Significance
Realization of a safe, low-cost rechargeable lithium battery of high energy density and long cycle life is needed for powering an electric road vehicle and for storing electric power generated by solar or wind energy. This urgent need has prompted efforts to develop a solid electrolyte with an alkali metal anode. Only now is it recognized that the key requirement is wetting of the electrolyte surface by the alkali-metal anode. We report a full rechargeable cell with a solid electrolyte that, although it is reduced by metallic lithium, forms a thin lithium–electrolyte interface that is wet by the anode and wets the electrolyte to give a small Li+ transfer resistance across the interface.
Keywords: solid electrolyte, lithium anode, polymer catholyte, interfaces, NASICON
Abstract
A solid electrolyte with a high Li-ion conductivity and a small interfacial resistance against a Li metal anode is a key component in all-solid-state Li metal batteries, but there is no ceramic oxide electrolyte available for this application except the thin-film Li-P oxynitride electrolyte; ceramic electrolytes are either easily reduced by Li metal or penetrated by Li dendrites in a short time. Here, we introduce a solid electrolyte LiZr2(PO4)3 with rhombohedral structure at room temperature that has a bulk Li-ion conductivity σLi = 2 × 10−4 S⋅cm−1 at 25 °C, a high electrochemical stability up to 5.5 V versus Li+/Li, and a small interfacial resistance for Li+ transfer. It reacts with a metallic lithium anode to form a Li+-conducting passivation layer (solid-electrolyte interphase) containing Li3P and Li8ZrO6 that is wet by the lithium anode and also wets the LiZr2(PO4)3 electrolyte. An all-solid-state Li/LiFePO4 cell with a polymer catholyte shows good cyclability and a long cycle life.
A rechargeable cell having a flammable organic liquid Li+ electrolyte has enabled the wireless revolution, but it is not able to power safely an electric road vehicle at a cost that is competitive with the gasoline-powered internal combustion engine (1–4). Safety concerns as well as cost, volumetric energy density, and cycle life have prevented realization of a commercially viable electric road vehicle. To address this problem, considerable effort is being given to the development of a solid Li+ or Na+ electrolyte that is wet by a metallic lithium or sodium anode and has an alkali ion conductivity σi > 10−4 S⋅cm−1 at the cell operating temperature Top, where a Top ≤ 25 °C is desired (5–10). Such a development would allow new as well as traditional strategies for the cathode. Wetting of the solid electrolyte surface is desired not only because it prevents dendrite formation and growth during plating of an alkali metal anode, but also because wetting constrains the anode volume change in a charge/discharge cycle to be perpendicular to the anode/electrolyte interface, thereby allowing a long cycle life. Therefore, the shear modulus of the electrolyte may not be critical where lithium wets the electrolyte surface.
Ceramic oxide electrolytes offer a large energy gap between their conduction and valence bands, which can allow realization of a battery cell with a large energy separation between the anode and cathode chemical potentials without either reduction or oxidation of the electrolyte by an electrode (2). However, if an alkali-metal anode reduces the solid electrolyte, formation of a stable solid-electrolyte interphase (SEI) that conducts the working Li+ or Na+ ion is acceptable if the Li+ or Na+ transfer across the SEI has a low resistance. Although many ceramic solid Li+ electrolytes have been investigated, they are easily reduced by Li metal and/or they have failed to block dendrite formation and growth into their grain boundaries (SI Appendix, Fig. S1). However, the rhombohedral structure of the Na electrolyte Na1+3xZr2(SixP1–xO4)3 (11), NASICON, which was developed over 45 y ago, has recently been used in a cell design in which a seawater cathode provides the sodium of the anode (12).
The stability of the solid electrolyte on contact with a lithium anode is a critical issue. If a lithium anode reduces the electrolyte, (i) the electrolyte may become an electronic conductor, (ii) an interface layer may form that blocks Li+ transfer, or (iii) an interface layer may form that conducts Li+ ions with a low impedance. The third situation forms with a LiZr2(PO4)3 electrolyte. In this paper, we report that a Li+ electrolyte with the NASICON structure, LiZr2(PO4)3, can be fabricated by using zirconium acetate as the precursor and spark plasma sintering (SPS); it forms a stable Li+-conducting SEI that is wet by a metallic lithium anode and also wets the electrolyte to provide a safe, all-solid-state Li/LiFePO4 cell operating at Top = 80 °C with a long cycle life; the LiFePO4 cathode particles are embedded in a polymer catholyte and carbon.
