Significance
Large, fibrous, and flexible extracellular matrix proteins are integral to development and maintenance of tissues in the body. Laminin is an extracellular matrix component that provides a physical substrate for cell adhesion and induces signaling pathways that maintain cell health and functionality. Despite the physiological importance of laminin, major gaps remain in our understanding of how its three subunits come together to form the characteristic cross-shaped laminin structure. Laminin was treated with chemicals that link amino acids close in space, providing a map of the subunit arrangement and correcting previous suppositions made on the basis of amino acid sequence inspection alone.
Keywords: coiled coil, extracellular matrix, laminin, cross-linking, mass spectrometry
Abstract
Laminin, an ∼800-kDa heterotrimeric protein, is a major functional component of the extracellular matrix, contributing to tissue development and maintenance. The unique architecture of laminin is not currently amenable to determination at high resolution, as its flexible and narrow segments complicate both crystallization and single-particle reconstruction by electron microscopy. Therefore, we used cross-linking and MS, evaluated using computational methods, to address key questions regarding laminin quaternary structure. This approach was particularly well suited to the ∼750-Å coiled coil that mediates trimer assembly, and our results support revision of the subunit order typically presented in laminin schematics. Furthermore, information on the subunit register in the coiled coil and cross-links to downstream domains provide insights into the self-assembly required for interaction with other extracellular matrix and cell surface proteins.
Laminins are network-forming constituents of the extracellular matrix (ECM) (1, 2). They interact with the cell surface and other ECM components to generate a physical and functional framework affecting cell viability, identity, and activity. The laminin family appears to have arisen during the evolution of multicellularity in animals (3), and laminins contribute to a diversity of basement membrane and connective tissue structures in mammals (2). Laminins are studied in the context of development (4), stem cell biology (5), tissue engineering (6), cancer (7), and aging (8). The remarkable structural organization of laminins underlies their important physiological functions.
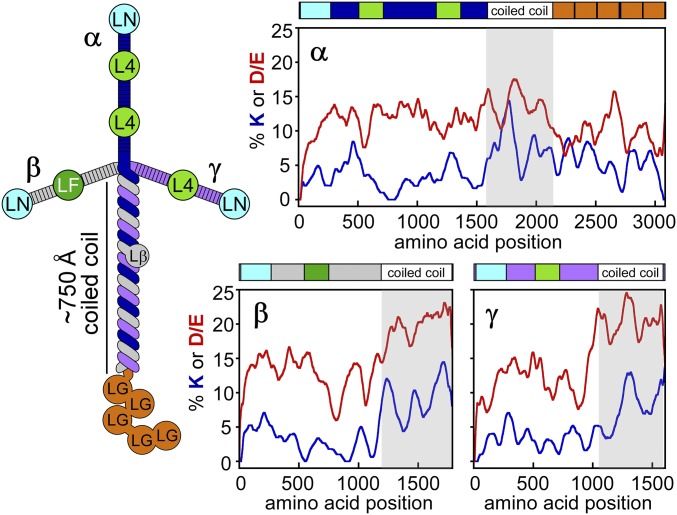
Laminins are composed of three subunits, α, β, and γ, that assemble into a roughly humanoid form as visualized using rotary shadowing electron microscopy (9). The individual subunits separately form the “head” and two “arms,” which are composed of epidermal growth factor (EGF)-like cysteine-rich repeats with globular domains embedded (10) (Fig. 1). Following the head and arms, the three subunits come together to form a long coiled coil, constituting the “body.” Additional globular domains unique to the α subunit are the “feet.” The laminin head and arms may splay to form a tripod (2), a feature not captured in rotary shadowing images or in diagrams of domain composition. Due to the centrality of laminin in cell–ECM interactions, efforts have been made to analyze the functional regions of the trimer, to determine which α, β, and γ paralogs associate into physiological heterotrimers and to understand how trimers self-assemble into higher-order networks. Laminin fragments have been generated to assess their binding properties (11, 12) and as targets for structure determination by X-ray crystallography (13–20).
Fig. 1.
Laminin domains and cross-linkable side chains. (Left) Laminin subunits are labeled α, β, and γ. Domains are labeled according to convention (10). (Right) Percentages of primary amine-bearing (K, blue) and carboxylic acid-bearing (D/E, red) side chains were calculated using a sliding window of 40 amino acids, smoothed over 100 amino acids. Coiled-coil regions are shaded.
Despite this progress, few insights into the overall 3D architecture of laminin have been made in the past few decades, and certain regions of the complex have been neglected as targets of structural techniques. In particular, no structure has been determined for any segment of the laminin coiled coil, which spans more than 750 Å and is the backbone of the laminin quaternary structure. Because the regions of high coiled-coil propensity in α, β, and γ are similar in length, their alignment can be roughly surmised. However, structural deviations in the coiled coil have been suggested to occur (21). More fundamentally, the order of the three subunits has remained ambiguous since evidence for a coiled coil in laminin was presented (22, 23). An analysis based on charged residues in laminin sequences led to the proposal that α, β, and γ are arranged in a clockwise manner when viewed from the carboxyl terminus (24); this order is widely adopted in seminal reviews on laminin structure and function (10, 25, 26). However, other investigators have interpreted biochemical results in the context of a model with the opposite order (27). Clearly, gaps remain in our appreciation of the laminin assembly.
Cross-linking analyzed by MS is a strategy for determining the spatial organization of protein quaternary structures (28, 29). This technique is particularly suitable for elongated protein assemblies such as coiled coils (30), which have high surface-to-volume ratios. The laminin coiled coil is rich in amino acids with cross-linkable functional groups (Fig. 1). Furthermore, coiled coils have well-defined degrees of freedom: oligomeric state, subunit register (i.e., the relative positions of the subunits along the coiled-coil axis), pitch, interface angle, and the order of chains in a hetero-oligomer. Benefiting from the parameterization of coiled-coil geometry, a computational model for laminin subunit association was evaluated under constraints from the cross-linking data. This analysis illuminated previously inaccessible aspects of the laminin quaternary structure. Coupled with information from low resolution electron microscopy and laminin fragment crystallization, a complete picture of the laminin structure is beginning to emerge.
Results
Cross-Linking of the Laminin Heterotrimer.
Mouse laminin-111 [laminin isotype (31) containing the α1, β1, and γ1 paralogs, hereafter laminin] was treated with isotopic mixtures, differing by 12.076 Da, of the cross-linkers bis(sulfosuccinimidyl)suberate (BS3) and suberic acid dihydrazide (SDH) to target primary amine and carboxylic acid groups, respectively. Cross-linked laminin was digested proteolytically and subjected to liquid chromatography and tandem MS. Parent ion spectra (MS1) of cross-linked peptides identified from the isotope fingerprint were selected for analysis of fragmentation (MS2) data, which were assigned using xQuest (32). All assigned MS2 spectra were then inspected manually. Criteria for rejecting assignments are given in the SI Materials and Methods. During SDH data analysis, we observed direct (zero-length) cross-linking of acidic side chains to lysines as a side effect of the coupling reagent, as reported previously (33). Therefore, we performed a reaction in the absence of SDH to obtain additional zero-length links intentionally. With no isotope coding in this case, all MS1 spectra were searched. In all (Dataset S1), we observed 177 reliable, nonredundant BS3 or SDH links, 134 of which were within the coiled-coil, and 47 of these were intersubunit links. We also observed 92 reliable, nonredundant zero-length links, 77 of which were in the coiled coil, and 22 of these were between subunits.
Cross-Link Validation.
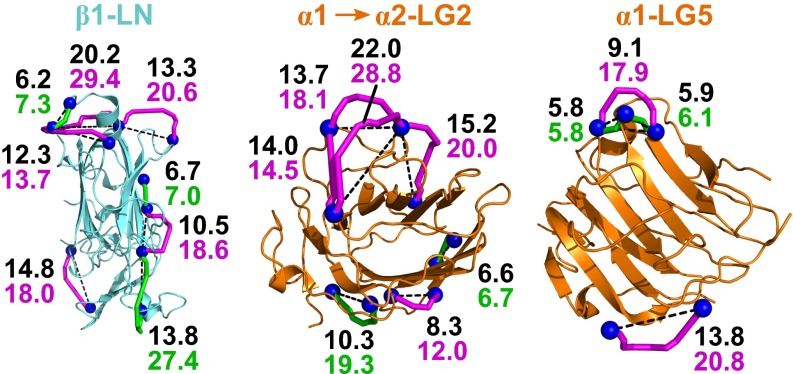
Most of the cross-linked peptides were in the coiled coil, but some were within or between globular domains and in the EGF-like repeats. Crystal structures of laminin fragments (14, 15, 17, 18) were used to assess the quality of the cross-linking results (Fig. 2). Cross-links within the β subunit LN domain, the LG4–LG5 domains, and an EGF-like repeat were validated based on Protein Data Bank (PDB) ID codes 4AQS, 2JD4, and 1NPE, respectively. Cross-links within the LG1, LG2, and LG3 domains of the α1 subunit were mapped onto the structure of the mouse α2 paralog (PDB ID code 2WJS), and links within the α1 L4b domain were mapped onto human α2 L4b (PDB ID code 4YEP). Using xWALK (34), the shortest solvent-accessible surface distances (SASDs) between Cβ atoms of linked residues were calculated from the experimentally determined structures listed above. All SASDs for BS3 and SDH links were under 30 Å (average, 17.9 ± 5.0 Å; range, 7.7–29.4 Å), which is less than the maximum of 34 Å for BS3 SASDs in xWALK. Eight SASDs for zero-length cross-links were below 7.3 Å, but three were 14.2, 19.3, and 27.4 Å. Structure inspection indicated that the linked residues are in all cases close in space (<14 Å Euclidean Cβ-Cβ distance) and suggested that minor loop motions or alternate rotamer sampling of intervening residues, expected to occur due to natural protein motions in solution, would permit these direct links to form. The consistency with available crystal structures supports the reliability of the cross-link set.
Fig. 2.
