Significance
During embryogenesis, a dense vascular network develops in the pituitary gland through the process of angiogenesis. In tandem, pituitary gland precursor cells differentiate into hormone-producing cells that will rely on the vasculature to carry out regulated endocrine function. Our data show that expression of the cell surface adhesion molecule, integrin β1, in the epithelial-derived precursor cells is required for development of the vasculature and coordinated terminal differentiation of endocrine cells.
Keywords: angiogenesis, integrin β1, pituitary gland, mice
Abstract
As a key component of the vertebrate neuroendocrine system, the pituitary gland relies on the progressive and coordinated development of distinct hormone-producing cell types and an invading vascular network. The molecular mechanisms that drive formation of the pituitary vasculature, which is necessary for regulated synthesis and secretion of hormones that maintain homeostasis, metabolism, and endocrine function, remain poorly understood. Here, we report that expression of integrin β1 in embryonic pituitary epithelial cells is required for angiogenesis in the developing mouse pituitary gland. Deletion of pituitary epithelial integrin β1 before the onset of angiogenesis resulted in failure of invading endothelial cells to recruit pericytes efficiently, whereas deletion later in embryogenesis led to decreased vascular density and lumen formation. In both cases, lack of epithelial integrin β1 was associated with a complete absence of vasculature in the pituitary gland at birth. Within pituitary epithelial cells, integrin β1 directs a large transcriptional program that includes components of the extracellular matrix and associated signaling factors that are linked to the observed non–cell-autonomous effects on angiogenesis. We conclude that epithelial integrin β1 functions as a critical and canonical regulator of developmental angiogenesis in the pituitary gland, thus providing insight into the long-standing systems biology conundrum of how vascular invasion is coordinated with tissue development.
The mammalian pituitary consists of glandular anterior and intermediate lobes that arise from invaginated oral ectoderm termed Rathke’s pouch and a neural posterior lobe that develops in tandem as an outgrowth of the ventral diencephalon (1). A dense vascular network delivers hypothalamic regulatory factors and target organ feedback signals that control pituitary endocrine function (2). Angiogenesis gives rise to this network beginning on mouse embryonic day 13.5 (e13.5) as epithelial cells in the expanding anterior wall of Rathke’s pouch delaminate and intercalate with invading endothelial and mesenchymal cells (3). As embryonic blood vessels branch and spread laterally throughout the expanding anterior lobe, they form fenestrated capillaries that surround developing endocrine cells (4). Before birth, portal vessels from the hypothalamus begin to deliver regulatory hormones, target organ feedback, and afferent blood flow to this capillary network (5). Anterior pituitary hormones secreted in response to these signals are then delivered into the general circulation via venous drainage (6).
Vascular development depends on integrins, a large family of glycoprotein type I transmembrane receptors that function as αβ heterodimers to mediate cell adhesion to the extracellular matrix (ECM) (7). In turn, integrin binding to ECM affects deposition, organization, and remodeling of individual ECM components into functional supramolecular structures (8). Integrin β1 pairs with twelve separate α-chains to form the largest integrin subfamily, and its role in vascular development has been the subject of significant investigation. Although integrin β1-null embryos died before assembly of vessel primordia (vasculogenesis), integrin β1-null teratomas and embryoid bodies suggested an endothelial cell-autonomous requirement for integrin β1 in angiogenesis [formation of new vessels from preexisting vessels (7)].
Targeted deletions proved directly that endothelial cell integrin β1 was dispensable for vasculogenesis but required for subsequent angiogenic sprouting, branching, adhesion, migration, and cell survival (9). Later in embryogenesis, integrin β1 was necessary in arterioles for Par3-dependent endothelial cell polarity and lumen formation (10). In postnatal retinal vasculature, integrin β1-mediated endothelial cell–ECM interactions were required for production and assembly of the ECM, as well as formation of stable, nonleaky blood vessels (11). Mural cells (pericytes and vascular smooth muscle cells) that encase blood vessels also must express integrin β1 for adhesion and spreading, as well as assembly of ECM proteins that stabilize blood vessel walls (12, 13).
Here, we report that expression of integrin β1 in embryonic pituitary gland epithelial cells is absolutely required for the actions of the stromal cells that constitute the developing vasculature. Based on the phenotypes of mice harboring two temporally distinct, tissue-specific disruptions of Itgb1 in embryonic pituitary gland epithelial cells, we conclude that the non–cell-autonomous function of integrin β1 is critical for pericyte recruitment, lumen formation, and persistence of the vasculature. Analysis of the epithelial cell transcriptome revealed an integrin β1-dependent gene expression program encoding ECM and associated signaling molecules that exert pleiotropic effects on the coordinated development of the pituitary gland and its invading vascular system during organogenesis.
Results
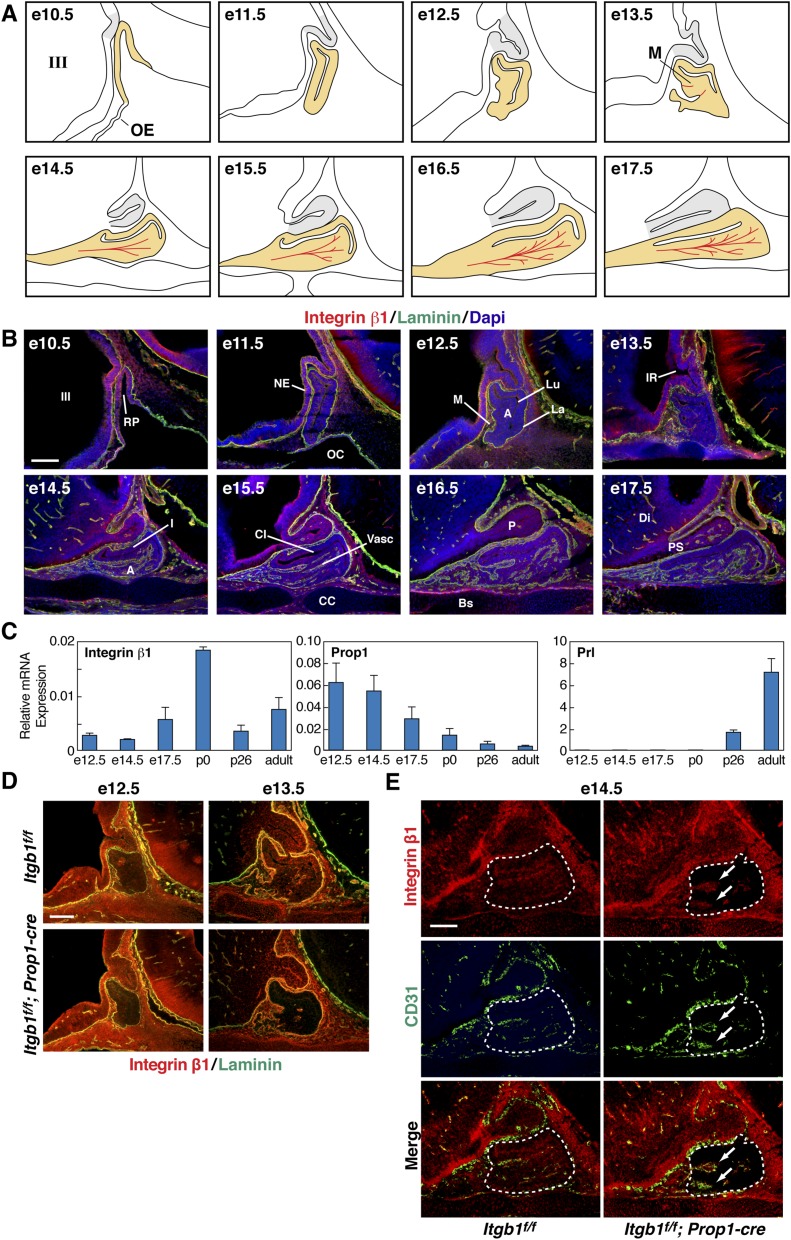
Integrin β1 Is Expressed in the Pituitary Gland Throughout Embryonic Development.
Integrin β1 protein was detected in all three lobes of the pituitary from e10.5 through birth, in oral ectoderm-derived epithelial cells that comprise the parenchyma of the developing gland, and in endothelial and supporting mesenchymal cells that form the vasculature (Fig. S1 A and B). Laminin, an ECM glycoprotein that is bound by a subset of integrin β1 heterodimers, was abundant in the basement membrane that separates the epithelial tissue of the early embryonic pituitary gland from adjacent mesenchyme (3, 14). As angiogenesis proceeds, this demarcation persists in the form of a vascular basement membrane that surrounds nascent vessels in the anterior lobe (3) (Fig. S1B). Quantitative PCR analyses of integrin β1 mRNA in the pituitary gland from e12.5 to adulthood detected continuous low levels, with a spike in expression at postnatal day 0 (p0). Validity of these measurements was provided by quantitation of previously characterized Prop1 and prolactin (Prl) mRNAs (15) (Fig. S1C).
Fig. S1.
