Significance
We explored the role of the medial temporal lobe (MTL) in autobiographical remembering and future imagining. Patients with MTL damage and controls produced narratives in four time periods (past and future). Findings showed that anterograde amnesia (forgetfulness) can interfere with narrative construction. Under conditions that minimized the effect of anterograde amnesia, patients produced as many details as controls in five different content categories (spatial and nonspatial) and in all time periods except the recent past. The results suggest that remembering the remote past and imagining the future are independent of the MTL. Comparisons among studies suggest that differences in performance likely depend on differences in the locus and extent of brain damage.
Keywords: hippocampus, remote memory, anterograde amnesia
Abstract
In two experiments, patients with damage to the medial temporal lobe (MTL) and healthy controls produced detailed autobiographical narratives as they remembered past events (recent and remote) and imagined future events (near and distant). All recent events occurred after the onset of memory impairment. The first experiment aimed to replicate the methods of Race et al. [Race E, Keane MM, Verfaellie M (2011) J Neurosci 31(28):10262–10269]. Transcripts from that study were kindly made available for independent analysis, which largely reproduced the findings from that study. Our patients produced marginally fewer episodic details than controls. Patients from the earlier study were more impaired than our patients. Patients in both groups had difficulty in returning to their narratives after going on tangents, suggesting that anterograde memory impairment may have interfered with narrative construction. In experiment 2, the experimenter used supportive questioning to help keep participants on task and reduce the burden on anterograde memory. This procedure increased the number of details produced by all participants and rescued the performance of our patients for the distant past. Neither of the two patient groups had any special difficulty in producing spatial details. The findings suggest that constructing narratives about the remote past and the future does not depend on MTL structures, except to the extent that anterograde amnesia affects performance. The results further suggest that different findings about the status of autobiographical memory likely depend on differences in the location and extent of brain damage in different patient groups.
Episodic memory affords the capacity to recollect past events that occurred at a particular time and place (2). In humans, episodic recollection allows for reexperiencing an event through a process of “mental time travel” (3). The hippocampus is known to be important for episodic memory, but its specific contribution is unclear. In one view, the hippocampus is needed for the formation and consolidation of long-term memory for a limited time after learning (4). This view finds support in reports that patients with hippocampal damage were intact at recollecting episodes from early life (and impaired only for more recent time periods) (5–7). Another view holds that episodic memories remain dependent on the hippocampus so long as they persist (8, 9). In support of this idea, patients with hippocampal damage were sometimes impaired at recollecting events from early life (1, 10). A third view follows from the suggestion that the same process that enables recollection of the past is also engaged when imagining the future (11–15). In two studies, patients with hippocampal damage were impaired at imagining new experiences or future events (1, 14, but also see ref. 6). This deficit has been proposed to be part of a broader impairment in the ability to construct spatially coherent scenes (15).
The present study explored these divergent views of hippocampal function by asking healthy controls and patients with hippocampal damage to remember past episodes (near past and distant past) and to imagine future episodes (near future and distant future). In the first experiment, we aimed to replicate the methods of Race et al. (1), where memory-impaired patients were impaired at recollecting the past and also at imagining the future. The original transcripts from the earlier study were made available to us, and we scored them with the same methods used to score our own data. In this way, it was possible to evaluate the importance of how narratives are elicited and scored. Our scoring largely reproduced the earlier findings. Our patients were marginally impaired at producing episodic details during narrative construction. The deficit was more severe in the patients from the earlier study. Both patient groups also tended to lose track of their narratives and to go on tangents. To explore the significance of this finding, in the second experiment, the experimenter helped keep participants on task during narrative construction through the frequent use of supportive questioning. With this method, the performance of our patients was intact for all time periods except the near past.
Results
Experiment 1.
