Significance
This work reports a processing route to fabricate hydrogel films through their rapid and spontaneous growth in aqueous solutions. This approach exploits the strong solvent depletion created at the surface of a polymer substrate swelling in a polymer solution. By immersing substrates that swell in associative polymer solutions, we showed that this local suction effect induces the gelation and growth of hydrogel films. Experiments and a simple model captured how the kinetics of this process can be deliberately controlled by handy processing parameters. Other solutes can be easily incorporated in the so-formed hydrogel films to provide other functionalities. We demonstrated this potential by coating complex shapes with bioceramic particles and by encapsulating mammalian cells in freestanding membranes.
Keywords: hydrogel film, hydrogel coating, associative polymers, cell culture, tissue engineering
Abstract
Hydrogel films used as membranes or coatings are essential components of devices interfaced with biological systems. Their design is greatly challenged by the need to find mild synthesis and processing conditions that preserve their biocompatibility and the integrity of encapsulated compounds. Here, we report an approach to produce hydrogel films spontaneously in aqueous polymer solutions. This method uses the solvent depletion created at the surface of swelling polymer substrates to induce the gelation of a thin layer of polymer solution. Using a biocompatible polymer that self-assembles at high concentration [poly(vinyl alcohol)], hydrogel films were produced within minutes to hours with thicknesses ranging from tens to hundreds of micrometers. A simple model and numerical simulations of mass transport during swelling capture the experiments and predict how film growth depends on the solution composition, substrate geometry, and swelling properties. The versatility of the approach was verified with a variety of swelling substrates and hydrogel-forming solutions. We also demonstrate the potential of this technique by incorporating other solutes such as inorganic particles to fabricate ceramic-hydrogel coatings for bone anchoring and cells to fabricate cell-laden membranes for cell culture or tissue engineering.
Hydrogel films are used in a number of biomedical applications like wound healing (1), drug delivery (1), antiadhesive membranes (2), soft-tissue reconstruction (3, 4), and cell culture (5). The quality of their interactions with biological tissues or entrapped cells is central to the performance of these systems. Although considerable advances have been made in synthesis (6) and processing (7) to tailor these interactions, the toxicity of by-products and the degradation caused by harsh processing conditions remain key issues (8), which can be overcome (9) but are often addressed at the cost of difficult synthesis or multistep processes. There is a great interest for simple, versatile, and in vivo-friendly methods to fabricate and modify hydrogels. We propose a strategy to design hydrogel films in mild conditions. This method could be used to produce freestanding hydrogel membranes or hydrogel coatings to functionalize other hydrogels.
Our approach relies on the understanding of transport phenomena occurring near the surface of a polymer network swelling in a polymer solution. As an illustration, let us consider a flat polymer network with thickness H0 immersed in a solution containing a volume fraction Φ0 of free polymer chains, as depicted in Fig. 1A. When the solvent of the solution is also a solvent for the network, the network swells in a diffusive process. For early immersion times t, the increase in thickness ΔH is given by the following (10, 11):
| [1] |
where H∞ is the thickness at equilibrium swelling in the polymer solution and D0 is a diffusivity coefficient depending on the dynamics of the network and of the solution. The systems of interest are those where the chains in solution do not penetrate the network during swelling, for example, for chains that are much larger than the network mesh size. In this case, free chains are pushed away by the swelling network, which creates an opposite flux of solvent into the network. As a result, a volume of free chains accumulates near the network surface and is redistributed by thermal diffusion within a layer of thickness , where Ds is the diffusivity coefficient of free polymer chains in the solution. The excess in polymer concentration averaged over this layer thus scales as . For polymer gels, the ratio is typically between 1 and 10. Therefore, with a proper selection of the diffusivity ratio , the concentration of a polymer solution might be amplified manyfold near the surface of a swelling polymer network. In particular, this regime might be achieved with solutions of associative polymers, in which diffusivity decays strongly with concentration. For instance, strong concentration gradients were reported at the surface of polyelectrolyte gels swelling in a solution of (hydroxypropyl)cellulose (12). Here, we propose to exploit this solvent depletion induced by swelling to produce hydrogel films and coatings in a soft and autonomous manner.
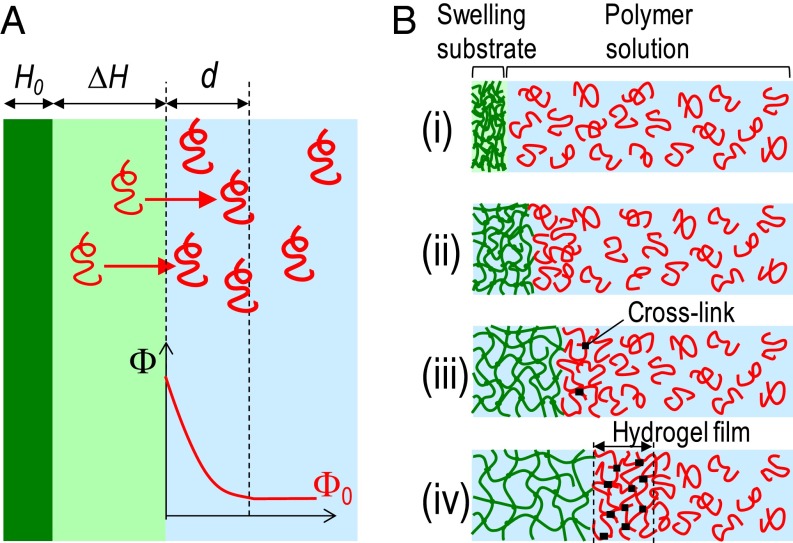
Fig. 1.
Formation of hydrogel films by swelling-induced gelation. (A) Schematic representation of the solvent depletion created near the surface of a swelling polymer network. Upon immersion in a polymer solution, the dry network (dark green) swells. Free polymer chains (red) are pushed away from the volume spanned by the swelling network (light green) and accumulate near its surface. This creates a local excess in polymer concentration in the solution, which is redistributed by thermal diffusion. (B) Illustration of the design strategy using swelling of a hydrophilic polymer substrate to induce the formation of hydrogel films.
The generic idea of our approach is depicted in Fig. 1B. It uses the property of some aqueous polymer solutions to form physical gels at high concentration. First, a substrate composed of a dry hydrophilic network is immersed into such a solution (i). As described above, substrate swelling causes the polymer concentration to increase in a layer near the substrate surface (ii). At a given level of swelling, this layer becomes so concentrated that free polymer chains associate and form physical cross-links (iii). As swelling proceeds, the cross-linking density increases until the gel point is reached and a hydrogel film is formed at the surface of the substrate (iv).
Aqueous solutions of associative polymers forming thermoreversible gels are good candidates to fabricate hydrogel films by the proposed method. In particular, we concentrate on poly(vinyl alcohol) (PVA), which undergoes a sol–gel transition in water at 20 °C for concentrations between 20 and 40 wt% (13, 14). This gelation results from the physical cross-linking of PVA chains that associate through hydrogen bonds and form crystallites (14). For high-enough cross-linking densities, the so-formed PVA hydrogels remain stable below the melting temperature of PVA crystallites, which can reach 80–90 °C. Practically, physical PVA hydrogels show a good biocompatibility, which makes them of high interest to the biomedical field. They have been widely studied as a constitutive material for biomembranes (15, 16), cell encapsulation (17, 18), or soft-tissue reconstruction (4, 19). In that respect, several methods have been developed to induce the gelation of PVA, using solvent evaporation (20), thermal treatments by freezing–thawing (19), coagulation in poor solvent (15), or salting out (21).
Results and Discussion
Formation of Hydrogel Films Induced by Swelling.
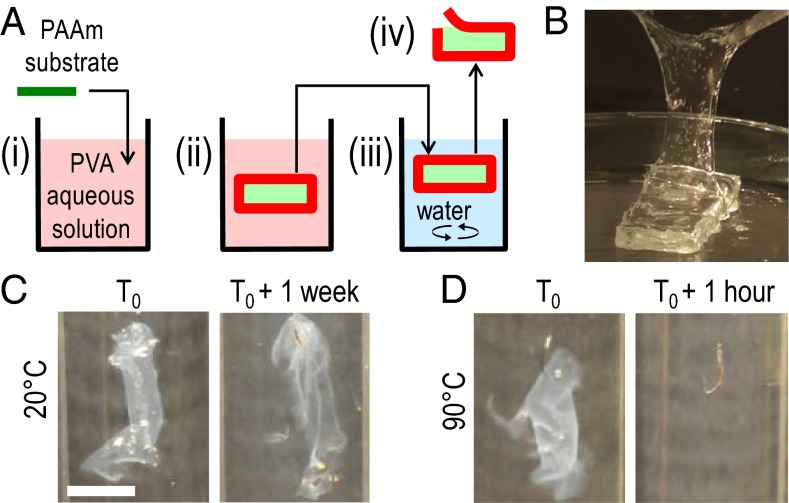
The formation of PVA hydrogel films induced by the swelling of a polymer substrate was demonstrated with a simple experiment described in Fig. 2A and Movie S1. Substrates consisted of 3-mm-thick films of covalently cross-linked poly(acrylamide) (PAAm). In water, they normally swell and reach equilibrium within 90 h to form 6-mm-thick hydrogel films. In this experiment, dry PAAm films were immersed in a 15 wt% PVA aqueous solution for 3 h and were then vigorously rinsed in water for 15 min. Films of PVA hydrogel could be peeled off from the substrates immediately after rinsing (Fig. 2B). These films remained insoluble in water at 20 °C for over a week (Fig. 2C) but dissolved in less than 1 h at 90 °C (Fig. 2D). This confirms that physical PVA hydrogels were formed, for which cross-linking crystallites melt in water above 85 °C (22, 23). Accordingly, the formation of PVA crystallites in these films was verified by X-ray scattering, which revealed the main Bragg peak of PVA crystals (Fig. S1).