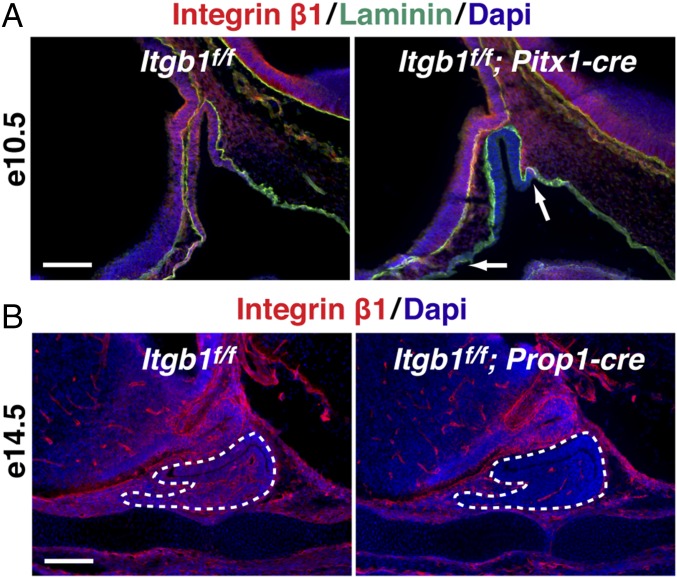
Integrin β1 is expressed in pituitary gland epithelial cells throughout embryonic development and is inactivated at e10.5 with Pitx1-cre and at e14.5 with Prop1-cre. (A) Embryonic development of the glandular anterior and intermediate lobes (yellow) beginning with invagination of the oral ectoderm at e10.5. Tandem outgrowth of the third ventricle gives rise to the neural posterior lobe (gray). At e13.5, the first evidence appears of nascent microvessels (M) in red. (B) Immunohistochemical staining of integrin β1 and laminin in sections of control embryonic pituitaries. (Top Left) e10.5 is the same image shown in Fig. 1A, Left. Laminin marks the basement membranes of the organ primordium and the developing vasculature. A, anterior lobe; Bs, basisphenoid bone; CC, condensing cartilage; CI, cleft between the intermediate and anterior lobes; DI, diencephalon; I, intermediate lobe; III, third ventricle of the diencephalon; IR, infundibular recess; La, laminin-rich basement membrane of the organ primordium; Lu, residual of Rathke’s pouch; M, rostral mesenchyme; NE, neuroepithelium; OC, oral cavity; P, posterior lobe; PS, pituitary stalk; RP, Rathke’s pouch; Vasc, vasculature. (Scale bar: 130 μm.) (C) Expression of integrin β1 mRNA throughout pituitary organogenesis. Quantitative real-time PCR of integrin β1, Prop1, and PRL mRNA transcripts isolated from dissected pituitaries. Data are normalized to GAPDH, and error bars represent SEM from triplicate quantitative PCR (qPCR) reactions. (D) Itgb1f/f; Prop1-cre pituitaries immunostained with integrin β1 and laminin show progressive decrease of integrin β1 protein from e12.5 to e13.5. (Scale bar: 130 μm.) (E) Expression of integrin β1 is unaffected in CD31(+) endothelial cells (arrows) in e14.5 Itgb1f/f; Prop1-cre pituitary glands (enclosed by dashed lines). (Scale bar: 62.5 μm.) Midsagittal sections are shown in B, D, and E.
Targeted Deletions of Integrin β1 in Embryonic Pituitary Gland Epithelial Cells at e10.5 and e14.5.
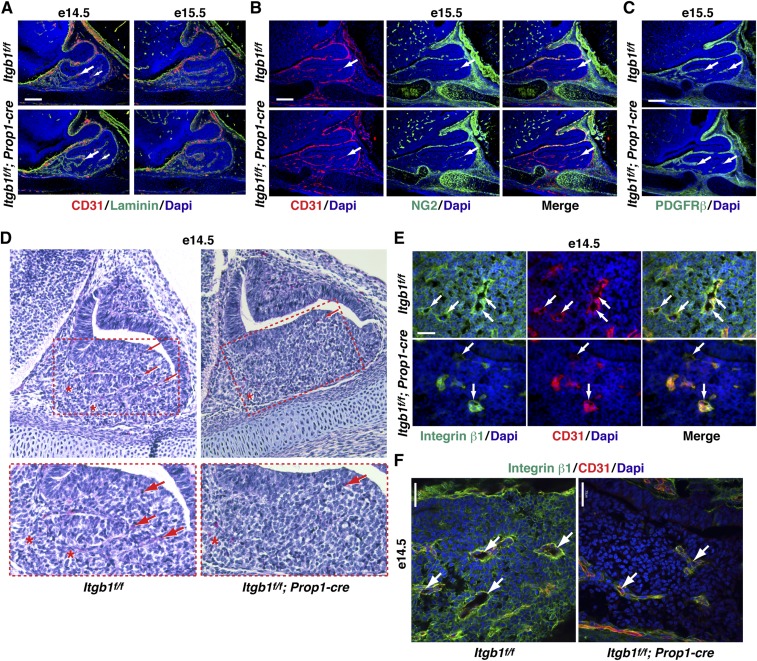
To examine integrin β1 function in pituitary epithelial cells before cell-type differentiation or angiogenesis, we crossed Pitx1-cre transgenic mice to Itgb1flox mice, resulting in complete loss of integrin β1 protein throughout Rathke’s pouch by e10.5 (16, 17) (Fig. 1A). To study the role of integrin β1 after the initiation of cell-type differentiation and angiogenesis, we crossed Prop1-cre transgenic mice to Itgb1flox mice, causing progressive loss of integrin β1 protein in the parenchyma of the developing anterior and intermediate lobes that began on e13.5 and was complete by e14.5 (18) (Fig. 1B and Fig. S1D). Importantly, coimmunostaining of integrin β1 and CD31 in Prop1-cre embryos demonstrated that expression of integrin β1 in invading endothelial cells was unaffected (Fig. S1E).
Fig. 1.
Targeted deletions of Itgb1 in the pituitary gland. (A) Pitx1-cre eliminates integrin β1 at e10.5 in Rathke’s pouch epithelium (Right, between arrows). Laminin marks basement membrane. (Scale bar: 130 μm.) Image on the left is shown again as the first panel in an ontogenic series in Fig. S1B. (B) Prop1-cre eliminates integrin β1 at e14.5 in pituitary gland epithelium (enclosed by dotted lines). (Scale bar: 130 μm.) Midsagittal sections are shown in A and B.
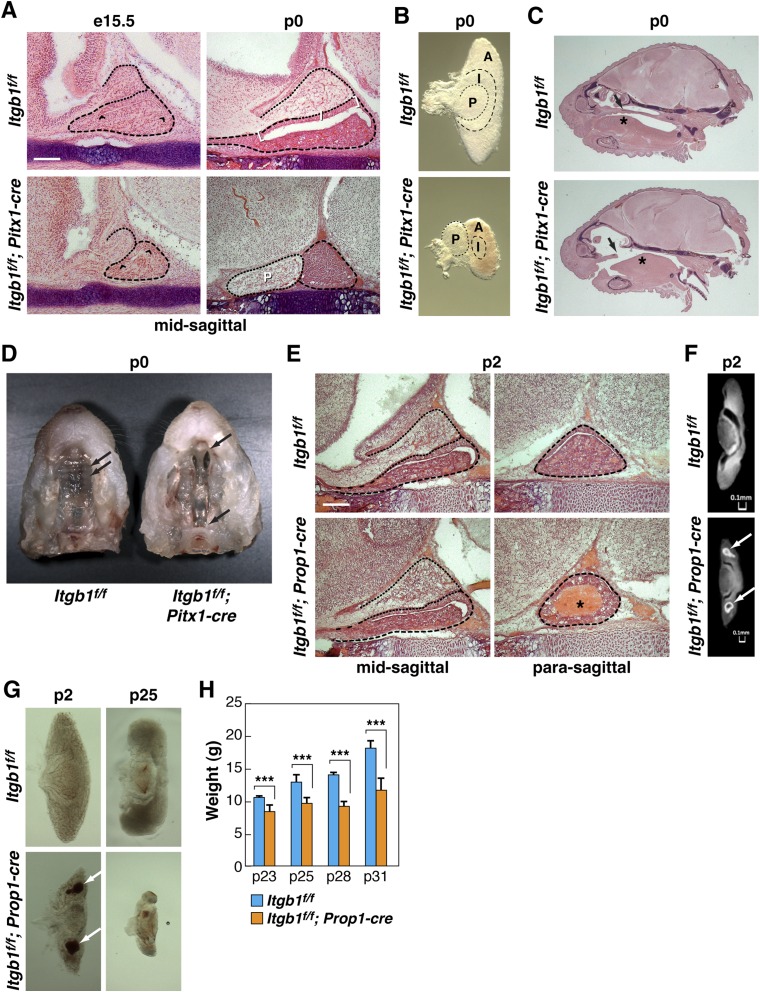
Itgb1f/f; Pitx1-cre Pups Die at Birth but Itgb1f/f; Prop1-cre Mice Are Viable.
Itgb1f/f; Pitx1-cre mice were born in Mendelian ratios, but all mutant pups died at birth. At e15.5, hematoxylin and eosin (H&E)-stained midline sagittal sections revealed a smaller gland with a shortened pituitary cleft. By p0, the anterior and intermediate lobes were significantly smaller and displayed altered morphology, the posterior lobe was displaced in the rostral direction, there was poor anatomical definition of the intermediate lobe because of progressive shortening of the cleft, red blood cells (RBCs) were absent from the anterior lobe, and the secondary palate had failed to fuse along the midline (Fig. S2 A–D).
Fig. S2.
Phenotypic defects in Itgb1f/f; Pitx1-cre embryos and Itgb1f/f; Prop1-cre embryos. (A) Shortened cleft (between arrowheads) in H&E-stained e15.5 Itgb1f/f; Pitx1-cre pituitary. By p0, the cleft has disappeared, intermediate lobe (I, between brackets in Itgb1f/f control) is indistinct, and posterior lobe (P) has shifted in the rostral direction. (Scale bar: 130 μm.) (B) Altered morphology and diminished size of anterior (A), intermediate (I), and posterior (P) lobes in Itgb1f/f; Pitx1-cre pituitaries dissected at p0. (C) H&E stained sections of p0 heads show that a secondary palate (arrow) fails to form and the tongue (asterisk) is misshapen in Itgb1f/f; Pitx1-cre pups. (D) In Itgb1f/f control, arrows indicate palatal ridges in the formed secondary palate. In Itgb1f/f; Pitx1-cre embryos, palatal shelves fail to join at the midline to form the secondary palate (between arrows). (E) Itgb1f/f; Prop1-cre p2 pituitaries show normal organ morphology but evidence of hemorrhage in lateral anterior lobes (asterisk). (Scale bar: 130 μm.) (F) MicroCT scans of pituitaries dissected at p2 show diminished size and evidence of hemorrhage (arrows) in anterior lobe of Itgb1f/f; Prop1-cre pituitary. (G) Hemorrhage (arrows) in lateral anterior lobes of pituitaries dissected from Itgb1f/f; Prop1-cre animals at p2. An increasingly diminished size of anterior lobe is visible at p25. (H) Itgb1f/f; Prop1-cre animals fail to gain weight following weaning. Average weights of control vs. knockout littermates in four separate litters (ranging in size from six to 11 animals) in the 10-d period following weaning at p21. Error bars indicate SD. ***P < 0.005 determined by t test. Midsagittal sections are shown in A, C, and E.
