Significance
New interventions are needed to improve bone health and reduce the risk for osteoporosis and fracture. Dysbiosis is increasingly linked to metabolic abnormalities, although the effect of the microbiota on skeletal health is poorly understood. Previous studies suggest microbiota are detrimental to bone by increasing resorption. In this report, we show that the gut resident microbiota promote bone formation, as well as resorption, with long-term exposure to microbiota resulting in net skeletal growth. Microbiota induce the hormone insulin-like growth factor 1 (IGF-1), which promotes bone growth and remodeling. Short-chain fatty acids (SCFAs), produced when microbiota ferment fiber, also induce IGF-1, suggesting a mechanism by which microbiota affect bone health. Manipulating the microbiome or its metabolites may afford opportunities to optimize bone health and growth.
Keywords: microbiota, bone, IGF-1, SCFA
Abstract
Appreciation of the role of the gut microbiome in regulating vertebrate metabolism has exploded recently. However, the effects of gut microbiota on skeletal growth and homeostasis have only recently begun to be explored. Here, we report that colonization of sexually mature germ-free (GF) mice with conventional specific pathogen-free (SPF) gut microbiota increases both bone formation and resorption, with the net effect of colonization varying with the duration of colonization. Although colonization of adult mice acutely reduces bone mass, in long-term colonized mice, an increase in bone formation and growth plate activity predominates, resulting in equalization of bone mass and increased longitudinal and radial bone growth. Serum levels of insulin-like growth factor 1 (IGF-1), a hormone with known actions on skeletal growth, are substantially increased in response to microbial colonization, with significant increases in liver and adipose tissue IGF-1 production. Antibiotic treatment of conventional mice, in contrast, decreases serum IGF-1 and inhibits bone formation. Supplementation of antibiotic-treated mice with short-chain fatty acids (SCFAs), products of microbial metabolism, restores IGF-1 and bone mass to levels seen in nonantibiotic-treated mice. Thus, SCFA production may be one mechanism by which microbiota increase serum IGF-1. Our study demonstrates that gut microbiota provide a net anabolic stimulus to the skeleton, which is likely mediated by IGF-1. Manipulation of the microbiome or its metabolites may afford opportunities to optimize bone health and growth.
Trillions of microorganisms inhabit the gut and have coevolved with the host to forge mutually beneficial relationships (1). These symbiotic microorganisms, known as microbiota, can be viewed as a multicellular organ that communicates with and influences the host in numerous diverse ways (2, 3). The role of gut microbiota in shaping host systemic immune function has been well described (4), and emerging evidence demonstrates that microbiota influence host metabolism (5); for example, altering hepatic lipogenesis (6). Microbiota may influence host physiology by production of specific metabolites or by inducing host response to symbiont-associated molecular patterns (7, 8). The best-studied microbial metabolites in this regard are short-chain fatty acids (SCFAs). SCFAs are produced by intestinal bacteria via fermentation of nondigestible polysaccharides and have been implicated in the maturation of the host immune system, including induction of peripheral regulatory T cells, protection from infection, and modulation of metabolic rate and energy homeostasis (8–12). The gut microbiome is indispensable for multiple aspects of host physiology, but reported effects on bone physiology have differed regarding whether microbiota promote bone resorption or bone formation (13, 14).
The axial skeleton forms by endochondral ossification, in which a cartilage anlage is replaced with mineralized bone. Postnatal bone growth depends on continued proliferation and hypertrophy of chondrocytes of the growth plate. Growth plate chondrocytes are highly regulated by circulating and local hormones and growth factors (15, 16). The mature skeleton is then remodeled throughout life, with ∼10% of the skeleton being replaced yearly (17). Bone remodeling is a highly regulated process mediated by the activities of bone-resorbing osteoclasts and bone-forming osteoblasts (18). Bone mass is determined by the balance between bone formation and resorption. If bone formation is more active than bone resorption, a net anabolic effect is observed. If resorption outpaces formation, the net effect is catabolic. Hormonal, immune, and metabolic pathways all affect this balance, and the gut microbiota could affect any of these pathways (19). Bone mass is the major determinant of fracture risk with aging (19), and a better understanding of how gut microbiota affect bone remodeling in adults may lead to new therapeutic approaches.
Recently, Schwarzer et al. showed that microbiota influence growth in the neonatal period (14). Neonatal rates of growth and weight gain were lower in germ-free (GF) mice than for conventionally raised mice (14). Interestingly, femur length and bone mass were also lower in GF mice, suggesting gut microbiota have an anabolic effect on bone (14). In contrast, exposure of conventional weanling mice to subtherapeutic concentrations of antibiotics resulted in a significant, but transient, increase in bone mineral density, suggesting microbiota can have catabolic effects on bone (20). Consistent with this, Sjogren et al. found that bacterial colonization of young adult GF animals resulted in loss of trabecular bone mass and an increase in bone-resorbing osteoclasts (13). However, other studies have suggested that treatment with beneficial microbes modestly increases bone mineral density (21–26) and protects against bone loss after sex steroid deficiency (27). Discrepant results in studies examining the effect of microbiota on bone may reflect differences in the composition of the resident microbiota, age of the animal at colonization and necropsy, mouse strain, and sex. An additional critical variable that has not been examined is the duration of colonization. Studies investigating how age and duration of colonization influence the effect of microbiota on bone are needed.
One candidate mechanism for microbiota effects on bone is IGF-1. Colonization of GF flies with conventional microbiota increased Drosophila insulin/IGF-like peptide activity, and IGF-1 was shown to mediate the microbiota effect on postnatal growth (28, 29). IGF-1 is a growth factor with both endocrine and paracrine/autocrine actions on bone (30–32). Exogenous IGF-1 promotes longitudinal femur growth (33), and cartilage-specific deletion of insulin-like growth factor I receptor (Igf1r) demonstrates an essential role for IGF-1 in growth plate maturation and formation of the secondary ossification center (34). Liver-specific IGF-1-deficient mice exhibit relatively normal growth and development, despite a 75% reduction in serum IGF-1, suggesting local IGF-1 also contributes significantly to bone growth (33). In addition, IGF-1 can promote both bone formation and resorption through direct effects on osteoblasts (35, 36) and osteoclasts (37), respectively. A recent report demonstrated lower IGF-1 levels in GF mice compared with mice with either conventional or defined microbiota (14), but whether IGF-1 levels change in response to alterations in microbiota is unknown, as is the mechanism underlying this association.
Here, we examine the effects of both short- and long-term colonization of adult GF mice with normal intestinal microbiota on bone remodeling. We demonstrate a previously unappreciated positive effect of gut microbiota on the skeleton of adult animals. The microbiota-mediated increase in bone formation is initially counteracted by a concurrent increase in resorption, resulting in an acute catabolic effect of colonization. With long-term colonization, however, effects on bone formation predominate, resulting in increased longitudinal and radial bone growth and equalization of bone mass. Circulating IGF-1 is increased in both short- and long-term colonized mice compared with GF mice. Conversely, antibiotic depletion of microbiota from conventionally raised mice reduced serum IGF-1 levels, resulting in a transient decrease in both bone resorption and formation. Feeding antibiotic-treated mice with SCFA increased IGF-1 levels and recapitulated the bone phenotype seen after short-term colonization. Our study is the first to describe that gut microbiota acutely increase both bone formation and bone resorption and to demonstrate that the effect of long-term colonization primarily reflects increased bone formation. SCFA represent a potential microbial metabolite mediating this effect, but additional mechanisms by which gut microbiota affect bone likely exist. This study provides new insights into microbiota-mediated bone remodeling in adult mice that may inform therapeutic approaches to treat and prevent low bone mass conditions.