Fig. 2.
Formation of PVA hydrogel films on swelling PAAm substrates. (A) Schematic representation of the experimental protocol: (i) a dry PAAm substrate is immersed in a PVA aqueous solution; (ii) the PAAm substrate swells inducing the formation of a PVA hydrogel film (dark red); (iii) the sample is collected and rinsed in water; (iv) the PVA hydrogel film can be peeled off from the swollen PAAm substrate. (B) Peeling of a PVA hydrogel film after 3-h immersion in a 15 wt% PVA aqueous solution. (C and D) Stability in water of the same films as in B: right after fabrication (C and D, Left) and after storage at 20 °C for 1 wk (C, Right) or after storage at 90 °C for 1 h (D, Right). (Scale bar: 1 cm.)
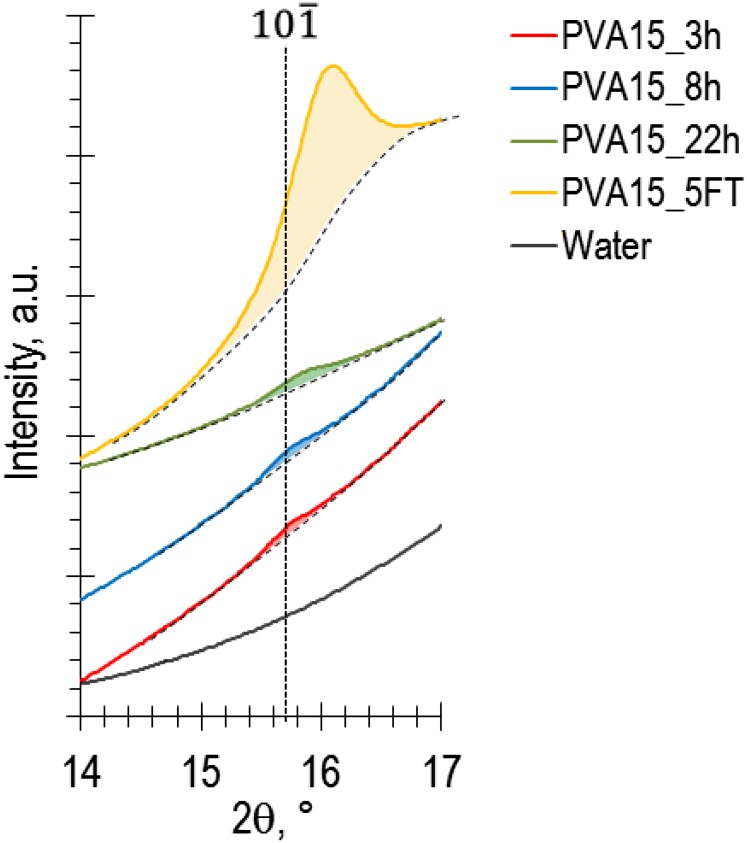
Fig. S1.
X-ray diffraction profile of PVA hydrogel films produced at the surface of 3-mm-thick PAAm films after immersion in a 15 wt% PVA solution for 3 h (red), 8 h (blue), and 22 h (green). The spectra are compared with those of water (black) and of a physical PVA hydrogel film prepared by five cycles of freezing at −18 °C and thawing at 20 °C. The dashed line indicates the known position of the main Bragg peak of PVA crystals calculated from Bunn (42).
Characterization of Hydrogel Film Growth as a Function of Substrate Swelling.
The relationship between substrate swelling and hydrogel film formation was investigated in other experiments, as illustrated in Fig. 3A. Spherical substrates were immersed in aqueous solutions and were observed from the top to monitor both substrate swelling and hydrogel film formation. Spheres of poly(acrylic acid)–poly(acrylamide) (PAAc–PAAm) hydrogels containing a green dye were used as the swelling substrates (Fig. 3B). These dry spheres had a radius of 1.20 ± 0.05 mm. During swelling in pure water, a sharp line at the sphere equator indicated the substrate–water interface, as shown in Fig. 3C for 1-h immersion. Equilibrium swelling was reached in 400 min with a radius of 13.0 ± 0.1 mm.
Fig. 3.
Observation of hydrogel film growth. (A) Schematic representation of the experimental setup enabling the simultaneous observation of the size of the substrate (light green) and of the thickness of the hydrogel film (dark red). (B) Spherical PAAc–PAAm substrates: dry (Left) and swollen to equilibrium in water (Right). (C–E) Pictures of the equatorial region of PAAc–PAAm spheres after 1-h immersion in different conditions: the sphere is immersed dry in water (C); the sphere is immersed dry in a 10 wt% PVA solution, which reveals the formation of a PVA hydrogel film (D); the sphere is first swollen to equilibrium in pure water and then immersed in a 10 wt% PVA solution (E). (F) Time series of a PAAc–PAAm sphere swelling in a 10 wt% PVA solution: substrate swelling (Top) and close-up showing film growth at the sphere surface (Bottom).
The growth of PVA hydrogel films induced by swelling was observed when the dry spheres were immersed in PVA solutions. In those cases, a second larger circle appeared concentric with the substrate equator, as shown in Fig. 3 D and F for 10 wt% PVA solutions (Fig. S2 for other concentrations). This line corresponds to the interface between the solution and a PVA hydrogel film, as seen when detaching the film under the microscope (Fig. S3 and Movie S2). The film thickness was measured from the radius difference between the two circles (Fig. S4). Control experiments confirmed that film formation was induced by swelling. For that, PAAc–PAAm spheres were swollen to equilibrium in pure water before immersion in a 10 wt% PVA solution. As expected, osmotic deswelling was observed after immersion in the PVA solution (24) but no hydrogel film was noticed, as shown in Fig. 3E for 1-h immersion.
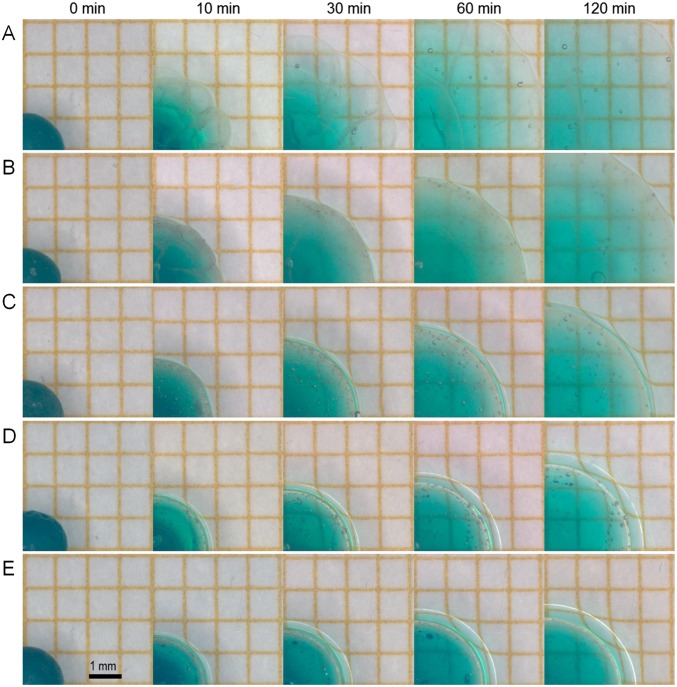
Fig. S2.
Time series showing the equator of PAAc–PAAm spheres swelling in PVA aqueous solutions with different concentrations: 1 wt% (A), 2.5 wt% (B), 5 wt% (C), 10 wt% (D), and 15 wt% (E).
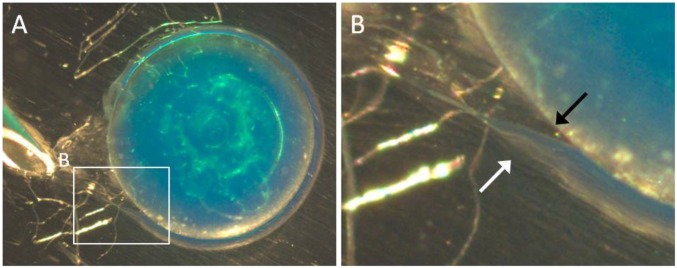
Fig. S3.