Results and Discussion
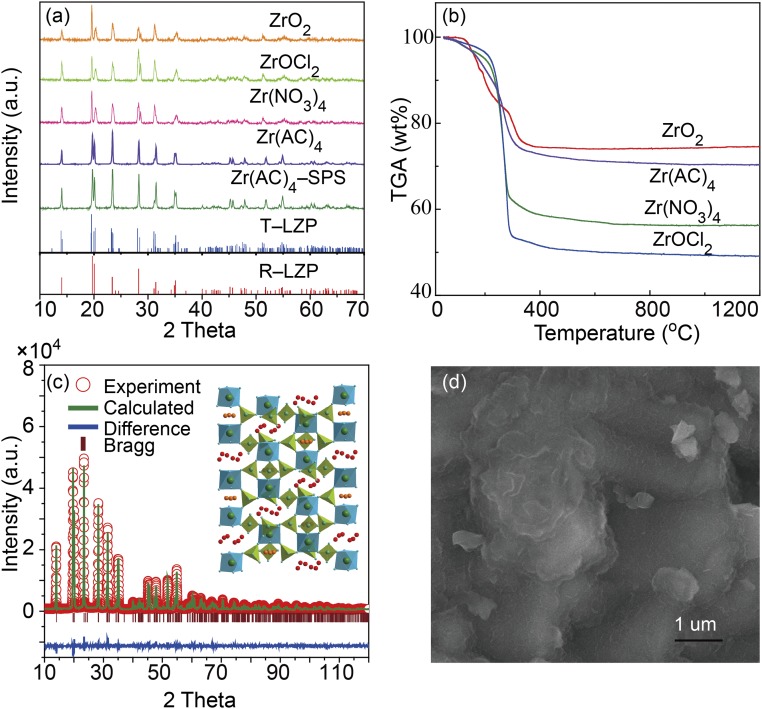
The X-ray diffraction (XRD) results of as-prepared LiZr2(PO4)3 are shown in Fig. 1A; the phase of LiZr2(PO4)3 depended on the starting materials. Although different zirconium salts decomposed at the same temperature T < 400 °C (Fig. 1B), a pure LiZr2(PO4)3 phase with rhombohedral structure could only be obtained with zirconium acetate; a triclinic phase with space group C-1 was obtained with other zirconium materials. LiZr2(PO4)3 prepared by solid-state reaction with ZrO2 as the starting material was reported to change from triclinic to rhombohedral structure at 60 °C (13, 14). The rhombohedral LiZr2(PO4)3 phase with high Li-ion conductivity was stable at room temperature with zirconium acetate as the starting zirconium salt. The XRD refinement of the rhombohedral LiZr2(PO4)3 phase is shown in Fig. 1C with reliability factors (Rwp = 11.0%; RB = 2.3%). The rhombohedral phase has a space group R-3c with lattice parameters a = 8.8442 Å and c = 22.2645 Å, which is very close to those of LiZr2(PO4)3 at 400 °C prepared by solid-sate reaction. A pure rhombohedral LiZr2(PO4)3 phase was also obtained by SPS. The electron-dispersive spectroscopy mapping in SI Appendix, Fig. S2 indicates a uniform distributions of Zr, P, and O elements in the LiZr2(PO4)3 pellet. The LiZr2(PO4)3 pellets fired at 1,150 °C for 20 h in a box furnace and fired at 1,000 °C for 100 min by SPS have a density of 85% (2.66 g⋅cm–3, SI Appendix, Fig. S3) and 99.9% (3.15 g⋅cm–3, Fig. 1D), respectively.
Fig. 1.
(A) XRD patterns of LiZr2(PO4)3 prepared with different starting materials. (B) Thermogravimetric analysis (TGA) curves of LiZr2(PO4)3 with different starting materials. (C) Rietveld analysis of the XRD data of rhombohedral LiZr2(PO4)3. (D) SEM image of rhombohedral LiZr2(PO4)3 prepared by SPS at 1,000 °C for 10 min.
