Significance
C-14 dating methods can be used to determine the time of death of wildlife products. We evaluate poaching patterns of elephants in Africa by using 14C to determine lag time between elephant death and recovery of ivory by law enforcement officials. Most ivory in recent seizures has lag times of less than 3 y. Lag times for ivory originating in East Africa are shorter, on average, than the lag times for ivory originating in the Tridom region (Cameroon–Gabon–Congo). The 14C data show little or no evidence that large-scale ivory shipments contained ivory stockpiled over long time periods. Little, if any, “old” ivory (i.e., >10 y) seems to contribute to large ivory shipments.
Keywords: wildlife, forensics, isotopes, Africa, genetics
Abstract
Carbon-14 measurements on 231 elephant ivory specimens from 14 large ivory seizures (≥0.5 ton) made between 2002 and 2014 show that most ivory (ca. 90%) was derived from animals that had died less than 3 y before ivory was confiscated. This indicates that the assumption of recent elephant death for mortality estimates of African elephants is correct: Very little “old” ivory is included in large ivory shipments from Africa. We found only one specimen of the 231 analyzed to have a lag time longer than 6 y. Patterns of trade differ by regions: East African ivory, based on genetic assignments of geographic origin, has a much higher fraction of “rapid” transit than ivory originating in the Tridom region of Cameroon–Gabon–Congo. Carbon-14 is an important tool in understanding patterns of movement of illegal wildlife products.
The illegal trade in elephant ivory has increased significantly in the past decade (1, 2), with studies estimating the current rate of decline of regional African elephant populations to be as high as 8%, primarily due to poaching (3, 4). Central African forest elephant populations decreased by ca. 62% from 2002 to 2011 (5). Forest elephants are particularly vulnerable to poaching because of their slow population growth rates compared with their savanna counterparts (6). Savanna elephants have also experienced massive population declines, particularly in Tanzania and northern Mozambique. The savanna elephant population in the Selous Wildlife Reserve in Tanzania saw a 66% decline from 2009 to 2013 (7). The rapid decline in elephants across Africa has been attributed to the high poaching rates and increased amount of ivory seized over the last decade or so (3). Total global seizures in excess of 40 tons of ivory have occurred in several years since 2010 (8), with over 70% of all ivory seizures exceeding 0.5 ton (hereafter termed “large seizures” or “large ivory seizures”).
We use “bomb 14C” to determine ages of ivory from 14 different large seizures intercepted by law enforcement officials between 2002 and 2014. These ages represent the date of death of those elephants whose tusks were sampled. This study answers several questions including whether “old” ivory—such as tusks from government stockpiles—is being incorporated into the illegal ivory stream, what the lag time is between animal death and seizure of ivory by law enforcement officials, and whether there are significant differences between the age of ivory originating in different parts of Africa.
We first establish a 14C-calibration relationship for animal tissues from Africa by using elephant hair of known age. We then report 231 14C-calibrated ages of seized ivory and discuss how these ages influence our understanding of the illegal ivory trade in Africa. We use specimens previously studied by Wasser et al. (2), who assigned ivory to its general location of origin using DNA assignment methods.
Results
Calibration Curve.
Carbon-14 is a naturally occurring isotope produced by cosmic radiation in the upper atmosphere. Aboveground nuclear weapons testing, primarily in the early 1960s, nearly doubled the concentration of 14C from the natural abundance level (Fig. 1B). Since the atmospheric concentration “spike” of 14C reached at that time, the 14C/12C ratio has been steadily declining from peak values of ca. 1.95 and ca. 1.8 F14C [fraction modern carbon (9)] in the Northern and Southern Hemispheres (NH and SH), respectively, to the current value of < 1.03; this “bomb-curve” record is well-documented (10–16). The overall higher concentration and peak value of atmospheric 14C in the NH was due to higher amounts of testing in the NH compared with the SH. Zonal heterogeneity in concentrations was observed in each hemisphere in the 1960s and early 1970s and, as a result, multiple calibration curves (e.g., NH1, NH2, and NH3 and SH1–2 and SH3) were established for each hemisphere (10–12). Atmospheric 14C is present primarily as 14CO2 and the mixing time of CO2 in the atmosphere is ∼10 y. Thus, by about 1973, the bomb pulse of 14CO2 was essentially mixed and zonal differences were insignificant; the NH and SH have been each treated as a single zone since 1973 (12).
Fig. 1.
(A) Relationship between F14C and date of collection for elephant hair and for the data used in NH3 and SH3 calibration curves (6). The elephant hair calibration curve is used to calculate date of death from 2001 to 2014. (B) The complete “bomb curve” from 1950 to the present for NH3 and SH3. We note that after 1973 all NH zones are treated as a single zone, and all SH zones are treated as a single zone.
However, differences between the NH and SH persist today due in part to the Suess effect, the dilution of 14C in the atmosphere by “dead carbon”—fossil fuels have no radiocarbon and combustion of those fuels dilutes the modern atmosphere with carbon (as CO2) with an F14C value of 0.0. Most of the dead carbon is produced in the NH and mixes across the equator with the SH in the Inter-Tropical Convergence Zone (ITCZ, shown in Fig. S1C); since 1990 this has been the principal driver for differences in F14C between the NH and SH (10–12). The natural exchange of 14CO2 between the ocean and atmosphere also contributes to the F14C gradient observed between the NH and SH. Atmospheric samples collected near the equator should have a time–F14C relationship between that of the NH and SH due to mixing across the ITCZ.
