Abstract
Objective
SCA12 is a progressive autosomal-dominant disorder, caused by a CAG/CTG repeat expansion in PPP2R2B on chromosome 5q32, and characterized by tremor, gait ataxia, hyperreflexia, dysmetria, abnormal eye movements, anxiety, depression, and sometimes cognitive impairment. Neuroimaging has demonstrated cerebellar and cortical atrophy. We now present the neuropathology of the first autopsied SCA12 brain and utilize cell models to characterize potential mechanisms of SCA12 neurodegeneration.
Methods
A fixed SCA12 brain was examined using gross, microscopic, and immunohistochemical methods. The effect of the repeat expansion on PPP2R2B Bβ1 expression was examined in multiple cell types by transient transfection of constructs containing the PPP2R2B Bβ1 promoter region attached to a luciferase reporter. The neurotoxic effect of PPP2R2B overexpression was examined in transfected rat primary neurons.
Results
Neuropathological investigation revealed enlarged ventricles, marked cerebral cortical atrophy and Purkinje cell loss, less-prominent cerebellar and pontine atrophy, and neuronal intranuclear ubiquitin-positive inclusions, consistent with Marinesco bodies, which did not stain for long polyglutamine tracts, alpha-synuclein, tau, or transactive response DNA-binding protein 43. Reporter assays demonstrated that the region of PPP2R2B containing the repeat functions as a promoter, and that promoter activity increases with longer repeat length and is dependent on cell type, repeat sequence, and sequence flanking the repeat. Overexpression of PPP2R2B in primary cortical neurons disrupted normal morphology.
Conclusions
SCA12 involves extensive, but selective, neurodegeneration distinct from Alzheimer’s disease, synucleinopathies, tauopathies, and glutamine expansion diseases. SCA12 neuropathology may arise from the neurotoxic effect of repeat-expansion–induced overexpression of PPP2R2B.
Keywords: ataxia, Purkinje, neurodegeneration, promoter, spinocerebellar
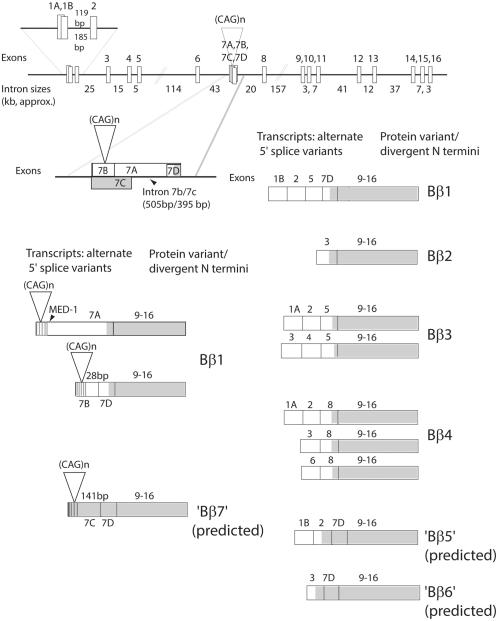
Spinocerebellar ataxia type 12 (SCA12) is an autosomal-dominant disorder characterized by head and arm tremor, gait ataxia, hyperreflexia, dysmetria, abnormal eye movements, psychiatric disorders, and, in older patients, dementia.1 MRIs of affected individuals reveal generalized atrophy of the brain, predominantly affecting the cerebral cortex and cerebellum.2 Age of onset ranges from 8 to 55 years of age, but the disease usually presents in the fourth decade of life. The disease is most prevalent in India, where it is one of the most common forms of SCA in populations thus far sampled,3-5 but it has also been identified in one American family of European descent1 and a few European and non-Indian Asian families.6-9 SCA12 is caused by a cytosine-adenine-guanine/cytosine thymine guanine (CAG/CTG) repeat expansion immediately flanking exon 7 on chromosome 5q32 in the gene for PPP2R2B.10 Repeat length in the normal population ranges from 4 to 32 triplets, with a mode of 10 triplets in all populations sampled to date. Repeat length associated with disease ranges from 46 to 78 triplets; repeats of intermediate length and of uncertain significance have also been detected.8,11 The multiple isoforms of PPP2R2B (Fig. 1) encode different Bβ regulatory units of the heterotrimer protein phosphatase 2A (PP2A). These regulatory units serve to direct PP2A toward specific targets, with specificity conferred by N-terminal sequence. Bioinformatic analysis at the time of mutation discovery indicated that the repeat, in the CAG orientation, was present 133 nucleotides upstream of the 5' end of the first exon of PPP2R2B (NM_004576; now NR_073526). Subsequent investigations identified six additional 5' exons, such that the exon associated with the repeat is now referred to as exon 7.2 The predominant protein product, Bβ1, is encoded by a transcript that usually begins with exon 7. The CAG repeat appears to be largely untranscribed,12 but does fall within the promoter region of the most common Bβ1-encoding transcripts.
FIG. 1.
PPP2R2B structure and transcription expression. Exons are not to scale. The length of each intron is indicated in kb. Total gene length is approximately 493 kb. Alternate transcripts and their encoded proteins are depicted. Shaded regions of transcripts indicate open reading frames. The predominant transcript begins at exon 7 and encodes Bβ1. The predominant initiation site for transcripts beginning with exon 7 appears to be 3' to the repeat, with much less frequently used start sites 5' to the repeat and within the repeat. Exon 7 includes a multiply spliced internal intron (IVS 7b/7c) and a MED-1 (multiple start-site element downstream 1) sequence (GCTCCC)13 65 bp 3' to the CAG repeat.10 Intron 7b/7c has two alternate donor sites (28 and 141 bp downstream from the repeat) spliced to a common acceptor site (127 bp upstream from the Bβ1 coding region). Use of the second donor site yields a transcript predicted to encode an alternate N-terminus in which the repeat encodes polyserine. Multiple combinations of upstream exons lead to protein variants with divergent N-termini; Bβ5-7 remain hypothetical. Edited and reprinted with permission from Elsevier.12
The pathogenic pathways involved in SCA12 remain unknown. One possibility, based on the location of the repeat within a predicted PPP2R2B promoter,10 is that repeat length may influence the expression level of the PPP2R2B isoform that encodes Bβ1,14,15 a phenomenon also observed at other repeat loci.16-22 Here, we describe, for the first time, neuropathology of an SCA12 brain, and we provide evidence that this neuropathology may derive from an effect of the SCA12 repeat expansion on Bβ1 expression.10,23
Materials and Methods
Neuropathology
The brain was fixed in 10% formalin. Paraffin block sections (10-μm) were stained with Cresyl violet (Nissl stain), hematoxylin and eosin (H & E), and silver by Hirano’s method.24 After deparaffinization, sections were immunostained for ubiquitin, expanded polyglutamine repeats (1C2 antibody25; see Supporting Table 1), glial fibrillary acidic protein (GFAP), synuclein-1, tau (PHF-1), and transactive response DNA-binding protein 43 (TDP-43). Sections were examined with an Olympus (Olympus, Tokyo, Japan) and a Leitz DMRB/E microscope and photographed with a Leica DC500 camera (Leica Microsystems GmbH, Wetzlar, Germany) (see Supporting Methods).
