Significance
BEST1 is a Ca2+-activated chloride channel found in a variety of cell types that allows chloride to traverse the plasma membrane. Mutations in BEST1 can cause macular degeneration. The mechanisms for anion selectivity and Ca2+-dependent activation of BEST1 are unknown. Here, we show that a hydrophobic “neck” region of the channel’s pore does not play a major role in ion selectivity but acts as an effective gate, responding to Ca2+ binding at a cytosolic sensor. Mutation of a cytosolic “aperture” dramatically affects relative permeabilities among anions. These insights help rationalize how disease-causing mutations in BEST1 affect channel behavior and contribute to a broader understanding of ion channel gating and selectivity mechanisms.
Keywords: calcium-activated chloride channel, CaCC, ion selectivity, channel gating, ion channel biophysics
Abstract
Cytoplasmic calcium (Ca2+) activates the bestrophin anion channel, allowing chloride ions to flow down their electrochemical gradient. Mutations in bestrophin 1 (BEST1) cause macular degenerative disorders. Previously, we determined an X-ray structure of chicken BEST1 that revealed the architecture of the channel. Here, we present electrophysiological studies of purified wild-type and mutant BEST1 channels and an X-ray structure of a Ca2+-independent mutant. From these experiments, we identify regions of BEST1 responsible for Ca2+ activation and ion selectivity. A “Ca2+ clasp” within the channel’s intracellular region acts as a sensor of cytoplasmic Ca2+. Alanine substitutions within a hydrophobic “neck” of the pore, which widen it, cause the channel to be constitutively active, irrespective of Ca2+. We conclude that the primary function of the neck is as a “gate” that controls chloride permeation in a Ca2+-dependent manner. In contrast to what others have proposed, we find that the neck is not a major contributor to the channel’s ion selectivity. We find that mutation of a cytosolic “aperture” of the pore does not perturb the Ca2+ dependence of the channel or its preference for anions over cations, but its mutation dramatically alters relative permeabilities among anions. The data suggest that the aperture functions as a size-selective filter that permits the passage of small entities such as partially dehydrated chloride ions while excluding larger molecules such as amino acids. Thus, unlike ion channels that have a single “selectivity filter,” in bestrophin, distinct regions of the pore govern anion-vs.-cation selectivity and the relative permeabilities among anions.
Eukaryotic bestrophin proteins constitute a family of Ca2+-activated chloride (Cl−) channels (CaCCs) (1–3). The human genome contains four bestrophin paralogs and other mammals have three or four paralogs (BEST1–4), all of which form CaCCs when expressed (4). Mutations in bestrophin channels cause retinal disease, and the broad tissue distribution of the channels (5) suggest additional, yet largely unresolved, physiological roles. BEST1, the most studied paralog, was first identified by genetic linkage to a juvenile-onset macular degeneration disease called “Best disease” (6). Nearly 200 mutations in BEST1 have been associated with retinal degenerative disorders, and the vast majority of those that have been characterized cause Cl− channel dysfunction (7–11). Studies have also indicated that certain bestrophin channels are important for cell volume regulation (12–14).
Electrophysiological studies of bestrophin proteins expressed in cells have elucidated ion selectivity and gating properties of the channels. The channels are activated by intracellular Ca2+ (2, 4, 15), selective for monovalent anions, and exhibit a lyotropic sequence of relative anion permeabilities where SCN− > NO3− > I− > Br− > Cl− (2, 5). However, the mechanisms for ion selectivity and Ca2+-dependent gating, and the regions of the channel involved in these processes, are not known. Making these matters more mysterious, a prokaryotic protein, KpBest, which shares 14% identity to BEST1 and is not Ca2+ activated, is cation selective despite a similar structural architecture to BEST1 (16, 17). Pharmacologically, BEST1 is inhibited by anion channel blockers, including 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate (DIDS), tannic acid, and NPPB (2, 18). The N-terminal region (amino acids 1–390) is highly conserved in eukaryotic bestrophin channels and has been shown to be sufficient for CaCC activity in whole-cell studies (19). The C-terminal remainder of the polypeptide (amino acids 391–585 in human BEST1) is predicted to be unstructured but could potentially be involved in aspects of channel regulation. We have shown that a purified chicken BEST1 construct encompassing the N-terminal region (amino acids 1–405), reconstituted into liposomes and assessed by a flux assay, exhibits activity consistent with a monovalent anion channel activated directly by Ca2+ (16), but no purified eukaryotic bestrophin channel has yet been studied by electrical recordings.
In 2014, the X-ray structure of chicken BEST1, which shares 74% sequence identity with human BEST1, revealed the architecture of the channel (16) (Fig. S1). The structure indicates that bestrophin is a pentamer and that it contains a single ion conduction pore along the channel’s axis of symmetry. Although the structure was suggestive, three fundamental properties of the channel’s function are unknown: Where is the Ca2+ sensor? What portion of the pore acts as a gate to control the flow of Cl− ions in a Ca2+-dependent manner? Finally, what endows the channel with selectivity not only for anions over cations but also its lyotropic permeability sequence among permeant anions?
Fig. S1.
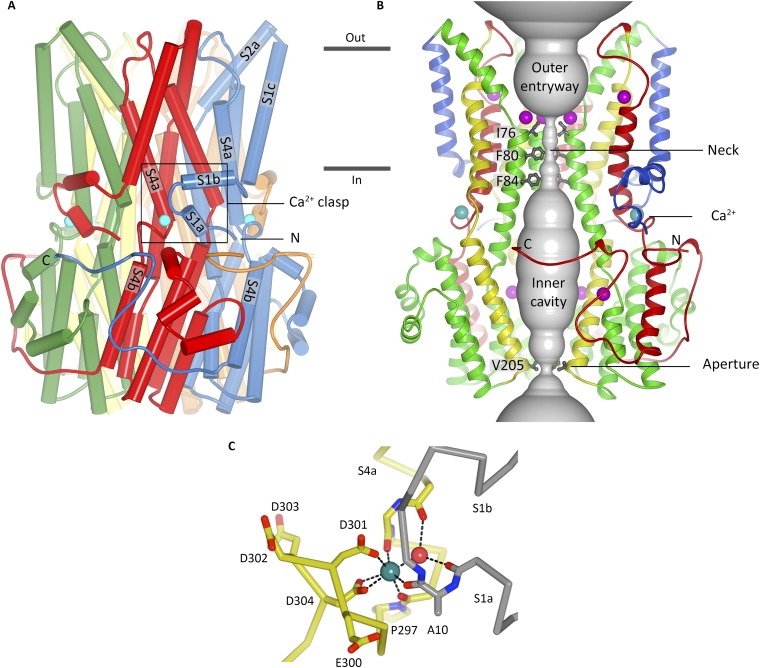
Overall structure, ion pore, and Ca2+ clasp of BEST1 (16). (A) Overall structure of BEST1 is shown where the perspective is from the membrane. Subunits are colored individually, α-helices are depicted as cylinders, and the approximate boundaries of the membrane are indicated. The boxed region highlights a Ca2+ clasp with bound Ca2+ (teal sphere). (B) Within a ribbon representation of three subunits of BEST1 is a representation (gray color) of the minimal radial distance from the center of the pore to the nearest van der Waals protein contact. Secondary structural elements are colored according to their four segments (S1, blue; S2, green; S3, yellow; S4 and C-terminal tail, red), which each contain one transmembrane region. Also depicted are sites for Ca2+ (teal) and Cl− (purple). (C) Coordination in the Ca2+ clasp. The acidic cluster and the backbone carbonyls that coordinate (dotted lines) the Ca2+ (teal sphere) are depicted as sticks on a Cα representation. Dotted lines also indicate hydrogen bonds between the water molecule (red sphere) and the protein (backbone carbonyls of Val9 and Glu292). Carbon atoms of one subunit are gray, and those from another are yellow.
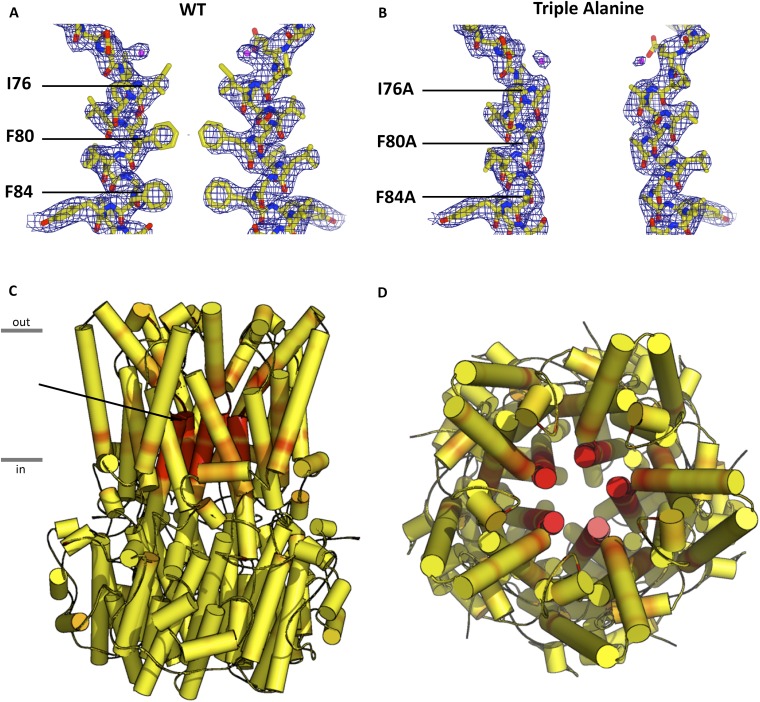
The pore of BEST1, which is an impressive ∼95 Å long, stretches from the extracellular side, traverses the membrane, and continues through a large cytosolic portion of the bestrophin pentamer (Fig. S1) (16). The diameter of the pore varies along its length and it includes two narrow hydrophobic constrictions, the “neck” and the “aperture,” either of which could potentially serve as an effective “gate” that opens and closes in response to Ca2+. Each constriction is just wide enough to accommodate a dehydrated Cl− ion, and although the structure of BEST1 is of a Ca2+-bound state, it is not yet clear whether it represents a conductive conformation of the channel or if one or both of these constrictions dilates further in the fully “open” state. The neck is ∼15 Å long, its location corresponds roughly to the inner leaflet of the membrane, and it is lined by 15 hydrophobic amino acids, which are contributed by residues I76, F80, and F84 of helix S2b from each subunit. These residues are highly conserved and are a hot spot among disease-causing mutations (10). The aperture, a much shorter section, is formed by the five symmetrical side chains contributed by amino acid V205 from each subunit and is located at the cytosolic end of the pore.
The X-ray structure of BEST1 also revealed five equivalent Ca2+ binding sites in the cytosolic region of the channel, corresponding to the pentameric architecture, which were termed “Ca2+ clasps.” Each Ca2+ clasp is formed by an N-terminal portion of one subunit, the S1a–S1b helix–turn–helix element, and a C-terminal portion of an adjacent subunit containing a cluster of acidic amino acids in the junction between helices S4a and S4b (Fig. S1). The Ca2+ ion is coordinated with pentagonal bipyramidal geometry, typical of Ca2+-binding proteins (20), by three backbone carbonyl oxygen atoms, a water molecule, and the side chains of D301 and D303, which are perfectly conserved among eukaryotic bestrophin proteins (Fig. S1C). Although the coordination is consistent with a high-affinity binding site, in line with the ∼100 nM K1/2 for Ca2+ activation reported for mammalian bestrophins (5, 15), the S1a–S1b helix–turn–helix element covers the Ca2+ ion and would need to be repositioned for its exit. It is unclear whether the Ca2+ clasp serves as the sensor that responds to intracellular Ca2+ levels, or whether there are other Ca2+ binding site(s) that are responsible for this.