Fig. S1.
(A) Map of Africa showing location assignments of specimens (from ref. 2) with measured F14C on ivory from the pulp cavity surface. (B) Map of Africa showing the locations of samples of elephant hair used for calibration of F14C for elephant tissues. (C) Global map showing locations of “clean-air” sites used for calibration of the NH zones (NH1, NH2, and NH3) and SH zones (SH1–2 and SH3) for the period from 2000 to 2013; NH3 and SH3 are bounded by the northern and southern limits of the Inter-Tropical Convergence Zone (following ref. 12 for NH and SH boundaries).
Atmospheric 14C (as 14CO2) enters the terrestrial biosphere by photosynthesis; 14C is subsequently incorporated into herbivore tissues as the animals ingest plants for food. The bomb-14C signal has been used in human forensic studies (17–21) and has also been used previously to date ivory and other animal tissues including hair, horn, and tooth enamel (22–25). Prior work on 14C dating of ivory has used the atmospheric 14C/12C historical record to determine the calibrated age of samples. Both Vogel et al. (23) and Uno et al. (25) note that the 14C/12C atmospheric record gives ivory ages that are, in general, 0–2 y earlier than the known age of the sample. This age mismatch likely results from several processes: (i) differing 14C/12C ratios between the NH and SH compounded by the lack of atmospheric 14CO2 measurements in the mixing zone between the hemispheres (i.e., equatorial Africa), (ii) the remobilization of nonstructural carbon during plant growth, (iii) the time lag between C fixation by plants and ingestion of plants by an elephant, and (iv) recycling of proteins in mammals. Each of these is discussed below.
(i) Differing 14C/12C ratios between hemispheres: Fig. 1A shows that the “clean-air” sites of the NH and SH have different values for F14C at any given time. Fig. S1C shows that the clean-air sites used to determine F14C of NH and SH are far from the African continent. (ii) Remobilization of nonstructural carbon during plant growth: Muhr et al. (26) have shown that nonstructural carbon in perennial tissues (e.g., branches, stems, and roots) can be mobilized and used for growth several years after fixation and thus F14C of dietary foodstuff may lag the F14C of the atmosphere; because elephants have a high component of perennial plants in their diets, this effect could be important in understanding any offset between atmospheric F14C and newly formed tissue F14C in herbivores. Ehleringer et al. (27) showed a significant range in F14C for plants collected at a single time from a single region. (iii) Time lag between C fixation by plants and ingestion of plants by an elephant: If carbon in the food that animals eat has been fixed previously, there is likely to be an offset of months or years between the date of ingestion and the date of carbon fixation. This is particularly significant in elephants, because they ingest large quantities of bark and wood, which—if even a small fraction of this carbon is digested and fixed in animal tissues—would skew the 14C-calibrated age to an earlier date. (iv) Recycling of proteins in mammals: Ayliffe et al. (28) showed that newly formed proteinaceous animal tissues (e.g., collagen and keratin) are composed of about 45% carbon with a turnover time (half-life) of ∼140 d; thus, proteins should have an “average age” skewed several months before tissue formation.
The combination of these processes may affect the accuracy of age-dating ivory when using the standard NH or SH zonal calibration curves over the past ca. 15 y where the slope of the bomb curve is relatively shallow. Therefore, we analyzed 14 elephant hairs with known collection dates between 2001 and 2013 (Methods, Table S1, and Fig. S1B) to develop a calibration curve specific to elephant tissues. Fig. 1 shows the elephant hair relationship for F14C versus date and also the data for the same time interval used to calculate the NH and SH regions NH3 and SH3, respectively, as reported in ref. 16. Dates were assigned to hairs using the segment length collected for measurement and assuming a growth rate of 0.8 mm/d. We use a linear regression for the period 2001–2013 to derive the relationship F14C = [−0.0049928 × year + 11.09197] (r2 = 0.988); we note that this linear relationship should not be used for dates before 1995 (Fig. 1B). We assume that the incorporation of 14C from diet into proteinaceous tissue is the same for elephant hair keratin as it is for elephant ivory collagen and thus use the calibration curve to determine the date of death of the elephant from which each seized tusk originated. If a calibration curve can be generated from ivory of known age—and differs significantly from the calibration curve generated from elephant hair—dates can be recalculated using such new data. All of the calibration samples, and the assigned locations of ivory, fall in (or very near to) the mixing zone across the ITCZ, which separate the NH and SH zones where the “clean-air” sites are located. Future calibrations for ivory, or for other wildlife products, to obtain “age” determinations using F14C measurements should focus on obtaining samples near regions of interest; such calibrations will further improve the age estimates of this and other studies.
Table S1.
