Abstract
Background
Natrelle 410 implants (Allergan, Inc., Irvine, CA) are approved in the United States for breast augmentation, reconstruction, and revision.
Objectives
To assess the risk of nipple and skin sensation changes and lactation issues in subjects receiving implants for primary breast augmentation and ascertain whether differences based on incision site exist.
Methods
We used 410 Continued Access study data to assess safety and effectiveness of devices implanted via inframammary or periareolar incision sites. Subjects were evaluated preoperatively and at 4 weeks, 6 months, and annually up to 10 years postoperatively. Lactation issues and nipple and skin sensation changes (hypersensitivity/paresthesia, loss of sensation) were assessed.
Results
The inframammary and periareolar cohorts comprised 9217 and 610 implanted devices, with mean follow-up of 4.1 years (range, 0-10.1 years) and 4.8 years (range, 0-10.1 years), respectively. In the inframammary cohort, risk of first occurrence of nipple sensation changes was 0.3% (95% CI: 0.2-0.5) at week 4 and month 6, and 0.4% (0.3-0.7) at year 10. Risk of skin sensation changes was 0.0% (95% CI: 0.0-0.2) at week 4, 0.1% (0.0-0.2) at month 6, and 0.1% (0.0-0.3) at all subsequent time points. No nipple or skin changes occurred in the periareolar cohort. Incidence of lactation issues was similar to that reported in postpartum women who did not have breast implants.
Conclusions
We found that the risk of nipple or skin sensation changes and lactation issues is low and provide long-term safety and effectiveness data on subjects receiving implants for primary breast augmentation.
Level of Evidence: 3
 Therapeutic
Therapeutic
Breast augmentation is among the most common cosmetic surgery procedures worldwide, with approximately 287,000 procedures performed in the United States alone in 2014.1 Silicone gel-filled implants have been used for breast augmentation for more than 50 years.2 Implant characteristics have changed greatly during that time, from having limited available sizes (ie, volume), shapes, and textures to having a variety of dimensional and silicone gel-filled styles. Over the years, surgical methodology and operational techniques have evolved from an experiential approach to analytical quantitative measurement.3,4
While rates of certain adverse events, such as capsular contracture, are well documented for many implant types,5 other potential complications of breast implants are not as well documented. Specifically, a limited number of articles have reported on the potential effect of breast implants on nipple or skin sensitivity and on lactation in women undergoing primary breast augmentation. This study reports on the risk of nipple and skin sensation changes and lactation issues in subjects receiving Natrelle 410 breast implants (Allergan plc, Dublin, Ireland) for primary breast augmentation.
METHODS
Study Design
This analysis, based on data collected through July 31 2014, in the 410 Continued Access study, assessed the safety and effectiveness of Natrelle 410 implant devices. Natrelle 410 breast implants are form-stable, teardrop-shaped devices filled with a highly cohesive silicone gel.3 Each implant style has a Biocell (Allergan plc, Dublin, Ireland) textured shell surface with irregularly spaced depressions and a mean pore diameter of 300 µm (range, 100-600 µm).6-8 Natrelle 410 breast implants were approved by the US Food and Drug Administration in 2013.9,10 Long-term safety, effectiveness, and subject satisfaction are supported by results from a 10 year, multicenter prospective study.11
Devices were implanted by surgeons certified by the American Board of Plastic Surgery with experience placing silicone-filled implants; investigators were required to have participated in the pivotal study.11 The study was conducted in compliance with the US Food and Drug Administration requirements.12 Before enrolling subjects, an institutional review board (IRB) at each site approved the study protocol (the full list of approving IRBs is available online as Supplementary material, at www.aestheticsurgeryjournal.com). All subjects provided written informed consent before surgery.