Validation of laminin cross-linking. Cross-linked positions (Cβ atoms, blue spheres) were mapped onto the structures of the β1 LN and α1 LG5 domains (PDB ID codes 4AQS and 2JD4, respectively) and by homology onto the structure of the α2 LG2 domain (PDB ID code 2WJS). Shortest SASDs are shown as magenta (BS3 and SDH links) or green tubes (zero-length links), and direct Cβ-Cβ distances are shown as black dashed lines. Measures in Ångstrom for the two routes are in the corresponding colors.
Assignment of the Subunit Order in the Laminin Coiled Coil.
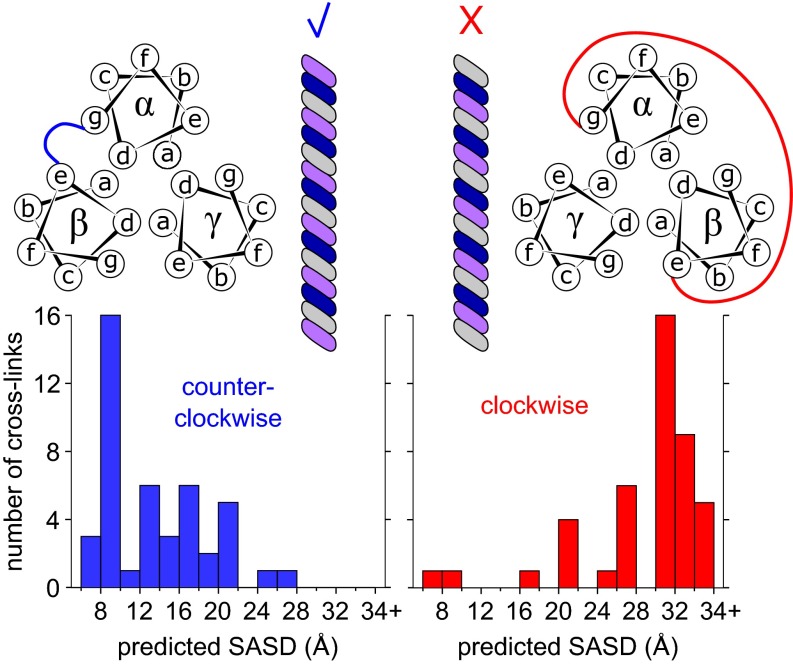
The α, β, and γ subunits can be arranged either clockwise or counter clockwise when viewed down the coiled-coil axis. The order of subunits in the trimer underlies the mechanism of combinatorial assembly of laminin isotypes (35) and therefore is key to understanding the complexity and diversification of the laminin family. We reasoned that the alternative subunit arrangements could be distinguished without knowledge of the register of the three strands. To begin, an ideal trimeric coiled coil was generated using Coiled-Coil Crick Parameterization (36), and the position of each amino acid in the heptad repeat (a, b, c, d, e, f, or g) was noted. SASDs with a 34-Å upper limit then were calculated in xWALK for all possible pairs of heptad positions between helices of the model coiled coil (Table S1). In parallel, each laminin coiled-coil residue was assigned its most likely position within a heptad repeat using MARCOIL (37). According to predicted heptad positions, each intersubunit BS3 or SDH cross-link in the coiled coil was given its corresponding SASDs for the clockwise and counter clockwise arrangements (Table S2), and histograms of expected distance were generated for the two competing models (Fig. 3). The cross-linking data overwhelming supported a counter clockwise arrangement of α, β, and γ as viewed from the carboxyl terminus.
Table S1.
SASDs between heptad positions in an ideal, parallel, trimeric coiled coil
| Position 1 | Position 2 | CCW* SASD (Å) | CW* SASD (Å) | Position 1 | Position 2 | CCW SASD (Å) | CW SASD (Å) |
| b9 | b16 | 22.4 | 27.6 | e12 | e5 | 31.2 | 25.7 |
| b9 | b9 | 21.3 | 21.6 | e12 | f13 | 32.5 | 14.9 |
| b9 | c10 | 31.8 | 13.1 | e12 | f6 | >34 | 15.9 |
| b9 | c17 | 32.1 | 22.9 | e12 | g14 | >34 | 8.3 |
| b9 | e12 | 19 | 29.4 | e12 | g7 | 31.1 | 9.3 |
| b9 | e5 | 21.5 | 26.6 | f13 | b16 | 16.3 | 29.4 |
| b9 | f13 | 25.5 | 20.5 | f13 | b9 | 20.6 | 27.1 |
| b9 | f6 | 28.1 | 16.6 | f13 | c10 | 28.2 | 17.7 |
| b9 | g14 | >34 | 15 | f13 | c17 | 24.5 | 22.6 |
| b9 | g7 | >34 | 9.3 | f13 | e12 | 15.4 | 32.7 |
| c10 | b16 | 12.3 | 32.6 | f13 | e5 | 23.6 | 33 |
| c10 | b9 | 13.4 | 33.2 | f13 | f13 | 20.5 | 22.9 |
| c10 | c10 | 21.7 | 23.7 | f13 | f6 | 28.3 | 21.8 |
| c10 | c17 | 21 | 29.1 | f13 | g14 | 30.7 | 17.3 |
| c10 | e12 | 9 | 31.7 | f13 | g7 | >34 | 16 |
| c10 | e5 | 14.7 | >34 | g14 | b16 | 8.7 | 30.2 |
| c10 | f13 | 15.9 | 30.6 | g14 | b9 | 15.9 | >34 |
| c10 | f6 | 20.5 | 27.6 | g14 | c10 | 24.2 | 27.9 |
| c10 | g14 | 26.3 | 24.3 | g14 | c17 | 19.4 | 30.9 |
| c10 | g7 | 30 | 21.8 | g14 | e12 | 8 | 33.8 |
| e12 | b16 | 28.1 | 22.4 | g14 | e5 | 19.3 | >34 |
| e12 | b9 | 30.8 | 19.5 | g14 | f13 | 15 | 32 |
| e12 | c10 | 32.1 | 9.7 | g14 | f6 | 23.9 | 32.3 |
| e12 | c17 | >34 | 15.1 | g14 | g14 | 24.7 | 27.6 |
| e12 | e12 | 26 | 24.2 | g14 | g7 | 31.5 | 25.9 |
Counterclockwise (CCW) and clockwise (CW) are as viewed from the carboxyl terminus.
Table S2.
Predicted SASDs between the assigned heptad positions of all reliable intersubunit cross-links in the laminin coiled coil
| BS3, SDH cross-links | Zero-length cross-links | ||||||
| Position 1 | Position 2 | CCW* SASD (Å) | CW* SASD (Å) | Position 1 | Position 2 | CCW SASD (Å) | CW SASD (Å) |
| α1614c | β1231e | 9, 14.7 | 31.7 | α1746c | β1372f | 15.9, 20.5 | 27.6, 30.6 |
| α1742f | β1368b | 16.3, 20.6 | 27.1, 29.4 | α1746c | β1379f | 15.9, 20.5 | 27.6, 30.6 |
| α1746c | β1368b | 12.3, 13.4 | 32.6, 33.2 | α1750g | β1372f | 15, 23.9 | 32, 32.3 |
| α1749f | β1379f | 20.5, 28.3 | 21.8, 22.9 | α2007f | β1659e | 15.4, 23.6 | 23.6, 32.7 |
| α1820g | β1476e | 8, 19.3 | 33.8 | α1950b | γ1416g | 9.3, 15 | >34 |
| α1896g | β1552c | 19.4, 24.2 | 27.9, 30.9 | α1950b | γ1419c | 13.1, 22.9 | 31.8, 32.1 |
| α1896g | β1558b | 8.7, 15.9 | 30.2 | β1218f | γ1074b | 16.3, 20.6 | 27.1, 29.4 |
| α1962g | β1610e | 8, 19.3 | 33.8 | β1263g | γ1123b | 8.7, 15.9 | 30.2 |
| α2007f | β1656b | 16.3, 20.6 | 27.1, 29.4 | β1373g | γ1233g | 24.7, 31.5 | 25.9, 27.6 |
| α2015g | β1659e | 8, 19.3 | 33.8 | β1492g | γ1306b | 8.7, 15.9 | 30.2 |
| α2015g | β1674f | 15, 23.9 | 32, 32.3 | β1513g | γ1327b | 8.7, 15.9 | 30.2 |
| α2035f | β1696f | 20.5, 28.3 | 21.8, 22.9 | β1517d | γ1327b | 11.4, 11.8 | 33.6, 33.9 |
| α2036g | β1691b | 8.7, 15.9 | 30.2 | β1718g | γ1537b | 8.7, 15.9 | 30.2 |
| α2036g | β1693c | 19.4, 24.2 | 27.9, 30.9 | β1725g | γ1544c | 19.4, 24.2 | 27.9, 30.9 |
| α2119f | β1773f | 20.5, 28.3 | 21.8, 22.9 | β1746g | γ1560e | 8, 19.3 | 33.8 |
| α1615d | γ1094a | >34 | 6.5, 7.9 | β1746g | γ1567e | 8, 19.3 | 33.8 |
| α1676b | γ1152c | 13.1, 22.9 | 31.8, 32.1 | ||||
| α1746c | γ1233g | 21.8, 24.3 | 26.3, 30 | ||||
| α1793a | γ1261g | 6.1, 13.2 | 31.5 | ||||
| α1869a | γ1325g | 6.1, 13.2 | 31.5 | ||||
| α1894e | γ1360g | 8.3, 9.3 | 31.1 | ||||
| α1898b | γ1360g | 9.3, 15 | >34 | ||||
| α1898b | γ1363c | 13.1, 22.9 | 31.8, 32.1 | ||||
| α1943b | γ1401f | 16.6, 20.5 | 25.5, 28.1 | ||||
| α1943b | γ1405c | 13.1, 22.9 | 31.8, 32.1 | ||||
| α1950b | γ1423g | 9.3, 15 | >34 | ||||
| α1961f | γ1423g | 16, 17.3 | 30.7 | ||||
| α2080b | γ1542g | 9.3, 15 | >34 | ||||
| α2094b | γ1555g | 9.3, 15 | >34 | ||||
| α2094b | γ1565c | 13.1, 22.9 | 31.8, 32.1 | ||||
| α2101b | γ1572c | 13.1, 22.9 | 31.8, 32.1 | ||||
| β1202f | γ1075c | 24.5, 28.2 | 17.7, 22.6 | ||||
| β1226g | γ1074b | 8.7, 15.9 | 30.2 | ||||
| β1226g | γ1091e | 8, 19.3 | 33.8 | ||||
| β1304f | γ1186b | 16.3, 20.6 | 27.1, 29.4 | ||||
| β1323c | γ1189e | 9, 14.7 | 31.7 | ||||
| β1492g | γ1303f | 15, 23.9 | 32, 32.3 | ||||
| β1714c | γ1546e | 9, 14.7 | 31.7 | ||||
| β1736d | γ1544c | 21.7, 23 | 33.4 | ||||
| β1736d | γ1548g | 26.2, 28.8 | 30.7, 33.1 | ||||
| β1757d | γ1582f | 16.8, 19.1 | >34 | ||||
| β1773f | γ1591a | 14.8, 18.1 | 20.7, 23.4 | ||||
| β1778d | γ1591a | 6.5, 7.9 | >34 | ||||
| β1778d | γ1592b | 11.4, 11.8 | 33.6, 33.9 | ||||
Counterclockwise (CCW) and clockwise (CW) are as viewed from the carboxyl terminus.