Carbon-14 data for calibration curve from elephant hair collected on known dates
| Sample ID | Date | F14C | ||||||||
| Field ID | Latitude | Longitude | IsoForensics ID | UCIAMS no. | Julian | Decimal | Tissue | F14C | (1 σ) | Average |
| STE-010709-Thoreau | 0.6 | 37.5 | ivory_214 | 171243 | 09.Aug.2001 | 2001.521 | 2001.482 | 1.1012 | 0.0018 | |
| STE-010709-Thoreau | 0.6 | 37.5 | ivory_214 | 171244 | 09.Aug.2001 | 2001.521 | 2001.482 | 1.1017 | 0.0016 | 1.1014 |
| STE-021030–148:490 | 0.0 | 38.3 | ivory_216 | 173827 | 30.Oct.2002 | 2002.830 | 2002.792 | 1.0930 | 0.0016 | 1.0930 |
| STE-030207-Loidaiga | 0.2 | 37.4 | ivory_217 | 173828 | 07.Feb.2003 | 2003.104 | 2003.066 | 1.0874 | 0.0016 | 1.0874 |
| STE-040722-Anastasia | 0.6 | 37.5 | ivory_218 | 173829 | 22.Jul.2004 | 2004.557 | 2004.519 | 1.0842 | 0.0017 | 1.0842 |
| KWS-050828–2 Shimba | −4.3 | 39.4 | ivory_219 | 171245 | 26.Aug.2005 | 2005.652 | 2005.614 | 1.0776 | 0.0016 | 1.0776 |
| STE-060419-Grace | 0.6 | 37.5 | ivory_220 | 173830 | 19.Apr.2006 | 2006.299 | 2006.260 | 1.0773 | 0.0016 | 1.0773 |
| STE-070425-Sora | 2.3 | 38.0 | ivory_221 | 173831 | 25.Apr.2007 | 2007.315 | 2007.277 | 1.0678 | 0.0016 | 1.0678 |
| STE-080613-Shadrack | 2.3 | 38.0 | ivory_224 | 173833 | 13.Jun.2008 | 2008.451 | 2008.412 | 1.0637 | 0.0016 | 1.0637 |
| STE-100221-Anabelle | 0.6 | 37.5 | ivory_226 | 173834 | 21.Feb.2010 | 2010.142 | 2010.104 | 1.0556 | 0.0016 | 1.0556 |
| STE-100807-Kisima | 0.1 | 37.6 | ivory_227 | 173836 | 07.Aug.2010 | 2010.600 | 2010.562 | 1.0551 | 0.0016 | 1.0551 |
| KWS-LFN-862 | −1.6 | 35.2 | ivory_228 | 173837 | 04.Feb.2011 | 2011.096 | 2011.058 | 1.0539 | 0.0018 | 1.0539 |
| KWS-LFN-853 | 0.5 | 36.6 | ivory_230 | 171247 | 11.Jan.2012 | 2012.030 | 2011.992 | 1.0442 | 0.0016 | |
| KWS-LFN-853 | 0.5 | 36.6 | ivory_230 | 171248 | 11.Jan.2012 | 2012.030 | 2011.992 | 1.0443 | 0.0016 | 1.0443 |
| KWS-LFN-847 | −3.4 | 38.0 | ivory_231 | 173838 | 25.Mar.2012 | 2012.232 | 2012.194 | 1.0428 | 0.0015 | 1.0428 |
| STE-131030-Mtn Bull | 0.3 | 36.8 | ivory_232 | 173839 | 30.Oct.2013 | 2013.830 | 2013.792 | 1.0395 | 0.0015 | 1.0395 |
Date (tissue) is the estimated age for the integrated sample used for F14C measurement (samples were from 10 to 12 mm from proximal end of hair; assume 0.8 mm/d growth). Data from elephant hair samples used for calibration to establish the relationship between F14C and year of tissue growth for African elephants.
Ivory for date-of-death determination was sampled from the pulp cavity of tusks (the proximal end of the tusk; Fig. 2 and Fig. S2), which is where new ivory (dentine) was forming when the elephant died and thus most accurately records the date of death.
Fig. 2.
Example of sample from pulp cavity section of specimen SGP-1-2. Arrows indicate the locations of the pulp cavity surface, a sampled area (∼2 mm total depth from the pulp cavity surface), outer dentine, and the cementum layer deposited on the outside of the tusk.
Fig. S2.
Schematic of elephant ivory showing longitudinal cross-section of a tusk with pulp cavity, dentine, and cementum. Samples (as shown in Fig. 2) were taken from the proximal end to include the pulp cavity, where the thickness from pulp cavity to the outer cementum sheath was less than 2 cm. Modified from Fisher and Fox (32) with permission from University Press of Colorado.
Radial Growth Rates of Tusks.
For a limited set of tusks we sampled the innermost dentine and also the outermost dentine (Fig. 2 and Fig. S2); in a few cases we also sampled the cementum, which forms outside the outermost dentine. The bomb curve has a “rising” and a “falling” limb: rapidly increasing in the late 1950s, peaking in the early 1960s with the crest in the SH occurring after that in the NH, then gradually diminishing to the current F14C value of ca. 1.02 (Fig. 1B). Thus, an age assignment using F14C of any postbomb ivory has three solutions, one entirely on the rising limb of the bomb curve (i.e., between ca. 1955 and 1965), one with the outer dentine on the rising limb (between 1955 and 1965) and the inner dentine on the falling limb (between 1965 and the present), and one entirely on the falling limb (both after 1965). For ivory entirely on the rising limb, the innermost dentine should have a higher F14C value than the outermost dentine; data from Table S2 show that this is not the case. For the second case, the outermost dentine would have formed between 1955 and 1965 and the innermost dentine would have formed in the last decade, giving unreasonably slow growth rates for ivory (ca. 1 cm thickening per 50 y). Our samples fall in the third case, where both the innermost and outermost dentine F14C values are on the falling limb, with the innermost dentine having lower F14C values than the outermost dentine.