Plasmid Constructs
The pCAT-Basic reporter vector (Promega, Madison, WI) was used to create PPP2R2B plasmid constructs with repeat lengths of CAG10, CAG24, CAG35, CAG53, and CAG78. A Huntingtin CAG80 construct and a C-G content of approximately 65% construct were generated. Luciferase constructs were then generated from the chloramphenicol acetyltransferase (CAT) constructs by removing the 1.7-kilobase (kb) promoter region and cloning into pGL3-Basic (luciferase vector; Promega) (Supporting Fig. 1).
Reporter Assay
LA-N-1, N2a, and SK-N-AS lines were maintained using standard conditions (see Supporting Methods). Primary cortical neurons for reporter assays were prepared from fetal rats at 16 days of gestation, as previously described.26 Forty-eight hours after transfection, cells were harvested, lysed, and assayed for promoter activity. Luciferase activity was normalized to the internal control by calculating the ratio of firefly luciferase activity to Renilla luciferase activity of each sample. Normalized firefly luciferase activity for each test construct was expressed relative to expression of pGL3-Control plasmid (luciferase driven by an SV40 promoter). CAT activity, as corroboration for the luciferase reporter assay, was measured using pCAT-basic, pCAT-control, and experimental constructs in cells harvested 48 hours after transfection (Promega). At least three biological replicates were performed in triplicate for each experiment.
Transcript Expression
Total RNA was extracted from transfected LA-N-1 cells and complementary DNA (cDNA) was synthe-sized using random hexamers (see Supporting Methods). Real-time polymerase chain reaction (RT-PCR) was performed according to ABI protocols. Quantitative PCR (qPCR) assays were performed for firefly and Renilla luciferase and 18S RNA. For assessment of transcript stability, LA-N-1 cells were transfected with PPP2R2B-CAG10, -CAG35, and -CAG78 plasmids along with phRL-TK plasmid as internal control. Twenty-four hours after transfection, one set of cells were treated with 10 μg/mL of actinomycin D (ActD). Cells were harvested at 0, 1, 2, 3, and 6 hours after ActD treatment. Levels of luciferase messenger RNA (mRNA) were measured by qPCR.
Primary Cortical Neuron Toxicity Assay
Primary cortical neurons were transfected with PPP2R2B-pcDNA or empty pcDNA vector constructs using the Nucleofector system (Amaxa/Lonza, Cologne, Germany). The fragment of PPP2R2B expressed was Bβ1 (exons 7 and 9–16; Fig. 1). Seventy-two hours after transfection, cells were fixed and stained with 1 μg/mL of Hoechst (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline and mounted. Images were taken by fluorescence microscopy (Carl Zeiss, Thornwood, NY). Neurite complexity was evaluated quantitatively using Sholl analysis.27 At least 40 neurons were counted per condition in each experiment. Three independent experiments were performed with embryos from different progenitors.
Results
Gross Neuropathology
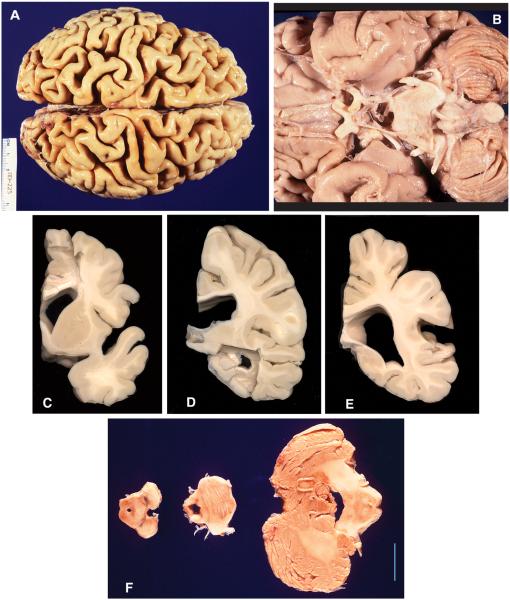
The clinical description of the index family proband has been reported previously.1 Genotyping revealed a 73 CAG triplet expansion in PPP2R2B.10 In brief, she developed action tremor of both upper extremities in her thirties, followed by head tremor, gait ataxia, urinary incontinence, and cognitive decline over the next 30 years. Neurological exam at age 64 was notable for marked dementia, left-sided neglect, impersistence of movements, primitive reflexes, diffuse hyperreflexia with extensor plantar responses, paucity of spontaneous movement, and mild, generalized bradykinesia. She exhibited action tremor of arms and head, mild limb dysmetria, saccadic and dysmetric eye movements, and left arm apraxia. Her gait had cerebellar and parkinsonian features. She died from urosepsis at age 66. Gross brain pathology was remarkable for diffuse cortical atrophy, particularly in the parietal and posterior frontal lobes, with mild atrophy in multiple other regions, including the cerebellar folia (Table 1; Fig. 2).
TABLE 1.