A pure rhombohedral LiZr2(PO4)3 phase can be obtained as a well-sintered ceramic at 1,200 °C by Y3+ or Ca2+ doping for Zr4+; the rhombohedral structure remains unchanged from –70 °C to 200 °C (15). The acetate route supplies particles with the rhombohedral phase in a single firing at 900 °C; it is a simpler route, but the product undergoes a reversible change from rhombohedral to triclinic symmetry on cooling through 15 °C (SI Appendix, Fig. S3). Both products give comparable ionic conductivities at 80 °C.
The impedance spectra of LiZr2(PO4)3 prepared with different starting materials are shown in Fig. 2 and SI Appendix, Fig. S3. The rhombohedral LiZr2(PO4)3 pellets fired by conventional sintering in a box furnace and by SPS at 1,000 °C for 10 min have, respectively, Li-ion conductivities of 2.2 × 10−5 S⋅cm–1 and 3.8 × 10−5 S⋅cm–1 at 25 °C, which is two to three orders of magnitude higher than those of the room-temperature triclinic phases; the pellet fired by SPS method has a Li-ion conductivity of 1.8 × 10−4 S⋅cm−1 at 80 °C. The bulk Li-ion conductivity of rhombohedral LiZr2(PO4)3, as calculated from the distance between the zero point and the left interception of the semicircle with the Z′′ axis, was 2 × 10−4 S⋅cm−1 at 25 °C (SI Appendix, Fig. S3). The activation energy of rhombohedral LiZr2(PO4)3 calculated from an Arrhenius plot over 300 K to 450 K in SI Appendix, Fig. S4 was 0.28 eV, which is smaller than that (0.40 eV) of LiZr2(PO4)3 prepared by solid-state reaction (15, 16). In rhombohedral LiZr2(PO4)3 (Fig. 1C, Inset), 90% and 10% Li ions occupy, respectively, the sixfold disordered 36f M1 sites and the threefold disordered 18e M2 sites (13). The interstitial space is large enough for Li-ion transport, and higher Li-ion conductivity may be obtained by doping with different valent ions to increase the Li-ion population inside the framework.
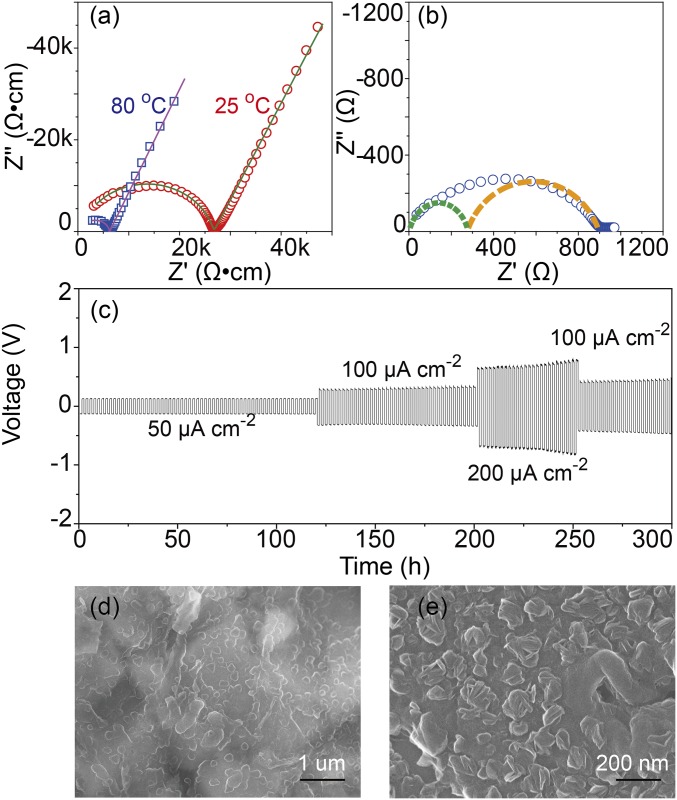
Fig. 2.
(A) The impedance plots of LiZr2(PO4)3 at 25 °C and 80 °C. (B) The impedance plots Li/LiZr2(PO4)3/Li symmetric cell. (C) Cyclability of the Li/LiZr2(PO4)3/Li symmetric cell for 300 h with different current densities. (D) Surface SEM image of a LiZr2(PO4)3 pellet after cycling the cell for 100 h. (E) SEM image of Li metal after charging the symmetric cell for 20 h at 150 µA⋅cm–2.
