Structured abstract
Purpose of review
This review article focuses on the most significant cardiovascular complications in dialysis patients (sudden cardiac death, acute coronary syndromes, heart failure, and atrial fibrillation).
Recent findings
Current and ongoing research aims to quantify the rate and pattern of significant arrhythmia in dialysis patients and to determine the predominant mechanism of sudden cardiac death. Preliminary findings from these studies suggest a high rate of atrial fibrillation and that bradycardia and asystole may be more frequent than ventricular arrhythmia as a cause of sudden death. A recently published matched cohort study in dialysis patients who received a defibrillator for primary prevention showed that there was no significant difference in mortality rates between defibrillator -treated patients and propensity matched controls. Two randomized controlled trials are currently recruiting participants and will hopefully answer the question whether implantable or wearable cardioverter-defibrillators can prevent sudden cardiac death. An observational study using USRDS data demonstrated how difficult it is to keep hemodialysis patients on warfarin, as more than two thirds discontinued the drug during the first year. The ISCHEMIA-CKD trial may provide answers about the optimal strategy for treatment of atherosclerotic coronary disease in patients with advanced CKD.
Summary
The article reviews diagnosis of acute coronary syndromes in dialysis patients, current literature on myocardial revascularization, and data on fatal and non-fatal cardiac arrhythmia. The new classification of heart failure in end-stage renal disease is reviewed. Finally, available cohort studies on warfarin for stroke prevention in dialysis patients with atrial fibrillation are reviewed.
Keywords: end-stage renal disease, sudden cardiac death, acute coronary syndromes, heart failure, atrial fibrillation
Introduction
Cardiovascular complications are the leading cause of morbidity and mortality among dialysis patients. This review article will focus on the most common and significant complications, namely sudden cardiac death (SCD), acute coronary syndromes (ACS), heart failure, and atrial fibrillation (AF).
Sudden cardiac death
SCD is traditionally defined as “death from a cardiac cause within one hour from symptom onset in an otherwise well individual” [1]. In practice, unwitnessed death without alternative cause is also frequently categorized as SCD. However, the latter category is likely to include many deaths that are sudden but that are caused by stroke, embolism, or causes other than a fatal, primary arrhythmia. Nevertheless, as commonly defined, SCD appears to be responsible for the majority of all deaths on dialysis. SCD accounted for approximately 25% of all deaths in dialysis patients in the 2010 United States Renal Data System (USRDS) report [2]. Findings in a post-hoc analysis of the EVOLVE trial, a large randomized study enrolling 3883 hemodialysis patients, were similar- cardiovascular causes were responsible for 54% of deaths and SCD accounted for 24.5% [3]. Similar event rates have been observed in the 4D trial and the CHOICE cohort [4–5], as well as in peritoneal dialysis patients [6].
Primary event in SCD
The pathophysiology of SCD in dialysis patients has not been elucidated. As noted above, an arrhythmic event may not be the underlying mechanism in at least a minority of cases. Furthermore, whether the majority of arrhythmic deaths are due to shockable rhythms such as ventricular tachycardia and ventricular fibrillation as opposed to non-shockable rhythms such as asystole or bradycardia is currently unknown. To wit, a retrospective observational study including hemodialysis patients using a wearable cardioverter defibrillator following an initial cardiac arrest identified 75 patients who experienced at least one SCD event [7]. Sixty-four percent of the initial rhythms were ventricular tachycardia and 14% ventricular fibrillation. However, ventricular events could be over-represented in this cohort due to selection bias. Conversely, a prospective study from Australia enrolled 50 stable hemodialysis patients with left ventricular ejection fraction (LVEF) ≥ 35% and no history of syncope or ventricular tachyarrhythmias [8]. An implantable cardiac monitor was used for continuous electrocardiographic monitoring and arrhythmic events were automatically recorded if they fulfilled certain predefined criteria. Five SCD occurred after a mean follow-up of 12 months. Although one patient experienced a sustained ventricular tachycardia, the others experienced severe bradycardia with ensuing asystole as the terminal event. This small study suggests that bradycardia and asystole (primary or secondary) rather than ventricular tachyarrhythmia is the electrical event underlying the majority of SCD. However, it should be recognized that non-cardiac deaths such as massive stroke, hemorrhage, or embolism ultimately lead to terminal asystole, and electronic monitoring alone cannot distinguish between primary asystole causing death and terminal asystole from non-cardiac death.