(A) Microscopic observation of the in situ detachment of a PVA hydrogel film formed at the surface of a PAAc–PAAm sphere after 1-h swelling in a 10 wt% PVA aqueous solution. (B) Close-up showing the interfaces of the PVA hydrogel film with the PAAc–PAAm substrate (black arrow) and with the PVA solution (white arrow).
Fig. S4.
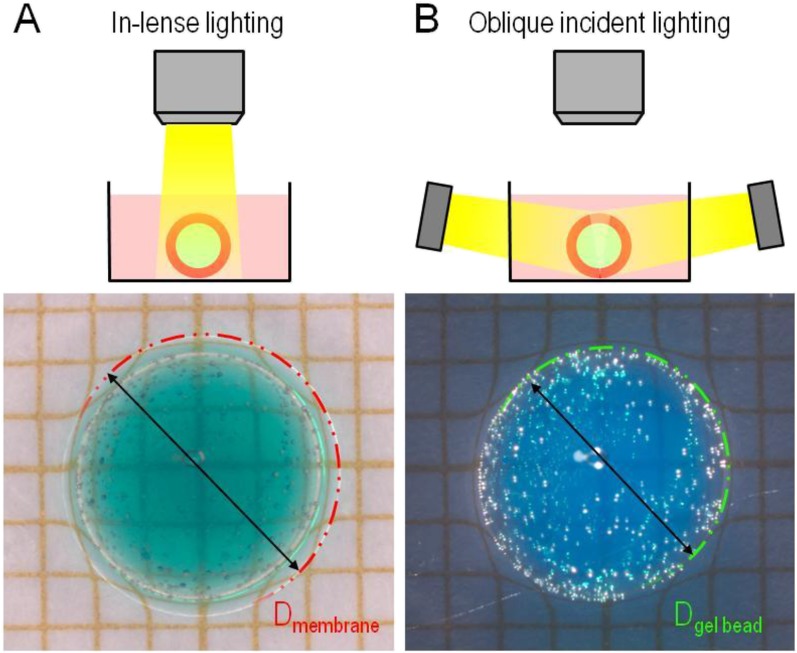
Measurement protocol to determine the radius of PAAc–PAAm spheres and the thickness of PVA hydrogel films. (A) The interface between the PVA hydrogel film and the PVA solution is revealed by in-lens lighting. The outer radius is measured by fitting with a circle. (B) The interface between the PVA hydrogel film and the PAAc–PAAm substrate is revealed by in-plane lighting. The inner radius is measured by fitting with a circle. The film thickness is determined from the difference between these two radii.
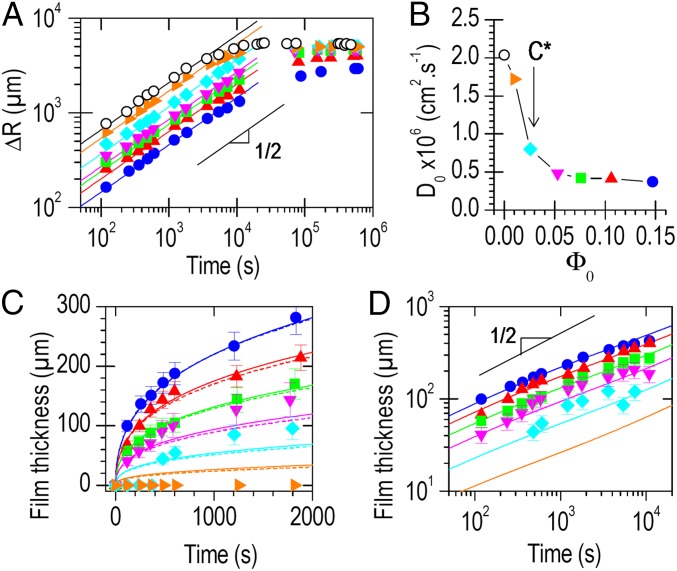
Fig. 4A shows the increase in radius of PAAc–PAAm spheres as a function of immersion time in solutions with PVA concentrations ranging from 0 to 15 wt%. Swelling decreased with increasing PVA concentration because of the osmotic pressure created by free PVA chains. For all of the studied concentrations, the curves were well fitted by Eq. 1. The diffusivity, D0, obtained from these fits is plotted in Fig. 4B. It drops sharply as the PVA concentration increases above the overlap concentration (C* = 2.6 wt%) (25). This transition suggests that, in the semidilute regime, the slow relaxation of overlapping PVA chains is the limiting process that dictates the kinetics of substrate swelling.
Fig. 4.
Relationship between substrate swelling and hydrogel film growth. (A) Increase in the radius of PAAc–PAAm spheres as a function of immersion time in aqueous solutions with different PVA concentrations: 0 wt% (○), 1 wt% (▸), 2.5 wt% (◆), 5 wt% (▼), 7.5 wt% (■), 10 wt% (▲), and 15 wt% (●). Full lines show best fits by Eq. 1 for the first hour of swelling. (B) Substrate diffusivity measured from fits as a function of PVA concentration. The overlap concentration C* indicates the transition from dilute to semidilute PVA solutions. (C) Thickness of PVA hydrogel films as a function of immersion time for the same experiments as in A. Full lines show model predictions with Eq. 4 for a spherical substrate (Φc = 0.35 wt%). Dashed lines show the results of numerical simulations. (D) Same as C in log-log scale. In all graphs, error bars represent the maximum SD in a time series. They are not shown when smaller than the symbols.
Fig. 4C shows the thickness of hydrogel films formed at the surface of the spheres as a function of immersion time. These results depend strongly on the solution concentration. In the dilute regime (1 wt%), no film was observed. Close to the overlap concentration (2.5 wt%), the formation of a 40-µm-thick film was noticed after 10 min, which may correspond to an incubation time or to the detection limit of our technique. In the semidilute regime (≥5 wt%), a film was detected within the first 100 s. For a given immersion time, higher PVA concentrations produced thicker films. In all cases, the thickness growth obeys a power law close to t1/2, as shown in Fig. 4D.
Mechanisms and Modeling of Swelling-Induced Gelation.
A microscopic picture explains the mechanisms of film growth. At the beginning of immersion in a PVA solution, the gelation of a PVA layer is induced by swelling as depicted in Fig. 1B: (i and ii) the PVA concentration increases near the surface of the swelling substrate; (iii) PVA chains cross-link by crystallization in this concentrated layer; (iv) above a critical gelation concentration Φc, a hydrogel film is formed. At this stage, a new regime is expected. Extraction of water from the newly formed hydrogel film into the substrate becomes difficult because it implies an elastic deformation of the cross-linked PVA network. Instead, it becomes more favorable for water to flow from the solution through the hydrogel film and into the substrate, as already described for gel membrane permeation (26). As a result, the solvent depletion process is displaced at the interface between the hydrogel film and the solution. The film builds up continuously through this process until substrate swelling no longer generates a concentration exceeding the gelation concentration.
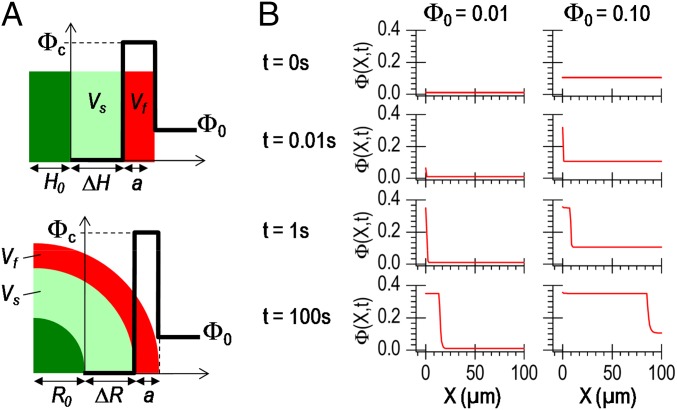
A simple model based on this picture captures quantitatively the kinetics of film growth. Let us consider that, after an immersion time t, a PVA hydrogel layer of thickness a is formed, as illustrated in Fig. 5A. The solution is in large excess and the PVA concentration far from the substrate remains constant, equal to Φ0. We assume that the hydrogel layer is homogeneous with a constant PVA concentration equal to Φc and that the PVA concentration decreases sharply from Φc to Φ0 in a negligible transition layer near the hydrogel surface. This latter assumption is supported by the measured values of substrate diffusivity D0, which are much larger than the values reported for the diffusivity of PVA in semidilute aqueous solutions, Ds < 10−8 cm2/s (27). In other words, the swelling surface advances much faster than the spreading of concentration fluctuations in the solution. Mass conservation implies that the amount of PVA pushed away during swelling is equal to the excess in PVA in the hydrogel film. This writes simply as follows:
| [2] |
where Vs is the volume spanned by the substrate during swelling and Vf is the volume of the film. Combination with Eq. 1 gives the expression for the hydrogel film thickness as a function of immersion time, for 1D swelling of a flat substrate with initial thickness H0:
| [3] |
and for swelling of a spherical substrate with initial radius R0 (see calculation details in SI Materials and Methods):
| [4] |
where H∞ and R∞ are the thickness and the radius of the substrate at equilibrium swelling in the PVA solution, respectively.
