Summary
Neurodevelopment is a complex process governed by both intrinsic and extrinsic signals. While historically studied by researching the brain, inputs from the periphery impact many neurological conditions. Indeed, emerging data suggests communication between the gut and the brain in anxiety, depression, cognition and autism spectrum disorder (ASD). The development of a healthy, functional brain depends on key pre- and post-natal events that integrate environmental cues, such as molecular signals from the gut. These cues largely originate from the microbiome, the consortium of symbiotic bacteria that reside within all animals. Research over the past few years reveals that the gut microbiome plays a role in basic neurogenerative processes such as the formation of the blood-brain-barrier, myelination, neurogenesis, and microglia maturation, and also modulates many aspects of animal behavior. Herein, we discuss the biological intersection of neurodevelopment and the microbiome, and explore the hypothesis that gut bacteria are integral contributors to development and function of the nervous system, and the balance between mental health and disease.
Introduction
The development of the mammalian brain is an intricate process that lasts through adolescence and into early adulthood in humans. Further, the process of brain development involves extraordinary, large-scale long distance migration of cells during fetal development to specific regions or layers, as well as navigation of their processes across even longer distances (often hundreds of cell body diameters) to build the specific circuits that underlie behavior (Geschwind and Rakic, 2013; Marín and Rubenstein, 2003). The complexity and protracted pre- and post-natal time course over which these events occur, makes them highly sensitive and even vulnerable to environmental factors. In fact, many of the processes governing brain development are driven by extrinsic cues and experiences that shape the developing brain through both generative and regressive events. As the gut is our largest portal to the molecular universe, various dietary components have been shown to interact directly with the developing brain and to induce functional alterations in the mature brain (Chang et al., 2009; Zeisel, 2004), and there is now mounting evidence for a role by the gut microbiome in directing and facilitating developmental processes in the brain with long term implications to health (Figure 1, Table 1).
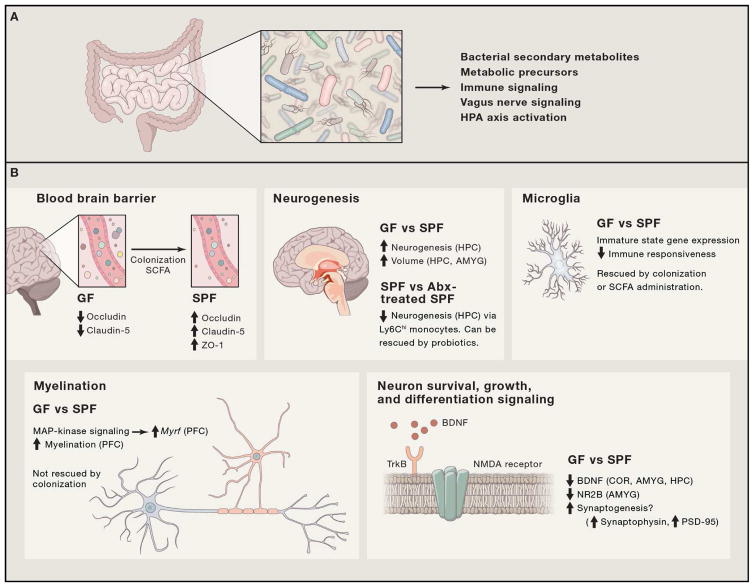
Figure 1. Intersections of gut microorganisms and basic developmental processes.
Basic developmental processes driven directly or indirectly by gut microbes and their products. (A) Gut microorganisms relay messages to the brain via various direct and indirect mechanisms. (B) Basic neurodevelopmental processes are modulated as a result of colonization of GF animals or depletion of gut bacteria by antibiotics. Specifically, the following processes are modulates: blood-brain barrier (BBB) formation and integrity (Braniste et al., 2014), neurogenesis (Möhle et al., 2016; Ogbonnaya et al., 2015), microglia maturation and ramification (Erny et al., 2015; Matcovitch-Natan et al., 2016), myelination (Gacias et al., 2016; Hoban et al., 2016), and expression of neurotrophins (Bercik et al., 2011a, 2011b; Desbonnet et al., 2015), neurotransmitters (Bercik et al., 2011a; O’Mahony et al., 2015), and their respective receptors.
Table 1.
Perturbation of the microbiome and microbial products can affect behavioral outcomes in mouse models and humans
| Treatment / pertubation to microbiome | Effects on behavior | Known/Persumed Mechanism (by correlation) | Reference | |
|---|---|---|---|---|
| Prenatal effects | Abx | Offspring exhibited anxiety-like behavior and hypoactivity, as well as decreased sociability | Dysbiotic microbiome | Tochitani et al., 2016; Degroote et al., 2016 |
|
| ||||
| High-fat diet | Social deficit and repetitive behavior in offsprings | Dysbiotic microbiome; deficient VTA synaptic plasticity and oxytocin | Buffington et al., 2016 | |
|
| ||||
| Peptidoglycan | Offspring show decreased cognitive function | TLR-2 mediated neuroprolifiration via FoxG1 induction in fetal cortex | Humann et al., 2016 | |
|
| ||||
| Prenatal stress | Change in offspring microbiome, gut and brain metabolome | Dysbiotic microbiome; altered free amino-acid levels in offspring brain | Jašarević et al., 2015b | |
|
| ||||
| Maternal immune activation by Poly(I:C) administration | Change in offspring microbiome and metabolome, increase repetitive behavior, anxiety-like behavior, social deficit, communication deficit | IL-6 and IL-17A mediated behavioral and cortical development abnormalities; Dysbiotic microbiome. | Smith et al., 2007; Hsiao et al., 2013; Choi et al., 2016 | |
|
| ||||
| Propionic acid | Offspring exhibited anxiety-like behavior | - | Foley et al., 2014 | |
|
| ||||
| Postnatal effects | Perinatal Abx | Visceral hypersensitivity | Dysbiotic microbiome; decreased expression of various genes involved in pain preception in the lumbosacral region of the spine | O’Mahony et al., 2014 |
|
| ||||
| Abx (short-term) | Short-term anxiolytic effect | Dysbiotic microbiome; increased BDNF levels in hippocampus, and decreased in the amygdala; phenotype independent of sympathetic and parasympathetic pathways | Bercik et al., 2011a | |
|
| ||||
| Abx (Long-term) | Anxiolytic effect, cognitive deficits | Dysbiotic microbiome; increased tryptophan and decreased kynurenine in serum; increased noradrenaline in hippocampus and increased L-DOPA in amygdala; decreased BDNF in the hippocampus and vasopressin expression in the hypothalamus | Desbonnet et al., 2015 | |
|
| ||||
| Deficits in memory formation | Decreased BDNF and c-Fos expression in the CA1 region of the hippocampus | Gareau et al., 2011 | ||
|
| ||||
| Colonization of GF Swiss Webster with BALB/c microbiome increased exploratory behavior, while the reciprocal colonization of GF BALB/c mice with Swiss Webster microbiome reduced exploration | GF Swiss Webster mice comlonize with Swiss Webster microbiota have higher levels of BDNF in the hippocampus, but not in the amygdala, compared to GF mice colonized with microbiota from BALB/c mice | Bercik et al., 2011a | ||
|
| ||||
| Germ-Free Animals | Increased motor activity and reduced anxiety-like behavior compared to SPF | GF mice, compared to SPF controls, show: increased turnover of noradrenaline, dopamine, and serotonin in the striatum ; decreased expression of NGFI-A in the frontal cortex, BDNF in the basolateral amygdala and CA1 region of hippocampus, and dopamine D1 receptor in the dendate gyrus; differences in gene expression in hippocampus, frontal cortex, and striatum; higher expression of synaptophysin and PSD-95 (increased synaptogenesis) | Heijtz et al., 2011 | |
|
| ||||
| Reduced anxiety-like behavior | GF mice had decreased expression of NR2B in the amygdala; increased expression of BDNF and decreased expression of 5HT1A in the dentate gyrus | Neufeld et al., 2011 | ||
|
| ||||
| Increased stress-induced HPA response | GF animals, compared to SPF controls, show: increased levels of stress-induced acetylcholine and corticosterone (HPA-axis); decreased expression of NR-1 in the cortex, and of NR-2a in the cortex and hippocampus; BDNF levels were lower in the cortex and hippocampus | Sudo et al., 2004 | ||
|
| ||||
| Reduced social behavior | - | Desbonnet et al., 2014 | ||
|
| ||||
| Increased social behavior | Decreased expression of specific BDNF transcripts in the amygdala | Arentsen et al., 2015 | ||
|
| ||||
| Clostridium butyricum administration restored cognitive function in mouse model for vascular dementia | Restoration correlated with increased levels of the SCFA butyrate in feces and brains, and accompanied by activation of the BDNF-PI3K/Akt pathway in the hippocampus | Liu et al., 2015 | ||
|
| ||||
| Clostridium butyricum protected from cerebral ischemia/reperfusion injury in diabetic mice | Anti-apoptotic effects via Akt activation; restoration of bacterial diversity | Sun et al., 2016 | ||
|
| ||||
| Lactobacillus farciminis prevented stress-induced intestinal permeability and neuroinflammation in mice | Decreased intestinal permeability that mediated the following stress-induced phenotypes: increased CRF expression in PVN; increased expression of proinflammatory cytokines in blood and increased plasma levels of corticosterone | Ait-Belgnaoui et al., 2012 | ||
|
| ||||
| Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 (Probio’Stick®) protected from effects of water avoidence stress in mice and decreased intestinal barrier dysfunction | Probiotic treatment attentuated plasma levels of HPA/ANS-related corticosterone, adrenaline, and noradrenaline, as a result of stress induction, as well as activation of nuclei in the PVN, amygdala, CA3 of the hippocampus, and the dentate gyrus. Additionally, The expression of various genes related to | Ait-Belgnaoui et al., 2014 | ||
|
| ||||
| Probiotic Administration | Lactobacillus rhamnosus (JB-1) reduced stress induced anxiety- and depression-like behaviors in mice | Probiotic decreased levels of stress-induced plasma corticosterone; changed expression levels of GABAB1b, GABAAa1, and GABAAa2 thorughout the brain in a vagus-dependent manner | Bravo et al., 2011 | |
|
| ||||
| Lactobacillus rhamnosus (R0011) and Lactobacillus helveticus (R0052) (Lacidofil) reduced anxiety-like behaviors in mice | Increased BDNF and c-Fos expression in the hippocampus | Gareau et al., 2011 | ||
|
| ||||
| Lactobacillus reuteri corrected social deficit in mice | Probiotic restored levels of oxytocin producing cells in the PVN and synaptic plasticity in the VTA | Buffington et al., 2016 | ||
|
| ||||
| Monoassociation with Bifidobacterium infantis reversed increased stress-induced HPA response in GF mice | Treatment of GF mice with probiotic or colonizing with SPF microbiome early in development normalized levels of stress-induced acetylcholine and corticosterone; normalized expression of NR1 in the cortex, and of NR2a in the cortex and hippocampus; normalized BDNF levels in the cortex and hippocampus | Sudo et al., 2004 | ||
|
| ||||
| Bifidobacterium longum NCC3001 reduced colitis-induced anxiety-like behaviors in mice | Probiotic decreased anxiety, but not pathology, in a vagus-dependant manner; excitability of enteric neurons incubated in probiotic-fermented media was lower than controls | Bercik et al., 2011b | ||
|
| ||||
| Fermented milk product affected brain activity in regions processing emotion and sensation in healthy human subjects | Probiotic reduced activity in response to an emotional faces attention task in the insula cortex and somatosensory cortex | Tillisch et al., 2013 | ||
|
| ||||
| Bacteroides fragilis corrected anxiety-like and repetitive behaviors in mice | Partial restoration of microbial community; restoration of intestinal barrier function | Hsiao et al., 2013 | ||
Abx - Antibiotics; GF - Germ-free ; SPF - Specific pathogen free; VTA - ventral tegmental area; BDNF - brain-derived neurotropic ;HPAfactor; NR2B - N-methyl-D-aspartate receptor subunit 2B; 5HT1A - serotonine receptor 1A; SCFA - short-chain fatty-acid; HPA - hypothalamus-pituitary-adrenal axis; ANS - autonomous nervous system; PVN - paraventricular nucleus of the hypothalamus;
The mammalian microbiome consists of unique assemblages of microorganisms (i.e., bacteria, archaea, fungi, and viruses) associated with various niches in and on the body. Research in animal models and humans has inextricably linked gut bacteria to the development and function of the immune system. The presence of entire immune cell types requires the microbiome, and specific microbes have been discovered that either promote or ameliorate immunologic disorders such as type 1 diabetes, asthma and inflammatory bowel disease (Round and Mazmanian, 2009). If the gut microbiome can so profoundly impact the immune system, why would its influence not reach the nervous system? Indeed, germ-free (GF) mice, devoid of all associated microorganisms, exhibit increased risk-taking behaviors and hyperactivity, while also displaying learning and memory deficits compared to conventional (specific pathogen free; SPF) mice (Clarke et al., 2013; Gareau et al., 2011; Heijtz et al., 2011; Neufeld et al., 2011) (Table 1). Further, GF mice show changes in expression of the 5-hydroxytryptamine receptor (5-HT1A), neurotrophic factors (e.g., BDNF), and NMDA receptor subunits in the hippocampus (Bercik et al., 2011a; Heijtz et al., 2011; Sudo et al., 2004), while also displaying impaired blood-brain barrier function, as well as increased myelination in the prefrontal cortex (Braniste et al., 2014; Hoban et al., 2016)(Table 1; Figure 1). There is also evidence, albeit preliminary and mostly from animal models, for a potential role for the microbiome in neuropsychiatric conditions, including depression and anxiety (Foster and McVey Neufeld, 2013), autism spectrum disorder (ASD) (Krajmalnik-Brown et al., 2015), schizophrenia (Severance et al., 2014) and even Parkinson’s (PD) and Alzheimer’s disease (AD) (Keshavarzian et al., 2015).
In this Review, we discuss the intersection between the mammalian microbiome and the brain in both humans and animal models. We explore developmental trajectories and the outcomes of interactions between microbes and the brain during prenatal development, as well as postnatally and to adulthood where microbial communities are established. Further, we highlight potential paradigms by which host-associated microorganisms may play an active role in both supporting health and potentiating disease states. By correlating microbial activities to progressive structural and functional events in the brain in mouse models and humans, we propose pathways whereby the gut microbiome may contribute to neurodevelopment and neurodegeneration. Uncovering microbial and host pathways that regulate these connections may provide novel approaches for addressing behavioral, psychiatric and neurodegenerative disorders.
Major processes in neurodevelopment coincide with changes in the maternal and neonatal gut microbiome
Prenatal brain development
On the third week after conception in humans, after gastrulation is complete, neural stem cells differentiate from the epiblast, marking the first event in a sequence that would eventually result in the adult brain. In the cerebral cortex, which is most elaborated in humans (Geschwind and Rakic, 2013), neural progenitor cells proliferate in the ventricular zone (NPCs; also known as radial glial cells) proliferate in the ventricular zone. Committed progenitors or neurons that migrate over a distance of many cell body diameters through the intermediate zone to the cortical plate, while NPCs remain in the proliferative zone (Kriegstein and Alvarez-Buylla, 2009; Rakic, 1988). While cortical neurogenesis is complete (for the most part) by mid gestation, gliogenesis is primarily a postnatal process. Anterior-posterior and dorsal-ventral patterning of the nervous system occurs via the same basic rules, factors and pathways that pattern the body, and the signature of the regulatory factor gradients that govern the “Protomap” that underlies the spatial and structural differentiation of the brain are present in germinal zones of the developing embryonic brain (Marín and Rubenstein, 2003; Rakic, 1988; Stiles and Jernigan, 2010).
Following a massive neuronal expansion and migration during cortical development, approximately 50% of neurons undergo apoptosis during the final weeks of gestation (38–41) and only those that have been integrated into networks, and supported by neurotrophic signals, survive (Ceni et al., 2014). Neurotrophins, amongst them brain-derived neurotrophic factor (BDNF), serve as signals for neuron survival, promote maintenance and differentiation of various cell populations (Ichim et al., 2012), and mediate various stages of neuronal circuitry establishment (Park and Poo, 2013). According to the neurotrophic hypothesis, the more connections a neuron makes, the higher the concentration of neurotrophins around it, and hence the higher chance of survival (Oppenheim, 1989). Neurogenesis is influenced by the presence of microorganisms. Specifically, neurogenesis in the dorsal hippocampus of adult GF mice is increased compared to conventional mice (Ogbonnaya et al., 2015). Interestingly, colonization of GF mice at weaning could not reverse this phenotype, indicating that microbial signals very early in life reduce rates of neurogenesis in the hippocampus. Moreover, adult GF mice exhibit increased volume of the amygdala and hippocampus (specifically CA2/3), and differ in dendrite morphology, while no differences in total brain volume were recorded between GF and SPF animals (Luczynski et al., 2016a). By tapping into pathways that govern neuronal differentiation and survival, via neurotrophins and their receptors, gut microbes can influence the fate of neurons in various regions of the brain and subsequently neurodevelopment and health.