Similarly, the MiD study (NCT01779856) enrolled 81 patients with stage 5 chronic kidney disease (CKD) on hemodialysis or expected to initiate hemodialysis within 2 months [9]. A loop recorder was implanted and follow-up continued for a maximum of 12 months. The primary objective was to estimate the incidence of clinically significant arrhythmias and characterize those arrhythmic events. Although final results are pending, preliminary data presented at the American Society of Nephrology meeting in 2014 suggested that bradycardia and asystole were much more frequently detected than the sustained ventricular tachycardia [10].
Risk factors
Risk factors like coronary artery disease (CAD), heart failure with reduced ejection fraction or left ventricular hypertrophy, are highly prevalent among dialysis patients [3,11] and may provide an ideal myocardial substrate for the propagation of arrhythmia when triggered by inciting factors that are common such as metabolic abnormalities, electrolyte and fluid shifts, and ischemia that occur during dialysis or during the inter-dialytic interval [1].
Dialysate potassium concentration was examined in a large case-control study [12]. Low potassium dialysate (<2 mmol/l) was independently associated with SCD in a multivariable logistic regression model. The higher risk became more pronounced as pre-dialysis serum potassium decreased. Dialysate calcium < 2.5 mmol/l was also independently associated with SCD in adjusted models [13].
Hemodialysis itself has also been associated with acutely reduced myocardial blood flow, even in patients without angiographically significant stenosis of the coronary vessels [14]. Whether dialysis-induced ischemic myocardial stunning can trigger a malignant arrhythmia or is rather a predisposing factor contributing in the development of heart failure in dialysis patients is uncertain but seems probable. Lastly, volume shifts may lead to atrial and ventricular stretch from pre-dialysis volume overload or ischemia when rapid ultrafiltration leads to under filling and hypotension. Whether volume shifts cause SCD is uncertain but associations between rapid ultrafiltration and the risk of death suggest this may be possible [15].
Prevention of SCD
Implantable cardioverter defibrillators (ICDs) are recommended in patients with preserved renal function for secondary or primary prevention of SCD (when left ventricular ejection fraction is severely reduced) [16]. Evidence is lacking in dialysis patients. However, use of ICDs is increasing in the United States, especially for primary prevention [17].
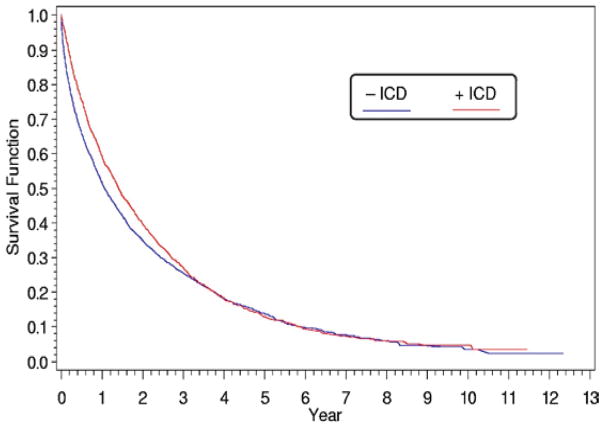
A retrospective evaluation of USRDS data showed that long-term dialysis patients who had an ICD for likely secondary prevention had an estimated 14% mortality decrease compared with propensity-matched controls [17]. However, the survival curves converged at 3 years of follow-up (Figure 1). Furthermore, the infection rate was elevated, 98.8 events per 100 patient-years during the first year and 63.9 thereafter, and bacteremia incidence was almost 52 cases per 100 patient-years. All-cause mortality was very high in this cohort (45 deaths per 100 patient-years) and 38% of deaths were attributed to arrhythmias despite ICD insertion.
Figure 1.
(previously published). Survival of patients who received an implantable cardioverter defibrillator for secondary prevention compared with matched cohort. Full source details: Figure 2, page 413 from Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis 2011; 58:409–417.
A retrospective cohort study including all dialysis patients who received an ICD between 2006 and 2007 for primary prevention for an evidence-based indication showed that there was no significant difference in one and three-year mortality rates between ICD-treated patients and propensity matched controls [18]. The ICD2 study is a randomized controlled trial (RCT) that will prospectively evaluate the use of ICDs for SCD prevention in dialysis patients (ISRCTN 20479861) [19]. The WED-HED study is an RCT evaluating the impact of a wearable cardioverter defibrillator for SCD prevention in incident dialysis patients (NCT02481206). Both studies are currently recruiting participants.
