Abstract
Objective
To evaluate the effectiveness of four commonly used induction methods.
Methods
This randomized trial compared four induction methods: Misoprostol alone, Foley alone, Misoprostol–cervical Foley concurrently, and Foley–oxytocin concurrently,. Women undergoing labor induction with full term (≥37 weeks), singleton, vertex presenting gestations, with no contraindication to vaginal delivery, intact membranes, Bishop score ≤6, and cervical dilation ≤2cm were included. Women were enrolled only once during the study period. Our primary outcome was time to delivery. Neither patients nor providers were blinded to assigned treatment group since examinations are required for placement of all methods; however, research personnel were blinded during data abstraction. A sample size of 123 per group (N=492) was planned to compare the four groups pairwise (P≤.008), with a 4-hour reduction in delivery time considered clinically meaningful.
Results
From May 2013 through June 2015, 997 women were screened and 491 were randomized and analyzed. Demographic and clinical characteristics were similar among the four treatment groups. When comparing all induction method groups, combination methods achieved a faster median time to delivery than single-agent methods, (misoprostol–Foley: 13.1 hours, Foley–oxytocin: 14.5 hours, misoprostol: 17.6 hours, Foley: 17.7 hours, p<0.001). When censored for cesarean and adjusting for parity, women who received misoprostol–Foley delivered almost twice as likely to deliver before women who received misoprostol alone (hazard ratio (HR, 95% CI) 1.92 [1.42–2.59]) or Foley alone (HR, 95%CI: 1.87 [1.39–2.52]), whereas Foley–oxytocin was not statistically different from single-agent methods.
Conclusion
After censoring for cesarean and adjusting for parity, misoprostol–cervical Foley resulted in twice the chance of delivering before either single-agent method.
Introduction
Four million women give birth each year in the United States with more than 20 percent of them undergoing an induction of labor.1 As such, induction is one of the most common procedures performed during a woman’s pregnancy. Despite this, the fastest and most effective method of inducing labor is unknown. 2, 3–5
Both mechanical and pharmacologic agents are used for induction of labor.3–5 These agents, when used individually, reduce the incidence of cesarean delivery in women undergoing induction. 3, 4 Plausibly, combining both mechanical and pharmacologic methods may have a synergistic effect in achieving labor. Some studies have shown promise in reducing labor time and risk of cesarean delivery with combination methods,6, 7 while others have not. 8–11
Therefore, the objective of our study was to compare the time to delivery among four different, routinely utilized cervical ripening methods for induction of labor, including two different combination methods. Our hypothesis was that women who undergo an induction with combined methods will have a shorter time to delivery than those who undergo induction with a single method.
MATERIALS AND METHODS
We conducted this randomized clinical trial (The ‘FOR MOMI’ trial: Foley or Misoprostol for the Management of Induction) at the Hospital of the University of Pennsylvania to compare time to delivery among four different methods of induction: misoprostol alone, Foley alone, misoprostol–cervical Foley concurrently, and cervical Foley–oxytocin concurrently. Prior to initiation of the study, approval was obtained from a convened Institutional Review Board at the University of Pennsylvania and registered with ClinicalTrials.gov (NCT01916681).
Participants were at least 18 years of age with a full term (≥37 weeks), singleton gestation in cephalic presentation. Both nulliparous and multiparous women were included. Women were required to have intact membranes, a Bishop score of ≤6 and cervical dilation ≤2cm to be eligible. Women were excluded if there was a contraindication to a vaginal delivery or to misoprostol, fetal demise, or major fetal anomaly. Non-English speaking women, women with HIV, and women with medical conditions requiring an assisted second stage were also excluded. Additional exclusion criteria were as follows: category 3 fetal heart rate tracing, hemolysis elevated liver enzymes and low platelets (HELLP) syndrome or eclampsia, growth restriction <10th percentile (based on Hadlock growth curves) with reversal of flow in umbilical artery Doppler studies, and growth restriction <5th percentile with elevated, absent, or reversal of flow in umbilical artery Doppler studies. No woman had a prior attempt at induction in this pregnancy. Women were included in the study only once. Gestational age was determined using routine obstetrical guidelines. 12
Patients were approached in the obstetrical unit by providers trained in the study consenting process prior to the start of their induction. Once written consent was obtained, the participants were randomized to one of the four treatment groups. An internet based clinical trial management system, Research Electronic Data Capture, REDCap, 13 was accessed to enter enrollment information, ensure eligibility, and request randomized treatment allocation. An independent consultant created a computer-generated randomization scheme that used balanced treatment allocation in blocks of 20. Randomization was stratified by parity.