Analysis of the structure also suggested regions that might be involved in ion selectivity. For example, 15 binding sites for Cl− (3 sites, each having fivefold symmetry; Fig. S1B) were identified within the ion pore (16). It was also proposed that phenylalanine residues within the neck (F80 and F84) could be involved in anion selectivity and/or could lower the barrier for anion permeation through the neck because the edges of their aromatic rings might interact with permeating Cl− ions via anion–quadropole (also known as anion–π) interactions (16). The hypothesis that the neck is a determinant of ion selectivity was also suggested by the X-ray structure of KpBest and associated electrophysiological studies because this channel is selective for cations-over-anions and contains an isoleucine amino acid in the position corresponding to F80 of BEST1 (17).
Here, we characterize the ion selectivity and Ca2+-dependence properties of chicken BEST1 using electrophysiological analyses of the purified protein. To study the potential involvement of the neck, aperture, and Ca2+ clasp in channel activation and ion selectivity, we use electrophysiology, X-ray crystallography, and flux assays to study properties of mutant channels. We find that the Ca2+ clasp serves as an intracellular Ca2+ sensor and that the neck, but not the aperture, controls the Ca2+-dependent flow of Cl− through the channel. Although ion permeation through the neck might be aided by electrostatic interactions with phenylalanine residues, we find that the neck is not a major determinant of ion selectivity. Rather, we find that the aperture is responsible for the lyotropic permeability sequence among anions and hypothesize that the anion binding sites underlie most of the anion-over-cation selectivity of eukaryotic bestrophin channels.
Results
Electrical Recordings of BEST1 in Bilayers.
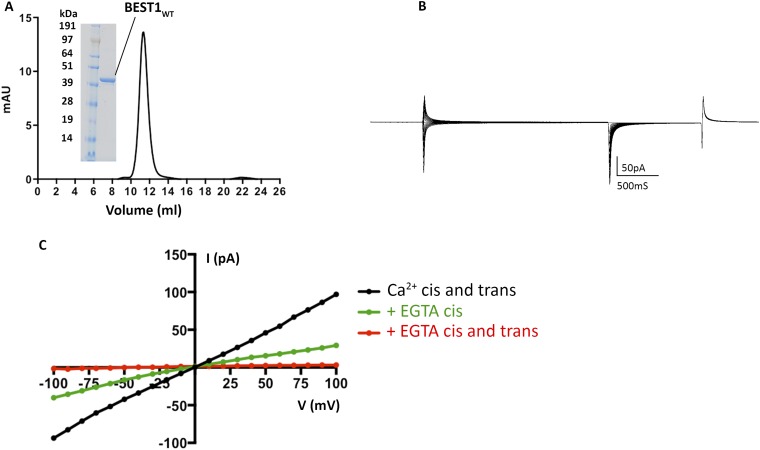
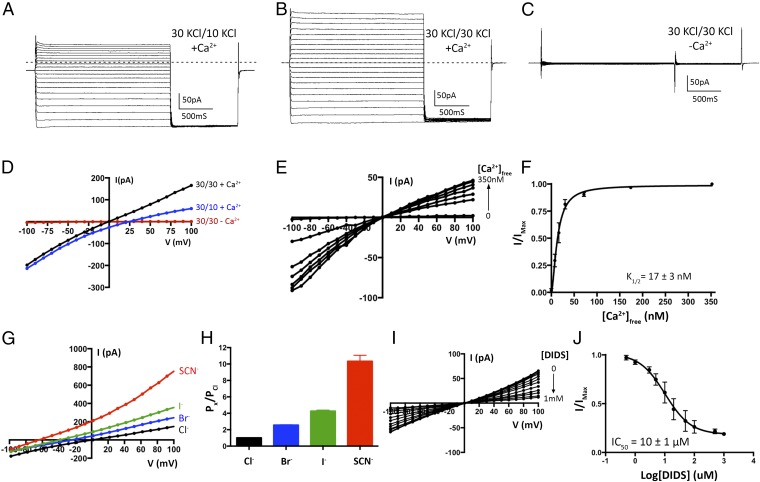
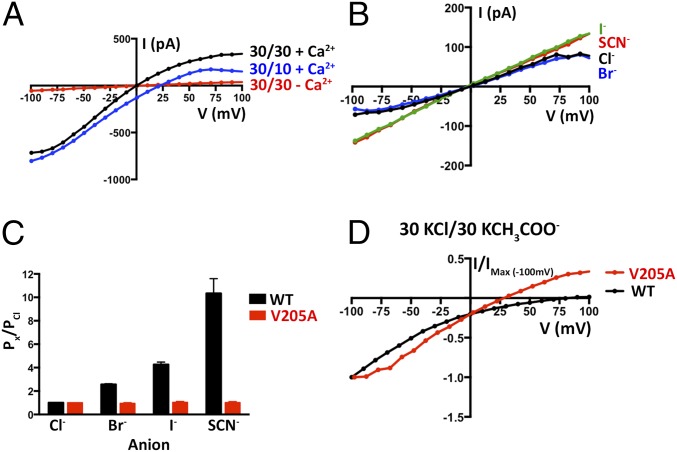
In order to make direct comparisons with the X-ray structure of chicken BEST1 (16), we performed functional analyses using the same ortholog. Electrical recordings, made using purified lipids and purified protein, allowed us to investigate ion selectivity, Ca2+ dependence and pharmacological properties of the channel, and to compare these properties with those observed previously for mammalian BEST1 in cellular contexts. Following expression and purification (Fig. S2A), the protein was reconstituted into liposomes containing a 3:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE):1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG) and applied to planar lipid bilayers of the same lipid composition for electrophysiological analysis following procedures established for other ion channels (21, 22). Macroscopic currents recorded from bilayers containing BEST1 in the presence of ∼300 nM free Ca2+ are shown in Fig. 1. Selectivity for Cl− over K+ is evidenced by the “reversal potential,” that is, the voltage needed to null the current when a transmembrane KCl gradient is present. When recorded using asymmetric conditions of 10 mM KCl on the “trans” side of the bilayer, which is held at ground, and 30 mM KCl on the “cis” side, the reversal potential is +25.1 ± 0.8 mV (n = 8), indicating selectivity for Cl− and a permeability ratio of PK/PCl = 0.05 ± 0.01 (Fig. 1 A and D and Methods). As has been observed for mammalian BEST1 from whole-cell measurements (1), the currents reach a steady-state level after relaxation of the transient capacitive current and do not exhibit noticeable voltage or time dependence on the timescale of the measurements (Fig. 1).
Fig. S2.
(A) Purified BEST1 protein. The elution profile of from a size exclusion column (Superdex 200; GE Healthcare; black UV trace) and SDS/PAGE analysis of the purified protein (Inset; molecular weight marker, Left; purified BEST1WT, Right) are shown. (B) Currents observed for an “empty” lipid bilayer without channels. A representative trace is shown using the same voltage pulse protocol described in Fig. 1. (C) BEST1 channels insert in both orientations in the bilayer. I–V relationships are shown for macroscopic currents recorded from bilayers containing purified chicken BEST1WT (using the same pulse protocol as in Fig. 1). The voltage is stepped to values between −100 and +100 mV under symmetrical conditions before (black; ∼300 nM Ca2+) and after addition of 10 mM EGTA to standard solutions on the cis (green) and then trans (red) sides. The stepwise reduction in currents observed by chelation of Ca2+ by EGTA on the cis and trans sides is indicative that channels are inserted in both orientations in the bilayer.
Fig. 1.
Electrical recordings of planar lipid bilayers containing purified chicken BEST1 protein. A–C are representative experiments using the same bilayer. The dashed lines represent the zero current level. (A) With ∼300 nM Ca2+ on both sides of the membrane, currents were measured using asymmetric KCl (30 mM cis and 10 mM trans) and the following protocol: from a holding potential of 0 mV, the voltage was stepped to test values between −100 and +100 mV, in 10-mV increments, for 2 s. After a further 1-s step to −100 mV, the voltage was returned to 0 mV. (B) Currents recorded using ∼300 nM Ca2+ and symmetric 30 mM KCl. (C) Currents recorded after 10 mM EGTA had been added to each side. In figures, values such as “30/10” indicate the concentrations of KCl, in millimolar, on the cis/trans sides. (D) Current–voltage (I–V) relationships of the data shown in A–C, which were obtained by plotting the test voltages vs. the average currents from 200-ms windows at the end of the 2-s pulses (connected filled circles). (E) Reactivation of BEST1 currents by increasing the free Ca2+ concentration ([Ca2+]free). After observing currents in standard symmetrical solutions, 10 mM EGTA was added to the cis side to deactivate channels with their cytosolic side facing that direction. Then, using perfusion, the [Ca2+]free in the trans solution was varied from ∼0 to 350 nM with a Ca-EGTA buffer system (Methods). I–V relationships are shown for data recorded using the same pulse protocol as A–C. (F) Ca2+ response curve. From three separate experiments as in E, average currents observed at −100 mV for different [Ca2+]free were plotted as the fraction of the current recorded at −100 mV from the [Ca2+]free = 350 nM condition (IMax). The data were fit to a one-site binding model. (G) Lyotropic series of anion permeability. After first recording using symmetric 30 mM KCl (black I–V trace), the solution on the trans side was replaced (by perfusion) with solutions containing 30 mM KBr, KI, or KSCN. I–V traces are shown for data collected using the standard voltage protocol. (H) Permeability ratios, determined using data from G by solving the Goldman–Hodgkin–Katz equation (SI Extended Data Analysis). Three separate experiments were used to compute the SE. (I and J) DIDS block. (I) I–V relationships show the decrease in current with increasing concentrations of DIDS. (J) Fit of the data from I and two analogous experiments to a standard dose–response curve. The data were plotted as the fraction of the current recorded at −100 mV in the absence of DIDS (IMax).
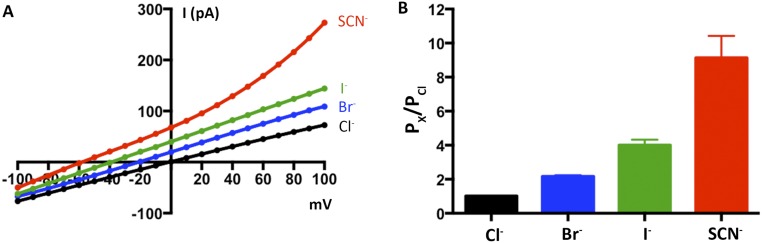
Chelation of Ca2+ using EGTA on both the cis and trans sides almost completely abolishes the currents (Fig. 1 C and D), and the addition of EGTA to either side partially reduces the currents, consistent with Ca2+-activated channels being inserted in the bilayer in both orientations (Fig. S2C). The subsequent addition of Ca2+, introduced by exchanging bath solutions with varying free [Ca2+], activates the currents in a dose-dependent manner, with an apparent K1/2 for Ca2+ of 17 ± 3 nM (n = 3) (Fig. 1 E and F). This value is smaller than reported for mammalian bestrophin channels (∼100 nM) (2, 5, 15) and may be due to specifics of the chicken ortholog and/or differences between working with purified protein in the simplified planar lipid bilayer system vs. whole-cell studies (e.g., differences in membrane lipid composition). Notably, basal intracellular Ca2+ concentrations in cultured chicken retinal photoreceptor cells are reported to be lower than in most mammalian cells, at ∼50 nM (23). Starting from symmetric 30 mM KCl on both sides and replacing KCl on the trans side with 30 mM Br−, I−, or SCN− shifts the reversal potential to increasingly negative values to reveal the hallmark lyotropic order of anion permeability for bestrophin channels: SCN− > I− > Br− > Cl− (Fig. 1 G and H). As has been observed for mammalian BEST1 channels studied in whole cells, currents are inhibited by DIDS (Fig. 1 I and J) with similar fractional inhibition and IC50 values (18). Together, these results indicate that channels formed from purified chicken BEST1 and inserted into synthetic lipid membranes have properties comparable to mammalian BEST1 channels in cells.