Whereas a garnet Li+ electrolyte can have a higher bulk σLi = 1 × 10−3 S⋅cm−1 at 25 °C (17), an insulating Li2CO3 surface layer forms on exposure to moist air; the interfaces of a symmetric Li/garnet/Li cell create a large resistance to Li+ transfer between the anode and the electrolyte (about 1,700 Ω⋅cm−2, SI Appendix, Fig. S5) that dominates the total resistance of the electrolyte. In contrast, the SEI in the Li/LiZr2(PO4)3/Li interfaces with a 400-µm-thick electrolyte pellet gave an interfacial resistance of 650 Ω⋅cm−2 at 80 °C in the absence of an applied pressure on the cell. The much lower interfacial resistance of the Li/LiZr2(PO4)3/Li cell makes LiZr2(PO4)3 a much better Li+ solid electrolyte than garnet.
The electrochemical compatibility and stability of LiZr2(PO4)3 with Li metal was evaluated further at 80 °C with symmetric Li/LiZr2(PO4)3/Li cells by subjecting them to different current densities from 50 µA⋅cm–2 to 350 µA⋅cm–2. The symmetric cell showed good cyclability at current densities less than 200 µA⋅cm–2, but the current densities above 200 µA⋅cm–2 increased the voltage a little after 50 h. At 50 µA⋅cm–2, the symmetric cell has a low overpotential of about 0.13 V and there is no evident voltage increase after 120 h; the interface between Li metal and LiZr2(PO4)3 is very stable. Li/ LiZr2(PO4)3/Li cells were tested at 80 °C for up to 500 h. At 80 °C, a sealing problem with the coin cells resulted in oxidation of the lithium, but there was no evidence of failure by dendrite formation and growth. After cycling a Li/LiZr2(PO4)3/Li cell for 10 h, a thin layer with black color formed only on the surface of the LiZr2(PO4)3 pellet (SI Appendix, Fig. S6). No new diffraction peaks except those of the rhombohedral LiZr2(PO4)3 were observed with this thin layer (SI Appendix, Fig. S7), and no Li dendrites were found on the LiZr2(PO4)3 pellet after cycling the symmetric cell at different current densities for 300 h. The Raman inactive spectra of the black thin layer in Fig. 3A indicated that the thin layer was amorphous. Fig. 2D shows the surface of LiZr2(PO4)3 after cycling the Li/LiZr2(PO4)3/Li cell at 50 µA⋅cm–2 for 100 h; the particle size was much smaller than that of fresh LiZr2(PO4)3, but it still kept a dense surface structure. There were no Li metal dendrites on the Li metal surface after cycling the symmetric cell at 150 µA⋅cm–2 for 20 h (Fig. 2E and SI Appendix, Fig. S8). The lithium metal wet well the surface of the thin surface layer on the LiZr2(PO4)3 electrolyte that formed on reaction with a Li electrode.
Fig. 3.
(A) Raman spectra of the black SEI layer on the surface of LiZr2(PO4)3 after cycling the Li/LiZr2(PO4)3/Li cell for 10 h. (B) XRD pattern of LiZr2(PO4)3 after reaction with Li metal at 350 °C for 0.5 h. (C−F) XPS data of LiZr2(PO4)3 before and after reaction with Li metal at 350 °C for 0.5 h.