Fig. 5.
Modeling of swelling-induced gelation. (A) Schematic representations of the analytical model predicting hydrogel film growth for 1D swelling (Top) and for swelling of a spherical substrate (Bottom). (B) Calculated concentration profiles as a function of the distance from the substrate surface at different times during swelling for two initial polymer concentrations: 1 vol% (Right) and 10 vol% (Left).
The prediction of Eq. 4 is applied to fit the experimental data with Φc as the only adjustable parameter and using the experimental values for D0 given in Fig. 4B. For concentrations greater than 5 wt%, an excellent agreement between model and experiment is obtained with Φc = 0.35 (full lines in Fig. 4 C and D). This is consistent with the value of 30 ± 10 wt% reported for the gelation concentration of PVA in water at 20 °C (13, 28). These results demonstrate that film growth can be tailored by adjusting the properties of the solution (Φ0, Φc) and substrate (R0) as well as the swelling parameters (R∞, D0), all of which are well characterized by standard techniques.
A more microscopic insight is given by numerical simulations. For that, we adapted a model of mass transport near an interface, where diffusion and convection are decoupled (29). The model was implemented for a spherical substrate swelling in a polymer solution. Substrate swelling and polymer diffusion in the solution are characterized by constant diffusivities D0 and Ds, respectively. A discrete profile Φ(X,t) describes the polymer concentration in the solution at time t between a distance X and X + ΔX from the substrate surface. The origin (X = 0) is attached to the moving substrate surface. We define a as the thickness of the hydrogel film formed at the surface of the substrate. Simulations started from a solution with uniform polymer concentration Φ0 and with no hydrogel film at the surface of the substrate (a = 0).
Between each time step t and t + Δt, the increase in substrate radius ΔR due to swelling was calculated from Eq. 1 and the origin (X = 0) was translated by ΔR. The accumulation of free polymer chains in solution near the surface of the substrate was simulated by injecting at X = 0 (or X = a if a hydrogel film is formed) the polymer chains that were pushed away during swelling. The concentration profile was then equilibrated by taking into account the coordinates moving with the substrate surface and by applying Fick’s law in the solution. The formation (respectively, growth) of a hydrogel film was simulated by following the solution concentration at the substrate (respectively, hydrogel film) surface: Φ(X = a,t). Whenever Φ(X = a,t) ≥ Φc, the solution near the swelling surface was considered to be gelled in a layer of thickness ΔX. In that case, the volume of the hydrogel film was increased by adding the gelled layer to it and the value of a was updated. A more detailed description of the calculations is given in SI Materials and Methods.
Simulations were performed with the same values for R0, R∞, and D0 as those measured experimentally with PAAc–PAAm spheres swelling in PVA solutions. The collective diffusivity of PVA in water decreases strongly with concentration. For the sake of simplicity, an upper bound value was taken as Ds = 2.10−8 cm2⋅s−1 (27). The critical gelation concentration was taken as Φc = 0.35. Calculated concentration profiles during swelling are shown in Fig. 5B for Φ0 = 1 vol% and 10 vol%. For both concentrations, it is found that swelling induces a strong local increase in PVA concentration in a thin layer close to the substrate surface. The critical concentration Φc was reached very rapidly within 1 µm of the substrate surface, after less than 1 and 0.1 s s for 1 vol% and 10 vol% solutions, respectively. For concentrations greater than 2.5 vol%, the predicted growth in hydrogel film thickness is in good agreement with the experiment (dashed lines in Fig. 4C) and is very similar to the results of the simple analytical model. For Φ0 = 1 vol%, simulations suggest that PVA hydrogel films still formed but were too thin to be detected with our setup.
Application Potential of Hydrogel Film Fabrication by Swelling-Induced Gelation.
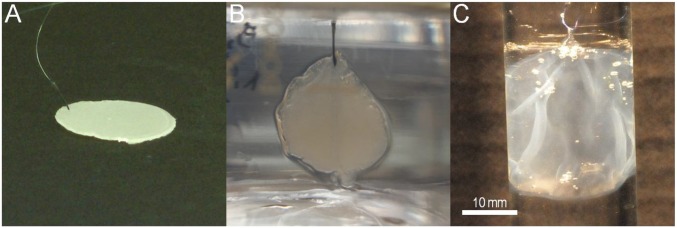
The versatility of the method was demonstrated by producing PVA hydrogel films with a variety of swelling polymer substrates: neutral chemical gels of PAAm, polyelectrolyte gels of PAAc–PAAm, as well as dry un–cross-linked films of poly(ethylene glycol) (PEG) (Fig. S5). For the latter, the substrate swells and eventually dissolves, which enables the formation of freestanding membranes of PVA hydrogel. To verify the generality of the approach, hydrogel films were also produced from aqueous solutions of poly(oxyethylene)-block-poly(oxypropylene)-block-poly(oxyethylene) (Pluronic F127), which form colloidal hydrogels above 15–20 wt% at 20 °C in water (Fig. S6A). Unlike with PVA, the so-obtained hydrogel films are not stable and eventually dissolve if they stay in solution. Alternatively, we verified that no hydrogel film is obtained if the polymer substrate swells in a polymer solution that does not exhibit a sol–gel transition like for PEG and poly(vinyl pyrrolidone) (PVP) solutions (Fig. S6 C and D).
Fig. S5.
Formation of a PVA hydrogel film by swelling-induced gelation around a hydrosoluble PEG substrate: (A) dry un–cross-linked PEG substrate obtained by melt pressing, (B) formation of a PVA hydrogel film during immersion of the substrate in a 10 wt% PVA aqueous solution, and (C) PVA hydrogel film after complete dissolution of the PEG substrate and immersion in water.
Fig. S6.
Equator of PAAc–PAAm spheres swelling in hydrogel forming (A and B) or nonhydrogel forming (C and D) solutions: (A) Pluronic F127 aqueous solution (10 wt%), (B) PVA aqueous solution (10 wt%), (C) PVP aqueous solution (10 wt%), and (D) PEG aqueous solution (10 wt%). (Immersion time, 20 min.)
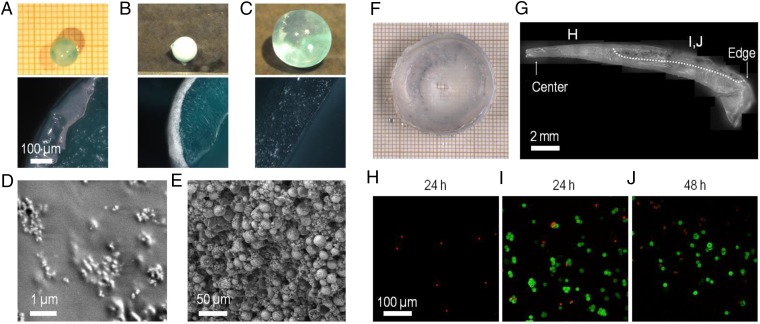
One major practical interest of this approach lies in the possibility to incorporate other solutes to make functional coatings or films. For instance, the incorporation of osteoconductive hydroxyapatite particles into PVA hydrogel films has been shown to enhance cell adhesion to PVA hydrogels and stimulate osteoblast proliferation (30, 31). As an illustration, coatings of hydroxyapatite particles were produced by swelling-induced gelation on the same spherical PAAc–PAAm substrates as the ones studied above. The dry spheres were immersed in 10 wt% PVA aqueous solutions containing suspensions of nanoparticles or microparticles of hydroxyapatite. After 30–60 min of immersion and rinsing in water, the spheres were homogeneously coated with a white film (Fig. 6 A and B). Scanning electron microscopy (SEM) shows that both coatings consist of nanoparticles or microparticles embedded in a PVA matrix (Fig. 6 D and E). Conversely, no coating was formed if the substrate was swollen to equilibrium in water before immersion in the PVA–hydroxyapatite solutions (Fig. 6C).
Fig. 6.