In contrast to neurons, astroglia, and oligodendrocytes, microglia are central nervous system (CNS)-resident innate immune cells derived from a primitive subset of macrophages in the yolk sac. Although still controversial, recent reports indicate that microglia are not derived from monocytes, but rather develop earlier and express distinct cell markers, different from bone marrow-derived macrophages (specifically Ly-6ChiCCR2+ monocytes) that replenish the CNS following brain injury (Bennett et al., 2016; Ginhoux et al., 2010; Nayak et al., 2014; Varvel et al., 2012). In contrast, microglia were shown to originate from CD45-c-kit+ erythromyeloid progenitor cells and mature as CD45+c-kit-CX3CR1+ cells in the CNS. In mice, studies show that microglia enter the brain through circulation by embryonic day 8.5 (E8.5), start expressing the microglial marker Tmem119 as early as postnatal day 6 and become fully ramified throughout the brain by postnatal day 28 (Bennett et al., 2016; Nayak et al., 2014). Microglia originate from yolk-sac progenitor cells, and can be replenished by bone-marrow derived macrophages upon insult; both cell types can be subjected to microbial signals during early development (Erny et al., 2015; Khosravi et al., 2014). A central role for the microbiota in the development and maturation of the microglia has recently emerged (Erny et al., 2015; Matcovitch-Natan et al., 2016). In the absence of the microbiota, mice harbor microglia with significantly altered developmental states. These microglia display morphological characteristics and a gene expression profile that indicate an arrest in their developmental maturation, and subsequently are maintained in an immature status (Erny et al., 2015; Matcovitch-Natan et al., 2016). Notably, microglia derived from GF mice display limited responses towards viral infection and microbially-associated molecular patterns (MAMPs). Such defective responses can be rescued by administration of short-chain fatty-acids (SCFAs)(Erny et al., 2015).
The blood-brain-barrier (BBB) forms during gestation and serves as a selective barrier between the brain and circulation. The importance of gut microbiome and microbial metabolites in the formation of the BBB has been exemplified in GF mice (Braniste et al., 2014). In the absence of gut microorganisms, the BBB is more permeable to macromolecules, compared to conventionally-raised animals, mediated by decreased expression of key tight-junction proteins in the brain endothelium. Furthermore, permeability decreased upon colonization of GF animals, or, alternatively, administration of the SCFA butyrate that is produced as a result of bacterial fermentation in the gut (Braniste et al., 2014). Correspondingly, the BBB in the sterile fetus is permeable, compared to the adult BBB (Møllgård and Saunders, 1986). Recently, a lymphatic vasculature of the brain that drains from the cerebrospinal fluid in the adjacent subarachnoid space and the intrastitial fluid, to the deep cervical lymph nodes was discovered (Aspelund et al., 2015). This network allows easy passage for various immune cells as well as macromolecules and metabolites in and out of the brain (Aspelund et al., 2015; Louveau et al., 2015). The BBB and the brain lymphatic vasculature serve as a gateway for various signals to the brain, such as circulating immune cells and soluble molecules (including hormones and neurotransmitters, both host and microbial in origin), and along with stimulation of the vagus nerve, represent mechanisms that facilitate direct and indirect transmission of microbial signals from the gut to the brain.
The maternal gut and vaginal microbiome during pregnancy and its effect on offspring behavior
The maternal microbiome is distinct and dynamically changing during pregnancy compared to other periods in female life (DiGiulio et al., 2015; Koren et al., 2012; MacIntyre et al., 2015; Nuriel-Ohayon et al., 2016; Romero et al., 2014a). With the progression of pregnancy, various taxa change in abundance with some becoming more dominant over others. For example, in the gut some Proteobacteria and Actinobacteria appear to increase in relative abundance in the third trimester, compared to the first (DiGiulio et al., 2015; Koren et al., 2012). The vaginal microbiome during pregnancy remains dominated by Lactobacillus species; however as pregnancy progresses the species composition varies between stable, common states termed community state types (CSTs). CSTs with higher microbial diversity in the vagina are associated with preterm birth (DiGiulio et al., 2015; Romero et al., 2014b). Some data suggest that the mammalian fetus is not necessarily sterile as commonly predicted. Studies advocate that the placenta and, at times, the amniotic fluid surrounding the fetus, harbor distinct bacterial populations (Aagaard et al., 2014; DiGiulio, 2012; Kuperman and Koren, 2016; Zheng et al., 2015a); however these findings remain controversial and require further investigation, as Lauder and colleagues (2016) demonstrated. Significant work is needed to validate and implement this information for therapeutic or diagnostic applications.
Variation in maternal microbial populations have been suggested to modulate the microbiome, neurodevelopment, and behavior of the offspring (Jašarević et al., 2015a). Perinatal administration of antibiotics can affect offspring health and immune status in both humans and mouse models (Russell et al., 2013; Stensballe et al., 2013). Administration of nonabsorbable antibiotics to rodent dams resulted in shifts of both the maternal and offspring gut microbiomes and induced hypoactivity compared to controls (Degroote et al., 2016; Tochitani et al., 2016). Moreover, offspring exhibited anxiety-like behavior and deficits in locomotion (Tochitani et al., 2016). Similarly, offspring Wistar rats exhibited reduced social behavior and increased anxiety as a result of nonabsorbable antibiotics administration to dams early in gestation (Degroote et al., 2016). A recent report indicates that maternal diet may also change both the microbial population and behavior of offspring (Buffington et al., 2016), following clinical data showing an association between maternal obesity and an ASD diagnosis in children (Krakowiak et al., 2012). A maternal high-fat diet in mice was sufficient to render offspring that are less social and exhibited repetitive behavior, compared to controls fed normal chow. The social deficit in these mice could be reversed by administration of Lactobacillus reuteri, found to be missing in the gut microbiome of high-fat diet offspring (Buffington et al., 2016). Similarly, behavioral deficits induced by antibiotic treatment during pregnancy could be rescued by cross-fostering offspring with control dams (Degroote et al., 2016; Tochitani et al., 2016). These examples indicate that maternal microbial populations can impact behavioral outcomes in the offspring. Whether these changes are mediated indirectly through effects on maternal behavior, or directly alter fetal brain development, remains to be determined.
While the fetal environment may or may not harbor a microbiome, the fetus is inarguably exposed to microbial products from the mother, such as secondary metabolites, fermentation products, LPS, and/or peptidoglycan (PG). MacPherson and colleagues (2016) elegantly demonstrated that microbial metabolites produced in the maternal gut during transient colonization of otherwise GF mice with an auxotrophic strain of Escherichia coli can reach the fetal compartment and induce a specific developmental program prenatally. Bacterial cell wall components can also affect offspring: PG can cross the placenta and reach the fetal brain, where it induces proliferation of neurons in the frontal cortex, via increased expression of FOXG1, a critical regulator of forebrain development and neurogenesis. Offspring exposed to PG prenatally exhibit decreased cognitive function (Humann et al., 2016). Exposure to other microbial products prenatally and neonatally has impacts on offspring behavior. Offspring to dams exposed to propionic acid or LPS, injected subcutaneously, exhibited anxiety-like behaviors (Foley et al., 2014). These effects were observed even when offspring were exposed neonatally, suggesting a direct effect on the offspring rather than on maternal behavior (Foley et al., 2014). The maternal microbiome, the microbiome transmitted to offspring, their metabolites, and other microbial products are important in driving a developmental program in a healthy trajectory, and when perturbed are sufficient to induce behavioral deficits in offspring.
The maternal immune system comes in close interaction with both gut microorganisms and the fetus. Immune activation during gestation has potentially severe implications on offspring physiology, neuropathology and behavior, as well as the microbiome (reviewed by Estes and McAllister, 2016; Knuesel et al., 2014). Large-scale epidemiological studies have demonstrated that prenatal infections significantly increase the risk for schizophrenia in offspring (Khandaker et al., 2013), and data support involvement in ASD as well, although less conclusively (Gardener et al., 2011). Based on these findings, rodent models for maternal immune activation (MIA) have been developed, where prenatal administration of the Toll-like receptor ligands LPS or Poly(inosine:cytosine) as surrogates for infection induced detrimental effects on offspring neuropathology and behavior (Estes and McAllister, 2016). Furthermore, changes in offspring microbiome following MIA have been reported with implications on the metabolomic profile in the serum of these offspring (Hsiao et al., 2013). Intervention with the human commensal bacterium Bacteroides fragilis corrected many of the adverse effects induced by MIA (Hsiao et al., 2013). Specifically, B. fragilis treatment decreased intestinal barrier permeability and lowered the concentration of potentially pathogenic metabolites (Hsiao et al., 2013). Furthermore, a recent study found that MIA phenotypes depend on Th17 cells and the production of IL-17A (Choi et al., 2016); Interestingly, the development of this T helper cell was previously shown to depend on gut bacteria (Ivanov et al., 2009). MIA models demonstrate one possible axis through which the gut microbiome and immune system act in concert to shape offspring physiology, behavior, and neuropathology.