Table S2.
Radial tusk growth rates calculated from multiple carbon-14 analyses of ivory
| Sample | UCIAMS no. | Distance, mm | F14C | 1 σ | Year | Rate, mm/y |
| S7.033_id | 167289 | 0.5 | 1.0574 | 0.0016 | 2009.8 | |
| S7.033_od | 167292 | 14.3 | 1.0671 | 0.0016 | 2007.9 | 7.2 |
| LAC3136_id | 169673 | 0.5 | 1.1007 | 0.0017 | 2001.2 | |
| LAC3136_od | 169675 | 16.0 | 1.1118 | 0.0018 | 2000.6 | 7.0 |
| SGP01.1_id | 169651 | 0.5 | 1.0477 | 0.0016 | 2011.7 | |
| SGP01.1_od | 169650 | 10.8 | 1.0513 | 0.0016 | 2011.0 | 14.4 |
| SGP19.2_id | 169664 | 0.5 | 1.0461 | 0.0017 | 2012.0 | |
| SGP19.2_od | 169663 | 13.0 | 1.0560 | 0.0016 | 2010.1 | 6.4 |
| TOGI037_id | 170313 | 0.5 | 1.0435 | 0.0020 | 2012.5 | |
| TOGI037_od | 170312 | 13.0 | 1.0514 | 0.0016 | 2011.0 | 8.0 |
| HKN159_id | 170292 | 0.5 | 1.0416 | 0.0019 | 2012.9 | |
| HKN159_od | 170291 | 11.5 | 1.0491 | 0.0016 | 2011.4 | 7.3 |
“id” and “od” represent the innermost and outermost dentine, respectively. F14C data of selected ivory samples with thickness >10 mm for innermost (along pulp cavity) and outermost (inside of cementum) dentine. Age of ivory formation calculated using the elephant hair calibration curve (Fig. 1; Table S1).
All tusks with multiple samples are on the falling limb of the bomb curve, and we thus assume that all other specimens analyzed in this study are on the falling limb. We use the tusks with multiple samples to determine the rate of radial growth of tusks. Radial growth rates were about 7 mm/y for both savanna and forest elephants, ranging from ca. 6–14 mm/y (complete results are presented in Table S2 and Fig. S3). Uno et al. (25) measured longitudinal growth rates of ca. 5 cm/y for two female African elephants, which correspond to radial growth rates of about 5 mm/y based on a longitudinal/radial growth rate ratio of about 10:1.
Fig. S3.
F14C and thickness relationships for innermost and outmost dentine measured for six elephant tusks in this study. Sample thicknesses were between 1 and 2.5 mm; absolute distances between innermost and outermost samples ranged from 10 to 16 mm.
Date-of-Death Determinations of Seized Ivory.
The F14C values and calculated date of death of 231 ivory specimens (tusks) from 14 large seizures from 2002 to 2014 are summarized in Table 1; complete data are presented in Dataset S1. Details for 12 of the seizures are given in Wasser et al. (2). Two additional seizures were included in our study and were not available at the time of the Wasser et al. (2) study.
Table 1.
Lag time in months for ivory samples from 14 different seizures, grouped by region of origin, as determined by 14C dating
| Name | Abbreviation | Date of seizure | Region of origin | N | Mean | ±1 σ | Mimimum | Maximum | Median |
| Avocado | AVO | August 22, 2010 | East Africa | 14 | 6 | 9 | −10 | 15 | 10 |
| HKTA | HKTA | October 17, 2012 | East Africa | 11 | 19 | 7 | 11 | 37 | 18 |
| HKTB | HKTB | October 17, 2012 | East Africa | 14 | 14 | 6 | 6 | 21 | 12 |
| Hong Kong-Kenya | HKK | January 26, 2013 | East Africa | 10 | 14 | 9 | 1 | 27 | 13 |
| Hong Kong-Nigeria | HKN | August 7, 2013 | Tridom | 11 | 21 | 7 | 8 | 30 | 23 |
| Hong Kong-Togo | HKI | July 18, 2013 | Tridom | 10 | 39 | 11 | 25 | 62 | 38 |
| Malawi | M | May 23, 2013 | East Africa | 11 | 20 | 9 | 5 | 35 | 21 |
| Malaysia* | MYS | December 11, 2012 | East Africa | 39 | 23 | 7 | 4 | 39 | 24 |
| Malaysia* | MYS | December 11, 2012 | Tridom | 28 | 35 | 40 | 5 | 231 | 27 |
| Malaysia* | MYS | December 11, 2012 | West Africa | 13 | 19 | 15 | 5 | 67 | 15 |
| Philippines7 | S7 | June 9, 2009 | East Africa | 14 | 6 | 6 | −4 | 18 | 6 |
| Pili | PIL | May 6, 2011 | East Africa | 10 | 18 | 8 | 3 | 31 | 20 |
| Singapore 2002 | LAC | June 28, 2002 | Zambia | 8 | 15 | 6 | 6 | 25 | 14 |
| Singapore 2014 | SGP | March 27, 2014 | East Africa | 10 | 28 | 7 | 20 | 44 | 27 |
| Taiwan1 | 53- | July 6, 2006 | East Africa | 12 | 15 | 20 | 1 | 69 | 8 |
| Togo | TOGI | January 29, 2014 | Tridom | 16 | 25 | 11 | 9 | 52 | 22 |
The Malaysia 2012 seizure had ivory assigned to three different geographic origins: East Africa, Tridom, and West Africa. Results from these different assignments are listed separately.
