Significance
Macropinocytosis and phagocytosis are two Ras-regulated, highly related processes of great physiological relevance collectively termed large-scale endocytosis. Both are actin-driven and entail engulfment of extracellular material by crown-like protrusions. Aside from the Arp2/3 complex, which serves as the main nucleator of branched actin filaments at the cup rim, the underlying mechanisms of actin assembly still remain elusive. Here, we analyzed the role of Diaphanous-related formin G (ForG) from Dictyostelium by biochemical, genetic, and imaging techniques. Our data demonstrate that this formin exhibits a rather weak nucleation activity and imply that ForG-mediated filament elongation synergizes with the Arp2/3 complex in actin assembly. Finally, we identify ForG as a Ras-regulated formin and show its significance for actin assembly in endocytic structures.
Keywords: Arp2/3 complex, formin, macropinocytosis, phagocytosis, Ras
Abstract
Phagocytosis and macropinocytosis are Ras-regulated and actin-driven processes that depend on the dynamic rearrangements of the plasma membrane that protrudes and internalizes extracellular material by cup-shaped structures. However, the regulatory mechanisms underlying actin assembly in large-scale endocytosis remain elusive. Here, we show that the Diaphanous-related formin G (ForG) from the professional phagocyte Dictyostelium discoideum localizes to endocytic cups. Biochemical analyses revealed that ForG is a rather weak nucleator but efficiently elongates actin filaments in the presence of profilin. Notably, genetic inactivation of ForG is associated with a strongly impaired endocytosis and a markedly diminished F-actin content at the base of the cups. By contrast, ablation of the Arp2/3 (actin-related protein-2/3) complex activator SCAR (suppressor of cAMP receptor) diminishes F-actin mainly at the cup rim, being consistent with its known localization. These data therefore suggest that ForG acts as an actin polymerase of Arp2/3-nucleated filaments to allow for efficient membrane expansion and engulfment of extracellular material. Finally, we show that ForG is directly regulated in large-scale endocytosis by RasB and RasG, which are highly related to the human proto-oncogene KRas.
The internalization of solid particles by phagocytosis and internalization of bulk fluid by macropinocytosis are closely related and evolutionary conserved clathrin-independent endocytic processes (1). In higher eukaryotes, as exemplified by neutrophils and macrophages, phagocytosis is a central part of the innate immune system responsible for the clearance of viral and bacterial pathogens (2), whereas the scavenging of nutrients and metabolites by macropinocytosis is considered increasingly important for tumor progression and cancer cell proliferation (3–5). Phagocytosis and macropinocytosis are actin-driven processes that entail rearrangements of the plasma membrane to engulf extracellular material, followed by delivery of the ingested material into lysosomes for extraction of nutrients (6). Morphologically, several stages can be distinguished, starting with the initiation of cup formation after detection of external cues via diverse G protein-coupled receptors, such as the Fc-γ and C3a receptors in mammalian cells (7), followed by membrane protrusion and the pursestring-like closure of the cup leading to the separation of the closed vesicle from the plasma membrane (6, 8).
The social amoeba Dictyostelium discoideum is a professional phagocyte that hunts bacteria by chemotaxis and ingests them by phagocytosis. Nevertheless, because cultivation on bacteria is difficult, most laboratories use axenic strains that can grow through uptake of liquid media by macropinocytosis (9). Recent work interestingly revealed that macropinocytosis in these strains is strongly improved due to a mutation in the axeB gene encoding the Ras GTPase-activating protein (RasGAP) neurofibromin 1 (NF1) (10). Due to its genetic tractability and ease of use, D. discoideum has become an attractive model organism that allows dissecting conserved mechanisms and signaling pathways of large-scale endocytosis (11). Comparable to mammalian cells, the initial steps of the underlying signaling cascades are quite well understood and are initiated by G protein-coupled folate receptor fAR1 activation upstream of heterotrimeric G proteins (12, 13). This step is followed by activation of small GTPases of the Ras family, which act as master regulators of diverse downstream signaling pathways, including target of rapamycin complex 2 (14) and class-I PI3-kinases (PI3Ks) (15), albeit Dictyostelium cells use ether-linked plasmanylinositides instead of phosphatidylinositides (16). PI3K1 and PI3K2 produce patches of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which are linked to actin-dependent cup formation, whereas PI3K4 is required for the conversion of the cups into intracellular vesicles (15, 17). The latter process is associated with rapid disassembly of the F-actin coat (18), and, consistently, a number of F-actin depolymerization factors, including coronin, actin-interacting protein (Aip1), and cofilin, were shown to participate in cup maturation and disassembly (18–20). Moreover, force generation by myosins was shown to play an important role in amoeba as well as in mammalian cells (17, 21, 22). However, it still remains elusive how Ras and PIP3 signaling are linked to actin assembly on the molecular level to drive extensive membrane deformations during cup formation.
Eukaryotic cells mainly use two types of actin assembly factors. The Arp2/3 complex is composed of seven subunits and operates downstream of SCAR/WAVE (Wiskott–Aldrich syndrome protein family verprolin-homologous protein) signaling to nucleate branches on the sides of existing mother filaments to generate a dense actin meshwork as illustrated by the actin architecture at the leading edge (23). Formins are dimeric multidomain proteins that nucleate and elongate linear actin filaments to form, for instance, filopodial bundles and cortical actin (24, 25). The proline-rich formin homology domain 1 (FH1) recruits profilin (PFN)–actin complexes for filament elongation, which is accomplished by the neighboring FH2 domain (24). Members of the subfamily of Diaphanous-related formins (DRFs) are tightly regulated. By virtue of additional regulatory sequences located in the N- and C-terminal regions, these proteins are intrinsically autoinhibited. Binding of Rho family GTPases to the N-terminal GTPase-binding domain (GBD) releases this autoinhibition and renders the protein active (24).
Here, we describe the role of Diaphanous-related formin G (ForG) from D. discoideum in large-scale endocytosis. We find ForG to be highly enriched in the cups of macropinosomes and phagosomes, and show that it is required for efficient uptake of fluid and solid particles. Our biochemical data further demonstrate that this formin exhibits rather weak nucleation activity in vitro, strongly suggesting that ForG mainly contributes to filament elongation during expansion and closure of the cups. Finally, our data reveal that ForG acts as a specific effector of active Ras-subfamily GTPases, demonstrating an unanticipated type of DRF regulation that, in turn, directly links Ras signaling to dynamic remodeling of the actin cytoskeleton in endocytosis.
Results
ForG Localizes to Nascent Macropinosomes and Phagosomes.
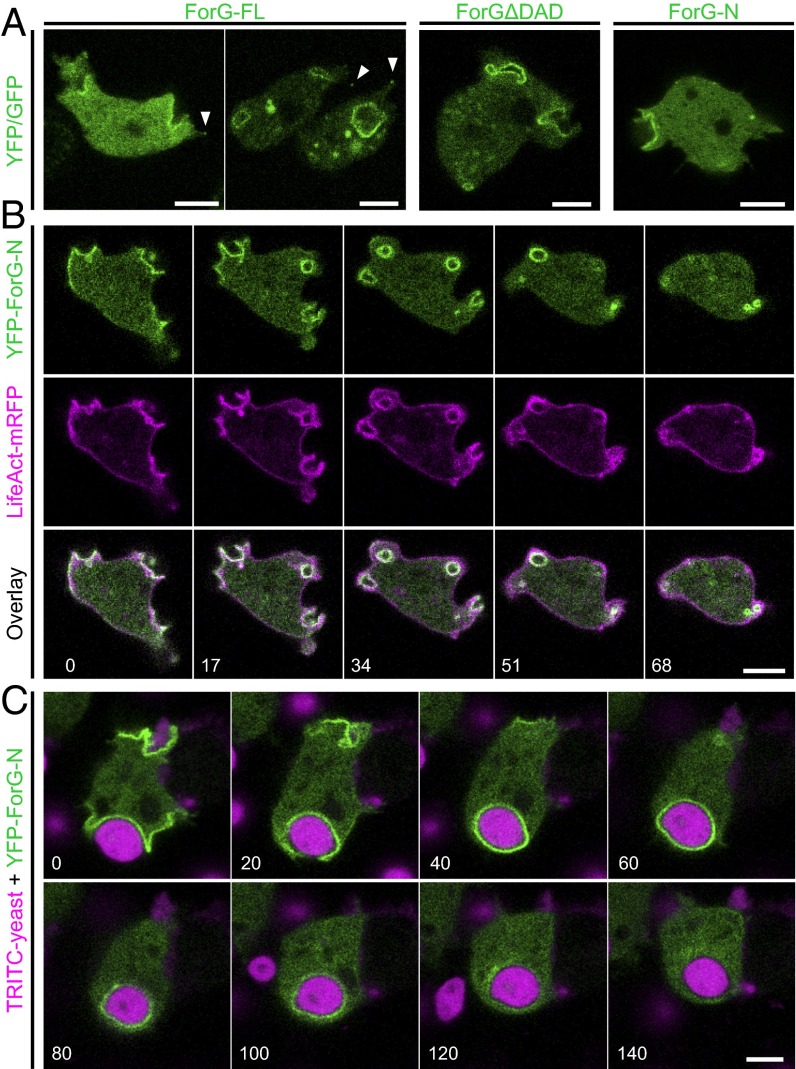
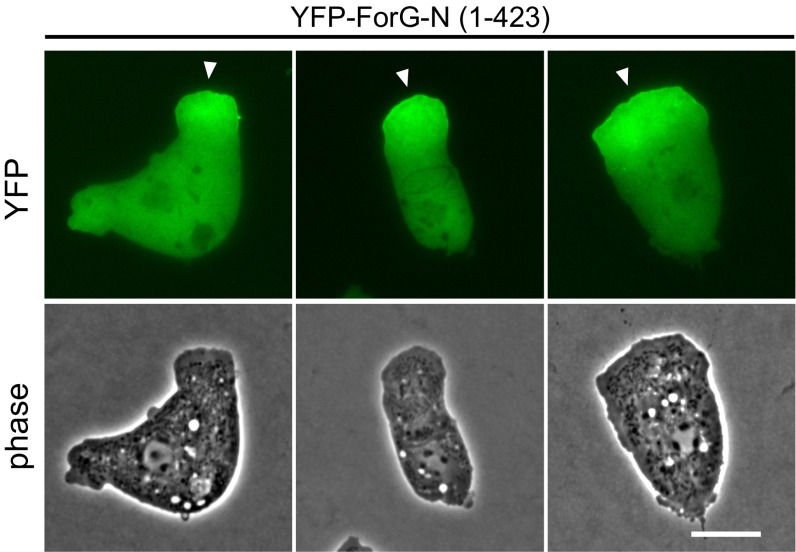
Cups, sometimes referred to as crown-shaped membrane protrusions, are formed during large-scale uptake of nutrients in macropinocytosis and phagocytosis, and depend on dynamic F-actin polymerization (6). In Dictyostelium cells, the Arp2/3 activator SCAR/WAVE was previously found to localize exclusively to the rim of macropinocytic cups, whereas F-actin was highly enriched in the entire structure beneath the plasma membrane (8). We therefore asked whether formins, the second class of actin assembly factors present in Dictyostelium, may contribute to the generation of this specialized F-actin network and operate in large-scale endocytosis. Previously, we reported that the developmentally regulated formin C (ForC) of Dictyostelium cells localizes to endocytotic structures when ectopically expressed in vegetative cells (26). However, endogenous ForC only begins to accumulate after 6 h of development, at a time point when micropinocytosis has largely ceased (27), strongly indicating that ForC is not involved in macropinocytosis. Thus, we focused our attention on the closely related formin ForG, because previous real-time PCR data indicated continuous expression of this protein in growth-phase cells and later developmental stages (28). ForG encompasses 1,074 residues (European Nucleotide Archive data bank accession no. LN901451) and fully conforms to the canonical domain organization of DRFs (28). To assess a potential role of ForG in endocytosis, we expressed full-length (FL) and two truncated ForG constructs tagged with either green or yellow fluorescent protein (GFP/YFP) in the AX2 laboratory wild-type (WT) strain and monitored protein localization by confocal microscopy (Fig. 1A and Table S1). ForG-FL localized prominently to crowns and early endosomes, and also faintly to filopodia tips. In strong overexpressors (Fig. 1A, Left), however, the localization was generally more diffuse, suggesting that endogenous GTPase levels became limiting to release ForG-FL from autoinhibition. Consistently, less diffuse distribution was observed with the constitutively active ForG variant (ForG∆DAD) lacking its C-terminal Diaphanous-autoregulatory domain (DAD). Because both constructs were formally able to catalyze excessive actin polymerization that might interfere with cell function, we additionally designed a truncated, N-terminal construct of ForG (ForG-N) encompassing the putative GBD at the very N terminus, followed by the FH3 domain (amino acids 1–423). Analogous constructs have previously been shown to be sufficient for intracellular targeting (25, 26, 29). As expected, ForG-N also faithfully localized to endocytotic structures but was no longer seen in filopodia tips. Moreover, when cup formation was physically inhibited by overlaying the cells with a thin sheet of agar, ForG-N accumulated at the leading edge of polarized cells (Fig. S1). Because we focused on endocytosis in the further course of this work, this construct was used for the localization studies.
Fig. 1.
ForG localizes to endocytic structures. (A) GFP-tagged ForG-FL, constitutively active ForGΔDAD (amino acids 1–1,040), and YFP–ForG-N (amino acids 1–423) ectopically expressed in vegetative Dictyostelium cells prominently localized to nascent macropinosomes. Faint enrichment at filopodia tips is indicated by white arrowheads. (B) Time-lapse imaging of cells coexpressing LifeAct-mRFP and YFP–ForG-N revealed that ForG localization is fairly distinct from cortical F-actin. (Bottom) Merged images illustrate striking colocalization of F-actin and ForG at macropinosomes. (C) YFP–ForG-N also accumulated at phagocytic cups throughout engulfment of the large TRITC-labeled yeast particles. Confocal sections are shown in B and C, and correspond to Movies S1 and S2. Time is given in seconds. (Scale bars: 5 μm.)