Gross brain pathology
| Brain Region | Gross Pathological Results |
|---|---|
| Cerebral cortex | Atrophic gyri and widened sulci (Fig. 2A). Diffuse cortical atrophy, marked at the parietal lobes and posterior aspect of the frontal lobes (Fig. 2A). Small, symmetric, cerebral hemispheres (Fig. 2C–E). Selective thinning of the cortical gray matter well demarcated from underlying white matter. The volume of cerebral white mat- ter was also reduced and the corpus callosum was thin (Fig. 2D), with normal myelination. |
| Ventricular system | Moderate hydrocephalus, more prominent in the lateral ventricles. |
| Hippocampus | Mild atrophy accompanied by widening of the temporal horns of the lateral ventricles. |
| Thalamus and hypothalamus | Proportionate to the size of the brain, but the medial walls appeared concave in association with a dilated third ventricle. Subthalamic region was normal. |
| Other cortical regions | The amygdalae and entorhinal cortices appeared normal. The caudate and putamen were of normal shape and their size was proportional to that of the brain. |
| Midbrain and medulla | Midbrain and medulla were slightly atrophic, but proportional to the size of the brain (Fig. 2F). The midbrain showed mild atrophy, but the substantia nigra (SN) was well pigmented. Cerebral peduncles were normal. |
| Pons | Mildly atrophic and the basis pontis was scalloped with mild atrophy (Fig. 2B,F). The tegmentum appeared nor- mal and the locus ceruleus was normally pigmented. |
| Cranial nerves | Cranial nerves were unremarkable (Fig. 2B). |
| Cerebellum | Mildly decreased in size. The deep nuclei and white matter were normal. The cerebellar folia, however, showed mild atrophy manifest by subtle widening of the sulci (Fig. 2F). |
| Other gross findings | At autopsy, body weight was 44.2 kg and body length was 160 cm. Brain weight was 1,045 g (normal, 1,300– 1,400 g). The cranial cavity was normal. The brain was covered with translucent meninges. Mild,nonocclu- sive, atherosclerotic plaques were present in the proximal portions of both middle cerebral arteries. The spinal cord was not recovered. |
FIG. 2.
SCA12 gross neuropathology. (A) Dorsal view of cerebral hemispheres showing diffuse cortical atrophy with enlarged cortical fissures, greatest in the parietal and posterior frontal lobes. Scale bar = 4.3 cm. (B) Inferior view, showing mild atrophy of the pons and cerebellum. Cranial nerves are unremarkable. (C) Coronal section of forebrain, showing moderate enlargement of the lateral ventricle, atrophy of the cerebral gray and white matter, and preservation of the caudate and putamen. (D) Coronal section, showing enlarged body and temporal horn of the lateral ventricle and thinning of the corpus callosum. (E) Posterior coronal section, showing enlarged trigone of the lateral ventricle, thinning of the corpus callosum, and diffuse cortical atrophy. (F) Slices of the midbrain, showing normal-appearing SN, mild atrophy of the base of the pons, and mild cerebellar atrophy. Scale bar = 2 cm.
Microscopic Neuropathology
Microscopic examination of the cerebral cortex revealed normal differentiation of gray and white matter with preservation of neuronal layers and cytoarchitectonics, with no apparent loss of any particular neuronal population or astrogliosis. Silver stains showed rare neuritic plaques in frontal and parietal cortex and a single neurofibrillary tangle in the subiculum. Immunostaining with antibodies against phosphorylated tau was negative. The cerebral white matter was unremarkable. Caudate, putamen, globus pallidum, hypothalamus, amygdala, and hippocampus were normal. There was mild focal gliosis in the dorsomedial thalamus. In the midbrain, the substantia nigra pars compacta (SNpc) showed a normal number of pigmented neurons, with minimal pigment incontinence and no Lewy bodies. In the pons, the locus ceruleus showed a normal number of well-pigmented neurons free of Lewy bodies or intranuclear inclusions. In the nuclei of the basis pontis and in the medulla, occasional degenerating neurons and axons were noted.
The three-layered neuronal pattern of cerebellar cortex was preserved without notable change in thickness. The deep cerebellar nuclei (fastigial, globosus, emboliform, and dentate) appeared normal in size and cytology (not shown). Moderate loss of Purkinje cells, greater than expected for age, was observed by H & E staining (Fig. 3A,B) in the vermis and cerebellar hemispheres and occurred in patches of up to approximately 10 cells. Occasional rounded swellings of the proximal Purkinje cell axon (torpedoes) were found in the cerebellar granule cell layer, indicative of injured Purkinje cells. Cerebellar white matter was mildly thinned. GFAP immunostaining did not reveal evidence of activated Bergmann glia in cerebellar cortex.
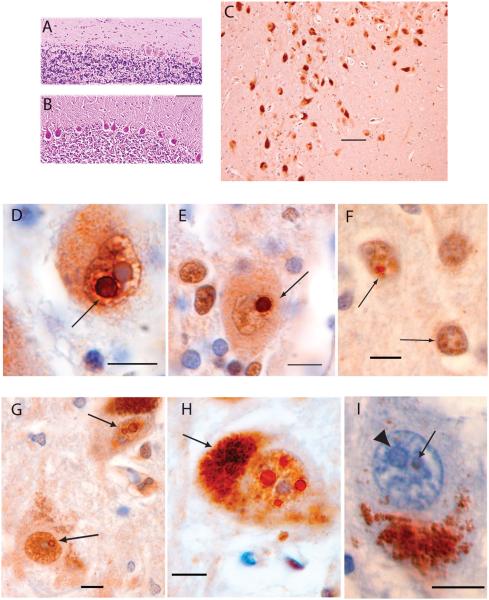
FIG. 3.
SCA12 histopathology and intranuclear neuronal inclusions. (A) SCA12 cerebellar cortex (H & E staining) showing Purkinje cell loss. (B) Cerebellar cortex, normal control: H & E staining. Scale bar = 200 μm. (C) SNpc, immunostained for ubiquitin, showing melanin-containing neurons that exhibit normal density, size, and appearance for a 66-year-old subject. Scale bar = 100 μm. (D) Purkinje cell with a ubiquitin positive, round, intranuclear inclusion (arrow) adjacent to a similarly sized pale blue nucleolus. (E) A different Purkinje cell containing a ubiquitin-positive, round, intranuclear inclusion (arrow). (F) Two neurons (arrows) in motor cortex with multiple, ubiquitin-positive, intranuclear inclusions distinct from the nucleolus (stained blue). (G) Two neurons in the SNpc. The upper neuron contains two ubiquitin-positive, intranuclear inclusions adjacent to each other and separate from the nucleolus. The lower neuron contains one ubiquitin-positive intranuclear inclusion adjacent to the nucleolus. (H) SNpc neuron with at least four round, ubiquitin-positive, intranuclear inclusions surrounding the nucleolus. Arrow points to cytoplasmic melanin. (I) SNpc neuron with cytoplasmic melanin inferiorly. Pale blue nucleolus indicated with arrowhead. Arrow points to a smaller, round inclusion that is negative for 1C2 immunostaining (expanded polyglutamine repeats) and is faintly eosinophilic. Sections D to I are immunostained for ubiquitin and counterstained with H & E. Section I is immunostained with 1C2 antibody and counterstained with H & E. Scale bars are 10 μm in sections D to I.