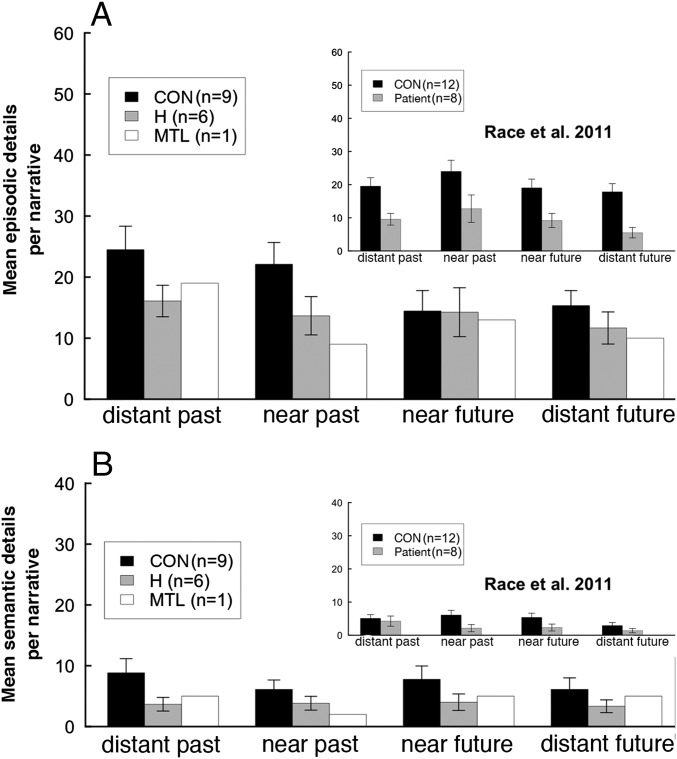
Fig. 1 shows the number of episodic and semantic details in experiment 1 as participants recalled the past and imagined the future. Repetitions, metacomments, and irrelevant details were not counted (Materials and Methods). Corresponding results from our independent analysis of data from Race et al. (1) are also illustrated. Data for episodic and semantic details in our study were analyzed using three-way, mixed-factorial ANOVA (patient vs. control, past vs. future, distant vs. near). For episodic details, the overall difference between patients and controls did not reach significance [F(1,13) = 3.3, P = 0.09]. However, relative to controls, patients had more difficulty with past time periods than with future time periods [interaction of group × temporal direction: F(1,13) = 12.5, P < 0.01]. Post hoc t tests revealed differences between patients and controls in both past time periods but not in future time periods. In addition, both groups produced more details for past time periods than for future time periods [F(1,13) = 41.5, P < 0.001]. None of the other main effects or interactions approached significance (P > 0.1). For semantic details, patients were marginally impaired overall [F(1,13) = 4.2, P = 0.06]. There were no other main effects or interactions (P > 0.1). The single patient with large medial temporal lobe (MTL) lesions performed similar to the patients with hippocampal lesions.
Fig. 1.
Number of episodic (A) and semantic (B) details (experiment 1). (Insets) Corresponding findings from our independent analysis of data from Race et al. (1). CON, control; H, patients with hippocampal lesions; MTL, a patient with large MTL lesions. Error bars show SEM.
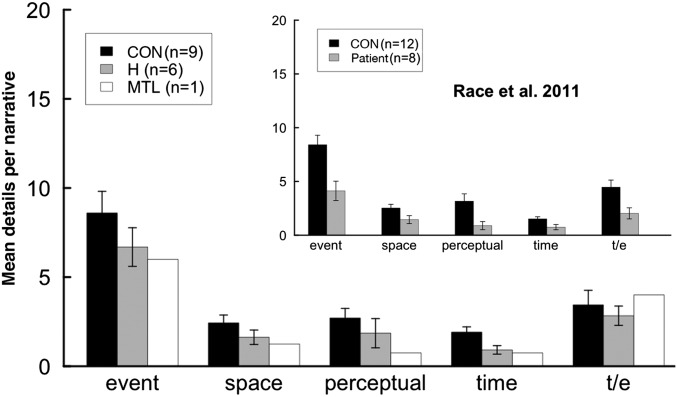
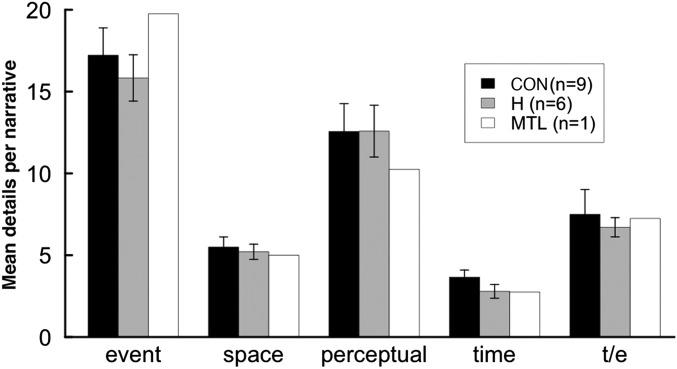
An important question is whether patients with hippocampal damage had particular difficulty in producing spatial details in their narratives in comparison to other kinds of details. Fig. 2 shows the number of details per narrative in all five categories of episodic content (Materials and Methods). The data were analyzed using a 2 × 5 mixed-factorial ANOVA (two groups, five content categories). Some categories contained more details than others [F(4,52) = 64.5, P < 0.01], but this effect was similar for the patients and controls (no interaction of group × category; P > 0.1), and there was no indication that patients had special difficulty in producing spatial details. Indeed, the largest difference between patients and controls was in time details (Cohen’s d = 1.1), and the difference in spatial details was among the smallest (Cohen’s d = 0.6). The findings were similar in the study by Race et al. (1). Although their patients were impaired at producing details in each of the five categories, the impairment in spatial details was the smallest (Cohen’s d = 0.6).