Each of the four treatment groups had a standardized protocol for induction and active phase labor management. A brief description of the protocols is described below. The complete algorithm for the induction groups and active labor management can be found in the supplemental information (Appendixes 1–5, available online at http://links.lww.com/xxx). Neither the patients nor the providers were blinded to the assigned treatment group as this would not be practical. However, trained research personnel were blinded to study group during data abstraction.
Women in the misoprostol only group received 25 micrograms of misprostol per vagina every 3 hours, repeated up to five additional times for a maximum of 24 hours. Oxytocin was initiated if there was a contraindication to another misoprostol dose or if additional cervical ripening was not indicated (Appendix 1, http://links.lww.com/xxx).
Women in the cervical Foley only group had a 18F Foley catheter with 30cc balloon inserted digitally or by direct visualization with a speculum. The Foley bulb was placed just above the level of the internal os and inflated with 60cc of sterile water.6, 7, 11, 14 The catheter was taped to the inner thigh with gentle traction and deflated and removed after 12 hours if still in place. The oxytocin protocol was initiated once the Foley bulb was no longer in place (Appendix 2, http://links.lww.com/xxx).
Women in the misoprostol–cervical Foley group had both misoprostol and a cervical Foley placed concurrently using the same procedures as noted for the individual groups (Appendix 3, http://links.lww.com/xxx).
Women in the cervical Foley–oxytocin group had a cervical Foley placed using the same protocol as described in the cervical Foley only group. Oxytocin was initiated concurrently at the start of induction (Appendix 4, http://links.lww.com/xxx).
For all participants, our hospital based oxytocin protocol was utilized. The protocol begins with 2 milliunits/minute of oxytocin increasing by 2 milliunits every 15 minutes until regular uterine contractions occur. Forty milliunits of oxytocin is considered the maximum dose with no limit as to the length of time a participant can remain at 40 milliunits.
Providers were able to perform an amniotomy at any point during the labor course. If the patient had not yet had membranes ruptured and cervix was ≥4 cm dilated, it was recommended that an amniotomy be performed at this time, if clinically feasible. Once cervical dilation was ≥5cm, providers were instructed to proceed with the active labor management protocol (Appendix 5, http://links.lww.com/xxx) although active labor itself was defined as cervical dilation ≥6cm. Labor interventions including amnioinfusion, fetal scalp electrode, tocolysis, and management of the second stage (including operative delivery) were at the discretion of the managing provider. All patients had continuous fetal monitoring throughout induction, labor and delivery. Cesarean delivery was at the discretion of the provider. Recommendations specific for this study included cesarean if not in active labor after 36 hours of cervical ripening, or if the patient was undelivered 12 hours after achieving active labor.
The primary outcome measure was time to delivery (hours) defined as time from initiation of induction method to delivery time, regardless of mode of delivery. Secondary outcome measures were Cesarean delivery rate, time to vaginal delivery (hours), time to delivery censored for cesarean section, time to active labor (defined as dilatation ≥5cm), delivery within 12 hours, delivery within 24 hours, and maternal length of stay (defined as length of time from admission for induction to discharge postpartum, days) and indication for cesarean delivery. A composite maternal morbidity outcome was pre-specified to include ≥1 of the following during labor, delivery, or in the 4 weeks postpartum: 3rd/4th degree perineal laceration, blood transfusion, endometritis, wound separation–infection (defined by the need for additional wound closure or the need for antibiotics), venous thromboembolism, hysterectomy, intensive care unit admission, or death. Other maternal secondary outcomes analyzed were chorioamnionitis (defined by the presence of maternal fever ≥100·4°F in the presence of maternal or fetal tachycardia or fundal tenderness), use of terbutaline, intrauterine pressure catheter, amnioinfusion, or analgesia use.
A composite neonatal morbidity outcome was pre-specified to include ≥1 of the following prior to neonatal discharge: severe respiratory distress syndrome (defined as intubation and mechanical ventilation for a minimum of 12 hours), culture proven–presumed neonatal sepsis, neonatal blood transfusion, hypoxic ischemic encephalopathy, intraventricular hemorrhage grade 3 or 4, necrotizing enterocolitis, or receipt of head cooling. Other neonatal outcomes analyzed were neonatal intensive care unit (NICU) admission, NICU admission >48 hours, and neonatal length of stay (days).
Trained research staff, uninvolved with the clinical care, collected all induction, labor and delivery information, maternal demographics, and maternal and neonatal outcomes.
A data safety monitoring board (DSMB) was established to independently evaluate the safety of the study. An interim safety analysis was performed for pre-defined adverse outcomes with recommendations to continue the study without changes.