The Neck.
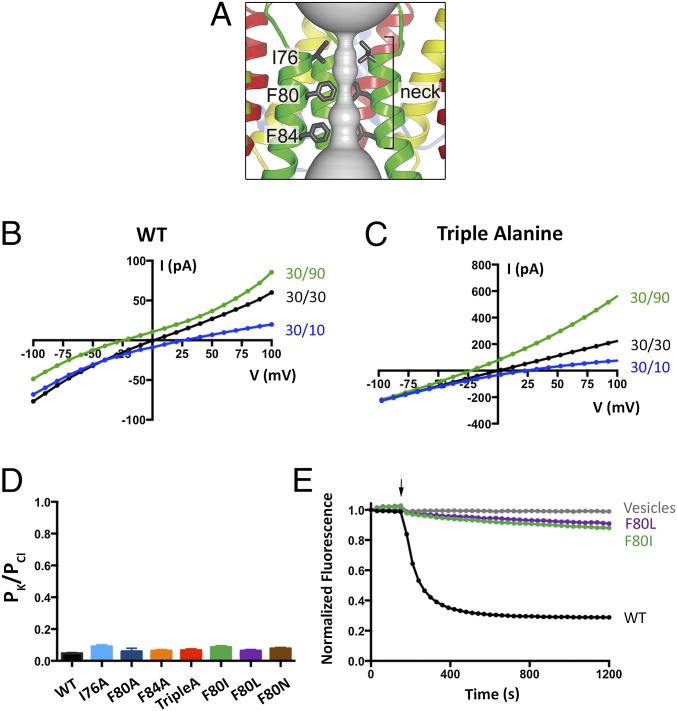
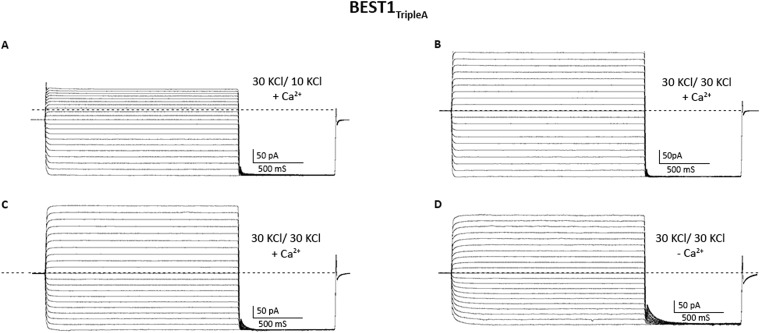
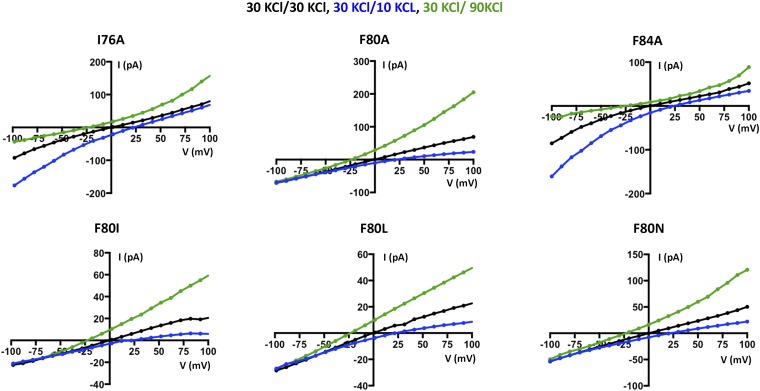
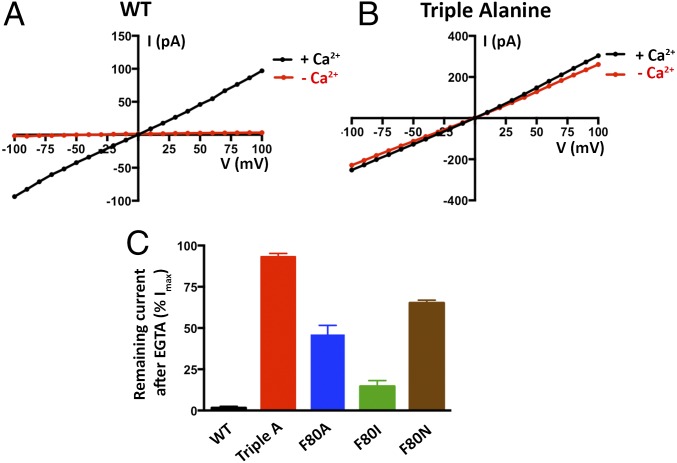
Having established a system to study the ion selectivity and Ca2+ activation properties of purified BEST1 channels, we wanted to address whether mutation of the neck region of the pore would have an effect on ion selectivity. The side chains of three highly conserved hydrophobic amino acids from each of the five subunits, I76, F80, and F84, form the neck. (Fig. 2A). We made a construct containing simultaneous substitutions of these residues with alanine (I76A, F80A, and F84A, which we will refer to as BEST1TripleA) and studied the purified channel. Bilayers containing BEST1TripleA, in the presence of ∼300 nM free Ca2+, yielded robust currents and I–V relationships with similar reversal potentials to those of the wild-type channel (BEST1WT) under both symmetric and asymmetric KCl conditions (Fig. 2 B and C and Fig. S3 A and B). For example, asymmetric conditions of 10 mM KCl on the trans side and 30 mM KCl on the cis side gave rise to a reversal potential of 24.8 ± 1.3 mV (n = 7) for BEST1TripleA in comparison with the value of +25.1 ± 0.8 mV (n = 8) for BEST1WT. Thus, the TripleA mutation did not noticeably alter PK/PCl of the channel. Not surprisingly, individual alanine substitutions of these amino acids also had essentially no effect on PK/PCl (Fig. 2D and Fig. S4). To study the ion selectivity of BEST1TripleA among anions, we measured currents starting with symmetric 30 mM KCl and then replaced Cl− by Br−, I−, or SCN− on the cis side using the same procedure that we used for BEST1WT (Fig. S5). The resulting I–V curves are similar to those obtained for BEST1WT, and the reversal potentials and resulting permeability ratios are statistically indistinguishable. Thus, both the anion-over-cation selectivity and the lyotropic sequence of permeability are essentially unaltered by the TripleA mutation in the neck.
Fig. 2.
Mutation of the neck does not affect anion-vs.-cation selectivity in BEST1. (A) Close-up view of the neck region of the pore (gray surface) of BEST1 (illustrated as in Fig. S1B) . (B and C) Mutation of amino acids in the neck does not noticeably shift reversal potentials. I–V relationships are shown for BEST1WT (B) and BEST1TripleA (C) recorded from −100- to +100-mV voltage steps under symmetric (black) and asymmetric (blue, green) KCl conditions using standard solutions. Labeled values (e.g., “30/90”) indicate the concentration of KCl (in millimolar) used for the cis/trans sides. (D) Permeability ratios (PK/PCl) for BEST1WT and neck mutants. Using reversal potentials measured from both 30/10 and 30/90 mM KCl conditions, the Goldman–Hodgkin–Katz voltage equation was solved (SI Extended Data Analysis) to compute an average PK/PCl permeability ratio per experiment: WT, 0.05 ± 0.01; I76A, 0.09 ± 0.01; F80A, 0.06 ± 0.03; F84A, 0.06 ± 0.01; TripleA, 0.07 ± 0.02; F80I, 0.09 ± 0.02; F80L, 0.06 ± 0.01; and F80N, 0.08 ± 0.01. Error bars denote the SEM from three separate experiments (using different bilayers). (E) Hydrophobic mutations at F80 reduce Cl− flux. Flux assay measurements for BEST1WT, BEST1F80I, and BEST1F80L, and for empty vesicles were made on the same day to compare channel activity. In this assay, a decrease in fluorescence is indicative of Cl− influx into liposomes. The arrow indicates when the assay was initiated by the addition of the proton ionophore CCCP (Methods).
Fig. S3.
Current traces for BEST1TripleA. (A and B) Current traces, using the same bilayer, from which I–V relationships presented in Fig. 1C are derived. The dashed line represents the zero current level. (A) Asymmetric (30 mM cis, 10 mM trans) and (B) symmetric (30 mM cis, 30 mM trans) KCl conditions. (C and D) Current traces, from the same bilayer, in the presence of ∼300 nM free Ca2+ (C) and after the addition of 10 mM EGTA to the cis and trans chambers (D). The protocols used are identical to those described for BESTWT in Fig. 1 A–C.
Fig. S4.
Example I–V traces for BEST1 neck mutants used to compute the permeability ratios (PK/PCl) presented in Fig. 2D. For each mutant, three separate experiments, using different bilayers, were used to compute PK/PCl. The bath solutions and pulse protocols were identical to those described in Fig. 2B.
Fig. S5.
The lyotropic sequence of anion permeability is unaltered in the BEST1TripleA mutant. (A) Example I–V relationships recorded using voltage steps from −100 to +100 mV. Following the procedure outlined in Fig. 1G, after first recording using symmetric 30 mM KCl (black I–V trace), the solution on the trans side was replaced (by perfusion) with solutions containing 30 mM KBr, KI, or KSCN. I–V traces are shown for data collected using the standard voltage protocol. (B) Permeability ratios were determined by solving the Goldman–Hodgkin–Katz equation (SI Extended Data Analysis) using three separate experiments to compute the SEM.
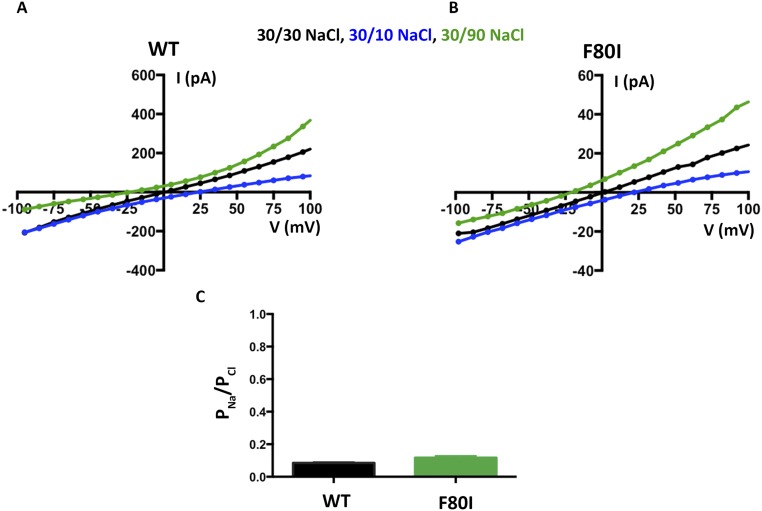
We wondered what effect more subtle mutations of F80, which is located in the middle of the neck, might have on the channel. F80 has been postulated to be a determinant of ion selectivity based on work stemming from the crystal structure of the prokaryotic KpBest channel, which is selective for cations over anions and has an isoleucine at the corresponding position in the neck of its pore (17). We find that mutation of F80 to isoleucine, or to leucine, or even to asparagine, does not significantly change PK/PCl of BEST1, as evidenced by the reversal potentials measured using transmembrane KCl gradients (Fig. 2D and Fig. S4). Among the neck mutants we tested, there is a slight trend toward elevated PK/PCl values (Fig. 2D), which is on the order of the error in the measurements, but these effects, if any, are subtle. To compare more directly with previous work that asserted that the neck determines the charge selectivity of the channel, and that assessed PNa/PCl (17), we studied the channels using NaCl gradients and find that both BEST1WT and BEST1F80I are selective for Cl− over Na+ and exhibit nearly identical permeability ratios (Fig. S6, PNa/PCl = 0.09 ± 0.01 and 0.12 ± 0.02 for BEST1WT and BEST1F80I, respectively). Contrary to previous implications, we conclude that the neck is not a major determinant of anion-vs.-cation selectivity in BEST1.