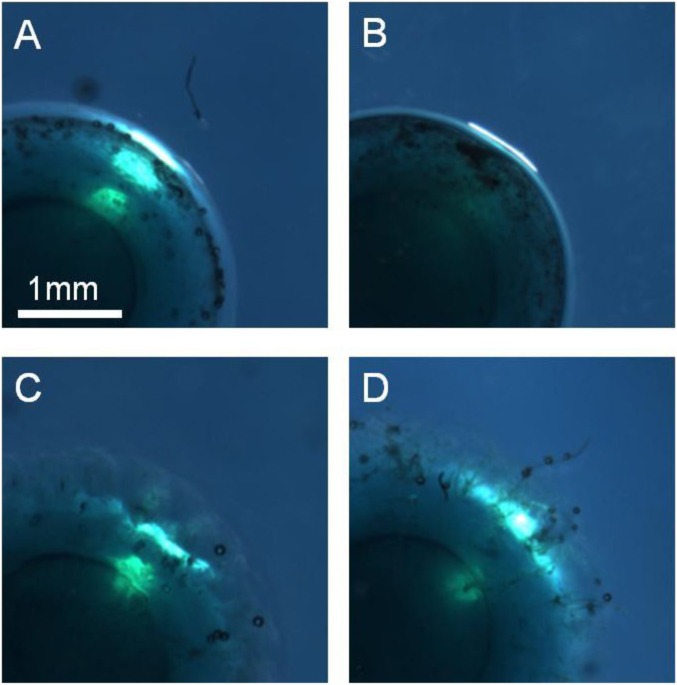
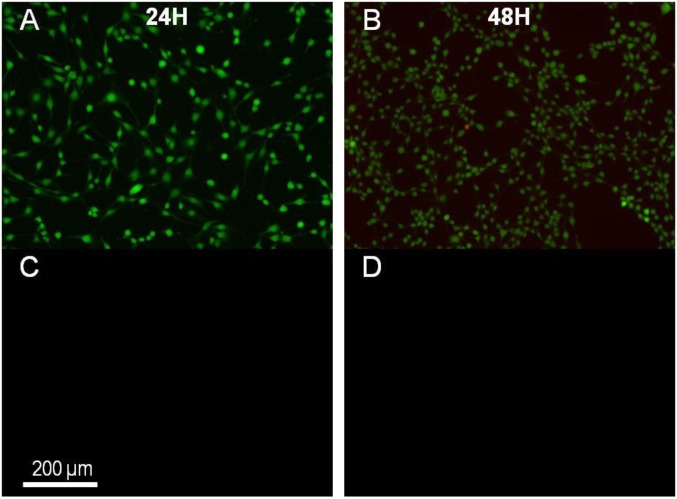
Fabrication of composite ceramic-hydrogel coatings and cell encapsulation. (A–E) Fabrication of composite hydroxyapatite–PVA hydrogel coatings. (A and B) Coating by immersion of dry PAAc–PAAm spheres in 10 wt% PVA solutions containing dispersions of hydroxyapatite nanoparticles (5 wt%) (A) or microparticles (20 wt%) (B, Top) Pictures of spheres after immersion (30 min for nanoparticles, 60 min for microparticles) and rinsing in water (15 min). (Bottom) Cross-section images by optical microscopy. (C) Same observations as B showing the absence of coating when the PAAc–PAAm sphere is swollen to equilibrium in pure water before immersion in the PVA solution with hydroxyapatite microparticles. (D and E) Low-vacuum SEM observations of the surface of the spheres coated with nanoparticles (D) and with microparticles (E). (F–J) Encapsulation of mouse fibroblasts NIH 3T3 in PVA hydrogel films using swelling of dry PEG films. (F) Cell-laden PVA hydrogel film obtained after the encapsulation protocol. (G) Cross-section of the PVA hydrogel film showing the gradient in film thickness. The picture is constructed by juxtaposing several images. The bottom surface was in contact with the PEG substrate, and the top surface with the cell suspension. Dotted line delimitates the upper region where clusters of living cells are revealed by MTT staining. (H–J) Confocal microscopy observations near the top surface of the hydrogel films for different film thicknesses: less than 1-mm thickness after 24 h (H); more than 1-mm thickness after 24 h (I) and 48 h (J). Green staining by calcein reveals the cytoplasm of living cells. Red staining by ethidium reveals the nucleus of dead cells.
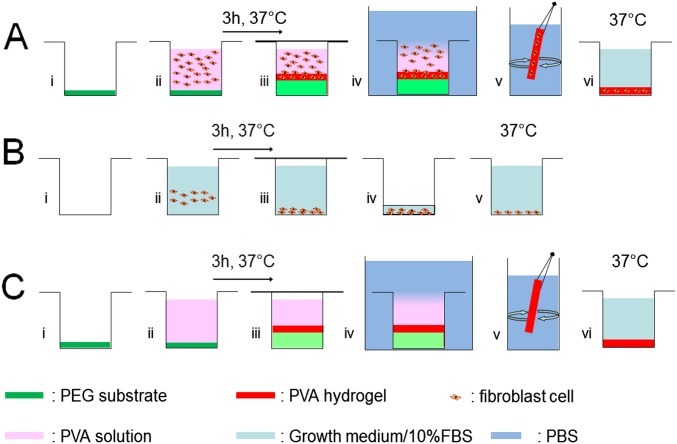
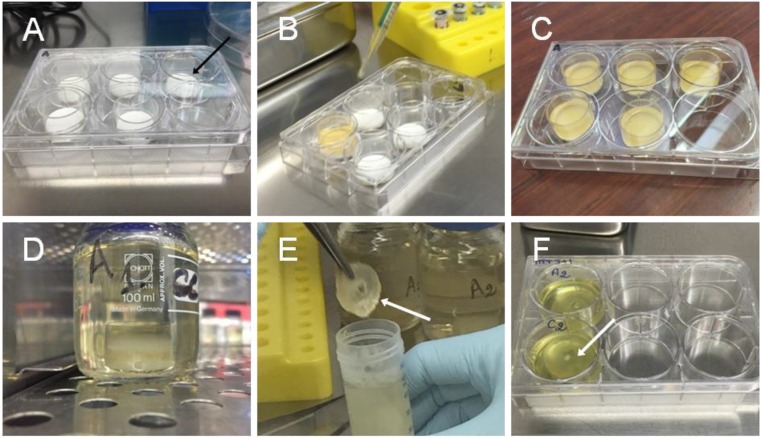
The potential for cell encapsulation was demonstrated in another experiment adjusted to cell culture (Fig. S7 and SI Materials and Methods). Mouse fibroblasts (NIH 3T3 cell line) were dispersed in growth medium containing 10% (vol/vol) FBS and 12 wt% PVA. The solution was poured in wells containing dry un–cross-linked PEG substrates, which swelled and dissolved to produce freestanding PVA hydrogel films (Fig. S8). Such soluble substrates were selected to reduce the number of direct manipulations and ensure aseptic conditions during membrane rinsing, collection, and incubation. After 3-h incubation at 37 °C, these films were rinsed and incubated in growth medium (Fig. 6F). Due to sticking to the walls of the glass wells during swelling, they exhibited a gradient in thickness from 2 mm at the edges to 0.5 mm in the center (Fig. 6G). Epifluorescence observations show that cells were efficiently encapsulated in the PVA films (see Fig. S9 for positive and negative controls). Cells located too close to the interface with the PEG substrate did not survive, probably because of hypertonic conditions created during swelling (Fig. 6H). On the contrary, cells encapsulated further than 1.0 ± 0.5 mm from the PEG substrate remained alive with a viability of 70 ± 20% after 24 and 48 h (Fig. 6 I and J). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) staining revealed clusters of living cells and indicated the extent of the viability zone (Fig. 6G).
Fig. S7.
Schematic representations of the encapsulation and control protocols. (A) Encapsulation protocol. (B) Positive control for cell viability. (C) Negative control for cell encapsulation.
Fig. S8.
Pictures of the encapsulation process. (A) PEG substrates (black arrow) are placed in sterile glass wells. (B) The PVA/cell solution is poured in the wells. (C) Glass wells after incubation at 37 °C for 3 h. (D) Glass wells are soaked in a large excess of growth medium. (E) The obtained PVA hydrogel films (white arrow) are collected and rinsed in PBS solutions. (F) The films (white arrow) are stored individually in cell culture wells and incubated in growth medium at 37 °C.
Fig. S9.
Epifluorescence microscopy observations of the positive control for cell viability (A and B) and of the negative control for cell encapsulation (C and D) after 24 and 48 h, respectively.
This experiment shows that there exists a range of conditions for which cells (fibroblasts) can be viably encapsulated by swelling-induced gelation. Depending on the cell type, sensitivity to hypertonic conditions and thus viability are expected to vary (32–34). The proposed approach offers many levers to modulate the microenvironment of encapsulated cells and therefore to control their viability and activity (35). For instance, the local gradient in osmotic pressure undergone during swelling can be reduced by changing the initial and equilibrium swelling ratios of the substrate as well as the PVA concentration; the cross-linking density and porosity of the PVA hydrogel might be adjusted by varying the PVA molecular weight or by mixing with other water-soluble polymers. More generally, incorporating bioactive additives in the PVA solution is another straightforward route to enhance the affinity of the encapsulated cells with the hydrogel matrix.