A 4 hour reduction in time to delivery was considered clinically meaningful. In the literature, the mean time to delivery for patients undergoing induction of labor was 18 hours ±8.5 6–8. Since there is not one method of induction that is thought to be standard of care, we chose to compare all four groups with 6 separate comparisons. Therefore, a type-I alpha error rate of 0.008 was selected. Assuming 80% power, equal group sizes, and a two sided p-value, we would need 112 patients in each group for a total sample size of 448. A crossover–dropout rate of 10% was assumed leading to a final desired sample size of 492.
Descriptive statistics for the primary and secondary labor outcomes are reported both overall and by parity. Statistical analyses were performed using an intention-to-treat principle. Bivariate analyses were carried out using ANOVA and Kruskal-Wallis for normally and non-normally distributed continuous variables, respectively, and Pearson Chi-square or Fisher exact for categorical variables, as appropriate. Time to event regression analysis for labor length (regardless of delivery mode) was modeled with a Cox proportional hazard model. Labor Length with censoring at the time of cesarean delivery was also modeled using a Cox proportional hazards model. Risk of cesarean delivery was estimated using a generalized linear model with robust error variance 15. Final models were adjusted for parity. Hazard ratios (HR) and risk ratios (RR) with the 95% confidence interval are reported. Statistical significance for the primary outcome was set at p<0.008.
A sensitivity analysis was also performed using the induction methods that the patient actually received in order to determine whether crossovers may have influenced the results.
RESULTS
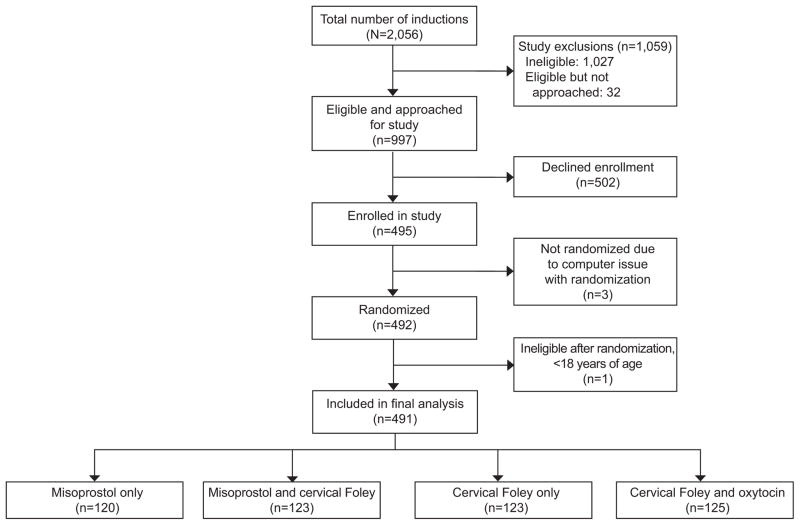
There were 2056 inductions of labor during the study period of May 2013 to June 2015.. Of the 997 women who met eligibility criteria and were approached for enrollment, 502 declined enrollment, and 492 were randomized into one of the four treatment groups (Figure 1). One woman was excluded post-randomization after it was discovered that she was <18 years old, yielding a final sample size of 491 women.
Figure 1.
Flowchart of patients enrolled.
Demographic and clinical characteristics were similar among the four treatment groups including Bishop score, cervical dilation at induction, or indication for induction among the groups (Table 1).
Table 1.