Fig. 2.
Number of episodic details per narrative, averaged across time periods (experiment 1). Details were assigned to one of five categories according to their content. (Inset) Corresponding findings from our independent analysis of data from Race et al. (1). t/e, thought/emotion. Error bars show SEM.
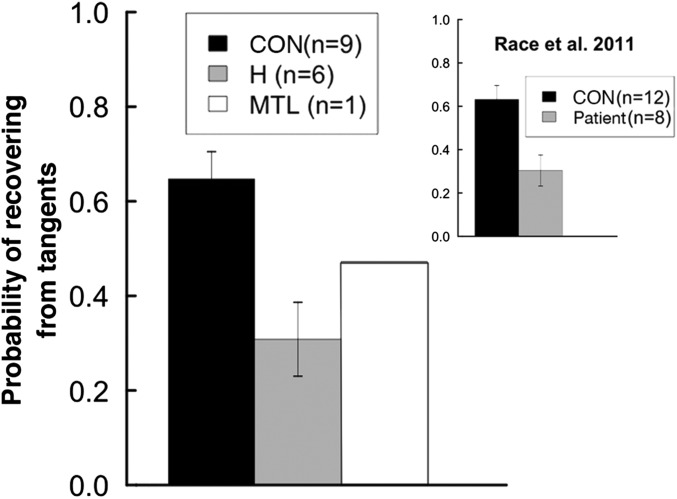
Anterograde amnesia may have contributed to task difficulty by impairing the ability to keep the organization of a narrative in mind during narrative construction. We tested for this possibility with a novel analysis by asking how often participants were able to return to the central event of their narratives after going on tangents (Fig. 3). Our patients were deficient at recovering from tangents [t(13) = 3.6, P < 0.01], and the same effect is evident in the data from Race et al. (1). Although our patients had difficulty in recovering from tangents, the frequency with which they went on tangents was similar for patients and controls (mean = 0.6 vs. 0.9 tangents per narrative; P > 0.2).
Fig. 3.
Tangents were defined as three or more consecutive details that were irrelevant to the narrative. Recovery from a tangent was defined as the production of one or more relevant episodic details following the tangent. (Inset) Corresponding findings from our independent analysis of data from Race et al. (1). Error bars show SEM.
Experiment 2.
If anterograde amnesia contributed to the impaired performance of the patients in experiment 1, then patient performance should be better in experiment 2. For experiment 2, the experimenter provided extensive support during narrative construction in the form of questioning and probing for details. This procedure helped keep participants on task as they developed their narratives.
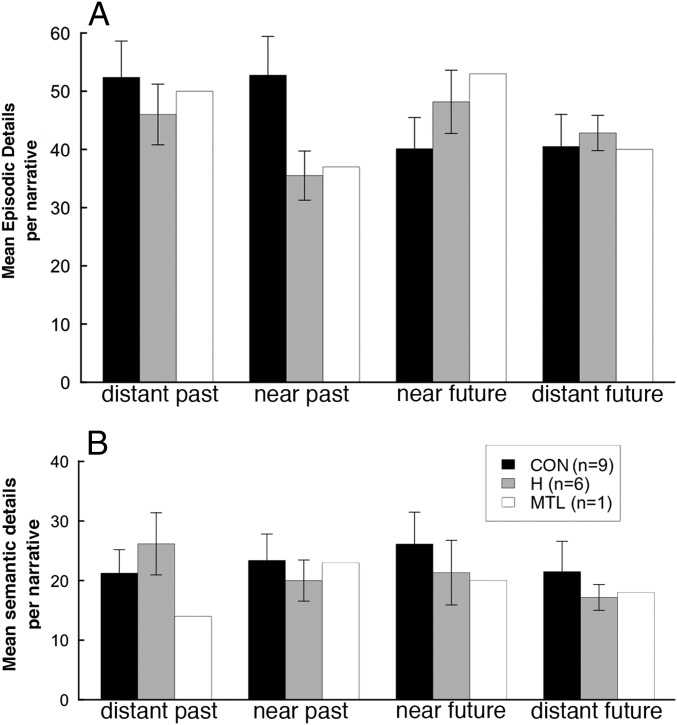
Fig. 4 shows the number of episodic and semantic details in experiment 2 as participants recalled the past and imagined the future. Participants produced many more details in experiment 2 than in experiment 1. The main finding was that patients were strikingly deficient at remembering episodic details from the near past, but they did well at remembering the distant past and imagining the future. An ANOVA (patient vs. control, past vs. future, distant vs. near) confirmed this specific deficit in the near past [interaction of group × temporal direction × temporal distance: F(1,13) = 7.6, P < 0.05]. Post hoc t tests also revealed a difference between patients and controls in the near past [t(13) = 3.2, P < 0.01] but not in other time periods (P > 0.1). Lastly, there was an interaction of group × temporal direction [F(1,13) = 33.4, P < 0.001], indicating that the patients did a little better imagining the future than remembering the past, whereas controls exhibited the opposite pattern. The patient with large MTL lesions performed like the patients with hippocampal lesions. With respect to semantic details, patients and controls performed similarly, and an ANOVA yielded no significant findings.