Inclusions
In the midbrain, no ubiquitin-positive inclusions were noted in neuronal cytoplasm, glial cells, astrocytes, microglia, or oligodendroglia. No ubiquitin-positive inclusions were observed in the striatum, thalamus, locus coeruleus, deep cerebellar nuclei, vestibular nuclei, hippocampus, pontine nuclei, or inferior olivary nuclei.
Ubiquitin immunostaining revealed large intranuclear inclusions in SNpc, consistent with Marinesco bodies,28 observed in multiple neurons within any 40× field (Fig. 3G). Cytoplasmic inclusions were not present. Many SNpc neurons contained one to four inclusions (Fig. 3H), were round, ranging from 1 to 5 μm in diameter (Fig. 3G–I), and typically adjacent to nucleoli. Ubiquitin staining was darkest in the outer rim of the inclusion (Fig. 3G–I). SNpc neurons with inclusions appeared morphologically normal, with intact plasma membranes and no neuronal swelling, nuclear condensation, or apparent depigmentation. Some of these inclusions were also H & E positive, appearing as round eosinophilic structures readily distinguishable from nucleoli (Fig. 3I).
Eosinophilic and ubiquitin-immunostained inclusions were found in rare Purkinje cells (Fig. 3D,E). These inclusions were consistently intranuclear, single, round, 1 to 5 μm in diameter, and were adjacent but separate from nucleoli. Ubiquitin-positive inclusions in Purkinje cells were more densely stained, but lacked the rim of increased ubiquitin observed in SNpc inclusions. The few Purkinje cells that contained ubiquitin-positive inclusions appeared morphologically normal, with intact nuclear and plasma membranes. Ubiquitin immunostaining revealed very rare 1- to 2-μm ubiquitin-positive neuronal intranuclear inclusions in layer 3 of the motor cortex (Fig. 3F). Multiple inclusions were found within one nucleus (Fig. 3F).
Immunostaining for expanded polyglutamine tracts (Fig. 3I), alpha-synuclein, tau, and TDP-43 was negative.
The Repeat-Containing Region of PPP2R2B Functions as a Promoter
We used a dual-luciferase cell assay to characterize the putative promoter region of Bβ1, as well as the effect of the CAG repeat and flanking sequence on promoter activity (Supporting Fig. S1). In recognition of the inherent limitation of this approach to promoter analysis,29 activity analysis was performed in four different cell types: LA-N-1 and SK-N-AS (human neuroblastoma); N2a (mouse neuroblastoma); and primary rat cortical neurons. Results of key experiments were confirmed using CAT assay (data not shown).
First, we confirmed the strong promoter activity of the PPP2R2B repeat region previously reported in human neuroblastoma lines.15 In LA-N-1 (Fig. 4A), in which PPP2R2B expression has been previously demonstrated,30 the PPP2R2B construct with CAG10, the modal repeat length in the human population, functions as a strong promoter, comparable to an SV40 promoter. Nearly identical results were obtained using the CAT reporter assay system (data not shown). A similar effect was detected in primary cortical neurons (Fig. 4A), but not in N2a or SK-N-AS cells, suggesting cell-type specificity of promoter activity.
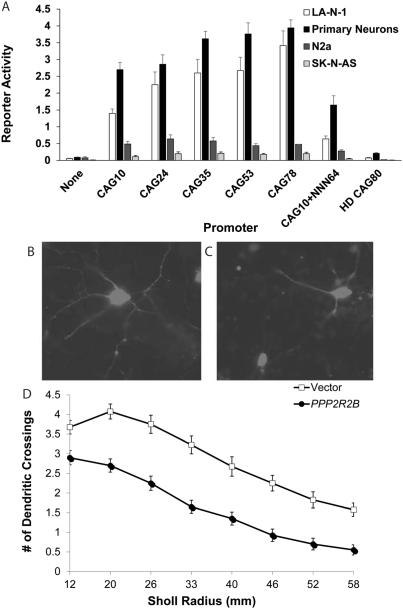
FIG. 4.
PPP2R2B promoter activity and PPP2R2B-induced neurotoxicity. (A) Constructs as depicted in Supporting Figure 1, with luciferase activity normalized to Renilla activity, and all values normalized to the SV40 construct (not shown on graph) to enable comparisons across experiments. This graph represents the mean and SE of five (LA-N-1 cell line and rat primary cortical neurons) or three (N2a and SK-N-AS cell lines) independent experiments, each performed in triplicate. For LA-N-1 cells, there was a strong correlation between repeat length and reporter expression (r2 = 0.90; P = 0.014), with CAG10 significantly different from CAG35, CAG53, and CAG78 (ANOVA: F = 24.51; P < 0.0001; Tukey’s post-hoc test: P < 0.05 for CAG35 and CAG53; P < 0.01 for CAG78). For primary rat cortical neurons, the same effect of increasing repeat length on reporter activity was evident (exponential curve: r2 = 0.81; P = 0.037; ANOVA: F = 58.08; P < 0.0001; post-hoc Tukey’s test: P < 0.05 for CAG35 and CAG53; P < 0.01 for CAG78), but no effect of repeat length was observed in N2a or SK-NAS cell lines. In LA-N-1 and rat primary cortical neurons, the reporter activity induced by the CAG10+(NNN)64 construct was equivalent to the PPP2R2B-CAG10 construct and was significantly less than activity induced by the CAG78 construct (P < 0.01). The reporter activity of the CAG80-HD construct was minimal; activity was significantly less than the CAG10 construct and substantially less than the CAG78 construct in LA-N-1 cells (post-hoc: P < 0.05 and P < 0.01, respectively) and primary rat cortical neurons (P < 0.01 for each comparison). (B and C) Primary cortical neurons cotransfected with DsRed and Bβ1 variant of PPP2R2B, fixed at 72 hours post-transfection, 100x magnification. Neurite outgrowth is markedly greater in cells transfected with vector (B) than in cells transfected with PPP2R2B encoding Bβ1 (C). (D) Sholl analysis demonstrating loss of neurite outgrowth after overexpression of PPP2R2B (ANOVA: F = 541.5; P < 0.0001). Experiment performed in triplicate; N = 25 to 40 neurons in each experiment per condition. ANOVA, analysis of variance.