Results
Short-Term Colonization of Adult GF Mice Promotes Both Bone Formation and Resorption.
Disparate effects of microbiota on bone have been reported in mouse studies (13, 14). To evaluate the short-term effects of microbiota on bone remodeling, GF female mice were colonized with microbiota from conventionally raised specific pathogen-free (SPF) mice at 2 mo of age and compared with GF siblings (Fig. 1A). One month after colonization, trabecular bone mass was decreased compared with GF controls (Fig. 1B). Body weight was also modestly reduced, but body length was similar (Fig. S1A). Trabecular bone mass reflects the balance between bone resorption and bone formation, and a net decrease in bone mass could be caused by increased resorption or decreased formation. The serum bone resorption marker C-terminal telopeptides of type I collagen (CTX-I) was increased in colonized mice (Fig. 1C), indicating that colonization increased bone resorption activity.
Fig. 1.
Microbiota promote bone turnover and endochondral ossification after short-term colonization in adulthood. (A) Experimental procedure schematic. Two-month-old GF CB6F1 mice were colonized with unfractionated feces from SPF mice and evaluated 1 mo later. (B) Trabecular bone mass determined by micro-CT (n ≥ 15), with representative images of trabecular bone from the femurs of GF or colonized (Col) siblings. (C and D) Serum bone turnover markers CTX-I and P1NP in GF and Col mice (n ≥ 15). (E) Dynamic BFR/BS and MAR, determined by histomorphometry on double-labeled GF and Col mice (n ≥ 10). Representative double labeling in trabecular bone (Left) and secondary ossification center (Right) is shown. White arrows indicate bone formation in the interval between labels. (F) Growth plate thickness measured on 3D reconstructions of micro-CT images and on histologic specimens (Left). Representative H&E and toluidine blue staining is shown (Right). White dashed lines indicate the growth plate. Black arrows indicate hypertrophic chondrocytes. *P < 0.05; ***P < 0.001.
Fig. S1.
Effect of colonization on macroscopic data, vertebral and cortical bone parameters. (A) Body weight and length of GF and colonized (Col) littermates, 1 mo after colonization. (B–H) Comparison of GF and colonized littermates, 8 mo after colonization. Only female mice were examined, unless otherwise specified. (B) L5 vertebrae length. (C and D) Cortical bone thickness and porosity for female (C) and male (D) mice. (E) Body weight. (F) Vertebral L5 trabecular bone parameters. (G and H) Cortical dynamic bone formation indices in periosteal (Peri) and endosteal (Endo) areas. *P < 0.05; ns, not statistically significant.
Interestingly, procollagen type I N-terminal propeptide (P1NP), a serum marker of bone formation, was also significantly increased in colonized mice (Fig. 1D). To further confirm that colonization increased bone formation, bone formation rates (BFR) were measured by dynamic histomorphometry. Colonization increased BFR per bone surface (BFR/BS) and mineral apposition rate (MAR) in the trabecular bone (Fig. 1E). In addition, the secondary ossification center displayed qualitatively more active mineral incorporation (Fig. 1E).
Unlike in humans, the mouse growth plate remains open after sexual maturity and longitudinal growth continues, although at a slower rate (38). We found that the growth plate of colonized mice was significantly thicker, as measured by micro-CT and histology, and had more hypertrophic chondrocytes (Fig. 1F). These data suggest there is more active endochondral ossification in colonized mice, which might alter longitudinal growth of long bones.
Long-Term Colonization Leads to Increased Bone Growth.
Because osteoporosis and growth retardation are chronic diseases, determining the effect of long-term colonization is relevant to any clinical intervention aimed at leveraging the microbiota to improve skeletal health, but the effect of long-term colonization on bone has not been reported. To examine this, we compared GF mice with colonized siblings 8 mo after colonization (Fig. 2A). Femur length was significantly longer in colonized mice of both sexes compared with littermate GF animals (Fig. 2B). The height of L5 vertebrae was also greater in colonized mice (Fig. S1B). Long-term colonization also increased the periosteal and endosteal area compared with GF siblings (Fig. 2C), without changing cortical thickness or porosity (Fig. S1 C and D), although the effect on radial growth was less pronounced in females. Together, these data suggest colonization promotes radial and longitudinal bone growth, consistent with the more active endochondral ossification seen after short-term colonization (Fig. 1 E and F). The observed increase in bone size after long-term colonization is accompanied by a modest increase in body weight (Fig. S1E). In contrast to short-term colonization, we found that long-term colonized mice had a trend toward increased trabecular bone mass in the femur (Fig. 2D) and have comparable bone mass in L5 vertebrae (Fig. S1F). This suggests that during longer periods of colonization, the bone formation promoting effects of colonization overtake the effect on bone resorption. Consistent with this, increased bone resorption in response to colonization appears to be transient, as serum CTX-I in long-term colonized and GF mice was similar (Fig. 2E). Eight months after colonization, bone formation determined either by serum P1NP (Fig. 2F) or by dynamic histomorphometry (Fig. S1 G and H) was low, and we were unable to detect significant differences in long-term colonized mice compared with GF littermates. Our data demonstrate that colonization affects bone formation and growth plate function, in addition to bone resorption. Although the net effect of short-term colonization is decreased bone mass, long-term colonization results in increased longitudinal and radial bone growth and equalization of bone mass.
Fig. 2.
Long-term colonization with gut microbiota promotes longitudinal and radial bone growth in adult mice. (A) Schematic diagram of the experimental procedure. Two-month-old GF CB6F1 female and male mice (n ≥ 6) were colonized with unfractionated feces from SPF mice and evaluated 8 mo later. (B–D) Bone parameters of GF and colonized (Col) mice determined by micro-CT. (B) Femur length (Left) with representative images (Right). (C) Periosteal (Left) and endosteal (Middle) area with representative 3D reconstructions of midshaft cortical bone (Right). (D) Trabecular bone mass. (E and F) Serum bone turnover markers CTX-I and P1NP in female GF and Col mice. *P < 0.05; **P < 0.01; ns, not statistically significant.
Microbiota Increase Systemic and Bone Marrow IGF-1.
IGF-1 is a potent regulator of bone (32, 37), and IGF-1 levels were recently shown to be higher in mice with either a conventional or defined microbiota compared with GF mice (14). However, it is not known whether colonization of adult GF mice with microbiota is sufficient to increase IGF-1 levels, nor whether IGF-1 levels remain persistently elevated in older colonized mice. We asked whether the bone phenotype seen in both short- and long-term colonized adult mice could be mediated by changes in IGF-1 levels. IGF-1 was significantly increased in colonized mice compared with GF controls at both 1 and 8 mo after colonization (Fig. 3 A and B), and a trend toward increased serum IGF-1 was observed as early as 7 d after colonization (Fig. S2A).
Fig. 3.
Microbiota increase systemic and local IGF-1. Serum IGF-1 and IGFBP3 levels in (A) long-term and (B) short-term colonized mice compared with GF siblings (n ≥ 6). (C) Relative expression of Runx2 in the epiphyseal bone tissue (n = 5). (D and E) Tissue IGF-1 production. (D) Liver and (E) abdominal fat pad total tissue IGF-1 (Left) was calculated by multiplying the IGF-1 production per gram tissue (Middle) by the weight of the tissue (Right). (F) Relative expression of Igf1 in the bone marrow (BM). *P < 0.05; **P < 0.01; ns, not statistically significant.
Fig. S2.