Maternal characteristics by randomized treatment group
| Characteristics | Misoprostol Only (n=120) | Misoprostol/ Foley (n=123) | Foley Only (n=123) | Foley/ Oxytocin (n=125) | |
|---|---|---|---|---|---|
| Maternal Age (years)† | 26.7 [21.8–31.0] | 28 [22.4–33.1] | 27.3 [22.3–32.9] | 26.5 [22.4–31.7] | |
| Maternal BMI (kg/m2)† | 27.5 [24.3–35.9] | 28.3 [23.9–32.6] | 29.1 [24.4–35.7] | 30.1 [24.6–35.8] | |
| Race, Black | 94 (78.3) | 93 (75.6) | 99 (80.5) | 95 (76.0) | |
| Insurance, Public | 80 (66.7) | 78 (63.4) | 81 (65.9) | 87 (69.6) | |
| Prenatal Care Provider, Clinic | 78 (65.0) | 72 (58.5) | 70 (56.9) | 77 (61.6) | |
| Number of prenatal visits† | 9.5 [7–11] | 10 [8–12] | 10 [8–12] | 10 [7–12] | |
| Nulliparous | 70 (58.3) | 73 (59.4) | 73 (59.4) | 74 (59.2) | |
| Gestational age at induction (weeks)† | 39.1 [37.9–40.1] | 39.6 [38.3–40.7] | 39.3 [38.3–40.4] | 39.1 [38.3–40.6] | |
| Bishop score at randomization† | 3 [2–4] | 3 [2–4] | 3 [2–4] | 3 [2–4] | |
| Dilation at randomization† | 1 [0.5–1.5] | 1 [1–1.5] | 1 [0.5–1.5] | 1 [0.5–1.5] | |
| Bishop score at induction† | 3 [2–4] | 3 [3–4] | 3 [2–4] | 3 [2–4] | |
| Dilation at induction† | 1 [1–1.5] | 1.5 [1–2] | 1 [1–1.5] | 1 [1–1.5] | |
| Dilation at amniotomy† | 4 [3–4] | 4 [4–5] | 4 [4–4.5] | 4 [4–5] | |
| Diabetes | |||||
| Gestational diabetes | 6 (5.0) | 8 (6.5) | 5 (4.1) | 14 (11.2) | |
| Pre-gestational | 3 (2.5) | 2 (1.6) | 4 (3.3) | 2 (1.6) | |
| Chronic hypertension | 7 (5.8) | 10 (8.1) | 10 (8.1) | 12 (9.6) | |
| Pregnancy related hypertension | |||||
| GHTN/ Mild preeclampsia | 22 (18.3) | 27 (22.0) | 33 (26.8) | 32 (25.6) | |
| Severe/ Superimposed preeclampsia | 13 (10.8) | 14 (11.4) | 12 (9.8) | 11 (8.8) | |
| History of other medical morbidity | 10 (8.3) | 19 (15.5) | 8 (6.5) | 17 (13.6) | |
| Tobacco use in pregnancy | 9 (7.5) | 9 (7.3) | 10 (8.1) | 15 (12.0) | |
| Induction scheduled | 55 (45.8) | 55 (44.7) | 47 (38.2) | 62 (49.6) | |
| Indication for induction | |||||
| Late term/post-term§ | 12 (10.0) | 20 (16.3) | 18 (14.6) | 17 (13.6) | |
| Maternal|| | 28 (23.3) | 37 (30.1) | 38 (30.9) | 44 (35.2) | |
| Fetal¶ | 64 (53.3) | 57 (46.3) | 54 (43.9) | 50 (40.0) | |
| Elective/Other # | 16 (13.3) | 9 (7.3) | 13 (10.6) | 14 (11.2) | |
| Female sex | 61 (50.8) | 65 (52.9) | 54 (43.9) | 60 (48.0) | 0.53 |
BMI, Body mass index; GHTN, gestational hypertension. Data are presented as n (%) unless otherwise indicated. Categorical comparisons with Chi-square or Fisher Exact tests and continuous comparisons with Kruskal-Wallis test unless otherwise indicated.
[median, IQR for continuous]
p-value for ANOVA
Defined as ≥41 weeks
Examples include: chronic hypertension, gestational hypertension, preeclampsia, diabetes, renal disease, history of venous thromboembolism, cardiac disease or other chronic medical condition where induction was recommended
Examples include: Oligohydramnios, intrauterine growth restriction, abnormality on fetal testing
Examples of “other” include: history of an intrauterine fetal demise, vaginal bleeding at term, cholestasis consider
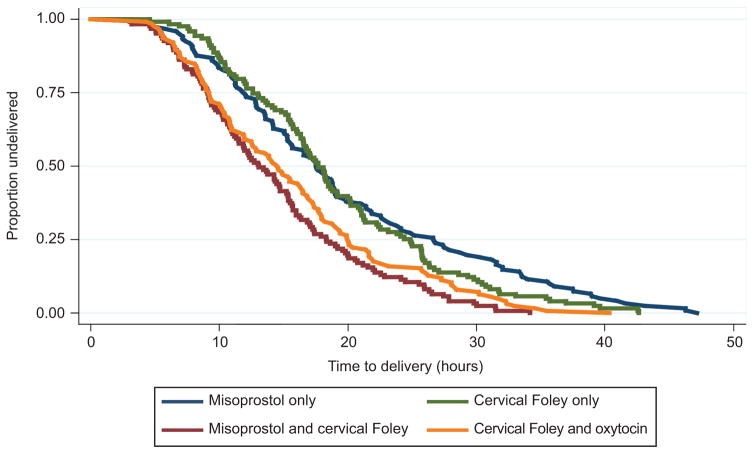
Overall, combination methods achieved a faster median time to delivery than the single agents, p<0.001, (misoprostol–Foley: 13.1 hours, Foley–oxytocin: 14.5 hours, misoprostol alone: 17.6 hours, Foley alone: 17.7 hours, Table 2, Figure 2). The faster time to delivery for combined methods was observed in both nulliparous and multiparous women and remained true when analysis was restricted to women who had a vaginal delivery (Table 2). The proportion of women delivered by 24 hours and by 12 hours was significantly greater for the combined induction methods.