Fig. S6.
The F80I mutation does not make BEST1 a sodium-selective ion channel. (A and B) Experiments are performed as in Fig. 2A except bath solutions contained NaCl instead of KCl. Example I–V relationships are shown for WT (A) and F80I (B). (C) Using reversal potentials measured from both the 30/10 and 30/90 mM KCl conditions, the Goldman–Hodgkin–Katz voltage equation was solved (SI Extended Data Analysis) to compute an average permeability ratio per experiment. Error bars are computed using the SEM from three separate experiments.
In the course of the experiments, we noticed that macroscopic currents for BEST1TripleA were typically larger than for BEST1WT when roughly the same amount of proteoliposomes were applied to the bilayer. Because it is difficult to determine the actual amount of protein incorporated in the bilayer, we measured Cl− flux into liposomes using an assay where the amount of protein being studied is known. Using proteoliposomes containing nominally equivalent amounts of BEST1WT or BEST1TripleA protein, we observed substantially more Cl− flux for BEST1TripleA than for BEST1WT (Fig. S7). This indicates that individual BEST1TripleA channels support a higher overall Cl− transport rate than BEST1WT channels. (We use the term “overall Cl− transport rate” to mean the time-averaged rate at which ions flow through a single ion pore, which is proportional to the product of the channel’s open probability and the conductance of a single open channel. Changes in the overall Cl− transport rate could be due to changes in the open probability and/or the single-channel conductance, but these possibilities cannot be distinguished by the present data.) In contrast, the F80I and F80L mutant channels consistently yielded smaller macroscopic currents than BESTWT for a given amount of proteoliposomes applied to the bilayer. Accordingly, for the electrical measurements of these latter mutants, we applied proteoliposomes to the bilayer until we obtained sufficient currents to accurately determine reversal potentials (>20 pA at 100 mV; Fig. S4). Using the flux assay, we found that BEST1F80I and BEST1F80L showed substantially reduced Cl− flux compared with BEST1WT for the same amount of protein, indicating that these mutant channels have lower overall Cl− transport rates than BEST1WT (Fig. 2E). This suggests that, although F80 does not determine ion selectivity, this phenylalanine residue is important for Cl− ion flow through the pore. These findings are consistent with F80L being described as a disease mutation that impairs BEST1 function (10, 24).
Fig. S7.
BEST1TripleA has a higher unitary Cl− transport rate than BEST1WT. To compare the activity of BEST1WT and BEST1TripleA, channel protein (purified and incorporated into liposomes in parallel) was incorporated into liposomes using a 1:1,000 protein:lipid ratio and the fluorescence-based Cl− flux assay was carried out as described in Fig. 2E (Methods). The arrow indicates when the CCCP proton ionophore was added to initiate the assay. In comparison to the other flux assays, 10-fold less protein was used in this assay in order to better compare the relative activities of BEST1WT and BEST1TripleA.
Ca2+-Dependent Activation.
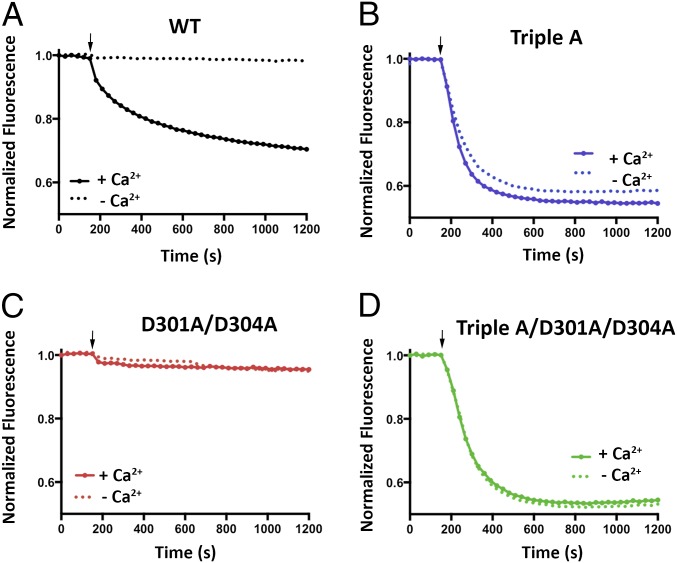
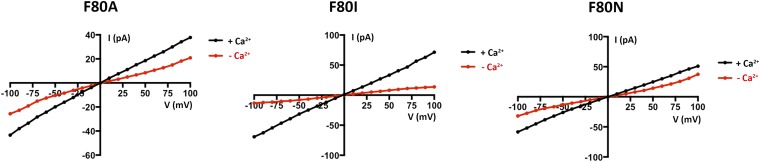
We next assessed whether mutations in the neck affect the Ca2+ dependence of the channel. We found that, unlike the currents observed for BEST1WT, which depend on Ca2+, chelation of Ca2+ in the bath solutions had almost no effect on the currents observed for BEST1TripleA (Fig. 3 A and B and Fig. S3 C and D). Analogous results were obtained using a flux assay. For BEST1WT, we observed Cl− flux with 2 µM Ca2+ present, but not when Ca2+ was chelated by EGTA (Fig. 4A). On the other hand, ion flux for BEST1TripleA was observed whether or not Ca2+ was present, indicating that the mutant channel is constitutively active and not dependent on Ca2+ (Fig. 4B). Studying single amino acid substitutions of F80, we found varying degrees of effects on the Ca2+ dependence of ionic currents (Fig. 3C and Fig. S8). When Ca2+ is chelated, the F80A mutant retains almost 50% of the current in comparison with when Ca2+ is present. The hydrophobic substitution F80I yields channels with currents that are nearly as Ca2+ dependent as BEST1WT. On the other hand, mutating F80 to asparagine, which is a similarly sized but hydrophilic amino acid, produces channels that retain ∼70% of their current in the absence of Ca2+. Evidently, we find that mutation of the neck has dramatic effects on the Ca2+ dependence of the channel.
Fig. 3.
Size and hydrophobicity of the amino acids in the neck affect Ca2+-dependent activation of BEST1. (A and B) Unlike BEST1WT, BEST1TripleA currents are almost completely independent of [Ca2+]. I–V relationships are shown for currents recorded by voltage steps to values between −100 and +100 mV under symmetric KCl (30/30) conditions before (“+ Ca2+”, [Ca2+]free ∼300 nM) and after (“− Ca2+”) the addition of 10 mM EGTA to both cis and trans chambers. The current traces for BEST1TripleA are shown in Fig. S3. (C) Mutations to the neck region have dramatic effects on Ca2+-dependent activation. Macroscopic currents were recorded as in A and B for each mutant. The fraction of current remaining at +100 mV after the addition of EGTA is plotted (Fig. S8 shows example I–V relationships). Error bars indicate the SEM from three separate experiments.
Fig. 4.
Cl− flux analysis of BEST1TripleA and Ca2+ clasp mutants. (A–D) For each assay, proteoliposomes were diluted 100-fold into flux assay buffer containing either 1 mM EGTA (- Ca2+) or 1 mM EGTA-CaSO4 (+ Ca2+, [Ca2+]free ∼ 2 μM). The arrow indicates when the assay was initiated by the addition of the proton ionophore CCCP. (A) Cl− flux of BEST1WT is Ca2+ dependent. (B) Cl− flux through BEST1TripleA is observed regardless of the presence or absence of Ca2+. (C) Mutation of aspartate amino acids that coordinate Ca2+ in the Ca2+ clasp to alanine (D301A/D304A) abolishes Cl− flux and makes the channel unresponsive to 2 µM Ca2+. (D) Combining the D301A/D304A and the BEST1TripleA mutations produces a constitutively active channel that is not responsive to the Ca2+ concentration. The increased flux observed for BEST1TripleA relative to BEST1WT is partially due to the nature of the assay, where Ca2+ is only added to the outside of the vesicles. For BEST1WT, only those channels with their Ca2+ sensors facing outside are reactivated, whereas Cl− flux through BEST1TripleA channels in both orientation is detected because these channels conduct Cl− in a Ca2+-independent manner.
Fig. S8.
I–V relationships for data presented in Fig. 3. Different mutations of F80 have varying effects on the Ca2+ dependency of BEST1 currents. Representative I–V relationships are shown; the SEM was calculated from three such experiments.
The X-ray structure of BEST1 identified a Ca2+ binding site, the Ca2+ clasp, in the channel’s cytosolic region and we wondered whether this binding site could be responsible for the channel’s regulation by intracellular [Ca2+] levels. The side chains of two conserved aspartate amino acids, D301 and D304, directly coordinate Ca2+ in the Ca2+ clasp (Fig. S1C) (16). In previous whole-cell patch-clamp recordings, no currents that could be attributed to BEST1 were observed when these amino acids were mutated to alanine (19). In accord with these results, we did not observe Cl− flux either in the presence or absence of Ca2+ for a construct in which both D301 and D304 are mutated to alanine (Fig. 4C). It is unlikely that gross protein misfolding is responsible for the lack of activity because the purified protein migrates on a size exclusion column as a single Gaussian peak with approximately the same elution volume as BEST1WT. We hypothesized that incorporation of the TripleA mutation within the neck might restore activity to the D301A/D304A mutant. Indeed, Cl− flux assay measurements indicate that the combination of these mutations yields a channel that is active in the presence and absence of Ca2+ (Fig. 4D).
X-Ray Structure of BEST1TripleA.
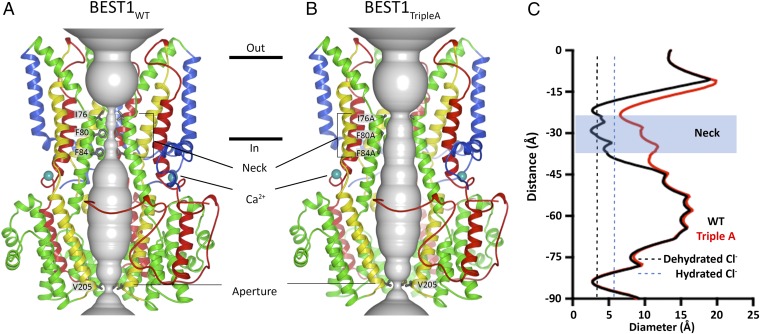
To better understand how mutations to the neck cause the channel to be constitutively active irrespective of Ca2+, we determined the X-ray structure of BEST1TripleA to 3.1-Å resolution (Fig. 5 and Table S1). The overall structure is practically indistinguishable from that of BEST1WT (Fig. 5 A vs. B, rmsd of 0.2 Å). In both structures, Ca2+ ions are bound within the channel’s five symmetry-related Ca2+ clasps. The entire cytosolic region of the channel, including the dimensions of the aperture, is unchanged. The only notable differences between the structures of BEST1WT and BEST1TripleA are within the neck region. The alanine substitutions (I76A, F80A, and F84A) increase the average diameter of the neck, measured between van der Waals surfaces, from ∼3.5 Å in BEST1WT, which is approximately the diameter of a dehydrated Cl− ion, to ∼9 Å in BEST1TripleA, which is amply wide to accommodate a hydrated Cl− (Fig. 5). There are also subtle shifts, on the order of ∼0.3 Å, in the backbone positions of the helices lining the neck and the surrounding area (Fig. S9). It is remarkable that the channel’s selectivity for anions and its lyotropic permeability sequence, which is an indication that ion dehydration occurs during permeation, are unaffected by the widening of the neck in BEST1TripleA. The structures of BEST1TripleA and BEST1WT, together with the electrophysiological and flux assay analyses of the neck and Ca2+ clasp mutants support the conclusion that the Ca2+ clasp is the sensor that responds to intracellular Ca2+ levels and that conformational changes within the neck permit the permeation of Cl− through BEST1 in a Ca2+-dependent manner.