Effects of colonization on serum IGF-1, GH, and tissue Igf1 expression. GF and colonized (Col) littermates were compared at the points indicated in the legend. (A) Serum IGF-1 concentration, 1 wk after colonization. (B) Serum GH concentration, 1 mo after colonization. (C and D) Relative expression of Igf1 and Igfbp3 in liver and adipose tissue, 1 mo after colonization. (E) Relative expression of Igf1 in muscle tissue, 1 mo after colonization. *P < 0.05; ns, not statistically significant.
The bioactivity of IGF-1 is modulated by IGF binding proteins (IGFBPs) that have higher affinity for IGF-1 than the IGF-1 receptor, and inhibit the biological effects of IGF-1 through sequestration (33). The majority of serum IGF-1 is bound to IGFBP3 (39). We found no difference in the level of serum IGFBP3 at either point after colonization (Fig. 3 A and B). Therefore, colonization likely increases free IGF-1. Consistent with this, the expression of runt-related transcription factor 2 (Runx2), a downstream target of the IGF-1 signaling pathway in bone, was significantly increased in epiphyseal bone samples from colonized mice (Fig. 3C). As circulating IGF-1 is regulated by growth hormone (GH) (40), we examined GH levels in colonized mice, but found no effect of colonization (Fig. S2B). However, GH release is circadian and pulsatile (40), so our ability to detect a difference is limited by sampling growth hormone levels at a single point.
To understand how colonization increases serum IGF-1, we examined whether tissue production of IGF-1 was altered by short-term colonization. Circulating IGF-1 is mainly produced by the liver (33). Total IGF-1 content of liver tissue was significantly higher in colonized mice (Fig. 3D, Left). IGF-1 content of liver tissue measured by ELISA on tissue homogenates increased significantly (Fig. 3E, Middle), whereas liver weight after colonization remained unchanged (Fig. 3D, Right). However, no change in liver Igf1 transcript levels was seen after colonization (Fig. S2C). This is in contrast to a report of higher liver Igf1 in conventional mice compared with GF mice (14). As with GH, liver Igf1 expression is circadian, and detection of differences may depend on sampling time (41). Although serum levels of IGFBP3 are unchanged (Fig. 3 A and B), liver tissue from colonized mice expressed significantly less Igfbp3 mRNA (Fig. S2C), leaving open the possibility that microbiota further regulate local IGF-1 activity by modulating the expression of IGF-1-binding proteins.
Recent studies reported that white-adipose-tissue–derived IGF-1 elicited systemic effects in models of diabetes (42) and colitis (43). We found that colonization increased total abdominal fat pad IGF-1 (Fig. 3E, Left), driven primarily by increased fat pad weight, rather than changes in tissue production of IGF-1 (Fig. 3E, Middle, Right), which is consistent with previous report showing that colonization increases abdominal fat pad weight (6). Neither Igf1 nor Igfbp3 expression were altered in the fat pad (Fig. S2D). Together, these data suggest that both liver and adipose tissue contribute to increasing circulating IGF-1 after colonization.
Myocytes also express IGF-1 and ectopic or increased expression of IGF-1 in muscle has been shown to increase bone mass (44–46). To explore the potential contribution of muscle-derived IGF-1, we isolated RNA from gastrocnemius muscle tissue and measured Igf1 gene expression levels by real-time PCR. We found that muscle tissue from colonized mice expressed significantly less Igf1 (Fig. S2E), suggesting muscle-derived IGF-1 may not contribute to the increased circulating IGF-1 pool.
IGF-1 produced by osteoclasts, osteoblasts, and osteocytes acting in an autocrine or paracrine manner also plays an important role in bone remodeling (47). Igf1 transcript levels are increased in bone marrow from colonized mice (Fig. 3F), but colonization status did not alter cortical or epiphyseal Igf1 or Igf1r (Fig. S3). Together, these data suggest that although colonization with gut microbiota may increase local IGF-1 production, the effect is not as strong as that on circulating IGF-1.
Fig. S3.
Igf1 and Igf1r expression in bone. Relative expression of (A) Igf1 and (B) Igf1r in epiphyseal and cortical bone tissue. ns, not statistically significant.
Effect of Microbiota on Inflammatory Cytokine Expression in Colon and Bone Marrow.
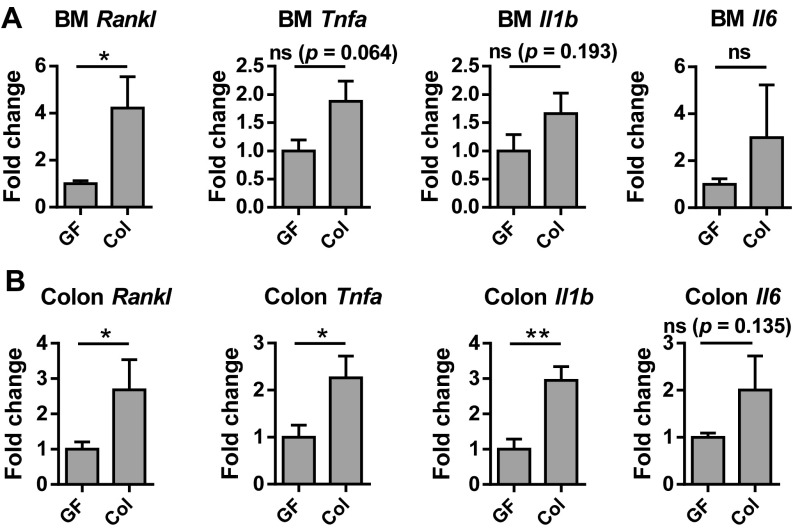
Although increased circulating IGF-1 after colonization could contribute to the transient increase in bone resorption through its promotion of osteoclast formation, effects of gut microbiota on inflammatory cytokine production may also contribute (48). Microbiota have been reported to increase TNF-α (13) and be required to increase osteoclastogenic cytokines in colon and bone marrow after sex steroid deprivation (48). Thus, we examined the effect of short-term colonization on colon and bone marrow expression of receptor activator of nuclear factor-kappaB ligand (Rankl), tumor necrosis factor alpha (Tnfa), interleukin 1 beta (Il1b), and interleukin 6 (Il6). RANKL is the master regulator of osteoclastogenesis, whereas TNF-α, IL-1β, and IL-6 are also potent stimulators of bone resorption (49, 50). Colonization significantly increased Rankl in the bone marrow, whereas increases in Tnfa, Il1b, and Il6 were not significant (Fig. S4A). In the colon, Rankl, Tnfa, and Il1b expression increased significantly after colonization (Fig. S4B). Thus, microbiota induction of pro-osteoclastogenic cytokines likely contributes to the transient increase in bone resorption seen after colonization. The relative contribution of inflammatory cytokines, IGF-1, and other mechanisms to bone loss in response to microbiota is an area for future investigation.
Fig. S4.
Effects of colonization on pro-osteoclastogenic cytokine expression in the bone marrow and colon. Relative expression of Rankl, Tnfa, Il1b, and Il6 in the (A) bone marrow and (B) colon from GF and colonized mice. *P < 0.05; **P < 0.01, ns, not statistically significant.
Broad-Spectrum Antibiotics or Vancomycin Decrease Serum IGF-1 Levels in SPF Mice.