Itgb1f/f; Prop1-cre animals were born in Mendelian ratios and survived normally into adulthood. H&E staining at p2 showed signs of hemorrhage or hematoma in the lateral wings of the anterior lobes that was confirmed by microCT scans (Fig. S2 E and F). Although H&E staining showed normal placement of the three pituitary lobes, Itgb1f/f; Prop1-cre pituitaries dissected at p2 revealed a decreased size of the anterior lobe that became dramatic by p25 (Fig. S2G). Given that normal postnatal growth relies on secretion of growth hormone (GH) from anterior lobe somatotropes, we compared the weights of Itgb1f/f; Prop1-cre mice with the weights of Itgb1f/f littermates in the 10-d period following weaning at p21. In four separate litters, all Itgb1f/f; Prop1-cre animals weighed less at each time point, and by p31, their weights were, on average, 66% of controls (Fig. S2H). These results suggested diminished somatotrope function due to decreased expression, secretion, and/or delivery of GH.
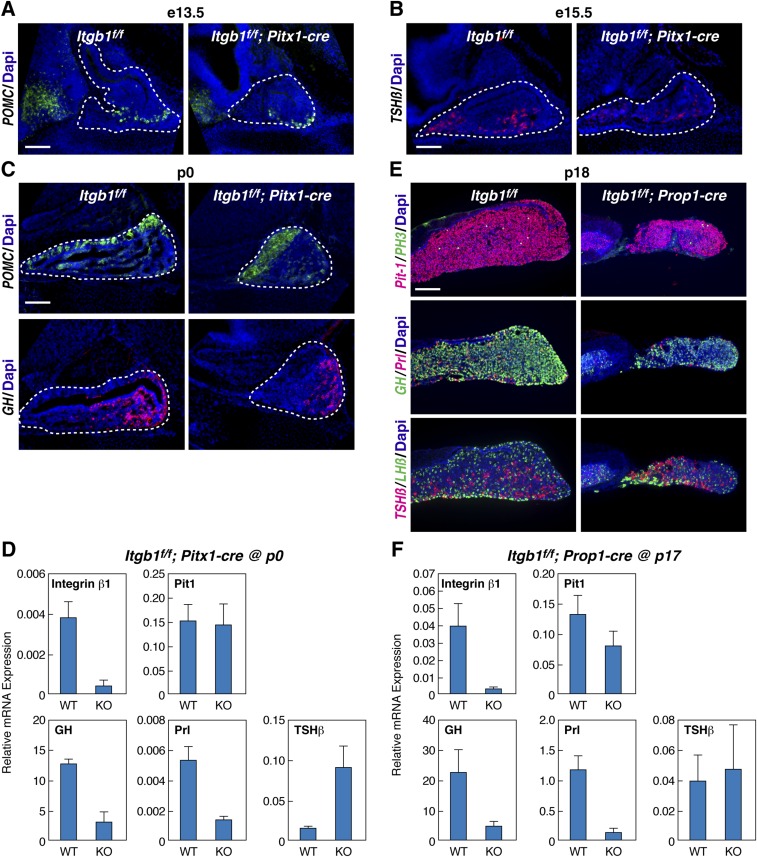
Endocrine Cell-Type Differentiation.
To assess the role of integrin β1 in endocrine cell-type differentiation, we examined expression of pituitary hormone genes. Pit-1 is a key transcription factor that is required for specification of the somatotrope, lactotrope, and thyrotrope cells that, upon terminal differentiation, express growth hormone (GH), Prl, and thyroid-stimulating hormone-β (TSH-β), respectively. These three hormone-encoding genes are also direct transcriptional targets of Pit-1, which functions in combination with hypothalamic regulatory signals and target organ feedback to achieve regulated physiological expression (15). Although normal temporal expression of pro-opiomelanocortin (POMC) at e13.5, TSH-β at e15.5, and POMC and GH at p0 was observed, differences in spatial expression patterns were noted at each stage in Itgb1f/f; Pitx1-cre embryos (Fig. S3 A–C). At p0, expression of Pit-1 mRNA was normal but GH and Prl were significantly down-regulated, whereas TSH-β was up-regulated (Fig. S3D). At p18, Itgb1f/f; Prop1-cre pituitary glands were significantly smaller, expressed less GH and Prl protein, and contained mislocalized dorsal thyrotropes (Fig. S3E). At p17, Pit-1 mRNA expression was normal but levels of GH and Prl were diminished (Fig. S3F). In both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre pituitaries, normal expression of Pit-1 was combined with significantly altered expression of its target genes, suggesting that failure to receive additional critical hypothalamic regulatory signals and target organ feedback via the circulatory system might be the explanation.
Fig. S3.
Timing of endocrine cell differentiation is normal, but spatial alterations and changes in gene expression levels occur in Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre embryos. (A) Ventral expression of POMC in Itgb1f/f; Pitx1-cre pituitaries at e13.5. (B) Thyroid-stimulating hormone-β (TSH-β) in Itgb1f/f; Pitx1-cre pituitaries at e15.5. (C) Altered spatial expression of POMC and GH in Itgb1f/f; Pitx1-cre pituitaries at p0. (D) qPCR of pituitary mRNA from Itgb1f/f; Pitx1-cre pituitaries at p0 revealed reduced GH and Prl but increased TSH-β. (E) Reduced density of GH cells and mislocalized dorsal TSH-β cells in Itgb1f/f; Prop1-cre pituitaries at p18 immunostained for Pit-1 and PH3, GH and Prl, TSH-β, and LH-β. (F) qPCR of pituitary mRNA from Itgb1f/f; Prop1-cre at p17 mimics GH and Prl data from Itgb1f/f; Pitx1-cre pituitaries at p0. Midsagittal sections are shown in A-C, and coronal sections are shown in E. (Scale bars: A–C and E, 130 μm.) KO, knockout; WT, wild type.
Blood Vessels Are Absent in Both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre Mice at Birth.
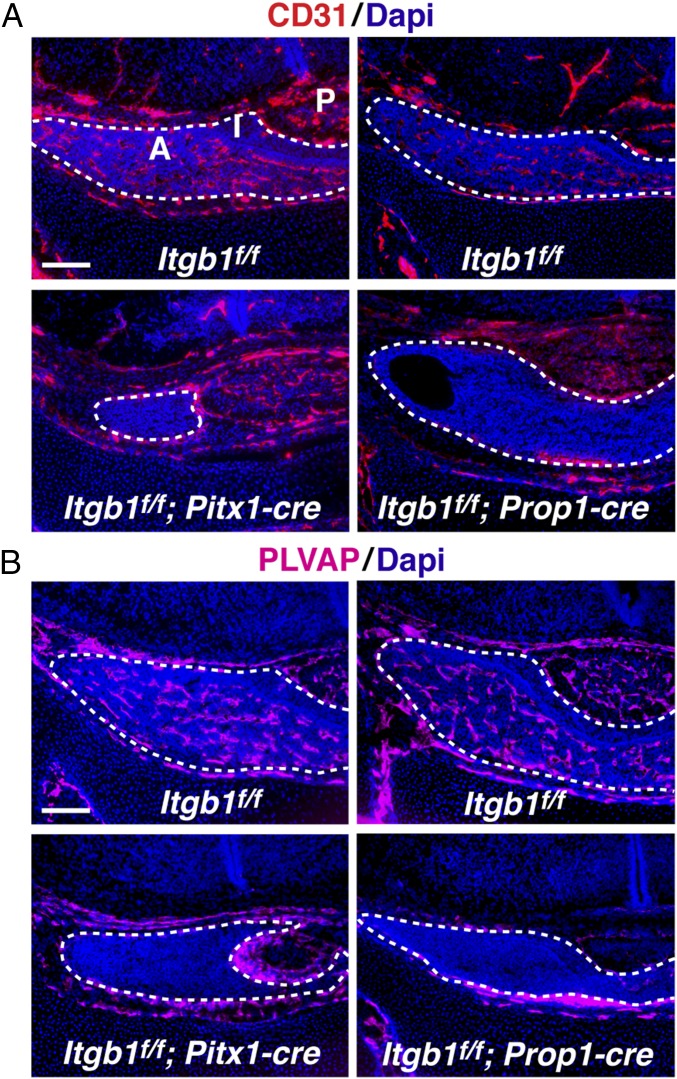
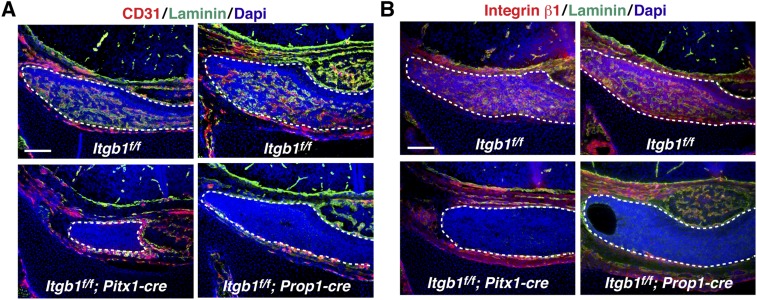
Development of the vascular network that delivers hypothalamic regulatory signals and target organ feedback to pituitary endocrine cell types was examined at p0. In control pituitaries, CD31 immunostained abundant blood vessels in the anterior lobe (the intermediate lobe is notably avascular). In contrast, both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre pituitaries displayed a remarkable absence of CD31 immunostaining, suggesting that the entire dense network of blood vessels had failed to form and/or stabilize in the absence of epithelial integrin β1 (Fig. 2A). This result was verified with a second, independent endothelial cell marker, PLVAP (plasmalemmal vesicle-associated protein) (19) (Fig. 2B). Coimmunostaining of CD31 and laminin showed laminin-rich vascular basement membranes in control animals but failed to detect laminin-rich “empty basement membrane sleeves” in either Itgb1f/f; Pitx1-cre or Itgb1f/f; Prop1-cre pituitary glands, suggesting that even if vessels had formed, they had been absent for several days (20) (Fig. S4A). Coimmunostaining of integrin β1 and laminin in control animals demonstrated that the highest level of integrin β1 expression occurred in cells within the laminin-rich vascular basement membrane (Fig. S4B).