Fig. 5.
Comparison of the X-ray structures of BEST1WT (A) and BEST1TripleA (B). Ribbon representations are shown for three subunits (two in the foreground are removed for clarity). The ion pore (gray tube) is depicted as a representation of the minimal radial distance from the central axis to the nearest van der Waals protein contact. Secondary structural elements are colored according to the four segments of the channel (S1, blue; S2, green; S3, yellow; S4 and C-terminal tail, red), which contain corresponding transmembrane regions. Approximate boundaries of a lipid membrane are indicated by horizontal black lines. (C) Plots of the diameters of the pores from A and B along the ion conduction pathway. Vertical dashed lines indicate the diameters of hydrated and dehydrated Cl− ions.
Table S1.
Data collection and refinement statistics for BEST1TripleA
| Data collection | |
| Space group | P21 |
| Wavelength, Å | 1.033 |
| Cell dimensions, Å | |
| a | 98.24 |
| b | 243.88 |
| c | 172.05 |
| α = ɣ = 90°, β =, o | 93.84 |
| Resolution, Å | 38–3.1 (3.15–3.10) |
| Rmerge | 0.25 (1.9) |
| Rpim | 0.086 (0.646) |
| CC1/2 in outer shell | 0.604 |
| I/σI | 9.7 (1.2) |
| Completeness, % | 99.9 (99.9) |
| Redundancy | 9.5 (9.7) |
| Refinement | |
| Resolution, Å | 38–3.1 |
| No. of reflections | 145,877 |
| No. atoms | 30,705 |
| Ions | 25 |
| Water | 10 |
| Rwork | 0.218 (0.334) |
| Rfree | 0.242 (0.358) |
| Average B factors, Å2 | 101.2 |
| Protein | 101.3 |
| Ions | 90.1 |
| Water | 72.82 |
| Ramachandran, % | |
| Favored | 95 |
| Outliers | 0.4 |
| Rms deviations | |
| Bond lengths, Å | 0.004 |
| Bond angles, Å | 0.95 |
| Rotamer outliers, % | 1.6 |
| Clash score | 7.6 |
Data collection statistics are taken from HKL3000 (39), refinement statistics are from PHENIX (43), and CC1/2 is defined in ref. 40. Numbers in parentheses indicate the highest resolution shells and their statistics. The 5% of reflections used for calculation of Rfree were derived from the search model PDB ID code 4RDQ.
Fig. S9.
Differences at the neck in BEST1WT and BEST1TripleA. 2Fo – FC electron density is shown for the neck region for WT (A) and BEST1tripleA (B) with two subunits displayed for simplicity. The density is contoured at 1.3σ in the context of the final atomic model, which is shown as sticks and spheres (Cl− is depicted as a purple sphere). (C) Side view and (D) top view of BEST1 channel with α-helices depicted as cylinders and colored on a yellow-to-red spectrum according to the displacement of Cα atoms between the refined atomic models of BEST1WT and BEST1TripleA. Yellow color represents displacements less than 0.15 Å, and red color represents displacements greater than 0.4 Å. An arrow indicates the neck of the pore.
The Aperture.
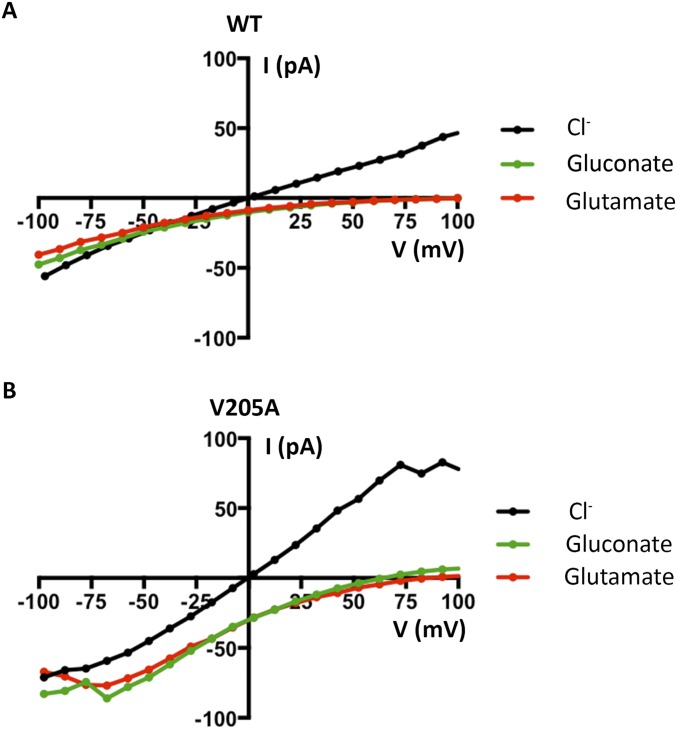
The permeability sequence of BEST1 (SCN− > I− > Br− > Cl−) is lyotropic, that is, it corresponds to the inverse sequence of the energy of dehydration of the anions, with SCN− being the most easily dehydrated and Cl− being the least easily dehydrated. Lyotropic permeability sequences are an indication that ion permeation involves ion dehydration (25–27). Because the structure of BEST1TripleA shows that it has a wide-enough neck region to accommodate hydrated anions and yet we observe that the BEST1TripleA channel exhibits a lyotropic permeability sequence, we reasoned that permeating anions become dehydrated at other region(s) in the pore. We turned our attention to the other hydrophobic constriction of the channel, the ∼3-Å-wide cytosolic aperture, which is formed from a ring of V205 side chains from the five subunits (Fig. 5A). To investigate the function of the aperture, we mutated V205 to alanine and studied the channel in the bilayer. Like BEST1WT, we find that the V205A mutant is Ca2+ dependent and retains selectivity for anions over cations (PK/PCl = 0.08 ± 0.01; n = 3; Fig. 6A). Strikingly, unlike BEST1WT, the V205A mutant is essentially equally permeable to SCN−, I−, Br−, and Cl− (Fig. 6 B and C). Therefore, the V205A mutant does not exhibit a lyotropic sequence among permeant anions. Hence, this single hydrophobic amino acid is responsible for this aspect of the channel’s ion selectivity. We also find that acetate, which is not detectably permeant through wild-type BEST1, is permeant through the V205A mutant (Fig. 6D). Like BEST1WT, BEST1V205A remains essentially impermeable to larger anions such as gluconate and glutamate (Fig. S10). Our data support a conclusion that the aperture forms a molecular filter that discriminates on the basis of size, allowing small ions (e.g., SCN−, I−, Br−, and Cl−) to pass, while excluding larger molecules such as amino acids.
Fig. 6.
Function of the aperture. (A) Observed BEST1V205A currents are Cl− selective and Ca2+ dependent. Example I–V relationships are shown for voltages stepped from −100 to +100 mV for BEST1V205A for the indicated standard conditions [cis/trans KCl concentrations in millimolar, and 300 nM Ca2+ (+ Ca2+) or 10 mM EGTA (-Ca2+)]. (B) The V205A mutation abolishes the lyotropic permeability sequence of BEST1. I–V traces are shown for procedures equivalent to Fig. 1G. After first recording using symmetric 30 mM KCl (black I–V trace), the solution on the trans side was replaced (by perfusion) with solutions containing 30 mM KBr, KI, or KSCN. (C) Comparison of PX/PCl for BEST1WT and BEST1V205A. Using data from experiments carried out as in B, permeability ratios were determined by solving the Goldman–Hodgkin–Katz equation (SI Extended Data Analysis). Three separate experiments were used to compute the SEM. (D) BEST1V205A shows increased permeability to acetate (CH3COO−) compared with BEST1WT. Representative I–V relationships using solutions containing 30 mM KCl on the cis side and 30 mM KCH3COO− on the trans side are shown for BEST1WT and the V205A mutant. For the comparison, the currents were normalized to the values recorded at −100 mV (Imax = −62 and −105 pA for BEST1WT and the V205A mutant, respectively, in this experiment).
Fig. S10.
BEST1WT and BEST1V205A are essentially impermeable to gluconate and glutamate anions. Example I–V relationships recorded using voltage steps from −100 to +100 mV are shown for BEST1WT (A) and BEST1V205A (B). For each channel, following measurements made from symmetric 30 mM KCl using the standard voltage protocol (Fig. 1) and standard bath solutions (black traces), the bath solution on the trans side was replaced, by perfusion, with either 30 mM potassium gluconate or potassium glutamate and the recordings were repeated. Neither gluconate or glutamate are perceptively permeable through BEST1WT. Glutamate (red trace) is not perceptively permeable through BEST1V205A, but gluconate (green trace) might be slightly permeable through BEST1V205A, as indicated by the small currents observed above approximately +75 mV, consistent with the conclusion that the aperture functions as a size-selective filter.
Discussion
The structure of chicken BEST1 provides a framework to investigate the mechanisms of ion selectivity and Ca2+-dependent activation in bestrophin channels (16). The structure identified two hydrophobic constrictions in the ion pore, a neck positioned at the inner leaflet of the membrane, and an aperture located at the cytosolic end of the channel. Potential roles for these constrictions are as gate(s) that prevent or permit ion passage, or as “selectivity filter(s)” that discriminate among ions. It has been proposed that the neck is responsible for ion selectivity (17). The structure of BEST1 also identified a binding site for Ca2+ on the channel’s cytosolic side, the Ca2+ clasp, which could conceivably be the sensor of intracellular Ca2+ that controls a variable constriction within the pore (16). To address the functions of the neck, aperture, and Ca2+ clasp, we studied purified wild-type and mutant BEST1 channels using electrophysiology and flux assays, and we determined the X-ray structure of a mutant of the neck that is constitutively active.
When reconstituted into planar lipid bilayers, we found that purified chicken BEST1 protein recapitulates electrophysiological properties that had previously been observed for mammalian bestrophin channels in cellular membranes (1, 2, 5, 15, 18, 19). These properties include monovalent anion-vs.-cation selectivity, a lyotropic anion permeability sequence, Ca2+-dependent activation, and inhibition by the channel blocker DIDS. The purified system allows for the study of properties of wild-type and mutant channels without potential complications arising from endogenous anion channels or potential accessory proteins in cells in which eukaryotic bestrophin proteins have been studied previously.
The Neck Is Not a Major Determinant of Ion Selectivity.