Fig. 4.
Number of episodic (A) and semantic (B) details (experiment 2). Error bars show SEM.
Fig. 5 shows the number of details per narrative in each of the five episodic content categories (as in experiment 1). The patients included spatial details in their narratives as frequently as controls. A 2 × 5 ANOVA (patient vs. control, five episodic content categories) yielded a main effect of content category [F(4,52) = 57.9, P < 0.001], reflecting the tendency of both groups to produce more event and perceptual details than other kinds of details. There was no group effect and no interaction. Post hoc t tests revealed no differences between patients and controls for any content category (P > 0.2).
Fig. 5.
Number of episodic details per narrative, averaged across time periods (experiment 2). Details were assigned to one of five categories according to their content. Error bars show SEM.
Although the support provided in experiment 2 successfully encouraged participants to produce long and detailed narratives, the question naturally arises as to whether these details, to any extent, reflected an influence of the experimenter on how patients and controls constructed their narratives. This possibility seems unlikely because the number of probes provided to participants was virtually identical for all time periods within each group. However, patients did receive more probes per narrative than controls did (29.6 vs. 18.3; P < 0.001). To test whether patients succeeded, in part, by incorporating information suggested by the experimenter into their narratives, we categorized all probes from the experimenter as general or specific (Materials and Methods). If patients often incorporated information suggested by the experimenter, then they should have produced more details in response to specific probes than in response to general probes. However, this pattern did not occur. An ANOVA (patient vs. control, general vs. specific) yielded only a main effect of group [F(1,13) = 19.3, P < 0.001] but no interaction. In other words, patients produced fewer details in response to each probe than did controls (2.1 vs. 3.5), but the number of details produced was about the same when the probe was general and nonspecific as when the probe was specific (even when the probe included an explicit suggestion about content). For controls, the average number of details in response to each probe was 3.7 and 3.4 for general and specific probes, respectively. For patients, these numbers were 2.2 and 2.2 for general and specific probes. Thus, although patients received more probes than controls, there was no evidence that these probes advantaged the patients by suggesting content that they could incorporate into their narratives. We suggest that the probes, whether general or specific, served mainly to keep participants engaged in the task.
Discussion
Participants were invited to construct detailed personal narratives as they remembered past events and imagined future events. We tested seven memory-impaired patients and nine controls, and also rescored transcripts kindly made available to us from an earlier, similar study (1). Our patients produced fewer episodic details from the past than controls but were intact at imagining the future. Patients from the earlier study exhibited a more severe deficit that affected all time periods (Fig. 1). In addition, both patient groups had difficulty in returning to the central event of a narrative after going on a tangent (Fig. 3), suggesting that anterograde amnesia may have affected narrative construction. To explore this possibility, in experiment, the experimenter provided participants with supportive questions to keep them on task and to reduce the burden on anterograde memory. This manipulation markedly increased the number of details produced by all participants and rescued the performance of our patients as they recollected memories from the distant past (Fig. 4). Patients were impaired only for the near past. In summary, in experiment 2, when patients with MTL damage recollected memories from the distant past and when they imagined the future, they produced narratives that contained as much detail as control narratives.
For the near past, all of the events that patients were asked about had occurred after the onset of their memory impairment. Accordingly, memory for events from the near past would not be expected to be available, with or without experimenter support. By contrast, for the distant past, memory was queried for events that occurred long before the onset of memory impairment. We suggest that without experimenter support in experiment 1, impaired anterograde memory challenged the ability to produce detailed and coherent narratives. In experiment 2, experimenter support diminished the effect of anterograde memory impairment and improved performance.