Other interventions have been evaluated in an attempt to lower the risk of SCD in end-stage renal disease (ESRD). Although beneficial in the general population, β-blockers were not associated with reduced incidence of SCD in a post-hoc analysis of the HEMO study [20], but a small randomized study of carvedilol in dialysis patients with heart failure did detect a reduction in cardiovascular deaths and a trend toward a reduction in SCD [21]. Other modifiable practices associated with SCD, as identified in the DOPPS trial, include the dialysate potassium concentration (>2.5 mmol/l), the dialysis prescription (treatment time ≥ 210 min, Kt/V ≥ 1.2), the ultrafiltration volume (≤5.7%), or amiodarone avoidance [15], but each remains unproven.
Acute coronary syndromes and myocardial revascularization
ACS is a frequent cardiovascular event in dialysis patients. According to USRDS data, approximately 17% of deaths in ESRD are attributable to ACS. CAD prevalence in the same population was 36%, but some estimates suggest that >60% of new dialysis patients have evidence of coronary atherosclerosis [22–23]. Results from the GRACE registry showed that non-ST segment elevation myocardial infarction is the most common presentation for ACS in dialysis patients [24]. Whether this represents demand ischemia (myocardial infarction type 2) or is related to atherosclerotic plaque rupture is still unclear. Despite the high prevalence of non-ST compared with ST segment elevation myocardial infarction, mortality rates are very high- 59% at one year, 73% at two years, and 90% at five years in one study [25]. Similarly, in the GRACE registry, in-hospital mortality and clinical outcomes at 6 months (death, recurrent infarction, or unplanned hospital readmission) after an ACS were significantly worse among dialysis patients compared with non-dialysis patients [24].
Diagnosis
ACS diagnosis may be challenging in dialysis patients [22]. Chest pain is absent on admission in more than 50% of dialysis patients who present with an ACS, likely due to autonomic and/or uremic neuropathy [26–27].
Interpretation of troponin values may also be problematic because these patients frequently have elevated troponin levels in the absence of clinical ischemia, significantly affecting the specificity for the diagnosis of acute infarction [28]. Nevertheless, elevated troponin levels in CKD patients with or without suspected ACS are associated with higher risk for subsequent major adverse cardiovascular events [28–29]. For the diagnosis of ACS among dialysis patients, the National Academy of Clinical Biochemistry recommends a dynamic increase in troponin levels of >20% within 9 hours and at least one value exceeding the 99th percentile [30]. Clearly a high index of suspicion may be necessary to avoid missing the diagnosis of ACS in this population.
Management
Secondary preventive measures, as use of aspirin, angiotensin converting enzyme inhibitors (ACEIs), β-blockers, or statins, are not applied in the majority of dialysis patients [31,32]. Coronary angiography and coronary revascularization are also underutilized across the spectrum of CKD, possibly in an attempt to avoid contrast-induced nephrotoxicity, an approach referred to as renalism [33,34,35]. However, conservative approaches appear to be associated with a significantly higher one-year mortality [34]. Although, confounding by indication cannot be ruled out in the majority of retrospective analyses, it seems reasonable to treat ACS in dialysis patients according to the standard guidelines used for non-dialysis patients (Table 1) [22].
Table 1.
Management of acute coronary syndromes in dialysis patients. STEMI, ST-segment elevation myocardial infarction; NSTE-ACS, non ST-segment elevation acute coronary syndrome
| For all patients | Oxygen |
| Aspirin | |
| β-blockers | |
| Statins | |
| Renin-angiotensin system blockade | |
| If STEMI | Reperfusion therapy: percutaneous coronary intervention (or thrombolytics) |
| ADP receptor blocker: clopidogrel, prasugrel, or ticagrelor | |
| If NSTE-ACS | Nitrates for symptom control |
| P2Y12 inhibition, preferably with ticagrelor | |
| Anticoagulation: fondaparinux, bivalirudin, heparin, or enoxaparin | |
| Consider coronary angiography +/− revascularization based on individual risk profile |
Myocardial revascularization
There is only one RCT comparing myocardial revascularization, either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), to medical treatment in ESRD [36]. The primary outcome, unstable angina, myocardial infarction, or cardiac death, occurred in 10/13 medically treated patients (3 deaths) and 2/13 revascularized patients (no deaths) (p=0.002). However, both medical therapy and revascularization techniques have radically changed in the decades since this small study was published raising questions about its contemporary relevance. The ISCHEMIA-CKD Trial (NCT01985360) may provide answers about the optimal strategy for treatment of atherosclerotic coronary disease in patients with advanced CKD.