Fig. 2.
Absence of vasculature at p0 in both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre pituitary glands. (A and B) Immunostaining of coronal sections with independent markers, CD31 and PLVAP, failed to detect endothelial cells in Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre pituitary glands at birth. A, anterior lobe; I, intermediate lobe; P, posterior lobe; PLVAP, plasmalemmal vesicle-associated protein. (Scale bars: 130 μm.)
Fig. S4.
Absence of vasculature at p0 in both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre pituitary glands. (A) Laminin-rich vascular basement membranes have disappeared from pituitary glands in both Itgb1f/f; Pitx1-cre and Itgb1f/f; Prop1-cre knockouts by birth. (B) Integrin β1 expression is higher in vascular cells surrounded by laminin-rich vascular basement membrane than in surrounding epithelial cell parenchyma in controls. Coronal sections are shown in A and B. (Scale bars: 130 μm.)
Angiogenesis Begins at e13.5 but Invading Endothelial Cells Fail to Recruit Pericytes in Itgb1f/f; Pitx1-cre Pituitaries.
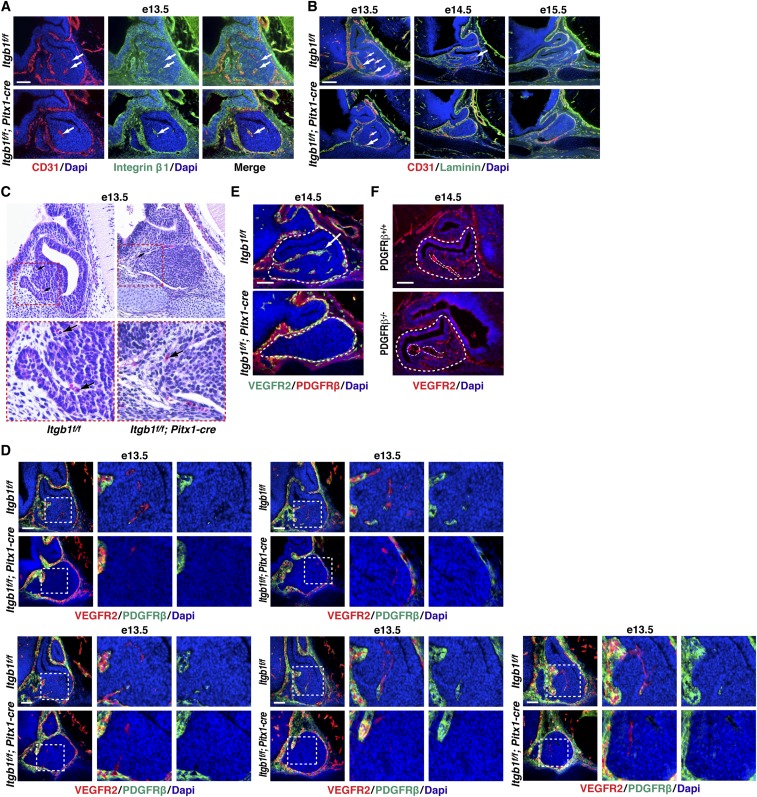
To assess how the initial steps in angiogenesis proceeded in the absence of epithelial integrin β1, we examined Itgb1f/f; Pitx1-cre pituitaries at e13.5, the time point at which we observed angiogenesis beginning in the anterior lobe. CD31 and integrin β1(+) endothelial cells were present in control and Itgb1f/f; Pitx1-cre pituitaries (Fig. S5A). Coimmunostaining with CD31 and laminin revealed that they deposited laminin-rich basement membranes at e13.5. By e14.5, however, neither endothelial cells nor their basement membranes were detected in Itgb1f/f; Pitx1-cre pituitaries (Fig. S5B).
Fig. S5.
Invading endothelial cells fail to recruit pericytes in Itgb1f/f; Pitx1-cre pituitary glands at e13.5 and are absent by e14.5. (A) CD31(+) endothelial cells (arrows) migrate into anterior lobes of both Itgb1f/f and Itgb1f/f; Pitx1-cre pituitaries at e13.5 as angiogenesis begins. Integrin β1 expression in endothelial cells (arrows) is not affected in Itgb1f/f; Pitx1-cre pituitaries. (Scale bar: 62.5 μm.) (B) At e13.5, endothelial cells deposit laminin-rich basement membranes in both Itgb1f/f and Itgb1f/f; Pitx1-cre pituitaries (arrows). By e14.5, endothelial cells and laminin-rich vascular basement membranes are absent from Itgb1f/f; Pitx1-cre pituitary glands. (Scale bar: 130 μm.) (C) H&E staining at e13.5 shows RBCs (arrows) in vessels surrounding both Itgb1f/f and Itgb1f/f; Pitx1-cre pituitaries, but not within the glands. (Magnification: Top, 200×.) (D) Magnified images of the same serial sagittal sections shown in Fig. 3 from e13.5 Itgb1f/f and Itgb1f/f; Pitx1-cre pituitary glands immunostained with VEGFR2 (endothelial cells) and PDGFRβ (pericytes). Endothelial cells recruited pericytes in Itgb1f/f but not Itgb1f/f; Pitx1-cre pituitary glands. (Scale bars: 62.5 μm.) (E) E14.5 Itgb1f/f and Itgb1f/f; Pitx1-cre pituitaries immunostained with VEGFR2 and PDGFRβ. Neither pericytes nor endothelial cells are present at e14.5 in Itgb1f/f; Pitx1-cre pituitary glands. (Scale bar: 62.5 μm.) (F) E14.5 PDGFRβ−/− pituitary gland (enclosed by large dashed lines) immunostained with VEGFR2 shows that blood vessels (small dashed lines) are present but dilated relative to vessels in PDGFRβ+/+ control pituitary gland. (Scale bar: 62.5 μm.) Midsagittal sections are shown in A–C, E, and F.
To determine whether lumen formation was related to the disappearance of endothelial cells, we looked for evidence of RBCs using H&E staining. In both control and Itgb1f/f; Pitx1-cre embryos at e13.5, RBCs were present in vessels surrounding the pituitary but were not detected within the gland, suggesting that lumen formation begins after e13.5 (Fig. S5C). Therefore, failure of vascular development in the Itgb1f/f; Pitx1-cre pituitaries likely preceded lumen formation.
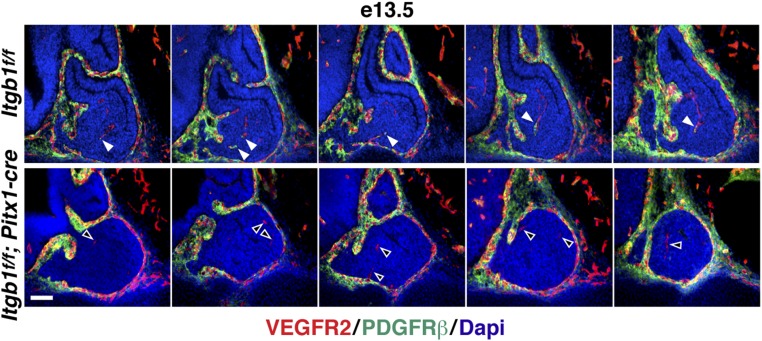
Next, we considered recruitment of pericytes that normally envelop nascent microvessels, span endothelial cell junctions, and embed within the vascular basement membrane to provide structural support and signals that control endothelial cell growth versus quiescence (21). In the pituitary, rostral neural crest-derived mesenchyme gives rise to pericytes that surround anterior lobe capillaries (22). At e13.5, we coimmunostained with the pericyte marker PDGF receptor β (PDGFRβ) and vascular endothelial growth factor receptor 2 (VEGFR2) which labels endothelial cells (21). We observed densely packed PDGFRβ(+) cells surrounding the developing glands, and in controls, pericytes were associated with invading endothelial cells. In Itgb1f/f; Pitx1-cre pituitaries, however, endothelial cells lacked associated pericytes at e13.5, and by e14.5, endothelial cells were also absent (Fig. 3 and Fig. S5 D and E).
Fig. 3.
Endothelial cells invade Itgb1f/f; Pitx1-cre pituitary glands at e13.5 but fail to recruit pericytes. Serial sagittal sections beginning at the midline and progressing through the lateral anterior lobes immunostained with VEGFR2 (endothelial cells) and PDGFRβ (pericytes) are shown. Pericytes recruited to endothelial cells in Itgb1f/f control pituitary glands (filled arrowheads) are shown. Endothelial cells that failed to recruit pericytes in Itgb1f/f; Pitx1-cre pituitary glands (empty arrowheads) are shown. Magnified images of the same panels are provided in Fig. S5D. (Scale bar: 62.5 μm.)