The possibility that anterograde amnesia might directly impair narrative constructions about the past or the future has been considered previously (16–19). Several observations support this idea. First, in an earlier study, patients repeated themselves more often than controls when they recalled the past (20). In another study, patient K.C. was able to distinguish true from false details about familiar fairy tales that he would have learned as a youth, but his narratives lacked detail when he tried to recount the stories himself (17). In still another study, memory-impaired patients were asked to imagine new experiences as well as to construct narratives about a picture that was presented to them (18). Descriptions of the scene were impoverished, and this impairment appeared to explain the difficulty that patients also had in imagining new experiences. Lastly, experiment 1 showed that both our patients and the patients from the earlier study (1) had difficulty in returning to their narratives after going on a tangent (Fig. 3).
Additional evidence for the impact of anterograde amnesia on narrative construction is that our patients frequently made statements discontinuous with what had been said earlier in the narrative (patients: 1.6 instances per narrative; controls: 0.2 instances per narrative; P < 0.05). For example when asked to imagine a future event, one patient described learning to play bridge with friends. However, in the midst of the narrative, the card game changed from bridge to pinochle. Another patient, while imagining the future graduation ceremony of a grandson from college, abruptly began to describe watching him in a soccer game. These and other examples suggest that due to their anterograde amnesia, patients forgot aspects of their narrative and introduced discontinuous content.
The question arises as to why impaired anterograde memory might sometimes affect the ability to recollect the past but not the ability to imagine the future (our data, experiment 1). One possibility is that anterograde memory impairment has a greater influence on narrative construction when narratives are relatively long. In our experiment 1, participant narratives about the distant past contained 46% more details than the narratives about the future. Another possibility follows from the fact that narratives about future events need not depend on any particular memory. As has been suggested (21), future imagining typically involves constructing a novel recombination of information from multiple different memories. By contrast, narratives about the past are based upon memory of an already experienced event. If one loses track of a narrative while recalling the past, one must remember what event to return to. However, if one loses track of a narrative while imagining the future, one can draw on any number of events to continue the narrative.
An analysis of narrative content indicated that impairments were similar across all five content categories (Figs. 2 and 5). Notably, neither our patients nor the patients from the earlier study (1) exhibited any special difficulty in the production of spatial details (also ref. 6). Similarly, in another study in which memory-impaired patients imagined scenes (14), the patients produced fewer details in all content categories, both spatial and nonspatial. Note, however, that in a different study from the same group, the number of spatial details was selectively reduced when patients described what might lie outside the boundaries of a photograph (15). In any case, in our patients and in the patients from the earlier study (1), there was little support for the proposal that the human hippocampus is specifically important for constructing spatially coherent mental images (15). Rather, whatever memory impairments occurred in particular time periods, there was a similar reduction in narrative content across all of the content categories that were examined.
Differences in findings among studies of narrative construction could arise for a number of reasons. One possibility is that there might be differences in the methods used to elicit narratives, including differences in experimenter style. For example, McKinlay et al. (22) reviewed the narratives from another study (14) and suggested that impaired scene construction might have as much to do with the nature of the experimenter–patient interaction as with the ability of the patients to imagine scenes. In our experiment 1, different experimenter methods seem unlikely to explain differences between our findings and the findings of Race et al. (1). First, we attempted to reproduce their methods as closely as possible. Second, experiment 1 involved minimal interaction between the experimenter and participants (only a request for more information after 3 min). Nevertheless, it is difficult to rule out altogether that some difference in experimenter behavior was important (e.g., quality of the rapport during the test sessions).
Another possibility is that there might be important differences in how narratives are scored. In our experiment 1, we evaluated the importance of scoring methods by rescoring the transcripts from Race et al. (1) and comparing our results with what was originally reported. The findings were similar, which rules out the importance of scoring methods in this case. In other cases, however, scoring methods can be an important issue. For example, in one study (14), results from an unpublished spatial coherence index suggested that patients had difficulty in constructing spatially coherent scenes. However, no indices were used to compare spatial coherence with other features of the narratives (e.g., temporal coherence). Without additional data, it is unclear that the patients had particular difficulty in generating spatial details in their narratives.
Lastly, differences in the extent and location of brain damage and in the severity of memory impairment might account for differences in the performance of different groups. Quantitative analysis of magnetic resonance images revealed that our patients (excluding G.P.) had a mean reduction in hippocampal volume of 42.5% and a mean reduction in the volume of the parahippocampal gyrus of 1.0%. In addition, two patients had damage in the basal ganglia (neither of these two patients had the worst score of the group in any time period).