One caveat is that the majority of new techniques have not been tested in the dialysis population. Drug eluting stents, for example, hold promise as an alternative to surgical revascularization or bare metal stents, but retrospective analyses are inconclusive about their benefits in uremia. For example, in a propensity score-matched cohort, 2-year mortality, myocardial infarction, and target vessel revascularization rates were similar with both types of stents [37]. Similarly, there is no RCT directly comparing CABG to PCI in ESRD. Nevis et al. systematically reviewed seventeen retrospective cohort studies conducted between 1977 and 2002 [38]. Although significant heterogeneity was identified, CABG was associated with a higher short-term mortality (30 days or in hospital) compared with PCI (10.6% vs. 5.4%, p<0.001). However, CABG was superior to PCI for long-term mortality (at least 1 year): 51.6% vs. 59.5%, p=0.01) and other cardiac events (defined as myocardial infarction or repeat revascularization) [38]. Although many of the included studies were completed prior to the adoption of modern practice patterns, a second meta-analysis, published in 2011 and including three more recent trials, yielded similar results [39]. Registry data in the United States (1997–2009) and the CREDO-Kyoto registry in Japan (2005–2007) have also confirmed the superiority of CABG to PCI for long-term clinical outcomes in dialysis patients, especially for multi-vessel procedures [40,41].
Given the absence of randomized data, the very high peri-operative risks of surgical revascularization in dialysis patients (surgical mortality rates may exceed 10% [42]), and the high overall mortality rates in the dialysis population, an individualized, patient-centered approach should be considered. CABG may not be the best option in those with significant comorbidities who are not expected to live long enough to reap a long-term benefit from CABG either due to comorbidities or peri-operative risk. These patients may be better with medical therapy or percutaneous options. Conversely, otherwise healthy patients may be best treated with CABG [43].
Heart failure in end-stage renal disease
Patients with ESRD have a high incidence of structural heart disease [44]. However, heart failure in dialysis patients is poorly characterized and not optimally treated because the dialytic cycle of volume accumulation between sessions and intra-dialytic extracorporeal ultrafiltration mask the clinical presentation of the underlying heart disease. In an attempt to classify heart failure in ESRD, the Acute Dialysis Quality Initiative XI Workgroup proposed a functional classification system based on the echocardiographic evidence of heart disease and the impact of renal replacement therapy (RRT) and/or ultrafiltration on symptoms. The classification is based on the New York Heart Association functional classes and divides each class into subgroups depending on whether symptoms persist or not after RRT [44]. This approach helps differentiate patients who present with volume overload in the absence of underlying cardiomyopathy from those who develop symptoms secondary to an underlying heart condition. In order to differentiate between patients with diastolic dysfunction and those with pure volume overload, the authors suggest assessing right atrial pressures by inferior vena cava imaging before and after ultrafiltration [44].
Left ventricular hypertrophy is the major mechanism of diastolic dysfunction in CKD. Other mechanisms implicated in the pathogenesis of diastolic dysfunction in ESRD patients include myocardial fibrosis, activation of the intracardiac renin-angiotensin system, anemia, or hyperphosphatemia [45]. A detailed description of the pathophysiology of heart failure in ESRD is beyond the scope of this article but has been reviewed elsewhere [46].
Heart failure is associated with significant morbidity in dialysis patients. A Canadian cohort found that heart failure is the most common reason for emergency department visit in hemodialysis patients recently discharged from the hospital [47]. Volume overload, as identified with assessment of inter-dialytic weight gain, has been associated with all-cause and cardiovascular mortality after multivariate adjustment for demographics, inflammation, and malnutrition [48]. Conversely, data from the HEMO study showed that ultrafiltration rates exceeding 13 ml/kg/hour are associated with higher all-cause or cardiovascular mortality compared to ultrafiltration rates up to 10 ml/kg/hour despite presumably better treatment of congestion [49]. The mechanism underlying this observation could be repetitive occult myocardial injury. Rapid fluid removal from the intravascular compartment could acutely reduce the effective circulating volume and cause transient myocardial ischemia and myocardial stunning. The long-term impact of repetitive myocardial stunning events on ventricular function may ultimately outweigh the short-term benefits of improved ultrafiltration [49].
Finally, it is well known that high output heart failure is occasionally associated with the creation of an arteriovenous fistula, particularly in those with limited myocardial function reserve [50]. Whether more subtle effects on ventricular function occur in the majority of patients with venous accesses is an unsolved question, but in patients with pre-existing congestive heart failure, careful estimation of the myocardial functional reserve before fistula creation may be advisable.