Subjects
The 410 Continued Access study enrolled female subjects aged 18 years or older, presenting for primary breast augmentation, primary breast reconstruction, or breast implant revision surgery. Inclusion and exclusion criteria were identical to those previously detailed in the published pivotal study.13 Here, we report results for subjects exclusively undergoing primary breast augmentation who underwent surgical implantation of devices via inframammary or periareolar incision sites, the two most common surgical approaches in clinical practice.14 Eligible subjects aged 18 years or older underwent primary breast augmentation because of dissatisfaction with breast size or shape, asymmetry, ptosis, or aplasia. All subjects were required to have adequate tissue available to cover the implants and be willing to follow all study requirements.
Data Analysis
Subjects were evaluated pre- and postoperatively at 0 to 4 weeks, 6 months, and annually for up to 10 years following primary implant surgery. Written case report forms (CRFs) were used for data collection in this study; investigators reviewed and signed the CRFs and carried the ultimate responsibility for the accuracy and completeness of the data provided. Nipple hypersensitivity/paresthesia or loss of nipple sensation changes and skin hypersensitivity/paresthesia or loss of skin sensation changes were captured prospectively on a complications CRF. Data on severity, treatment, and resolution status were collected. Those reported as moderate, severe, or very severe, but not as mild or very mild, were counted. Overall nipple and skin sensation changes were compiled from the data collected on the CRF. Risk of first occurrence of overall nipple sensation changes (ie, nipple hypersensitivity/paresthesia plus loss of nipple sensation) was calculated; risk of first occurrence, resolution status, and time to resolution were also calculated for individual variables (nipple hypersensitivity/paresthesia and loss of nipple sensation). Similarly, risk of first occurrence of overall skin sensation changes was calculated; risk of first occurrence, resolution status, and time to resolution were also calculated for individual variables (skin hypersensitivity/paresthesia and loss of skin sensation). For subjects experiencing nipple or skin sensation changes, time to resolution was reported for those with resolution.
Some subjects became pregnant following implantation and opted to breastfeed their babies. Lactation issues were assessed before and after implantation as reported on the medical history or follow-up CRF, as applicable. Information collected included whether breastfeeding was attempted, the number of children for whom breastfeeding was attempted, the number of children who were successfully breastfed, and any lactation problems that occurred. Lactation problems were defined as inadequate milk production, excessive milk production, mastitis, pain, and “other.” Device assessments at year 8 were conducted by asking physicians if the shape of the breast reflected the shape of the implant (yes or no) and if the breast implant maintained its original position (yes or no).
Statistical Analysis
Kaplan-Meier analyses of the rates of sensation problems were performed to assess the risk of first occurrence of the development of changes in nipple and skin sensation. The cumulative risk ratio and its 95% confidence interval (CI) were calculated for assessments of risk. No hypothesis tests of predictors were performed because of the low incidence of occurrences. Descriptive statistics were used in all other analyses.
RESULTS
All enrolled subjects were female. Among the subjects in the primary breast augmentation cohort, 4621 subjects received implants via inframammary incisions, and 306 subjects received implants via periareolar incisions (Table 1). Demographics were similar among subjects in the inframammary and periareolar cohorts. Median age was 36 years (range, 18-72 years) for the inframammary cohort and 36 years (range, 18-82 years) for the periareolar cohort. The majority of subjects in both cohorts were white, married, and had professional occupations. Most subjects had at least some college education. Subjects in the inframammary group had a similar median body mass index compared with those in the periareolar group: 20.8 kg/m2 (range, 15.4-47.0 kg/m2) and 21.7 kg/m2 (range, 16.5-40.4 kg/m2), respectively. Mean follow-up was 4.1 years (range, 0-10.1 years) for subjects in the inframammary group and 4.8 years (range, 0-10.1 years) for subjects in the periareolar group. Following implantation, 455 live births occurred in the inframammary group, with 34 live births in the periareolar group. Subjects in the lactation sub-cohort were younger than those in the primary augmentation cohort. The median age of the 294 subjects attempting to breastfeed after inframammary implantation was 28 years (range, 18-52 years) and for the 19 subjects who attempted to breastfeed after periareolar implantation was 32 years (range, 26-46 years) (Table 1).
Table 1.