In the earlier study (1), MRI data were reported for four of the eight patients. Of these four, two had damage limited to the MTL and two had damage that extended into the lateral temporal cortex (MTL+). By our scoring, the number of episodic details per narrative, averaged across time periods, was 15.5 and 6.8 for the MTL and MTL+ patients, respectively. The two MTL patients scored, on average, 1 SD below controls, and the two MTL+ patients scored, on average, 2.4 SDs below controls. These data suggest that differences in the severity of retrograde memory impairment between patient groups may arise as a result of differences in the extent of brain lesions. In particular, several studies have demonstrated that when damage extends into the lateral temporal cortex, retrograde amnesia for autobiographical memory affects both recent and remote memory (23–25). In a comprehensive review of studies finding impaired autobiographical memory (26), 54% found that the impairment extended into the remote past. When patients were excluded if they had damage beyond the MTL, 9% of studies found such an extended impairment and 91% found retrograde amnesia to be temporally graded.
In conclusion, in experiment 1, our memory-impaired patients produced marginally fewer episodic details than controls. Patients from an earlier study (1) were more impaired than our patients. Patients in both groups had difficulty in returning to their narratives after going on tangents, suggesting that anterograde memory impairment may have interfered with narrative construction. In experiment 2, the experimenter used supportive questioning to help keep participants on task. This procedure rescued the performance of our patients for all time periods except the near past. Notably, neither our patients nor patients from the earlier study (1) exhibited any special difficult in producing spatial details. These findings suggest that MTL structures, including the hippocampus, have no special role in constructing narratives, spatial or nonspatial (except narratives about the recent past), so long as anterograde amnesia does not interfere with performance. The results further suggest that conflicting findings in different patient groups about the status of autobiographical memory likely depend on differences in the locus and extent of brain damage.
Materials and Methods
Participants.
Seven memory-impaired patients participated (Table 1), six with bilateral lesions limited to the hippocampus (CA fields, dentate gyrus, and subicular complex) and one with larger MTL lesions. Patients R.S., G.W., and D.A. became amnesic in 1998, 2001, and 2011, respectively, following drug overdose and associated respiratory failure. Patient K.E. became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. Patient L.J. (the only female) became amnesic in 1988 during a 6-mo period with no known precipitating event. Her memory impairment has been stable since that time. Patient J.R.W. became amnesic in 1990 following an anoxic episode associated with cardiac arrest. Patients K.E., R.S., J.R.W., L.J., G.W., and D.A. have an average bilateral reduction in hippocampal volume of 49%, 33%, 44%, 46%, 48%, and 35%, respectively (methods are described in ref. 27). All values are more than 2.9 SDs from the control mean. On the basis of two patients (L.M. and W.H.) with similar bilateral volume loss in the hippocampus for whom detailed postmortem neurohistological information was obtained (28), the degree of volume loss in these six patients may reflect nearly complete loss of hippocampal neurons. The volume of the parahippocampal gyrus (including temporopolar, perirhinal, entorhinal, and parahippocampal cortices) is reduced by 11%, −5%, 12%, −17%, 10%, and −5% for K.E., R.S., J.R.W., L.J., G.W., and D.A., respectively (all values within 2 SDs of the control mean). These values are based on published guidelines for identifying the boundaries of the parahippocampal gyrus (29, 30). The negative values indicate instances where the volume was larger for a patient than for controls.
Table 1.
Characteristics of memory-impaired patients
| WMS-R | ||||||||
| Patient | Age, y | Education, y | WAIS-III IQ | Attention | Verbal | Visual | General | Delay |
| D.A. | 31 | 12 | 95 | 104 | 90 | 91 | 90 | 56 |
| K.E. | 73 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 77 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| R.S. | 58 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| G.W. | 55 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| J.R.W. | 51 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
| G.P. | 68 | 16 | 90 | 102 | 79 | 62 | 66 | 50 |
The Wechsler Adult Intelligence Scale (WAIS)-III and the Wechsler Memory Scale-Revised (WMS-R) yield mean scores of 100 in the normal population with an SD of 15. The WMS-R does not provide numerical scores for individuals who score below 50. Intelligence quotient (IQ) scores for patients R.S. and J.R.W. are from the WAIS-Revised. The IQ score for patient D.A. is from the WAIS-IV.
Patient G.P. has severe memory impairment resulting from viral encephalitis in 1987. This patient has demonstrated virtually no new learning since the onset of his amnesia, and during repeated testing over many weeks, he did not recognize that he had been tested before (31). Patient G.P. has an average bilateral reduction in hippocampal volume of 96%. The volume of the parahippocampal gyrus is reduced by 94%. Eight coronal magnetic resonance images from each patient, together with a detailed description of the lesions, can be found in a study by Knutson et al. (32).
Nine healthy volunteers also participated (two female, mean age = 60.8 y, mean education = 13.8 y; for patients, mean age = 59.0 y, mean education = 12.8 y). All procedures were approved by the Institutional Review Board at the University of California, San Diego, and participants gave written informed consent before participation. All participants completed both experiments 1 and 2.
Experiment 1: Future Imagining and Past Remembering Without Experimenter Support.