Table S1.
Oligonucleotides used in this work
| Primer | Sequence | Orientation |
| Dictyostelium constructs | ||
| GFP-ForG-FL | ||
| ForG-BU | 5′-CGCGGGATCCGCATGATATTATCAATTACATTTCAATTAGATC-3′ | Forward |
| ForG-2600-BglD | 5′-ATCAAAGATCTCCATGGAAAAGTATCCAAAAACTATG-3′ | Reverse |
| ForG-800-NsiUp | 5′-AAGTGAATGCATTGATTTTGGTTTCCAAATC-3′ | Forward |
| ForG-SD-FL | 5′-CGCGTCGACTTATTTATTTAAATTTAATTGTGATCC-3′ | Reverse |
| GFP-ForG∆DAD | ||
| ForG-PstI-DWN | 5′-AGGACCTGCAGCAATTCAACC-3′ | Forward |
| ForG-DelDAD-SD | 5′-CGCGTCGACTTATGGATCAGCACCACCAGCAATCTTTTTA-3′ | Reverse |
| pDM304-YFP | ||
| DYFP-BamHI-F1 | 5′-ACCGGATCCAAAAATGAGTAAAGGTGAAGAACTTTTC-3′ | Forward |
| DYFP-BglII-R1 | 5′-AATAGATCTGAGTCCGGATTTGTATAGTTCATCCATG-3′ | Reverse |
| YFP–ForG-N | ||
| ForG(1–423)-BglII-F1 | 5′-ATTAGATCTATGATATTATCAATTACATTTC-3′ | Forward |
| ForG(1–423)-SpeI-R1 | 5′-AATACTAGTTTAAATTTTCTTTTCAAATTCATTAA-3′ | Reverse |
| YFP-ForG-N∆RBD | ||
| ForG-delRBD-BU | 5′-ACTGGATCCTCTTCAAAATGGGTAAAAGC-3′ | Forward |
| ForG(423)-SpeD | 5′-ACAACTAGTTTAAATTTTCTTTTCAAATTCATTAA-3′ | Reverse |
| YFP-ForG∆RBD∆DAD | ||
| ForG-delRBD-BU | 5′-ACTGGATCCTCTTCAAAATGGGTAAAAGC-3′ | Forward |
| ForG(1–1,040)-SpeI-R2 | 5′-CGCACTAGTTTATGGATCAGCACCACCAGC-3′ | Reverse |
| LifeAct-mRFP | ||
| Lifeact_N-BglII-F1 | 5′-GATCTAAAAATGGGTGTCGCTGACCTGATAAAGAAGTTTGAAAGC | Forward |
| ATCTCCAAGGAAGAGA-3′ | ||
| Lifeact_N-SpeI-R1 | 5′-CTAGTCTCTTCCTTGGAGATGCTTTCAAACTTCTTTATCAGGTCAGC | Reverse |
| GACACCCATTTTTA-3′ | ||
| ForG-KO | ||
| ForG BU KO | 5′-CGCGGGATCCGCATGATATTATCAATTACATTTCAATTA-3′ | Forward |
| ForG PstD KO | 5′-CGCCTGCAGCATTATATCTTTTTCATTTG-3′ | Reverse |
| ForG HU KO | 5′-GCCAAGCTTCTTCCTCTTCTTCAAATACTTC-3′ | Forward |
| ForG Sal KO | 5′-CGCGTCGACTTGGAATAACCAAGCTACTAAACGTT-3′ | Reverse |
| Bsr-AU | 5′-CAGTTACTCGTCCTATATACG-3′ | Forward |
| KO-AD4 | 5′-GTTCTTGAGCGACATTCATAG-3′ | Reverse |
| RasB-KO | ||
| RasB-5KO-BU | 5′-CGCGGATCCCAATTCCAATAGTAAAAAGTC-3′ | Forward |
| RasB-5KO-PD | 5′-GCGCTGCAGGATAGTAAGTGCACTCTTACTG-3′ | Reverse |
| RasB-3KO-H3U | 5′-GCGAAGCTTGTCAAGATGATTACAGTGCTA-3′ | Forward |
| RasB-3KO-SD | 5′-CGCGTCGACCTTTGATCTGCCTGGCTCTTTG-3′ | Reverse |
| RasB-KO-AD | 5′-CTAAAGGATTAAACAATCACCACCT-3′ | Reverse |
| YFP-RasB | ||
| RasB-BglU | 5′-GCGAGATCTATGTCAGTTTCAAATGAATATAAAT-3′ | Forward |
| RasB-SpeD | 5′-GCGACTAGTCTAAAGGATTAAACAATCACCACC-3′ | Reverse |
| YFP-RasG | ||
| RasG-BglU | 5′-GCGAGATCTATGACAGAATACAAATTAG-3′ | Forward |
| RasG-SpeD | 5′-GCGACTAGTTTATAAAAGAGTACAAGCTTTTAATGG-3′ | Reverse |
| VN210-RasB-G15V/-S20N | ||
| RasB-BglU | 5′-GCGAGATCTATGTCAGTTTCAAATGAATATAAA-3′ | Forward |
| RasB-SpeD | 5′-GCGACTAGTCTAAAGGATTAAACAATCACCACC-3′ | Reverse |
| YFP-Raf1-RBD | ||
| mRaf(RBD)_BglU | 5′-GTAAGATCTGATTCTTCTAAGACAAGCAATACT-3′ | Forward |
| mRaf1(RBD)_SpeD | 5′-GATACTAGTTTAATCCAAAAAATCCACTTGCAG-3′ | Reverse |
| E. coli constructs | ||
| ForG-3P (562–1,074) | ||
| ForG BamHI 3P | 5′-CGCGGATCCCCAATTTCTGGTGGTGGTGCA-3′ | Forward |
| ForG SD | 5′-GCGGTCGACTTATTTATTTAAATTTAATTGTGATCCTCCAGATGAATTTTCAGG-3′ | Reverse |
| ForG-1P (599–1,074) | ||
| ForG BamHI 1P opt | 5′-GCGCGGATCCGGAGCACCGCCACCGCCCCCTCCGCCACCGCC | Forward |
| TCCGGGTGGTAAAAAAGCAGGAGCACCA-3′ | ||
| ForG SD | 5′-GCGGTCGACTTATTTATTTAAATTTAATTGTGATCCTCCAGATGAATTTTCAGG-3′ | Reverse |
| ForE-4P (1,009–1,561) | ||
| ForE-C_BU | 5′-GGATCCATTTCTGGTGCTCCACCCCCACCCCCA-3′ | Forward |
| ForE CProFull_SD | 5′-GGCGTCGACTTAATTTTTATTTGGACTTGT-3′ | Reverse |
| S. cerevisiae constructs | ||
| AD–ForG-N (1–423) | ||
| ForG pGADT7 EcoRI | 5′-GCCGAATTCATGATATTATCAATTACATTTC-3′ | Forward |
| ForG pGADT7 BamHI | 5′-GGCGGATCCTTAAATTTTCTTTTCAAATTCATTAA-3′ | Reverse |
| AD-Raf1-RBD (50–132) | ||
| mRaf1-GAD-RU | 5′-CGCGAATTCGGA GATTCTTCTAAGACAAGCAATACT-3′ | Forward |
| mRaf1-GAD-BD | 5′-CGCGGATCCTTAATCCAAAAAATCCACTTGCAG-3′ | Reverse |
AD, Gal4-activation domain; BD, Gal4-binding domain.
Fig. S1.
ForG accumulates at the leading edge in 2D confinement. To visualize ForG localization in the absence of endocytic cups, the formation of these structures was physically suppressed by overlaying the cells with a thin sheet of agar. In the agar overlay, YFP-tagged ForG-N expressed in WT growth-phase cells accumulated at the leading edge. (Scale bar: 10 μm.)
Vegetative-phase cells, coexpressing LifeAct-mRPF as a marker for F-actin (30), revealed YFP–ForG-N to colocalize exclusively with F-actin–rich structures that formed macropinosomes (Fig. 1B and Movie S1). As opposed to the actin marker, which visualized actin-driven cell protrusions as well as the overall cortical F-actin, ForG was only enriched at the plasma membrane during the formation and maturation of macropinocytotic cups. Within a minute, these structures developed into endocytic vesicles filled with extracellular fluid that remained transiently decorated with ForG and F-actin before disassembly.
Next, we investigated whether phagocytosis of solid particles showed a similar spatiotemporal distribution of ForG. To assess this assumption, the uptake of TRITC-labeled yeast particles by Dictyostelium cells expressing YFP–ForG-N was monitored. Again, ForG-N localized to nascent phagocytic cups and remained associated with the membrane over the entire course of engulfment and internalization (Fig. 1C and Movie S2). In contrast to macropinosomes, however, the formation of phagosomes spanning several micrometers in diameter took considerably longer due to the large size of the yeast particles. Taken together, these localization experiments pointed toward a contribution of ForG in macropinocytosis and phagocytosis.
ForG Is Maximally Expressed in Vegetative Cells.
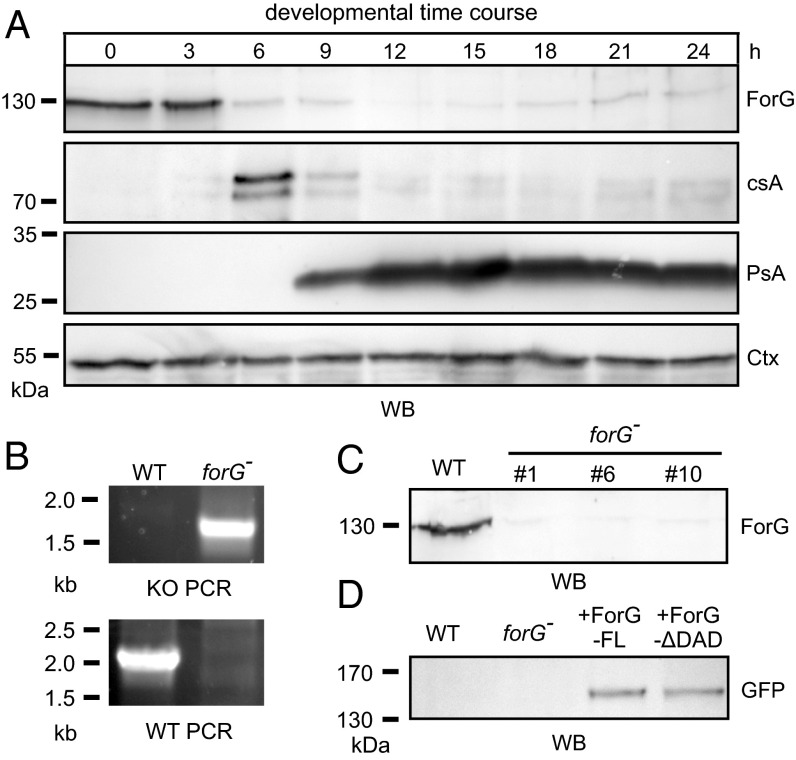
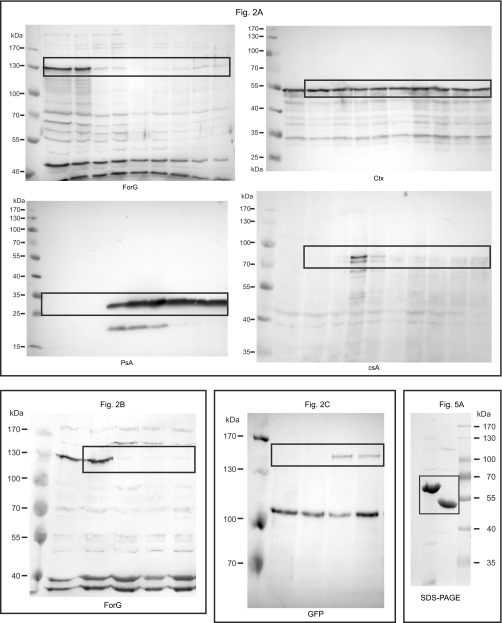
Large-scale endocytosis occurs mainly in vegetative cells and during early development (27). To validate our hypothesis, that ForG is involved in endocytosis, we set out to monitor its expression profile on the protein level and raised ForG-specific polyclonal antibodies directed against the C-terminal half of the protein (amino acids 562–1,074). Western blot analyses with total lysates from WT cells, collected at 3-h intervals from an entire developmental time course on filter pads, were probed for ForG and a battery of other marker proteins. ForG has a calculated molecular mass of 118.6 kDa but migrated at an apparent molecular mass of ∼130 kDa, most likely due to the presence of its proline-rich FH1 region as seen previously with other formins (31). Of note, ForG was maximally expressed in growth-phase cells and at the onset of starvation but then drastically dropped to low levels and remained more or less constant throughout the rest of the development (Fig. 2A, Upper). The drop occurred concomitantly with the appearance of the early developmental marker protein contact site A (csA) and clearly before the appearance of the prespore-specific antigen A (PsA) (32, 33). Taken together, these data therefore supported the notion that ForG is implicated in endocytosis.
Fig. 2.
ForG is expressed during vegetative growth and onset of development. (A) Expression of ForG during the development of the D. discoideum in the WT strain. Growth-phase cells (0 h) or developing cells (3–24 h) were harvested every 3 h and analyzed by SDS/PAGE and immunoblotting. ForG was maximally expressed during growth (0 h) and the onset of starvation (3 h). Thereafter, ForG protein levels sharply declined and remained at a low but constant level. The contact site A (csA) protein served as an early aggregation marker, and the prespore antigen A (PsA) specified the onset of differentiation. The constitutively expressed cortexillin (Ctx) was used as a loading control. (B) Inactivation of the forG gene was validated by two PCR assays to screen for disruption (KO) or the presence of the WT allele using specific primer pairs. (C) Absence of ForG in independent mutants was confirmed by Western blotting using anti-ForG antibodies. (D) Reconstitution of forG− cells with GFP-tagged ForG-FL and ForGΔDAD was confirmed by Western blotting (WB) using anti-GFP antibodies.