Reporter Activity Is Correlated With CAG Repeat Length
We next investigated the effect of different CAG repeat lengths on reporter activity. In addition to CAG10, we examined CAG24 (upper range of normal repeat length), CAG35 (an intermediate range of unknown clinical significance), CAG53 (low end of the pathogenic range), and CAG78 (high end of the pathogenic range). In LA-N-1 cells (Fig. 4A), there was a strong correlation between repeat length and reporter expression, with CAG10 significantly different from CAG35, CAG53, and CAG78. Similar results were observed using a CAT reporter assay (data not shown). These results are consistent with previous findings in these neuroblastoma cells.15 The same effect of increasing repeat length on reporter activity was evident in primary cortical neurons (Fig. 4A), but not in N2a or SK-N-AS lines. These findings support our hypothesis that repeat length is correlated with promoter activity, including in untransformed neurons.
Sequence Specificity of the Effect of the Repeat Expansion
Preliminary evidence from insertion of A-T repeats in place of the PPP2R2B CAG repeat suggested that repeat sequence may be an important factor influencing the efficiency of the PPP2R2B promoter.15 We therefore asked the more specific question of whether the effect of repeat expansions on reporter activity was sequence specific (e.g., can replacement of the CAG repeat with a random sequence of equal G-C content also increase reporter activity?). We inserted a 192-bp sequence from a fetal brain cDNA clone, with G-C content of approximately 65%, immediately 3' to the CAG10 repeat in the PPP2R2B-CAG10 construct, generating a total length equivalent to 74 CAG triplets. Reporter activity induced by the CAG10+(NNN)64 construct was equivalent to that induced by the PPP2R2B-CAG10 construct in LA-N-1 cells and rat primary cortical neurons (Fig. 4A) and was significantly less than activity induced by the CAG78 construct. Thus, increased reporter activity observed with expanded CAG repeats appears to be, at least partially, sequence specific.
The Effect of CAG Repeat Expansion Is Dependent on Flanking Sequence
To address the question of whether the sequence flanking the CAG repeat modulates reporter activity in our assay, we utilized the CAG80-HD construct, whose insert was of similar length to that of the PPP2R2B construct (Supporting Fig 1). Reporter activity driven by this construct was minimal (Fig. 4A); activity was significantly less than the CAG10 construct and substantially less than the CAG78 construct in LA-N-1 cells, as well as primary rat cortical neurons. These results indicate that sequence context (regions flanking the repeat) is an important determinant of reporter expression. This is potentially related to the presence of cyclic 3',5'-adenosine monophosphate response element-binding protein (CREB)1 and specificity protein 1 (Sp1) recognition sites in sequence flanking the repeat, previously shown to influence PPP2R2B expression.15
Reporter Activity Level Correlates With Transcript Level
Genomic analysis of PPP2R2B exon 7 suggests that the CAG repeat is occasionally included in transcripts (Fig. 1). We determined, by RT-PCR and sequencing of the product, that the transcripts expressed in our assay do include the repeat and flanking regions. We also determined that a 505-bp region of the transcript, beginning 28 bp 3' to the end repeat, is excised. Whereas reporter assays are typically interpreted to indicate the promoter strength of the attached sequence, the presence of the repeat within the transcript in our assay could also have influenced reporter activity by altering translational efficiency. We therefore used qPCR (designed against luciferase mRNA) to examine construct transcript levels in LA-N-1 cells (Supporting Fig. 2) transfected with constructs containing different CAG repeat lengths. The results show that transcript levels correlate with promoter activity, indicating that the primary effect of the repeat expansion is not at the level of translation. Transcript degradation (measured by qPCR in the presence and absence of ActD) does not appear to be accelerated by increasing repeat lengths in the luciferase assay (data not shown), providing additional evidence that greater luciferase activity with longer repeats reflects enhanced promoter activity.
Overexpression of PPP2R2B Reduces Neurite Outgrowth
To assess the potential impact of PPP2R2B overexpression on neurons, the predominate transcript, encoding Bβ1, was transfected into rat primary cortical neurons with the transfection marker, DsRed. Sholl analysis of transfected neurons revealed clear evidence of impaired neurite outgrowth, a marker of neuronal toxicity (Fig. 4B–D).31
Discussion
Here, we describe the first neuropathological study of an SCA12 brain. Gross examination revealed a small brain with generalized cerebral cortical atrophy, most prominently affecting the superior parietal and posterior frontal lobules bilaterally. The cerebellum and pons were mildly reduced in size. The ventricular system was moderately dilated throughout and the corpus callosum was abnormally thin, suggesting loss of commissural axons. Evidence of cell loss was not obvious in the cerebral cortex. Purkinje cell loss, but not granule cell loss, was readily apparent in the cerebellum. Ubiquitin immunoreactive, intraneuronal, intranuclear inclusions, consistent with Marinesco bodies, were abundant in SN neurons. Whereas Marinesco bodies are a common finding in the aged brain, they may be associated with motor dysfunction28 and suggest possible SN dysfunction in this patient consistent with her parkinsonism. Similar appearing inclusions were found in rare Purkinje cells and in layer 3 neurons of prefrontal cortex. Inclusions were not detected in cytoplasm or glia. Inclusions did not stain with antibodies against long polyglutamine tracts, alpha-synuclein, tau, or TDP-43. Neuronal plaques and tangles were rare.