It is possible that the differences in IGF-1 levels and bone phenotype observed in GF mice compared with colonized mice result from abnormal immune system development in GF mice (51). Further, colonization experiments do not address whether endogenous microbiota contribute to the regulation of bone remodeling under homeostatic conditions. Therefore, we tested whether broad-spectrum antibiotic treatment, which alters resident microbiota, modulates IGF-1 and bone mass. Two-month-old female SPF mice were treated with broad-spectrum antibiotics for 4 wk (Fig. 4A). Antibiotic treatment reduced levels of fecal bacteria, as determined by real-time PCR, using universal 16S rRNA primers (Fig. S5). Depletion of microbiota with antibiotics significantly reduced serum IGF-1 levels (Fig. 4B). Analogous to the reduced bone mass observed after short-term colonization, trabecular bone mass was lower in control-treated SPF mice compared with antibiotic-treated mice (Fig. 4C). Bone formation, as reflected by the serum marker P1NP, was significantly higher in SPF mice than in antibiotic-treated mice (Fig. 4D). Thus, the increased bone mass in antibiotic-treated mice is likely a result of decreased resorption, analogous to GF mice. However, no difference was seen in CTX-I levels after 1 mo (Fig. 4E). We hypothesized that alteration of microbiota affects resorption transiently, as we observed with colonization. We then measured serum CTX-I levels at baseline and 1 wk after antibiotic treatment (Fig. 4F) and found that antibiotic treatment did decrease CTX-I levels acutely (Fig. 4G).
Fig. 4.
Antibiotic treatment decreases IGF-1 and increases bone mass. (A) Experimental procedure schematic. Two-month-old BALB/c mice raised under SPF conditions were treated with either broad-spectrum antibiotics (B–E) or vancomycin (H–K) for 1 mo. Serum levels of IGF-1 after (B) antibiotic mixture (Abx) or (H) vancomycin treatment. Trabecular bone mass after treatment with (C) Abx or (I) vancomycin was determined by micro-CT. Serum P1NP concentrations after treatment with (D) Abx or (J) vancomycin. Serum CTX-I concentrations after treatment with (E) Abx or (K) vancomycin. (F) Serum was collected from SPF mice before and 1 wk after antibiotic treatment and (G) CTX-I concentration. *P < 0.05; **P < 0.01; ns, not statistically significant.
Fig. S5.
Validation of bacterial depletion efficiency after antibiotic treatment. Genomic DNA was isolated from equal amounts of fecal material of SPF and antibiotic-treated mice. Relative quantification of 16S rRNA gene copies of total bacteria were determined by qPCR, using universal 16S rRNA primers. *P < 0.05.
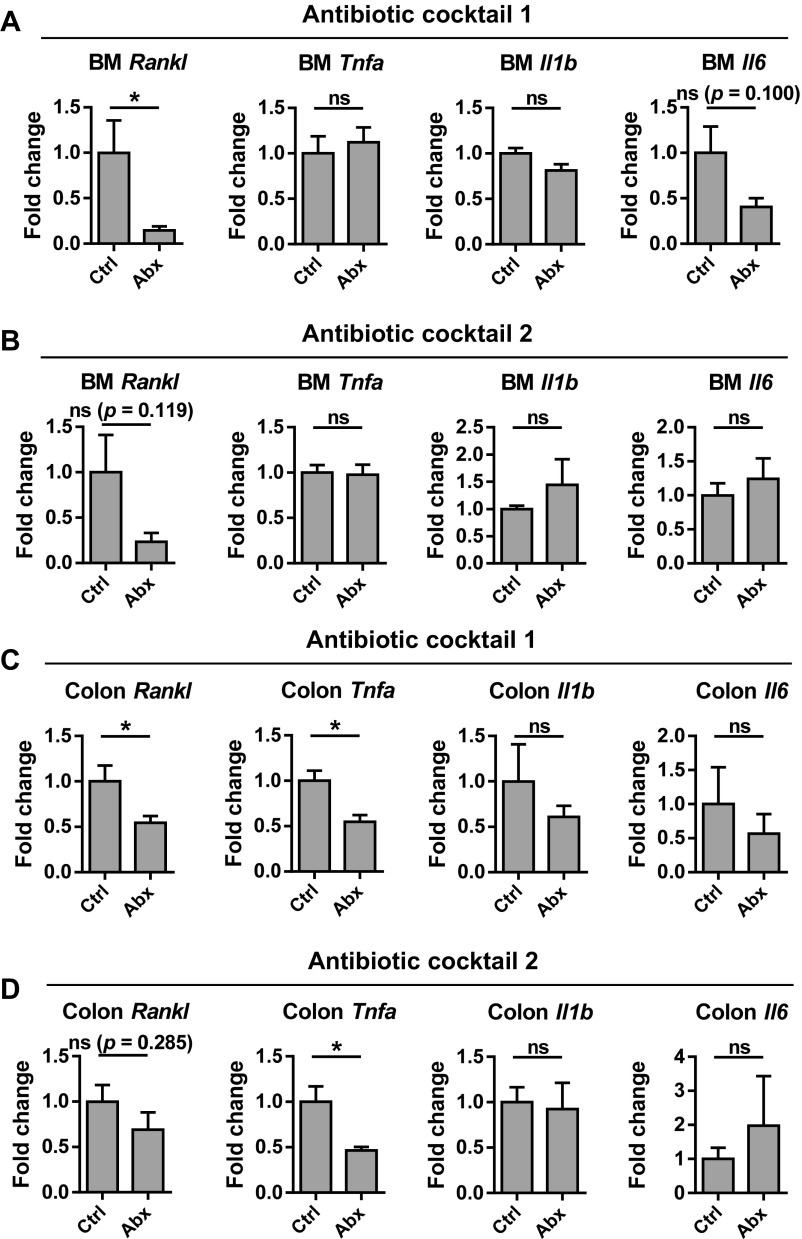
We examined the effect of broad-spectrum antibiotics on pro-osteoclastogenic cytokine expression in the colon and bone marrow and found that treatment with either of two broad-spectrum antibiotic mixtures significantly decreased the expression of the Rankl in both bone marrow and gut, whereas Tnfa expression was only altered in the gut (Fig. S6). These data suggest that regulation of these cytokines by microbiota is reversible.
Fig. S6.
Effects of antibiotic treatment on pro-osteoclastogenic cytokine expression in the bone marrow and colon. Relative expression of Rankl, Tnfa, Il1b, and Il6 in the bone marrow (A and B) and colon (C and D) of SPF mice treated with either of two antibiotic mixtures (Abx) or control treated (Ctrl). *P < 0.05; ns, not statistically significant.
Broad-spectrum antibiotics could potentially affect bone turnover in a nonspecific manner. However, treatment with oral vancomycin, which is poorly systemically absorbed, for 1 mo also reduced serum IGF-1 levels (Fig. 4H), increased bone mass (Fig. 4I), and decreased P1NP (Fig. 4J), strongly suggesting that changes in IGF-1 and bone mass in antibiotic-treated mice are a result of elimination of resident microbiota, rather than a systemic effect of antibiotics. As in mice treated with broad-spectrum antibiotics, CTX-I level in vancomycin-treated mice was similar to SPF mice after 1 mo of vancomycin (Fig. 4K). As vancomycin directly targets only Gram-positive bacteria, these results suggest that Gram-positive resident bacteria may play important roles in regulation of bone remodeling by microbiota. Together, these results demonstrate that gut microbiota contribute to the normal regulation of bone turnover and that the modulation of bone formation by microbiota is reversible in adult mice.
Cecal SCFA Concentration Is Altered by Colonization and Antibiotic Treatment.