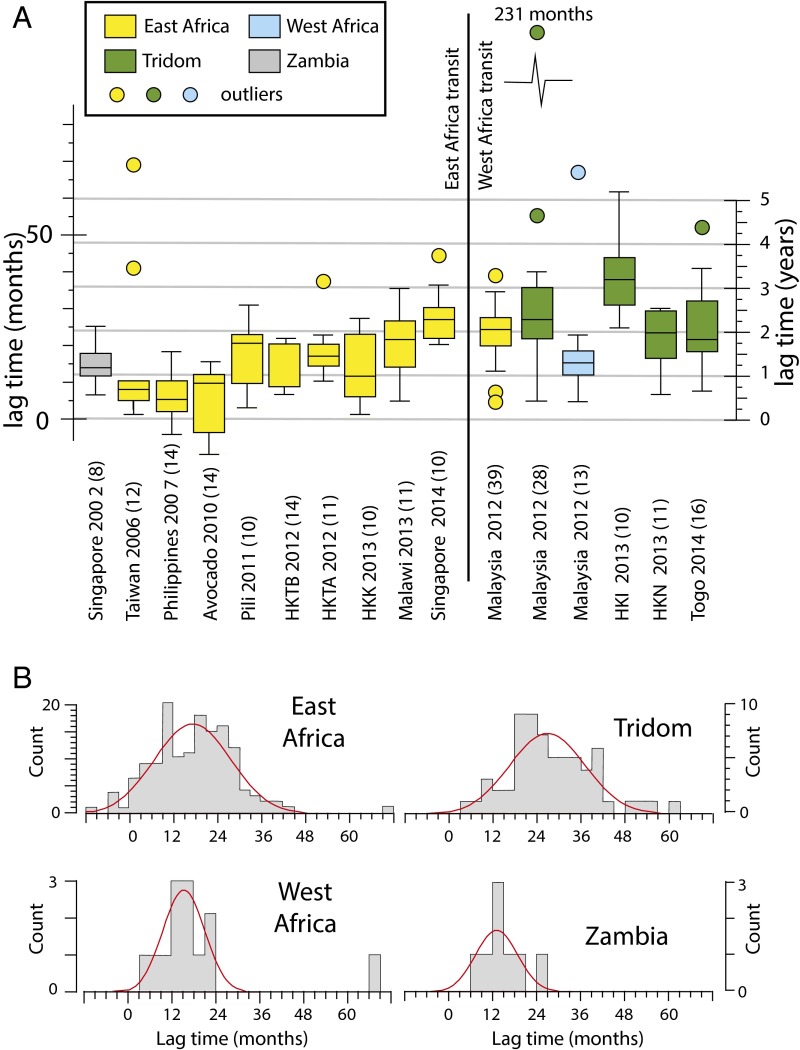
Ivory specimens are discussed in the context of lag time, which is the time between when the animals died as determined by F14C using the calibration discussed above and the time of seizure by law enforcement officials. The lag times for each seizure are shown in Fig. 3, where they are grouped by geographic origin based on DNA analysis (2). Uncertainty for the calculated lag time, defined here as the expanded uncertainty (U), incorporates uncertainty in the calibration curve (Fig. 1B) and measurement reproducibility (Dataset S1 and Methods).
Fig. 3.
(A) Box and whisker diagram for median lag times (difference between date of death and seizure date) of ivory specimens analyzed in this study; the number of specimens with F14C measurements for each grouping is given in parentheses. (B) Histograms showing lag times for specimens from East Africa, Tridom, West Africa, and Zambia. Red lines are the normal distributions for each regions fit to the data (Methods).
Wasser et al. (2) assigned individual ivory specimens to different geographic regions based on genetic data (Fig. S1A). Those assignments have an estimated accuracy of approximately ±300 km for both latitude and longitude. Based on those assignments, we group specimens into four distinct regions of origin: East Africa (southern Kenya to northern Mozambique), the Tridom (Cameroon, Republic of Congo, and Gabon, but also including Southwest Central African Republic), West Africa, and Zambia. The earliest and largest seizure analyzed (6.5 tons seized in Singapore in 2002) seemed to have primarily a Zambian origin. A seizure from Malaysia (December 2012), the second-largest seizure on record (6 tons), had specimens with assigned origins in West Africa, East Africa, and the Tridom; tusks from different regions within that Malaysian seizure will be discussed in a regional context along with other specimens from those respective regions.
Very Old Ivory in Seizures.
Of the 231 individual ivory specimens studied, only one specimen had a lag time greater than 6 y (72 mo) (Fig. 3); this specimen was from the Tridom region and had an estimated lag time of 231 mo (>19 y; uncertainty range is 211–252 mo). Only three other specimens had lag times greater than 5 y (60 mo). In contrast, we would expect ivory originating from long-term stockpiles to date from the present to 1989 or earlier (i.e., with possible lag times of 27 y or greater), coinciding with the international trade ban; such ivory would have a broad distribution of lag times that should range from near zero to tens of years. Thus, it is unlikely that “old” ivory from government or other stockpiles was present in the large ivory seizures we analyzed.