Fig. 3.
Cross-linking supports a counterclockwise arrangement of α, β, and γ, viewed from the carboxyl terminus. Schematics of the competing coiled-coil subunit arrangements are shown above. Predicted SASD values based on the likely heptad positions of BS3- and SDH-linked residues are plotted below for each model.
Zero-length cross-linking confirmed the subunit order. Many compatible zero-length intersubunit cross-links were observed for a counter clockwise arrangement of α, β, and γ, whereas no compatible links were found for the clockwise arrangement (Table S2). Some cross-links appearing incompatible with either arrangement may involve a rare misassigned cross-link or, more likely, a misassigned heptad position.
Coiled-Coil Register Determination.
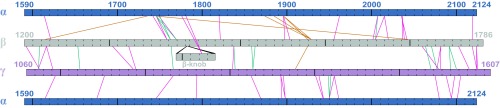
In addition to the order of subunits around the coiled-coil axis, other fundamental aspects of laminin quaternary structure are the subunit register and the extent of coiled-coil disruptions (21). A primary structural map revealed that most of the intersubunit cross-links are mutually compatible with a continuous coiled coil (Fig. 4). The only major disruption is about 45 residues comprising the Lβ knob (Lβ; Fig. 1), which had to be excised from the alignment to accommodate zero-length cross-links between β and γ on either side of this region (Fig. 4). Two cross-links were observed from Lβ to the α subunit, aligning the knob with the α chain. Another, smaller register shift appears to occur between α1750 and α2007. Specifically, a set of direct cross-links (α1746–β1372, α1746–β1379, and α1750–β1372) is separated from another direct link (α2007–β1659) by 257–261 residues in α and 280–287 residues in β, suggesting a readjustment of two to four heptads in the intervening region (Fig. 4). No other gross register shifts appear necessary to account for the data.
Fig. 4.
Map of observed cross-links in the laminin coiled coil. The α subunit is represented twice to show links to both β and γ. BS3/SDH and zero-length cross-links are represented by magenta and green lines, respectively. Orange cross-links appear reliable according to MS data evaluation but are inconsistent with the prevailing register. Figure generated using xiNET (49).
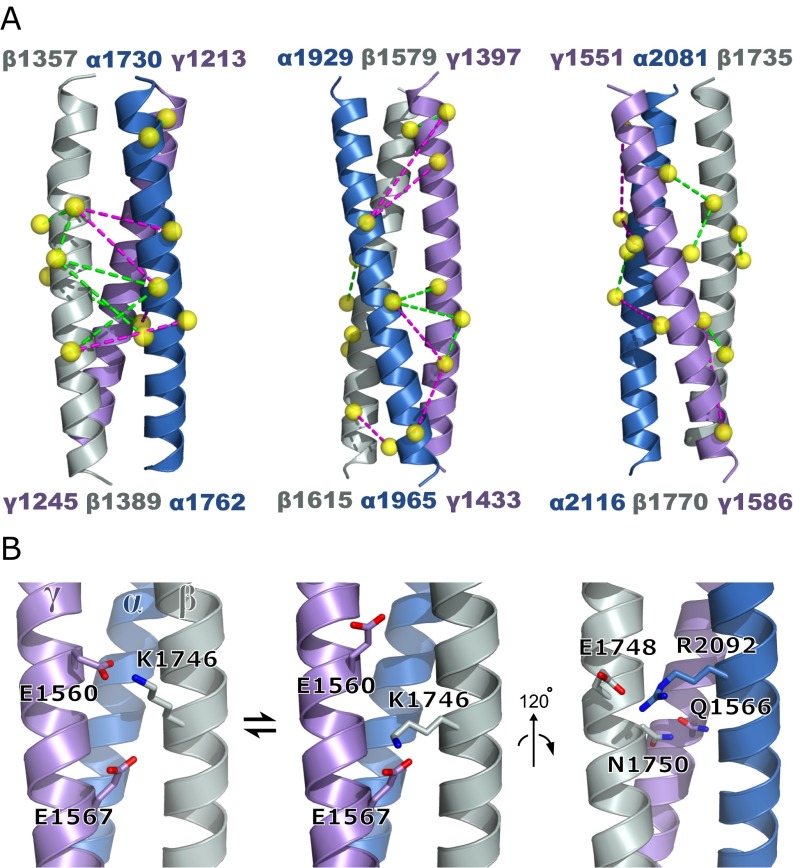
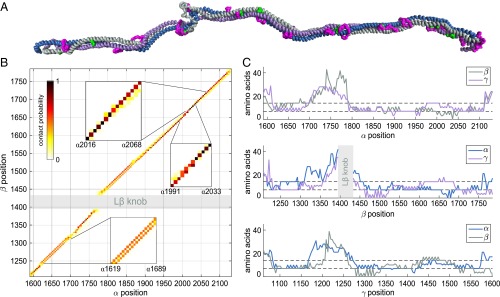
Although some heavily cross-linked regions could be directly modeled (Fig. S1 and Table S3), coarse-grained molecular dynamics simulations restrained by the experimental cross-links were used to obtain a global assessment of the subunit register (Fig. 5A). The amino acid sequence and MARCOIL heptad position assignments were first threaded onto an ideal trimeric coiled coil. A bead representing each amino acid was placed at the coordinates of its Cα atom in this structure. The resulting model was allowed to relax subject to a force field based on coiled-coil geometry, together with a Lennard–Jones-type potential applied generically between MARCOIL a and d positions on different chains. This latter feature of the force field accounted for predicted irregularities (e.g., stutters, stammers, and skips) (38) in the heptad repeat by pulling predicted core residues to the core. Following relaxation, the cross-links were applied as bead chains mimicking the dimensions and extensibility of the physical links. Next, multiple simulations were conducted for each of seven different starting alignments differing by four-heptad displacements of individual subunits (for a representative trajectory, see Movie S1). Nearest-neighbor interactions between residues at a and d positions in each helix pair were extracted from the simulations, yielding the most likely chain register based on the cross-linking data evaluated in the context of the MARCOIL predictions (Fig. 5B and Dataset S2). The above procedures were validated using the 120-residue coiled coil of fibrinogen, the current best example of a heterotrimeric coiled coil with known structure (39, 40). Specifically, an artificial set of cross-links compatible with the native fibrinogen structure was able to rectify a register-shifted variant.
Fig. S1.
Models of coiled-coil segments. (A) Three well-constrained regions of the laminin trimer. Yellow spheres are Cβ atoms of cross-linked residues (listed in Table S4). Cross-links are indicated by dashed lines (BS3 and SDH: magenta; zero-length: green). Zero-length intrasubunit cross-links are shown along with all intersubunit cross-links. The subunit register used to construct this image is given in Table S3. (B) (Left and Center) Model of residues engaging in zero-length links in the C-terminal region of the coiled coil (corresponding to the rightmost image in A; Fig. 6). (Right) Putative interactions implied by the resulting register.
Table S3.