Generation of ForG-Null Mutants.
To study the physiological role of ForG in endocytosis, we next generated genetic KO mutants by homologous recombination. Successful gene disruption was initially assessed by diagnostic PCR using specific primer pairs and subsequently confirmed by Western blotting using the ForG antibodies (Fig. 2 B and C). This procedure allowed us to isolate multiple independent clones; however, because their phenotypes were indistinguishable, we used clone 10 in all later experiments, and henceforth refer to it as forG− cells. Based on this null mutant, we additionally generated reconstituted cell lines expressing ForG-FL and ForGΔDAD fused to GFP for further analyses (Fig. 2D).
ForG Is Required for Efficient Endocytosis.
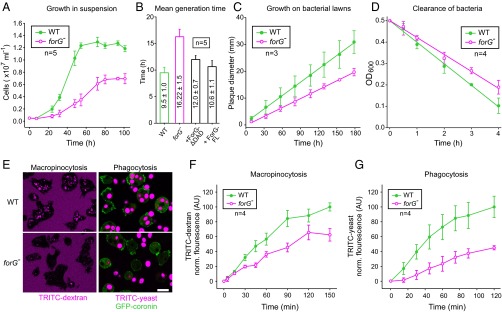
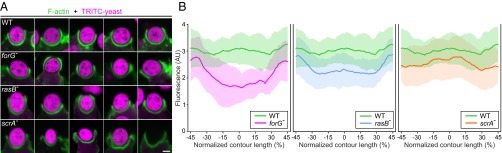
Next, we compared forG− and WT cells in a variety of nutrient uptake-dependent assays to evaluate the physiological role of ForG in endocytosis. Analysis of cell growth in shaken suspension with liquid medium revealed an almost doubled mean generation time for forG− cells of 16.2 ± 1.5 h (mean ± SD, n = 5) compared with WT cells, which required 9.5 ± 1.0 h (n = 5). Moreover, forG− cells only reached half-maximal cell density compared with control (Fig. 3 A and B). This effect was ForG-specific, because in forG− reconstituted cell lines expressing GFP-tagged ForG-FL or ForG∆DAD, the poor growth phenotype was largely reverted to WT values (Fig. 3B). We then assessed the growth of cells on bacterial lawns, which is mainly dependent on phagocytosis and subsequent digestion of nutrients. Notably, plaque growth as assessed by measuring its diameter over time was reduced by ∼33% to 0.111 ± 0.002 mm⋅h−1 in forG− cells as opposed to 0.166 ± 0.002 mm⋅h−1 for the WT (mean ± SEM, n = 3; Fig. 3C). Consistently, we observed diminished clearance of bacteria from shaken suspension culture by the mutant by about 20% (Fig. 3D). Impaired growth in shaken suspension or on bacterial lawns as seen with the forG− cells can occur due to poor uptake of nutrients as previously observed in PI3K multinull mutants and in KOs lacking their upstream activator Ras (15, 34), but it can also be caused by defects in cell division as shown for myosin II- or IBARa-null mutants (35, 36). However, due to the clear accumulation of ForG in macropinosomes and phagosomes, as well as the normal size of forG− cells in suspension, we reasoned that reduced nutrient uptake in the mutant cells was the most likely cause, and we therefore investigated fluid-phase and particle uptake in more detail. To this end, we first challenged forG− cells with the fluid-phase marker TRITC-dextran. Notably, after 90 min of incubation, a time point when equilibrium between TRITC-dextran accumulation via clathrin-independent endocytosis and exocytosis is normally reached (37), we observed substantially fewer and less brighter TRITC-dextran–containing vesicles in forG− cells as opposed to the WT (Fig. 3E, Left). Concurrently, we also examined phagocytosis of TRITC-labeled yeast particles by cells expressing GFP-coronin as a phagosome marker to visualize particle uptake in real time (18). Consistent with the reduced growth on bacteria, forG−-derived cells accumulated only one to two yeast particles within a period of 30 min, whereas most WT cells were fully packed, containing multiple yeast particles (Fig. 3E, Right). Quantification of both macropinocytosis and phagocytosis over time corroborated these findings. The fluid-phase uptake rate in the forG− cells was reduced by 46% compared with controls (Fig. 3F). Moreover, the steady-state plateau was decreased by about one-third in the mutant. Quantification of phagocytosis in shaken suspension with TRITC-labeled yeast particles revealed similar results. The uptake rate of the forG− mutant cells was markedly reduced and reached only 55% of the WT value (Fig. 3G). Collectively, these data define ForG as a decisive factor of large-scale endocytosis.
Fig. 3.
Macropinocytosis and phagocytosis are impaired in forG− cells. (A and B) Growth of forG− cells in liquid medium was strongly reduced and resulted in an almost doubled mean generation time. Rescue with GFP–ForG-FL or GFP-ForG∆DAD almost restored WT rates (mean ± SD). (C) Plaque diameter on bacterial lawn was observed for several days and indicated a phagocytosis defect (mean ± SEM). (D) Clearance of bacteria from shaken suspension in the presence of WT or mutant cells revealed a 20% reduction of bacterial phagocytosis by forG− cells. (E) Accumulation of TRITC-dextran by macropinocytosis after 90 min of incubation and of TRITC-labeled yeast particles by phagocytosis after 30 min of incubation was visualized by confocal microscopy. (Scale bar: 10 μm.) (F) Quantification of macropinocytosis revealed decreased TRITC-dextran uptake in the forG− cells (mean ± SD). (G) Quantification of phagocytosis with TRITC-labeled yeast revealed markedly reduced uptake of large particles (mean ± SD). n, number of independent experiments.
ForG Does Not Substantially Affect Cell Migration.
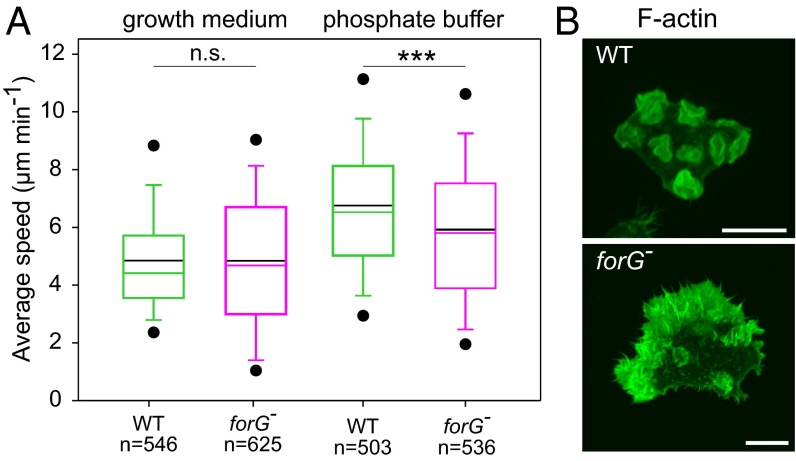
Previous work implied that endocytosis and cell migration are antagonistic processes because they require the same core machinery ultimately driving membrane protrusion (18, 38). To examine whether ForG is also implicated in migration, we compared the motility rates of WT and forG− cells in random motility assays. Surprisingly, the motile behavior of vegetative forG− cells was rather unaffected and did not noticeably deviate from WT motility regardless of the experimental conditions (Fig. 4A). In growth medium, the average speed of WT cells at 4.9 ± 1.9 μm⋅min−1 (mean ± SD, n = 546) was highly similar to forG− cells at 4.8 ± 2.5 μm⋅min−1 (n = 625). In low-osmolarity buffer, which is known to promote cell migration due to increased hydrostatic pressure (25), vegetative WT cells migrated only slightly faster (6.8 ± 2.6 μm⋅min−1, n = 503) compared with forG− cells (6.0 ± 2.8 μm⋅min−1, n = 536). These findings therefore argued against a general concept of inverse correlation between endocytosis and cell migration at that point. However, phalloidin labeling and phase-contrast time-lapse imaging of the cells revealed that forG− cells formed massively exaggerated actin-rich protrusions from large regions of their periphery as opposed to the WT, which formed mainly crowns and one or two pseudopods at a given time (Fig. 4B and Movie S3). Thus, although the loss of ForG strongly promotes the formation of actin-rich protrusions, this behavior does not seem to translate into increased cell motility, due to their excessive and uncoordinated nature.
Fig. 4.
Lack of ForG does not enhance motility but drives excessive formation of actin-rich cell protrusions. (A) Box plot summarizing quantitative analysis of random cell migration of WT and forG− cells in growth medium and low-osmolarity Sorensen’s buffer. Note similar random motility rates for both strains in both conditions. Boxes indicate the 25th through 75th percentiles, the whiskers mark the 10th and 90th percentiles, and the outliers mark the fifth and 95th percentiles. Black solid lines indicate mean values. n, number of tracked cells. ***P < 0.001 (Mann–Whitney U test). n.s., nonsignificant. (B) Loss of ForG results in strikingly exaggerated actin-rich protrusion (Movie S3). The cells indicated were cultivated in growth medium, fixed, and stained for F-actin with ATTO488-conjugated phalloidin. (Scale bar: 10 μm.)
ForG Promotes F-Actin Assembly.
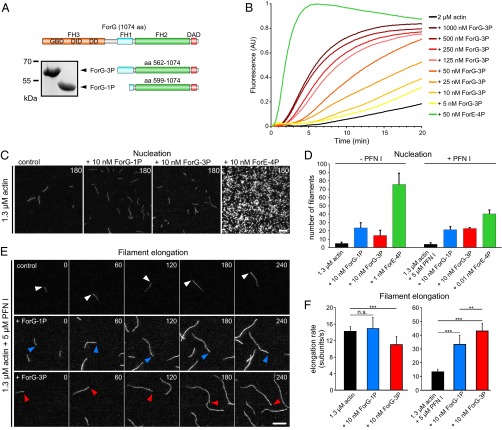
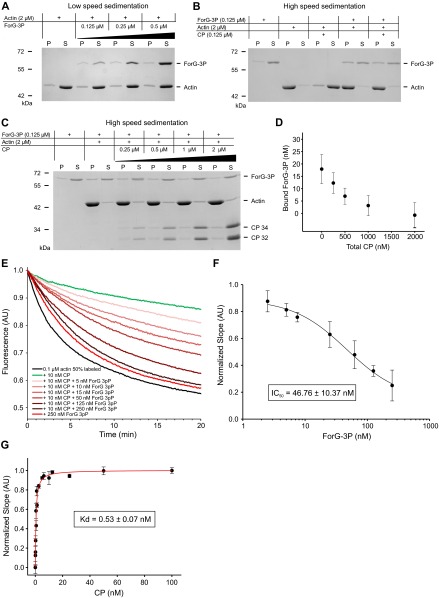
The core FH1-FH2 domains of canonical formins catalyze the nucleation and processive elongation of linear F-actin filaments, but their specific activities can be highly diverse (39). Thus, to assess the potential function of ForG in endocytosis, we expressed and purified two recombinant C-terminal ForG fragments for biochemical analysis. The larger construct comprises all three polyproline stretches of the FH1 domain (ForG-3P), and the shorter ForG-1P construct contains just the last stretch adjacent to the FH2 domain (Fig. 5A). Bulk polymerization assays with pyrene-actin and increasing amounts of ForG-3P in the nanomolar range revealed that ForG stimulated spontaneous actin assembly in a concentration-dependent manner (Fig. 5B), albeit less strongly compared with a C-terminal ForE fragment (ForE-4P), which was used as a reference (31). Next, both ForG fragments and ForE-4P were analyzed with regard to their nucleation activity by total internal reflection fluorescence microscopy (TIRFM) on the single-filament level (Fig. 5C). As opposed to other formins, such as Dictyostelium ForE or mDia2 (26), at 10 nM concentrations, both ForG fragments only moderately enhanced the birth of new actin filaments, indicative of a rather weak nucleation activity (Fig. 5D). Then, we analyzed the filament elongation in the TIRFM assay. In the presence of Dictyostelium PFN I, both ForG-1P and ForG-3P promoted barbed-end elongation by about threefold to 33.4 and 43.1 subunits per second, respectively, compared with the actin control with 13.4 subunits per second (Fig. 4 E and F and Movie S4). The higher activity of ForG-3P emphasized more efficient PFN-actin recruitment by its two additional polyproline stretches and is consistent with previous work (31, 40). In the absence of PFN, formins can reside as leaky cappers on the growing filament barbed ends and may suppress their growth significantly (41). Interestingly, impaired growth was not seen with ForG-1P, and ForG-3P showed just slightly reduced filament elongation at a concentration of 10 nM from 14.2 to 11 subunits per second (Fig. 5F). Extended biochemical analyses by additional pyrene, as well as low-speed and high-speed sedimentation assays, further revealed that ForG efficiently competes with Dictyostelium capping protein Cap32/34 (CP) for filament barbed ends. By determining the dissociation constant of CP for filament ends (0.53 ± 0.07 nM; Fig. S2), we were able to calculate the affinity of ForG for filament barbed ends from competition experiments in presence of CP to 4.4 ± 1.1 nM (Fig. S2). Thus, ForG is a leaky capper that allows incorporation, as well as the release, of monomers even when tightly bound to barbed ends. Because ForG also neither bundles filaments nor binds to their sides (Fig. S2), these data therefore pointed toward a central role of ForG in filament elongation during large-scale endocytosis.
Fig. 5.