To explore the composition of the thin interfacial layer that formed during cycling at 80 °C, a Li metal foil on the surface of a LiZr2(PO4)3 pellet was heated from 25 °C to 350 °C (SI Appendix, Fig. S6). For temperature below 300 °C, there was no change of the LiZr2(PO4)3 pellet; a dense, black thin layer on the surface of LiZr2(PO4)3 was formed when the pellet was heated at 350 °C for 30 min. The XRD result of the pellet confirmed the decomposition of LiZr2(PO4)3 to trigonal Li8ZrO6 and hexagonal Li3P phases by the reaction
The XPS result of the black thin layer is shown in Fig. 3 C−F and SI Appendix, Fig. S9; the main peak at 284.8 eV in the C 1s spectrum corresponds to adventitious carbon, and the small peak at 286.2 eV in SI Appendix, Fig. S9 may be from the residue of the polymer glue during the polishing process. No Li2CO3 peak at 288 eV was observed; LiZr2(PO4)3 is more stable against moist air than garnet Li7La3Zr2O12, which forms a Li-ion insulating Li2CO3 layer on the particle surfaces during cooling in the firing process. The NASICON structure with strong P–O bonds usually has high stability against moist air; for example, NASICON Li1.3Al0.3Ti1.7(PO4)3 is stable in air and in water (18, 19). A Li2CO3-related peak in the C 1s spectrum was from the reaction of Li metal with organic electrolyte in the glove box. The Zr4+ 3d3/2 and 3d5/2 peaks at 185.5 eV and 183.13 eV in LiZr2(PO4)3 shifted, respectively, to 184.8 eV and 181.7 eV after reaction, which is similar to the binding energy of Zr4+ in ZrO2. After reaction, one more peak at 127.13 eV in the P 2p spectrum corresponds to P3– ions in Li3P, and the peak at 132.8 eV is from the P5+ ions in LiZr2(PO4)3. The O2– 2p peaks in LiZr2(PO4)3 shifted from 531.25 eV to 530.3 eV after reaction, which is the same as the O2– 2p binding energy in Li8ZrO6. No clear Li 1s binding energy difference was observed in LiZr2(PO4)3 before and after reaction. Li3P in the thin layer is a good Li-ion conductor, and Li8ZrO6 with a layer structure may also have some Li-ion conductivity. After the reaction of LiZr2(PO4)3 with Li metal, a Li/LiZr2(PO4)3/Au cell was assembled with Au contacting the black thin layer; the cyclic voltammogam of the cell in SI Appendix, Fig. S10 showed that the black thin layer was unstable at high voltage. The Li3P was reported to decompose at voltage above 0.7 V, which resulted in a voltage increase in the Li/LiZr2(PO4)3/Li cell at high current densities. For the application of LiZr2(PO4)3 in Li metal batteries, the thin layer only forms at the Li metal side, so the electrolyte will be stable during the charge/discharge process of an all-solid-state Li metal battery.
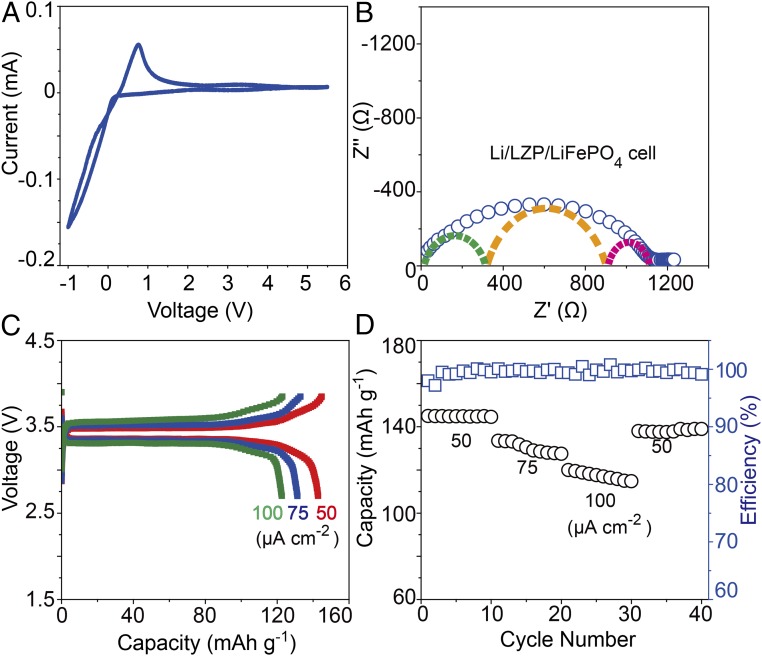
The electrochemical stability window of LiZr2(PO4)3 is shown in Fig. 4A; the two peaks near 0 V versus Li+/Li correspond to Li metal deposition and dissolution; there were no other redox peaks up to 5.5 V. An all-solid-state Li/LiZr2(PO4)3/LiFePO4 battery was fabricated with the LiFePO4 cathode embedded in a Li-ion polymer electrolyte and carbon in a loading of 2 mg⋅cm–2. The polymer membrane with a melting point of 240 °C has a Li-ion conductivity of 1 × 10−4 S⋅cm−1 and an electrochemical stability up to 4.7 V at 65 °C. The Li/LiZr2(PO4)3/LiFePO4 battery had a small interfacial resistance with LiFePO4 of about 300 Ω⋅cm−2 and a total resistance of 1,100 Ω⋅cm−2 at 80 °C (Fig. 4B), which is smaller than those of previously reported all-solid-state batteries (20). Fig. 4C shows the charge/discharge voltage profiles at current densities of 50 µA⋅cm–2, 75 µA⋅cm–2, and 100 µA⋅cm–2 at 80 °C; the cells had a discharge capacity of 140 mAh⋅g–1 and 120 mAh⋅g–1 with a cell polarization of 0.1 V and 0.2 V at 50 µA⋅cm–2 and 100 µA⋅cm–2, respectively. A high coulombic efficiency of 99.5 ± 0.5% over 40 cycles was obtained, which indicates that the Li/LiZr2(PO4)3 and LiZr2(PO4)3/LiFePO4 interfaces were stable during cycling.