Atrial fibrillation and stroke risk in dialysis patients
Atrial fibrillation (AF) is a common arrhythmia in dialysis patients. Its prevalence was estimated to be 10.7% in 2006 and had significantly risen between 1992 and 2006 [51]. The absolute number of hemodialysis patients with AF increased from 3620 patients in 1992 to 23,893 patients in 2006, also reflecting the increasing prevalence of ESRD in the US population. The incidence of AF similarly increased from 11.3% of incident dialysis patients in 1995 to 14.5% in 2007 [52]. This study used Medicare data and enrolled only patients aged 67 or more. Preliminary results from the MiD trial showed that up to 40% of individuals without known AF at baseline had AF detected with an implantable loop recorder during follow-up [53]. Therefore, AF may be even more common but under-diagnosed in the dialysis population.
AF is associated with significant morbidity and mortality. One–year mortality rates were significantly higher in dialysis patients with atrial fibrillation compared with those without (hazard ratio 1.72 after adjustment for age, gender, and race) [51]. ESRD patients are at increased risk of stroke compared with the general population (relative risk of 4.4 to 9.7) [54]. The excess risk of embolic stroke attributable to AF in ESRD patients was found to be 6 strokes per 1000 patient-years [55]. In a Taiwan nationwide cohort study, the incidence of ischemic stroke increased with higher CHADS2 and CHA2DS2-VASc scores (C-statistic of 0.608 and 0.682 respectively) [56].
Unfortunately whether warfarin is protective against embolic stroke in patients with AF and ESRD remains unclear. No RCTs exist directly comparing vitamin K antagonists with placebo in dialysis patients. Evidence comes from several well-conducted cohort studies that are summarized in Table 2 but the findings are contradictory [57–62]. Many of those studies did not show any clear benefit or harm from warfarin (hazard ratios in the 0.7 to 1.7 range). Shen et al. also demonstrated how difficult it is to keep hemodialysis patients on warfarin, as more than two thirds discontinued the drug during the first year [62]. The American Heart Association and American College of Cardiology guideline for the management of patients with AF still recommends warfarin for dialysis patients with a CHA2DS2-VASc score >1 (class IIa) [63]. However, in the absence of randomized data and given the conflicting results of the cohort studies, routine anticoagulation of all dialysis patients with atrial fibrillation cannot be unequivocally recommended. Better data is sorely needed. In the meantime, an individualized approach should be considered taking into account the stroke and bleeding risks and the patient’s preference.
Table 2.
Warfarin and risk of stroke in dialysis patients with atrial fibrillation (AF)
| Study | Chan et al.57 | Wizemann et al.58 | Winkelmayer et al.59 | Olesen et al.60 | Shah et al.61 | Shen et al.62 |
|---|---|---|---|---|---|---|
| Cohort | 1300 Fresenius clinics, North America | DOPPS I & II cohorts | Medicare (New Jersey & Pennsylvania) | Denmark | Canada | USRDS data |
| Design | Retrospective cohort study, covariate & propensity score-adjusted | Observational cohort study, multi-adjusted HR | Propensity score- matched cohort | Cohort study; HRs adjusted for the CHA2DS2-VASc score, antithrombotic treatment, and inclusion year | Retrospective cohort study; HRs adjusted for the CHA2DS2 score, and sex | Retrospective observational cohort study |
| Population | Incident dialysis patients | Prevalent dialysis patients | Incident dialysis patients >65 years | All patients discharged with a diagnosis of AF requiring renal replacement therapy | Dialysis patients ≥ 65 years admitted with a diagnosis of AF | Dialysis patients with a new diagnosis of AF |
| Number of patients | 1400 | 2188 | 2313 | 901 | 1626 | 12284 |
| Outcome definition | Death or hospitalization from new stroke | Death or hospitalization from new stroke | Any stroke (ischemic or hemorrhagic) | Stroke or systemic thromboembolism | First hospital admission or emergency visit for stroke | Any stroke or stroke death |
| Hazard ratio (HR) in warfarin users vs. non-users (95% CI) | 1.74 (1.11–2.72) | <65: 1.29 (0.45–3.68) 66–75: 1.35 (0.69–2.63) >75: 2.17 (1.04–4.53) |
1.08 (0.76–1.55) | 0.44 (0.26–0.74) | 1.14 (0.78–1.67) | 0.83 (0.61–1.12) |
All the clinical trials with the novel anticoagulants have excluded patients with ESRD. However, given their pharmacological profile, apixaban, and potentially edoxaban, could theoretically be considered with some dose adjustment, as they are not primarily renally excreted [64]. However, experience with these medications in dialysis is minimal, and more evidence is required before such a recommendation can be made.