SCFAs are the major metabolite produced by gut bacteria during fermentation of dietary fiber (52). Several Gram-positive bacteria such as Clostridium cluster XIVa species, Lactobacillus reuteri and Bifidobacterium longum, produce SCFAs as a major fermentation product (11, 52–54). Cecal content SCFA concentrations are higher in conventionally raised mice compared with in GF mice (9, 10). However, the effect size and timing of alterations in cecal SCFA after colonization is unknown. We found a trend toward increased acetate and butyrate levels in cecal contents by 1 mo after colonization (Fig. S7A) that was sustained at 8 mo (Fig. S7B). As expected, two different mixtures of broad-spectrum antibiotics significantly decreased acetate and butyrate concentrations (ref. 10 and Fig. S7C). We find that vancomycin alone was sufficient to decrease SCFA to a similar extent as broad-spectrum antibiotic therapy (Fig. S7C).
Fig. S7.
Cecal content SCFA concentration is increased by colonization and decreased by antibiotic treatment. Acetate, propionate, and butyrate concentrations in cecal contents were determined after colonization of GF mice (A and B) and after antibiotic treatment (C). (A and B) Cecal SCFA concentrations in GF mice compared with littermates colonized for 1 mo (A) or 8 mo (B). (C) Cecal SCFA concentrations in SPF mice compared with SPF mice treated with either of two broad-spectrum antibiotic mixtures (Abx 1, Abx 2) or vancomycin. *P < 0.05; ***P < 0.001; ns, not statistically significant.
SCFA Increases Systemic IGF-1 Levels and Reduces Bone Mass in Antibiotic-Treated Mice.
Because antibiotic therapy decreased SCFA, as well as IGF-1 and P1NP levels, we examined whether supplementation of antibiotic-treated SPF mice with SCFA could recapitulate the effects of colonization on IGF-1. We treated SPF mice with broad-spectrum antibiotics in drinking water for 2 wk, followed by supplementation of the antibiotic water with SCFA or control water (matched for sodium content and pH) for another 4 wk (Fig. 5A). Antibiotic treatment decreased serum IGF-1, as previously observed, whereas SCFA supplementation restored circulating IGF-1 levels to nonantibiotic-treated levels (Fig. 5B). Serum IGFBP3 was unchanged (Fig. 5B); thus, SCFA supplementation increased free IGF-1. Moreover, trabecular bone mass decreased after 4 wk of SCFA supplementation (Fig. 5C), analogous to short-term colonization. No changes in bone formation and bone resorption markers in response to SCFA were detected at this time (Fig. S8).
Fig. 5.
SCFA supplementation increases IGF-1 and decreases bone mass in antibiotic-treated mice. (A) Experimental procedure schematic. Mice were treated with broad-spectrum antibiotics for 2 wk to perturb microbiota, followed by supplementation of antibiotic water with either SCFA (Abx+SCFA) or sodium chloride control (Abx) for another 4 wk. Control (Ctrl) group received water with NaCl for 6 wk. (B) Serum IGF-1 and IGFBP3 levels. (C) Trabecular bone mass was determined by micro-CT. (D and E) Tissue IGF-1 production. (D) Abdominal fat pad and (E) liver total tissue IGF-1 (Left) was calculated by multiplying the IGF-1 production per gram tissue (Middle) by the weight of the tissue (Right). *P < 0.05; **P < 0.01; ns, not statistically significant.
Fig. S8.
SCFA supplementation of antibiotic-treated mice does not affect bone turnover markers. Bone turnover markers were measured in serum collected from the mice described in Fig. 5. (A) P1NP and (B) CTX-I levels at termination of the experiment. **P < 0.01; ns, not statistically significant.
We next examined the effect of SCFA supplementation on tissue IGF-1 production. As in Fig. 3, tissue IGF-1 is calculated by multiplying the IGF-1 content per gram tissue by the tissue weight. SCFA supplementation increased fat pad IGF-1 production compared with antibiotic-treated mice, restoring IGF-1 to the level of control mice (Fig. 5D, Left). A similar trend was seen in IGF-1 produced by the liver (Fig. 5E, Left), although the difference is not statistically significant. Similar effects of SCFA supplementation on serum and tissue IGF-1 were seen in mice treated with a second combination of broad-spectrum antibiotics (Fig. S9). No significant effect of antibiotics or SCFA supplementation on muscle Igf1 expression was seen (Fig. S10). Although other mechanisms by which gut microbiota influence bone remodeling likely exist, SCFAs are sufficient to mimic the effects of short-term colonization, resulting in increased IGF-1 and decreased bone mass.
Fig. S9.
Effects of antibiotics and SCFA on IGF-1 levels are consistent across antibiotic mixtures. SPF mice were treated as outlined in Fig. 5A, but antibiotic treatment was with antibiotic mixture 2, described in the Materials and Methods. (A) Serum IGF-1 and IGFBP3 levels. (B) Trabecular bone mass determined by micro-CT. (C and D) Tissue IGF-1 production (Left) was calculated by multiplying IGF-1 production per gram tissue (Middle) by the weight of the tissue (Right) for both liver (C) and fat pad (D). *P < 0.05; **P < 0.01; ns, not statistically significant.
Fig. S10.
Antibiotic treatment does not alter muscle Igf1 expression. Relative expression of Igf1 in the muscle of SPF mice treated with either of two antibiotic mixtures (A and B) or vancomycin (C) compared with control treated mice. ns, not statistically significant.
Discussion
Here, we demonstrate that microbiota stimulate bone formation in adult animals and report both the short- and long-term effects of gut microbiota on bone remodeling, using gnotobiotic models. Previous investigators found that colonization of GF mice with resident fecal microbiota at weaning decreased bone mass and increased osteoclastogenesis (13), suggesting that exposure to microbiota promotes bone resorption. In contrast, conventionally raised neonatal mice have higher bone mass, cortical thickness, and femur length compared with GF mice (14), which would suggest that microbiota have anabolic effects on bone. We show that exposure of GF mice to microbiota promotes both bone resorption and formation, and that the net effect of colonization varies with the duration of colonization.
We found that colonization of the adult mouse gut with SPF microbiota acutely increased bone resorption and reduced bone mass, which confirms earlier findings (13). However, colonization also increased bone formation rates and growth plate thickness within 1 mo after colonization. With longer duration of colonization, this lead to increased longitudinal and radial bone growth in colonized compared with GF mice. The increased bone resorption seen after short-term colonization resolves, and with longer duration of colonization trabecular bone mass recovers. This suggests that increased bone formation persists during long-term colonization. However, we were not able to detect increased bone formation rate by dynamic histomorphometry 8 mo after colonization. It is possible our methods are not sensitive enough to detect a true difference in bone formation, as double-label incorporation was sparse in these older animals. Alternatively, bone formation may eventually return to baseline after long-term colonization. However, the increase in femur length, circumference, and bone mass would argue that the positive effect of colonization on bone formation endures longer than its effect on bone resorption. Antibiotic treatment experiments provide supportive evidence that microbiota can affect both bone resorption and formation, as depletion of gut microbiota with antibiotics acutely decreased serum CTX-I, resulting in increased bone mass, but also decreased serum P1NP, suggesting that eliminating microbiota inhibits both bone resorption and formation. Thus, the seemingly discrepant findings of previous investigators (13, 14, 20) may be explained by our observations that microbiota affect both bone formation and resorption, and that effects of bacterial colonization are duration dependent.