Postnatal brain development
Postnatal brain development is predominantly governed by synaptic development and plasticity, including the overproduction and elimination of synapses during the first decade of human life (Paolicelli et al., 2011; Zuchero and Barres, 2015). Although neurogenesis is highly limited postnatally and is confined to the subventricular zone of the lateral ventricle and the subgranular zone of the hippocampal dentate gyrus, glial cells continue proliferating, migrating and differentiating throughout postnatal development and partially throughout life. Glial progenitors proliferate in the subventricular zone of the forebrain and migrate to various regions of the brain, where they differentiate to oligodendrocytes and astrocytes (Menn et al., 2006). Oligodendrocyte progenitor cells extend processes to neighboring axons and differentiate to oligodendrocytes and myelinate these axons, a process that extends over the first 2–3 decades of life in the frontal lobes of the cerebral cortex and that is crucial to development of higher cognitive function in humans.
Long-term antibiotic treatment of adult mice is sufficient to induce decreased neurogenesis in the hippocampus of adult mice, and results in deficits in the novel object recognition task (Möhle et al., 2016). Voluntary exercise and probiotic treatment are sufficient to rescue these phenotypes (Möhle et al., 2016). Reduced numbers of CD45+CD11b+Ly-6chiCCR2+ monocytes, but not microglia, were observed in the brains of antibiotic-treated animals. Remarkably, CCR2−/− knock-out animals, as well as Ly-6chi-monocyte depleted animals, show reduced hippocampal neurogenesis. Lastly, adoptive transfer of Ly-6chi monocytes into antibiotic-treated animals was sufficient to rescue the neurogenesis phenotype in the hippocampus, indicating that circulating monocytes play an important role in adult neurogenesis (Möhle et al., 2016). These reports suggest that neurogenesis, apoptosis, and synaptic pruning may be regulated by signals from the microbiome. However, more research is needed to mechanistically study the role of the microbiome in these processes in both animal models and humans. Adult neurogenesis can also be promoted by serotonin (Alenina and Klempin, 2015), and gut bacteria have been shown to play a role in serotonergic pathways both in the gut and in various regions of the brain (reviewed by O’Mahoni et al., (2015).
During postnatal brain development, astrocytes and microglia are thought to facilitate pruning of weak neuronal synapses by complement activation and subsequent phagocytosis (Hong and Stevens, 2016). In the absence of microglia, for example, the adult brain harbors significantly more synapses, exemplifying that these glial cells are necessary for synaptic pruning (Paolicelli et al., 2011; Zhan et al., 2014). While complement components are secreted by multiple cells in the CNS, astrocytes and microglia are major producers of complement (Bahrini et al., 2015; Stephan et al., 2012). Microglia produce copious amounts of C1q, the first protein in the complement activation cascade, and express various complement receptors (Schafer et al., 2012; Stephan et al., 2012). Synaptic pruning, as well as other processes, shape neuronal connections after cell differentiation and migration, refining neuronal networks following major events in postnatal brain development.
Proper conductance in neuronal axons is essential for information and signal relay, and myelination is a critical process in the development of a healthy brain that continues well into adolescence (Davison and Dobbing, 1966). Two reports demonstrated that the presence of an intact gut microbiome modulates myelination. In these studies, myelin-related transcripts were increased in the prefrontal cortex, but not other brain regions, as a result of antibiotic-treatment (Gacias et al., 2016) or in GF mice (Hoban et al., 2016). Interestingly, while antibiotic treatment was sufficient to induce elevated expression of myelin-related genes in non-obese-diabetic (NOD) mice and could be transferred by microbiome transplantation from these mice to C57Bl/6 mice (Gacias et al., 2016), colonization of GF animals with SPF microbiota did not rescue the myelin phenotype (Hoban et al., 2016). These observations suggest that early-life exposure to the microbiome is necessary for dynamic response to changes in the microbiome later in life. In addition, Oligodendrocyte development and differentiation relies on various signals, among them are the chemokine CXCL1 and its receptor CXCR2. Notably, CXCL1 expression was shown to be differentially induced in the brains of an ischemic stroke mouse model, in mice with different microbiotas, suggesting a potential role during homeostasis and development, although further investigation is needed (Benakis et al., 2016).
The early life gut microbiome and its impact on brain development and behavior
Under the assumption that the fetus is sterile of bacteria, the first direct encounter an infant has with the microbial world is during birth. Infants born via vaginal delivery are colonized by microbial populations that are closely related to maternal vaginal populations, dominated by Lactobacillus and Prevotella species (Dominguez-Bello et al., 2010). In contrast, infants born by cesarean-section (C-section) are exposed to and colonized by skin microbes such as Staphylococcus and Corynebacterium (Bäckhed et al., 2015; Dominguez-Bello et al., 2010). This initial exposure to such distinct microbial populations has various implications over the health and development of a newborn, with long-term consequences. In addition, prenatal stress changes the vaginal microbiome, and has been shown to subsequently remodel the gut microbiome and metabolome of the offspring(Jašarević et al., 2015b). It has been documented that children born by C-section are at a higher risk for autoimmune diseases (Sevelsted et al., 2015). However, some restoration towards vaginal-derived microbial status can be achieved by exposing newborns to vaginal microorganisms derived from their mothers (Dominguez-Bello et al., 2016). The infant microbiome is highly sensitive to various perturbations like changes in diet and antibiotic treatment (Bäckhed et al., 2015; Koenig et al., 2011; Yassour et al., 2016). Moreover, infants are exposed to vertical acquisitions of novel microorganisms through intimate interaction with parents and siblings, as well as exposure to new environments (Bäckhed et al., 2015; Bordenstein and Theis, 2015; Rosenberg and Zilber-Rosenberg, 2016). Bäckhed and colleagues (2015) have identified signature taxa for various stages during the first year of life. While the newborns’ gut is predominantly aerobic and inhabited by Bifidobacterium, Enterococcus, Escherichia/Shigella, Streptococcus, Bacteroides, and Rothia, gut bacterial populations are significantly closer to those of mothers, and more anaerobic, by the age of one. At this stage, children are already colonized with Clostredium, Ruminococcus, Veilonella, Roseburia, Akkermansia, Alistipes, Eubacterium, Faecalibacterium and Prevotella, in addition to other bacteria. The gut microbiome during the first three years of life is more amenable and prone to perturbations. Interestingly, as some bacteria can be transmitted from mother to newborn, probiotic administration to mothers during pregnancy can transfer specific species to newborns (Dotterud et al., 2015). Hence, this period of life is critical for the establishment of a healthy, stable microbiome (Lloyd-Price et al., 2016a; Yatsunenko et al., 2012).
Disruption of the microbiome, by administration of antibiotics or drastic changes in diet, or conversely, augmentation with probiotic microorganisms, has profound effects on the microbial community and its trajectory through life. Perturbations of the microbial community may have significant impacts on the developing individual, with long lasting effects on metabolism, physiology and immune status, as has been suggested in animal studies (Blaser, 2016; Kuperman and Koren, 2016; Zeissig and Blumberg, 2014). Others have reported that antibiotic administration during the first year of life was correlated with depression and behavioral difficulties later in life (Slykerman et al., 2016). The administration of antibiotics pre- or post-natally changes the physiological status of the mother or its offspring in animal models, and subsequently may impact the developmental trajectory of the offspring’s brain, or alternatively modulate behavior via primary effects on maternal behavior. Partial depletion of an animal’s associated microbiota for a short period of time may not always impact behavior; a short-term treatment of rat neonates with vancomycin had no effect on anxiety- and depressive-like behaviors in adulthood. However, during adulthood these rats showed visceral hypersensitivity, indicating that gut microbes can impact nociception (Amaral et al., 2008; O’Mahony et al., 2014). In adult mice, a seven-day course of nonabsorbable antibiotics was sufficient to decrease anxiety-like behavior. Interestingly, this effect was short-lasting, and behavior normalized to baseline within two weeks (Bercik et al., 2011a), as the microbiome likely returned to its initial state. Long-term broad-spectrum antibiotic treatment from weaning through adulthood restructured the microbiome and subsequently modulated brain chemistry and behavior (Desbonnet et al., 2015). Correspondingly, GF animals display various behavioral and developmental phenotypes, compared to SPF animals (Arentsen et al., 2015; Bercik et al., 2011a; Desbonnet et al., 2014; Gareau et al., 2011; Heijtz et al., 2011; Luczynski et al., 2016b; Neufeld et al., 2011) (Table 1, Fig. 1). These observations indicate a close connection between the microbiome and behavior, and suggest possible pathways through which they interact.