Subunit registers and heptad positions corresponding to Fig. S1A
| α | β | γ | α | β | γ | α | β | γ | |||||||||||
| L1730 | a | E1357 | e | L1213 | a | A1929 | b | K1579 | b | A1397 | b | D2081 | c | S1735 | c | D1551 | c | ||
| K1731 | b | D1358 | f | L1214 | b | D1930 | c | R1580 | c | E1398 | c | M2082 | d | K1736 | d | L1552 | d | ||
| A1732 | c | L1359 | g | R1215 | c | A1931 | d | A1581 | d | A1399 | d | E2083 | e | L1737 | e | D1553 | e | ||
| A1733 | d | M1360 | a | T1216 | d | H1932 | e | S1582 | e | N1400 | e | M2084 | f | Q1738 | f | R1554 | f | ||
| K1734 | e | L1361 | b | L1217 | e | K1933 | f | K1583 | f | E1401 | f | Q2085 | g | L1739 | g | K1555 | g | ||
| D1735 | f | E1362 | c | A1218 | f | T1934 | g | S1584 | g | K1402 | g | A2086 | a | L1740 | a | V1556 | a | ||
| L1736 | g | R1363 | d | G1219 | g | A1935 | a | A1585 | a | T1403 | a | N2087 | b | E1741 | b | S1557 | b | ||
| L1737 | a | E1364 | e | E1220 | a | N1936 | b | T1586 | b | R1404 | b | L2088 | c | D1742 | c | D1558 | c | ||
| S1738 | b | S1365 | f | N1221 | b | K1937 | c | D1587 | c | E1405 | c | L2089 | d | L1743 | d | L1559 | d | ||
| R1739 | c | F1367 | g | Q1222 | c | T1938 | d | V1588 | d | A1406 | d | L2090 | e | E1744 | e | E1560 | e | ||
| I1740 | d | P1366 | a | T1223 | d | D1939 | e | K1589 | e | Q1407 | e | D2091 | f | R1745 | f | S1561 | f | ||
| Q1741 | e | K1368 | b | A1224 | e | L1940 | f | V1590 | f | L1408 | f | R2092 | g | K1746 | g | E1562 | g | ||
| K1742 | f | E1369 | c | L1225 | f | I1941 | g | T1591 | g | A1409 | g | L2093 | a | Y1747 | a | A1563 | a | ||
| R1743 | g | Q1370 | d | E1226 | g | S1942 | a | A1592 | a | L1410 | a | K2094 | b | E1748 | b | R1564 | b | ||
| F1744 | a | Q1371 | e | I1227 | a | E1943 | b | D1593 | b | G1411 | b | P2095 | c | D1749 | c | K1565 | c | ||
| Q1745 | b | E1372 | f | E1228 | b | S1944 | c | M1594 | c | N1412 | c | L2096 | d | N1750 | d | Q1566 | d | ||
| K1746 | c | E1373 | g | E1229 | c | L1945 | d | V1595 | d | A1413 | d | K2097 | e | Q1751 | e | E1567 | e | ||
| P1747 | d | Q1374 | a | L1230 | d | A1946 | e | K1596 | e | A1414 | e | T2098 | f | K1752 | f | A1568 | f | ||
| Q1748 | e | A1375 | b | N1231 | e | S1947 | f | E1597 | f | A1415 | f | L2099 | g | Y1753 | g | A1569 | g | ||
| E1749 | f | R1376 | c | R1232 | f | R1948 | g | A1598 | g | D1416 | g | E2100 | a | L1754 | a | I1570 | a | ||
| K1750 | g | L1377 | d | K1233 | g | G1949 | a | L1599 | a | A1417 | a | E2101 | b | E1755 | b | M1571 | b | ||
| L1751 | a | L1378 | e | Y1234 | a | K1950 | b | E1600 | b | T1418 | b | N2102 | c | D1756 | c | D1572 | c | ||
| K1752 | b | D1379 | f | E1235 | b | A1951 | c | E1601 | c | E1419 | c | L2103 | d | K1757 | d | Y1573 | d | ||
| A1753 | c | E1380 | g | Q1236 | c | V1952 | d | A1602 | d | A1420 | d | S2104 | e | A1758 | e | N1574 | e | ||
| L1754 | d | L1381 | a | A1237 | d | L1953 | e | E1603 | e | K1421 | e | R2105 | f | Q1759 | f | R1575 | f | ||
| K1755 | e | A1382 | e | K1238 | e | Q1954 | f | K1604 | f | N1422 | f | N2106 | g | E1760 | g | D1576 | g | ||
| E1756 | f | G1383 | f | N1239 | f | R1955 | g | A1605 | g | K1423 | g | L2107 | a | L1761 | a | I1577 | a | ||
| A1757 | g | K1384 | g | I1240 | g | S1956 | a | Q1606 | a | A1424 | a | S2108 | b | V1762 | b | A1578 | b | ||
| N1758 | a | L1385 | a | S1241 | a | S1957 | b | V1607 | b | H1425 | b | E2109 | c | R1763 | c | E1579 | c | ||
| S1759 | b | Q1386 | b | Q1242 | b | R1958 | c | A1608 | c | E1426 | c | I2110 | d | L1764 | d | I1580 | d | ||
| L1760 | c | S1387 | c | D1243 | c | F1959 | d | A1609 | d | A1427 | d | K2111 | e | E1765 | e | I1581 | e | ||
| L1761 | d | L1388 | d | L1244 | d | L1960 | e | E1610 | e | E1428 | e | L2112 | f | G1766 | f | K1582 | f | ||
| S1762 | e | D1389 | e | E1245 | e | K1961 | f | K1611 | f | R1429 | f | L2113 | g | E1767 | g | D1583 | g | ||
| E1962 | g | A1612 | g | I1430 | g | I2114 | a | V1768 | a | I1584 | a | ||||||||
| S1963 | a | I1613 | a | A1431 | a | S2115 | b | R1769 | b | H1585 | b | ||||||||
| V1964 | b | K1614 | b | S1432 | b | R2116 | c | S1770 | c | N1586 | c | ||||||||
| G1965 | c | Q1615 | c | A1433 | c | ||||||||||||||
Heptad positions are given for each residue. Residues participating in cross-links (Table S4) are styled in boldface.
Fig. 5.
Laminin coiled-coil register obtained from molecular dynamics simulations subject to cross-linking restraints. (A) Snapshot from a simulation (last frame of Movie S1). BS3/SDH and zero-length cross-links are magenta and green, respectively. (B) Intersubunit contact map between α and β (α-γ and β-γ maps are in Fig. S2). The map is colored according to the probability for pairs of residues to be found in closest contact from the simulations. Contiguous diagonal contacts indicate a continuous register (e.g., α2016 to α2068). Parallel diagonal sets indicate alternative registers (e.g., α1619 to α1689). A register shift appears as a step between two parallel diagonals (e.g., between α1991 and α2033). (C) The uncertainty of the predicted register is estimated for each amino acid residue (x axes) by the residue range in another subunit that comprises 95% of observed nearest-neighbor contacts (y axes). Dashed lines mark one and two heptads.
Table S4.
| BS3/SDH | Zero length | BS3/SDH | Zero length | BS3/SDH | Zero length | ||
| α1742-β1368 | α1746-β1372 | α1943-γ1401 | α1950-γ1416 | α2094-γ1555 | β1746-γ1560 | ||
| α1746-β1368 | α1746-β1379 | α1943-γ1405 | α1950-γ1419 | α2094-γ1565 | β1746-γ1567 | ||
| α1746-γ1233 | α1750-β1372 | α1950-γ1423 | β1596-β1600 | α2101-γ1572 | α2097-α2101 | ||
| α1749-β1379 | β1373-γ1233 | α1962-β1610 | β1603-β1604 | β1757-γ1582 | β1749-β1752 | ||
| α1734-α1735 | α1961-γ1423 | γ1401-γ1402 | β1757-β1760 | ||||
| β1368-β1369 | γ1419-γ1423 | ||||||
| β1368-β1372 |
The residues involved in cross-links are styled in boldface in Table S3.
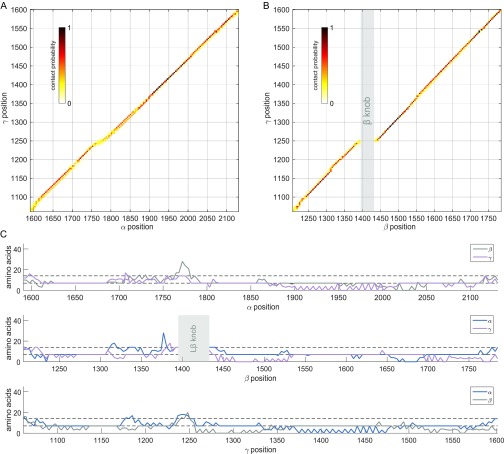
During the laminin simulations, the subunit alignment downstream of the Lβ knob, including segments not particularly rich in observed cross-links, converged to a narrow register range. Specifically, 95% of the nearest-neighbor observations fell within a range of two heptads and 80% within one heptad in this region of about 300 amino acids (Fig. 5C, Fig. S2, and SI Materials and Methods). Another segment of about 100 residues upstream of Lβ also converged with similar precision. The coiled-coil register in the immediate vicinity of Lβ was somewhat more variable in the simulations, tending to absorb the two- to four-heptad register readjustment between the α subunit and the other chains noted above. Variability in the Lβ knob region may reflect real flexibility or may be due to insufficient structural information on the knob itself as input to the simulations. Overall, the simulations are consistent with the manual modeling of specific coiled-coil regions (Fig. S1) and extended the register prediction over most of the laminin coiled coil.
Fig. S2.
Laminin coiled-coil register obtained from molecular dynamics simulations subject to cross-linking restraints. Contact maps between (A) α and γ and (B) β and γ were generated as for the contact map between α and β presented in Fig. 5B. (C) The uncertainty of the predicted register is shown as in Fig. 5C but using a range comprising 80% of the observed close contacts.
Functional Implications for Laminin Coiled-Coil Register Assignment.
The binding site of the heparan sulfate proteoglycan agrin was mapped to residues γ1329 to γ1348 in the laminin coiled coil (11). A coiled-coil framework was necessary for binding activity (11), but further progress in understanding the physical basis for this interaction has been hampered by the unknown register of the three subunits (41). The laminin coiled-coil simulations, restrained by one BS3 and two zero-length links in this region, place the agrin-binding stretch of the γ subunit opposite residues 1863–1880 of the α subunit and 1517–1533 of the β subunit, with a precision of about one heptad. Interestingly, a missing c position in the heptad prediction for the α subunit in this region suggests that an irregularity in the coiled coil may contribute to the structural signature recognized by agrin.