Procedure.
Experiment 1 intended to reproduce the methods from an earlier study (1). Participants were asked to recollect 10 specific personal events from the past (e.g., a wedding) and to imagine 10 specific personal events in the future (e.g., winning the lottery). For the past events, five were to be drawn from the past 2 y (near past) and five were to be drawn from more than 20 y ago (distant past) (>10 y ago for patient D.A.). For the future events, five were to be from the next 2 y (near future) and five were to be from more than 20 y in the future (distant future). Data were collected in two sessions. One session asked about distant future events and then near past events, and the other session asked about near future events and then distant past events. The order of the sessions was counterbalanced.
For each of 20 recollections, participants were first given a prompt and then asked to describe the event in as much detail as possible (e.g., “Please tell me about your wedding or a wedding that you attended as a young adult. Describe in as much detail as you can what this event was like. Describe where and when the event took place, who was there, how you felt, and what you were thinking.”). The 20 prompts were the same as in the study by Race et al. (1). After the prompt, participants had up to 3 min to describe the event without interruption. After 3 min, or after a natural ending point, the experimenter provided a single, general probe to elicit additional details (i.e., “Can you tell me any more about where and when the event took place, who was there, how you felt, and what you were thinking?”). Following the general probe, participants were given an additional 3 min, again without interruption.
Transcripts from an earlier study.
We obtained transcripts of the narratives collected by Race et al. (1) and independently analyzed them. The analyses described next were carried out for both these data and our own data.
Narrative scoring.
Narratives were first partitioned into details, as has been done previously (1, 14, 20, 33). Following Race et al. (1), each detail was then scored as episodic memory, semantic memory, repetition, or metacomment. Episodic details described aspects of specific events. Semantic details described facts that contextualized events. Repetitions were details that repeated information from earlier in the narrative. Metacomments were details that referred to the task itself (e.g., “It’s difficult to remember that”), and were not analyzed further.
Next, each episodic detail was categorized according to its content: event, spatial, time, perceptual, or thought/emotion. Event details described persons or actions. Spatial details described places or spatial relationships between objects or persons. Time details described specific temporal information about an event. Perceptual details described objects, colors, weather, or other sensory information. Thought/emotion details described introspective commentary or internal states. Each semantic detail was also categorized according to its content: general, personal, place, and time. General details described widely known facts. Personal details described facts particular to the participant. Place details described facts about locations. Time details described the broad time period in which events occurred.
Lastly, each detail was scored (range: 1–4) for relevance to the central theme of the narrative (1 = highly relevant, 4 = irrelevant). Relevance ratings made it possible to analyze the effect of tangents on narrative construction. Tangents occur in narratives when the narrative moves off topic from the central event being described. To return to the central event of a narrative after going on a tangent, participants must remember what the central event of the narrative was. Thus, memory-impaired patients might be expected to return from a tangent to the central event less frequently than controls. Tangents were defined as the production of three or more consecutive details that were irrelevant to the central event of the narrative (relevance rating of 4). A participant was said to have returned from a tangent to the central event of the narrative if, following a tangent, he/she produced one or more relevant episodic details before either completing the narrative or receiving the probe from the experimenter.
One of the authors (A.J.O.D.) was the primary rater for both scoring methods (detail content and relevance). Interrater reliability was assessed for each rating method with a second rater blinded to group membership. For the content category ratings, the second rater scored a randomly selected 20% of the data from the present study (four narratives from each participant for a total of 64 narratives). For the relevance ratings, a different second rater, also blinded to group membership, scored a randomly selected 20% of the data from both studies (four narratives from each participant for a total of 140 narratives). Across participants and content ratings, the correlation between raters was 0.73 and Cronbach’s α was 0.84. For the relevance scores, the correlation between raters was 0.93 and Cronbach’s α was 0.96. For the narratives of the study by Race et al. (1), A.J.O.D. was blinded to group membership during rating and served as the only rater for the content ratings.
Experiment 2: Future Imagining and Past Remembering with Experimenter Support.
Procedure.
Experiments 1 and 2 were separated by at least 1 y. The procedure was the same as in experiment 1, with one key difference. In experiment 2, the experimenter provided support during narrative construction in the form of extensive probing for detail (5). New prompts were used to elicit the narratives, and participants could speak for up to 5 min. The probes offered by the experimenter were both general and specific. General probes simply asked for more detail and did not direct the participant in any way. Specific probes oriented participants to types of content (e.g., “What time of day was it?”, “How far away will he be from you?”). Specific probes sometimes suggested possible content (e.g., “Was it evening?”). Whereas probing in experiment 1 (and in ref. 1) was limited to a single general probe, in experiment 2, the experimenter used as many probes as needed to keep the participant on task for 5 min per narrative.