Table 2.
Time to delivery outcomes among treatment groups
| Outcome | Misoprostol Only (n=120) | Misoprostol/ Foley (N=123) | Foley Only (n=123) | Foley/ Oxytocin (n=125) | P* |
|---|---|---|---|---|---|
| Time to delivery (hours) | 17.6 [11.9–26.7] | 13.1 [9.1–18.3] | 17.7 [12.6–24.9] | 14.5 [9.3–20.0] | <0.001 |
| Nulliparous | 21.4 [15.6–33.3] | 16.2 [11.5–21.6] | 21.0 [15.8–26.4] | 17.7 [11.9–22.0] | <0.001 |
| Multiparous | 12.9 [9.9–18.7] | 9.3 [6.7–13.0] | 15.5 [10.1–18.2] | 10.4 [6.8–14.8] | <0.001 |
| Time to vaginal delivery (hours) | 16.6 [11.2–23.8] | 11.0 [8.0–15.5] | 16.3 [11.2–21.0] | 11.0 [8.4–16.5] | <0.001 |
| Nulliparous | 19.1 [15.1–28.7] | 15.3 [10.2–17.9] | 18.2 [13.4–24.4] | 15.2 [9.7–20.0] | <0.001 |
| Multiparous | 12.9 [9.9–18.2] | 9.1 [6.6–12.6] | 14.8 [10.1–17.7] | 10.1[6.6–13.5] | <0.001 |
| Time to active labor (hours) | 13.3 [9.1–22.4] | 7.4 [4.4–10.7] | 11.0 [7.9–14.9] | 8.1 [5.3–11.2] | <0.001 |
| Nulliparous | 17.7 [10.1–25.7] | 8.8 [5.5–11.5] | 12.2 [8.3–18.1] | 9.0 [7.2–13.7] | <0.001 |
| Multiparous | 10.9 [7.8–16.0] | 5.7 [3.9–8.5] | 9.4 [7.6–11.8] | 6.4 [4.2–9.9] | <0.001 |
| Deliver within 24hrs, n (%) | 84 (70.0) | 108 (87.8) | 90 (73.2) | 105 (84.0) | 0.001 |
| Nulliparous | 38 (54.3) | 59 (80.8) | 42 (57.5) | 58 (78.4) | <0.001 |
| Multiparous | 46 (92.0) | 49 (98.0) | 48 (96.0) | 47 (92.2) | 0.52 |
| Deliver within 12hrs, n (%) | 31 (25.8) | 55 (44.7) | 27 (22.0) | 51 (40.8) | <0.001 |
| Nulliparous | 9 (12.9) | 20 (27.4) | 8 (11.0) | 19 (25.7) | 0.02 |
| Multiparous | 22 (44.0) | 35 (70.0) | 19 (38.0) | 32 (62.8) | 0.003 |
| Cesarean delivery, n (%) | 29 (24.2) | 34 (27.6) | 35 (28.5) | 38 (30.4) | 0.74 |
| Nulliparous | 23 (32.9) | 32 (43.8) | 30 (41.1) | 30 (40.5) | 0.58 |
| Multiparous | 6 (12.0) | 2 (4.0) | 5 (10.0) | 8 (15.7) | 0.27 |
Data are presented as median hours [inter-quartile range], unless otherwise indicated. Categorical variables were compared with chi-square or Fisher Exact tests and continuous variables were compared with Kruskal-Wallis tests.
P-values are significance level for four-group comparisons.
Figure 2.
Estimated time to delivery by study group. This figure displays the Kaplan-Meier survival curves for time to delivery for the four induction method groups, P<.001.
Assumptions for proportional hazard were met for all analyses. When censoring for cesarean delivery and adjusting for parity, the Foley–oxytocin group is no longer significantly faster than single agent methods and the misoprostol–Foley group is superior (Table 3). When using misoprostol alone or cervical Foley alone as the reference group, women with combined misoprostol–Foley are twice as likely to deliver earlier (HR 1.92, HR 1.87, respectively, Table 3). In other words, among women who have not yet delivered, women with misoprostol only or Foley only were almost 50% less likely to deliver before women with misoprostol–Foley concurrently (HR 0.52, HR 0.53, respectively). There was no significant difference between Foley–oxytocin and any of the three other groups.