These findings demonstrate that SCA12 affects multiple brain regions, with changes most evident in the cerebral cortex and in the cerebellum, consistent with previous neuroimaging results.1,9 Pathology involves neuronal loss and, potentially, changes in the neuropil. This neuropathology is consistent with the main clinical features of the patient: action tremor, neglect, dementia, mild parkinsonism, and cerebellar dysfunction. Though many of the clinical and neurological findings observed in SCA12 are suggestive of multiple system atrophy (MSA), the neuropathological hallmarks of MSA, argyrophilic glial cytoplasmic inclusions staining for ubiquitin and synuclein,32,33 were not detected. The absence of staining for long polyglutamine tracts also provides strong evidence that SCA12 is not a polyglutamine disease, particularly given that the repeat in this case, 73 triplets, is relatively long. The absence of detectable polyglutamine tracts is consistent with the infrequency with which the SCA12 repeat is transcribed. Even in rare transcripts containing the repeat (Bβ7, NM_181675), the repeat is in frame to encode serine rather than glutamine. In addition, neuropathological findings are not consistent with Alzheimer’s disease, tauopathies, synucleinopathies, or frontotemporal lobar degeneration with ubiquitin-positive inclusions.34
The neuropathology reported here, in conjunction with previously reported neuroimaging and clinical findings,1,9 demonstrates that SCA12 is quite distinct from other neurodegenerative diseases, including other SCAs, with practical implications for differential diagnosis and genetic testing of individuals presenting with an atypical neurodegenerative disorder. An important caveat is that the neuropathology findings reported here are from an individual with an expansion of 73 triplets, at the high end of the pathogenic range observed in SCA12, who had an unusually severe clinical course. It is therefore possible that the neuropathology in this individual is quantitatively or qualitatively different than individuals with more-modest repeat expansions. However, neuroimaging findings of other individuals with more modest expansions have also demonstrated atrophy in cortical and subcortical regions,9 suggesting that SCA12, regardless of repeat length, is a diffuse disease not limited to the cerebellum.
The SCA12 repeat expansion may lead to SCA12 pathogenesis by multiple, nonmutually exclusive roles in regulation of PPP2R2B expression, as previously outlined for other repeat expansion diseases,35,36 including RNA toxicity from transcripts containing the expanded repeat from either strand,37,38 splicing dysregulation,39 dysfunction of transcription or translation,40-43 cryptic expression of a protein from a small open reading frame,44 or repeat-associated non-ATG translation (RAN translation).45 However, given the location of the SCA12 repeat immediately 5' to PPP2R2B exon 7, the first exon in the most common PPP2R2B transcript, we chose to test the hypothesis that the repeat expansion influences transcript expression.40-42
The results of our cell experiments, consistent with previous findings,14,15 suggest that the SCA12 repeat locus, flanking and partially including exon 7, serves as a functional promoter, and that the strength of promoter activity increases with increasing CAG/CTG repeat length, so that repeats in the expanded range lead to marked increase in activity. In addition, the cell type, the sequence of the repeat itself, and the region flanking the repeat all are important features in promoter activity. Promoter activity, and the influence of repeat length, was detectable in one of three neuroblastoma lines, as well as in primary cortical neurons. This result is opposite to that observed in progressive myoclonus epilepsy type 1, in which expansion of a dodecamer repeat causes disease by reducing transcription.46-48 The effect of the SCA12 repeat expansion is similar to that observed in FMR1, with repeats of an intermediate length (56–200 triplets), which is associated with premature ovarian failure and the fragile X-associated tremor/ataxia syndrome. The mechanism by which these repeat expansion mutations influence promoter function remains to be determined, but presumably involves quantitative or qualitative changes in binding efficiency of transcription factors at the promoter locus.49
The conclusion that the SCA12 repeat expansion increases expression of the PPP2R2B isoform encoding Bβ1 must be tempered by the complex alternative splicing of PPP2R2B, and the likelihood that PPP2R2B expression is regulated by multiple promoters, each with their own complex regulation. For instance, CREB1 and SP1 binding 5' of the PPP2R2B exon 7 repeat upregulate PPP2R2B expression, whereas TFAP4, binding 3' to the repeat, downregulates PPP2R2B expression.15 It is possible that within the full-length PPP2R2B gene, or within other cell types or at different stages of cell development, repeat length might have a different or additional effect on gene expression, such as an influence on splicing. The availability of only a single fixed SCA12 brain, and the lack of PPP2R2B expression in lymphoblastoid lines, has limited the ability to compare the levels of PPP2R2B variants in SCA12 patients with controls. Future availability of brain or biopsy tissue, particularly the generation of induced pluripotent stem (iPS) cells with the PPP2R2B expansion, may help validate the predictions of our model.
The findings in our study have potential pathogenic implications for SCA12. PPP2R2B is one of 18 known genes encoding PP2A-regulatory subunits.12,50,51 PPP2R2B encodes a brain-specific regulatory subunit, Bβ (also termed PR55β), of PP2A, a ubiquitous serine/ threonine phosphatase implicated in numerous normal and abnormal cellular processes, including apoptosis and tau phosphorylation.30,52 PP2A is a heterotrimer with a structural, catalytic, and a regulatory B subunit.53 The multiplicity of available B subunits results in dozens of different PP2A holoenzyme possibilities, with B subunits providing key information for differential substrate specificity, subcellular targeting, and PP2A enzyme kinetics.52,54-56 PPP2R2B is spliced to encode multiple different protein isoforms, Bβn, which differ at their N-termini, the region that provides targeting information for the PP2A holoenzyme.55 Increased activity of the exon 7–associated promoter would presumably increase levels of isoform Bβ1, potentially offsetting the normal decline in Bβ1 observed (in the rat) during normal postnatal development.54,55 We speculate that overexpression of Bβ1 may shift the normal ratio of regulatory subunits forming the PP2A complex, resulting in a quantitative shift in PP2A targeting with eventual neurotoxic effects. Given that overexpression of Bβ1 in PC12 cells does not lead to marked changes in cell viability,55 it is possible that Bβ1-associated neurotoxicity is a slow or cell-specific process or involves properties such as neurite outgrowth more prominently than cell loss. Overexpression of Bβ2 causes mitochondrial fragmentation leading to apoptosis in transgenic Drosophila,57 as well as neuroblastoma and primary hippocampal neurons,58 confirming the potential cytotoxicity of relative or absolute excess expression of PPP2R2B isoforms. Future cell and mammalian models, including the generation of SCA12 iPS cells, may lead to illumination of this and other mechanisms of pathogenesis.