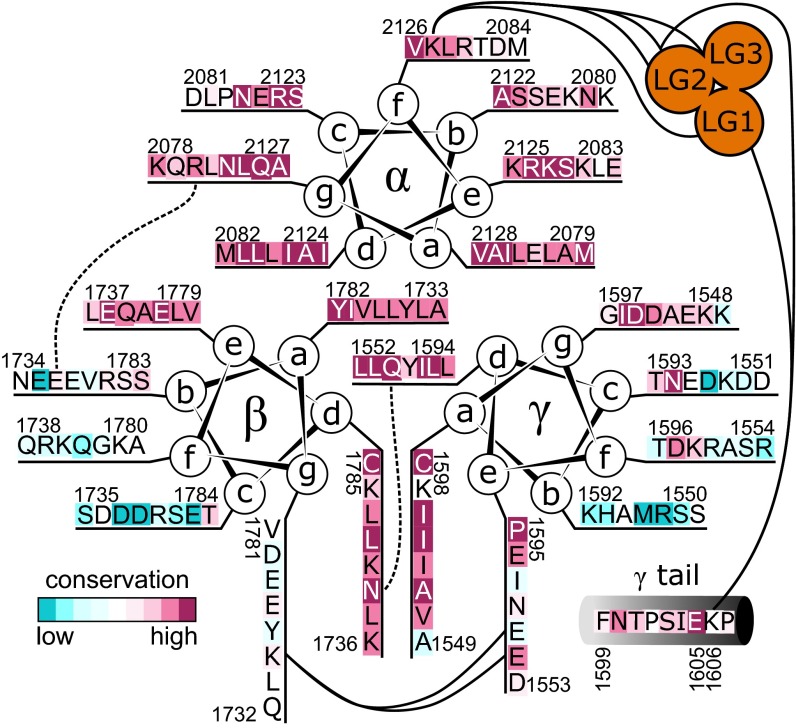
Another functionally important region of the laminin coiled coil is its carboxyl terminus, which may guide oligomerization and alignment of the three subunits (42). An accurate register assignment of this region is essential for understanding the physical basis of laminin assembly. We note that the preferred MARCOIL designation for the laminin carboxyl terminus differs by half of a heptad from a previous assignment (27, 42). Approximately 40 amino acids from the carboxyl terminus, we observed zero-length links between β K1746 and both γ E1560 and E1567 (Figs. S1 and S3). To form these links, K1746 would have to be in a g position, midway between the two glutamates at e positions (Fig. 6). The overall effect is that the β subunit is rotated such that what were previously considered d and g positions (27) become a and d positions (Fig. 6). The heptad assignment and register fixed by the direct links in turn place a conserved glutamate, β E1748, previously identified as critical for trimer assembly (42), adjacent to a conserved basic residue, α R2092 (Fig. 6 and Fig. S1). In addition, the new heptad assignment places at a d position the conserved asparagine β N1750, where it would be adjacent to the highly conserved glutamine γ Q1566, also at a d position. It is noteworthy that N1750 is substituted by glycine in laminin β3 chains. It has been observed that β3 forms heterodimeric assembly intermediates with γ2 but not with the γ1 or γ3 paralogs (35). The γ2 subunit differs from γ1 and γ3 at the residue immediately following the conserved Q1566, possessing an arginine instead of a glutamate. Speculatively, this γ2 arginine may compensate for the missing β3 asparagine, a potential factor in formation of β3/γ2 complexes.
Fig. S3.
MS2 spectra of β subunit K1746 linked to γ subunit E1560 and E1567.
Fig. 6.
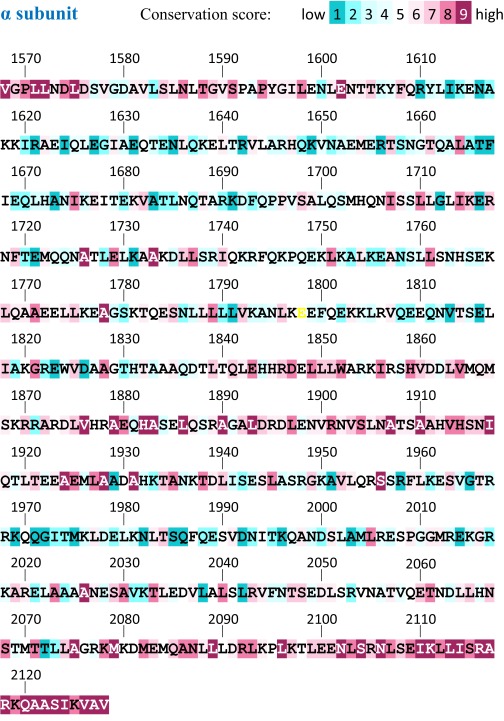
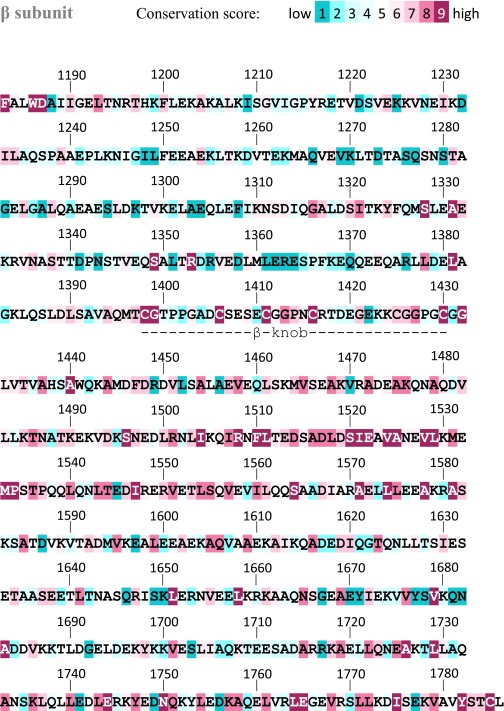
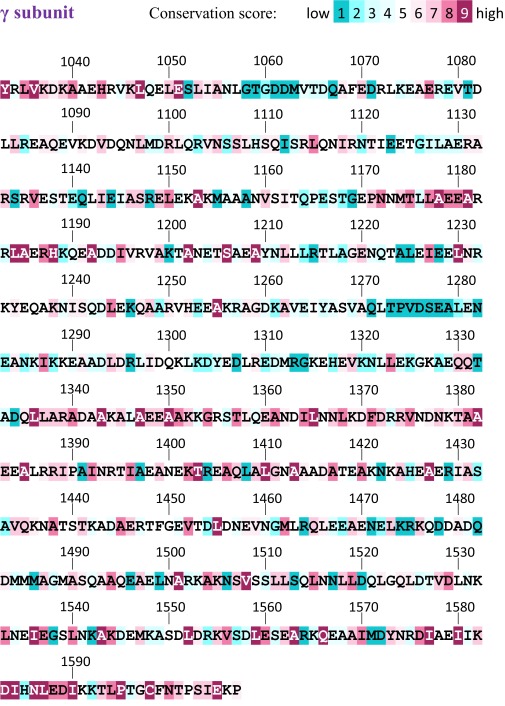
Carboxyl terminus of the laminin coiled coil. Helical wheels with residues colored according to conservation (50). The γ subunit segment downstream of the coiled coil (γ tail) is also displayed. Solid curves indicate cross-links, and dashed curves indicate proposed interactions discussed in the text. Conservation scores for the entire laminin coiled coil are in Figs. S7–S9.
Fig. S7.
Conservation scores for the laminin coiled-coil α subunit. Multiple sequence alignments were prepared and scored as described in SI Materials and Methods. The yellow glutamate is a residue for which insufficient information was available from the alignment to assign a score.
Fig. S8.
Conservation scores for the laminin coiled-coil β subunit.
Fig. S9.
Conservation scores for the laminin coiled-coil γ subunit.
Additional Quaternary Structural Insights from Laminin Cross-Links.
Many cross-links were observed between β and γ in the carboxyl-terminal region of the coiled coil, but few cross-links were seen coupling α to either β or γ (Fig. 4). Over the last 75 coiled coil residues, the α subunit has fewer cross-linkable residues and is more hydrophobic than the comparable regions of β and γ. The α sequence is also more conserved than the other subunits in noncore positions (Fig. 6). Hydrophobicity and conservation suggest constraints on α chain evolution in addition to maintenance of the coiled coil. Notably, one function of the coiled-coil terminus may be to stabilize the quaternary structural arrangement of the LG domains and support their adhesion functions.
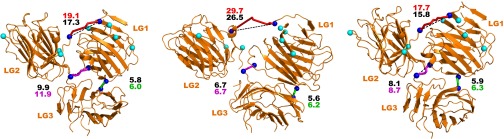
The structures of all five laminin LG domains were previously determined in two sets (14, 15). In the structure of LG1 through LG3, LG1 makes a domain swapping interaction to the LG2–LG3 pair from a different molecule (15), as the tight cloverleaf association of contiguous LG1, LG2, and LG3 domains suggested by electron microscopy (43) was lost. We observed two cross-links between LG1 and LG2–LG3: a zero-length link between LG1 and LG2, and an SDH link between LG1 and the LG2–LG3 junction. By use of these links as restraints, docking with HADDOCK (44) restored the cloverleaf arrangement (Fig. S4). In addition to the cross-links between LG domains, four distinct cross-links tethered the LG domains to the coiled coil. The four links affixed LG1, two positions in LG2, and the LG2/LG3 junction to a single α subunit residue, α K2119, indicating that much of the cloverleaf is near the α side of the coiled-coil base but does not bury position K2119.
Fig. S4.
Three possible structures of LG1, LG2, and LG3 domains as suggested by molecular docking under constraints from observed cross-links. Cβ atoms of linked side chains for cross-links between the LG domains are indicated by blue spheres (Cα for glycine). Residues found to be linked to the carboxyl-terminal region of the coiled coil are indicated by cyan spheres. Cross-links were mapped by homology onto the structure of the α2 LG domains (extracted from PDB ID code 2WJS). Solvent accessible surface distances between Cβ atoms of linked residues are shown as a magenta tube (SDH link), green tube (zero-length link), or red tube (polypeptide link). Direct Cβ-Cβ distances are indicated by dashed lines. Distances in Ångstrom calculated according to the two routes are shown for each cross-link in the appropriate color.
No cross-links between the LG domains and the β and γ coiled-coil regions were observed, but a short carboxyl-terminal tail of the γ subunit downstream of the coiled coil was cross-linked to LG domains. Specifically, residue γ K1606, the second to last amino acid of the γ subunit, was linked to LG1 (α K2277) and LG2 (α K2467). K1606 is adjacent to a conserved glutamate (γ E1605) (Fig. 6), shown previously to be crucial for integrin binding (45). The cross-linking results support a model in which the γ chain tail is in the vicinity of the LG1/LG2 interface and may assist in forming the cloverleaf association, explaining why the crystal structure of LG1 through LG3, which lacked the coiled coil and γ chain tail, did not form the cloverleaf and why removal of γ E1605 undermined integrin binding (45). The LG docking models and observed interactions with residues near the coiled-coil terminus, while yielding only approximate orientations, nevertheless provide molecular insight into self-assembly of cell-binding regions of laminin.
SI Materials and Methods
Cross-Linking and Sample Preparation for LC-MS/MS.