A well-characterized mechanism of pericyte recruitment involves secretion of PDGF-B by endothelial tip cells in angiogenic sprouts. Subsequent tethering of secreted PDGF-B to heparan sulfate and heparan sulfate proteoglycans (HSPGs) in the ECM retains it at relatively high local concentrations for binding to PDGFRβ on pericytes. In the absence of PDGF-B signaling, lack of pericyte recruitment has been correlated with increased vessel diameter, hemorrhage, and edema in many tissues (21). In the pituitary, genetic ablation of neural crest-derived pericytes resulted in vessels that survived and formed lumens but were dilated and leaky (22, 23). Based on these findings, we examined vascular development in the pituitary glands of PDGFRβ−/− mice (24). At e14.5, immunostaining with VEGFR2 revealed that blood vessels were present but highly dilated, suggesting that PDGF-B signaling plays a role in pericyte recruitment in the embryonic pituitary (Fig. S5F). By extension, defective PDGF-B signaling may explain failure of pericyte recruitment in e13.5 Itgb1f/f; Pitx1-cre pituitaries, but it does not fully explain the absence of endothelial cells observed at e14.5.
Reduced Vessel Density and Lumen Formation in Itgb1f/f; Prop1-cre Pituitaries at e14.5.
The role of epithelial integrin β1 in later steps in angiogenesis was examined in Itgb1f/f; Prop1-cre embryos. Coimmunostaining of CD31 and laminin at e14.5 and e15.5 demonstrated that endothelial cells invaded the anterior lobe and deposited laminin-rich basement membranes equivalently in control and Itgb1f/f; Prop1-cre pituitaries (Fig. S6A). Immunostaining of CD31 and NG2 (Fig. S6B), or PDGFRβ (Fig. S6C), demonstrated that pericytes were associated with endothelial cells in Itgb1f/f; Prop1-cre pituitary glands at e15.5. We next examined lumen formation. H&E staining at e14.5 detected RBCs in both control and Itgb1f/f; Prop1-cre pituitaries in the rostral anterior lobes where endothelial cells first invade. RBCs were also present within the central and caudal anterior lobe in control pituitaries, thus identifying e14.5 as the time at which lumen formation normally begins. In Itgb1f/f; Prop1-cre pituitaries, however, RBCs were rarely observed in the central and caudal anterior lobe, suggesting compromised lumen formation and/or reduced vessel number (Fig. S6D).
Fig. S6.
Reduced lumen formation in Itgb1f/f; Prop1-cre pituitaries at e14.5. (A) Endothelial cells are present (arrows) and surrounded by laminin-rich vascular basement membrane in e14.5 and e15.5 Itgb1f/f; Prop1-cre pituitary glands. (Scale bar: 130 μm.) (B) Pericytes immunostained with NG2 are associated with endothelial cells in e15.5 Itgb1f/f; Prop1-cre pituitaries. (Scale bar: 130 μm.) (C) Pericytes immunostained with PDGFRβ are associated with endothelial cells in midsagittal sections of e15.5 Itgb1f/f; Prop1-cre pituitaries. (Scale bars: 130 μm.) (D) H&E staining of parasagittal pituitary sections showed fewer RBCs (arrows) within the anterior lobe (red dashed lines) of Itgb1f/f; Prop1-cre pituitaries at e14.5. RBCs are present along the rostral aspect of both the Itgb1f/f and Itgb1f/f; Prop1-cre pituitary glands (asterisks). (Magnification: Top, 200×.) (E) Higher magnification images of lumens (arrows) from the section presented in Fig. 4A (Itgb1f/f, Top) and a section adjacent to the one shown in Fig. 4A (Itgb1f/f; Prop1-cre, Bottom). (Scale bar: 32.5 μm.) (F) Confocal images of lumens (arrows) in Itgb1f/f and Itgb1f/f; Prop1-cre pituitary glands at e14.5. (Scale bars: 30 μm.) Midsagittal sections are shown in A–C, parasagittal sections are shown in D, and coronal sections are shown in E and F.
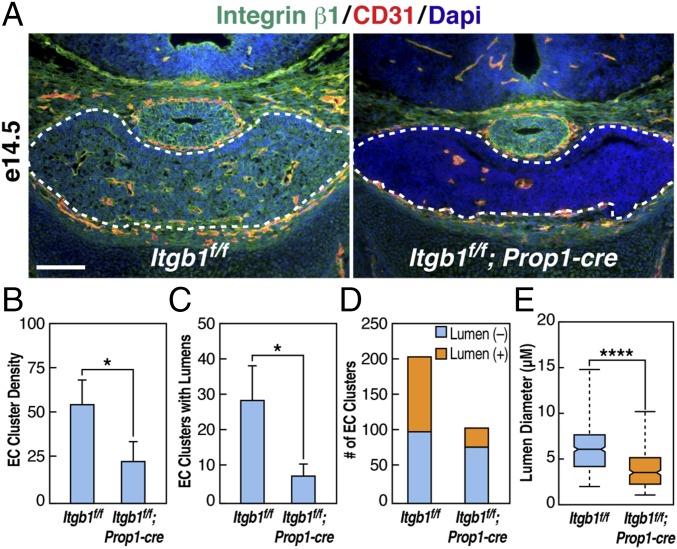
To investigate vessel number and lumen formation further, e14.5 coronal sections were immunostained with integrin β1 and CD31. At this stage, cords of invading endothelial cells appeared as tight clusters with or without visible lumens (Fig. 4A and Fig. S6E). In Itgb1f/f; Prop1-cre pituitaries, endothelial cell cluster number was reduced by >50%, suggesting a defect in vessel branching (Fig. 4 B and D), and the number with a detectable lumen was ∼25% of normal (Fig. 4 C and D). Within clusters that formed lumens at e14.5, median lumen diameter in controls was 6.0 μm, with a range from 2.0 μm to 14.9 μm, but it was only 3.5 μm, with a range from 1.1 μm to 10.1 μm in Itgb1f/f; Prop1-cre pituitary glands (Fig. 4E and Fig. S6 E and F). Hence, loss of epithelial integrin β1 by e14.5 resulted in a decreased number of nascent blood vessels with compromised lumen formation.
Fig. 4.
Endothelial cell (EC) cluster number and lumen formation are reduced in Itgb1f/f; Prop1-cre pituitary glands at e14.5. (A) Coronal sections immunostained with integrin β1 and CD31 reveal that EC cluster number is reduced in Itgb1f/f; Prop1-cre pituitary glands at e14.5. (Scale bar: 130 μm.) Higher magnification images of the panel on the left and of a section adjacent to the panel on the right are shown in Fig. S6E. (B) Average number of EC clusters in coronal sections of e14.5 pituitary glands. Three sections of equivalent size and anatomical position from each of four Itgb1f/f and Itgb1f/f; Prop1-cre embryos were analyzed. The sum of EC clusters counted in the three sections for each animal is shown. Error bars represent SEM. *P = 0.0408 determined by t test. (C) Average number of EC clusters with lumens in the coronal sections analyzed in B. Error bars represent SEM. *P = 0.0266 determined by t test. (D) Sum of all EC clusters counted without lumens (blue) and with lumens (orange) as shown in B and C. (E) Mean diameter of blood vessel lumens determined using the straight-line tool in ImageJ (NIH) to measure the shortest distance across lumens in four Itgb1f/f and four Itgb1f/f; Prop1-cre pituitary glands at e14.5. Measurements were made in three sections from each pituitary for a total of 104 lumens in Itgb1f/f and 27 in Itgb1f/f; Prop1-cre. Whisker ends represent minimum and maximum data points. ****P < 0.0001 determined by Mann–Whitney test. Magnified images are provided in Fig. S5 E and F.
RNA-Sequencing Identified an Integrin β1-Dependent Transcriptional Program Encoding ECM and Associated Molecules.