Supplementary Material
Acknowledgments
The authors dedicate this work to the memory of our colleague, Dr. Elizabeth O’Hearn. The authors thank the index family for their participation and assistance, Robert Seeger for the gift of LA-N-1 cells, and Mark Moliver, Abdul Bachani, Zachary Kaminsky, Colleen Callahan, Daniel Gorelick, BI Bae, Rebekah Zann, and Erica Allen for experimental assistance.
Funding agencies: This work was funded by the National Institutes of Health (NS38054 and NS042930) and the National Ataxia Foundation.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.O’Hearn E, Holmes SE, Calvert PC, et al. SCA-12: tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology. 2001;56:299–303. doi: 10.1212/wnl.56.3.299. [DOI] [PubMed] [Google Scholar]
- 2.O’Hearn E, Holmes SE, Margolis RL. Spinocerebellar ataxia type 12. Handb Clin Neurol. 2012;103:535–547. doi: 10.1016/B978-0-444-51892-7.00034-6. [DOI] [PubMed] [Google Scholar]
- 3.Bahl S, Virdi K, Mittal U, et al. Evidence of a common founder for SCA12 in the indian population. Ann Hum Genet. 2005;69:528–534. doi: 10.1046/j.1529-8817.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujigasaki H, Verma IC, Camuzat A, et al. SCA12 is a rare locus for autosomal dominant cerebellar ataxia: a study of an indian family. Ann Neurol. 2001;49:117–121. [PubMed] [Google Scholar]
- 5.Srivastava AK, Choudhry S, Gopinath MS, et al. Molecular and clinical correlation in five indian families with spinocerebellar ataxia 12. Ann Neurol. 2001;50:796–800. doi: 10.1002/ana.10048. [DOI] [PubMed] [Google Scholar]
- 6.Brussino A, Graziano C, Giobbe D, et al. Spinocerebellar ataxia type 12 identified in two italian families may mimic sporadic ataxia. Mov Disord. 2010;25:1269–1273. doi: 10.1002/mds.22835. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Tan EK, Law HY, et al. Prevalence and ethnic differences of autosomal-dominant cerebellar ataxia in Singapore. Clin Genet. 2002;62:478–481. doi: 10.1034/j.1399-0004.2002.620610.x. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Wu JJ, Wu ZY. Identification of 46 CAG repeats within PPP2R2B as probably the shortest pathogenic allele for SCA12. Parkinsonism Relat Disord. 2015;21:398–401. doi: 10.1016/j.parkreldis.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Ma J, Zhang X. Diffusion tensor imaging of spinocerebellar ataxia type 12. Med Sci Monit. 2014;20:1783–1791. doi: 10.12659/MSM.891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmes SE, O’Hearn EE, McInnis MG, et al. Expansion of a novel CAG trinucleotide repeat in the 5' region of PPP2R2B is associated with SCA12. Nat Genet. 1999;23:391–392. doi: 10.1038/70493. [DOI] [PubMed] [Google Scholar]
- 11.Musova Z, Sedlacek Z, Mazanec R, et al. Spinocerebellar ataxias type 8, 12, and 17 and dentatorubro-pallidoluysian atrophy in czech ataxic patients. Cerebellum. 2013;12:155–161. doi: 10.1007/s12311-012-0403-5. [DOI] [PubMed] [Google Scholar]
- 12.Holmes SE, O’Hearn E, Cortez-Apreza N. Spinocerebellar Ataxia 12 (SCA12) Elsevier Academic; San Diego, CA: 2006. [Google Scholar]
- 13.Ince TA, Scotto KW. A conserved downstream element defines a new class of RNA polymerase II promoters. J Biol Chem. 1995;270:30249–30252. doi: 10.1074/jbc.270.51.30249. [DOI] [PubMed] [Google Scholar]
- 14.Chen CM, Hou YT, Liu JY, et al. PPP2R2B CAG repeat length in the Han Chinese in Taiwan: association analyses in neurological and psychiatric disorders and potential functional implications. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:124–129. doi: 10.1002/ajmg.b.30785. [DOI] [PubMed] [Google Scholar]
- 15.Lin CH, Chen CM, Hou YT, et al. The CAG repeat in SCA12 functions as a cis element to up-regulate PPP2R2B expression. Hum Genet. 2010;128:205–212. doi: 10.1007/s00439-010-0843-2. [DOI] [PubMed] [Google Scholar]
- 16.Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274:13176–13180. doi: 10.1074/jbc.274.19.13176. [DOI] [PubMed] [Google Scholar]
- 17.Itokawa M, Yamada K, Yoshitsugu K, et al. A microsatellite repeat in the promoter of the N-methyl-D-aspartate receptor 2A subunit (GRIN2A) gene suppresses transcriptional activity and correlates with chronic outcome in schizophrenia. Pharmacogenetics. 2003;13:271–278. doi: 10.1097/00008571-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Iwashita S, Koyama K, Nakamura Y. VNTR sequence on human chromosome 11p15 that affects transcriptional activity. J Hum Genet. 2001;46:717–721. doi: 10.1007/s100380170006. [DOI] [PubMed] [Google Scholar]
- 19.Syagailo YV, Okladnova O, Reimer E, et al. Structural and functional characterization of the human PAX7 5'-flanking regulatory region. Gene. 2002;294:259–268. doi: 10.1016/s0378-1119(02)00798-9. [DOI] [PubMed] [Google Scholar]
- 20.Shimajiri S, Arima N, Tanimoto A, et al. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999;455:70–74. doi: 10.1016/s0014-5793(99)00863-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamada N, Yamaya M, Okinaga S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Soria JC, Chang YS, et al. Association of a functional tandem repeats in the downstream of human telomerase gene and lung cancer. Oncogene. 2003;22:7123–7129. doi: 10.1038/sj.onc.1206852. [DOI] [PubMed] [Google Scholar]
- 23.Holmes SE, Hearn EO, Ross CA, Margolis RL. SCA12: an unusual mutation leads to an unusual spinocerebellar ataxia. Brain Res Bull. 2001;56:397–403. doi: 10.1016/s0361-9230(01)00596-2. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Hirano A. A comparative study of modified bielschowsky, bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986;12:3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 25.Trottier Y, Lutz Y, Stevanin G, et al. Polyglutamine expansion as a pathological epitope in Huntington’s disease and four dominant cerebellar ataxias. Nature. 1995;378:403–406. doi: 10.1038/378403a0. [DOI] [PubMed] [Google Scholar]