Narrative scoring.
Narratives were transcribed and partitioned into episodic and semantic details and then categorized by content as described for experiment 1. A.J.O.D. served as the primary rater. Interrater reliability was assessed using a blinded second rater who did not participate in experiment 1. The second rater rated a randomly selected 20% of the data (four events from each participant for a total of 64 events). Across participants and content ratings, the correlation between raters was 0.74 and Cronbach’s α was 0.85.
Acknowledgments
We thank Jennifer Frascino, Ryan Ward, Reina Mizrahi, Katherine Ahn, Christine Smith, Meilinne Hancock, and Soyun Kim for assistance and Elizabeth Race and Mieke Verfaellie for sharing their data. This work was supported by the Medical Research Service of the Department of Veterans Affairs CX-000359-05A1, and National Institute of Mental Health Grant 24600. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of interest statement: In 2013, Dr. Kirwan kindly administered some tests to two patients he had access to. Subsequently, he was a middle author on the resulting PNAS paper [Smith CN, et al. (2014) When recognition memory is independent of hippocampal function. Proc Natl Acad Sci 111(27):9935–9940]. The authors do not regard this as a conflict of interest, as Dr. Kirwan had no part in the planning, interpretation, or writing, and did not participate in any discussions about the project.
References
- 1.Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31(28):10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. Oxford Univ Press; New York: 1983. [Google Scholar]
- 3.Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Kirwan CB, Bayley PJ, Galván VV, Squire LR. Detailed recollection of remote autobiographical memory after damage to the medial temporal lobe. Proc Natl Acad Sci USA. 2008;105(7):2676–2680. doi: 10.1073/pnas.0712155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107(44):19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopelman MD, Bright P. On remembering and forgetting our autobiographical pasts: Retrograde amnesia and Andrew Mayes’s contribution to neuropsychological method. Neuropsychologia. 2012;50(13):2961–2972. doi: 10.1016/j.neuropsychologia.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17(5):766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum RS, et al. Patterns of autobiographical memory loss in medial-temporal lobe amnesic patients. J Cogn Neurosci. 2008;20(8):1490–1506. doi: 10.1162/jocn.2008.20105. [DOI] [PubMed] [Google Scholar]
- 11.Tulving E. Memory and consciousness. Can Psychol. 1985;26(1):1–12. [Google Scholar]
- 12.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 13.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullally SL, Intraub H, Maguire EA. Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr Biol. 2012;22(4):261–268. doi: 10.1016/j.cub.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zola-Morgan S, Cohen NJ, Squire LR. Recall of remote episodic memory in amnesia. Neuropsychologia. 1983;21(5):487–500. doi: 10.1016/0028-3932(83)90005-2. [DOI] [PubMed] [Google Scholar]
- 17.Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47(11):2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Zeman AZJ, Beschin N, Dewar M, Della Sala S. Imagining the present: Amnesia may impair descriptions of the present as well as of the future and the past. Cortex. 2013;49(3):637–645. doi: 10.1016/j.cortex.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Caspari I, Parkinson SR. Effects of memory impairment on discourse. J Neurolinguist. 2000;13:15–36. [Google Scholar]
- 20.Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38(1):135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 21.Addis DR, Schacter DL. The hippocampus and imagining the future: Where do we stand? Front Hum Neurosci. 2012;5(173):173. doi: 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinlay A, McVittie C, Della Sala S. Imaging the future: Does a qualitative analysis add to the picture? J Neuropsychol. 2010;4(Pt 1):1–13. doi: 10.1348/174866409X468395. [DOI] [PubMed] [Google Scholar]
- 23.Bright P, et al. Retrograde amnesia in patients with hippocampal, medial temporal, temporal lobe, or frontal pathology. Learn Mem. 2006;13(5):545–557. doi: 10.1101/lm.265906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayley PJ, Gold JJ, Hopkins RO, Squire LR. The neuroanatomy of remote memory. Neuron. 2005;46(5):799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayley PJ, Hopkins RO, Squire LR. The fate of old memories after medial temporal lobe damage. J Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lah S, Miller L. Effects of temporal lobe lesions on retrograde memory: A critical review. Neuropsychol Rev. 2008;18(1):24–52. doi: 10.1007/s11065-008-9053-2. [DOI] [PubMed] [Google Scholar]
- 27.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Insausti R, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- 30.Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2014;35(1):248–256. doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436(7050):550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutson AR, Hopkins RO, Squire LR. A pencil rescues impaired performance on a visual discrimination task in patients with medial temporal lobe lesions. Learn Mem. 2013;20(11):607–610. doi: 10.1101/lm.032490.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]