Baseline Demographics and Characteristics
| Characteristic | Inframammary Incision | Periareolar Incision |
|---|---|---|
| Primary augmentation cohort | ||
| Number of subjects | 4621 | 306 |
| Age, years, median (range) | 36 (18-72) | 36 (18-82) |
| Body mass index, kg/m2 | ||
| Median (range) | 20.8 (15.4-47.0) | 21.7 (16.5-40.4) |
| Race,a no. (%) | ||
| White | 4121 (88.9) | 260 (83.6) |
| Asian | 215 (4.6) | 11 (3.5) |
| Hispanic | 125 (2.7) | 18 (5.8) |
| Black | 51 (1.1) | 6 (1.9) |
| Other | 47 (1.0) | 7 (2.3) |
| Not provided | 74 (1.6) | 9 (2.9) |
| Marital status,a no. (%) | ||
| Married | 2753 (59.6) | 185 (60.5) |
| Single | 1186 (25.7) | 68 (22.2) |
| Divorced | 535 (11.6) | 37 (12.1) |
| Separated | 90 (1.9) | 12 (3.9) |
| Widowed | 54 (1.2) | 4 (1.3) |
| Not provided | 4 (0.1) | 0 (0) |
| Education, no. (%) | ||
| College graduate | 2070 (44.8) | 98 (32.0) |
| Some college | 1342 (29.0) | 109 (35.6) |
| Post-college | 729 (15.8) | 52 (17.0) |
| High school graduate | 437 (9.5) | 42 (13.7) |
| Less than high school | 20 (0.4) | 4 (1.3) |
| Not provided | 23 (0.5) | 1 (0.3) |
| Occupation,a no. (%) | ||
| Professional | 2139 (46.3) | 139 (45.4) |
| Housewife | 935 (20.2) | 58 (19.0) |
| Clerical/sales | 507 (11) | 40 (13.1) |
| Student | 370 (8.0) | 18 (5.9) |
| Service industry | 319 (6.9) | 22 (7.2) |
| Other | 281 (6.1) | 25 (8.2) |
| Trade | 62 (1.3) | 4 (1.3) |
| Not provided | 10 (0.2) | 0 (0.0) |
| Lactation sub-cohort | ||
| Number of subjects | 294b | 19 |
| Age, years, median (range) | 28 (18-52) | 32 (26-46) |
| Pregnancies/live births in subjects with no prior pregnancies | 145 | 7 |
| Pregnancies/live births in subjects with prior pregnancies | 159 | 12 |
aSubjects were allowed to select more than one response. bIn the inframammary subgroup, 294 subjects attempted breastfeeding after 304 pregnancies.
Surgical Characteristics
In total, 9217 devices were implanted via inframammary incision, and 610 devices were implanted via periareolar incision. In the inframammary cohort, full height, moderate projection (FM) was the most common implant style, while the most common style in the periareolar cohort was moderate height, full projection (MF; Table 2). For both cohorts, the placement of most devices was partial submuscular (bi-planar) (88.7% in the inframammary cohort; 83.0% in the periareolar cohort).
Table 2.
Surgical Characteristics
| Characteristic | Inframammary Incision, n (%) | Periareolar Incision, n (%) |
|---|---|---|
| Number of subjects | 4621 | 306 |
| Number of devices implanted | 9217 | 610 |
| Product style | ||
| Full height | ||
| Extra-full projection (FX) | 852 (9.2) | 26 (4.3) |
| Full projection (FF) | 1398 (15.2) | 132 (21.6) |
| Moderate projection (FM) | 2950 (32.0) | 69 (11.3) |
| Low projection (FL) | 39 (0.4) | - |
| Moderate height | ||
| Extra-full projection (MX) | 653 (7.1) | 31 (5.1) |
| Full projection (MF) | 1888 (20.5) | 212 (34.8) |
| Moderate projection (MM) | 1247 (13.5) | 127 (20.8) |
| Low projection (ML) | 24 (0.3) | - |
| Low height | ||
| Extra-full projection (LX) | 118 (1.3) | 5 (0.8) |
| Full projection (LF) | 32 (0.3) | 5 (0.8) |
| Moderate projection (LM) | 16 (0.2) | 3 (0.5) |
| Implant location | ||
| Submuscular (partial) | 8175 (88.7) | 506 (83.0) |
| Submuscular (complete) | 207 (2.2) | 24 (3.9) |
| Subglandular | 825 (9.0) | 80 (13.1) |
| Subcutaneous | 6 (0.1) | 0 (0) |
| Unknown | 4 (0) | - |
F, full; L, low; M, moderate; X, extra full.