- 26.Pletnikov MV, Ayhan Y, Xu Y, et al. Enlargement of the lateral ventricles in mutant DISC1 transgenic mice. Mol Psychiatry. 2008;13:115. doi: 10.1038/sj.mp.4002144. [DOI] [PubMed] [Google Scholar]
- 27.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 28.Beach TG, Walker DG, Sue LI, et al. Substantia nigra Marinesco bodies are associated with decreased striatal expression of dopaminergic markers. J Neuropathol Exp Neurol. 2004;63:329–337. doi: 10.1093/jnen/63.4.329. [DOI] [PubMed] [Google Scholar]
- 29.Cirulli ET, Goldstein DB. In vitro assays fail to predict in vivo effects of regulatory polymorphisms. Hum Mol Genet. 2007;16:1931–1939. doi: 10.1093/hmg/ddm140. [DOI] [PubMed] [Google Scholar]
- 30.Mayer RE, Hendrix P, Cron P, et al. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: Evidence for a neuronal-specific isoform. Biochemistry. 1991;30:3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- 31.Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: Models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–376. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 33.Dickson DW, Lin W, Liu WK, Yen SH. Multiple system atrophy: a sporadic synucleinopathy. Brain Pathol. 1999;9:721–732. doi: 10.1111/j.1750-3639.1999.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudnicki DD, Margolis RL. Repeat expansion and autosomal dominant neurodegenerative disorders: consensus and controversy. Expert Rev Mol Med. 2003;5:1–24. doi: 10.1017/S1462399403006598. [DOI] [PubMed] [Google Scholar]
- 36.Rudnicki DD, Margolis RL, Pearson CE, Krzyzosiak WJ. Diced triplets expose neurons to RISC. PLoS Genet. 2012;8:e1002545. doi: 10.1371/journal.pgen.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudnicki DD, Holmes SE, Lin MW, et al. Huntington’s disease-like 2 is associated with CUG repeat-containing RNA foci. Ann Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- 38.Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in drosophila. Nature. 2008;453:1107–1111. doi: 10.1038/nature06909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanadia RN, Shin J, Yuan Y, et al. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(-CUG) model for myotonic dystrophy. Proc Natl Acad Sci U S A. 2006;103:11748–11753. doi: 10.1073/pnas.0604970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 41.Oostra BA, Willemsen R. A fragile balance: FMR1 expression levels. Hum Mol Genet. 2003;12:R249–57. doi: 10.1093/hmg/ddg298. Spec No 2. [DOI] [PubMed] [Google Scholar]
- 42.Tassone F, Beilina A, Carosi C, et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chutake YK, Costello WN, Lam C, Bidichandani SI. Altered nucleosome positioning at the transcription start site and deficient transcriptional initiation in friedreich ataxia. J Biol Chem. 2014;289:15194–15202. doi: 10.1074/jbc.M114.566414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilburn B, Rudnicki DD, Zhao J, et al. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington’s disease-like 2 mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zu T, Gibbens B, Doty NS, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alakurtti K, Virtaneva K, Joensuu T, et al. Characterization of the cystatin B gene promoter harboring the dodecamer repeat expanded in progressive myoclonus epilepsy, EPM1. Gene. 2000;242:65–73. doi: 10.1016/s0378-1119(99)00550-8. [DOI] [PubMed] [Google Scholar]
- 47.Lalioti MD, Antonarakis SE, Scott HS. The epilepsy, the protease inhibitor and the dodecamer: progressive myoclonus epilepsy, cystatin b and a 12-mer repeat expansion. Cytogenet Genome Res. 2003;100:213–223. doi: 10.1159/000072857. [DOI] [PubMed] [Google Scholar]
- 48.Lalioti MD, Scott HS, Buresi C, et al. Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature. 1997;386:847–851. doi: 10.1038/386847a0. [DOI] [PubMed] [Google Scholar]
- 49.Lalioti MD, Scott HS, Antonarakis SE. Altered spacing of promoter elements due to the dodecamer repeat expansion contributes to reduced expression of the cystatin B gene in EPM1. Hum Mol Genet. 1999;8:1791–1798. doi: 10.1093/hmg/8.9.1791. [DOI] [PubMed] [Google Scholar]
- 50.Holmes SE, O’Hearn E, Margolis RL. Why is SCA12 different from other SCAs? Cytogenet Genome Res. 2003;100:189–197. doi: 10.1159/000072854. [DOI] [PubMed] [Google Scholar]
- 51.Schild A, Schmidt K, Lim YA, et al. Altered levels of PP2A regulatory B/PR55 isoforms indicate role in neuronal differentiation. Int J Dev Neurosci. 2006;24:437–443. doi: 10.1016/j.ijdevneu.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Strack S, Ruediger R, Walter G, et al. Protein phosphatase 2A holoenzyme assembly: identification of contacts between B-family regulatory and scaffolding A subunits. J Biol Chem. 2002;277:20750–20755. doi: 10.1074/jbc.M202992200. [DOI] [PubMed] [Google Scholar]
- 54.Strack S, Zaucha JA, Ebner FF, et al. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J Comp Neurol. 1998;392:515–527. [PubMed] [Google Scholar]
- 55.Dagda RK, Zaucha JA, Wadzinski BE, Strack S. A developmentally regulated, neuron-specific splice variant of the variable subunit bbeta targets protein phosphatase 2A to mitochondria and modulates apoptosis. J Biol Chem. 2003;278:24976–24985. doi: 10.1074/jbc.M302832200. [DOI] [PubMed] [Google Scholar]
- 56.Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- 57.Wang YC, Lee CM, Lee LC, et al. Mitochondrial dysfunction and oxidative stress contribute to the pathogenesis of spinocerebellar ataxia type 12 (SCA12) J Biol Chem. 2011;286:21742–21754. doi: 10.1074/jbc.M110.160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dagda RK, Merrill RA, Cribbs JT, et al. The spinocerebellar ataxia 12 gene product and protein phosphatase 2A regulatory subunit Bbeta2 antagonizes neuronal survival by promoting mitochondrial fission. J Biol Chem. 2008;283:36241–36248. doi: 10.1074/jbc.M800989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.