To assay for a contribution of the neck to charge selectivity, we determined cation-vs.-anion permeability ratios and found that, among the mutants that we made, including simultaneous substitution of all three amino acids lining the neck (I76, F80, and F84) by alanine (BEST1TripleA), no mutant altered PK/PCl in a substantial way. Previous studies have shown that replacing the hydrophobic amino acids lining the neck with acidic amino acids, or with cysteine residues that were subsequently modified with the negatively charged methanethiosulfonate (MTSES−) reagent, can increase the permeability of cations relative to Cl− (17, 24). In BEST2, mutation of F80 to cysteine also has modest effects on the relative permeabilities among anions (28, 29). That the permeation properties were perturbed by such modifications is an indication that this region of the channel contributes to the ion pore, as was predicted before structural knowledge (2, 17, 24, 28, 29). However, the effects of dramatic alterations of the chemical environment within the neck, such as the introduction of charged amino acids, do not necessarily support a conclusion that the neck underlies charge selectivity in the wild-type channel, as has been postulated (17). The X-ray structure of BEST1TripleA shows that the neck is made dramatically wider by the I76A, F80A, and F84A mutations and that the remainder of the channel is essentially unperturbed. However, in the absence of phenylalanine residues in the neck of BEST1TripleA, the channel’s selectivity for anions-over-cations is maintained. In addition, despite the widened neck of BEST1TripleA, which could conceivably alter the hydration properties of anions passing through it, we found that the lyotropic sequence of permeability among monovalent anions (SCN− > I− > Br− > Cl−) was also unaltered. We conclude that the neck does not contribute to ion selectivity of BEST1 in a substantial way for either the anion-vs.-cation selectivity or the relative permeabilities among permeant anions.
Nevertheless, our data suggest that the particular amino acids lining the neck, which are highly conserved, facilitate anion permeation through the channel. For example, we find that mutating F80 to the similarly sized leucine or isoleucine amino acids dramatically diminishes ion flux through the channel. This suggests that a property of phenylalanine other than its size or hydrophobicity is important for Cl− flux. In addition to the well-known negative electrostatic potential associated with the face of their π system, the aromatic rings of phenylalanine residues have positive electrostatic potential associated with their edges. If positioned correctly, the ring edges can favorably interact with negatively charged species through anion–quadrupole interactions, and it has been observed that such interactions commonly occur in proteins (30, 31). As was proposed on the basis of the initial structure of BEST1, we envisage that the aromatic side chains of phenylalanines F80 and F84 within the neck interact with anions flowing through the pore via encircling rings of anion–quadrupole interactions (16). In accord with this hypothesis, anion–quadrupole interactions have been observed in a bacterial fluoride channel and mutation of the interacting phenylalanine residues to isoleucine also reduced ion flux through that channel (32). Anion–quadrupole interactions mediated by the phenylalanines may reduce the energy barriers for Cl− and other anions to pass through the neck relative to other hydrophobic amino acids, but these interactions must not be the determining factor for charge selectivity in eukaryotic bestrophin channels, because their removal has almost no effect on anion-vs.-cation selectivity.
The Ca2+-Dependent Activation Apparatus.
Our data indicate that the primary function of the neck is in Ca2+-dependent activation. We find that Cl− flux through BEST1TripleA, which we measure electrically and using a flux assay, is almost completely independent of the presence or absence of Ca2+. Additionally, single amino acid substitutions in the neck yield channels with varying degrees of “leakiness” in the absence of Ca2+. Comparison of the structures of BEST1WT and BEST1TripleA reveals that the differences are confined to the local environment of the neck. The TripleA mutations (I76A, F80A, and F84A) have not caused changes elsewhere in the protein, and, tellingly, they do not alter the diameter of the aperture, which is the other constriction in the pore that could form a barrier to ion permeation. We conclude that, in the wild-type channel, the neck functions as a gate that permits or prevents anion permeation depending on the Ca2+ concentration. This conclusion is supported by the observation that channels that are made constitutively closed by eliminating the Ca2+ sensor (see below) can be made constitutively active by the introduction of the TripleA mutation of the neck. When the 15 hydrophobic amino acids lining the neck (I76, F80, and F84 from the five subunits) are truncated to alanine (the TripleA mutant), the neck constriction found in BEST1WT is eliminated and it is no longer able to prevent anion permeation in the absence of Ca2+. This implies that the neck undergoes a conformational change that depends on the Ca2+ concentration to permit anion flux in the presence of Ca2+ and prevent anion flux in its absence.
Evidence supports the conclusion that the Ca2+ clasps, which were identified by the X-ray structure, serve as sensors of intracellular [Ca2+] that are responsible for the Ca2+ dependence of the channel. Each of the five symmetry-related Ca2+ clasps is located on a cytosolic surface of the channel and mutation of amino acids in the Ca2+ clasp (D301A/D304A) yields channels that are constitutively closed. As such, these mutants may mimic the Ca2+-free state of BEST1. However, as we showed using an assay that detects Cl− flux, a combination of the Ca2+ clasp mutant with the TripleA mutation of the neck yields channels that are constitutively active. We conclude that there is structural coupling between the Ca2+ clasp and the neck such that, when Ca2+ is bound within the clasp, the neck is more optimal for Cl− permeation than when Ca2+ is absent.
Although Ca2+ is bound in the Ca2+ clasp in the X-ray structure of BEST1, and a Cl− ion could physically fit in the neck, because no Cl− ion is observed there, it is not yet clear if the structure represents an open state, a nearly open state, or, possibly, an inactivated state (16). [It has been observed using patch-clamp experiments, for example, that human BEST1 currents slowly dissipate, perhaps by inactivation of the channels, when the intracellular [Ca2+] is in the micromolar range (19).] A comparison of two nearly identical X-ray structures that were determined with Ca2+ bound but using slightly different crystallization conditions gave a hint that motions near the Ca2+ clasps, which were small in this instance (∼0.3-Å displacements), are coupled to corresponding motions in the neck (16). For Ca2+-dependent gating, we envisage that the neck can dilate and constrict and that Ca2+ binding in the clasps directs these movements, favoring channel opening when Ca2+ is present and favoring channel closing when it is absent. The BEST1TripleA mutant circumvents this change because the amino acid side chains that form the constriction of the neck are truncated, and so Cl− ions can leak through even when Ca2+ is absent. In wild-type BEST1, the transition between an “open” neck and a “closed” neck may be subtle from a structural perspective, perhaps involving only a slight change in orientation of the phenylalanine residues such that their quadrupole edges transition between favorable and unfavorable interactions with anions. Through mutagenesis of F80, we found that both the size and hydrophobic nature of the neck contribute to its role as an ion barrier when Ca2+ is absent. In this regard, the neck appears similar to the so-called “hydrophobic gates” identified in other ion channels (33–35). By this theory, the bulky hydrophobic side chains at the neck region would prevent “wetting” of the channel pore by forming a “vapor lock” (33), blocking the passage of ions. Relieving this restriction either by widening the region or making it more hydrophilic would disable the gate. Analogous to experiments performed with the K2P1 (TWIK-1) potassium channel (35), we find that making a single hydrophobic to hydrophilic mutation (F80N) can considerably disrupt the neck’s ability to act as a seal in the absence of Ca2+, consistent with this notion of hydrophobic gating.
The Aperture Is a Size-Selective Filter.
The other constriction in the pore, the cytosolic aperture, which is formed by V205, was also identified from the structure as a potential barrier in BEST1. Mutations to an analogous aperture in a prokaryotic homolog seemed to alter that channel’s open probability (17). Although it is feasible that the aperture of BEST1 may act as a gate in some way that has yet to be determined, our results show that current flow through BEST1V205A is still Ca2+ dependent. Because mutations in the neck abolish the Ca2+ dependence of the channel whereas the V205A mutation had no detectable effect on it, we conclude that the aperture does not function as a Ca2+-dependent gate in BEST1. The BEST1V205A channel also maintains selectivity for Cl− over K+ with approximately the same permeability ratio as wild-type BEST1. On the other hand, we find that ions that have dramatically different relative permeabilities in wild-type BEST1 and follow a lyotropic sequence (SCN− > I− > Br− > Cl−), have essentially equal permeabilities in the BEST1V205A aperture mutant. This indicates that BEST1 has a “division” of ion selectivity properties; one portion of the channel, the aperture, determines relative permeabilities among anions, whereas other region(s) give rise to anion-over-cation selectivity. Thus, unlike many ion channels, such as those belonging to the superfamily of tetrameric cation channels whose pore architecture is typified by a potassium channel (36), BEST1 does not contain a single canonical selectivity filter that determines both the anion/cation selectivity and the selectivity among permeant ions. For the most part, these properties are divided among distinct regions of the pore in BEST1.
The BEST1 channel’s lyotropic permeability sequence among anions is a hallmark that dehydration occurs during permeation. That the lyotropic permeability sequence is abolished by the V205A mutation is an indication that anions become partially or fully dehydrated as they pass through the aperture of wild-type BEST1. The diameter of the aperture in the structure of BEST1 (∼3 Å) is consistent with this: it is too small for a hydrated ion to pass, but wide enough to permit the passage of Cl− and similarly sized anions (e.g., Br−, I−, SCN−) when they are dehydrated (Fig. 5C). We envisage that anions permeating through the aperture are dehydrated “laterally” so as to fit through the aperture but retain coordination to preceding and trailing water molecules. The necessity for anion dehydration while traversing the aperture gives BEST1 its lyotropic permeability sequence. Anions that are more easily dehydrated (e.g., SCN−) are more permeable than those that are less easily dehydrated (e.g., Cl−). Our data suggest that the aperture forms a constriction that discriminates on the basis of size and not charge: the aperture is a “size-selective filter.” It allows small ions such as SCN−, I−, Br−, and Cl− (and K+ and Na+) to pass, but it excludes larger molecules. Accordingly, we find that the V205A mutation, which would be expected to widen the aperture, also makes the channel permeable to acetate, whereas the wild-type channel is not detectably so. In a cellular context, the aperture would prevent large biological anions (e.g., nucleic acids, proteins, ATP, etc.) from accessing the ion pore. Although it is typically hydrophobic, the amino acid at position 205 varies somewhat among bestrophin channels. The corresponding residue is I205 in human BEST1 and is S205 in human BEST2. Amino acid differences at this position may engender distinctions between bestrophin channels regarding permeabilities among ions that are available to the experimentalist, and they may impart biologically relevant distinctions in anion permeabilities in vivo.
Perspective and Conclusions.
Upon inspection of the diameter of the pore along its length (Fig. 5A), it is understandable that anions become partially or fully dehydrated as they pass through the aperture, but surprising that the V205A mutant alone eliminates the lyotropic sequence, given that the neck is also too narrow to accommodate a hydrated Cl− ion. One might expect that both the aperture and the neck would contribute to the lyotropic permeability sequence, whereas our data indicate that the aperture is essentially responsible for this property. Two hypotheses, which are not mutually exclusive, could account for this. Phenylalanine residues within the neck may favorably interact with permeating anions through anion–quadropole interactions, as our data suggest, and these interactions may reduce the energy cost of dehydration. It is also possible that the structure of BEST1WT determined to date, although Ca2+ bound (16), may represent an inactivated state and that the neck may become more dilated when the channel is fully open.