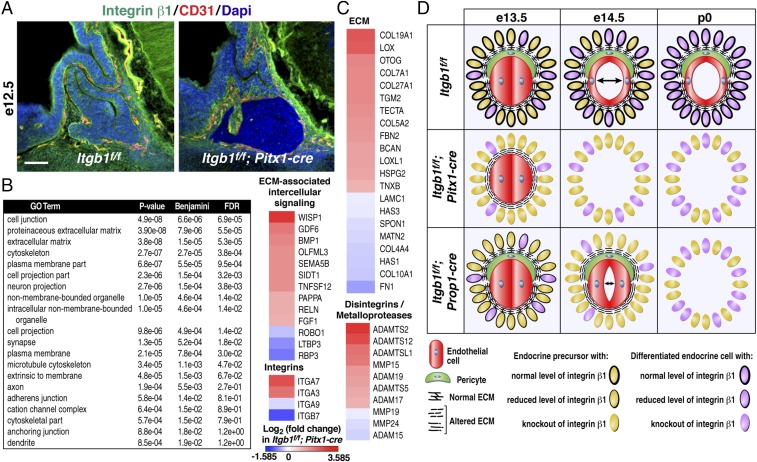
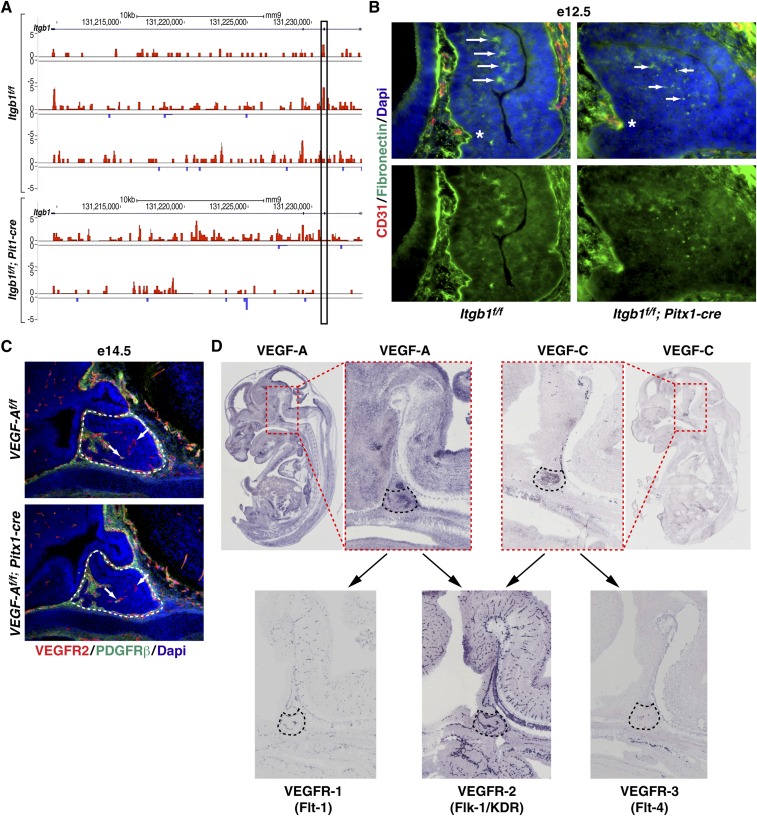
In Itgb1f/f; Pitx1-cre mice, e10.5 inactivation of integrin β1 provided a homogeneous population of epithelial cells before initiation of angiogenesis at e13.5. We hypothesized that profiling gene expression at e12.5 in cells that uniformly lacked integrin β1 might provide insight into why angiogenesis failed (Fig. 5A). Massively parallel RNA-sequencing (RNA-seq) was used to compare the transcriptomes of pituitaries dissected at e12.5 from Itgb1f/f and Itgb1f/f; Pitx1-cre littermate embryos. Deletion of exon 3 in the Itgb1f/f; Pitx1-cre RNA samples was validated by viewing sequence tags mapped to the Itgb1 locus (Fig. S7A).
Fig. 5.
Before initiation of angiogenesis in the pituitary gland, integrin β1-dependent transcriptome encodes ECM and associated factors critical for angiogenesis. (A) Midsagittal sections of e12.5 Itgb1f/f and Itgb1f/f; Pitx1-cre pituitaries immunostained with integrin β1 and CD31 1 d before initiation of angiogenesis. (Scale bar: 62.5 μm.) (B) RNA-seq analysis of pituitaries dissected from Itgb1f/f and Itgb1f/f; Pitx1-cre embryos at e12.5 identified 496 down-regulated and 642 up-regulated genes. Gene ontology analysis revealed significant changes in categories of genes encoding intrinsic and extrinsic factors. (C) Heat maps with identity of genes that encode ECM and related proteins. (D) Model for function of pituitary gland epithelial cell integrin β1 in developmental angiogenesis.
Fig. S7.
Deletion of epithelial integrin β1 alters ECM. (A) RNA-seq reads mapped to the Itgb1 locus on the UCSC Genome Browser indicate absence of exon 3-encoded sequence in RNA from e12.5 Itgb1f/f; Pitx1-cre dissected pituitaries. (B) FN deposition and reduced fibril formation (arrows) in e12.5 Itgb1f/f; Pitx1-cre pituitary glands. Asterisks indicate rostral aspect of the anterior lobe where endothelial cells first invade. (Scale bar: 32.5 μm.) (C) VEGFR2 and PDGFRβ immunostaining at e14.5 in VEGF-Af/f control and VEGF-Af/f; Pitx1-cre knockout littermate pituitaries showed comparable endothelial cell invasion and recruitment of pericytes (arrows). (D) RNA in situ hybridization images from the genepaint.org website showing e14.5 pituitary gland expression of VEGF-A and VEGF-C ligands and VEGF receptors (VEGFR-1, VEGFR-2, and VEGFR-3). Arrows indicate that both VEGF-A and VEGF-C bind to the highly expressed VEGFR2.
In Itgb1f/f; Pitx1-cre pituitaries, RNA-seq data revealed significantly altered expression (>1.5-fold change) of 1,138 genes (P ≤ 0.01). Expression of 496 genes decreased, and expression of 642 genes increased. Gene ontology analysis revealed changes in both cell-intrinsic and cell-extrinsic gene programs (Fig. 5B). Heat maps representing selected genes showed explicit membership in four categories of extrinsic factors that may be involved in the non–cell-autonomous effect of epithelial cells on angiogenesis. Genes in these categories included components of the ECM, ECM-associated intercellular signaling molecules, several integrin subunits that heterodimerize with integrin β1 (α3, α7, and α9), and a number of disintegrins/metalloproteinases (Fig. 5C). Curiously, integrins α3β1 and α7β1 bind specifically to laminin, a major component of vascular basement membranes in the embryonic pituitary (3, 14).
In the ECM category, expression of the core matrisome components fibronectin (FN), six of 28 collagens, and laminin C1 were significantly altered (8) (Fig. 5C). FN, which is important for embryonic blood vessel morphogenesis, was the most down-regulated, as seen in mice null for integrin α5 (25, 26). In addition to stimulating expression of FN, integrin α5β1 is required to bind to FN to initiate assembly of the fibrillar matrix, which functions as a reservoir for locally secreted growth factors such as VEGF-A (8). Immunohistochemical staining of FN in e12.5 Itgb1f/f; Pitx1-cre pituitaries revealed diminished expression along the rostral aspect of the anterior lobe where endothelial cells first invade and a punctate, rather than fibrillar, pattern, suggesting diminished integrin β1-induced conversion of compact FN into extended fibrils (Fig. S7B).
In the retina, FN deposited by astrocytes localized VEGF-A ahead of migrating tip cells in angiogenic sprouts (27). Interestingly, VEGF-A is highly expressed in e14.5 embryonic pituitaries (GenePaint.org) and has been implicated in vascular development in the mouse pituitary gland (28). Based on these findings, we asked if deletion of VEGF-A might phenocopy the absence of endothelial cells observed in e14.5 Itgb1f/f; Pitx1-cre pituitaries. VEGF-Af/f; Pitx1-cre mice were generated, and pituitaries were immunostained with VEGFR2 and PDGFRβ, but angiogenesis appeared normal at e14.5 (29) (Fig. S7C). One explanation is that VEGF-A may have escaped deletion in a small percentage of cells such as folliculostellate cells (30). However, given that Pitx1-cre deleted Itgb1flox alleles with complete penetrance in the mouse pituitary gland, it seems unlikely. A more probable explanation is functional redundancy with VEGF-C, which is also highly expressed in the e14.5 pituitary gland (GenePaint.org) and can bind to VEGFR2 (Fig. S7D).
Discussion
Our findings provide unambiguous evidence of critical non–cell-autonomous roles for integrin β1 in vascular development that contrast with numerous reports of other tissue-specific integrin β1 knockouts (31–34), and complement established vascular cell-autonomous functions of integrin β1 (9–13). Our data support the idea that there are distinct tissue and temporal context-dependent requirements for specific integrin heterodimers in vascular development (11). Indeed, the only other well-defined example of a non–cell-autonomous role played by integrins in vascular development involves integrin αvβ8 in the CNS and retina, where failure to activate latent ECM-bound TGF-β was an underlying cause of the observed vascular defects (35, 36). Interestingly, the palate defect in the Pitx1-cre; Itgb1f/f embryos phenocopies double-knockouts of integrin β6/β8 and single-knockouts of TGF-β3, suggesting integrin β1-dependent TGF-β signaling in the palate (37, 38).
We observed that multiple steps in developmental angiogenesis required expression of epithelial integrin β1 in the embryonic pituitary gland. In its absence, expression of genes encoding core components of the ECM, ECM-associated signaling factors, and remodeling molecules are changed, suggesting that alterations in ECM function might underlie the observed phenotypes. In addition, integrin β1 initiates self-assembly of secreted FN dimers into a multimeric fibrillar matrix that subsequently affects formation of collagen fibrils (8). Ultimately, changes in the deposition and assembly of the ECM in the absence of epithelial integrin β1 may have altered its ability to provide structural support, and to function as a scaffold that binds and presents secreted growth factors to vascular cells (8).
PDGF-B, VEGF, and TGF-β are secreted growth factors that regulate blood vessel development through binding to the ECM in a manner that controls their local concentration and bioavailability (8). Examination of PDGFRβ-null mice indicated that PDGF-B signaling plays a role in pericyte recruitment in the embryonic pituitary gland. Failure to recruit pericytes, however, led to dilated and leaky vessels but not to the loss of endothelial cells observed in the e14.5 Pitx1-cre; Itgb1f/f pituitaries (21). Therefore, additional endothelial cell migration, retention, and/or survival signals must also be compromised. Candidate molecules with such function include VEGF-A and VEGF-C, both of which are highly expressed in the embryonic pituitary gland at e14.5. Deletion of VEGF-A alone in VEGF-Af/f; Pitx1-cre pituitaries surprisingly showed no effect on endothelial cell invasion or pericyte recruitment, and so raised the possibility of compensation by VEGF-C (39). Interestingly, vascularization defects in the embryonic pituitaries of Propdf/df mice (Ames dwarf) that lack the transcription factor, Prop1, have been attributed to altered expression of VEGF-A protein (28). Although this finding appeared to contrast with the apparent lack of vascular phenotype in our deletion of VEGF-A, it was separately noted that VEGF-C mRNA was also decreased more than threefold in pituitaries from e12.5 Prop1 knockout mice (Fig. S8). Together, these data suggest that simultaneous down-regulation of both VEGF-A and VEGF-C may cause the vascular defects seen in the embryonic pituitary glands of mice lacking Prop1. In the case of Pitx1-cre; Itgb1f/f mice, and perhaps Prop1-cre; Itgb1f/f mice, interaction of both VEGF-A and VEGF-C with the ECM would likely be compromised. This outcome suggests that the absence of vasculature observed with inactivation of pituitary epithelial integrin β1 might result from the failure of a series of signals, including PDGF-B, VEGF-A, and VEGF-C, that, together, orchestrate vascular development.