In conclusion, these results demonstrate that swelling of a hydrophilic polymer substrate in a polymer solution is a soft, autonomous, and finely tunable process to induce the growth of hydrogel films. Both experiments and simulations show that, even for rather dilute solutions (∼1 wt%), high polymer concentrations are produced near the swelling surface. New synthesis or analytical methods based on such phenomenon could provide interesting low-energy alternative for polymer concentration and extraction, for example, without energy-intensive centrifugation steps. Furthermore, this autonomous mechanism opens fabrication routes, which may prove particularly valuable for biomedical and biological applications. The possibility to coat hydrogels with complex shapes and to incorporate bioactive compounds is a strong asset to tailor the biointegration of implants. The numerous advances in the design of autoassociative polymers (36), especially PVA modification (21, 37–40), and of nanocomposite hydrogels (41) are a boon to expand the range of attainable properties. With the possibilities to encapsulate cells and to produce freestanding membranes, one may also envision cost-effective and easy-to-implement methods for tissue engineering and 3D cell culture.
SI Materials and Methods
Materials.
For the composite coatings, two types of hydroxyapatite particles were used: a powder of nanoparticles of hydroxyapatite with diameters less than 200 nm (Sigma-Aldrich; 677418) and a medical-grade powder of microparticles of hydroxyapatite (Science Applications Industries, Medical Group) with particle diameters ranging from 5 to 25 µm.
For the cell encapsulation experiments, substrates were made from high–molecular-weight poly(ethylene glycol) (PEG) (100,000 g⋅mol−1) (Sigma-Aldrich; 181986). Studied cells were mouse fibroblasts from the NIH 3T3 cell line (ATCC; CRL-1658) gratefully given by Suzie Lefebvre (INSERM, UMR-S 1124, Paris, France). Growth medium was composed of DMEM at 1 g/L glucose (Invitrogen; 11880-028) plus 1 mL of penicillin–streptomycin (PS) (10,000 U/mL penicillin/10,000 µg/mL streptomycin; Invitrogen; 15140) and 10 mL of glutamine (200 mM) (Invitrogen; 25030-024) per 500 mL of growth medium. FBS was used at 10% (vol/vol) (Lonza; DE14-801E). Buffer solution was PBS− 1× (Invitrogen; 14190-094), named PBS in the rest of the manuscript. Cells were harvested using a solution of trypsin-EDTA (0.05%) (Invitrogen; 25300-054). Viability assays were performed on unfixed cells. A viability/cytotoxicity kit for mammalian cells (Molecular Probes; L3224) was used for staining. It was composed of two dyes, calcein AM and ethidium homodimer-1.
Wide-Angle X-Ray Scattering of Hydrogel Films.
The 2D scattering pattern of hydrogel films were measured on the DiffAbs beamline at the French National Synchrotron Facility SOLEIL (Gif-sur-Yvette, France). The sample holder consisted in a 2.7-mm-thick chamber between two 25-µm-thick sheets of mica. Hydrogel films were inserted between the sheets so as to fill all of the space. The samples were illuminated with a monochromatic X-ray beam tuned at 10 keV with a wavelength λ = 1.2424 Å. An area detector (MAR345 image plate) was placed at 188 mm downstream of the sample. The exposure time was 30 s. A Pb beam stop was used to block the intense transmitted beam and a Si diode placed in the center of the beam stop served to record the transmitted beam intensity through the sample. Synchrotron diffraction images were analyzed using Fit2D software.
Several samples were observed: PVA hydrogel films were fabricated by the swelling-induced gelation method, using the immersion of dry 3-mm-thick rectangular PAAm substrates in a 15 wt% PVA aqueous solution for 3, 8, and 22 h. After immersion, the swollen substrates were removed from the PVA solution and were vigorously washed in a large excess of ultrapure water for 5 min. Hydrogel films were then peeled off from the substrate. For comparison, PVA hydrogel films were also fabricated by the freezing–thawing method: a 10 wt% PVA aqueous solution was cast in Petri dishes; physical gelation was achieved by repeating five cycles of freezing at −20 °C for 20 h and thawing at room temperature for 5 h.
Analytical Model for Hydrogel Film Growth on Spherical Substrate.
Developing Eq. 2 for a spherical substrate gives the following:
| [S1] |
where R0 is the initial radius of the substrate and ΔR is the increase in substrate radius after immersion time t. The thickness of the hydrogel film writes as follows:
| [S2] |
Introducing the time dependence of ΔR with Eq. 1, the growth of the hydrogel film for a spherical substrate is given by the following:
| [S3] |
where R∞ is the radius of the substrate at equilibrium swelling in the PVA solution.
Numerical Modeling.
Simulations were performed for a spherical substrate swelling in a polymer solution. The concentration in the solution during swelling is described by a discrete profile Φ(X,t) giving the volume fraction of polymer in solution in a spherical shell between a distance X and X + ΔX from the substrate surface. We define a as the thickness of the hydrogel film formed at the surface of the substrate. At t = 0, the solution is homogeneous and Φ(X,0) = Φ0 for all X and no hydrogel layer is formed (a = 0). During the simulation, the origin of the coordinate system (X = 0) is attached to the moving substrate surface. Each simulation proceeds as follows.
At each time step between time t and t + Δt, the increase in substrate radius ΔR is obtained from ΔR = R(t + Δt) − R(t), where R(t) and R(t + Δt) are the radii of the substrate at t and t + Δt, respectively, and are calculated from Eq. 1. For each solution layer, we calculate the change in solution concentration caused by substrate swelling and thermal diffusion as follows:
In the first liquid layer (X = a) at the substrate–solution interface (a = 0) or at the hydrogel film–solution interface (a > 0),
| [S4] |
Far from the interface in the solution (X > a),
| [S5] |
The first term in the right-hand side of Eqs. S4 and S5 corresponds to the amount of polymer that was already in the layer between X and X + ΔX at time t. The second term is the transport between layers caused by the displacement of the substrate surface. It is this second term in Eq. S4 that simulates the accumulation of polymer chains at the surface of the swelling substrate. The third term accounts for the thermal diffusion between adjacent layers. Note that, in Eq. S4, the diffusion of polymer chains is restricted to the solution (X > 0).
The growth of a hydrogel film is simulated whenever the polymer concentration in the first liquid layer close to the swelling substrate exceeds the critical gelation concentration [Φ(a,t) ≥ Φc]. In this case, the volume of the hydrogel film Vf(t) is updated by adding the newly gelled layer to it:
| [S6] |
In any case, the hydrogel film thickness is updated at the end of each time step to account for substrate swelling as follows:
| [S7] |
For the simulations to be stable and independent on time step size, the following relations must be verified at all times: and . Here, we chose Δt = 10−3 s and ΔX = 1 µm, which satisfies the above conditions.
Fabrication and Characterization of Composite Ceramic-Hydrogel Coatings.
Suspensions of hydroxyapatite particles in PVA solutions were prepared by adding the hydroxyapatite particles to a 10 wt% PVA aqueous solution. The amount of added particles was 5 wt% for the nanoparticles and 20 wt% for the microparticles. Dispersion was performed by mechanical stirring for 5 min at 20 °C. The solutions were put in ultrasonic bath to remove air bubbles. Dry PAAc–PAAm beads were immersed in these solutions for 60 and 30 min for microparticles and nanoparticles, respectively. Coated beads were collected and rinsed in excess of ultrapure water under magnetic stirring for 15 min. Immediately after rinsing, coated PAAc–PAAm beads were cut at the equator using a razor blade and characterized by optical microscopy and scanning electron microscopy (SEM).
Optical microscopy was performed with an Axio Scope A1 (Zeiss) apparatus on hydrated samples. Observations were made in dark-field reflection mode at a 5× magnification. SEM observations were performed on a Nova NanoSEM 450 (FEI) apparatus in low-vacuum mode at 90 Pa and 5 kV using secondary electron detector. Low-vacuum mode does not require sample metallization and enables observation in semihydrated state for the first 10 min.
Cell Encapsulation.
PEG substrates were obtained by pressing PEG powder in a manual hydraulic press (Specac). Before pressing, PEG powder was spread on a sterile plate and exposed to UV light (253.7 nm) for 30 min to be sterilized. Substrates were prepared by pressing 1 g of powder into an aluminum mold with a diameter of 22.1 ± 0.1 mm, previously sterilized by dry heat at 180 °C for 30 min. The powder was pressed under 1 ton of hydraulic pressure for 1 min. Substrate dimensions were 22 mm in diameter and 2 mm in thickness. Substrates were packed and stored in sterile bags (Whirl-Pak; Nasco).
A mouse fibroblast NIH 3T3 (ATCC; CRL-1658) cell line was used. Cells were cultured in growth medium/10% FBS at 37 °C in a humidified 95% air/5% CO2 incubator. Confluent cells from 10 T175 flasks were trypsinized by adding 5 mL of trypsin/EDTA (0.05%) per flask. The number of cells, their viability, and their diameter were estimated using an automated cell counter (Luna; Logos Biosystems). The total cell concentration was 27 × 106 per mL (in 5 mL of growth medium/10% FBS) with a viability of 94% and an average cell diameter of 15 µm.