If neither the neck constriction nor the aperture constriction gives BEST1 its anion-over-cation selectivity, what is responsible for this? A comparison between the X-ray structures of BEST1 and KpBest, which is more permeable to cations than anions, may provide clues (Fig. 7). In BEST1, 3 Cl−/Br− binding sites per subunit (15 total sites per channel) were observed within the ion pore (16). Two of the sites (sites 1 and 2) are situated in the “outer entryway” of the pore, and the other site (site 3) is located within the “inner cavity” (Fig. S1). None of the sites is buried; all of them are exposed to wide sections of the pore such that the dissociation or association of anions could occur readily. Each site is formed by a break in an α-helix that exposes a backbone amino nitrogen that interacts with the anion (sometimes referred to as a helix–dipole interaction). Strikingly, analogous sites are absent in KpBest because this channel contains continuous helices through what correspond to the anion binding sites of BEST1 (Fig. 7) (17). Akin to a proposal that was based on the structure of a glutamate-gated Cl− channel (37), we hypothesize that the anion binding sites in the pore of BEST1 contribute to ion selectivity by increasing the local concentration of anions that can bind in them (e.g., Cl−, Br−, etc.) relative to other ions.
Fig. 7.
Cl− binding sites observed in BEST1 are absent in prokaryotic KpBest. Structures are depicted similarly to Fig. 5A with two subunits shown. Cl− binding sites (magenta spheres, the three sites for each subunit are shown) in BEST1 (Left) are located at the N-terminal ends of α-helices. KpBest (Right) has continuous α-helices in the corresponding locations (arrows) and does not contain these sites. Ca2+ ions in BEST1 are depicted as teal spheres.
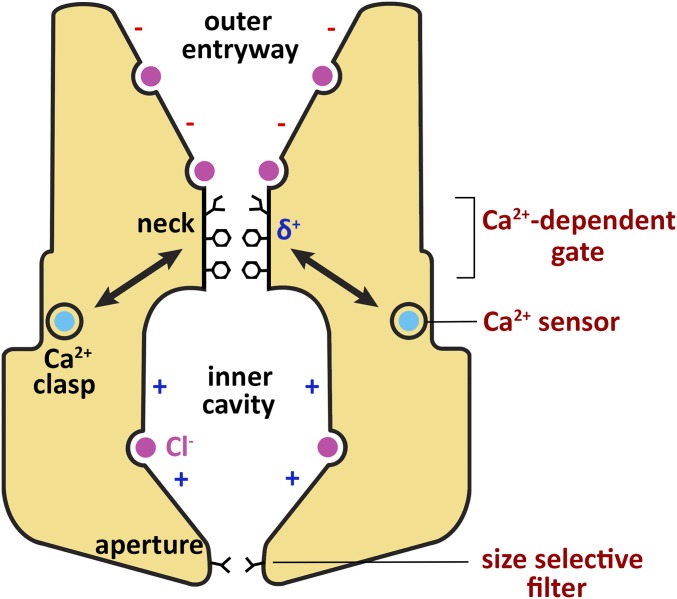
In conclusion, we have identified roles that the neck, aperture, and Ca2+ clasp play in ion selectivity and Ca2+-dependent activation of BEST1 (Fig. 8). The Ca2+ clasp and the neck are the principal components of the Ca2+-dependent gating apparatus. The Ca2+ clasp serves as a sensor of intracellular [Ca2+] and the neck functions as an effective gate that restricts the flow of Cl− and other anions when Ca2+ is absent from the clasp and permits their passage when Ca2+ is bound. Altering the size or hydrophobicity of amino acids within the neck can significantly alter the channel’s Ca2+ dependence, highlighting the importance of these properties for closure of this seal in the absence of Ca2+. The physicochemical properties of phenylalanines within the neck appear finely tuned in their abilities to form a seal in the closed state and permit anion conduction in the open state, perhaps by interacting with permeating anions via anion–quadropole interactions with the edges of their aromatic rings. Nevertheless, we find that the neck is not the determinant of anion-vs.-cation selectivity nor of the channel’s lyotropic permeability sequence among permeant anions. We find that the cytosolic aperture does not function as a Ca2+-dependent gate. Mutation of the aperture also has essentially no effect on the anion-over-cation selectivity of the channel, but we find that the aperture is responsible for establishing relative permeabilities among permeant anions. Thus, BEST1 does not contain a single selectivity filter because distinct regions of the channel are responsible for different aspects of ion selectivity. We conclude that the aperture functions as a size-selective filter that allows small molecules such as dehydrated Cl− ions to pass, while excluding larger entities. We hypothesize that anion-binding sites within the pore are primarily responsible for selectivity of anions over cations. Further studies are needed to understand the conformational changes in the neck and Ca2+ clasp that transition the channel from conductive to nonconductive states. Remarkably, however, it is clear that distinct regions in BEST1, and by analogy in BEST2–4, govern Ca2+-dependent activation, size discrimination among permeant species, and anion-over-cation selectivity.
Fig. 8.
Regions of BEST1 involved in Ca2+-dependent activation and ion selectivity. Structural features identified from the X-ray structure of BEST1 are labeled in black (16), and the mechanistic functions of these regions identified in this study are labeled in red (Right). Binding of intracellular Ca2+ (teal) to the Ca2+ clasp, which acts as a Ca2+ sensor, is coupled to conformational changes at the neck, which functions as a Ca2+-dependent gate. The neck is lined by three conserved hydrophobic residues (I76, F80, F84, stick representations). Phenylalanine residues within the neck (F80 and F84) may contribute favorable anion–quadropole interactions (δ+) to traversing Cl− ions, but these interactions do not determine ion selectivity. The cytosolic aperture, which is lined by the V205 residue, acts as a size-selective filter and endows BEST1 with its characteristic lyotropic permeability sequence. Selectivity for Cl− and other monovalent anions over cations may be determined by anion binding sites observed in the pore of BEST1 (purple) (16). The minus (−) and plus (+) signs in the pore indicate that, aside from the anion binding sites, the walls of the outer entryway and inner cavity have predominately negative and positive electrostatic potentials, respectively.
Methods
Cloning, Expression, and Purification of BEST1.
Chicken BEST1 (UniProt E1C3A0) constructs (amino acids 1–405 followed by a Glu–Gly–Glu–Glu–Phe tag) were expressed in Pichia pastoris and purified as described previously (16). Mutations were made using standard molecular biology techniques.
Reconstitution into Liposomes for Planar Lipid Bilayer Recordings.
Purified protein [in gel filtration buffer: 150 mM NaCl, 20 mM Tris⋅HCl, pH 8.5, 3 mM n-decyl-β-d-maltoside (Anatrace), and ∼1 μM Ca2+, as determined using Fura-2] was reconstituted into liposomes using slight modifications to methods described previously (16). A 3:1 (wt/wt) mixture of POPE (Avanti) and POPG (Avanti) lipids was prepared at 20 mg⋅mL−1 in reconstitution buffer (10 mM Hepes-NaOH, pH 7.0, 450 mM NaCl or KCl, 0.2 mM EGTA, 0.19 mM CaCl2), and the lipids were solubilized by adding 8% (wt/vol) n-octyl-β-d-maltopyranoside (Anatrace) and incubated with rotation for 30 min at room temperature. Purified protein was either concentrated using a 100-kDa molecular weight cutoff concentrator (Amicon Ultra; EMD Millipore) or diluted in gel filtration buffer before mixing with an equal volume of the solubilized lipids to give a final protein concentration of 0.2–1 mg⋅mL−1 and a lipid concentration of 10 mg⋅mL−1. The sample (<2 mL) was dialyzed (8,000-Da molecular mass cutoff) overnight at 4 °C against 2 L of reconstitution buffer to remove detergent. The resulting liposomes were flash frozen, as 50-μL aliquots, in liquid nitrogen and stored at −80 °C.
Electrophysiological Recordings.
Frozen liposomes were thawed and sonicated for approximately 10 s using an Ultrasonic Cleaner (Laboratory Supplies Company). All data are from recordings made using components analogous to the Warner planar lipid bilayer workstation (Warner Instruments) (21, 22). Briefly, two aqueous chambers (4 mL) were filled with bath solutions. The standard bath solutions consisted of the following: 30 mM KCl (cis side) or 10 mM KCl (trans side), 20 mM Hepes-NaOH, pH 7.6, 0.21 mM EGTA, and 0.19 mM CaCl2 (free [Ca2+], ∼300 nM, as determined using Fura-2). Chlorided silver (Ag/AgCl) wires were used as electrodes, submerged in 3 M KCl, and connected to the bath solutions via agar–KCl salt bridges [2% (wt/vol) agar, 3 M KCl]. The bath solutions were separated by a polystyrene partition with a ∼200-µm hole across which a bilayer was painted using POPE:POPG in n-decane [3:1 (wt/wt) ratio at 20 mg⋅mL−1]. Liposomes containing BEST1 were added, 1 µL at a time, to the cis chamber above the partition to a preformed bilayer until currents were observed. Solutions were stirred using a stir plate (Warner Instruments) to aid vesicle fusion to the bilayer. For recordings using 30 or 90 mM KCl on the trans side, KCl was added to the trans side (from a 3.5 M KCl stock) as necessary after vesicle fusion. NaCl was substituted for KCl in the bath solutions for experiments using NaCl. Unless noted, all reagents were purchased from Sigma-Aldrich. All electrical recordings were taken at room temperature (22–24 °C).
For Ca2+ reactivation experiments, after recording currents from standard symmetric KCl (30/30 mM) bath solutions, 10 mM EGTA was added to the cis chamber to deactivate channels with their cytosolic side facing this direction. Using a perfusion system (Nanion Technologies), the trans chamber was then perfused with 20 mL (5 chamber volumes) of bath solutions containing EGTA-Ca buffers (mixtures of 1 mM EGTA and 1 mM EGTA-Ca made according to ref. 38) to yield varying amounts of free [Ca2+] from ∼0 to ∼350 nM. Free [Ca2+] in these solutions was estimated using the maxchelator software (maxchelator.stanford.edu/webmaxc/webmaxcS.htm) and was verified using standard solutions (Invitrogen; pH adjusted to 7.6 with NaOH) and Fura-2 (Invitrogen) according to the manufacturer’s instructions. For experiments comparing the Ca2+ dependency of BEST1WT and channel mutants (Fig. 3), currents were recorded using standard symmetric (30/30 mM KCl) bath solutions before and after the addition of 10 mM EGTA (to both chambers, from a 0.5 M EGTA, pH 7.6, stock, followed by 4 min of stirring).
To assess permeabilities to anions other than Cl−, currents were first recorded in asymmetric (10/30 mM) and symmetric (30/30 mM) KCl conditions. The bath solution in the trans chamber was then replaced by perfusion, as described above, with standard bath solutions in which KCl was replaced by various potassium salts (30 mM). For experiments with DIDS (Sigma-Aldrich), freshly made solutions of DIDS (up to 100 mM in DMSO, stored at −20 °C before the experiment, and kept in the dark) were added to both the cis and trans bath solutions (with mixing for 1 min) to yield the desired final concentrations. Control experiments showed that the amount of DMSO in the bath solutions (maximally 2% for the 1 mM DIDS concentration) did not noticeably alter currents.
Currents were recorded using the Clampex 10.4 program (Axon Instruments) with an Axopatch 200B amplifier (Axon Instruments) and were sampled at 200 μs and filtered at 1 kHz. Data were analyzed using Clampfit 10.4 (Axon Instruments), and graphical display and statistical assessment were carried out using GraphPad Prism 6.0 software. In all cases, currents from bilayers without channels are subtracted. A representative bilayer recording is shown in Fig. S2C. Measurements were corrected for any offset voltage (typically <1 mV) at the end of the experiments by breaking the bilayer and using the Axopatch 200B pipette offset control. Error bars represent the SEM of at least three separate experiments, each in a separate bilayer. We define the side to which the vesicles are added as the cis side and the opposite trans side as electrical ground, so that transmembrane voltage is reported as Vcis–Vtrans. It is important to note that ion channels are inserted in both orientations in the bilayer.