Fig. 4.
(A) A cyclic voltammogram of LiZr2(PO4)3 at a scanning rate of 0.5 mV⋅s–1. (B) The impedance plots of all-solid-state Li/LiZr2(PO4)3/LiFePO4 cell. (C) Charge and discharge voltage profiles and (D) cycling performance of an Li/LiZr2(PO4)3/LiFePO4 all-solid-state battery at 80 °C with different current densities.
Conclusions
We have prepared a stable rhombohedral NASICON LiZr2(PO4)3 electrolyte at room temperature. A thin amorphous interfacial layer containing Li8ZrO6 and Li3P formed on the LiZr2(PO4)3 surface by reaction with Li metal; this layer is wet by Li metal, which suppresses Li dendrite formation. LiZr2(PO4)3 has a Li-ion conductivity of 2 ⋅ 10−4 S⋅cm−1 at 80 °C, a small interfacial resistance against Li metal and a LiFePO4 cathode, and a large electrochemical window up to 5.5 V. An all-solid-state Li metal battery with LiZr2(PO4)3 as a solid electrolyte contacting a Li metal anode showed no Li dendrite formation and good cycling performance with a Li insertion cathode embedded in a polymer catholyte.
Materials and Methods
The stoichiometric amounts of Li2CO3, (NH4)2HPO4, and different zirconium salts [Zr(AC)4, ZrOCl2, Zr(NO4)3 and ZrO2] were fired at 900 °C for 10 h, and the obtained powders were ground and fired at 1,150 °C for 20 h in a Pt crucible. The SPS pellets were obtained by firing the powders after 900 °C at 1,000 °C for 10 min with a pressure of 50 Mpa. Powder XRD was used to monitor the phase formation with a step size of 0.02°. A field emission scanning electron microscope was used to obtain the fracture surface microstructure of the pellet, and the distribution of elements was measured by energy dispersive spectroscopy. Ionic conductivity was measured from 298 K to 450 K with a Solarton Impedance Analyzer. Cyclic voltammetry was carried out on an Auto Lab workstation at a scan rate of 0.5 mV⋅s−1. The Li/LiZr2(PO4)3/Li symmetric cell was prepared by putting lithium foil on both sides of the LiZr2(PO4)3 pellet, and the cell was cycled with different current densities in a Land instrument. The LiFePO4 preparation process was the same as in our previous report (20). To prepare the cathode of an all-solid-state LiFePO4/Li cell, the active material LiFePO4 was mixed with carbon black, cross-linked polyethylene oxide, and LiTFSI (60:12:20:8 by weight) and ground in a mortar. The mixture was then dispersed in dimethylfluoride and stirred overnight. The obtained slurry was spread evenly on a carbon-coated aluminum foil to produce an electrode film with an active material loading of 2 mg⋅cm–2, which was dried at 90 °C for 12 h under vacuum. Then, 2,032 coin cells were fabricated in an argon-filled glove box with lithium foil as the anode. The cell was tested between 2.7 V and 3.8 V vs. Li+/Li in a Land instrument.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation (NSF) Grant CBET-1438007 and the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under Award DESC0005397. The SPS processing at The University of Texas at Austin was conducted with an instrument acquired with the support of NSF Award DMR-1229131. The work at Los Alamos National Laboratory was performed, in part, at the Center for Integrated Technologies, an Office of Science User Facility operated for the US Department of Energy Office of Science.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1615912113/-/DCSupplemental.