ForG promotes F-actin assembly. (A) Domain organization of ForG and derived C-terminal constructs used for biochemical analyses. (Inset) Purified ForG-1P and ForG-3P fragments. (B) ForG-3P promoted polymerization of pyrene-labeled G-actin (2 μM, 5% labeled) in a concentration-dependent manner. Note markedly higher assembly rates by 50 nM ForE-4P. AU, arbitrary units. (C) ForG exhibits rather weak actin filament nucleation compared with ForE-4P. Representative frames from TIRFM time-lapse recordings of actin filament assembly are shown. (D) Nucleation efficacies of ForG and ForE constructs in the absence or presence of PFN I (mean ± SD, n = 3). Note that due to the strong nucleation efficacy, and for technical reasons, 10- or 1,000-fold less of ForE-4P had to be used for the quantifications. (E) Filament elongation visualized by TIRFM in the presence of 5 μM PFN I alone or in combination with either 10 nM ForG-1P or 10 nM ForG-3P. Barbed ends are marked by arrowheads. Time is given in seconds. (F) Elongation rates of growing filaments were calculated from time-lapse movies as shown in E. (Left) In the absence of PFN I, addition of 10 nM ForG-3P led only to slightly reduced elongation compared with rates of control and ForG-1P. (Right) In the presence of PFN I, ForG-1P– and ForG-3P–associated filaments were elongated about threefold faster than control filaments (mean ± SD). At least 20 individual filaments were tracked. ***P ≤ 0.001, **P ≤ 0.01 (Mann-Whitney U test). Note that the scale differs between the left and right graphs. (Scale bars: C, 5 μm; E, 10 μm.)
Fig. S2.
ForG competes with capping protein, but neither bundles nor interacts with the sides of actin filaments. (A) ForG does not bundle actin filaments in low-speed sedimentation assays. After polymerizing 2 μM G-actin in presence of varying concentrations of ForG-3P for 2 h, negligible but constant amounts of the formin were found in the pellet fraction over the whole concentration range. P, pellet; S, supernatant. (B) ForG efficiently competes with CP for barbed-end binding. Two micromolar actin was polymerized in the presence of the proteins indicated for 2 h before high-speed sedimentation. In the controls, most actin was found in the pellet, whereas most ForG was found in the supernatant. In the presence of CP, most of the F-actin was found in the supernatant, demonstrating efficient barbed-end capping, resulting in many short filament species that were not pelletable at 150,000 × g. Notably, in presence of CP and the formin, most actin was found in the pellet, again demonstrating very efficient displacement of CP by the formin at filament barbed ends. (C and D) ForG at filament barbed ends can be displaced only by excess CP. (C) Two micromolar actin was prepolymerized for 2 h before addition of 125 nM ForG-3P. Following 30 min of incubation, varying concentrations of CP were added to the mixture and incubated for another 30 min before high-speed sedimentation. (D) Densitometric analysis of ForG-3P bands in the pellet fraction from experiments as shown in C. Notably, complete displacement of the formin was only seen at 16-fold excess CP, suggesting high-affinity binding of ForG to filament barbed ends (n = 4). (E–G) ForG also efficiently displaces CP from filament barbed ends in dilution-induced depolymerization assays. (E) Depolymerization of 0.1 μM F-actin in the presence of a fixed concentration of CP (10 nM) and varying amounts of ForG (5–500 nM). As opposed to CP, ForG-3P alone only weakly inhibited F-actin disassembly, suggesting that it operates as a leaky capper. However, already in the presence of substoichiometric concentrations (5 nM), ForG efficiently began to replace CP from barbed ends as evidenced by the increased depolymerization rates. (F) Normalized and averaged initial slopes of depolymerization curves from experiments shown in E were plotted against the concentration of ForG-3P to calculate the IC50 value for competition with CP for filament barbed ends assuming one-site competition (n = 4, mean ± SD). (G) Assuming one-site saturation kinetics, normalized and averaged initial slopes of F-actin depolymerization curves in the presence of varying concentration of CP alone yielded a dissociation constant (Kd) of CP for barbed ends (n = 4, mean ± SD). This constant, in turn, allowed us to calculate the Kd of ForG-3P for filament barbed ends, which is about 4.4 ± 1.1 nM (Materials and Methods). AU, arbitrary units. In A–G, all concentrations for ForG-3P and CP refer to the dimers.
ForG Interacts with Active Ras Proteins.
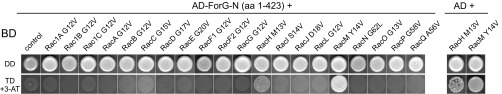
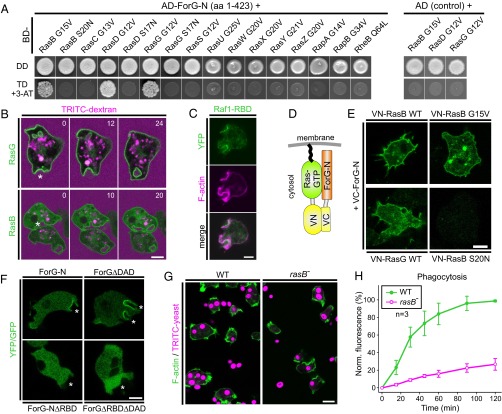
To date, DRFs have been shown to act exclusively as effectors of the Rho-family GTPases, such as Cdc42, Rho, and Rac (24). We therefore explored which GTPase could be responsible for activation of ForG by a yeast two-hybrid (Y2H) screen, using the N-terminal ForG fragment ForG-N encompassing the GBD and the FH3 region (amino acids 1–423) as bait. Although Dictyostelium cells lack genuine Rho and Cdc42 homologs, they express 20 Rac-subfamily GTPases (42). Thus, we systematically screened ForG-N with all these Rac GTPases in their constitutively activated forms obtained by amino acid substitutions corresponding either to position 12 within the P-loop or to position 61 within switch II of human Rac1, respectively (43, 44) (Table S2). However, despite false-positive hits with RacM and RacH, we failed to find any interaction with a Rac GTPase in this Y2H screen (Fig. S3). Of note, ForG contains a putative ubiquitin-like fold that, despite lack of sequence homology, represents structurally conserved topology of many Ras-binding domains (RBDs) in Ras-effectors, such as proteins from the MAP kinase pathway (45) or PI3Ks (46). Because Ras proteins are also intimately linked to endocytosis (3, 15), in a second Y2H screen, we used all members of the annotated Ras GTPase subfamily as prey except for RasV, which bears an N-terminal poly-N stretch, and RapC, which lacks the conserved nucleotide-binding sites (47). Strikingly, we found ForG-N to interact specifically with the activated forms of RasB, RasG, and RasD, but not with their dominant-negative variants (Fig. 6A). All these Ras proteins show high sequence homologies to the human KRas and HRas proto-oncogenes, but because RasD is exclusively expressed at later developmental stages (48) during which endocytosis has been largely shut down, these data imply that ForG is only regulated by RasG and RasB in vivo. Taken together, these unexpected results pointed toward a direct activation of a DRF by Ras proteins, which have long been considered important orchestrators of endocytosis and cell migration (15).
Table S2.
GTPase variants used for Y2H in this study
| GTPase | Inactive | Amino acids | Dictybase gene ID |
| RasB G15V ∆CAAX | Yes | 1–193 | DDB_G0292998 |
| RasB S20N ∆CAAX | |||
| RasC G13V ∆CAAX | 1–185 | DDB_G0281385 | |
| RasD G12V ∆CAAX | Yes | 1–183 | DDB_G0292996 |
| RasD S17N ∆CAAX | |||
| RasG G12V ∆CAAX | Yes | 1–185 | DDB_G0293434 |
| RasG S17N ∆CAAX | |||
| RasS G12V ∆CAAX | 1–190 | DDB_G0283537 | |
| RasU G25V ∆CAAX | 1–209 | DDB_G0270138 | |
| RasW G20V ∆CAAX | 1–212 | DDB_G0270122 | |
| RasX G20V ∆CAAX | 1–209 | DDB_G0270124 | |
| RasY G21V ∆CAAX | 1–212 | DDB_G0270126 | |
| RasZ G20V ∆CAAX | 1–210 | DDB_G0270140 | |
| RapA G14V ∆CAAX | 1–182 | DDB_G0291237 | |
| RapB G34V ∆CAAX | 1–201 | DDB_G0272857 | |
| RheB Q64L ∆CAAX | 1–181 | DDB_G0277041 | |
| Rac1A G12V ∆CAAX | 1–190 | DDB_G0277869 | |
| Rac1B G12V ∆CAAX | 1–190 | DDB_G0268622 | |
| Rac1C G12V ∆CAAX | 1–189 | DDB_G0282365 | |
| RacA G12V ∆C | 1–319 | DDB_G0286555 | |
| RacB G12V ∆CAAX | 1–189 | DDB_G0279605 | |
| RacC G15V ∆CAAX | 1–188 | DDB_G0293526 | |
| RacD G17V | 1–254 | DDB_G0291976 | |
| RacE G20V ∆CAAX | 1–219 | DDB_G0280975 | |
| RacF1 G12V ∆CAAX | 1–189 | DDB_G0269176 | |
| RacF2 G12V ∆CAAX | 1–189 | DDB_G0276967 | |
| RacG G12V ∆CAAX | 1–197 | DDB_G0269178 | |
| RacH M13V ∆CAAX | 1–196 | DDB_G0269240 | |
| RacI S14V ∆CAAX | 1–201 | DDB_G0277897 | |
| RacJ D18V ∆CAAX | 1–205 | DDB_G0292560 | |
| RacL G12V ∆CAAX | 1–192 | DDB_G0292816 | |
| RacM Y14V ∆CAAX | 1–185 | DDB_G0289103 | |
| RacN G62L ∆CAAX | 1–215 | DDB_G0278009 | |
| RacO G13V ∆C | 1–213 | DDB_G0277791 | |
| RacP G58V | 1–376 | DDB_G0285453 | |
| RacQ A56L ∆CAAX | 1–181 | DDB_G0278011 |
Fig. S3.
ForG does not interact with Rho family GTPases. (Left) ForG-N does not interact specifically with any of the 20 Rac proteins in the Y2H assay. Yeast were transformed with the indicated constructs and selected for the presence of prey and bait plasmids by growth on double-dropout (DD) media lacking leucine and tryptophan. Interactions were assayed by growth on stringent TD media additionally lacking histidine in the presence of 3 mM 3-AT. (Right) Two putative hits obtained with RacH and RacM were found to be unspecific because these GTPases also grew on TD media in the absence of the ForG-N bait. AD, Gal4-activation domain; 3-AT, 3-amino-1,2,4-triazole; BD, Gal4-binding domain.
Fig. 6.
ForG interacts with active Ras. (A, Left) ForG-N interacts specifically with the active forms of RasB, RasG, and RasD in the Y2H assay. Yeast was transformed with the indicated constructs and selected for the presence of prey and bait plasmids by growth on double-dropout (DD) media lacking leucine and tryptophan. Interactions were assayed by growth on stringent triple-dropout (TD) media additionally lacking histidine in the presence of 3 mM 3-AT. (A, Right) Three identified Ras proteins showed no genetic interaction in experiments using the dominant-negative Ras variants or empty AD plasmids as negative controls. AD, Gal4-activation domain; 3-AT, 3-amino-1,2,4-triazole; BD, Gal4-binding domain. (B) YFP-tagged RasG and RasB also localize to macropinosomes during the uptake of TRITC-dextran–labeled medium. Confocal sections are shown and correspond to Movies S5 and S6. Time is given in seconds. Macropinosomes are indicated by stars. (C) Macropinosomes contain active Ras proteins as evidenced by accumulation of the pan-Ras probe Raf1-RBD. A confocal section of a live-cell coexpressing YFP-tagged Raf1-RBD and the F-actin probe LifeAct-mRFP is shown. (D) Scheme of the BiFC strategy. VC, Venus C-terminal fragment; VN, Venus N-terminal fragment. (E) ForG-N interacted with active or WT forms of RasB and RasG, but not with the dominant-negative control of RasB in the BiFC assay. Representative confocal sections are shown. (F) Removal of the putative RBD in ForG-N and the constitutively active ForGΔDAD variant abolished localization of the fusion proteins at endocytic cups (white stars). (G) Strongly impaired yeast phagocytosis of rasB− cells. Accumulation of TRITC-labeled yeast particles by phagocytosis after 45 min of incubation on glass coverslips. Confocal sections are shown. (H) Quantification of phagocytosis with TRITC-labeled yeast revealed strikingly reduced uptake of large particles (mean ± SD, n = 3 for each cell line). Norm., normalized. (Scale bars: B–F, 5 μm; G, 10 μm.)
Although the role of RasG in endocytosis is well established (15), much less is known about RasB. We therefore compared the in vivo localization and dynamics of fluorescently tagged RasB and compared it with an analogous RasG fusion construct. Both GTPases localized prominently to the plasma membrane and also to invaginations that eventually developed into macropinosomes as assessed by the capture of the fluid-phase marker TRITC-dextran (Fig. 6B). To visualize active Ras in cells, we then used the RBD of Raf1 fused to YFP and found it to colocalize with F-actin in endocytic cups (Fig. 6C). The RBDs of mammalian Raf1 or of yeast Byr2 kinases are commonly used pan-Ras probes (49), and they consistently also interact with numerous active Dictyostelium GTPases, including RasB and RasG as evidenced by Y2H assays (Fig. S4). These findings therefore supported the notion that active RasG and RasB accumulate in the cups and act as regulators of ForG in endocytosis.
Fig. S4.