Average Lag Times and Distributions of Ivory Age in Seizures.
We consider the lag time for three transit periods: rapid (<12 mo), intermediate (12–24 mo), and slow (>24 mo). Only seizures of ivory originating in East Africa have rapid transit times; 5 of the 10 East African groups had 50% or more of specimens with lag times in the “rapid” category. No seizures from other regions had more than 25% of tested specimens in the rapid transit category (Fig. 4). In contrast, seizures originating in the Tridom were dominated by specimens with intermediate to slow transit times (Fig. 4). Overall, the rapid:intermediate:slow (R:I:S) pattern for lag times in East Africa is 35:41:24 (n = 145), whereas R:I:S is 8:35:57 (n = 65) for the Tridom region. With only a single subgroup from the Malaysian seizure originating in West Africa, and only a single (early) seizure originating in Zambia, it is difficult to make generalizations about the relative proportions of transit times based on the lag time determinations for those regions. Nevertheless, the short lag time of the West Africa ivory is noteworthy given the few remaining elephants there.
Fig. 4.
Lag time distribution shown as a ternary plot for rapid transit (<12 mo lag time), intermediate transit (12–24 mo), and long transit (>24 mo).
Our results suggest that there may be regional differences in lag times at the shipping container level of distinction. However, we note that the radiocarbon calibration and related uncertainties have large associated errors and that refined regional radiocarbon calibrations may provide better constraints on regional differences in lag times.
Changes in Lag Times from 2002 to 2014.
The median lag time for seizures between 2002 and August 2010 ranges from 7 to 13 mo. Lag time increases from 2011 onward, ranging from 13 to 32 mo (Fig. 5). This trend of increasing lag time is most evident in East African seizures where there are a total of nine seizures made over 9 y. The combined dataset from the regions of West Africa (n = 1), Southern Africa (Zambia, n = 1), and the Tridom (n = 4) is smaller than the East Africa dataset but supports the trend of increasing lag time since 2011. The reason for the general increase in lag time since 2011 is unclear. It is likely related to the overall decline in African elephant populations, particularly from 2010 to 2012, where data show population decline due to unsustainable poaching rates, especially in Central and East Africa (3, 4). As a result, it may take longer to accumulate enough large tusks to make a large shipment more profitable. Considering the markedly slower growth rate of tusks of forest elephants, this may also explain the longer lag times we observed for Central African ivory compared with East African ivory. West African forest ivory generally made up a small portion of the tusks in all seizures analyzed and, based on its short lag time, seemed to be added opportunistically at the time of shipment. Factors related to the dynamics of the illegal network of trade or perhaps to changes in the demand for ivory (29, 30) may have also contributed to these trends.
Fig. 5.
Median lag time (months) plotted versus seizure date from 2002 to 2014. There seems to be an increase in lag time beginning in 2011.
Conclusions
A calibration curve for F14C of modern wildlife tissues in Africa shows that modern elephant hair samples (keratin) have an offset with respect to the bomb curve between about 1 and 2 y from both the NH zone 3 (NH3) and SH zone 3 (SH3) relationships, with the tissue samples having higher F14C values than either the NH3 or SH3 curves. This is likely due to elephant diets that include carbon that was fixed some months to years before ingestion, and also due to recycling of some proteins during keratin or collagen synthesis. The elephant hair calibration curve can be used to determine the date of death of elephants if the F14C of ivory is measured along the pulp cavity of the tusk. It may be necessary to establish taxon-specific calibration curves for date-of-death determinations based on radiocarbon dating of other wildlife products, such as rhinoceros horn, pangolin scales, pelts or furs, or timber. Issues specific to different dietary (i.e., carbon) sources, tissue types, and geographic regions need to be considered for related studies that use F14C measurements to determine the age of wildlife products.
Examination of 231 ivory samples from 14 seizures made between 2002 and 2014 shows that the lag time between elephant death and seizure by law enforcement officials has median values generally ranging between 6 and 35 mo. Specimens originating from East Africa—previously determined via genetic analysis—have the shortest lag times, with ca. 35% of specimens having lag times <12 mo; by comparison, only 7% of ivory specimens originating from the Tridom region of Africa have lag times <12 mo. Only a single specimen analyzed in this study has a lag time >10 y: We find no evidence that long-term government or other stockpiles have been contributing significant amounts of ivory to the illegal ivory trade, emphasizing that poached ivory is being rapidly moved into the illegal trade. This study demonstrates the power of bomb-curve radiocarbon dating for revealing lag times between date of death and seizure, a technique that can be applied to seizures of other wildlife parts, providing critical information to law enforcement, conservation, government, and policy organizations and agencies.
