Abstract
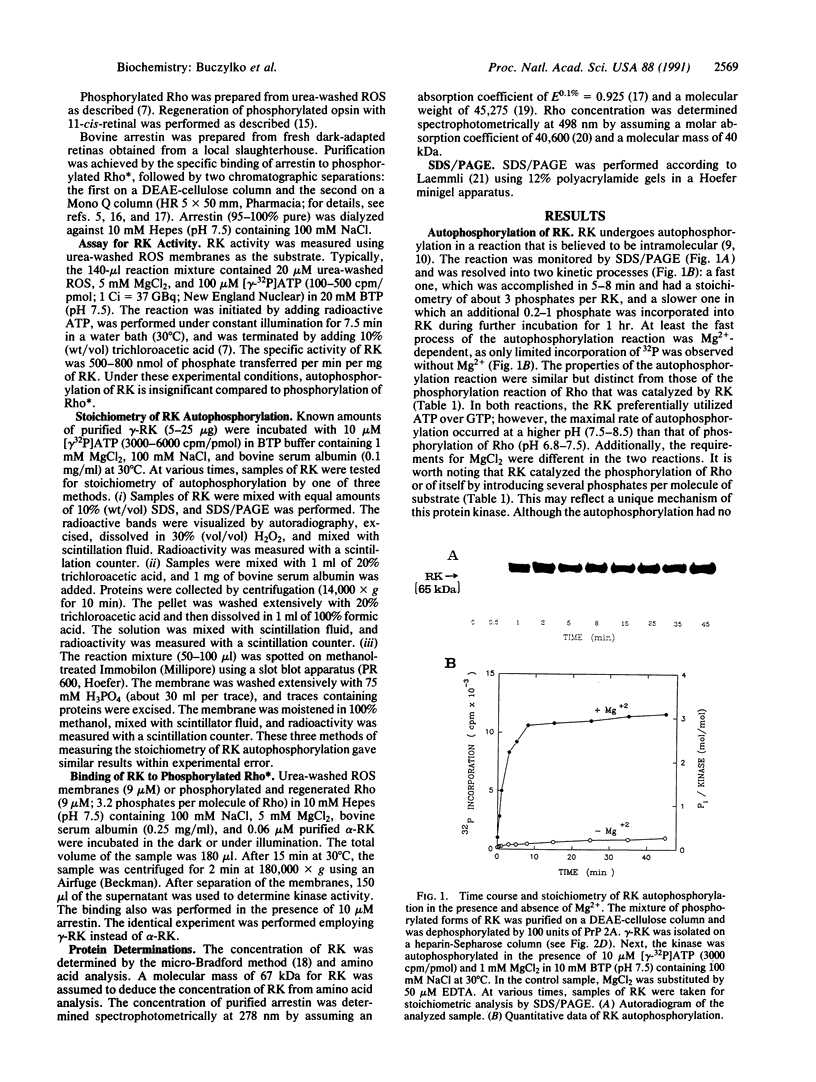
Rhodopsin kinase (RK) catalyzes the phosphorylation of rhodopsin (Rho) as one of the steps in quenching photoactivated Rho. In this work, we investigated the autophosphorylation of RK and how it affects the interaction between RK and Rho. RK undergoes intramolecular phosphorylation, resulting in the incorporation of three or four phosphates per RK molecule. Phosphorylated RK subsequently is a substrate for protein phosphatases 2A and 2B. We isolated three forms of RK based on their differential interactions with heparin-Sepharose. Fully phosphorylated RK (alpha-RK) binds tightly to Rho but has significantly lower affinity to phosphorylated Rho, whereas unphosphorylated RK (gamma-RK) binds avidly to both forms of Rho. The heterogenous intermediately phosphorylated RK (beta-RK) was not studied. Our data support the hypothesis that RK dissociates from Rho when both Rho and RK become phosphorylated, thereby allowing the binding of arrestin to phosphorylated Rho. These results suggest that autophosphorylation plays an important role in regulating the binding of RK to Rho and that the binding sites of RK and arrestin overlap at least partially.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Johnson G. L. Characterization of rhodopsin kinase purified from bovine rod outer segments. J Biol Chem. 1990 Feb 15;265(5):2632–2639. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Brown B. M., Lolley R. N. Autophosphorylation of rhodopsin kinase from retinal rod outer segments. Biochemistry. 1982 Jul 6;21(14):3303–3307. doi: 10.1021/bi00257a009. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Arendt A., McDowell J. H., Hargrave P. A. Substrate recognition determinants for rhodopsin kinase: studies with synthetic peptides, polyanions, and polycations. Biochemistry. 1989 Oct 31;28(22):8764–8770. doi: 10.1021/bi00448a013. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Hargrave P. A., Folta E. J., Kochman M. Affinity labeling of rabbit muscle fructose-1,6-bisphosphate aldolase with 5'-[p-(fluorosulfonyl)benzoyl]-1,N6-ethenoadenosine. Eur J Biochem. 1985 Jan 15;146(2):309–314. doi: 10.1111/j.1432-1033.1985.tb08654.x. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Hargrave P. A., McDowell J. H., Ingebritsen T. S. The catalytic subunit of phosphatase 2A dephosphorylates phosphoopsin. Biochemistry. 