Three protocols were performed: the protocol of cellular encapsulation (Fig. S7A), a protocol of positive control of cell viability (Fig. S7B), and a protocol of negative control where hydrogel films were formed in the absence of cells (Fig. S7C).
A 12 wt% PVA solution was prepared by dissolving 12 g of PVA in 88 g of growth medium without FBS. The mixture was autoclaved at 121 °C for 1 h and stored at 37 °C until use.
In the encapsulation protocol, 40 mL of the 12 wt% PVA/growth medium solution was mixed to 4.2 mL of FBS and placed in ultrasonic bath to degas. Thirty-eight milliliters of the mixture was mixed with 2.2 mL of cellular suspension at 27 × 106 cells per mL and 0.1 mL of PS. The whole mixture was homogenized by five linear up-and-down motions in a 50-mL pipette. Glass wells of 23 mm in diameter were placed in a six-well plate and PEG substrates were inserted into the glass wells (Fig. S8A). Then 3 mL of cellular suspension in PVA/FBS solution (containing 4.5 × 106 cells) were poured into the glass wells (Fig. S8B). The six-well plate was covered to prevent evaporation and drying (Fig. S8C) and was placed for 3 h in incubator at 37 °C. The wells were then removed from the six-well plate and dropped into a large excess of growth medium/10% FBS for 24 h in an incubator at 37 °C (Fig. S8D). After that, the obtained PVA hydrogel films were detached from the wall of the wells, vigorously rinsed in growth medium/10% FBS (Fig. S8E), and placed in a new six-well plate containing 4 mL of growth medium/10% FBS (Fig. S8F). Five hydrogel films were obtained: three films were observed 24 h after fabrication and two films after 48 h.
In the positive control experiment, cells were plated in a six-well plate at three different concentrations, 75 × 103, 150 × 103, and 300 × 103 cells per well in 4 mL of growth medium/10% FBS. Cells were then left in an incubator at 37 °C. After 24 h, the growth medium/10% FBS was replaced by a fresh one. Three wells were observed after 24 h and three wells after 48 h.
The negative control experiment consisted of the exact same procedure as the encapsulation protocol without adding the cells to the PVA solution. PVA hydrogel films were obtained from PVA/PBS solution and were placed in a six-well plate filled with growth medium/10% FBS. Four hydrogel films were obtained: two films were observed 24 h after fabrication and two films after 48 h.
Cell Viability.
Cell viability was assessed using a viability/cytotoxicity kit for mammalian cells according to the manufacturer’s instructions. Fluorescent images were obtained using an epifluorescence microscope (Eclipse TE2000E; Nikon). The staining procedure was as follows. Both fluorescence dyes were allowed to thaw at room temperature in darkness. Sixty microliters of 2 mM EthD-1 and 15 µL of 4 mM calcein were added to 30 mL of PBS, and the mixture was vortexed. Hydrogel films were rinsed with PBS. Three milliliters of the fluorescence solution were added in wells containing the hydrogel films. It was put in an incubator at 37 °C for 30 min. Last, hydrogel films were rinsed three times with PBS to remove unfixed fluorescent dyes. The calcein dye was retained in the cytoplasm of living cells, producing an intense uniform green fluorescence in living cells (excitation/emission 495/515 nm). The ethidium produced a red fluorescence (excitation/emission, 495/635 nm), when binding to nucleic acid of cells with damaged plasma membranes.
Living and dead cells were counted using the Cell Counter plugin of the ImageJ software for each of the epifluorescence microscopic observations. Viability was estimated as the ratio of the number of living cells over the total number of counted cells. Viability was assessed in 17 different regions of each hydrogel film. For each region, a number of cells between 20 and 300 were analyzed in a volume of 0.13 ± 0.07 mm3.
The same staining and observations were done for the positive and negative controls after 24 and 48 h. For the positive control, a good viability of the cells indicated that cellular death is not the result of some effect unrelated to the encapsulation protocol (Fig. S9 A and B). For the negative control, no fluorescent signal was detected, therefore confirming that the fluorescent staining corresponds to cells and not to some artifact (Fig. S9 C and D).
MTT assays were performed on hydrogel films both with and without cells. MTT (Sigma; 2128) was at 5 mg/mL in DMEM (70 mg of MTT plus 14 mL of DMEM-l-glutamine-PS). Four hundred microliters of this solution was added to the wells containing hydrogel films in 4 mL of growth medium/10% FBS. Wells were then put in an incubator at 37 °C for 2 h. After that, the supernatant was removed, and 1 mL of DMSO was added. The staining of living cells by MTT was assessed by macroscopic observations because spectrophotometric measurements were not possible due to the insolubility of the obtained PVA hydrogel films in DMSO at room temperature.
Materials and Methods
Materials.
The chemicals used in this work were used as purchased without further purification. PVA aqueous solutions were fabricated from high–molecular-weight (89,000–98,000 g·mol−1) and 99% hydrolyzed PVA (Sigma-Aldrich; 341584). The PAAm networks used as flat substrates were made from a 40 wt% aqueous solution of acrylamide (AAm) as the monomer, N,N′-methylenebisacrylamide (MBAAm) as the cross-linker with a ratio AAm/MBAAm of 37.5:1 (Sigma-Aldrich; A7168). Ammonium persulfate (APS) was used as the thermal initiator (Amresco; 0486) and N,N,N′,N′-tetramethylethylenediamine (TEMED) as the cross-linking accelerator (Sigma-Aldrich; T9281). Spherical substrates were made from commercially available PAAc–PAAm beads (Casa; Perles d’Eau). They were purchased in a swollen state composed of distilled water (99 wt%), sodium salt (0.2 wt%), and a network of acrylic acid (0.6 wt%) and acrylamide (0.2 wt%). Aqueous polymer solutions (10 wt%) were prepared from PEG (Mn = 3,400 g⋅mol−1; Sigma-Aldrich), PVP (Mn = 40,000 g⋅mol−1; Sigma-Aldrich), and Pluronic F127 (Mn = 12,600 g⋅mol−1; Sigma-Aldrich). For more details on the materials, please see SI Materials and Methods.
Preparation of PVA Solutions.
The PVA aqueous solutions were fabricated by dissolving PVA powder under mechanical stirring in ultrapure water using a silicon oil bath heated to 110 °C for 60 min. The weight of the solutions was measured before and after dissolution, and water was added to correct from evaporation. After stirring, the solutions were covered and let cool down and degas. Six solutions were prepared with theoretical PVA concentration of 1, 2.5, 5, 7.5, 10, and 15 wt%. Actual concentrations measured a posteriori by water evaporation were 1.0, 2.6, 5.3, 7.6, 10.6, and 14.7 wt%, respectively. These latter values were used for fitting by the model.
Fabrication of Swelling Substrates.
The flat PAAm substrates were prepared from a 12 vol% aqueous solution of AAm–MBAAm obtained by dilution of the commercial 40 wt% solution in ultrapure water. Synthesis was done by mixing this solution with of a 10 wt% aqueous solution of APS at 2 vol% and TEMED at 0.2 vol%. The whole solution was stirred by magnetic stirrer and poured in a glass mold of 100 × 130 × 5 mm3. The mold was kept at 20 °C for 2 h to obtain cross-linking. The obtained PAAm gel film was cut into 2 × 4 cm2 rectangular pieces and dried at 20 °C in air under extractor hood for 24 h. The spherical PAAc–PAAm substrates were obtained from swollen PAAc–PAAm beads that were dried under extractor hood for 24 h.
Stability of Hydrogel Films.
PVA hydrogel films were fabricated by immersing dry 3-mm-thick rectangular PAAm substrates in a 15 wt% PVA aqueous solution for 3 h. After immersion, the swollen substrates were removed from the PVA solution and were vigorously washed in a large excess of ultrapure water for 15 min. Hydrogel films were then peeled off from the substrate surface, attached to fishhooks, and stored in glass tubes filled with ultrapure water. Pictures of the films were taken with a camera (D300s; Nikon) right after fabrication, after 1-h and 1-wk storage at 20 °C or after 1-h storage in an oil bath at 90 °C.
Measurement of Hydrogel Film Growth.
The formation and growth of hydrogel films at the surface of swelling spherical substrates was observed with a macroscope (M420 Wild-Leitz; Leica). Spherical PAAc–PAAm substrates were placed in Petri dishes filled with ultrapure water or PVA aqueous solutions with different concentrations. The Petri dishes were placed on a X–Y translation stage. Two lighting methods were used separately to distinguish the hydrogel film and the substrate surface boundaries (Fig. S4). In-lens lighting was used to reveal the surfaces of the hydrogel film. Lighting in the plane of the image revealed the boundary of the substrate surface only. The formation and growth of hydrogel films was recorded during 24 h with a first measurement after 2 min of immersion. At each time point, images were recorded with the two types of lighting. Film thickness was estimated by subtracting the radius of the PAAc–PAAm sphere (Fig. S4B) to the radius of the film around the PAAc–PAAm sphere (Fig. S4A). Measurements were repeated on two to five beads for each time point. Data points in Fig. 4 are represented as the mean with SD as the error bar.