Chloride Flux Assay.
Protocols for the fluorescence-based Cl− flux assays are the same as previously described (16). Briefly, for assays in the presence of ∼1 μM free Ca2+ (Fig. 2), purified BEST1 was reconstituted into liposomes using the reconstitution buffer: 100 mM Na2SO4, 10 mM Hepes-NaOH, pH7.0, 0.2 mM EGTA, and 0.19 mM CaCl2. For flux assays that were used to monitor Ca2+ activation (Fig. 4), the reconstitution buffer was 100 mM Na2SO4, 10 mM Hepes-NaOH, pH 8.2, and 1 mM EGTA. Protein was mixed with solubilized lipids to give a final protein concentration of 0.1 mg⋅mL−1 (0.01 mg⋅mL−1 was used for Fig. S7) and a lipid concentration of 10 mg⋅mL−1. Empty vesicles were prepared in parallel in the same manner in the absence of protein. The samples were dialyzed for 4–6 d in 2 L of reconstitution buffer with daily buffer changes. Following dialysis, the liposomes were sonicated for ∼20 s in a water bath, divided into 50-µL aliquots, and flash frozen in liquid nitrogen for storage at −80 °C. On the day of the experiment, liposomes were thawed, incubated for ∼1 h at room temperature, and then sonicated briefly (∼10 s) before use. Vesicles were diluted 100-fold into flux assay buffer, which contained 125 mM NaCl, and Cl− influx into the vesicles was detected from the fluorescence decrease of 9-amino-6-chloro-2-methoxyacridine (ACMA), which is a pH-sensitive indicator, after initiation of the assay by addition of the proton ionophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) (16). For comparative analyses (e.g., data presented on the same graph) BEST1WT and mutant proteins were purified and reconstituted into liposomes in parallel and the flux assays were performed on the same day.
Crystallization and Structure Determination of BEST1TripleA.
Crystals of BEST1TripleA were obtained using the methods described previously for BEST1WT (16). A complex between BEST1TripleA and a Fab monoclonal antibody fragment (designated 10-D10) was formed by mixing the two in a molar ratio of 1:1.2, concentrated (Vivaspin 15R; Sartorius; 10-kDa molecular weight cutoff), and purified using size exclusion chromatography (SEC) (Superdex-200; GE Healthcare). The SEC buffer contained 10 mM Tris, pH 7.5, 75 mM NaCl, 75 mM KCl, and 0.5 mM 2,2-bis(3′-cyclohexylbutyl) propane-1,3-bis-β-d-maltopyranoside (Anatrace). Crystals of the BEST1TripleA–Fab complex were obtained by vapor diffusion at 20 °C [300 nL of protein plus 300 nL of well solution: 6% (wt/vol) PEG 4000, 50 mM sodium acetate, pH 4.0, and 20% (vol/vol) glycerol]. Although not explicitly added, Ca2+ is present at ∼1 μM in the solutions, as determined using Fura-2 calcium indicator (16). Crystals were harvested after 5–10 d and flash-cooled in liquid nitrogen. Diffraction data were collected at 100 K and processed with HKL-3000 (39). Resolution limits were assessed using the CC1/2 statistic (40). Phases were determined using molecular replacement (BEST1WT, PDB ID code 4RDQ, as a search model), and the atomic coordinates were improved by model building in Coot (41) and refinement using CNS and Phenix (42, 43). Data collection and refinement statistics are shown in Table S1. Molecular graphics figures were prepared using the programs PyMOL (www.pymol.org) and HOLE (44).
SI Extended Data Analysis
To determine permeability ratios for chloride vs. potassium/sodium, the Goldman–Hodgkin–Katz equation was used in the following form:
where X represents potassium or sodium, EREV is the measured reversal potential, and all other terms have their usual meanings.
The Goldman–Hodgkin–Katz equation was also solved to determine permeability ratios between monovalent anions using a derivation for biionic conditions of the following form:
where EREV is the measured reversal potential, X is the permeant anion applied to the trans solution in equimolar concentrations with chloride in the cis solution, and all other terms have their usual meanings.
Acknowledgments
We are very grateful to David Gadsby (Rockefeller University) for discussions about experimental design and the manuscript. We thank the staff of beamlines 24-ID and 23-ID at the Advanced Photon Source, where X-ray data were collected (these facilities were supported by NIH Grants ACB-12002, AGM-12006, P41 GM103403, and S10 RR029205, under Department of Energy Contract DE-AC02-06CH11357). G.V. received funding and mentorship from the Boehringer Ingelheim Fonds Predoctoral Fellowship Program. This work was supported, in part, by NIH Grant R01 GM110396 (to S.B.L.) and a core facilities support grant to Memorial Sloan Kettering Cancer Center (P30 CA008748).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID code 5T5N (for X-ray structure of BEST1TripleA)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614688113/-/DCSupplemental.
References
- 1.Sun H, Tsunenari T, Yau K-W, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci USA. 2002;99(6):4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qu Z, Fischmeister R, Hartzell C. Mouse bestrophin-2 is a bona fide Cl− channel: Identification of a residue important in anion binding and conduction. J Gen Physiol. 2004;123(4):327–340. doi: 10.1085/jgp.200409031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qu Z, Wei RW, Mann W, Hartzell HC. Two bestrophins cloned from Xenopus laevis oocytes express Ca2+-activated Cl− currents. J Biol Chem. 2003;278(49):49563–49572. doi: 10.1074/jbc.M308414200. [DOI] [PubMed] [Google Scholar]
- 4.Tsunenari T, et al. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278(42):41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien L-T. Molecular physiology of bestrophins: Multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88(2):639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 6.Petrukhin K, et al. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19(3):241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- 7.Marquardt A, et al. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best’s disease) Hum Mol Genet. 1998;7(9):1517–1525. doi: 10.1093/hmg/7.9.1517. [DOI] [PubMed] [Google Scholar]
- 8.Davidson AE, et al. Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. Am J Hum Genet. 2009;85(5):581–592. doi: 10.1016/j.ajhg.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Q, Hartzell HC, Yu K. Bestrophins and retinopathies. Pflugers Arch. 2010;460(2):559–569. doi: 10.1007/s00424-010-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milenkovic VM, Röhrl E, Weber BHF, Strauss O. Disease-associated missense mutations in bestrophin-1 affect cellular trafficking and anion conductance. J Cell Sci. 2011;124(Pt 17):2988–2996. doi: 10.1242/jcs.085878. [DOI] [PubMed] [Google Scholar]
- 11.Kinnick TR, et al. Autosomal recessive vitelliform macular dystrophy in a large cohort of vitelliform macular dystrophy patients. Retina. 2011;31(3):581–595. doi: 10.1097/IAE.0b013e318203ee60. [DOI] [PubMed] [Google Scholar]
- 12.Fischmeister R, Hartzell HC. Volume sensitivity of the bestrophin family of chloride channels. J Physiol. 2005;562(Pt 2):477–491. doi: 10.1113/jphysiol.2004.075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stotz SC, Clapham DE. Anion-sensitive fluorophore identifies the Drosophila swell-activated chloride channel in a genome-wide RNA interference screen. PLoS One. 2012;7(10):e46865. doi: 10.1371/journal.pone.0046865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milenkovic A, et al. Bestrophin 1 is indispensable for volume regulation in human retinal pigment epithelium cells. Proc Natl Acad Sci USA. 2015;112(20):E2630–E2639. doi: 10.1073/pnas.1418840112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsunenari T, Nathans J, Yau K-W. Ca2+-activated Cl− current from human bestrophin-4 in excised membrane patches. J Gen Physiol. 2006;127(6):749–754. doi: 10.1085/jgp.200609527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane Dickson V, Pedi L, Long SB. Structure and insights into the function of a Ca2+-activated Cl− channel. Nature. 2014;516(7530):213–218. doi: 10.1038/nature13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T, et al. Structure and selectivity in bestrophin ion channels. Science. 2014;346(6207):355–359. doi: 10.1126/science.1259723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, et al. Characterization of the effects of Cl⁻ channel modulators on TMEM16A and bestrophin-1 Ca²⁺ activated Cl⁻ channels. Pflugers Arch. 2015;467(7):1417–1430. doi: 10.1007/s00424-014-1572-5. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Q, Prussia A, Yu K, Cui Y-Y, Hartzell HC. Regulation of bestrophin Cl channels by calcium: Role of the C terminus. J Gen Physiol. 2008;132(6):681–692. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405(2):199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 21.Miller C. Ion Channel Reconstitution. Springer Science & Business Media; New York: 2013. [Google Scholar]
- 22.Lee S, Zheng H, Shi L, Jiang Q-X. Reconstitution of a Kv channel into lipid membranes for structural and functional studies. J Vis Exp. 2013;2013(77):e50436. doi: 10.3791/50436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida K, Iuvone PM. Intracellular Ca2+ concentrations in cultured chicken photoreceptor cells: Sustained elevation in depolarized cells and the role of dihydropyridine-sensitive Ca2+ channels. Mol Vis. 1999;5:1. [PubMed] [Google Scholar]
- 24.Chien L-T, Hartzell HC. Rescue of volume-regulated anion current by bestrophin mutants with altered charge selectivity. J Gen Physiol. 2008;132(5):537–546. doi: 10.1085/jgp.200810065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu Z, Hartzell HC. Anion permeation in Ca2+-activated Cl− channels. J Gen Physiol. 2000;116(6):825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 27.Hille B. Ion Channels of Excitable Membranes. Sinauer Associates; Sunderland, MA: 2001. [Google Scholar]
- 28.Qu Z, Hartzell C. Determinants of anion permeation in the second transmembrane domain of the mouse bestrophin-2 chloride channel. J Gen Physiol. 2004;124(4):371–382. doi: 10.1085/jgp.200409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu Z, Chien L-T, Cui Y, Hartzell HC. The anion-selective pore of the bestrophins, a family of chloride channels associated with retinal degeneration. J Neurosci. 2006;26(20):5411–5419. doi: 10.1523/JNEUROSCI.5500-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philip V, et al. A survey of aspartate-phenylalanine and glutamate-phenylalanine interactions in the protein data bank: Searching for anion-π pairs. Biochemistry. 2011;50(14):2939–2950. doi: 10.1021/bi200066k. [DOI] [PubMed] [Google Scholar]
- 31.Jackson MR, et al. A preference for edgewise interactions between aromatic rings and carboxylate anions: The biological relevance of anion-quadrupole interactions. J Phys Chem B. 2007;111(28):8242–8249. doi: 10.1021/jp0661995. [DOI] [PubMed] [Google Scholar]
- 32.Stockbridge RB, et al. Crystal structures of a double-barrelled fluoride ion channel. Nature. 2015;525(7570):548–551. doi: 10.1038/nature14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aryal P, Sansom MSP, Tucker SJ. Hydrophobic gating in ion channels. J Mol Biol. 2015;427(1):121–130. doi: 10.1016/j.jmb.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science. 2008;321(5893):1179–1183. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aryal P, Abd-Wahab F, Bucci G, Sansom MSP, Tucker SJ. A hydrophobic barrier deep within the inner pore of the TWIK-1 K2P potassium channel. Nat Commun. 2014;5:4377. doi: 10.1038/ncomms5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 37.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- 39.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: The integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 8):859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 40.Karplus PA, Diederichs K. Linking crystallographic model and data quality. Science. 2012;336(6084):1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2(11):2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- 43.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph. 1996;14(6):354–360. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]