Interactions of activated Dictyostelium Ras family GTPases with commonly used pan-Ras probes. (Left) Pan-Ras probes Raf1-RBD and Byr2-RBD interact with a whole variety of active Ras family members in the Y2H assay. (Right) Specificity of relevant interaction was confirmed by the use of dominant-negative variants. Yeast were transformed with the indicated constructs and selected for the presence of prey and bait plasmids by growth on DD media lacking leucine and tryptophan. Interactions were assayed by growth on stringent TD media additionally lacking histidine in the presence of 3 mM 3-AT or highly stringent QD media lacking leucine, tryptophan, histidine, and adenine.
Unfortunately and despite numerous attempts, we were not able to purify a soluble N-terminal ForG fragment for in vitro interaction studies with recombinant RasB and RasG. Nevertheless, to corroborate the interaction of RasG and RasB with ForG in an independent assay, we first adapted in vivo bimolecular fluorescence complementation (BiFC) of the YFP variant Venus for use in Dictyostelium (Fig. 6 D and E). To this end, an extrachromosomal vector for the simultaneous expression of two proteins was constructed with split Venus fragments that eliminate self-complementation, and therefore provide high specificity as reported by Ohashi et al. (50), who conducted systematic fragment pair optimization and demonstrated active H-Ras and Raf1 interaction. When the BiFC construct was expressed in forG− mutants to circumvent unconstructive interaction of ectopic RasB with endogenous ForG, most cells showed rather weak but distinct fluorescence enrichment at the plasma membrane. We assumed this weak fluorescence enrichment was due to the maturation of split Venus, which takes minutes to hours (51) and evidently exceeds the rather short time frame of Ras activation during macropinocytosis, which is completed within 1 min (15) (Fig. 1B). Because Venus is quite stable once assembled, some cells accumulated a stronger fluorescence at the plasma membrane several days after transfection, as shown in Fig. 6E. Notably, fluorescence complementation was seen with WT as well as constitutively active RasB, but never with dominant-negative RasB (Fig. 6E). Because similar results were also obtained with RasG-WT, these data demonstrated that active RasB and RasG specifically interact with ForG in vivo. Finally, we asked whether Ras binding is required for ForG localization to endocytic cups. To this end, we expressed truncated variants of ForG-N and ForGΔDAD additionally lacking the putative RBD (amino acids 1–119). Notably, as shown in Fig. 6F, cup localization was abolished with both of these constructs, demonstrating that the RDB is essential for subcellular targeting.
RasB-Null Cells Exhibit Severe Defects in Phagocytosis.
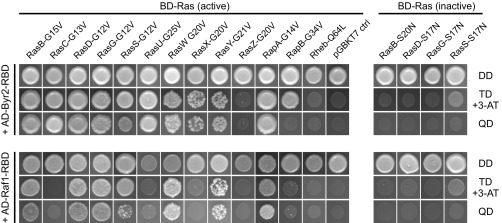
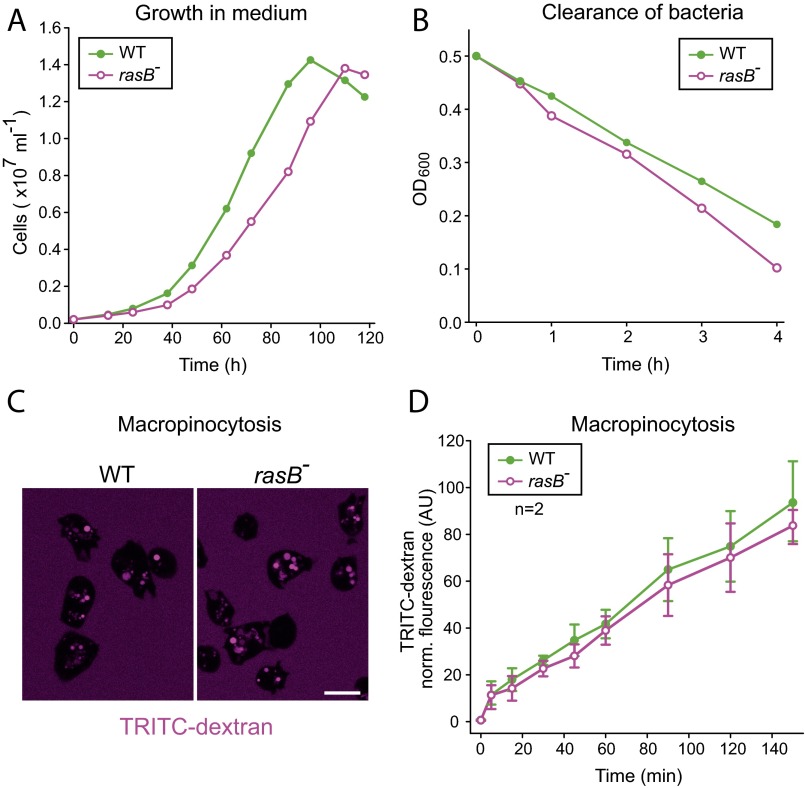
As opposed to RasG, the function of RasB in endocytosis has not yet been examined. Thus, we generated a cell line devoid of RasB by homologous recombination (Fig. S5) and assayed a number of growth parameters depending on large-scale endocytosis. In comparison to WT cells, rasB− cells grew slightly slower in shaken suspension containing growth medium, but exhibited only weakly diminished pinocytosis rates (Fig. S6). However, the uptake of large particles was substantially impaired (Fig. 6 G and H), although the phagocytosis of bacteria was only moderately reduced (Fig. S6). Therefore, rasB− cells especially appear to exhibit major defects in phagocytosis of large objects.
Fig. S5.
Genetic inactivation of the rasB gene. Inactivation of the rasB gene was confirmed by two diagnostic PCR assays to screen for disruption (KO, Left) or the presence of the WT allele (Right) using specific primer pairs.
Fig. S6.
RasB− cells display no major defects in macropinocytosis and phagocytosis of bacteria. (A) Growth of rasB− cells in liquid medium was moderately impaired compared with WT cells. A representative growth curve of three independent experiments is shown. (B) Impaired phagocytosis of bacteria by rasB− cells as assessed by clearance of E. coli strain B/r in the supernatant. A representative curve of three independent experiments is shown. (C) Accumulation of TRITC-dextran by macropinocytosis after 90 min of incubation was visualized by confocal microscopy. (Scale bar: 10 μm.) (D) Quantification of TRITC-dextran uptake by macropinocytosis revealed no major defects in the rasB− mutant (mean ± SD, n = 2).
Impaired F-Actin Accumulation in Phagosomes of forG− Mutants.
To assess potential defects in actin assembly during large-scale endocytosis, we then visualized the F-actin distribution in semiclosed phagosomes of forG− and rasB− mutants engulfing yeast and compared them with control cells as well as the phagocytosis-impaired mutant lacking the Arp2/3 complex activator SCAR (52, 53). Whereas F-actin was more or less evenly distributed in WT cells around the entire phagocytic cup engulfing the yeast particles, we observed striking abnormalities in the F-actin distribution in forG− cells (Fig. 7A). The latter characteristically contained a markedly weaker F-actin signal at the base of forming phagosomes and occasionally also displayed asymmetries in the distribution of F-actin in the tip region. Because all images were obtained with identical microscope settings, we plotted the phalloidin fluorescence intensities along the contour of the nascent phagosomes to quantify the F-actin content in these structures. This analysis revealed a 47% reduction of F-actin in the base of the phagosomes of forG− cells (Fig. 7B, Left), which is consistent with ForG-mediated actin assembly in vitro. The analysis of the rasB− mutant revealed an intermediate actin reduction in the cup base by 26% (Fig. 7B, Middle) compared with the WT and forG− cells. Thus, it appears that ForG is only partly activated in this cell line, which is consistent with the notion that ForG is most likely also activated by RasG. Analysis of scrA− cells interestingly revealed only a minor reduction by 13% in the base, but the strongest F-actin reduction of all analyzed cell lines by about 24% at the rim of the cups (Fig. 7B, Right). Moreover, scrA− cells displayed aberrant cup morphology, as if these mutants were not able to grasp the yeast particles tightly. Consistently, and as opposed to all other cell lines, we frequently found many empty cups in fixed cells (Fig. 7A, Lower Right). Finally, we performed confocal time-lapse imaging with the GFP-tagged F-actin probe LimEΔcoil to monitor F-actin dynamics throughout the phagocytosis cycle in live cells. These recordings are consistent with the still images of Fig. 7A and corroborated, in particular, the problem of scrA− cells initiating cup formation and constricting the rim during phagosome maturation (Movies S7–S10). Together, these findings suggest a synergy between the Arp2/3 complex and ForG-generated actin networks to drive efficient engulfment of large particles.
Fig. 7.
Aberrant F-actin distribution in phagosomes of cells impaired in phagocytosis. (A) Representative phagosomes from WT cells (first row) and the forG− mutant cells (second row), rasB− cells (third row), and scrA− cells (fourth row). Growth-phase cells phagocytosing TRITC-labeled yeast particles were fixed and stained for F-actin with ATTO488-conjugated phalloidin. Note impaired F-actin distribution in the mutant cells and empty cups in scrA− cells (fourth row) suggesting loose association with the yeast particles. (Scale bar: 2 μm.) (B) Quantification of the relative F-actin contents in the phagosomes of WT and mutant cells. Fluorescence intensities of the ATTO488 phalloidin-labeled F-actin structure along the contour lengths of phagosomes are shown (mean ± SD). The base of the phagosomes was set to 0% (n = 25 for each cell line). AU, arbitrary units.
Discussion
D. discoideum is a professional phagocyte that feeds on bacteria or yeast in the soil. For the ease of cultivation, axenic laboratory strains have been engineered that are also able to internalize large quantities of bulk fluid due to greatly enhanced macropinocytosis (10). Nevertheless, both processes share many similarities and components because, in both cases, the dynamic assembly of actin filaments together with myosins generates forces that drive internalization of fluid or large particles. Moreover, they are of high medical relevance because, in the immune defense, phagocytosis is a major mechanism to remove pathogens (7), whereas many cancer cells thrive by excessive macropinocytosis (3, 5). Recent work with Dictyostelium showed that the nucleation-promoting factor SCAR/WAVE localizes at the very rim of macropinosomes, suggesting that the Arp2/3 complex acts as a potent nucleator of the underlying actin filament network (38). Consistently, the Arp2/3 complex was also shown to be required for large-scale endocytosis in higher eukaryotes (54). Formins, such as the DRFs mDia1, FMNL1/FRL1, and Daam1 (29, 55–57), have also been implicated in phagocytosis in immune cells, albeit the molecular function of these actin assembly factors is less clear.
In this study, we aimed to characterize the function of the Dictyostelium DRF ForG that regulates specifically the dynamic reorganization of the actin cytoskeleton in large-scale endocytosis. Interestingly, we found ForG to decorate the entire actin-rich base of nascent phagosomes and macropinosomes throughout their entire lifetime. This observation is reminiscent of the localization of coronin, which is an indicative marker of highly dynamic actin filament networks, such as phagosomes or lamellipodia (18, 58), in which it acts as a potent enhancer of cofilin-mediated filament disassembly (20). More importantly, our in vitro experiments revealed a rather weak nucleation activity by ForG, whereas filament elongation in the presence of PFN was increased about threefold at 1.3 μM actin in the TIRFM assay. Given the much higher actin concentration in cells, these data imply that ForG acts as an effective actin filament elongator that contributes to the generation of a functional F-actin network in endocytosis. Consistently, we observed substantially reduced amounts of F-actin, particularly at the base of phagocytic cups in forG− cells, which correlated with severe defects in large-scale endocytosis. Similarly, scrA− cells also have reduced F-actin levels in the cups and were previously shown to have significantly reduced rates of macropinocytosis (52). Most notably, deletion of SCAR in the PFN-null background resulted in a further substantial decrease in endocytosis compared with either mutant alone, suggesting that PFN and SCAR both functionally contribute to regulate endocytosis (52). However, at that time, the molecular mechanism of formins was not well enough understood to interpret these findings. However, in light of this study and our current knowledge about formin function, these data can now be easily explained by the synergistic action of the nucleating Arp2/3 complex and the filament-elongating activity of a formin, because the latter requires PFN to achieve fast filament elongation (39, 40, 59). Thus, ForG may operate in elongation of Arp2/3 complex-nucleated filaments to establish a robust actin meshwork allowing efficient membrane protrusion and internalization of extracellular material. This synergistic mode of action between different actin assembly factors has been proposed for actin polymerization in lamellipodia, in which Arp2/3 complex-generated filaments are captured by the DRF FMNL2 to promote their elongation in a PFN/actin-dependent manner to drive efficient cell migration (60). Of note, the Arp2/3 complex and the DRFs mDia1 and FMNL1 also seem to synergize during a specialized form of internalization of Borrelia burgdorferi by macrophages, termed coiling phagocytosis (56), meaning that this type of cooperation between different actin assembly factors may be a general concept in various actin-based processes.
However, if filament elongation is equally critical for macropinocytosis and phagocytosis, and provided that ForG is the only formin involved, why then is large-scale endocytosis in forG− cells not entirely blocked? Pioneering work on the requirement of the minimal set of proteins in actin-based cell motility in reconstituted systems led to the identification of actin, capping protein, ADF/cofilin, and the Arp2/3 complex as the only essential actin assembly factors (61). However, when the filament elongator VASP was added to these critical components, the speed of Listeria was accelerated 2.5-fold in vitro. These findings are therefore consistent with the ∼50% reduction of large-scale endocytosis in forG− cells and illustrate that coupling of an actin filament nucleator with an actin polymerase optimizes efficiency.
The initial steps of phagocytosis have been extensively studied and seem to be triggered by the recently identified folate receptor fAR1 (12) and its associated heterotrimeric G protein (13) to activate a subset of Ras proteins, which, in turn, leads to activation of their class-1 PI3K downstream effectors and production of PIP3 (34, 62). Due to a mutation of the RasGAP neurofibromin NF1, in axenic Dictyostelium strains, excessive Ras signaling appears to bypass the requirement for fAR1-receptor activation and makes macropinocytosis constitutive, allowing the cells to grow efficiently in liquid medium (10). The links between PIP3 and the cytoskeleton are not yet fully resolved, but several downstream effectors of this phosphoinositide, including RacGEFs and Akt, have been proposed to lead to activation of Arp2/3 complex-mediated actin assembly (63, 64).