Table 3.
Hazard Ratios for pairwise comparisons of time to delivery*
| Group | Miso Only | Foley Only | Miso/Foley | Foley/Oxytocin |
|---|---|---|---|---|
| Miso | ----- | 1.03 [0.76–1.38] (P=0.87) | 1.92 [1.42–2.59] (p<0.001) | 1.39 [1.03–1.87] (p=0.03) |
| Foley | 0.9 [0.72–1.31] (p=0.87) | ------- | 1.87 [1.39–2.52] (p<0.001) | 1.35 [1.00–1.82] (p=0.047) |
| Miso/Foley | 0.52 [0.39–0.74] (p<0.001) | 0.53 [0.40–0.72] (p<0.001) | ------ | 0.72 [0.54–0.97] (p=0.03) |
| Foley/ Oxytocin | 0.72 [0.54–0.97] (p=0.03) | 0.74 [0.55–1.00] (p=0.047) | 1.38 [1.03–1.87] (p=0.03) | -------- |
The references groups are listed on the left side of the table. Results that are statistically significant (p≤0.008) are bolded in the table.
Censored for cesarean delivery and adjusted for parity
There was no statistical difference in the cesarean delivery rate among the groups, both overall and when stratified by parity (Table 2). There were no statistically significant differences in the indication for cesarean delivery or the composite maternal morbidity among the four groups. There were no cases of thromboembolism, hysterectomy, intensive care unit admission, or death (Table 4).
Table 4.
Secondary outcomes among treatment groups
| Maternal outcomes | Misoprostol Only (n=120) | Misoprostol/ Foley (N=123) | Foley Only (n=123) | Foley/ Oxytocin (n=125) | P* |
|---|---|---|---|---|---|
| Indication for cesarean delivery† | |||||
| Failed induction | 10 (34.5) | 12 (35.3) | 16 (45.7) | 14 (36.8) | 0.76 |
| Arrest of Dilation | 4 (13.8) | 9 (26.5) | 8 (22.9) | 12 (31.6) | 0.40 |
| Arrest of Descent | 5 (17.2) | 5 (14.7) | 0 (0) | 4 (10.5) | 0.05 |
| NRFHT | 15 (51.7) | 19 (55.9) | 20 (57.1) | 16 (42.1) | 0.56 |
| Elective/ Other | 5 (17.2) | 2 (5.9) | 5 (14.3) | 4 (10.5) | 0.52 |
| Intrauterine pressure catheter | 53 (44.2) | 53 (43.1) | 70 (56.9) | 69 (55.2) | 0.05 |
| Amnioinfusion | 27 (22.5) | 23 (18.7) | 36 (29.3) | 29 (23.2) | 0.27 |
| Regional Analgesia | 111 (92.5) | 114 (92.7) | 114 (92.7) | 121 (96.8) | 0.43 |
| IV narcotics | 9 (7.5) | 11 (8.9) | 19 (15.5) | 18 (14.4) | 0.14 |
| Terbutaline used | 32 (26.7) | 19 (15.5) | 23 (18.7) | 25 (20.0) | 0.17 |
| Oxytocin use in active labor | 72 (68.6) | 82 (70.7) | 98 (89.9) | 115 (98.3) | <0.001 |
| Chorioamnionitis | 9 (7.5) | 15 (12.2) | 17 (13.8) | 20 (16.0) | 0.22 |
| Maternal morbidity | 8 (6.7) | 5 (4.1) | 13 (10.6) | 10 (8.0) | 0.26 |
| Endometritis | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0.24 |
| 3rd or 4th degree laceration | 2 (2.2) | 1 (1.1) | 6 (6.8) | 3 (3.5) | 0.20 |
| Blood transfusion | 2 (1.7) | 1 (0.8) | 5 (4.1) | 4 (3.2) | 0.36 |
| Wound separation/ infection | 1 (0.8) | 1 (0.8) | 1 (0.8) | 3 (2.4) | 0.73 |
| Readmission | 2 (1.7) | 2 (1.6) | 3 (2.4) | 7 (5.6) | 0.24 |
| Total maternal length of stay (days) ‡ | 3 [3–4] | 3 [3–4] | 3 [3–4] | 3 [3–4] | 0.20 |
| Postpartum length of stay (days) ‡ | 2 [2–2] | 2 [2–2] | 2 [2–2] | 2 [2–3] | 0.59 |
|
| |||||
| Neonatal Outcomes | |||||
|
| |||||
| Birth weight (grams) ‡ | 3178 [2635–3625] | 3240 [2875–3600] | 3230 [2855–3575] | 3240 [2920–3595] | 0.16§ |
| Apgar at 1 min‡ | 8 [7.5–9] | 8 [8–9] | 8 [8–9] | 8 [8–9] | 0.06 |
| Apgar at 5 min‡ | 9 [9–9] | 9 [9–9] | 9 [9–9] | 9 [9–9] | 0.26 |
| Neonatal length of stay (days) ‡ | 2 [2–3] | 2 [2–3] | 2 [2–3] | 2 [2–3] | 0.82 |
| NICU admission | 15 (12.5) | 10 (8.1) | 17 (13.8) | 11 (8.8) | 0.40 |
| NICU admission | |||||
| >48hours | 6 (5.0) | 4 (3.3) | 5 (4.1) | 2 (1.6) | 0.46 |
| Severe RDS | 1 (0.8) | 0 (0) | 2 (1.6) | 0 (0) | 0.29 |
| Neonatal sepsis | 2 (1.7) | 1 (0.8) | 1 (0.8) | 1 (0.8) | 0.82 |
NRFHT, non-reassuring fetal heart tracing; NICU, neonatal intensive care unit; RDS, Respiratory distress syndrome. Data are presented as n (%) unless otherwise indicated. Categorical variables are compared with chi-square and Fisher exact tests and continuous variables are compared with Kruskal-Wallis tests unless otherwise indicated.