Laminin-111 from Engelbreth–Holm–Swarm murine sarcoma (Gibco 23017-015) at a concentration of ∼1 mg/mL in 50 mM Tris, pH 7.5, and 150 mM NaCl, was dialyzed against 20 mM Hepes, pH 8.3, or PBS, pH 7.0, for amine or carboxylic acid cross-linking, respectively. For amine cross-linking, laminin was mixed with 2.5 mM BS3 H12/D12 cross-linker (Creative Molecules) dissolved in water. For carboxylic acid cross-linking, laminin was mixed with ∼50 mM SDH H12/D12 cross-linker (Creative Molecules) and 50 mM coupling reagent DMTMM [4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride; Sigma-Aldrich, St. Louis, MO]. The reactions were incubated for 1 h at 37 °C, and then quenched with 100 mM Tris, pH 8.0, or 100 mM sodium acetate, pH 7.0, for BS3 or SDH, respectively. The cross-linked samples were reduced with DTT and then applied to a 7.5% (wt/vol) SDS/PAGE gel, which, after electrophoresis, was stained with Coomassie Brilliant Blue. The band migrating at the appropriate size for a laminin trimer was selected for analysis by MS. Additional cross-linked samples were prepared and proteolytically cleaved in solution for MS analysis, without the SDS/PAGE step.
Gel bands were excised, sliced into 1- to 2-mm pieces, and placed into a 0.65-mL silicon tube (PGC Scientific). Gel bands were destained with 25 mM NH4HCO3 in 50% (vol/vol) acetonitrile in water and then completely dried in a rotary evaporator. Disulfide bonds were reduced by saturating the dry gel bands with 10 mM DTT (Sigma-Aldrich) in 25 mM NH4HCO3 at 56 °C for 1 h. Sulfhydryls were then alkylated with 55 mM iodoacetamide (Sigma-Aldrich) in 25 mM NH4HCO3 in the dark for 45 min at room temperature. After washing with 25 mM NH4HCO3 in 50% (vol/vol) acetonitrile and drying in a rotary evaporator, proteins were digested by rehydrating the gel pieces with 12.5 ng/μL trypsin (Promega) in 25 mM NH4HCO3 at 4 °C for 10 min followed by overnight incubation at 37 °C. Peptides were then extracted by addition of 50% (vol/vol) acetonitrile/5% (vol/vol) formic acid, vortexing, sonicating, centrifuging, and collecting the supernatant. Samples were dried and stored in −80 °C until further analysis.
Cross-linked laminin in solution was reduced by incubation with 5 mM DTT for 30 min at 60 °C, followed by alkylation with 10 mM iodoacetamide in the dark for 30 min at 21 °C. Proteins were then subjected to digestion with trypsin [1:50 (wt/wt) trypsin/protein] overnight at 37 °C. The digestion was stopped by 1% trifluoroacetic acid. In other experiments, digestion of cross-linked laminin was performed overnight with chymotrypsin [1:50 (wt/wt)] or Glu-C protease [1:50 (wt/wt)] at 37 °C, at which point trypsin was added, and the reactions were incubated for a further 4 h. Following digestion, peptides were desalted using solid-phase extraction columns (Oasis HLB; Waters), rotary evaporated to dryness, and then stored at −80 °C until further analysis.
LC and MS.
ULC/MS grade solvents were used for all chromatographic steps. Each sample was dissolved in 25 μL 3% (vol/vol) acetonitrile/0.1% formic acid and analyzed using nano-Ultra Performance Liquid Chromatography (10 kpsi nanoAcquity; Waters). The mobile phase was as follows: (A) H2O/2% (vol/vol) DMSO/0.1% formic acid and (B) acetonitrile/2% (vol/vol) DMSO/0.1% formic acid. Desalting (trapping) of the peptides was performed online using a reversed-phase C18 trapping column (180-µm internal diameter, 20 mm length, 5-µm particle size; Waters). The peptides were then separated using a HSS T3 nano-column (75-µm internal diameter, 250 mm length, 1.8-µm particle size; Waters) at 0.35 µL/min. Peptides were eluted from the column into the mass spectrometer using the following gradient: 4–35% B in 65 min, 35–90% B in 5 min, maintained at 90% for 5 min, and then back to initial conditions.
The nanoUPLC was coupled online through a nanoESI emitter (10-μm tip; New Objective) to a quadrupole orbitrap mass spectrometer (Q Exactive +; Thermo Scientific) using a FlexIon nanospray apparatus (Proxeon). Data were acquired in data dependent acquisition (DDA) mode using a Top20 method. MS1 resolution was set to 60,000 (at 400 m/z), and maximum injection time was set to 20 ms. MS2 resolution was set to 17,500 and maximum injection time of 60 ms. Quadrupole isolation was set to 1.6 m/z.
Cross-Link Identification.
Cross-links were identified with Rosetta Elucidator System, version 3.3 (Rosetta Biosoftware) and the web version of xQuest. The stand-alone version 2.1.1 of xQuest (33, 51) was used to analyze multienzyme digestion and zero-length cross-linking experiments. Elucidator was used to pair MS1 peaks based on the mass shift of the H12 vs. D12 cross-linkers. Parameter settings: retention time tolerance ±0.35 min, m/z tolerance ±10 ppm, z > 2, and light/heavy ratio 0.67–1.5. The web version of xQuest was run with the appropriate cross-linker mass parameters, MS1 tolerance of 10 ppm, MS2 range of 100–1600 m/z with 0.8 m/z tolerance, peptide size of 3–40 amino acids, and oxidation of methionine as a variable modification. The installable version of xQuest was run in enumeration mode with similar parameters except for peptide size of 4–40 and a minimum common peak number of 10. To identify zero-length cross-links, xQuest was run with light-only parameters. A cross-linker molecular weight of −18.0153 g/mol was used to account for the lost water upon amide bond formation between side chains.
Hits from xQuest were manually inspected to distinguish good and poor matches. MS spectra with low fragment ion coverage were rejected regardless of their score. MS spectra assigned to more than one peptide pair with similar coverage and score were also rejected. Finally, spectra with low scores were rejected without inspection. Because xQuest mis-assigned a few spectra as representing direct cross-links between acidic residues, we manually reassigned these links to involve a nearby lysine, yielding assignments that were both chemically reasonable and compatible with the observed data. In a few instances, a single MS spectrum was assigned to represent two cross-links, because fragmentation data provided convincing evidence for both pairings being represented.
Coiled-Coil Modeling.
An ideal three-stranded parallel coiled coil was generated using the Coiled-Coil Crick Parameterization (CCCP) program (36). Default values for a three-stranded coiled coil were used: rise per residue of 1.51 Å, superhelical radius 6.12 Å, superhelical frequency −2.94° per amino acid (negative value indicates left-handed superhelix), pitch angle −12.01°, α-helical radius 2.26 Å, α-helical frequency 102.857° per amino acid, α-helical phase −9°, −9°, −9°, superhelical phase offset 120°, 240°.
Chain Arrangement Determination.
An ideal model of a three-heptad-long (21 residues), parallel, trimeric coiled coil was generated. SASDs were calculated between exposed positions of the middle heptad (b9, c10, e12, f13, g14) of one chain and exposed positions of the other two chains. The SASDs are summarized in Tables S1 and S2. The heptad positions of the observed cross-linked residues were predicted with MARCOIL.
Construction of Multiple Sequence Alignments.
For each laminin subunit (LAMA1_MOUSE, LAMB1_MOUSE, and LAMC1_MOUSE in UniProt), the coiled-coil segment was used as the query for a sequence similarity search. PSI-BLAST (52) with a maximum of three iterations was performed to search for homolog sequences between each subunit query sequence and all of the protein sequences in vertebrates that were downloaded from UniprotKB on 24 June 2015. Sequence hits with at least 35% coverage to the query sequence (i.e., positive matches divided by query length) were considered for manual curation of their annotations. Finally, a multiple sequence alignment (MSA) was constructed for each subunit based on the relevant sequences from the PSI-BLAST search using the MUSCLE software tool, version 3.8.31 (53). MSAs were used as input to ConSurf (50) for estimating and visualizing the evolutionary conservation of amino acid positions in the predicted coiled-coil regions.
Coarse-Grained Molecular Dynamics Simulations.
A coarse-grained model was used in which each amino acid in the ideal, symmetric, trimeric coiled coil with the geometrical parameters given above was represented as a single bead. The beads were assigned to laminin α chain residues 1556–2133, β chain residues 1174–1396 and 1433–1786, and γ chain residues 1038–1603, according to our best guess of the register based on visual inspection of the observed cross-links. Five beads in the ideal coiled-coil model were left unassigned between β chain residues 1396 and 1433, the region corresponding to the Lβ knob. To model the knob, the five unassigned beads were removed, and a loop of 36 beads was inserted into the gap. The purpose of including the Lβ knob in this manner was to provide very weak restraints on the ends of the β subunit helices to either side of the knob rather than to accurately model the knob, whose structure and disulfide connectivity are unknown. In addition to the initial model described above, six more starting models were generated by displacing each strand individually four heptads in the amino- or carboxyl-terminal direction.
Simulations were carried out based on methods described in Azia et al. (54). To generate the force field, bonds and angles between the beads were given Hookian potentials and dihedral angles were given a cosine potential around the minimum-energy values of ideal coiled-coil geometry. Proline and glycine were treated as any other amino acid. Contacts between beads were assigned a Lennard–Jones-like potential. Only intrasubunit contacts were based on ideal coiled-coil distance values; interchain contacts were treated separately as described below. Excluded volume repulsion was set at 4 Å between beads not forming bonds or contacts. Force constants for the potentials were set at 100 kcal/mol for bonds, 20 kcal/mol for angles, and 1 kcal/mol for dihedrals, contacts, and repulsions. The dihedral term was not applied to the Lβ knob. Beads in the knob were linked at 3.8 Å and 109.5° with force constants of 1 and 20 kcal/mol, respectively.