1989 Jan 24;28(2):415–419. doi: 10.1021/bi00428a001. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Hargrave P. A. Purification and characterization of rhodopsin kinase. J Biol Chem. 1988 Oct 5;263(28):14067–14073. [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Hargrave P. A. Rhodopsin kinase: substrate specificity and factors that influence activity. Biochemistry. 1988 Apr 5;27(7):2306–2313. doi: 10.1021/bi00407a010. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Jakes S., Ingebritsen T. S., Hargrave P. A. Regulation of rhodopsin dephosphorylation by arrestin. J Biol Chem. 1989 Sep 25;264(27):15770–15773. [PubMed] [Google Scholar]
- Resink T. J., Hemmings B. A., Tung H. Y., Cohen P. Characterisation of a reconstituted Mg-ATP-dependent protein phosphatase. Eur J Biochem. 1983 Jun 15;133(2):455–461. doi: 10.1111/j.1432-1033.1983.tb07485.x. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Dietzschold B., Craft C. M., Wistow G., Early J. J., Donoso L. A., Horwitz J., Tao R. Primary and secondary structure of bovine retinal S antigen (48-kDa protein). Proc Natl Acad Sci U S A. 1987 Oct;84(20):6975–6979. doi: 10.1073/pnas.84.20.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Tung H. Y., Resink T. J., Hemmings B. A., Shenolikar S., Cohen P. The catalytic subunits of protein phosphatase-1 and protein phosphatase 2A are distinct gene products. Eur J Biochem. 1984 Feb 1;138(3):635–641. doi: 10.1111/j.1432-1033.1984.tb07962.x. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker W. B., Donoso L. A., Kalsow C. M., Yankeelov J. A., Jr, Organisciak D. T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977 Dec;119(6):1949–1958. [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- Wilden U., Wüst E., Weyand I., Kühn H. Rapid affinity purification of retinal arrestin (48 kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett. 1986 Oct 27;207(2):292–295. doi: 10.1016/0014-5793(86)81507-1. [DOI] [PubMed] [Google Scholar]
- Wolf M., Cuatrecasas P., Sahyoun N. Interaction of protein kinase C with membranes is regulated by Ca2+, phorbol esters, and ATP. J Biol Chem. 1985 Dec 15;260(29):15718–15722. [PubMed] [Google Scholar]
- Zigler J. S., Jr, Mochizuki M., Kuwabara T., Gery I. Purification of retinal S-antigen to homogeneity by the criterion of gel electrophoresis silver staining. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):977–980. [PubMed] [Google Scholar]