Methods
We report elephant hair and ivory 14C measurements in this paper. Ivory specimens were obtained from seizures in Africa and Asia; specimens were ∼10-cm2 blocks obtained from the proximal end of the tusk [see ref. 31 and as illustrated in Fig. S2 (32)]. We sampled the innermost dentine in the pulp cavity for samples used to assign date of death to animals (Fig. 2 and Fig. S2); for selected specimens, we also dated the outermost dentine, which often had an outer sheath of cementum that was sampled. Powder was obtained using a high-speed rotary Dremel tool and double-cut carbide bits from the innermost 1–2 mm of the ivory block. Hair samples were obtained during collaring or translocation operations conducted by Save the Elephants and by the Kenya Wildlife Service. The basal 1 cm of hair was analyzed for DNA markers; the subsequent 2 mm (i.e., from 10 to 12 mm) was used for F14C measurements. Dates were corrected to ca. 14 d before the date of collection assuming an average growth rate of 0.8 mm/d (33).
Dentine powder was demineralized with hydrochloric acid to isolate the collagen component; ∼1 mg of carbon was used for a single accelerator mass spectrometry (AMS) measurement (ca. 10 mg ivory powder). Delipified hair or demineralized collagen was placed into a precombusted quartz tube with precombusted reagents (cupric oxide and silver), sealed under vacuum, and combusted at 850 °C to convert all organic-bound carbon to carbon dioxide gas (CO2). CO2 was cryogenically purified and reduced to graphite; graphite from each sample was thereafter pressed into a target for radiocarbon (14C) measurement. The graphite targets were sent to the W. M. Keck Carbon Cycle Accelerator Mass Spectrometry Laboratory at the University of California, Irvine (UCIAMS) for radiocarbon measurement. Radiocarbon results are reported as fraction modern F14C (9), which specifically includes δ13C normalization to –25% using the AMS measured δ13C value.
Expanded uncertainty in lag time incorporates uncertainty in the calibration curve (Fig. 1B) and in measurement reproducibility. The latter is estimated by specimen “ivory_Modern 207,” a homogenized powdered ivory sample prepared multiple times (e.g., separate rounds of collagen extraction, combustion, graphitization, and F14C measurement) and analyzed within each analytical sequence. Ten analyses of ivory_Modern 207 gave an average F14C value of 1.1021 ± 0.0032 (1 σ) (Dataset S1). U is calculated from a linear combination of this measurement uncertainty and predicted values from the elephant hair calibration curve, multiplied by a coverage factor (k) of 2, which represents ca. 95% uncertainty for the range in lag times. For seized specimens with measured F14C values similar to those of the elephant hair used to build the calibration curve (e.g., F14C ≤1.1043), U was ± 1.55 y (± 18.5 mo). One seized ivory specimen had an F14C value of 1.1379; U for this specimen (“ivory_200;” see Dataset S1) was calculated as ± 1.71 y (± 20.6 mo). Collagen extracted from a fossil bone was used as a blank for AMS measurement (Dataset S1).
Normal distributions to histograms of lag times (Fig. 3) were fit using JMP (SAS Inst., Inc.).
Supplementary Material
Acknowledgments
We thank Tara Wilson for help processing the seizures for analysis, Mark Franklin for help with sampling of ivory specimens for radiocarbon dating, and John Howa and Michael Lott for help with expanded uncertainty calculations. We also thank Edouard Bard, Daryl Codron, and Susan Trumbore for helpful comments on the manuscript. This work was funded by Paul G. Allen Family Foundation Grant 11811 and by the Save the Elephants and Wildlife Conservation “Elephant Crisis Fund.” Hair calibration samples were imported with CITES permits US831854, US053837/9, US159997/9, US08996A/9; ivory samples were imported under permits 04US082567/9 and US03172A/9. The following governments agreed to provide samples from their ivory seizures: Malaysia, Thailand, the Philippines, Singapore, Cameroon, Taiwan, Hong Kong, Kenya, Uganda, Sri Lanka, Malawi, and Togo.
Footnotes
Conflict of interest statement: I.D.-H. is chief executive officer of Save the Elephants, which provided partial funding for this research, and K.S.B. is employed by Vulcan, Inc., which provided partial funding for this research through the Paul G. Allen Family Foundation.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614938113/-/DCSupplemental.