Previous work demonstrated that mutants lacking PI3Ks and the small GTPases RasG and RasS display a significantly decreased large-scale endocytosis, thus supporting the view that these proteins operate in a common signaling pathway (15, 34). However, the specific impact of these proteins on the two types of large-scale endocytosis is not always identical (15). In addition, seemingly unrelated phenotypes, such as the cytokinesis defect of rasG− cells (65), make the dissection of the underlying pathways a challenging task. Notwithstanding these findings, a shared inventory of core components must be at play, which can be complemented by additional factors that preferentially engage in either macropinocytosis or phagocytosis. In this work, we identify a formin that interacts with RasG and RasB, thus directly linking Ras GTPase signaling with actin polymerization in large-scale endocytosis. Because forG− cells displayed severe defects in both fluid-phase and solid particle uptake, we propose ForG to be part of the core machinery. Based on a rather weak impairment of macropinocytosis in rasB− cells but a strong impact on phagocytosis, this study further suggests that RasB is mainly responsible for activation and recruitment of ForG during phagocytosis of large particles, whereas RasG is the main regulator in macropinocytosis.
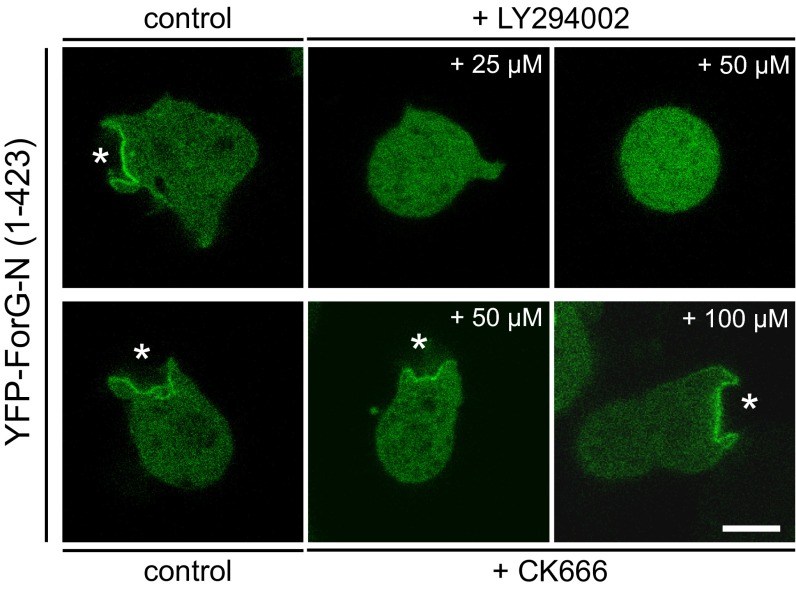
The interaction of active Ras with the N-terminal GBD of ForG is expected to recruit ForG to crown-shaped protrusions and release the intrinsic autoinhibition to drive elongation of actin filaments after their nucleation by the Arp2/3 complex. Consistent with previous localization studies with mDia1 (29), the GBD of ForG was found to be critical for subcellular targeting. Moreover, based on the close sequence relationship of the ForG-GBD with the N terminus of the developmentally regulated DRF ForC (26), which, in turn, was shown to interact with PIP3 (66), we hypothesized that PIP3 may additionally support targeting of ForG to endocytic cups. Unfortunately, we were not able to purify a soluble ForG fragment from E. coli to test Ras and lipid interactions in vitro. Nevertheless, treatment of cells with the PI3K inhibitor LY2904002 at 25 μM or 50 μM was sufficient to delocalize ForG-N completely from the membrane and render it entirely cytosolic, whereas the Arp2/3 inhibitor CK666 had no effect (Fig. S7). Together, these findings support the view that PIP3, together with active Ras but not the Arp2/3 complex or interaction with F-actin (Fig. 1A), is required for ForG targeting.
Fig. S7.
Recruitment of ForG-N is PIP3- but not Arp2/3 complex-dependent. Vegetative WT cells expressing YFP–ForG-N were seeded on glass-bottom dishes in low-osmolarity phosphate buffer and imaged either in the absence or presence of the inhibitors indicated. Notably, treatment of cells with the PIP3 kinase inhibitor LY2904002 at 25 μM or 50 μM was sufficient to delocalize ForG-N completely from the membrane and render it entirely cytosolic, supporting the view that PIP3, together with Ras signaling, is required for ForG targeting (also Fig. 6E). By contrast, treatment with the Arp2/3 complex inhibitor CK666 showed no significant effect at 50 μM and 100 μM, indicating that ForG does not require the Arp2/3 complex for targeting to the cups (white stars). Confocal sections of live cells are shown. (Scale bar: 5 μm.)
Of note, the tertiary structure of the ForC-GBD was shown to adapt an ubiquitin-like fold (66), which is, when combined with positively charged patches at the interaction surface, a hallmark of RBDs (45). In higher eukaryotes, the closest homologs of ForG are members of the FHOD family (28), which are actin-bundling proteins mainly found in stress fibers (67). Intriguingly, the crystal structure of the FHOD1-GBD also adapts an ubiquitin-like superfold (68). However, because FHOD1 lacks the positively charged residues, it fails to interact with active Ras, so that insertion of one lysine residue (P41K) is required to allow for moderate interaction with Ras (68). Moreover, FHOD1 was previously shown to be regulated by the Rho-dependent protein kinase ROCK, which releases the autoinhibition by phosphorylation of the DAD (67). Except for the dedicated phagocytes of the innate immune system, which clear pathogens, most vertebrate cells do not perform large-scale endocytosis. These findings therefore suggest that the ancestral FHOD-like formin in vertebrates might have evolved from a Ras-regulated formin to a Rho-regulated factor implicated in novel activities, such as stress fiber formation (67), whereas in Dictyostelium cells, the Ras regulation of this formin remained evolutionary conserved to preserve a direct link to actin assembly in large-scale endocytosis. Interestingly however, hyperactive Ras signaling in cancer from various origins might awaken the ancestral feeding mechanisms of large-scale endocytosis from dormancy in the form of excessive macropinocytosis. Thus, although the core machineries appear conserved, the unraveling of similarities and differences between amoeba and mammalian cells poses a challenging but fascinating task for the future.
Materials and Methods
A complete description of the methods is provided in SI Materials and Methods. This description includes construction of plasmids and Dictyostelium mutants, protein purification and labeling, actin assembly assays, antibodies and immunoblots, fluorescence microscopy and imaging, analyses of the F-actin distribution in phagocytic cups, endocytosis assays, analyses of cell migration, Y2H assays, and statistical analysis.
SI Materials and Methods
Plasmids.
To obtain a ForG-FL (amino acids 1–1,074) GFP-fusion expression vector, cDNA from vegetative Dictyostelium discoideum AX2 laboratory WT cells was amplified by PCR and assembled in two steps into plasmid pDGFP-MCS-Neo (69) as indicated in Table S1. After sequence verification (European Nucleotide Archive accession no. LN901451), this vector served as a template for all ForG-derived expression plasmids. For expression of ForG∆DAD (amino acids 1–1,040), the 3′ half of the coding sequence was substituted with a PCR PstI/SalI fragment using the unique PstI site in the forG gene. For expression of YFP–ForG-N fusion protein in Dictyostelium, ForG-N (amino acids 1–423) was amplified by PCR as a BglII/SpeI fragment and inserted into the corresponding sites of the extrachromosomal expression plasmid pDM304-YFP. This plasmid carries an N-terminal YFP tag that was obtained by PCR from pDEXRH-MCS-DYFP and carried BamHI/BglII sites (70) for insertion into the single BglII site of pDM304 (71). Similarly, the constructs YFP-ForG-N∆RBD (amino acids 119–423) and YFP-ForG∆RBD∆DAD (amino acids 119–1,040), both of which lack the putative N-terminal RBD, were obtained by PCR and subsequent insertion as BamHI/SpeI fragments into the BglII/SpeI sites of pDM304-YFP. For coexpression with mRFP-tagged LifeAct (30), the corresponding oligonucleotide sequences were synthesized and ligated into the shuttle vector pDM330 (71). Subsequently, the entire LifeAct-mRFP expression cassette was excised by NgoMIV and inserted into the same site of pDM304–YFP–ForG-N. For expression of RasB or RasG fusion protein, the respective coding sequences were amplified by PCR and inserted into BglII/SpeI sites of pDM304-YFP. The pan-probe for active Ras YFP-Raf1-RBD (amino acids 50–132; UniProt Q99N57) containing just the RBD of the RAF protooncogene serine/threonine protein kinase from mouse was generated by PCR amplification from cDNA and was inserted as a BglII/SpeI fragment into the respective sites of pDM304-YFP. Construction of the F-actin probe LimEΔcoil-GFP for life cell imaging has been described (72).
Double-expression plasmids for in vivo interaction experiments of ForG-N with RasB or RasG by BiFC of the YFP variant Venus were adapted from Ohashi et al. (50) and constructed as follows. The coding sequence for the C-terminal beta-sheet of Venus (amino acids 211–238), followed by a 30-aa linker, a BglII site, and ForG-N sequences, was synthesized by GenArt (Life Technologies) and inserted as a BamHI/SpeI fragment into BglII/SpeI sites of expression vector pDM304 or pDM326 (71) to obtain pVC–ForG-N. This approach allowed selection of clones either with G418 (pDM304) or Blasticidin S (pDM326), respectively. The major part of Venus (amino acids 1–210) was synthesized with a 10-aa linker, followed by the RasB-WT coding sequence. This fragment was inserted into the BglII/SpeI sites of the expression cassette of the pDM344. Subsequently, the entire expression cassette was excised by NgoMIV and inserted into the same site of pVC–ForG-N to obtain pBiFC-ForG + RasB. Variants of RasB and RasG were generated by replacement of the RasB-WT coding sequence with corresponding BglII/SpeI fragments. RasB-S20N served as a negative control.
For expression of ForG constructs in Escherichia coli, ForG-3P (amino acids 562–1,074) and ForG-1P (amino acids 599–1,074) were amplified by PCR and inserted into the BamHI and SalI sites of pGEX-6P-1. The ForE (Dictybase gene ID DDB_G0269626) construct ForE-4P (amino acids 1,009–1,561), previously used as a control (31), was generated in two steps due to the highly repetitive sequence elements encoding at its 5′ end. First, a fragment encoding just two proline-rich stretches (amino acids 1,042–1,561) was amplified by PCR from genomic DNA and inserted into BamHI and SalI sites of pGEX-6P-1. Then, a synthetic fragment encoding the entire FH1 domain with four proline-rich stretches was used to exchange the shorter FH1 domain by using BamHI/HindIII restriction sites.
The forG KO vector was constructed by amplification of a 411-base pair 5′ PstI/BamHI fragment and a 633-base pair 3′ SalI/HindIII fragment of the forG gene from genomic DNA, and the fragments were subsequently inserted into the corresponding sites of pLPBLP (73). The rasB targeting vector was constructed by amplification of a 566-base pair 5′ PstI/BamHI fragment and a 503-base pair 3′ SalI/HindIII fragment of the rasB gene from genomic DNA, and the fragments were subsequently inserted into the corresponding sites of pLPBLP (73). Sequences of all used oligonucleotides are provided in Table S1. All original pDM vectors were obtained from the Dicty Stock Center (74). The sequences of all generated constructs were verified by sequencing.
Cell Culture and Transfections.
D. discoideum AX2-214 cells were cultivated at 21 °C in HL5-C medium with glucose (Formedium) and transfected by electroporation using routine protocols. Electroporation was performed with an Xcell gene pulser (Biorad) using the preset protocol 4-6 for Dictyostelium. The forG− clone 10 (strain JFL110) and rasB− (strain JFL111) cells were obtained by transfection of AX2 WT cells with the respective KO vectors linearized with BamHI and SalI. Stably transfected cells were selected with 10 μg⋅mL−1 Blasticidin S (InvivoGen). Gene disruption was confirmed by PCR and, in the case of forG−, also by immunoblotting. In the same way, cell lines ectopically expressing fluorescence fusion proteins were obtained by transfection with the appropriate plasmids and subsequent selection with either 10 μg⋅mL−1 Blasticidin S or 10 μg⋅mL−1 G418 (InvivoGen). ScrA− (strain JFL100) cells have been described previously (53).
Protein Purification.
Expression of GST-tagged proteins was induced in E. coli strain Rosetta 2 (Novagen) with 1 mM isopropyl-β-d-thiogalactoside at 21 °C for 12 h. The proteins were subsequently purified from bacterial extracts by affinity chromatography using glutathione-conjugated agarose (Macherey–Nagel), followed by size exclusion chromatography. In case of ForG-1P, ForG-3P, and ForE-4P, the GST-tag was cleaved off by PreScission protease (GE Healthcare) and removed, together with uncleaved protein, by size exclusion chromatography using an Äkta Purifier System equipped with a HiLoad 26/600 Superdex 200 column (GE Healthcare). The fractions containing the respective formin construct were pooled and dialyzed against storage buffer [150 mM KCl, 1 mM DTT, 60% glycerol, and 20 mM Hepes (pH 7.4)] and stored at −20 °C. Recombinant, tag-free Dictyostelium PFN I was purified by poly-l-proline affinity chromatography (75). Purification of recombinant heterodimeric D. discoideum Cap32/34 (referred to as CP in this work) was performed as described (76). Actin was purified from acetone powder of rabbit skeletal muscle according to standard procedures (77) and labeled on Cys374 with ATTO 488 maleimide (ATTO-TEC) or with N-(1-Pyrenyl)maleimide (Invitrogen), respectively.