In addition to the potential governing intrasubunit geometry, interactions between subunits and representations of the experimental intersubunit cross-links were incorporated as follows. A favorable contact potential, described by a Lennard–Jones term, was applied among coiled-coil core amino acids (i.e., a and d positions) on separate subunits with no regard to chain register. Residues predicted above a 10% cutoff to participate in a coiled coil and to be at a or d positions according to MARCOIL were included. These interchain contacts, totaling 68,059 in number, were set to values of 6.9, 7.7, and 7.3 Å between a and a, d and d, and a and d, respectively. It should be noted that, due to predicted irregularities in the heptad repeat, the a and d positions in the starting model did not necessarily fall at core positions in the ideal coiled. Thus, a first stage of the simulation was performed to allow the ideal coiled coil to relax under the core-contact potential. This step in the simulation minimized topological conflicts on introduction of the cross-link restraints. Cross-links were added to the model by representing atoms in the cross-linker backbone and linked side chains: BS3 and SDH links were given 20 atomic beads between the appropriate amino acid beads, and direct cross-links were given eight atoms. The force field for cross-linker beads included bond and angle terms with optimal values of 1.5 Å and 109.5° and force constants of 1 and 20 kcal/mol, respectively. Disulfides at the amino and carboxyl termini of the coiled coil were represented by placing a restraint with an optimal distance value of 5.6 Å and a very weak force constant of 0.01 kcal/mol between the relevant beads to prevent unraveling of chain termini without affecting chain register.
Simulations were carried out using the Langevin equation. The temperature term was set at 0.6 arbitrary units. For each of the seven starting models, 100 simulations with different random seed values were performed for 106 time steps each. For each of the 700 simulations, the nearest core-position neighbors in the other two subunits were determined for each core position in each subunit. Nearest neighbors were calculated for the final 105 time steps of each simulation, as steady state was reached by this point. Nearest neighbors were selected as the median amino acid position calculated from 100 coordinate sets (the last 105 time steps sampled every 103 steps). Nearest-neighbor contacts depended somewhat on the reference subunit, such that, for example, the set of contacts from subunit α to β was similar but not identical to the set of contacts from subunit β to α. Therefore, both sets were merged to generate nearest-neighbor histograms. The residue ranges containing 95% and 80% (Fig. 5C and Fig. S2) of nearest-neighbor contacts were calculated from the resulting merged set.
Molecular Docking with HADDOCK.
The HADDOCK2.2 webserver was used in multibody interface mode with default parameters. One thousand structures for rigid body docking, 200 structures for semiflexible and solvent refinement, and an RMSD of 7.5 Å were used for clustering. The input structures for the three LG domains were based on PDB ID code 2WJS: LG1, residues 2142–2324; LG2, residues 2333–2519; LG3, residues 2520–2707. Unambiguous distance restraints had upper bounds of the following: residues 2259 and 2543, 8 Å; residues 2251 and 2520, 34 Å; residues 2324 and 2333, 29 Å; residues 2519 and 2520, 4 Å.
Discussion
To map the laminin quaternary structure, we sought to obtain intersubunit cross-links densely and extensively over the assembly. By use of three cross-linking chemistries, we obtained 69 cross-links between subunits in the coiled coil. It is likely that more cross-links formed but remained undetected by MS because of nonoptimal peptide properties, such as length or posttranslational modification (Figs. S5 and S6). From the data acquired, certain key regions, such as the carboxyl terminus of the coiled coil, were directly highly constrained (Fig. 6 and Fig. S1). In contrast, other regions were somewhat underdetermined. Due to the BS3 and SDH linker lengths, each cross-link fixes the register within only about three heptads to either direction. Greater insight was obtained, however, by developing new tools to integrate information from multiple cross-links. Coarse-grained molecular dynamics simulations in the context of coiled-coil geometry and heptad prediction maximized the information extractable from the entire experimental dataset (Fig. 5). This approach yielded a chain register prediction to within two heptads over most of the coiled-coil length, which was sufficient to achieve insights into laminin assembly and will help guide future studies of laminin interactions with other proteins.
Fig. S5.
Sequence coverage by MS of the laminin α subunit. Amino acids observed in reliable peptide identifications, whether cross-linked or not, are highlighted in yellow. Predicted N-linked glycosylation sites that were detected as unmodified in at least one reliably identified MS spectrum are highlighted in green. Predicted N-linked glycosylation sites that were not detected in unmodified form, and thus may be glycosylated, are highlighted in cyan.
Fig. S6.
Sequence coverage by MS of the laminin β and γ subunits. See legend to Fig. S5.
One of the central issues underlying laminin structure is the order of the three subunits in the coiled coil. In the laminin assembly mechanism, β and γ are proposed to form a preliminary heterodimer, onto which α docks to complete the trimer (46). Therefore, the subunit order is determined by which side of the β-γ interface the α subunit binds. Two major determinants of the preferred order are the geometric packing of residues at a and d positions and electrostatic interactions among other positions, primarily e and g. A previous attempt at assigning the chain order was based on electrostatics; in the absence of information on subunit register, the alignment was adjusted to optimize intersubunit ionic interactions (24). Our approach, which was independent of subunit register and relied on experimental data, led to the opposite conclusion regarding the subunit order: a counterclockwise arrangement of α, β, and γ when viewed from the carboxyl terminus (Fig. 3).
The chain order established herein supports the subunit arrangement accompanying a suggested mechanism for the multistep assembly of laminin trimers (27). However, the heptad position assignment in the suggested mechanism differs somewhat from our prediction (Fig. 6). In particular, the β subunit is rotated by one position and shifted by half a heptad. It is noteworthy that the tension between these two heptad and register assignments may reflect a key aspect of laminin structure. Whereas both models for the carboxyl terminus of the laminin coiled coil were based on an ideal, symmetric trimer (27) (Fig. 6), the laminin heterotrimer is most probably asymmetric, leading to deviations from the convention in which only a and d positions participate directly in coiled-coil core interactions.
Irregularities in hetero-oligomeric coiled coils may be the rule rather than the exception and may contribute both to function and to specificity of assembly. The blood clotting factor fibrinogen, which contains a coiled coil spanning about 160 Å, is currently the most extensive source of structural information on heterotrimeric coiled-coil assembly. X-ray crystal structures of fibrinogen reveal kinks, asymmetric superhelix crossing angles, and short, nonhelical segments looping out of the coiled coil (39, 40). Notably, glycines are accommodated with no disruption to coiled-coil helices, single prolines are accommodated with a minor kink, and two prolines separated by six intervening residues are observed to locally break the helix. Laminin may accommodate such sequence features in a similar manner. The laminin γ subunit contains a moderately conserved proline pair separated by four to seven amino acids, whereas β contains a highly conserved proline pair separated by two intervening amino acids. Although paired proline residues are indeed likely to cause local disruptions in the helix, fragmentation in laminin coiled-coil potential predictions (21) may not always indicate actual breaks in the helical assembly. Fibrinogen demonstrates how local aberrations may produce kinks or bends without undermining the coiled-coil framework.
The analyses presented in this study were based on a threefold symmetric, ideal coiled coil as a starting model. Notably, the two competing chain arrangements for α, β, and γ could be distinguished robustly using the cross-linking data interpreted on the backdrop of the ideal trimer (Fig. 3). For more subtle insights into heptad position assignments and register determination of specific coiled-coil regions, conflict between the cross-linking data and the ideal model can be exploited. For example, we found two neighboring zero-length cross-links between β and γ that were inconsistent with a continuous heptad repeat between them. These cross-links were between β subunit g-position residues E1725 and K1746 and γ residues K1544 and E1560, assigned by MARCOIL as c and e positions, respectively. According to standard coiled-coil geometry, g positions can form direct links to e or possibly b positions, but not c positions. Thus, a conflict between cross-linkability and ideal coiled-coil geometry arises. We interpret the cross-linking results to indicate deviations from ideal geometry and heptad continuity in this region.
In addition to the cross-links introduced into laminin in this study, a natural cross-link clamps the coiled-coil base: a disulfide bond links the β and γ subunits near their carboxyl termini (46). A disulfide similarly bridges two subunits at the fibrinogen coiled coil (39, 40). With the caveat that the laminin disulfide may lie outside the region that is formally a coiled coil, it should constrain the β/γ subunit register. Indeed, the introduction of cysteine residues into coiled coils has been used as an experimental strategy to determine coiled-coil register (47). In the fibrinogen structure, the bonded cysteines are at d and a positions. The corresponding laminin cysteines were previously proposed to be at g and a positions in β and γ (27). If the laminin coiled coil includes the disulfide, our cross-linking data suggest that the cysteines would be at d and a positions in β and γ, respectively, comparable to the fibrinogen positioning.
We present here a definitive experimental determination of the subunit order and a prediction of the subunit register for the laminin coiled coil. In addition, we obtained insights into the location of the Lβ knob with respect to the other coiled-coil subunits and into the association of LG domains with the carboxyl terminus of the coiled coil, a requirement for integrin binding (45). The functional importance of the laminin protein family in interaction and signaling between cells and the ECM warrants continued efforts to explore the structure and dynamics of this remarkable macromolecule. The data and analyses presented herein will facilitate future high-resolution structural studies of additional laminin segments. The methods described in this work extend the application and impact of cross-linking and mass spectrometry to proteins with repetitive structures that can be parameterized, including many proteins in the cell microenvironment. These proteins have been the focus of numerous molecular and cell biology studies, but they continue to be recalcitrant to high-resolution structure determination in their full-length, native assemblies.
Materials and Methods
Laminin-111 was purchased from Gibco and cross-linkers from Creative Molecules. Cross-linking reactions, MS data acquisition, MS and amino acid sequence analyses, and the design and execution of cross-link–constrained simulations are described in SI Materials and Methods. MS data have been deposited in the ProteomeXchange Consortium via the PRIDE (48) partner repository with the dataset identifier PXD004898.
Supplementary Material
Acknowledgments
We thank E. Levy for critical reading of the manuscript and helpful suggestions. D. Merhav and D. Elinger prepared samples for MS. H. Greenblatt and K. Levy group members provided infrastructure for simulations. This work was supported by a grant from the Israel Science Foundation, the European Research Council under the European Union’s Seventh Framework Programme, Grant 310649, and the Israeli Center of Research Excellence (I-CORE) in Structural Cell Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (dataset identifier PXD004898).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608424113/-/DCSupplemental.
