D. were supported by fellowships from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). E.K. and F.F.D. received fellowships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). M.S.S. was supported by a fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Work in the L.H.T., L.A.M.C., and M.T.B. laboratories was supported by grants from FAPERJ, CAPES, and CNPq.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608928113/-/DCSupplemental.
References
- 1.Dutra FF, Bozza MT. Heme on innate immunity and inflammation. Front Pharmacol. 2014;5:115. doi: 10.3389/fphar.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen R, Gouveia Z, Soares MP, Gozzelino R. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front Pharmacol. 2012;3:77. doi: 10.3389/fphar.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: Role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28(2):289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 4.Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988;265(2):539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- 5.Aft RL, Mueller GC. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984;259(1):301–305. [PubMed] [Google Scholar]
- 6.Aft RL, Mueller GC. Hemin-mediated DNA strand scission. J Biol Chem. 1983;258(19):12069–12072. [PubMed] [Google Scholar]
- 7.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 8.Soares MP, Bozza MT. Red alert: Labile heme is an alarmin. Curr Opin Immunol. 2016;38:94–100. doi: 10.1016/j.coi.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Barcellos-de-Souza P, et al. Heme modulates intestinal epithelial cell activation: Involvement of NADPHox-derived ROS signaling. Am J Physiol Cell Physiol. 2013;304(2):C170–C179. doi: 10.1152/ajpcell.00078.2012. [DOI] [PubMed] [Google Scholar]
- 10.Dutra FF, et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci USA. 2014;111(39):E4110–E4118. doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belcher JD, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyoumi S, et al. Heme and acute inflammation role in vivo of heme in the hepatic expression of positive acute-phase reactants in rats. Eur J Biochem. 1999;261(1):190–196. doi: 10.1046/j.1432-1327.1999.00254.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagener FA, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216(3):456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo RT, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro AP, et al. Leukotriene B4 mediates neutrophil migration induced by heme. J Immunol. 2011;186(11):6562–6567. doi: 10.4049/jimmunol.1002400. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123(11):4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortes GB, et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood. 2012;119(10):2368–2375. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinchi F, et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317–1329. doi: 10.1161/CIRCULATIONAHA.112.130179. [DOI] [PubMed] [Google Scholar]
- 20.Lim SK, et al. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92(6):1870–1877. [PubMed] [Google Scholar]
- 21.Carneiro LA, Travassos LH. The interplay between NLRs and autophagy in immunity and inflammation. Front Immunol. 2013;4:361. doi: 10.3389/fimmu.2013.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lelouard H, et al. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 2002;417(6885):177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 23.Szeto J, et al. ALIS are stress-induced protein storage compartments for substrates of the proteasome and autophagy. Autophagy. 2006;2(3):189–199. doi: 10.4161/auto.2731. [DOI] [PubMed] [Google Scholar]
- 24.Pankiv S, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 25.Fujita K, Maeda D, Xiao Q, Srinivasula SM. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc Natl Acad Sci USA. 2011;108(4):1427–1432. doi: 10.1073/pnas.1014156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez PL, et al. Heme amplifies the innate immune response to microbial molecules through spleen tyrosine kinase (Syk)-dependent reactive oxygen species generation. J Biol Chem. 2010;285(43):32844–32851. doi: 10.1074/jbc.M110.146076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu DT, et al. Correlation of membrane lipid peroxidation with oxidation of hemoglobin variants: Possibly related to the rates of hemin release. Free Radic Biol Med. 1996;21(1):89–95. doi: 10.1016/0891-5849(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 28.Moraes JA, et al. Heme modulates smooth muscle cell proliferation and migration via NADPH oxidase: A counter-regulatory role for heme oxygenase system. Atherosclerosis. 2012;224(2):394–400. doi: 10.1016/j.atherosclerosis.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, et al. Role of reactive oxygen species-dependent protein aggregation in metabolic stress-induced necrosis. Int J Oncol. 2010;37(1):97–102. doi: 10.3892/ijo_00000657. [DOI] [PubMed] [Google Scholar]
- 30.Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36(9):1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 31.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20(6):1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paiva CN, et al. Oxidative stress fuels Trypanosoma cruzi infection in mice. J Clin Invest. 2012;122(7):2531–2542. doi: 10.1172/JCI58525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem. 2010;285(29):22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Q, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282(28):20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- 36.García-Arencibia M, Hochfeld WE, Toh PP, Rubinsztein DC. Autophagy, a guardian against neurodegeneration. Semin Cell Dev Biol. 2010;21(7):691–698. doi: 10.1016/j.semcdb.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]