References
- 1.United Nations Office of Drugs and Crime . Guidelines for methods and procedures of ivory sampling and laboratory analysis. United Nations Office of Drugs and Crime; New York: 2014. [Google Scholar]
- 2.Wasser SK, et al. Genetic assignment of large seizures of elephant ivory reveals Africa’s major poaching hotspots. Science. 2015;349(6243):84–87. doi: 10.1126/science.aaa2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittemyer G, et al. Illegal killing for ivory drives global decline in African elephants. Proc Natl Acad Sci USA. 2014;111(36):13117–13121. doi: 10.1073/pnas.1403984111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chase MJ, et al. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ. 2016;4:e2354. doi: 10.7717/peerj.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisels F, et al. Devastating decline of forest elephants in central Africa. PLoS One. 2013;8(3):e59469. doi: 10.1371/journal.pone.0059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turkalo AK, Wrege PH, Wittemyer G. Slow intrinsic growth rate in forest elephants indicates recovery from poaching will require decades. J Appl Ecol. 2016 doi: 10.1111/1365-2664.12764. [DOI] [Google Scholar]
- 7.Bennett EL. Legal ivory trade in a corrupt world and its impact on African elephant populations. Conserv Biol. 2015;29(1):54–60. doi: 10.1111/cobi.12377. [DOI] [PubMed] [Google Scholar]
- 8.CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) 2014 doi: 10.1159/000459796. Elephant conservation, illegal killing and ivory trade. SC65 Doc. 42.1. Available at cites.org/sites/default/files/eng/com/sc/65/E-SC65-42-01_2.pdf. Accessed July 1, 2016. [DOI] [PubMed]
- 9.Reimer PJ, Brown TA, Reimer RW. Discussion: Reporting and calibration of post-bomb 14C data. Radiocarbon. 2004;46(3):1299–1304. [Google Scholar]
- 10.Hua Q, Barbetti M. Review of tropospheric bomb C-14 data for carbon cycle modeling and age calibration purposes. Radiocarbon. 2004;46(3):1273–1298. [Google Scholar]
- 11.Hua Q, Barbetti M. Influence of atmospheric circulation on regional 14CO2 differences. J Geophys Res. 2007;112(D19):D19102. [Google Scholar]
- 12.Hua Q, Barbetti M, Rakowski AZ. Atmospheric radiocarbon for the period 1950-2010. Radiocarbon. 2013;55(4):2059–2072. [Google Scholar]
- 13.Levin I, et al. Observations and modelling of the global distribution and long – term trend of atmospheric 14CO2. Tellus B Chem Phys Meterol. 2010;62(1):26–46. [Google Scholar]
- 14.Levin I, Kromer B, Hammer S. Atmospheric Δ14CO2 trend in Western European background air from 2000 to 2012. Tellus B Chem Phys Meterol. 2013;65:1–7. [Google Scholar]
- 15.Reimer PJ, et al. IntCal13 and Marine13 radiocarbon age calibration curves 0-50,000 years cal BP. Radiocarbon. 2013;55(4):1869–1887. [Google Scholar]
- 16.Reimer PJ, Reimer R. 2016 CALIBomb. Available at calib.qub.ac.uk/CALIBomb/. Accessed June 25, 2016.
- 17.Spalding KL, Buchholz BA, Bergman LE, Druid H, Frisén J. Forensics: Age written in teeth by nuclear tests. Nature. 2005;437(7057):333–334. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 18.Cook GT, Dunbar E, Black SM, Sheng X. A preliminary assessment of age at death determination using the nuclear weapons testing 14C activity of dentine and enamel. Radiocarbon. 2006;48(3):305–313. [Google Scholar]
- 19.Ubelaker DH, Buchholz BA, Stewart JEB. Analysis of artificial radiocarbon in different skeletal and dental tissue types to evaluate date of death. J Forensic Sci. 2006;51(3):484–488. doi: 10.1111/j.1556-4029.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang N, et al. Improved application of bomb carbon in teeth for forensic investigation. Radiocarbon. 2010;52(2):706–716. [Google Scholar]
- 21.Alkass K, et al. Analysis of radiocarbon, stable isotopes and DNA in teeth to facilitate identification of unknown decedents. PLoS One. 2013;8(7):e69597. doi: 10.1371/journal.pone.0069597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geyh MA. Bomb radiocarbon dating of animal tissues and hair. Radiocarbon. 2001;43(2B):723–730. [Google Scholar]
- 23.Vogel JC, Fuls A, Visser W. Accurate dating with radiocarbon from the atom bomb tests. S Afr J Sci. 2002;98:437–438. [Google Scholar]
- 24.Sideras-Haddad E, Brown T. 2002. Dating studies of elephant tusks using accelerator mass spectrometry. Proceedings of the Ninth International Conference on Accelerator Mass Spectrometry (Elsevier, New York)
- 25.Uno KT, et al. Bomb-curve radiocarbon measurement of recent biologic tissues and applications to wildlife forensics and stable isotope (paleo)ecology. Proc Natl Acad Sci USA. 2013;110(29):11736–11741. doi: 10.1073/pnas.1302226110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muhr J, et al. How fresh is maple syrup? Sugar maple trees mobilize carbon stored several years previously during early springtime sap-ascent. New Phytol. 2016;209(4):1410–1416. doi: 10.1111/nph.13782. [DOI] [PubMed] [Google Scholar]
- 27.Ehleringer JR, et al. 14C calibration curves for modern plant material from tropical regions of South America. Radiocarbon. 2011;53(4):585–594. [Google Scholar]
- 28.Ayliffe LK, et al. Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia. 2004;139(1):11–22. doi: 10.1007/s00442-003-1479-x. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Clark S. Elephant ivory trade in China: Trends and drivers. Biol Conserv. 2014;180:23–30. [Google Scholar]
- 30.Gao Y, et al. Rhino horn trade in China: An analysis of the art and antiques market. Biol Conserv. 2016;201:343–347. [Google Scholar]
- 31.Wasser SK, et al. Using DNA to track the origin of the largest ivory seizure since the 1989 trade ban. Proc Natl Acad Sci USA. 2007;104(10):4228–4233. doi: 10.1073/pnas.0609714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher DC, Fox DL. Season of death of the Dent mammoths. In: Brunswig RH, Pitblado BL, editors. From the Dent Prairie to the Peaks of the Rockies: Recent Paleoindian Research in Colorado. Univ of Colorado Press; Boulder, CO: 2007. pp. 123–153. [Google Scholar]
- 33.Wittemyer G, Cerling TE, Douglas–Hamilton I. Establishing longitudinal diet chronologies from isotopic profiles in serially collected animal tissues: An example using tail hairs from African elephants. Chem Geol. 2009;267:3–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.