Pyrene-Actin Assays.
Recombinant ForG-3P and ForE-4P were diluted in KMEI buffer [1× KMEI: 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0)] to reach a final concentration of 1.18× KMEI in 170-μL aliquots that were placed in black, nontreated, 96-well microtiter plates (Brand GmbH). Anti-foaming solution Extran AP33 (Merck) was added to a final concentration of 0.05%. Actin polymerization was initiated by injection of 30 μL of a 13.33 µM solution of 10% pyrene-labeled G-actin in G-buffer [5 mM Tris⋅HCl (pH 8.0), 0.2 mM ATP, 0.1 mM CaCl2, 0.5 mM DTT] into the protein mixture to reach a final concentration of 2 μM actin in 1× KMEI by the automated dispenser of a Synergy 4 fluorescence microplate reader (BioTek). After a 2-s mixing step, actin polymerization was monitored by measuring the increase of fluorescence of pyrene-actin at 364-nm excitation and 407-nm emission wave lengths for 20 min.
Dilution-induced depolymerization assays were essentially performed as described before (26). Data analysis was performed using SigmaPlot 11.2 (Systat Software, Inc.) and assuming one-site competition of CP and ForG-3P for filament barbed ends to calculate the IC50 for ForG-3P. The Kd of CP for filament barbed ends was calculated assuming one-site saturation kinetics and allowed us to calculate the apparent Kd of ForG-3P by the law of mass action at half-maximal displacement of CP using the equation:
[ForG]0 and [CP]0 are the initial concentrations at half-maximal displacement of CP.
TIRFM.
TIRFM was performed on an Olympus IX-81 inverted microscope equipped with an Apo N 60× TIRF objective and cooled Hamamatsu Orca-R2 CCD camera (Hamamatsu). The flow chambers consisted of 24 × 40-mm glass coverslips (Menzel–Gläser) that were fixed to microscope slides by double-sided adhesive tape. The flow chambers were preincubated with 10% (mol/vol) fish gelatin in 1× KMEI for 10 min and rinsed with 1× KMEI containing 10 mg/mL BSA (Sigma). Proteins were prediluted in 1× TIRF buffer [20 mM imidazole (pH 7.4), 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 50 mM DTT, 0.2 mM ATP, 15 mM glucose, 2.5 mg/mL methylcellulose, 20 μg/mL catalase, 100 μg/mL glucose-oxidase]. The assays were started by adding G-actin (1.3 μM final concentration, 23% ATTO488-labeled) to these mixtures and flushing them into the flow chambers. Images were captured every 2 s, with exposure times of 50–100 ms for at least 600 s. The pixel size corresponded to 0.21 μm due to the operation in 2 × 2 binning mode. The elongation rates of filaments were determined by manual tracking of growing barbed ends after processing the time-lapse movies by background subtraction using a 50-pixel rolling ball radius in ImageJ software (NIH). At least 20 filaments from three movies per condition were measured. The nucleation efficacies were obtained by counting and averaging the number of actin filaments in an area of 80 × 80 μm 180 s after the polymerization reaction was initiated.
F-Actin Binding and Cross-Linking Assays.
For high-speed sedimentation assays, G-actin in G-Buffer, ForG-3P, and CP dilutions in 1× KMEI were cleared for 60 min at 4 °C at 150,000 × g in a Beckman Optima TL-100 ultracentrifuge. The reaction mixtures were incubated for 2 h at room temperature in 1× KMEI. Subsequently, the samples were centrifuged for 60 min as mentioned above, and the pellets were brought to the original volume in 3× SDS sample buffer diluted with 1× KMEI. To quantitate the amount of ForG-3P that cosedimented with actin, the amounts of the proteins in the pellet and supernatant fractions were determined densitometrically from four independent experiments after SDS/PAGE and Coomassie Blue staining using ImageJ software. Calculation of free and bound ForG-3P was based on determining the ratio of the band intensities of the 56-kDa band from the pellet and supernatant fractions. For low-speed sedimentation, 2 μM G-actin was polymerized in presence of various ForG-3P concentrations for 2 h at room temperature in 1× KMEI, sedimented for 60 min at 15,800 × g in an Eppendorf centrifuge, and analyzed on SDS/PAGE in supernatant and pellet fractions as described above.
Antibodies and Immunoblots.
Polyclonal antibodies against ForG were raised by immunizing a female New Zealand White rabbit with recombinant ForG-1P (amino acids 562–1,074) following standard protocols. Immunoblotting was performed according to standard protocols using undiluted hybridoma supernatants of PsA-specific mAb MUD1 (78), contact site A-specific mAb 33-294-17 (79), cortexillin-specific mAb 241-438-1 (80), or polyclonal anti-ForG antibody serum (1:250 dilution). Primary antibodies in immunoblots were visualized with phosphatase-coupled anti-mouse (1:1,000, no. 115-055-62; Dianova) or anti-rabbit IgG (1:1,000; no. 111-055-046; Dianova). Uncropped scans of immunoblots are shown in Fig. S8.
Fig. S8.
Uncropped images of blots and Coomassie Blue-stained gels.
Fluorescence Microscopy and Imaging.
For life-cell imaging, growth-phase cells expressing fluorescent fusion proteins were seeded onto 3.5-cm-diameter glass-bottom dishes (Ibidi) and allowed to adhere on the glass surface for 20 min. The cells were then washed several times with PB buffer [17 mM Na-K-phosphate (pH 6.0]. Mutants expressing BiFC-Venus constructs were grown in LoFlo-medium (Formedium) for at least 3 h to reduce background signal from HL5-C medium, and PB was additionally supplemented with (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox; Sigma) antioxidant to counteract bleaching. Confocal imaging was performed using either an LSM510Meta confocal microscope (Zeiss) equipped with a 63×/1.3 Plan-Neofluar objective using the 488-nm and 543-nm laser lines or a Leica TCS SP8 X microscope equipped with an HC PL AP CS2 63×/1.4 oil objective (Leica Microsystems) using the 488-nm, 496-nm, 511-nm, 565-nm, and 577-nm laser lines. Qualitative analysis of cell morphology of growth-phase cells in HL5-C medium or PB was monitored by time-lapse imaging with an inverted Olympus IX-81 microscope equipped with 100× phase-contrast optics (Olympus) and a CoolSnap EZ camera (Photometrics). Localization of YFP-tagged ForG 1–423 in agar overlay assays was performed as described (25). LY294002 (Sigma) or CK666 (Sigma) dissolved in dimethyl sulfoxide (DMSO) was added to adherent cells in PB 5–10 min before confocal imaging; the corresponding amount of DMSO was added to control cells. Data were processed using ImageJ and CorelDraw software (Corel Corporation).
Analyses of F-Actin Distribution in Phagocytic Cups.
Dictyostelium cells engulfing TRITC-labeled yeast particles were fixed and stained for F-actin with Alexa Fluor 488 Phalloidin (Thermo Fisher Scientific). Confocal sections of cells with partially engulfed yeast were recorded using identical settings at the LSM510Meta confocal microscope. Fluorescence intensity profiles of the F-actin signal at phagocytic cups were then measured in ImageJ with a four-pixel-wide segmented line. Due to deviations of yeast size, the exact engulfment stage, and for reasons of comparability, the measured intensity profiles of the events were resampled to 100 data points, corresponding to 100% of the contour length with the resample function of MATLAB (MathWorks). Finally, the data were processed with Excel and SigmaPlot 11.2 (Systat Software, Inc.).
Macropinocytosis Assays.
Quantitative measurement of fluid-phase uptake was performed by standard protocol (58). In short, cells were shaken at 150 rpm for 1 h in a 30-mL HL5-C medium at a density of 6 × 106 mL−1, and TRITC-dextran (molecular mass of 65–85 kDa; Sigma) was then added to a final concentration of 2 mg/mL. Aliquots of 1 mL were taken at each time point and mixed briefly with 100 μL of Trypan Blue solution (4 mg⋅mL−1; Sigma) to quench fluorescence. The cells were quickly pelleted, washed twice with ice-cold PB, and lysed in 1 mL of lysis buffer [0.2% Triton X-100, 50 mM Na2HPO4 (pH 9.3)]. Fluorescence was measured directly after lysis at 544-nm excitation and 574-nm emission wavelengths using a Synergy 4 microplate reader (BioTek). Obtained values were normalized to protein content, which was assessed in the microplate reader using a Pierce 660 protein assay (Thermo Fisher Scientific).
For qualitative confocal microscopy sections at the Leica TCS SP8 X, the fluorescence settings were optimized with one batch of AX2 cells that were preincubated for 90 min with tracer-containing medium (1 mg⋅mL−1 TRITC-dextran) in glass-bottom dishes (Ibidi) at a cell density of 1.5 × 104 cm−2. After this setup step, the actual experiment with null mutants and WT control was started in parallel, and images were taken every 30 min to record tracer enrichment in the cells. Contrast of images was adjusted uniformly using ImageJ and CorelDraw software.
Phagocytosis Assays.
Phagocytosis of TRITC-labeled yeast particles was quantified following a standard protocol (18). In short, cells were grown overnight in a shaken suspension at 150 rpm in HL5-C medium with glucose and adjusted to a density of 2 × 106 cells per milliliter in 20 mL of medium. Subsequently, TRITC yeast was added at a sixfold excess. Aliquots of 1 mL were taken at each time point and incubated on a cooled shaker for 3 min with 100 μL of Trypan Blue solution (4 mg/mL; Sigma). Subsequently, cells were pelleted, washed once with ice-cold PB, and resuspended in 1 mL of PB for immediate measurement in a Jasco Fluo FP-6500 fluorescence spectrophotometer at 544-nm excitation and 574-nm emission wavelengths. Obtained values were normalized to respective protein content (macropinocytosis). For qualitative comparison of phagocytosis, WT and mutant cells were seeded on glass-bottom dishes (Ibidi) at a cell density of 5 × 104 cells per square centimeter and a fivefold excess of TRITC-labeled yeast particles in PB, and were imaged after 30 min of incubation with the LSM510 Meta confocal microscope.
To measure phagocytosis of bacteria, a clearance assay of Witke et al. (81) was performed with modifications. Dictyostelium cells were grown overnight in shaken suspension, washed with ice-cold PB, and adjusted to a suspension 2 × 106 cells per milliliter in 10 mL of PB shaken at 150 rpm. After 30 min, 10 mL of an E. coli strain B/r suspension in PB at an optical density of around 1.4 at 600 nm (OD600) was added. At the indicated time points, 1.5-mL samples were withdrawn, supplemented with Na-Azide to a final concentration of 5 mM, and vortexed for 30 s to remove surface-bound bacteria (82). Subsequently, Dictyostelium cells were pelleted for 1 min at 700 × g, and the OD600 of the supernatant containing the bacteria was measured to assess the clearance rate. Individual experiments were normalized to an initial OD600 of 0.5, and the clearance rates were additionally adjusted to protein content.
Analyses of Cell Migration.
Quantitative analysis of random cell motility was performed as follows: Growth-phase cells in PB buffer were monitored every 10 s for 15 min by time-lapse imaging using an inverted Olympus IX-81 microscope equipped with 10× phase-contrast optics (Olympus) and a CoolSnap EZ camera (Photometrics). Single-cell tracks were obtained from recordings with the Track Objects plug-in of Metamorph 7 software (Molecular Devices). Total data samples of each cell line contain more than 500 tracks from three independent experiments with at least 150 individual cells each. These data were further processed to obtain the average speed of single cells using a custom-built protocol operated in Excel (Microsoft). Average speed distributions of analyzed samples are presented in box plot representations using SigmaPlot 11.2 (Systat Software, Inc.). Boxes indicate the 25th through 75th percentiles, whiskers mark the 10th and 90th percentiles, and the outliers are the fifth and 95th percentiles.
Y2H Assays.
For protein–protein interaction studies the MATCHMAKER GAL4 Two-Hybrid System 3 (Clontech Laboratories) was used. ForG-N (amino acids 1–423) was inserted into the pGADT7 prey vector to yield Gal4-activation domain–ForG-N. Likewise, Raf1-RBD (amino acids 50–132) and Byr2-RBD (amino acids 1–236; UniProt P28829) were inserted into pGADT7 as established positive controls for active Ras (49).
All GTPases were inserted into the EcoRI and BamHI sites of the pGBKT7 bait vector. The coding sequences of the constitutively active and inactive forms were synthesized by GenArt (Life Technologies) and encode for Rho- and RasGTPases corresponding to the Dictybase entries listed in Table S2. Silent mutations of internal EcoRI and BamHI sites and codon optimizations were performed when necessary. The AH109 yeast strain was cotransfected with bait and prey vector, and grown on synthetic double-dropout agar lacking leucine and tryptophan according to the manufacturer’s instructions. Several colonies were collected and grown in synthetic dropout liquid media overnight and then subjected to dropout agar plates. Protein interactions were scored by growth of cells on agar plates for 4 d at 25 °C on triple-dropout (TD) or quadruple-dropout (QD) agar plates. TD plates lacked leucine, tryptophan, and histidine, and were supplemented with 3 mM 3-amino-1,2,4-triazole to suppress the leaky HIS3-reporter gene according to the manufacturer’s instructions. QD plates lacked leucine, tryptophan, histidine, and adenine for highest stringency.
Statistical Analysis.
Statistical analysis was performed using SigmaPlot 11.2 software (Systat Software). Statistical significance of differences between nonnormally distributed populations was determined by the Mann–Whitney U test. When data fulfilled the criteria of normality (Shapiro–Wilk test) and equal variance (Levene’s test), statistical differences were analyzed with a two-tailed, unpaired Student’s t test. Statistical differences are reported as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and nonsignificant.