Probiotic administration augments the population with a specific microbe, either transiently or permanently, and can change the microbiome profile and function, as well as interact with the host. Probiotic bacteria have been demonstrated to alter, reverse, or prevent various conditions in mouse models and humans. In addition, experiments with reconstitution of the gut microbial population with healthy consortia by fecal microbiota transplantation (FMT) are underway (Borody and Khoruts, 2012; Hourigan and Oliva-Hemker, 2016). Beneficial bacteria were found to reduce responses to stress and anxiety, depressive-like behavior, promote social behavior, decrease repetitive behavior, and improve cognitive function and communication in animals (Ait-Belgnaoui et al., 2012, 2014; Bercik et al., 2011b; Bravo et al., 2011; Buffington et al., 2016; Gareau et al., 2011; Hsiao et al., 2013; Sudo et al., 2004; Sun et al., 2016). This concept has been also expanded to humans where healthy volunteers that consumed a fermented milk product (containing several different probiotic bacteria) showed different brain activity during an emotional faces attention task, as measured by fMRI, in brain regions that control processing of sensation and emotion (Tillisch et al., 2013).
Mounting evidence suggests that the communication between the brain and gut microbial populations is bi-directional (Bailey et al., 2011; Carabotti et al., 2015; Moussaoui et al., 2014; Park et al., 2013). Using the maternal separation model in mice, De Palma et al. (2015) demonstrated profound differences in the gut microbiome in response to early-life stress resulted in an anxiety-like phenotype. Moreover, gene expression in the amygdala differs between GF and SPF animals (Stilling et al., 2015). A reciprocal effect by gut bacteria has been reported as well, where specific bacteria, or complete microbial assemblages, had effects on host stress- and depression-like behaviors (Bercik et al., 2011a; Gacias et al., 2016; Sudo et al., 2004). While it is yet unclear if these examples are driven by a direct gut-brain interaction, or mediated by other physiological factors induced by the disease state, these reports and others exemplify potential interactions between the microbiome, the gastrointestinal tract, and the brain.
The adult “steady-state” microbiome
In adulthood, the microbiome reaches a relative equilibrium in terms of bacterial abundance and diversity, and does not change significantly under stable environmental or health conditions. Known determinants that shape the microbiome are genetics (Goodrich et al., 2016), diet (Carmody et al., 2015; David et al., 2014), lifestyle (Allen et al., 2015; Kang et al., 2014) and geography (Rampelli et al., 2015; Yatsunenko et al., 2012). Health is defined rather ambiguously as “..the absence of any overt disease” (Aagaard et al., 2013; Lloyd-Price et al., 2016a). The healthy human microbiome was recently reviewed extensively by Huttenhower and colleagues (2016a). The human microbiome is niche-specific, with microbial diversity and abundance differing significantly from niche to niche. Each of these niches is colonized by specific microbial assemblages, with the phyla Bacteroidetes and Firmicutes dominating the intestine, Streptococcus sp. dominating the oral cavity, Corynebacterium, Propionibacterium, and Staphylococcus dominating the skin, and Lactobacillus dominating the vagina (Lloyd-Price et al., 2016a). Higher microbial diversity is correlated with health and functional redundancy (Lozupone et al., 2012; Moya and Ferrer, 2016). Importantly, the healthy microbiome is temporally stable, even when subjected to recurrent mild disturbances (Dethlefsen et al., 2008; Oh et al., 2016; Schloissnig et al., 2013). Decreased diversity, or lack of redundancy, in the microbiome has been reported in multiple diseases (Lloyd-Price et al., 2016a).
The microbiome in neurodevelopmental and mood disorders
Neurodevelopmental disorders are classically studied from a genetic perspective (Parikshak et al., 2015; de la Torre-Ubieta et al., 2016). However, gastrointestinal comorbidities and food allergies are common in neurodevelopmental disorders, suggesting a role for the gut microbiome (de Theije et al., 2014). Thus, an appreciation for a microbial role in these conditions has been gained through profiling bacterial populations in fecal samples of patients and controls. Recent reports support the notion that the microbiome, or its disruption, can contribute to the pathology of various neurologic disorders, using mouse models and intervention studies. Evidence in rodent models suggests a direct link between the gut microbiota and stress and anxiety (reviewed by (Foster and McVey Neufeld, 2013). These observations in animal models is supported by data in human subjects that associates the gut microbiome in IBD to stress disorders (Bonaz and Bernstein, 2013; Fond et al., 2014).
Autism spectrum disorder (ASD)
The gut microbiome of ASD children has been studied in multiple different cohorts using various methodologies, and a consensus between studies was rarely reported. It appears as though these small studies fail to generate a coherent picture, although differences in species richness and their diversity between ASD and controls have been repeatedly reported (De Angelis et al., 2013; Finegold et al., 2002, 2010; Kang et al., 2013; Parracho et al., 2005; Son et al., 2015; Williams et al., 2011, 2012). The gut microbiome of ASD patients have increased abundance and diversity of Clostridia species, and a general increase in non-spore forming anaerobes and microaerophilic bacteria, compared to neurotypical controls (De Angelis et al., 2013; Finegold et al., 2002, 2010; Parracho et al., 2005). Gastrointestinal comorbidities are significantly more prevalent in children with ASD compared to controls (Mannion et al., 2013; McElhanon et al., 2014). These comorbidities often coincide with differences in the gut microbiome of these children (Son et al., 2015; Williams et al., 2011, 2012). Interestingly, Sutterella was found in close association with the intestinal epithelium of ASD children presenting gastrointestinal symptoms, while it was absent in controls (Williams et al., 2012). Interestingly, Kang et al. (2013), reported the absence of specific probiotic members of the community such as Prevotella from the ASD gut bacterial population, suggesting that augmenting the microbiome with a specific microbe may be beneficial. These studies, and others, indicate a potential relationship between the microbiome (or specific microbes) and the brain in autism. Large-scale cross-center studies, using standardized methodologies, would help delineate what are the significant differences in the gut microbiome of ASD patients. Subsequent functional studies into the properties of the gut microbiome in ASD will shed light on mechanism by which the gut microbiome contributes to pathology and behavior. Understanding interactions between specific microbial species and the brain during development may help unravel the etiology of this enigmatic disorder.
Schizophrenia
To date, few studies of the schizophrenia microbiome exist. Higher incidence of lactic-acid bacteria in the oropharyngeal microbiome was reported, compared to controls (Castro-Nallar et al., 2015). Interestingly, a Lactobacillus specific phage was also discovered in high abundance (Yolken et al., 2015). Another study reported a blood-specific microbiome in schizophrenia patients, compared to controls, with higher alpha and beta diversity (Mangul et al., 2016; Severance et al., 2013). Further research in large populations is needed to show whether these differences are consistent throughout the population, further profiles microbiomes associated with this condition, and test the potential roles for gut microbes in the etiology of schizophrenia.
Depression (Major Depressive Disorder; MDD)
Evidence in rodent models suggest that the gut microbiome plays a role in depressive-like behaviors (Bravo et al., 2011; Desbonnet et al., 2010; Ferreira Mello et al., 2013). Gastrointestinal symptoms are associated with depression, with approximately 20% of patients reporting such symptoms (Mussell et al., 2008). One hypothesis claims that depression, or subsets of this disorder, is a microglial disorder, as the onset of depression often follows intense inflammatory episodes in the brain, or conversely, decline in microglial function (Yirmiya et al., 2015). Interestingly, minocycline, a tetracycline antibacterial agent known to inhibit the activation of microglia, can diminish depressive behaviors in rodents (Molina-Hernández et al., 2008; Zheng et al., 2015b) and humans (Miyaoka et al., 2012), and has been suggested as a potent antidepressant. In light of recent evidence on the role of the microbiome in microglia maturation and activation (Erny et al., 2015; Matcovitch-Natan et al., 2016), it is compelling to speculate that the microbiome impacts depression by influencing microglial maturation and activation. It should be noted, however, that it is yet to be determined whether the antidepressive effects of minocycline are due to its antimicrobial properties, inhibition of microglial activation, or a combination of the two. Recently, Zheng et al. (2016) have shown that the beta-diversity of the gut microbiome in MDD patients is significantly different from that of healthy controls, with significantly more Actinobacteria and less Bacteroidetes in MDD associated microbial populations. Further, the authors transplanted human samples from MDD and control samples to GF mice, and show that recipients of MDD samples exhibit depressive-like phenotypes, compared to controls (Zheng et al., 2016). These findings were reproduced by another group, where decreased bacterial richness and diversity were reported in depression, and depressive-like phenotypes could be transmitted by fecal transplantation into rats (Kelly et al., 2016). Mouse and human studies now show that the microbiome plays an active role in driving depressive-like behaviors, suggesting potential new avenues for therapeutic development.