Although the duration of colonization is one explanation for the seemingly paradoxical effects of colonization seen in previous studies (13, 14), differences in bacterial composition could also contribute. For example, Schwarzer et al. found that mice monoassociated with one Lactobacillus plantarum strain had femur length comparable to conventionally raised mice, whereas mice monoassociated with a different L. plantarum strain more closely resembled GF mice (14). In addition, the sex of the mice used may influence results. Sex-specific effects of treatment with antibiotics from birth have been observed. Antibiotic-treated male mice had reduced bone mineral content, whereas female mice surprisingly showed improved bone mineral content compared with controls (20, 55). Similarly, although we found increased bone growth after long-term colonization in both sexes, a more dramatic effect was observed in male mice. It is possible that differences in either microbiota composition or host response to microbiota are driven by sex hormones or other sex-specific factors.
One possible mechanism by which microbiota could promote bone formation in adult mice is by increasing serum IGF-1 levels. We found that colonization of adult mice was sufficient to increase serum IGF-1 twofold compared with GF siblings. As many factors, including age and nutrition (56, 57), affect IGF-1 levels, we used sibling controls fed identical diets to demonstrate that microbiota colonization increases serum IGF-1. This is further supported by our antibiotic treatment studies, in which sterilization of the gut decreased IGF-1. Although circulating IGF-1 is made primarily by the liver, IGF-1 synthesis is ubiquitous. Colonization increased both liver and adipose tissue IGF-1 production in our studies. In Drosophila, colonization enhanced activity of Drosophila insulin/IGF-like peptide in the fat body, a liver- and adipose-like organ (29), suggesting that the pathways by which microbiota influence IGF-1 production are likely highly conserved. It is possible that paracrine/autocrine actions of locally produced IGF-1 are also modulated by colonization, as we also detected increased Igf1 transcription in the bone marrow. Our study strengthens the link between gut microbiota and IGF-1 by demonstrating that manipulation of the microbiota in adult mice alters serum IGF-1 levels, with durable effects observed after long-term colonization.
IGF-1 may contribute to the acute increase in bone resorption seen after colonization, as IGF-1 promotes osteoclast differentiation (37). Consistent with this, SCFA supplementation of antibiotic-treated mice both increased IGF-1 and reduced bone mass similarly to colonization. Additional possible mediators of the acute increase in bone resorption after colonization include serotonin and sex steroids. It has been reported by several groups that microbiota induce serotonin in the gut (3, 13). However, the majority of reports suggest that the absence of gut serotonin production in Tph1−/− mice has little effect on bone physiology (58–60). Using uterus weight as a surrogate for estrogen status, no difference was observed between GF and conventionally raised mice (13). Alternatively, microbiota may affect bone resorption indirectly, via modulation of the immune system. Pro-osteoclastogenic TNF-α- and IL-17-producing CD4 T cells are associated with inflammatory-bowel-disease–induced bone loss (61), and it has been reported that conventionally raised mice have more bone marrow CD4 T cells and TNF-α expression than GF mice (13, 48). Moreover, microbiota might alter bone resorption through effects on B-cell production of osteoprotegerin (62), an inhibitor of osteoclast formation, as microbiota are known to affect B-cell development (63). The most consistent effect we observed on pro-osteoclastogenic cytokine expression was the increase in Rankl expression in bone marrow and colon after colonization, which is consistent with Li et al. (27). We also found that colonization and antibiotic treatment altered inflammatory cytokine expression in the colon, although we observed less dramatic effects in the bone marrow. The extent to which the immune system contributes to bone remodeling after colonization can be examined by testing the requirement for specific immune cells or inflammatory cytokines in the response of bone to microbiota.
Colonization increased IGF-1 and bone formation, measured both by P1NP and dynamic histomorphometry, whereas depletion of microbiota with either broad-spectrum antibiotics or vancomycin reduced IGF-1 levels and decreased serum P1NP. These data suggest that IGF-1 may link microbiota and bone formation. Supporting this hypothesis, Schwarzer et al. found that treating GF neonates with recombinant IGF-1 was sufficient to mimic the effects of colonization on skeletal growth, whereas an IGF-1 receptor inhibitor inhibited postnatal bone growth in colonized animals (14), but no mechanism linking exposure to microbiota and changes in IGF-1 levels was proposed.
Because many Gram-positive bacteria produce SCFA as a major fermentation product (52–54), and perturbation of the gut microbiota with vancomycin was sufficient to decrease IGF-1 and P1NP levels, we focused on SCFA production as a candidate mediator of these effects. We found that antibiotic treatment, including treatment with vancomycin alone, significantly decreased SCFA concentrations in the cecum, although there was a trend toward increased SCFA within 1 mo after colonization of GF animals. SCFA supplementation of antibiotic-treated mice recapitulated the effects of colonization on serum and tissue IGF-1, as well as bone mass. SCFA do not completely recapitulate the bone phenotype caused by colonization with resident bacteria, however, as serum P1NP was not significantly changed at the point examined. Further studies using GF mice treated with SCFA or mice lacking SCFA receptors may facilitate a better understanding of the effects of SCFA on bone. Taken together, our data suggest that gut resident-bacteria-derived SCFAs mediate microbiota induced changes in host IGF-1 levels and contribute to the effects of colonization on bone turnover.
The gut microbiota have pleiotropic effects on host physiology across the life course. We demonstrate that microbiota affect bone remodeling in the adult, with effects on both bone formation and resorption. Our findings have clinical relevance in understanding the potential for deleterious effects on bone from repeated or prolonged antibiotic exposure or dysbiosis. In addition, manipulation of the microbiome with pre- and probiotics is gaining public attention, and fecal transplants and other more selective therapeutic interventions targeting the gut microbiome are likely to become increasingly common as understanding of the role of microbiome in disease advances. Elucidating the connection between gut microbiota and bone turnover is an important contribution to our understanding of how these manipulations may affect bone. Ultimately, determining the mechanism by which microbiota promotes bone formation could lead to new therapeutic strategies to promote bone health.
Materials and Methods
Animals.
For colonization experiments, 2-mo-old sex matched GF F1 hybrid CB6F1 mice were generated from female BALB/c and male C57BL/6 mice housed in isolators in the National Gnotobiotic Rodent Resource Center, University of North Carolina. Littermates were used when possible, or siblings from multiple breeders were combined and randomly assigned before assignment to experimental groups. For antibiotic and SCFA treatments, 2-mo-old female BALB/c mice (Jackson) were used and housed under SPF conditions. All animal procedures were approved by the Institutional Animal Care and Use Committee, Harvard Medical School, and the University of North Carolina for the gnotobiotic experiments.
Colonization of GF Mice.
Feces were collected from 3-mo-old male CB6F1 SPF mice from the National Institute on Aging (Charles River), aliquoted, snap frozen, and stored at −80 °C. Testing for mouse pathogens was negative (Charles River Labs). A slurry of 10 fecal pellets in 5 mL sterile PBS was used for colonization. Two-month-old GF mice were transferred to a SPF barrier facility on the day of colonization. Sterile cotton swabs were used to inoculate the mouth and anus with fecal material, and additional slurry was wiped on the abdomen of the mouse.
Antibiotics and SCFA Treatment.