Conclusion
Cardiovascular morbidity and mortality remain exceedingly common in dialysis patients. Despite recent progress in the understanding of cardiovascular disease in patients with advanced renal failure, its pathophysiology has not been fully elucidated. The global burden from cardiovascular disease in ESRD is expected to rise given the population aging and the increasing prevalence of diabetes and hypertension in the general population. Current preventive and management strategies are lacking the efficiency demonstrated in patients with preserved renal function. Future research could focus on the impact of optimizing the dialysis prescription with attention to volume and electrolyte management, on the potential role of the novel anticoagulants in patients with atrial fibrillation, and the role of defibrillators in this patient group.
Key points.
Bradycardia and asystole (primary or secondary) rather than ventricular tachyarrhythmias may be the dominant electrical event underlying sudden cardiac death.
Retrospective data on ICDS for primary prevention in dialysis patients do not show definitive benefits.
CABG is associated with a higher short-term mortality (30 days or in hospital) compared with PCI but appears to be superior to PCI for long-term mortality (at least 1 year) and for cardiac events (defined as myocardial infarction or repeat revascularization), at least in those with acceptable surgical risks.
Heart failure is common in dialysis but dialysis related heart failure should be distinguished from structural-causes. The Acute Dialysis Quality Initiative XI Workgroup proposed a functional classification system for heart failure in ESRD based on the New York Heart Association functional classes that may be useful in this regard.
AF is extremely common in dialysis patients but whether anti-coagulation should be broadly recommended is uncertain. Better data on the role of traditional an novel anti-coagulants is sorely needed.
Acknowledgments
Financial support and sponsorship
Dr. Charytan is funded by NIH grant HL HL118314.
Footnotes
Conflicts of interest
Dr. Charytan has received research funding from Medtronic and Janssen pharmaceuticals for work on the MiD and CREDENCE trials, consulting fees from Zoll Medical for work on the steering committee of the WEDHEAD trial and consulting fees from Medtronic.
References
Papers of special interest have been highlighted *
- 1.Passman R, Herzog CA. Sudden cardiac death: stratifying risk in dialysis patients. Nat Rev Nephrol. 2011;7:133–135. doi: 10.1038/nrneph.2010.166. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data system. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End Stage Renal Disease in the United States, national institutes of Health, national institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD, USA: 2010. [Google Scholar]
- 3.Wheeler DC, London GM, Parfrey PS, et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. J Am Heart Assoc. 2014;3:e001363. doi: 10.1161/JAHA.114.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drechsler C, Grootendorst DC, Pilz S, et al. Wasting and sudden cardiac death in hemodialysis patients: a post hoc analysis of 4D (Die Deutsche Diabetes Dialyse Studie) Am J Kidney Dis. 2011;58:599–607. doi: 10.1053/j.ajkd.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74:1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 6.Wang AY, Lam CW, Chan IH, et al. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension. 2010;56:210–216. doi: 10.1161/HYPERTENSIONAHA.110.151167. [DOI] [PubMed] [Google Scholar]
- 7.Wan C, Herzog CA, Zareba W, et al. Sudden cardiac arrest in hemodialysis patients with wearable cardioverter defibrillator. Ann Noninvasive Electrocardiol. 2014;19:247–257. doi: 10.1111/anec.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Wong MCG, Kalman JM, Pedagogos E, et al. Bradycardia and asystole is the predominant mechanism of sudden cardiac death in patients with chronic kidney disease. J Am Coll Cardiol. 2015;65:1263–1265. doi: 10.1016/j.jacc.2014.12.049. This study highlights the importance of bradycardia and asystole as the main arrhythmic event responsible for sudden cardiac death in the hemodialysis population. [DOI] [PubMed] [Google Scholar]
- 9*.Charytan DM, Foley R, McCullough PA, et al. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clin J Am Soc Nephrol. 2016;11:721–734. doi: 10.2215/CJN.09350915. This article reviews current literature on arrhythmias in dialysis patients and presents the protocol of the MiD study, a clinical trial using implantable loop recorders in an attempt to estimate the true burden of arrhythmia in the dialysis population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charytan DM, Koplan BA, Podoll SA, et al. Greater frequency of clinically significant bradycardia than ventricular tachycardia in hemodialysis patients: preliminary results of the Monitoring in Dialysis (MiD) study. Abstract TH-OR145, Kidney Week; Philadelphia PA. November 2014. [Google Scholar]