For more details about the methods, in particular, X-ray scattering, fabrication of ceramic coatings, and cell encapsulation, please see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Auriac for technical assistance on experiment design, M. Betbeder and F. Gaslain for assistance in electron microscopy, C. Mocuta and H. Proudhon for assistance on synchrotron experiments, and S. Tencé-Girault for X-ray analysis. We also thank L. Tsagris for assistance on cell encapsulation experiments and J.-M. Petit for epifluorescence microscopy. We thank L. Leibler for encouragement and interpretations on gel swelling, M. Cloître and R. Fournier for discussions on associative polymers, and D. J. Pine and R. Nicolaÿ for discussions and critical review of the manuscript. D.M. acknowledges a PhD scholarship from MINES ParisTech. The financial support of MINES ParisTech, ESPCI Paris, and Institut Carnot Mines (Grant HAP-Process 2012) is acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609603113/-/DCSupplemental.
References
- 1.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: A review. J Pharm Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 2.Lih E, Oh SH, Joung YK, Lee JH, Han DK. Polymers for cell/tissue anti-adhesion. Prog Polym Sci. 2015;44:28–61. [Google Scholar]
- 3.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32-33):3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker MI, Walsh SP, Schwartz Z, Boyan BD. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res B Appl Biomater. 2012;100(5):1451–1457. doi: 10.1002/jbm.b.32694. [DOI] [PubMed] [Google Scholar]
- 5.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 7.Malda J, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater. 2013;25(36):5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 8.Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14(2):149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertaniemi H, et al. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials. 2016;82:208–220. doi: 10.1016/j.biomaterials.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Peters A, Candau SJ. Kinetics of swelling of spherical and cylindrical gels. Macromolecules. 1988;21(7):2278–2282. [Google Scholar]
- 11.Doi M. Gel dynamics. J Phys Soc Jpn. 2009;78(5):52001. [Google Scholar]
- 12.Budtova T, Navard P. Swelling dynamics of cross-linked poly(acrylic acid) and neutralized poly(acrylate-co-acrylic acid) in aqueous solutions of (hydroxypropyl) cellulose. Macromolecules. 1996;29(11):3931–3936. [Google Scholar]
- 13.Packter A, Nerurkar M. Crystallization in films of polar vinyl polymers—I. Crystallinity of polyvinyl alcohol films prepared by evaporation of aqueous solutions. Eur Polym J. 1968;4(6):685–693. [Google Scholar]
- 14.Kawanishi K, Komatsu M, Inoue T. Thermodynamic consideration of the sol-gel transition in polymer solutions. Polymer (Guildf) 1987;28(6):980–984. [Google Scholar]
- 15.Young T-H, Yao N-K, Chang R-F, Chen L-W. Evaluation of asymmetric poly(vinyl alcohol) membranes for use in artificial islets. Biomaterials. 1996;17(22):2139–2145. doi: 10.1016/0142-9612(96)00043-9. [DOI] [PubMed] [Google Scholar]
- 16.Barzin J, Madaeni SS, Pourmoghadasi S. Hemodialysis membranes prepared from poly(vinyl alcohol): Effects of the preparation conditions on the morphology and performance. J Appl Polym Sci. 2007;104(4):2490–2497. [Google Scholar]
- 17.Cascone MG, Laus M, Ricci D, Del Guerra RS. Evaluation of poly(vinyl alcohol) hydrogels as a component of hybrid artificial tissues. J Mater Sci Mater Med. 1995;6(2):71–75. [Google Scholar]
- 18.Vrana NE, et al. Cell encapsulation and cryostorage in PVA-gelatin cryogels: Incorporation of carboxylated ε-poly-l-lysine as cryoprotectant. J Tissue Eng Regen Med. 2012;6(4):280–290. doi: 10.1002/term.431. [DOI] [PubMed] [Google Scholar]
- 19.Hassan CM, Peppas NA. Biopolymers · PVA Hydrogels, Anionic Polymerisation Nanocomposites. Springer; Berlin: 2000. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods; pp. 37–65. [Google Scholar]
- 20.Otsuka E, Suzuki A. A simple method to obtain a swollen PVA gel crosslinked by hydrogen bonds. J Appl Polym Sci. 2009;114(1):10–16. [Google Scholar]
- 21.Jensen BEB, et al. Poly(vinyl alcohol) physical hydrogels: Noncryogenic stabilization allows nano- and microscale materials design. Langmuir. 2011;27(16):10216–10223. doi: 10.1021/la201595e. [DOI] [PubMed] [Google Scholar]
- 22.Watase M, Nishinari K. Rheological and DSC changes in poly(vinyl alcohol) gels induced by immersion in water. J Polym Sci Polym Phys Ed. 1985;23(9):1803–1811. [Google Scholar]
- 23.Willcox PJ, et al. Microstructure of poly(vinyl alcohol) hydrogels produced by freeze/thaw cycling. J Polym Sci B Polym Phys. 1999;37(24):3438–3454. [Google Scholar]
- 24.Bastide J, Candau S, Leibler L. Osmotic deswelling of gels by polymer solutions. Macromolecules. 1981;14(3):719–726. [Google Scholar]
- 25.Bercea M, Morariu S, Rusu D. In situ gelation of aqueous solutions of entangled poly(vinyl alcohol) Soft Matter. 2013;9(4):1244–1253. [Google Scholar]
- 26.Barrière B, Leibler L. Kinetics of solvent absorption and permeation through a highly swellable elastomeric network. J Polym Sci B Polym Phys. 2003;41(2):166–182. [Google Scholar]
- 27.Hara C, Matsuo M. Phase separation in aqueous poly(vinyl alcohol) solution. Polymer (Guildf) 1995;36(3):603–609. [Google Scholar]
- 28.Holloway JL, Lowman AM, Palmese GR. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter. 2013;9(3):826–833. [Google Scholar]
- 29.Cussler E. Diffusion: Mass Transfer in Fluid Systems. 3rd Ed Cambridge Univ Press; Cambridge, UK: 2009. [Google Scholar]
- 30.Hou R, et al. Magnetic nanohydroxyapatite/PVA composite hydrogels for promoted osteoblast adhesion and proliferation. Colloids Surf B Biointerfaces. 2013;103:318–325. doi: 10.1016/j.colsurfb.2012.10.067. [DOI] [PubMed] [Google Scholar]
- 31.Hayami T, Matsumura K, Kusunoki M, Nishikawa H, Hontsu S. Imparting cell adhesion to poly(vinyl alcohol) hydrogel by coating with hydroxyapatite thin film. Mater Lett. 2007;61(13):2667–2670. [Google Scholar]
- 32.Woods EJ, Zieger MAJ, Lakey JRT, Liu J, Critser JK. Osmotic characteristics of isolated human and canine pancreatic islets. Cryobiology. 1997;35(2):106–113. doi: 10.1006/cryo.1997.2029. [DOI] [PubMed] [Google Scholar]
- 33.Zawlodzka S, Takamatsu H. Osmotic injury of PC-3 cells by hypertonic NaCl solutions at temperatures above 0 °C. Cryobiology. 2005;50(1):58–70. doi: 10.1016/j.cryobiol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Cai Q, et al. Pax2 expression occurs in renal medullary epithelial cells in vivo and in cell culture, is osmoregulated, and promotes osmotic tolerance. Proc Natl Acad Sci USA. 2005;102(2):503–508. doi: 10.1073/pnas.0408840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 36.Tsitsilianis C. Responsive reversible hydrogels from associative “smart” macromolecules. Soft Matter. 2010;6(11):2372–2388. [Google Scholar]
- 37.Nuttelman CR, Henry SM, Anseth KS. Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials. 2002;23(17):3617–3626. doi: 10.1016/s0142-9612(02)00093-5. [DOI] [PubMed] [Google Scholar]
- 38.Schmedlen RH, Masters KS, West JL. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23(22):4325–4332. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 39.Sailaja GS, Sreenivasan K, Yokogawa Y, Kumary TV, Varma HK. Bioinspired mineralization and cell adhesion on surface functionalized poly(vinyl alcohol) films. Acta Biomater. 2009;5(5):1647–1655. doi: 10.1016/j.actbio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Arai K, Okuzono M, Shikata T. Reason for the high solubility of chemically modified poly(vinyl alcohol)s in aqueous solution. Macromolecules. 2015;48(5):1573–1578. [Google Scholar]
- 41.Schexnailder P, Schmidt G. Nanocomposite polymer hydrogels. Colloid Polym Sci. 2009;287(1):1–11. [Google Scholar]
- 42.Bunn CW. Crystal structure of polyvinyl alcohol. Nature. 1948;161(4102):929–930. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.