Mice were pooled and randomly assigned to treatment group to minimize cage effects. For antibiotic treatment experiments, mice were fed with antibiotics in the drinking water for 1 mo. Antibiotic treatment was either a mixture of antibiotics (1 mg/mL ampicillin, 0.5 mg/mL vancomycin, 1 mg/mL metronidazole, and 1 mg/mL neomycin) or vancomycin (0.5mg/mL) alone. Perturbation of microbiota was verified by quantitative real-time PCR, using universal 16S rRNA primers. In all antibiotic-mixture-treated groups, ∼99% of fecal bacteria were eliminated by antibiotic treatment. For the SCFA supplementation experiments, mice were first treated with an antibiotic mixture for 2 wk to deplete microbiota, and then either SCFA or sodium control (150 mM sodium chloride) was added to the antibiotic water for another 4 wk. This SCFA mixture (67.5 mM acetate, 40 mM butyrate, 25.9 mM propionate) has been proven to be physiologically relevant (9). A second mixture of antibiotics (0.2 mg/mL gentamicin, 0.15 mg/mL ciprofloxacin, 2 mg/mL streptomycin, and 1 mg/mL bacitracin) (12) was used to validate the effects of antibiotics and SCFA treatment on IGF-1. Following the policy of the animal facility, 3% (g/100 mL) sucrose was added to all water to ensure the palatability. Water solutions were prepared freshly and were changed twice a week (9).
Micro-CT.
After serial fixation in 4% (g/100 mL) paraformaldehyde and 70% (vol/vol) ethanol, femurs were scanned using a μCT 35 (Scanco Medical AG). Scans were conducted in 70% ethanol using a voxel size of 7 µm, X-ray tube potential of 55 kVp, intensity of 0.145 Ma, and integration time of 600 ms. A region beginning 0.35 mm proximal to the growth plate and extending 1 mm proximally was selected for trabecular bone analysis. A second region 0.6 mm in length and centered at the midpoint of the femur was used to calculate diaphyseal parameters. The region of interest was thresholded using a global threshold that set the bone/marrow cutoff at 352.3 mg HA/cm3 for trabecular bone and 589.4 mg HA/cm3 for cortical bone. 3D microstructural properties of bone were calculated using software supplied by the manufacturer. Bone length was measured using the scout view feature.
Histomorphometry.
Dynamic bone formation parameters were evaluated by histomorphometry after sequential skeletal labeling with calcein (20 mg/kg) and demeclocycline (40 mg/kg) at 7 and 2 d before harvest. For histomorphometry, the left femur was dissected and fixed in 70% ethanol. Undecalcified bones were processed for plastic embedding with four changes of infiltration solution [95% (vol/vol) methyl methacrylate and 5% (vol/vol) dibutyl phthalate] at 3-d intervals. After infiltration, samples were embedded in a solution of 95% methyl methacrylate and 5% dibutyl phthalate, with 0.25% Perkodox as the initiator and exposed to UV light for polymerization. Analysis of bone formation after short-term colonization was performed on 5-µm-thick coronal sections obtained from fully polymerized (plasticized) sample blocks. Dynamic bone formation indices after long-term colonization were determined for both the endosteal and periosteal envelopes of cortical bone, using a 30-μm ground section from the tibial–fibular junction. Dynamic bone parameters were measured using the Bioquant Osteo (BIOQUANT Image Analysis Corporation) by two observers who were blinded to treatment. Histomorphometric analyses were performed according to the guidelines of the American Society of Bone and Mineral Research histomorphometry nomenclature committee (64) by the Histomorphometry and Molecular Analysis Core in University of Alabama. MAR is the average distance between the two labels divided by the time interval between labeling. BFR/BS is the volume of mineralized bone formed per unit time and per unit bone surface. Mineralizing surface per bone surface (MS/BS) represents the percentage of bone surface that is actively mineralizing. BFR/BS is the product of MAR and MS/BS [BFR = MAR × (MS/BS)]. For the short-term colonization, reported MAR and BFR/BS represent data pooled from two independent experiments normalized to the mean value of the GF group within each experiment.
Growth Plate Thickness Measurement and Toluidine Blue Staining.
The growth plate thickness was measured in 3D reconstructions of micro-CT images and in histologic sections, using the image pro plus software (Media Cybernetics). Hematoxylin and eosin staining was used to show the histology of the growth plate, and 0.4% (g/100 mL) Toluidine blue staining was used to stain glycoproteins and visualize growth plate.
RNA Isolation and Real-Time PCR.
Frozen liver, fat pad, gastrocnemius muscle, bone, and bone marrow were homogenized in ice-cold TRIzol, using bullet blender beads (Next Advance), and RNA was isolated using a standard TRIzol protocol. For bone RNA, samples obtained after TRIzol extractions were further purified using the RNeasy Plus Micro Kit with on-column genomic DNA-digest (Qiagen). An Affinity Script quantitative PCR (qPCR) cDNA Synthesis Kit (Agilent Technologies) was used to generate cDNA. Real-time PCR was performed on StepOne Plus Realtime PCR Machine (Applied Biosystems), using Fast SYBR Green Master Mix (Thermo Fisher Scientific). Gene expression was determined relative to the housekeeping gene TATA box binding protein (Tbp). Fold change of experiment groups compared with control group was calculated by the delta-delta-Ct method. Real-time PCR primer sequences are listed in Table S1.
Table S1.
Sequences of real-time PCR primers
| Gene | Forward primer | Reverse primer |
| Igf1 | GTCGTCTTCACACCTCTTCTACCT | GCACAGTACATCTCCAGTCTCCT |
| Igfbp3 | CGATTCCAAGTTCCATCCACTCCAT | ACCGTATTCTGTCTCCCGCTTAGA |
| Igf1r | CTCAGGCTTCATCCGCAACAG | GTTCTCCAACTCCGAGGCAATG |
| Runx2 | TACAACCATACCCAGTCCCTGTTT | AGTGCTCTAACCACAGTCCATGCA |
| Rankl | CAGCATCGCTCTGTTCCTGTA | CTGCGTTTTCATGGAGTCTCA |
| Tnf | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Il1b | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| Il6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| Tbp | CAGCCTCAGTACAGCAATCAACAT | CAGCCAAGATTCACGGTAGATACAA |
| Eubacteria (all bacteria) | ACTCCTACGGGAGGCAGCAGT | ATTACCGCGGCTGCTGGC |
Tissue IGF-1 Detection.
Tissues from liver and fat pad were weighed and homogenized in lysis buffer (PBS, 1% Triton ×100 containing a protease inhibitor mixture; Roche), using bullet blender beads as described earlier. Lysates were centrifuged, and supernatants were used for IGF-1 ELISA.
ELISA for Serum IGF-1, IGFBP3, GH, CTX-I, and P1NP.
Serum was prepared from blood samples collected by cardiac puncture, using serum separation tubes (Becton Dickinson) and assayed for IGF-1 and IGFBP3, using the Murine IGF-1 Standard ABTS ELISA Development Kit (PeproTech) and the IGFBP3 duoset ELISA kit (R&D systems), respectively. GH levels were measured with the Rat/mouse growth hormone ELISA kit (EMD Millipore). Serum C-terminal telopeptides (CTX-I) and Procollagen type 1 propeptides (PINP) were quantified using EIA assays (Immunodiagnostics Systems). All ELISAs were read with Synergy H1 Hybrid Reader (Biotek).
SCFA Measurement.
Cecal contents were collected immediately after animals were killed, flash frozen, and stored at −80 °C until extraction. Thawed cecal contents were homogenized in HPLC-grade H2O, centrifuged to remove debris, and passed through a 0.22-μm filter. Extraction of SCFA was performed as in ref. 44. Briefly, volatile compounds, including the internal standard (valeric acid), were acidified, extracted with diethyl ether, and back-extracted into sodium phosphate buffer. SCFAs in reacidified samples were then analyzed, using an Agilent 1200 series HPLC equipped with a Poroshell 120 SB C18 column and 0.01 M sulfuric acid as the mobile phase, and quantified as described in ref. 9.
Bacterial Genomic DNA Extraction and Real-Time PCR.