- 11.Foley RN, Curtis BM, Randell EW, et al. Left ventricular hypertrophy in new hemodialysis patients without symptomatic cardiac disease. Clin J Am Soc Nephrol. 2010;5:805–813. doi: 10.2215/CJN.07761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pun PH, Lehrich RW, Honeycutt EF, et al. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 13.Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:797–803. doi: 10.2215/CJN.10000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis patients in the dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol. 2012;7:765–774. doi: 10.2215/CJN.08850811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013;10:e11–e58. doi: 10.1016/j.hrthm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58:409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 18*.Pun PH, Hellkamp AS, Sanders GD, et al. Primary prevention implantable cardioverter defibrillators in end-stage kidney disease patients on dialysis: a matched cohort study. Nephrol Dial Transplant. 2015;30:829–835. doi: 10.1093/ndt/gfu274. A retrospective cohort study including all dialysis patients who had an ICD between 2006 and 2007 for primary prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bie MK, Lekkerkerker JC, van Dam B, et al. Prevention of sudden cardiac death: rationale and design of the Implantable Cardioverter Defibrillators in Dialysis patients (ICD2) Trial- a prospective pilot study. Curr Med Res Opin. 2008;24:2151–2157. doi: 10.1185/03007990802237343. [DOI] [PubMed] [Google Scholar]
- 20.Tangri N, Shastri S, Tighiouart H, et al. β-Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO study. Am J Kidney Dis. 2011;58:939–945. doi: 10.1053/j.ajkd.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 22.Surana SP, Riella LV, Keithi-Reddy SR, et al. Acute coronary syndrome in ESRD patients. Kidney Int. 2009;75:558–562. doi: 10.1038/ki.2008.233. [DOI] [PubMed] [Google Scholar]
- 23.Joki N, Hase H, Nakamura R, et al. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant. 1997;12:718–723. doi: 10.1093/ndt/12.4.718. [DOI] [PubMed] [Google Scholar]
- 24.Gurm HS, Gore JM, Anderson FA, et al. Comparison of Acute Coronary Syndrome in Patients Receiving Versus Not Receiving Chronic Dialysis (from the Global Registry of Acute Coronary Events [GRACE] Registry) Am J Cardiol. 2012;109:19–25. doi: 10.1016/j.amjcard.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 25.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 26.Herzog CA, Littrell K, Arko C, et al. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: a collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation. 2007;116:1465–1472. doi: 10.1161/CIRCULATIONAHA.107.696765. [DOI] [PubMed] [Google Scholar]
- 27.Sosnov J, Lessard D, Goldberg RJ, et al. Differential symptoms of acute myocardial infarction in patients with kidney disease. Am J Kidney Dis. 2006;47:378–384. doi: 10.1053/j.ajkd.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Stacy SR, Suarez-Cuervo C, Berger Z, et al. Role of troponin in patients with chronic kidney disease and suspected acute coronary syndrome: a systematic review. Ann Intern Med. 2014;161:502–512. doi: 10.7326/M14-0746. [DOI] [PubMed] [Google Scholar]
- 29*.Michos ED, Wilson LM, Yeh HC, et al. Prognostic value of cardiac troponin in patients with chronic kidney disease without suspected acute coronary syndrome: a systematic review and meta-analysis. Ann Intern Med. 2014;161:491–501. doi: 10.7326/M14-0743. A systematic review summarizing all cohort studies evaluating the prognostic value of troponin T and I in stable outpatients undergoing hemodialysis. [DOI] [PubMed] [Google Scholar]
- 30.Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem. 2007;53:2086–2096. doi: 10.1373/clinchem.2007.095679. [DOI] [PubMed] [Google Scholar]
- 31.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin- converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 32.Winkelmayer WC, Charytan DM, Levin R, et al. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47:301–308. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Charytan D, Mauri L, Agarwal A, et al. The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J. 2006;152:558–564. doi: 10.1016/j.ahj.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chertow GM, Normand SLT, McNeil BJ. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 35.Weisbord SD. AKI and medical care after coronary angiography: renalism revisited. Clin J Am Soc Nephrol. 2014;9:1823–1825. doi: 10.2215/CJN.09430914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manske CL, Wang Y, Rector T, et al. Coronary revascularization in insulin-dependent diabetic patients with chronic renal failure. Lancet. 1992;340:998–1002. doi: 10.1016/0140-6736(92)93010-k. [DOI] [PubMed] [Google Scholar]