Neurodegeneration and the microbiome in old age
Throughout aging, mammals undergo physiological changes that increase susceptibility to disease. Interestingly, the incidence of some gastrointestinal diseases increase with age (Britton and McLaughlin, 2013), and prevalence of diagnosed GI disorders is approximately 24% in people over 65 (Alameel et al., 2012). Notably, the enteric nervous system degenerates with age, starting in adulthood. Cholinergic nerves, as well as enteric glia cells, are lost in both the myenteric plexus and the submucosal plexus (Phillips and Powley, 2007). This degeneration is partially responsible for the increase in bowel motility symptoms prevalent in the elderly (O’Mahony et al., 2002).
The microbiome also undergos profound remodeling in elderly populations (over 65 years old)(Biagi et al., 2011; O’Toole and Claesson, 2010; Salazar et al., 2014). Major shifts in bacterial taxa have been reported in the fecal microbiome of elderly people, compared to infants and young adults, and these shifts correlate with health status and frailty in elderly people (Claesson et al., 2011; van Tongeren et al., 2005). Notably, while in adulthood Firmicutes outnumber Bacteroidetes in the gut, it appears as though the ratio shifts in favor of Bacteroidetes in the elderly (Claesson et al., 2011; Mariat et al., 2009). Specifically, Bacteroides, Alistipes, Parabacteroides, and Proteobacteria (gamma-proteobacteria, specifically) are significantly more prevalent in the elderly compared to younger adults (Claesson et al., 2011; Mariat et al., 2009). Moreover, various studies show that the microbiome of elderly living in the community have a similar microbial diversity to that of younger adults than that of elderly people staying in short or long term care facilities (Claesson et al., 2012). Langlie et al. (2014) reported that similar differences occur in older mice, compared to a younger group, and is highly correlated with mouse frailty. A clinical study, testing the effects of Lactobacillus rhamnosus GG consumption in an elderly cohort, found that the probiotic restructured the bacterial population towards an anti-inflammatory phenotype that favors the survival and growth of beneficial microbes and elevated SCFA production (Eloe-Fadrosh et al., 2015). The microbiome in the elderly population is significantly different from that of younger adults, is less diverse and resilient, and can be modulated by environmental factors and interventions.
Remarkably, the gut microbiome of centenarians differs significantly from that of other adults (Biagi et al., 2010, 2016). Biagi and colleagues found that a core gut microbiome, comprised of species in the Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae families, is associated with the human host throughout life, with decreasing cumulative abundance. Specifically, Coprococcus, Roseburia, and Faecalibacterium, were found to be negatively correlated with age (Biagi et al., 2016). Other genera are positively correlated with age; among these are Oscillospira and Akkermansia (Biagi et al., 2016). Interestingly, certain subdominant bacterial species are enriched in centenarians, some of which are known to exert beneficial functions on their host, and may play a role in maintenance of health in old age (Biagi et al., 2016). Insights into the microbiome of centenarians may unravel specific microbes with beneficial and protective effect on the host.
The microbiome in neurodegenerative diseases
How initial interactions with gut microbes alter events later in life, such as during neurodegenerative diseases, is still unclear. A small number of studies to date have demonstrated different gut microbial populations in both human and animal models of neurodegenerative disease. Preliminary observations in the APP/PS1 mouse model of Alzheimer’s disease (AD) indicate that these animals harbor decreased Allobaculum and Akkermansia, with an increase in Rikenellaceae compared to wild-type controls (Harach et al., 2015). Concurrently, individuals afflicted with Parkinson’s disease (PD) display significantly different fecal and mucosal microbial populations (Hasegawa et al., 2015; Keshavarzian et al., 2015; Scheperjans et al., 2015). Prevotellaceae show decreased, while Lactobacilliaceae have increased, abundance compared to controls (Hasegawa et al., 2015; Scheperjans et al., 2015). Relative levels of Enterobacteraceae in feces is sufficient to discriminate between specific forms of PD; patients displaying the tremor-dominant form of PD had significantly lower Enterobacteraceae relative abundance than those with the more severe postural and gait instability (Scheperjans et al., 2015). In fact, intestinal biopsies of PD patients have indicated increased tissue-associated E. coli compared to healthy controls (Forsyth et al., 2011), further demonstrating the presence of an altered gut microbial community in individuals diagnosed with neurodegenerative diseases. However, this research correlating changes in the microbiome and neurodegeneration remain largely descriptive; how different microbial populations arise, and their physiological consequences, if any, remain unknown.
Neuroinflammation is postulated to play a key role in the pathology of neurodegenerative diseases (Cappellano et al., 2013; Glass et al., 2010). Proinflammatory cytokines produced both in the brain and periphery modulate neuronal function and can initiate pathologic cell death (Koprich et al., 2008; McCoy and Tansey, 2008). Given the importance of microglia functions in both the prevention and promotion of neurodegenerative processes, it is tempting to speculate that the gut microbiota may influence these inflammatory diseases of the aging brain. As discussed above, bacterial fermentation products, namely SCFAs, can drive the maturation of microglia and are needed for maintenance of mature microglia (Erny et al., 2015). Interestingly, decreased SCFA concentrations in feces from PD patients, compared to controls, were recently reported (Unger et al., 2016). Passage of MAMPs from the intestine and into the brain may produce low levels of inflammation (Pal et al., 2015). Such persistent, proinflammatory signaling has been linked to the severity of neurodegenerative disease (Cappellano et al., 2013; Glass et al., 2010). Interestingly, a recent report suggests that a physiologic role of the amyloid protein Aβ, known to form pathogenic plaques in AD, is as an antimicrobial agent to eliminate bacterial infection in the brain (Kumar et al., 2016). Studies to understand the long-term consequences of neurophysiological changes by the microbiota are critical.
While dysfunction in BBB integrity and maturation of microglia could have global effects, a recent study has revealed a specific example of neurodegenerative disease mediated by gut microbes. In a mouse model of sporadic uveitis, an inflammation of the middle layer of the eye, animals exhibit increased inflammation and loss of cells in the neuroretina leading to vision dysfunction. This inflammation is mediated by autoreactive T cells that recognize antigens present in the retina, a typically immune-privileged tissue. The activation of autoreactive T cells in these mice is dependent on the microbiota (Horai et al., 2015). In fact, not only are these T cells reactive towards retinal proteins, they are also capable of recognizing microbial antigens present in the gut (Horai et al., 2015). At this time, however, the microbial antigen driving this autoimmune neuroinflammation is unknown. Nonetheless, this observation suggests that “molecular mimicry” of host molecules present in the microbiota can trigger autoimmune responses that promote neurodegeneration. Other microbial molecules that mimic host structures have been suggested to play roles in promoting immune responses during AD and PD (Friedland, 2015; Hill and Lukiw, 2015). Therefore, one might consider the early developmental presence of certain gut microbes that produce these molecular mimics to potentially act as risk factors for specific immune and neurodegenerative diseases. However, no direct observation of this hypothesis has been reported to date, and the link between age-dependent influences to the microbiome and neuropathology are an active area of research.
Perspective
The microbiome plays a significant role in the well-being of its host. While much of the research on this topic to date has demonstrated that different bacterial populations are associated with certain clinical conditions, it is unclear for the most part whether these differences are causative, promote and/or enhance disease, or instead are a consequence of otherwise unrelated pathophysiology. Future research should tackle this challenging question in order to understand the intricate interaction between mammals (or any other host) and their associated microbial community. We must not continue exercises in simply cataloging bacterial populations. Rather, we must extend this foundational research approach to test the functional and ecological roles that a given microbial population plays, as well as decipher the physiological effects individual bacteria or consortia of bacteria have on their animal hosts. It is of importance to address questions of cause and effect: are changes in the microbiome underlying the pathophysiology or are they a result thereof? Are the effects on behavior direct, or a result of other fundamental physiological changes? Are there defined microbial features that are necessary and sufficient to support proper neurodevelopment and prevent neurodegeneration? The use of animal models is a great tool for studying basic processes in health and disease. However, we must use caution in extrapolating results to the human condition, and strive to use preclinical findings as one of several approaches to inform human health and disease.