P-values are significance level for four-group comparison.
If reported as the primary or secondary indication, it was counted in this summary. Therefore, percentages do not sum to 100%.
Median [Inter-quartile range].
Comparison performed with ANOVA.
There were no statistically significant differences in neonatal outcomes among the four groups. There were no cases of neonatal blood transfusion, hypoxic ischemic encephalopathy, intraventricular hemorrhage grade 3 or 4, necrotizing enterocolitis, or need for head cooling and therefore the initial a priori composite neonatal outcome was not evaluated (Table 4).
There were 31 (6.3%) women that received a different induction method than assigned by randomization. The main reasons for starting with a different agent were inability to place the cervical Foley or patient unable to tolerate Foley placement (n=9) and rapid advancement in cervical dilation from randomization to induction of labor, precluding the need for cervical ripening (n=10). There were 11 (2.2%) women that received the correct initial induction agent but had their method changed prior to active labor. When analyses were repeated using the “as treated” method, results were unchanged (data available upon request).
DISCUSSION
This randomized clinical trial compares four different labor induction methods in a head to head trial. After censoring for cesarean and adjusting for parity, we demonstrated misoprostol–cervical Foley to be the superior method, with women twice as likely to deliver before those who received either misoprostol alone (HR 1.92) or cervical Foley alone (HR 1.87). Additionally, there were a higher proportion of women delivered by 12 hours and by 24 hours with combination methods.
Our findings of a faster delivery with combined methods are consistent with other studies 6,7. However, these studies only included two treatment groups (one combination method, one single agent method). The optimal way to determine whether there is a superior method is to evaluate all four methods under the same conditions, in a head to head trial, as we have in our study. Additionally, in contrast to our study, previous studies that did not find a difference in time to delivery were limited by small sample size, different dosing of misoprostol, and heterogeneous labor management. 8–11
Our study has significant clinical implications for obstetrical care. Induction of labor is one of the most common procedures among pregnant women. Most recently, the induction rate in the United States was estimated to be 23.3%, 1 which equates to 932,000 women undergoing an induction annually. If combination methods were used for all of these women, there would be more than 3 million fewer hours, or more than 125,000 fewer days that women spend in labor in the US alone, which has large implications for healthcare utilization and delivery. The ability to shorten the length of time women spend in labor without increasing morbidity has large clinical and financial implications given the cost and known maternal–neonatal risks associated with both prolonged labor and cesarean delivery. Additionally, this may have an important impact on patient satisfaction as a recent survey of women’s experiences after an induction showed that 40% of women who underwent an induction stated that the speed of induction was the most important aspect of their induction they would have liked to have changed. 16
Strengths of this study include that it was a large, appropriately powered, randomized trial that compared, head to head, four common methods of induction. The management of induction and active labor was standardized so differences among the four methods can be attributed to the methods themselves and not the labor management. Our study took place at one institution that limits practice variation and more easily ensures compliance with the labor protocol. Additionally, we excluded very few indications for induction, increasing the generalizability of our findings.