- 37.Charytan DM, Varma MR, Silbaugh TS, et al. Long-term clinical outcomes following drug-eluting or bare-metal stent placement in patients with severely reduced GFR: results of the Massachusetts Data Analysis Center (Mass-DAC) State registry. Am J Kidney Dis. 2011;57:202–211. doi: 10.1053/j.ajkd.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Nevis IF, Mathew A, Novick RJ, et al. Optimal method of coronary revascularization in patients receiving dialysis: systematic review. Clin J Am Soc Nephrol. 2009;4:369–378. doi: 10.2215/CJN.02640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Xue S, Lian F, et al. Meta-analysis of clinical studies comparing coronary artery bypass grafting with percutaneous coronary intervention in patients with end-stage renal disease. Eur J Cardiothorac Surg. 2013;43:459–467. doi: 10.1093/ejcts/ezs360. [DOI] [PubMed] [Google Scholar]
- 40.Chang TI, Shilane D, Kazi DS, et al. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marui A, Kimura T, Nishiwaki N, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with end-stage renal disease requiring dialysis (5-year outcomes of the CREDO-Kyoto PCI/CABG registry cohort-2) Am J Cardiol. 2014;114:555–561. doi: 10.1016/j.amjcard.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 42.Charytan DM, Kuntz RE. Risks of coronary artery bypass surgery in dialysis-dependent patients analysis of the 2001 National Inpatient Sample. Nephrol Dial Transplant. 2007;22:1665–1671. doi: 10.1093/ndt/gfl835. [DOI] [PubMed] [Google Scholar]
- 43.Charytan DM. How is the heart best protected in chronic dialysis patients? Between Scylla and Charybdis: what is the appropriate role for percutaneous coronary revascularization and coronary artery bypass grafting in patients on dialysis? Semin Dial. 2014;27:325–328. doi: 10.1111/sdi.12181. [DOI] [PubMed] [Google Scholar]
- 44.Chawla LS, Herzog CA, Costanzo MR, et al. Proposal for a functional classification system of heart failure in patients with end-stage renal disease. Proceedings of the Acute Dialysis Quality Initiative (ADQI) XI Workgroup J Am Coll Cardiol. 2014;63:1246–1252. doi: 10.1016/j.jacc.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Pecoits-Filho R, Bucharies S, Barberato SH. Diastolic heart failure in dialysis patients: mechanisms, diagnostic approach, and treatment. Semin Dial. 2012;25:35–41. doi: 10.1111/j.1525-139X.2011.01011.x. [DOI] [PubMed] [Google Scholar]
- 46.Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–86. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 47*.Harel Z, Wald R, McArthur E, et al. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. doi: 10.1681/ASN.2014060614. A large cohort study characterizing readmissions and emergency department visits in hemodialysis patients within 30 days after hospital discharge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaalantar-Zadeh K, Regidor DL, Kovesdy CP, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S, Elramah M, Allana SS, et al. A case series of real-time hemodynamic assessment of high output heart failure as a complication of arteriovenous access in dialysis patients. Semin Dial. 2014;27:633–638. doi: 10.1111/sdi.12241. [DOI] [PubMed] [Google Scholar]
- 51.Winkelmayer WC, Patrick AR, Brookhart MA, et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol. 2011;22:349–357. doi: 10.1681/ASN.2010050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein BA, Arce CM, Hlatky MA, et al. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation. 2012;126:2293–2301. doi: 10.1161/CIRCULATIONAHA.112.099606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tumlin JA, Charytan DM, Williamson DE, et al. Frequency and distribution of dialysis-associated atrial fibrillation: results of MiD study. J Am Soc Nephrol. 2014;25:35A. (TH-OR144) [Google Scholar]
- 54.Seliger SL, Gillen DL, Longstreth WT, Jr, et al. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64:603–609. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 55.Wetmore JB, Ellerbeck EF, Mahnken JD, et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol. 2013;23:112–118. doi: 10.1016/j.annepidem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chao T-F, Liu C-J, Wang K-L, et al. Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm. 2014;11:1752–1759. doi: 10.1016/j.hrthm.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 59.Winkelmayer WC, Liu J, Setoguchi S, et al. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 61.Shah M, Tsadok MA, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–1203. doi: 10.1161/CIRCULATIONAHA.113.004777. [DOI] [PubMed] [Google Scholar]
- 62**.Shen JI, Montez-Rath ME, Lenihan CR, et al. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–688. doi: 10.1053/j.ajkd.2015.05.019. An observational cohort study of dialysis patients with newly diagnosed AF studying the impact of warfarin on stroke and mortality rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 64*.Mavrakanas TA, Samer C, Fontana P, et al. Direct oral anticoagulants: efficacy and safety in patients subgroups. Swiss Med Wkly. 2015;145:w14081. doi: 10.4414/smw.2015.14081. A review article presenting current experience with the novel anticoagulants in different patients subgroup, including patients with CKD. [DOI] [PubMed] [Google Scholar]