While research on the gut-brain axis is still in relative infancy, certain basic rules have begun to emerge. It appears as though specific neurological pathways evolved to respond to the effect of microbial population, while others are unaffected by microbiome “instruction” and subject to purely genomic or other environmental cues. Interaction with host-associated microbial communities, either directly via microbial metabolites or indirectly by the immune, metabolic or endocrine systems, can supply the nervous system with real-time information about the environment. These cues converge to control basic developmental processes in the brain such as barrier function, immune surveillance, and neurogenesis. The mechanistic understanding of how different microbial populations, beneficial or pathogenic, govern these and other functions related to health and disease holds promise in the diagnosis, treatment, and prevention of specific neuropathologies. Determining how a microbiome, changing with Westernization and other environmental factors, impacts a human population with growing rates of neurodevelopmental disorders and increasing life expectancy represents an urgent challenge to biomedical research, and to society.
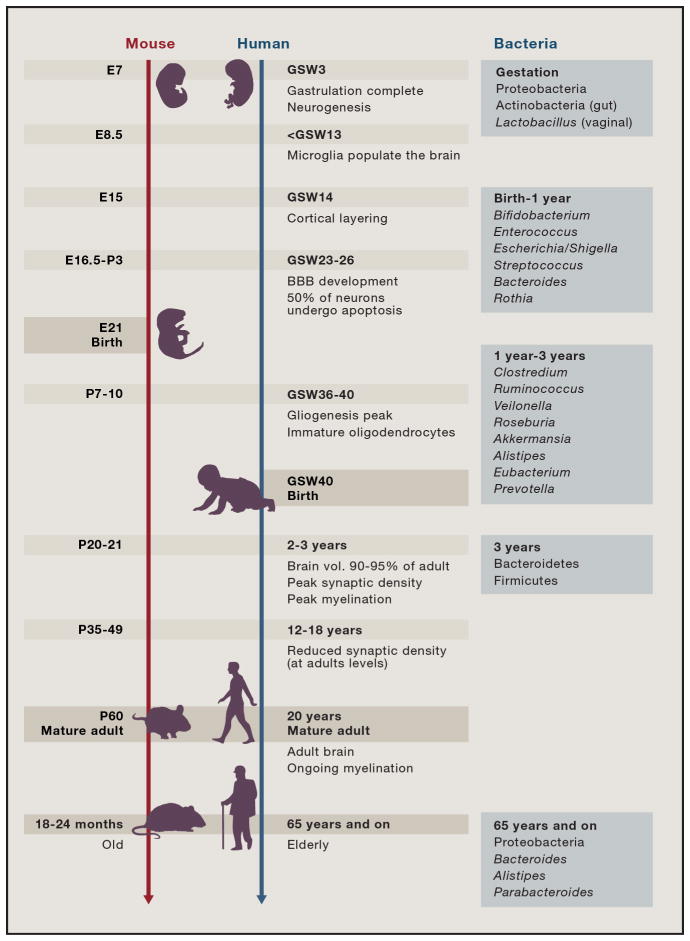
Figure 2. Major events in mammalian brain development.
Developmental trajectories and key neurodevelopmental events in mice and humans (adapted from(Knuesel et al., 2014; Pressler and Auvin, 2013; Semple et al., 2013). E-embryonic age, P-postnatal age, GSW-gestational week. Bacterial taxa on the right panel are the dominant ones at each life stage (Bäckhed et al., 2015; Lloyd-Price et al., 2016b; Nuriel-Ohayon et al., 2016).
Acknowledgments
The authors apologize to colleagues whose work could not be included in this Review. We thank Drs. Hiutung Chu and Wei-li Wu, as well as Carly Stewart for critical reading of this manuscript. The authors are supported by the Meixner Postdoctoral Fellowship in Translational Research (to G.S.) and the Larry L. Hillblom Foundation Postdoctoral Fellowship (to T.R.S). Research in the Mazmanian laboratory is funded by grants from the National Institutes of Health (MH100556, DK078938, GM099535 and NS085910), the Department of Defense, the Heritage Medical Research Institute, and the Simons Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard K, Petrosino J, Keitel W, Watson M, Katancik J, Garcia N, Patel S, Cutting M, Madden T, Hamilton H, et al. The Human Microbiome Project strategy for comprehensive sampling of the human microbiome and why it matters. FASEB J. 2013;27:1012–1022. doi: 10.1096/fj.12-220806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology. 2012;37:1885–1895. doi: 10.1016/j.psyneuen.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Colom A, Braniste V, Ramalho L, Marrot A, Cartier C, Houdeau E, Theodorou V, Tompkins T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol Motil. 2014;26:510–520. doi: 10.1111/nmo.12295. [DOI] [PubMed] [Google Scholar]
- Alenina N, Klempin F. The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res. 2015;277:49–57. doi: 10.1016/j.bbr.2014.07.038. [DOI] [PubMed] [Google Scholar]
- Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD, Woods JA. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol. 2015;118:1059–1066. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- Amaral FA, Sachs D, Costa VV, Fagundes CT, Cisalpino D, Cunha TM, Ferreira SH, Cunha FQ, Silva TA, Nicoli JR, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A. 2008;105:2193–2197. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis. 2015;26:29719. doi: 10.3402/mehd.v26.29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Bahrini I, Song J-H, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep. 2015;5:7989. doi: 10.1038/srep07989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal [gamma][delta] T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011a;141:599–609. 609.e1–e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut–brain communication. Neurogastroenterology & Motility. 2011b;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al. Gut Microbiota and Extreme Longevity. Curr Biol. 2016;26:1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Bordenstein SR, Theis KR. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton E, McLaughlin JT. Ageing and the gut. Proc Nutr Soc. 2013;72:173–177. doi: 10.1017/S0029665112002807. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellano G, Carecchio M, Fleetwood T, Magistrelli L, Cantello R, Dianzani U, Comi C. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:89–107. [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol Hepatol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Pérez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceni C, Unsain N, Zeinieh MP, Barker PA. Neurotrophins in the Regulation of Cellular Survival and Death. In: Lewin GR, Carter BD, editors. Neurotrophic Factors. Springer; Berlin Heidelberg: 2014. pp. 193–221. [DOI] [PubMed] [Google Scholar]
- Chang C-Y, Ke D-S, Chen J-Y. Essential fatty acids and human brain. Acta Neurol Taiwan. 2009;18:231–241. [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AN, Dobbing J. Myelination as a vulnerable period in brain development. Br Med Bull. 1966;22:40–44. doi: 10.1093/oxfordjournals.bmb.a070434. [DOI] [PubMed] [Google Scholar]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote S, Hunting D, Baccarelli AA, Takser L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016 doi: 10.1016/j.pnpbp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015;6:7735. doi: 10.1038/ncomms8735. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19:146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DSA, Wong RJ, Shaw G, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterud CK, Avershina E, Sekelja M, Simpson MR, Rudi K, Storrø O, Johnsen R, Øien T. Does Maternal Perinatal Probiotic Supplementation Alter the Intestinal Microbiota of Mother and Child? J Pediatr Gastroenterol Nutr. 2015;61:200–207. doi: 10.1097/MPG.0000000000000781. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira Mello BS, Monte AS, McIntyre RS, Soczynska JK, Custódio CS, Cordeiro RC, Chaves JH, Mendes Vasconcelos SM, Nobre HV, Júnior, Florenço de Sousa FC, et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res. 2013;47:1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Molitoris D, Song Y, Liu C, Vaisanen M-L, Bolte E, McTeague M, Sandler R, Wexler H, Marlowe EM, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35:S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Foley KA, Ossenkopp KP, Kavaliers M, Macfabe DF. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One. 2014;9:e87072. doi: 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651–660. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld K-A. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J Alzheimers Dis. 2015;45:349–362. doi: 10.3233/JAD-142841. [DOI] [PubMed] [Google Scholar]
- Gacias M, Gaspari S, Santos P-MG, Tamburini S, Andrade M, Zhang F, Shen N, Tolstikov V, Kiebish MA, Dupree JL, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016:5. doi: 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, Davenport ER, Waters JL, Clark AG, Ley RE. Cross-species comparisons of host genetic associations with the microbiome. Science. 2016;352:532–535. doi: 10.1126/science.aad9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Dutilleul N, Cheatham V, Mc Coy KD, Neher JJ, Jucker M, Fåk F, Lasser T, et al. Reduction of Alzheimer’s disease beta-amyloid pathology in the absence of gut microbiota 2015 [Google Scholar]
- Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Lukiw WJ. Microbial-generated amyloids and Alzheimer’s disease (AD) Front Aging Neurosci. 2015;7:9. doi: 10.3389/fnagi.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Stevens B. Microglia: Phagocytosing to Clear, Sculpt, and Eliminate. Dev Cell. 2016;38:126–128. doi: 10.1016/j.devcel.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, Jittayasothorn Y, Chan CC, Yamane H, Honda K, et al. Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourigan SK, Oliva-Hemker M. Fecal microbiota transplantation in children: a brief review. Pediatr Res. 2016;80:2–6. doi: 10.1038/pr.2016.48. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, Farr A, Hu Y, Durick-Eder K, Fillon SA, et al. Bacterial Peptidoglycan Traverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe. 2016;19:388–399. doi: 10.1016/j.chom.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichim G, Tauszig-Delamasure S, Mehlen P. Neurotrophins and cell death. Exp Cell Res. 2012;318:1221–1228. doi: 10.1016/j.yexcr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Rodgers AB, Bale TL. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol Stress. 2015a;1:81–88. doi: 10.1016/j.ynstr.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL. Alterations in the Vaginal Microbiome by Maternal Stress Are Associated With Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology. 2015b;156:3265–3276. doi: 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Jeraldo PR, Kurti A, Miller MEB, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’Brien C, Patterson E, el Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016 doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–257. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1$\beta$ increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:1–12. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajmalnik-Brown R, Lozupone C, Kang D-W, Adams JB. Gut bacteria in children with autism spectrum disorders: challenges and promise of studying how a complex community influences a complex disease. Microb Ecol Health Dis. 2015;26:26914. doi: 10.3402/mehd.v26.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DKV, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. 2016;14:91. doi: 10.1186/s12916-016-0636-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, Leite R, Elovitz MA, Parry S, Bushman FD. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016a;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016b;8:51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016a doi: 10.1111/ejn.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, McVey Neufeld K-A, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int J Neuropsychopharmacol. 2016b doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre DA, Chandiramani M, Lee YS, Kindinger L, Smith A, Angelopoulos N, Lehne B, Arulkumaran S, Brown R, Teoh TG, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangul S, Olde Loohuis LM, Ori A, Jospin G, Koslicki D, Yang HT, Wu T, Boks MP, Lomen-Hoerth C, Wiedau-Pazos M, et al. Total RNA Sequencing reveals microbial communities in human blood and disease specific effects 2016 [Google Scholar]
- Mannion A, Leader G, Healy O. An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with Autism Spectrum Disorder. Res Autism Spectr Disord. 2013;7:35–42. [Google Scholar]
- Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet J-P. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016 doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Wake R, Furuya M, Liaury K, Ieda M, Kawakami K, Tsuchie K, Taki M, Ishihara K, Araki T, et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:222–226. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, et al. Ly6Chi Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- Molina-Hernández M, Tellez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Saunders NR. The development of the human blood-brain and blood-CSF barriers. Neuropathol Appl Neurobiol. 1986 doi: 10.1111/j.1365-2990.1986.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Moussaoui N, Braniste V, Ait-Belgnaoui A, Gabanou M, Sekkal S, Olier M, Théodorou V, Martin PGP, Houdeau E. Changes in intestinal glucocorticoid sensitivity in early life shape the risk of epithelial barrier defect in maternal-deprived rats. PLoS One. 2014;9:e88382. doi: 10.1371/journal.pone.0088382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya A, Ferrer M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016;24:402–413. doi: 10.1016/j.tim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Mussell M, Kroenke K, Spitzer RL, Williams JBW, Herzog W, Löwe B. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J Psychosom Res. 2008;64:605–612. doi: 10.1016/j.jpsychores.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–264. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth and infancy. Front Microbiol. 2016:7. doi: 10.3389/fmicb.2016.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O’Leary OF. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78:e7–e9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M, Kong HH, Segre JA NISC Comparative Sequencing Program. Temporal Stability of the Human Skin Microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony D, O’Leary P, Quigley EMM. Aging and intestinal motility: a review of factors that affect intestinal motility in the aged. Drugs Aging. 2002;19:515–527. doi: 10.2165/00002512-200219070-00005. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014;277:885–901. doi: 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12:252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- Pal GD, Shaikh M, Forsyth CB, Ouyang B, Keshavarzian A, Shannon KM. Abnormal lipopolysaccharide binding protein as marker of gastrointestinal inflammation in Parkinson disease. Front Neurosci. 2015;9:306. doi: 10.3389/fnins.2015.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parikshak NN, Gandal MJ, Geschwind DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet. 2015;16:441–458. doi: 10.1038/nrg3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo M-M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Park AJ, Collins J, Blennerhassett PA, Ghia JE, Verdu EF, Bercik P, Collins SM. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho HMRT, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Pärtty A, Kalliomäki M, Wacklin P, Salminen S, Isolauri E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr Res. 2015;77:823–828. doi: 10.1038/pr.2015.51. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136:1–19. doi: 10.1016/j.autneu.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler R, Auvin S. Comparison of Brain Maturation among Species: An Example in Translational Research Suggesting the Possible Use of Bumetanide in Newborn. Front Neurol. 2013;4:36. doi: 10.3389/fneur.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rampelli S, Schnorr SL, Consolandi C, Turroni S, Severgnini M, Peano C, Brigidi P, Crittenden AN, Henry AG, Candela M. Metagenome Sequencing of the Hadza Hunter-Gatherer Gut Microbiota. Curr Biol. 2015;25:1682–1693. doi: 10.1016/j.cub.2015.04.055. [DOI] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014a;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014b;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, Zilber-Rosenberg I. Microbes Drive Evolution of Animals and Plants: the Hologenome Concept. MBio. 2016:7. doi: 10.1128/mBio.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SL, Gold MJ, Willing BP, Thorson L, McNagny KM, Finlay BB. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 2013;4:158–164. doi: 10.4161/gmic.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- Schloissnig S, Arumugam M, Sunagawa S, Mitreva M, Tap J, Zhu A, Waller A, Mende DR, Kultima JR, Martin J, et al. Genomic variation landscape of the human gut microbiome. Nature. 2013;493:45–50. doi: 10.1038/nature11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelsted A, Stokholm J, Bønnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135:e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148:130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykerman RF, Thompson J, Waldie KE, Murphy R, Wall C, Mitchell EA. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2016 doi: 10.1111/apa.13613. [DOI] [PubMed] [Google Scholar]
- Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, Litcher-Kelly L, Gadow KD, Gathungu G, Robertson CE, et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLoS One. 2015;10:e0137725. doi: 10.1371/journal.pone.0137725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensballe LG, Simonsen J, Jensen SM, Bønnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–838.e3. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, Dinan TG, Cryan JF. Microbes & neurodevelopment--Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–220. doi: 10.1016/j.bbi.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- de Theije CGM, Bavelaar BM, Lopes da Silva S, Korte SM, Olivier B, Garssen J, Kraneveld AD. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr Allergy Immunol. 2014;25:218–226. doi: 10.1111/pai.12149. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. 1401.e1–e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS One. 2016;11:e0138293. doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tongeren SP, Slaets JPJ, Harmsen HJM, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71:6438–6442. doi: 10.1128/AEM.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann K-U, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer K-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016 doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012:3. doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Vatanen T, Siljander H, Hämäläinen A-M, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8:343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–658. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LAB, Savage CLG, et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutritional importance of choline for brain development. J Am Coll Nutr. 2004;23:621S– 626S. doi: 10.1080/07315724.2004.10719433. [DOI] [PubMed] [Google Scholar]
- Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15:307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zheng J, Xiao X, Zhang Q, Mao L, Yu M, Xu J. The Placental Microbiome Varies in Association with Low Birth Weight in Full-Term Neonates. Nutrients. 2015a;7:6924–6937. doi: 10.3390/nu7085315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L-S, Kaneko N, Sawamoto K. Minocycline treatment ameliorates interferon-alpha-induced neurogenic defects and depression-like behaviors in mice. Front Cell Neurosci. 2015b;9:5. doi: 10.3389/fncel.2015.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–796. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–3809. doi: 10.1242/dev.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]