References
- 1.Tarascon JM, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414(6861):359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 2.Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010;22(3):587–603. [Google Scholar]
- 3.Goodenough JB, Park KS. The Li-ion rechargeable battery: A perspective. J Am Chem Soc. 2013;135(4):1167–1176. doi: 10.1021/ja3091438. [DOI] [PubMed] [Google Scholar]
- 4.Ellis BL, Lee KT, Nazar LF. Positive electrode materials for Li-Ion and Li-batteries. Chem Mater. 2010;22(3):691–714. [Google Scholar]
- 5.Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew Chem Int Ed Engl. 2007;46(41):7778–7781. doi: 10.1002/anie.200701144. [DOI] [PubMed] [Google Scholar]
- 6.Adachi GY, Imanaka N. Aono H fast Li+ conducting ceramic electrolytes. Adv Mater. 1996;8(2):127–135. [Google Scholar]
- 7.Catti M. First-principles modeling of lithium ordering in the LLTO (LixLa2/3-x/3TiO3) superionic conductor. Chem Mater. 2007;19(16):3963–3972. [Google Scholar]
- 8.Thangadurai V, Narayanan S, Pinzaru D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem Soc Rev. 2014;43(13):4714–4727. doi: 10.1039/c4cs00020j. [DOI] [PubMed] [Google Scholar]
- 9.Lü X, et al. Li-rich anti-perovskite Li3OCl films with enhanced ionic conductivity. Chem Commun (Camb) 2014;50(78):11520–11522. doi: 10.1039/c4cc05372a. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, et al. Fluorine-doped antiperovskite electrolyte for all-solid-state lithium-ion batteries. Angew Chem Int Ed Engl. 2016;55(34):9965–9968. doi: 10.1002/anie.201604554. [DOI] [PubMed] [Google Scholar]
- 11.Goodenough JB, Hong HYP, Kafalas JA. Fast Na+-ion transport in skeleton structures. Mater Res Bull. 1976;11(2):203–220. [Google Scholar]
- 12.Kim Y, Kim H, Park S, Seo I, Kim Y. Na ion conducting ceramic as solid electrolyte for rechargeable seawater batteries. Electrochim Acta. 2016;191:1–7. [Google Scholar]
- 13.Catti M, Comotti A, Di Blas S. High-temperature lithium mobility in α-LiZr2(PO4)3 NASICON by neutron diffraction. Chem Mater. 2003;15(8):1628–1632. [Google Scholar]
- 14.Catti M, Morgante N, Ibberson RM. Order–disorder and mobility of Li+ in the β′-and β-LiZr2(PO4)3 ionic conductors: A neutron diffraction study. J Solid State Chem. 2000;152(2):340–347. [Google Scholar]
- 15.Li Y-T, Liu M-J, Liu K, Wang C-A. High Li+ conduction in NASICON-type Li1+xYxZr2−x(PO4)3 at room temperature. J Power Sources. 2013;240:50–53. [Google Scholar]
- 16.Xie H, Goodenough JB, Li Y-T. Li1.2Zr1.9Ca0.1(PO4)3, a room-temperature Li-ion solid electrolyte. J Power Sources. 2011;196(18):7760–7762. [Google Scholar]
- 17.Li Y-T, Han J-T, Wang C-A, Xie H, Goodenough JB. Optimizing Li+ conductivity in a garnet framework. J Mater Chem. 2012;22(30):15357–15361. [Google Scholar]
- 18.Hasegawa S, et al. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass–ceramics with water. J Power Sources. 2009;189(1):371–377. [Google Scholar]
- 19.Shimonishi Y, et al. A study on lithium/air secondary batteries—Stability of the NASICON-type lithium ion conducting solid electrolyte in alkaline aqueous solutions. J Power Sources. 2011;196(11):5128–5132. [Google Scholar]
- 20.Zhou W, et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte. J Am Chem Soc. 2016;138(30):9385–9388. doi: 10.1021/jacs.6b05341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.