Supplementary Material
Acknowledgments
We thank Annette Breskott for technical assistance and all members of the group for stimulating discussions. Furthermore, we are grateful to Matthias Preller for suggestions in BiFC linker design, Sarah Körber for assistance in Y2H screens, Ludwig Eichinger for providing prespore antigen A antibody, the Dicty Stock Center, and depositor Douwe Veltman for supplying us with pDM plasmids. This work was supported by Deutsche Forschungsgemeinschaft Grants FA 330/6-2 and FA 330/10-1 (to J.F.). V.F. was supported by the FP7-REGPOT-2012-2013-1 Grant Agreement 316289–InnoMol.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the European Nucleotide Archive sequence data bank (accession no. LN901451).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611024113/-/DCSupplemental.
References
- 1.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 2.Rougerie P, Miskolci V, Cox D. Generation of membrane structures during phagocytosis and chemotaxis of macrophages: Role and regulation of the actin cytoskeleton. Immunol Rev. 2013;256(1):222–239. doi: 10.1111/imr.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamphorst JJ, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA. 2013;110(22):8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomfield G, Kay RR. Uses and abuses of macropinocytosis. J Cell Sci. 2016;129(14):2697–2705. doi: 10.1242/jcs.176149. [DOI] [PubMed] [Google Scholar]
- 6.Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9(8):639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin R, Grinstein S, Canton J. The life cycle of phagosomes: Formation, maturation, and resolution. Immunol Rev. 2016;273(1):156–179. doi: 10.1111/imr.12439. [DOI] [PubMed] [Google Scholar]
- 8.Veltman DM, Lemieux MG, Knecht DA, Insall RH. PIP₃-dependent macropinocytosis is incompatible with chemotaxis. J Cell Biol. 2014;204(4):497–505. doi: 10.1083/jcb.201309081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sussman R, Sussman M. Cultivation of Dictyostelium discoideum in axenic medium. Biochem Biophys Res Commun. 1967;29(1):53–55. doi: 10.1016/0006-291x(67)90539-6. [DOI] [PubMed] [Google Scholar]
- 10.Bloomfield G, et al. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife. 2015;4:1–25. doi: 10.7554/eLife.04940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniak M. Conserved features of endocytosis in Dictyostelium. Int Rev Cytol. 2002;221:257–287. doi: 10.1016/s0074-7696(02)21014-1. [DOI] [PubMed] [Google Scholar]
- 12.Pan M, Xu X, Chen Y, Jin T. Identification of a chemoattractant G-protein-coupled receptor for folic acid that controls both chemotaxis and phagocytosis. Dev Cell. 2016;36(4):428–439. doi: 10.1016/j.devcel.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeller O, et al. Gβ regulates coupling between actin oscillators for cell polarity and directional migration. PLoS Biol. 2016;14(2):e1002381. doi: 10.1371/journal.pbio.1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai H, et al. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J Cell Biol. 2010;190(2):233–245. doi: 10.1083/jcb.201001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeller O, et al. Two distinct functions for PI3-kinases in macropinocytosis. J Cell Sci. 2013;126(Pt 18):4296–4307. doi: 10.1242/jcs.134015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark J, et al. Dictyostelium uses ether-linked inositol phospholipids for intracellular signalling. EMBO J. 2014;33(19):2188–2200. doi: 10.15252/embj.201488677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke M, et al. Curvature recognition and force generation in phagocytosis. BMC Biol. 2010;8(1):154. doi: 10.1186/1741-7007-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: Dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83(6):915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 19.Konzok A, et al. DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J Cell Biol. 1999;146(2):453–464. doi: 10.1083/jcb.146.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikati MA, Breitsprecher D, Jansen S, Reisler E, Goode BL. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. J Mol Biol. 2015;427(19):3137–3147. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson JA, et al. A contractile activity that closes phagosomes in macrophages. J Cell Sci. 1999;112(Pt 3):307–16. doi: 10.1242/jcs.112.3.307. [DOI] [PubMed] [Google Scholar]
- 22.Brzeska H, Koech H, Pridham KJ, Korn ED, Titus MA. Selective localization of myosin-I proteins in macropinosomes and actin waves. Cytoskeleton (Hoboken) 2016;73(2):68–82. doi: 10.1002/cm.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinzenz M, et al. Actin branching in the initiation and maintenance of lamellipodia. J Cell Sci. 2012;125(Pt 11):2775–2785. doi: 10.1242/jcs.107623. [DOI] [PubMed] [Google Scholar]
- 24.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 25.Ramalingam N, et al. A resilient formin-derived cortical actin meshwork in the rear drives actomyosin-based motility in 2D confinement. Nat Commun. 2015;6:8496. doi: 10.1038/ncomms9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junemann A, et al. ForC lacks canonical formin activity but bundles actin filaments and is required for multicellular development of Dictyostelium cells. Eur J Cell Biol. 2013;92(6-7):201–212. doi: 10.1016/j.ejcb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Katoh M, Chen G, Roberge E, Shaulsky G, Kuspa A. Developmental commitment in Dictyostelium discoideum. Eukaryot Cell. 2007;6(11):2038–2045. doi: 10.1128/EC.00223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivero F, et al. A comparative sequence analysis reveals a common GBD/FH3-FH1-FH2-DAD architecture in formins from Dictyostelium, fungi and metazoa. BMC Genomics. 2005;6:28. doi: 10.1186/1471-2164-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the Diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174(5):701–713. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riedl J, et al. Lifeact: A versatile marker to visualize F-actin. Nat Methods. 2008;5(7):605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winterhoff M, et al. The Diaphanous-related formin dDia1 is required for highly directional phototaxis and formation of properly sized fruiting bodies in Dictyostelium. Eur J Cell Biol. 2014;93(5-6):212–224. doi: 10.1016/j.ejcb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Noegel A, Gerisch G, Stadler J, Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986;5(7):1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krefft M, Voet L, Gregg JH, Mairhofer H, Williams KL. Evidence that positional information is used to establish the prestalk-prespore pattern in Dictyostelium discoideum aggregates. EMBO J. 1984;3(1):201–206. doi: 10.1002/j.1460-2075.1984.tb01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chubb JR, Wilkins A, Thomas GM, Insall RH. The Dictyostelium RasS protein is required for macropinocytosis, phagocytosis and the control of cell movement. J Cell Sci. 2000;113(Pt 4):709–719. doi: 10.1242/jcs.113.4.709. [DOI] [PubMed] [Google Scholar]
- 35.De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 36.Linkner J, et al. The inverse BAR domain protein IBARa drives membrane remodeling to control osmoregulation, phagocytosis and cytokinesis. J Cell Sci. 2014;127(Pt 6):1279–1292. doi: 10.1242/jcs.140756. [DOI] [PubMed] [Google Scholar]
- 37.Klein G, Satre M. Kinetics of fluid-phase pinocytosis in Dictyostelium discoideum amoebae. Biochem Biophys Res Commun. 1986;138(3):1146–1152. doi: 10.1016/s0006-291x(86)80402-8. [DOI] [PubMed] [Google Scholar]
- 38.Veltman DM. Drink or drive: Competition between macropinocytosis and cell migration. Biochem Soc Trans. 2015;43(1):129–132. doi: 10.1042/BST20140251. [DOI] [PubMed] [Google Scholar]
- 39.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124(2):423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 40.Paul AS, Pollard TD. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr Biol. 2008;18(1):9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovar DR, Kuhn JR, Tichy AL, Pollard TD. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J Cell Biol. 2003;161(5):875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlahou G, Rivero F. Rho GTPase signaling in Dictyostelium discoideum: Insights from the genome. Eur J Cell Biol. 2006;85(9-10):947–959. doi: 10.1016/j.ejcb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- 44.Steffen A, Koestler SA, Rottner K. Requirements for and consequences of Rac-dependent protrusion. Eur J Cell Biol. 2014;93(5-6):184–193. doi: 10.1016/j.ejcb.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Wohlgemuth S, et al. Recognizing and defining true Ras binding domains I: Biochemical analysis. J Mol Biol. 2005;348(3):741–758. doi: 10.1016/j.jmb.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 46.Pacold ME, et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103(6):931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 47.Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435(7038):43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robbins SM, Williams JG, Jermyn KA, Spiegelman GB, Weeks G. Growing and developing Dictyostelium cells express different ras genes. Proc Natl Acad Sci USA. 1989;86(3):938–942. doi: 10.1073/pnas.86.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kae H, Lim CJ, Spiegelman GB, Weeks G. Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep. 2004;5(6):602–606. doi: 10.1038/sj.embor.7400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohashi K, Kiuchi T, Shoji K, Sampei K, Mizuno K. Visualization of cofilin-actin and Ras-Raf interactions by bimolecular fluorescence complementation assays using a new pair of split Venus fragments. Biotechniques. 2012;52(1):45–50. doi: 10.2144/000113777. [DOI] [PubMed] [Google Scholar]
- 51.Iizuka R, Yamagishi-Shirasaki M, Funatsu T. Kinetic study of de novo chromophore maturation of fluorescent proteins. Anal Biochem. 2011;414(2):173–178. doi: 10.1016/j.ab.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Seastone DJ, et al. The WASp-like protein scar regulates macropinocytosis, phagocytosis and endosomal membrane flow in Dictyostelium. J Cell Sci. 2001;114(Pt 14):2673–2683. doi: 10.1242/jcs.114.14.2673. [DOI] [PubMed] [Google Scholar]
- 53.Steffen A, et al. Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell. 2006;17(6):2581–2591. doi: 10.1091/mbc.E05-11-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Innocenti M, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7(10):969–976. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- 55.Brandt DT, et al. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178(2):193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naj X, Hoffmann AK, Himmel M, Linder S. The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages. Infect Immun. 2013;81(5):1683–1695. doi: 10.1128/IAI.01411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann AK, Naj X, Linder S. Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages. FASEB J. 2014;28(7):3075–3089. doi: 10.1096/fj.13-247049. [DOI] [PubMed] [Google Scholar]
- 58.Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110(Pt 2):105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- 59.Romero S, et al. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119(3):419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 60.Block J, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22(11):1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401(6753):613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 62.Chattwood A, Bolourani P, Weeks G. RasG signaling is important for optimal folate chemotaxis in Dictyostelium. BMC Cell Biol. 2014;15:13. doi: 10.1186/1471-2121-15-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121(Pt 5):551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devreotes P, Horwitz AR. Signaling networks that regulate cell migration. Cold Spring Harb Perspect Biol. 2015;7(8):a005959. doi: 10.1101/cshperspect.a005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuxworth RI, et al. Dictyostelium RasG is required for normal motility and cytokinesis, but not growth. J Cell Biol. 1997;138(3):605–614. doi: 10.1083/jcb.138.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dames SA, et al. Structure, dynamics, lipid binding, and physiological relevance of the putative GTPase-binding domain of Dictyostelium formin C. J Biol Chem. 2011;286(42):36907–36920. doi: 10.1074/jbc.M111.225052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27(4):618–628. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulte A, et al. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure. 2008;16(9):1313–1323. doi: 10.1016/j.str.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Dumontier M, Höcht P, Mintert U, Faix J. Rac1 GTPases control filopodia formation, cell motility, endocytosis, cytokinesis and development in Dictyostelium. J Cell Sci. 2000;113(Pt 1):2253–2265. doi: 10.1242/jcs.113.12.2253. [DOI] [PubMed] [Google Scholar]
- 70.Filić V, Marinović M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125(Pt 2):387–398. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
- 71.Veltman DM, Akar G, Bosgraaf L, Van Haastert PJM. A new set of small, extrachromosomal expression vectors for Dictyostelium discoideum. Plasmid. 2009;61(2):110–118. doi: 10.1016/j.plasmid.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Schneider N, et al. A Lim protein involved in the progression of cytokinesis and regulation of the mitotic spindle. Cell Motil Cytoskeleton. 2003;56(2):130–139. doi: 10.1002/cm.10139. [DOI] [PubMed] [Google Scholar]
- 73.Faix J, Kreppel L, Shaulsky G, Schleicher M, Kimmel AR. A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 2004;32(19):e143. doi: 10.1093/nar/gnh136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fey P, Dodson RJ, Basu S, Chisholm RL. One stop shop for everything Dictyostelium: DictyBase and the Dicty Stock Center in 2012. Methods Mol Biol. 2013;983:59–92. doi: 10.1007/978-1-62703-302-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breitsprecher D, et al. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008;27(22):2943–2954. doi: 10.1038/emboj.2008.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7(6):619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 77.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971;246(15):4866–4871. [PubMed] [Google Scholar]
- 78.Gregg JHJH, Krefft M, Haas-Kraus A, Williams KLKL. Antigenic differences detected between prespore cells of Dictyostelium discoideum and Dictyostelium mucoroides using monoclonal antibodies. Exp Cell Res. 1982;142(1):229–233. doi: 10.1016/0014-4827(82)90427-x. [DOI] [PubMed] [Google Scholar]
- 79.Bertholdt G, Stadler J, Bozzaro S, Fichtner B, Gerisch G. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 1985;16(3):187–202. doi: 10.1016/0045-6039(85)90516-0. [DOI] [PubMed] [Google Scholar]
- 80.Faix J, et al. Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell. 1996;86(4):631–642. doi: 10.1016/s0092-8674(00)80136-1. [DOI] [PubMed] [Google Scholar]
- 81.Witke W, Schleicher M, Noegel AA. Redundancy in the microfilament system: abnormal development of Dictyostelium cells lacking two F-actin cross-linking proteins. Cell. 1992;68(1):53–62. doi: 10.1016/0092-8674(92)90205-q. [DOI] [PubMed] [Google Scholar]
- 82.Maselli A, Laevsky G, Knecht DA. Kinetics of binding, uptake and degradation of live fluorescent (DsRed) bacteria by Dictyostelium discoideum. Microbiology. 2002;148(Pt 2):413–420. doi: 10.1099/00221287-148-2-413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.