References
- 1.Miner JH. Laminins and their roles in mammals. Microsc Res Tech. 2008;71(5):349–356. doi: 10.1002/jemt.20563. [DOI] [PubMed] [Google Scholar]
- 2.Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adhes Migr. 2013;7(1):56–63. doi: 10.4161/cam.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO. The evolution of metazoan extracellular matrix. J Cell Biol. 2012;196(6):671–679. doi: 10.1083/jcb.201109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards MM, Lefebvre O. Laminins and retinal vascular development. Cell Adhes Migr. 2013;7(1):82–89. doi: 10.4161/cam.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laperle A, et al. α-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Rep. 2015;5(2):195–206. doi: 10.1016/j.stemcr.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradhan S, Farach-Carson MC. Mining the extracellular matrix for tissue engineering applications. Regen Med. 2010;5(6):961–970. doi: 10.2217/rme.10.61. [DOI] [PubMed] [Google Scholar]
- 7.Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12(3):197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 8.Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29(5):402–410. doi: 10.1016/j.matbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Engel J, et al. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- 10.Aumailley M, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Kammerer RA, et al. Interaction of agrin with laminin requires a coiled-coil conformation of the agrin-binding site within the laminin γ1 chain. EMBO J. 1999;18(23):6762–6770. doi: 10.1093/emboj/18.23.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colognato-Pyke H, et al. Mapping of network-forming, heparin-binding, and α 1 β 1 integrin-recognition sites within the α-chain short arm of laminin-1. J Biol Chem. 1995;270(16):9398–9406. doi: 10.1074/jbc.270.16.9398. [DOI] [PubMed] [Google Scholar]
- 13.Tisi D, Talts JF, Timpl R, Hohenester E. Structure of the C-terminal laminin G-like domain pair of the laminin α2 chain harbouring binding sites for α-dystroglycan and heparin. EMBO J. 2000;19(7):1432–1440. doi: 10.1093/emboj/19.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison D, et al. Crystal structure and cell surface anchorage sites of laminin α1LG4-5. J Biol Chem. 2007;282(15):11573–11581. doi: 10.1074/jbc.M610657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carafoli F, Clout NJ, Hohenester E. Crystal structure of the LG1-3 region of the laminin α2 chain. J Biol Chem. 2009;284(34):22786–22792. doi: 10.1074/jbc.M109.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain SA, Carafoli F, Hohenester E. Determinants of laminin polymerization revealed by the structure of the α5 chain amino-terminal region. EMBO Rep. 2011;12(3):276–282. doi: 10.1038/embor.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carafoli F, Hussain SA, Hohenester E. Crystal structures of the network-forming short-arm tips of the laminin β1 and γ1 chains. PLoS One. 2012;7(7):e42473. doi: 10.1371/journal.pone.0042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran T, Gat Y, Fass D. Laminin L4 domain structure resembles adhesion modules in ephrin receptor and other transmembrane glycoproteins. FEBS J. 2015;282(14):2746–2757. doi: 10.1111/febs.13319. [DOI] [PubMed] [Google Scholar]
- 19.Stetefeld J, Mayer U, Timpl R, Huber R. Crystal structure of three consecutive laminin-type epidermal growth factor-like (LE) modules of laminin γ1 chain harboring the nidogen binding site. J Mol Biol. 1996;257(3):644–657. doi: 10.1006/jmbi.1996.0191. [DOI] [PubMed] [Google Scholar]
- 20.Takagi J, Yang Y, Liu JH, Wang JH, Springer TA. Complex between nidogen and laminin fragments reveals a paradigmatic β-propeller interface. Nature. 2003;424(6951):969–974. doi: 10.1038/nature01873. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman T, Blanco FJ. The coiled-coil structure potential of the laminin LCC domain is very fragmented and does not differentiate between natural and non-detected isoforms. J Biomol Struct Dyn. 2007;24(4):413–420. doi: 10.1080/07391102.2007.10507129. [DOI] [PubMed] [Google Scholar]
- 22.Barlow DP, Green NM, Kurkinen M, Hogan BL. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil α-helix. EMBO J. 1984;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsson M, et al. Evidence for coiled-coil α-helical regions in the long arm of laminin. EMBO J. 1985;4(2):309–316. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck K, Dixon TW, Engel J, Parry DAD. Ionic interactions in the coiled-coil domain of laminin determine the specificity of chain assembly. J Mol Biol. 1993;231(2):311–323. doi: 10.1006/jmbi.1993.1284. [DOI] [PubMed] [Google Scholar]
- 25.Yurchenco PD. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3(2):a004911. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. doi: 10.1146/annurev-cellbio-101011-155750. [DOI] [PubMed] [Google Scholar]
- 27.Nomizu M, et al. Mechanism of laminin chain assembly into a triple-stranded coiled-coil structure. Biochemistry. 1996;35(9):2885–2893. doi: 10.1021/bi951555n. [DOI] [PubMed] [Google Scholar]
- 28.Walzthoeni T, Leitner A, Stengel F, Aebersold R. Mass spectrometry supported determination of protein complex structure. Curr Opin Struct Biol. 2013;23(2):252–260. doi: 10.1016/j.sbi.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Merkley ED, Cort JR, Adkins JN. Cross-linking and mass spectrometry methodologies to facilitate structural biology: Finding a path through the maze. J Struct Funct Genomics. 2013;14(3):77–90. doi: 10.1007/s10969-013-9160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barysz H, et al. Three-dimensional topology of the SMC2/SMC4 subcomplex from chicken condensin I revealed by cross-linking and molecular modelling. Open Biol. 2015;5(2):150005. doi: 10.1098/rsob.150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aumailley M. The laminin family. Cell Adhes Migr. 2013;7(1):48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinner O, et al. Identification of cross-linked peptides from large sequence databases. Nat Methods. 2008;5(4):315–318. doi: 10.1038/nmeth.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitner A, et al. Chemical cross-linking/mass spectrometry targeting acidic residues in proteins and protein complexes. Proc Natl Acad Sci USA. 2014;111(26):9455–9460. doi: 10.1073/pnas.1320298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahraman A, Malmström L, Aebersold R. Xwalk: Computing and visualizing distances in cross-linking experiments. Bioinformatics. 2011;27(15):2163–2164. doi: 10.1093/bioinformatics/btr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdonald PR, Lustig A, Steinmetz MO, Kammerer RA. Laminin chain assembly is regulated by specific coiled-coil interactions. J Struct Biol. 2010;170(2):398–405. doi: 10.1016/j.jsb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grigoryan G, Degrado WF. Probing designability via a generalized model of helical bundle geometry. J Mol Biol. 2011;405(4):1079–1100. doi: 10.1016/j.jmb.2010.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delorenzi M, Speed T. An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics. 2002;18(4):617–625. doi: 10.1093/bioinformatics/18.4.617. [DOI] [PubMed] [Google Scholar]
- 38.Lupas AN, Gruber M. The structure of α-helical coiled coils. Adv Protein Chem. 2005;70:37–78. doi: 10.1016/S0065-3233(05)70003-6. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Kollman JM, Pandi L, Doolittle RF. Crystal structure of native chicken fibrinogen at 2.7 A resolution. Biochemistry. 2001;40(42):12515–12523. doi: 10.1021/bi011394p. [DOI] [PubMed] [Google Scholar]
- 40.Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal structure of human fibrinogen. Biochemistry. 2009;48(18):3877–3886. doi: 10.1021/bi802205g. [DOI] [PubMed] [Google Scholar]
- 41.Mascarenhas JB, et al. Mapping of the laminin-binding site of the N-terminal agrin domain (NtA) EMBO J. 2003;22(3):529–536. doi: 10.1093/emboj/cdg041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utani A, Nomizu M, Timpl R, Roller PP, Yamada Y. Laminin chain assembly. Specific sequences at the C terminus of the long arm are required for the formation of specific double- and triple-stranded coiled-coil structures. J Biol Chem. 1994;269(29):19167–19175. [PubMed] [Google Scholar]
- 43.Bruch M, Landwehr R, Engel J. Dissection of laminin by cathepsin G into its long-arm and short-arm structures and localization of regions involved in calcium dependent stabilization and self-association. Eur J Biochem. 1989;185(2):271–279. doi: 10.1111/j.1432-1033.1989.tb15112.x. [DOI] [PubMed] [Google Scholar]
- 44.de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5(5):883–897. doi: 10.1038/nprot.2010.32. [DOI] [PubMed] [Google Scholar]
- 45.Ido H, et al. The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin γ chains in integrin binding by laminins. J Biol Chem. 2007;282(15):11144–11154. doi: 10.1074/jbc.M609402200. [DOI] [PubMed] [Google Scholar]
- 46.Hunter I, Schulthess T, Engel J. Laminin chain assembly by triple and double stranded coiled-coil structures. J Biol Chem. 1992;267(9):6006–6011. [PubMed] [Google Scholar]
- 47.Weitzel CS, Waldman VM, Graham TA, Oakley MG. A repeated coiled-coil interruption in the Escherichia coli condensin MukB. J Mol Biol. 2011;414(4):578–595. doi: 10.1016/j.jmb.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Vizcaíno JA, et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016;44(D1):D447–D456. doi: 10.1093/nar/gkv1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Combe CW, Fischer L, Rappsilber J. xiNET: Cross-link network maps with residue resolution. Mol Cell Proteomics. 2015;14(4):1137–1147. doi: 10.1074/mcp.O114.042259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glaser F, et al. ConSurf: Identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19(1):163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]
- 51.Leitner A, Walzthoeni T, Aebersold R. Lysine-specific chemical cross-linking of protein complexes and identification of cross-linking sites using LC-MS/MS and the xQuest/xProphet software pipeline. Nat Protoc. 2014;9(1):120–137. doi: 10.1038/nprot.2013.168. [DOI] [PubMed] [Google Scholar]
- 52.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azia A, Levy Y. Nonnative electrostatic interactions can modulate protein folding: Molecular dynamics with a grain of salt. J Mol Biol. 2009;393(2):527–542. doi: 10.1016/j.jmb.2009.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.