Fig. S8.
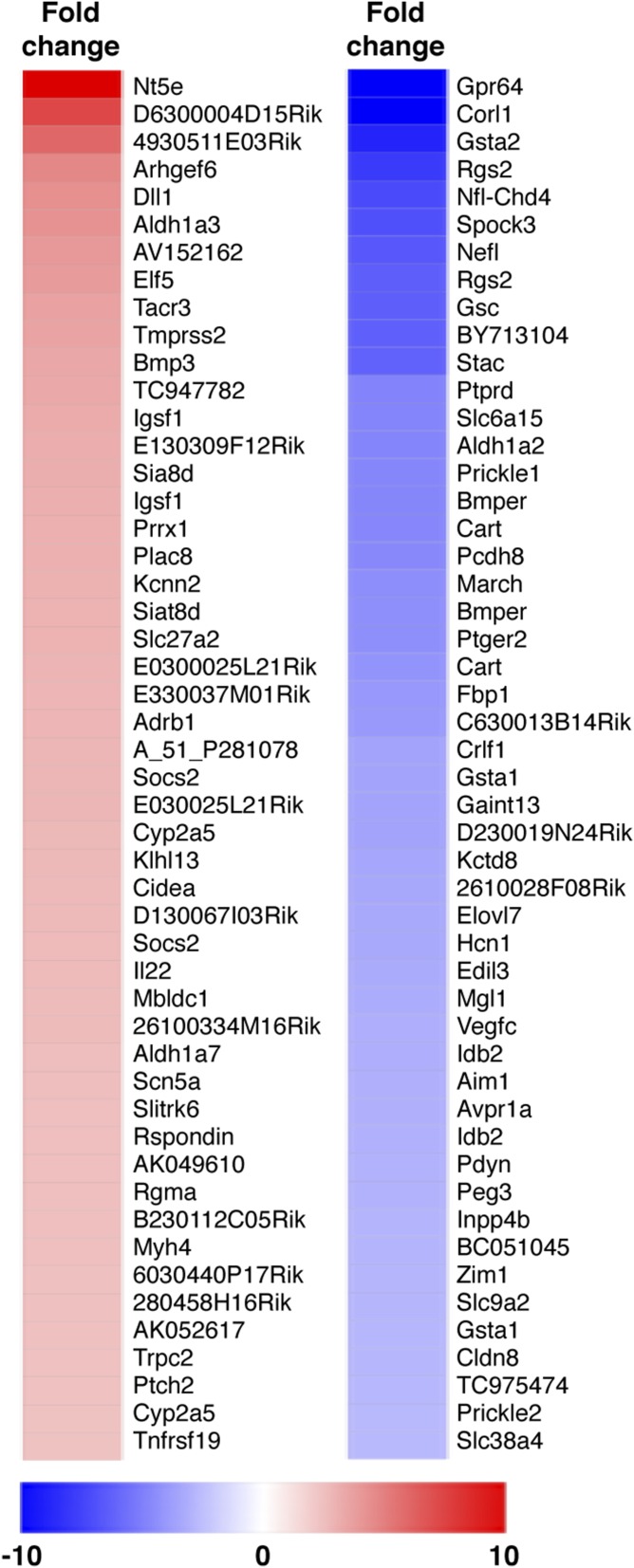
VEGF-C is down-regulated in Prop1 knockout pituitaries at e12.5. Heat maps of the top 50 up- and down-regulated transcripts based on microarray analysis of pituitaries dissected from e12.5 Prop1−/− and control littermate pituitaries (43]).
In total, our data support the conclusion that integrin β1 in the epithelial cells of the embryonic pituitary gland is required for normal expression of numerous ECM and associated molecules that function in critical non–cell-autonomous roles during vascular development. In addition, the cell-autonomous function of integrin β1 in epithelial cells is required for normal expression of endocrine cell terminal differentiation markers such as GH and Prl. Together, these effects reveal that epithelial integrin β1 coordinates development of the embryonic pituitary gland and its invading vascular system. Whether reciprocal heterotypic interactions between the parenchyma and nascent vasculature extend beyond development of a circulatory system that delivers regulatory signals to hormone-producing cells remains an open question.
Materials and Methods
Conditional Deletions of the Itgb1 and VEGF-A Genes.
The mouse strain B6;129-Itgb1tm1Efu/J (Itgb1flox) was obtained from The Jackson Laboratory (17). VEGF loxP mice (VEGF-Aflox) were provided by Genentech (29). Itgb1f/f and VEGF-Aflox mice were bred to Pitx1-cre (16) or Prop1-cre transgenic mice (18). PDGFRβ−/− embryos were generously provided by Lorin Olson, Oklahoma Medical Research Foundation, Oklahoma City (24). All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Accession Number.
For RNA-seq data deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus databank, the accession number is GSE89171 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89171).
Additional information is provided in SI Materials and Methods.
SI Materials and Methods
Generation of Conditional Deletions of the Itgb1 and VEGF-A Genes.
All mice were housed in strict accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals (40). All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee. The mouse strain B6;129-Itgb1tm1Efu/J (common name Itgb1flox), donated to The Jackson Laboratory by Elaine Fuchs, harbors loxP sites on either side of exon 3 of the Itgb1 gene. Deletion of exon 3 by Cre-recombinase results in frame shift and premature termination of translation (17). Itgb1f/f mice were bred to transgenic mice harboring cre-recombinase under the control of 8 kb of mouse pitx-1 5′ sequence (16) or 2 kb of mouse prop-1 5′ sequence, including exon 1 (18). Itgb1f/f; Pitx1-cre knockout pups die soon after birth with palate defects that preclude suckling. To generate littermate Itgb1f/f; Pitx1-cre knockout and Itgb1f/f controls, heterozygous Itgb1f/+; Pitx1-cre animals were crossed to Itgb1f/f animals. Primers used to genotype these animals were CRE5′ GGAAATGGTTTCCCGCAGAAC and CRE3′ ACCCTGATCCTGGCAATTTCG, which amplify an ∼400-bp product, and β1-integrin oIMR1906 CGGCTCAAAGCAGAGTGTCAGTC-3′ and oIMR1907 CCACAACTTTCCCAGTTAGCTCTC, which amplify ∼160 bp of the wild-type Itgb1 allele and ∼280 bp of the Itgb1f/f allele (genotyping by The Jackson Laboratory). Itgb1f/f; Prop1-cre animals were viable and fertile. Itgb1f/f; Prop1-cre knockouts and Itgb1f/f littermate controls were generated by breeding Itgb1f/f; Prop1-cre to Itgb1f/f animals. VEGF loxP mice (herein referred to as VEGF-Af/f) were generously provided by Genentech. These mice harbor loxP sites on either side of exon 3, which is common to all isoforms of VEGF-A. Primers used to genotype these animals were muVEGF419.F CCTGGCCCTCAAGTACACCTT and muVEGF567.R TCCGTACGACGCATTTCTAG. They amplify 148 bp of loxP alleles and ∼108 bp of wild-type alleles (29). VEGF-Af/f; Pitx1-cre animals were viable and fertile. VEGF-Af/f; Pitx1-cre knockouts and VEGF-Af/f littermate controls were generated by breeding VEGF-Af/f; Pitx1-cre to VEGF-Af/f animals. PDGFRβ−/− and littermate control embryos were generously provided by Lorin Olson, Oklahoma Medical Research Foundation, Oklahoma City (24).
Quantitative Real-Time PCR.
Quantitative real-time (RT) PCR was carried out with RNA extracted using TRIzol (Life Technologies) from control and Itgb1 gene-deleted pituitaries from stages as indicated in the figures. RNA samples were DNase I-treated, and all RT-PCR reactions were accompanied by RT(−) controls. Experiments were performed with three biological replicates and three technical replicates. The data were normalized to GAPDH.
Computed Tomography, Data Processing, and Image Generation.
All samples were processed using a Numira Biosciences proprietary staining technique. The samples were scanned on a high-resolution, volumetric microCT scanner (μCT40; ScanCo Medical). The image data were acquired with the following parameters: 6-μm isotropic voxel resolution at 200-ms exposure time, 2,000 views, and 10 frames per view. The microC-generated DICOM (Digital Imaging and Communication) files were used to create images of the samples. The raw data files were viewed using Microview (GE Healthcare). Using Numira’s software, the files were converted into frames for planar movies and used to generate rotating 3D movies. The movie frames were then converted into QuickTime (Apple, Inc.) movies for viewing and screen capture.
Histology and Immunofluorescence Assays.