Bacterial genomic DNA was extracted from 100 mg fecal material, using the Qiagen stool kit, according to the manufacturer’s instructions, with the optional high-temperature step. The abundance of commensal bacteria was quantified by real-time PCR, using the universally conserved 16S rRNA primer pair listed in Table S1.
Statistical Analysis.
All data are shown as mean ± SEM. Paired t test was used for Fig. 4G. For all other figures, differences between groups were compared by Mann–Whitney U test or one-way ANOVA with Kruskal-Wallis post hoc test, as appropriate. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not statistically significant.
Acknowledgments
We thank the Pathology Core Research Laboratory at the Department of Pathology, University of Alabama at Birmingham, for histomorphometric analysis, Wenhan Chang for helpful discussions, and Hank Kronenberg for comments on the manuscript. This work was supported by NIH grants K08 AR062590 (to J.F.C.), R01 AG046257 (to J.F.C. and A.O.A.), and R01 CA154426 (to W.S.G.). Additional support was provided by the Rheumatology Research Foundation (J.F.C.), the Bettina Looram Fund (J.F.C.), and a Hoffman-LaRoche research grant (W.S.G.). C.A.B. is the Dennis and Marsha Dammerman fellow of the Damon Runyon Cancer Research Foundation (DRG-2205-14). Gnotobiotic studies were performed at the National Gnotobiotic Rodent Resource Center at University of North Carolina at Chapel Hill (funding agencies 5-P30-DK034987 and 5-P40-OD010995 and Crohn’s and Colitis Foundation of America).
Footnotes
Conflict of interest statement: W.S.G. is a Scientific Advisory Board member of Evelo Therapeutics and Synlogic and consults for Janssen Pharmaceuticals. J.F.C. receives author royalties from Up To Date, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607235113/-/DCSupplemental.
References
- 1.Bäckhed F, et al. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Clarke G, et al. Minireview: Gut microbiota: The neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieuwdorp M, Gilijamse PW, Pai N, Kaplan LM. Role of the microbiome in energy regulation and metabolism. Gastroenterology. 2014;146(6):1525–1533. doi: 10.1053/j.gastro.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Bäckhed F, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharon G, et al. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014;20(5):719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjogren K, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzer M, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 15.Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackie EJ, Tatarczuch L, Mirams M. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211(2):109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 17.Martes C, Karampouta E, Lazarides N. [Indications for the use of saggittal splitting (based on 148 cases)] Stomatologia (Athenai) 1975;36(4):183–190. [PubMed] [Google Scholar]
- 18.Charles JF, Aliprantis AO. Osteoclasts: More than ‘bone eaters’. Trends Mol Med. 2014;20(8):449–459. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charles JF, Ermann J, Aliprantis AO. The intestinal microbiome and skeletal fitness: Connecting bugs and bones. Clin Immunol. 2015;159(2):163–169. doi: 10.1016/j.clim.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228(8):1793–1798. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britton RA, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229(11):1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCabe L, Britton RA, Parameswaran N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr Osteoporos Rep. 2015;13(6):363–371. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, et al. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology. 2015;156(9):3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. Scientific World Journal. 2014;2014:595962. doi: 10.1155/2014/595962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–130. doi: 10.1007/s11914-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyun S. Body size regulation and insulin-like growth factor signaling. Cell Mol Life Sci. 2013;70(13):2351–2365. doi: 10.1007/s00018-013-1313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erkosar B, Storelli G, Defaye A, Leulier F. Host-intestinal microbiota mutualism: “learning on the fly”. Cell Host Microbe. 2013;13(1):8–14. doi: 10.1016/j.chom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Yakar S, Courtland HW, Clemmons D. IGF-1 and bone: New discoveries from mouse models. J Bone Miner Res. 2010;25(12):2543–2552. doi: 10.1002/jbmr.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulzele K, Clemens TL. Novel functions for insulin in bone. Bone. 2012;50(2):452–456. doi: 10.1016/j.bone.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, et al. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011;26(7):1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, et al. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22(9):1329–1337. doi: 10.1359/jbmr.070517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulzele K, et al. Disruption of the insulin-like growth factor type 1 receptor in osteoblasts enhances insulin signaling and action. J Biol Chem. 2007;282(35):25649–25658. doi: 10.1074/jbc.M700651200. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, et al. Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res. 2006;21(9):1350–1358. doi: 10.1359/jbmr.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jilka RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juul A, et al. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: The relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab. 1995;80(8):2534–2542. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y. Essential roles of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the liver. Endocr J. 2012;59(11):955–962. doi: 10.1507/endocrj.ej12-0322. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111(45):16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klöting N, et al. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes. 2008;57(8):2074–2082. doi: 10.2337/db07-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schieber AM, et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science. 2015;350(6260):558–563. doi: 10.1126/science.aac6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamrick MW. The skeletal muscle secretome: An emerging player in muscle-bone crosstalk. Bonekey Rep. 2012;1:60. doi: 10.1038/bonekey.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banu J, Wang L, Kalu DN. Effects of increased muscle mass on bone in male mice overexpressing IGF-I in skeletal muscles. Calcif Tissue Int. 2003;73(2):196–201. doi: 10.1007/s00223-002-1072-z. [DOI] [PubMed] [Google Scholar]
- 46.Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18(1):221–223. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Bikle DD, Chang W. Autocrine and Paracrine Actions of IGF-I Signaling in Skeletal Development. Bone Res. 2013;1(3):249–259. doi: 10.4248/BR201303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamrick B. RSO interview with Barbara Hamrick. Interview with a radiation safety officer. Health Phys. 2012;103(2) Suppl 2:S121–S123. doi: 10.1097/HP.0b013e31825bd4c2. [DOI] [PubMed] [Google Scholar]
- 49.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: Cytokines and the skeletal system. BMB Rep. 2008;41(7):495–510. doi: 10.5483/bmbrep.2008.41.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J Clin Invest. 2011;121(7):2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 52.den Besten G, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 54.Stewart ML, Savarino V, Slavin JL. Assessment of dietary fiber fermentation: Effect of Lactobacillus reuteri and reproducibility of short-chain fatty acid concentrations. Mol Nutr Food Res. 2009;53(Suppl 1):S114–S120. doi: 10.1002/mnfr.200700523. [DOI] [PubMed] [Google Scholar]
- 55.Cox LM, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashpole NM, et al. IGF-1 regulates vertebral bone aging through sex-specific and time-dependent mechanisms. J Bone Miner Res. 2016;31(2):443–454. doi: 10.1002/jbmr.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steves CJ, Bird S, Williams FM, Spector TD. The microbiome and musculoskeletal conditions of aging: A review of evidence for impact and potential therapeutics. J Bone Miner Res. 2016;31(2):261–269. doi: 10.1002/jbmr.2765. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chabbi-Achengli Y, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci USA. 2012;109(7):2567–2572. doi: 10.1073/pnas.1117792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brommage R, et al. Adult Tph2 knockout mice without brain serotonin have moderately elevated spine trabecular bone but moderately low cortical bone thickness. Bonekey Rep. 2015;4:718. doi: 10.1038/bonekey.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciucci T, et al. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut. 2015;64(7):1072–1081. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Terauchi M, Vikulina T, Roser-Page S, Weitzmann MN. B cell production of both OPG and RANKL is significantly increased in aged mice. Open Bone J. 2014;6:8–17. doi: 10.2174/1876525401406010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wesemann DR. Microbes and B cell development. Adv Immunol. 2015;125:155–178. doi: 10.1016/bs.ai.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Dempster DW, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]