Limitations are as follows: neither patients nor providers were blinded to the assigned treatment since examinations are required for placement of all methods. However, the use of labor protocols for all enrolled patients should have reduced variations in practice by the un-blinded providers. Another important limitation of this study is that although we were powered to detect differences in time to delivery, for most outcomes, including cesarean delivery and maternal and neonatal adverse outcomes, we lacked statistical power to discern potentially important differences across groups.
Future studies should focus on validating our findings in studies where protocols or patient populations may be different than those in the current study and should be large enough to evaluate maternal and neonatal outcomes which could not be adequately evaluated in this study.
Supplementary Material
Acknowledgments
Funded in part by a career development award in Women’s Reproductive Health Research: K12-HD001265-15 and the Maternal and Child Health Research fund from the University of Pennsylvania.
Footnotes
Financial Disclosure: Sindhu K. Srinivas MD, MSCE has provided expert testimony in a case on behalf of Pfizer, which is unrelated to this manuscript. She also has a research grant from Bayer through the American College of Obstetricians and Gynecologists. The other authors did not report any potential conflicts of interest.
Presented at the Annual Meeting for the Society of Maternal Fetal Medicine in Atlanta, Georgia on February 4, 2016.
Clinical Trial Registration: ClinicalTrials.gov, https://clinicaltrials.gov, NCT01916681.
References
- 1.Centers for Disease Control and Prevention. Recent declines in induction of labor. [cited Accessed March 1, 2016 ]; Available from: http://www.cdc.gov/nchs/data/databriefs/db155.htm.
- 2.World Health Organization. WHO Recommendations for Induction of labour. [cited Accessed May 15, 2016]; Available from: http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf.
- 3.Jozwiak M, Bloemenkamp KW, Kelly AJ, Mol BW, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2012;14(3) doi: 10.1002/14651858.CD001233.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev. 2014;19(6) doi: 10.1002/14651858.CD003101.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2009;7(4) doi: 10.1002/14651858.CD003246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone JF, Tuuli MG, Fogertey PJ, Roehl KA, Macones GA. Combination of Foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstet Gynecol. 2013;121(2 Pt 1):247–52. doi: 10.1097/AOG.0b013e31827e5dca. [DOI] [PubMed] [Google Scholar]
- 7.Hill JB, Thigpen BD, Bofill JA, Magann E, Moore LE, Martin JN., Jr A randomized clinical trial comparing vaginal misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. Am J Perinatol. 2009;26(1):33–8. doi: 10.1055/s-0028-1091396. [DOI] [PubMed] [Google Scholar]
- 8.Chung JH, Huang WH, Rumney PJ, Garite TJ, Nageotte MP. A prospective randomized controlled trial that compared misoprostol, Foley catheter, and combination misoprostol-Foley catheter for labor induction. Am J Obstet Gynecol. 2003;189(4):1031–5. doi: 10.1067/s0002-9378(03)00842-1. [DOI] [PubMed] [Google Scholar]
- 9.Pettker CM, Pocock SB, Smok DP, Lee SM, Devine PC. Transcervical Foley catheter with and without oxytocin for cervical ripening: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1320–6. doi: 10.1097/AOG.0b013e31817615a0. [DOI] [PubMed] [Google Scholar]
- 10.Barrilleaux PS, Bofill JA, Terrone DA, Magann EF, May WL, Morrison JC. Cervical ripening and induction of labor with misoprostol, dinoprostone gel, and a Foley catheter: a randomized trial of 3 techniques. Am J Obstet Gynecol. 2002;186(6):1124–9. doi: 10.1067/mob.2002.123821. [DOI] [PubMed] [Google Scholar]
- 11.Rust OA, Greybush M, Atlas RO, Jones KJ, Balducci J. Preinduction cervical ripening. A randomized trial of intravaginal misoprostol alone vs. a combination of transcervical Foley balloon and intravaginal misoprostol. J Reprod Med. 2001;46(10):899–904. [PubMed] [Google Scholar]
- 12.American College of Obstetrics and Gynecology Practice Bulletin. Ultrasonography in pregnancy. 2009 Feb;101 doi: 10.1097/AOG.0b013e31819930b0. Reaffirmed 2014. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaney S, Shaffer BL, Cheng YW, Vargas J, Sparks TN, Paul K, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL: a randomized controlled trial. Obstet Gynecol. 2010;115(6):1239–45. doi: 10.1097/AOG.0b013e3181dec6d0. [DOI] [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Shetty A, Burt R, Rice P, Templeton A. Women’s perceptions, expectations and satisfaction with induced labour--a questionnaire-based study. Eur J Obstet Gynecol Reprod Biol. 2005;123(1):56–61. doi: 10.1016/j.ejogrb.2005.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.