For immunohistochemistry with anti-PECAM/CD31 and anti–PV-1 antibodies, fresh-frozen tissue sections were mounted on slides, fixed at room temperature in acetone for 2 min, and dried before staining. For all other histological analyses, including H&E staining, and indirect immunofluorescence assays, tissues were fixed with 10% (vol/vol) phosphate-buffered formalin or 4% (wt/vol) paraformaldehyde by transcardial perfusion and/or immersion. Primary antibodies directed against pituitary hormones were provided by A. F. Parlow (Harbor–UCLA Medical Center) and the National Hormone and Pituitary Program (National Institute of Diabetes and Digestive and Kidney Diseases). They included rabbit anti-human ACTH (adrenocorticotropic hormone) (1:500, AFP-39032082); rabbit anti-rat TSH-β (1:1,000, AFP-1274789); monkey anti-rat GH (1:1,000, AFP 411S); guinea pig anti-rat LH-β (1:1,000, AFP-2223879OGPOLHB); and rabbit anti-rat αGSU (1:500, AFP-66P9986). Other primary antibodies used were rabbit anti-human PRL (1:250, DAKO A0569); rabbit anti-rat Pit-1 [1:250, Simmons et al. (41)]; mouse antiphosphohistone H3 serine 10 (pH 3) (1:200, Cell Signaling Technology 9706S); rat anti-mouse integrin β1 clone MB1.2 (1:100, MAB1997; Chemicon/Millipore); rat anti-mouse CD31/PECAM-1 clone MEC13.3 (1:20, 550274; BD Pharmingen); rabbit anti-mouse EHS laminin (1:200, L9393; Sigma–Aldrich); rabbit anti-rat NG2 (1:200, AB5320; Chemicon/Millipore); rat anti-mouse CD140b/PDGFRβ clone APB5 (1:500, 14-1402-82; eBioscience); rat anti-mouse PV-1 IgG2a mAb (1:500), which was affinity-purified by FPLC on an anti-rat IgG column (GE Healthcare) from the supernatant of the clone MECA-32 hybridoma (Developmental Studies Hybridoma Bank, University of Iowa) grown in serum-free hybridoma medium (Invitrogen), and then labeled with Alexa-647 fluorophore (Molecular Probes/Invitrogen), as per the manufacturer’s instructions (19). Secondary antibodies were labeled with Alexa fluorphores-488, -594, or -647 (1:500; Molecular Probes/Invitrogen). Images were obtained using a Zeiss Axioplan 2 microscope and a Leica SP5 confocal microscope.
Pericyte Recruitment.
Sixteen-micrometer serial sagittal sections beginning at the midline and progressing through the lateral anterior lobes were collected and immunostained with VEGFR2 (endothelial cells) and PDGFRβ (pericytes). Four pairs of e13.5 Itgb1f/f; Pitx1-cre and control Itgb1f/f littermate embryos were examined in this manner. Each pair was from an independent litter.
Quantification of EC Clusters, Presence of Lumens, and Lumen Diameter.
Four Itgb1f/f and four Itgb1f/f; Prop1-cre were analyzed at e14.5. Each pair was from an independent litter. Coronal sections were immunostained with integrin β1 and CD31 antibodies and imaged on a Zeiss Axioplan 2 MOT Microscope System and a Leica SP5 confocal microscope. CD31(+) EC clusters were counted in three equivalently sized sections from each animal. The fraction of EC clusters with lumens was established on the same sections. Lumen diameters were measured using the straight-line tool in ImageJ. The shortest distance across each lumen was recorded to eliminate error based on the angle at which each vessel was cut. Statistical analysis was performed with Prism 7 software (GraphPad Software).
RNA-Sequencing.
Embryonic mouse pituitaries were dissected at e12.5 and frozen individually. Following genotyping, total RNA from separate biological replicates of three control and two knockout pituitaries was isolated using TRIzol (Life Technologies), chloroform extraction, and isopropanol precipitation. Ribosomal RNA was removed using a Ribo-Zero Magnetic Kit (Epicentre), a strand-specific library was constructed with ScriptSeq v2 RNA-Seq Library Preparation Kit (Epicentre), and deep sequencing was carried out on an Illumina HiSeq 2000. The RNA-seq reads were aligned to the mouse genome mm9 using Bowtie2 with ultrasensitive parameters. Because the number of assignable reads in the samples ranged from 11 × 106 to 38 × 106, Homer (homer.salk.edu) was used to select an equal number of reads randomly from each sample. RNA-seq reads were counted over gene exons and reads for the same exons were combined allowing only two exact duplicate tags in any one position. Genes with less than eight tags per kilobase were filtered out. edgeR software (www.bioconductor.org/packages/release/bioc/vignettes/edgeR/inst/doc/edgeRUsersGuide.pdf) was used for statistical analysis, set with a P value ≤0.01 and a minimum fold change of 1.5 either up or down. Pairwise comparisons of each control to each knockout sample were used to generate correlation plots that identified genes with a consistent pattern of differential expression in control vs. knockout pituitaries.
Microarray Analysis.
RNA was prepared from individual microdissected e12.5 pituitaries from Prop1 knockout and mutant animals. RNA quality was assessed using the Agilent Bioanalyzer 6000 Pico LabChip. One hundred nanograms of total RNA was labeled with Cy-3 or Cy-5 using the Agilent Low RNA Input Fluorescent Linear Amplification Kit. Labeled cDNA was hybridized to the Agilent 44K Whole-Mouse Genome Array. Data were collected using the Agilent Microarray Scanner and Feature Extraction Software, using a Lowess option with spatial detrend. Normalized data were imported into Focus to extract genes of interest with more confidence than through the use of fold-change only (42). Experiments were performed in triplicate, with litter-matched wild-type and mutant samples.
Acknowledgments
We thank Marilyn Farquhar, Eugene Tkachenko, Mark Ginsberg, Lorin Olson, and Xiaoyan Zhu for discussion; Jennifer Santini (University of California, San Diego School of Medicine Microscopy Core Grant P30 NS047101); Kenny Ohgi and Jie Zhang for RNA sequencing; and Janet Hightower for figure preparation. This work was supported by NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grants R37DK09949 and R01DK0184477 (to M.G.R.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE89171).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614970113/-/DCSupplemental.
References
- 1.Kerr T. The development of the pituitary of the laboratory mouse. Q J Microsc Sci. 1946;87:3–29. [PubMed] [Google Scholar]
- 2.Szabó K, Csányi K. The vascular architecture of the developing pituitary-median eminence complex in the rat. Cell Tissue Res. 1982;224(3):563–577. doi: 10.1007/BF00213753. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto H, Ishikawa H, Kusakabe M. Three-dimensional analysis of the developing pituitary gland in the mouse. Dev Dyn. 1998;212(1):157–166. doi: 10.1002/(SICI)1097-0177(199805)212:1<157::AID-AJA14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Farquhar MG. Fine structure and function in capillaries of the anterior pituitary gland. Angiology. 1961;12:270–292. doi: 10.1177/000331976101200704. [DOI] [PubMed] [Google Scholar]
- 5.Dearden NM, Holmes RL. Cyto-differentiation and portal vascular development in the mouse adenohypophysis. J Anat. 1976;121(Pt 3):551–569. [PMC free article] [PubMed] [Google Scholar]
- 6.Schaeffer M, et al. Influence of estrogens on GH-cell network dynamics in females: A live in situ imaging approach. Endocrinology. 2011;152(12):4789–4799. doi: 10.1210/en.2011-1430. [DOI] [PubMed] [Google Scholar]
- 7.Plow EF, Meller J, Byzova TV. Integrin function in vascular biology: A view from 2013. Curr Opin Hematol. 2014;21(3):241–247. doi: 10.1097/MOH.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4(1):a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malan D, et al. Endothelial beta1 integrins regulate sprouting and network formation during vascular development. Development. 2010;137(6):993–1002. doi: 10.1242/dev.045377. [DOI] [PubMed] [Google Scholar]
- 10.Zovein AC, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18(1):39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, et al. Integrin β1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6:6429. doi: 10.1038/ncomms7429. [DOI] [PubMed] [Google Scholar]
- 12.Turlo KA, et al. An essential requirement for β1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev Biol. 2012;365(1):23–35. doi: 10.1016/j.ydbio.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham S, Kogata N, Fässler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102(5):562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: Signaling and transcriptional networks. Physiol Rev. 2007;87(3):933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
- 16.Treier M, et al. Hedgehog signaling is required for pituitary gland development. Development. 2001;128(3):377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- 17.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150(5):1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu X, Tollkuhn J, Taylor H, Rosenfeld MG. Notch-dependent pituitary SOX2(+) stem cells exhibit a timed functional extinction in regulation of the postnatal gland. Stem Cell Rep. 2015;5(6):1196–1209. doi: 10.1016/j.stemcr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stan RV, et al. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23(6):1203–1218. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163(5):1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128(7):1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- 23.Davis SW, et al. β-catenin is required in the neural crest and mesencephalon for pituitary gland organogenesis. BMC Dev Biol. 2016;16(1):16. doi: 10.1186/s12861-016-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8(16):1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- 25.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12(2):165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis SE, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22(6):927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 27.Stenzel D, et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 2011;138(20):4451–4463. doi: 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20(6):1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 29.Gerber HP, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 31.Diaferia GR, et al. β1 integrin is a crucial regulator of pancreatic β-cell expansion. Development. 2013;140(16):3360–3372. doi: 10.1242/dev.098533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taddei I, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10(6):716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plosa EJ, et al. Epithelial β1 integrin is required for lung branching morphogenesis and alveolarization. Development. 2014;141(24):4751–4762. doi: 10.1242/dev.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graus-Porta D, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31(3):367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 35.Hirota S, et al. The astrocyte-expressed integrin αvβ8 governs blood vessel sprouting in the developing retina. Development. 2011;138(23):5157–5166. doi: 10.1242/dev.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnold TD, et al. Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Development. 2014;141(23):4489–4499. doi: 10.1242/dev.107193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tudela C, et al. TGF-beta3 is required for the adhesion and intercalation of medial edge epithelial cells during palate fusion. Int J Dev Biol. 2002;46(3):333–336. [PubMed] [Google Scholar]
- 38.Aluwihare P, et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122(Pt 2):227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 40.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Natl Inst Health; Bethesda: 1996. DHHS Publ No (NIH) 85-23. [Google Scholar]
- 41. Simmons DM, et al. (1990) Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev 4:695–711. [DOI] [PubMed]
- 42.Cole SW, Galic Z, Zack JA. Controlling false-negative errors in microarray differential expression analysis: A PRIM approach. Bioinformatics. 2003;19(14):1808–1816. doi: 10.1093/bioinformatics/btg242. [DOI] [PubMed] [Google Scholar]
- 43.Olson LE, et al. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125(3):593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]