The most common sizes for most implant styles were in the mid-range of the available sizes (Table 3). The sizes used for full height, full projection (FF) ranged from 185 to 740 cc, and for MF ranged from 165 to 640 cc; the most common size used in this study was at the higher end of the range (400 cc).
Table 3.
Most Common Implant Sizes
| Implant Style | Size Range (cc) | Inframammary Incision |
Periareolar Incision |
||
|---|---|---|---|---|---|
| Size (cc) | n (%) | Size (cc) | n (%) | ||
| Full height | |||||
| Extra-full projection (FX) | 185-615 | 360 | 223 (26.2) | 410 | 10 (38.5) |
| Full projection (FF) | 185-740 | 375 | 583 (41.7) | 375 | 57 (43.2) |
| Moderate projection (FM) | 155-550 | 310 | 1022 (34.6) | 350 | 26 (37.7) |
| Low projection (FL) | NA | - | - | - | - |
| Moderate height | |||||
| Extra-full projection (MX) | 195-620 | 325 | 193 (29.6) | 445 | 10 (32.3) |
| Full projection (MF) | 165-640 | 335 | 530 (28.1) | 375 | 77 (36.3) |
| Moderate projection (MM) | 160-450 | 280 | 323 (25.9) | 400 | 34 (26.8) |
| Low projection (ML) | NA | - | - | - | - |
| Low height | |||||
| Extra-full projection (LX) | 225-515 | 365 | 36 (30.5) | 405 | 2 (40.0) |
| Full projection (LF) | 240-440 | 270 | 11 (34.4) | 390 | 2 (40.0) |
| Moderate projection (LM) | 140-320 | 250 | 7 (43.8) | 320 | 3 (100.0) |
F, full; L, low; M, moderate; X, extra full; NA, not available.
Nipple Sensation Changes
Nipple sensation changes were categorized on the CRF as hypersensitivity/paresthesia and loss of nipple sensation, and further compiled as overall nipple sensation changes. In the inframammary cohort, the risk of first occurrence of overall nipple sensation changes was 0.3% (95% CI: 0.2-0.5) at week 4 and month 6, and 0.4% (95% CI: 0.3-0.7) at annual time points from years 1 through 10. Nipple sensation changes occurred in 19 subjects who received various implant styles: 6 received FM, 5 received moderate height, moderate projection (MM), 3 received MF, 2 received moderate height, extra-full projection (MX) and FF, and 1 received low height, extra-full projection (LX) implants. Mean implant volumes for subjects with nipple sensation changes ranged from 291.7 cc for FM implants to 375 cc for FF implants. Overall, the mean volume for all subjects receiving FM and FF implants was 302.3 cc and 365.9 cc, respectively. Of the 19 nipple sensation changes reported in the inframammary cohort, 9 (47.4%) resolved without treatment or with nonsurgical treatment; of the 10 unresolved cases, 7 were undergoing continued treatment at the time of analysis, and treatment was not possible for 3 subjects. The median time to resolution for nipple sensation changes in subjects with resolution was 241 days (range, 6-406 days). In the periareolar cohort, no nipple sensation changes were reported.
When analyzed separately for the specific complications of nipple hypersensitivity/paresthesia and loss of nipple sensation, in the inframammary cohort, the risk of first occurrence of nipple hypersensitivity/paresthesia was 0.1% (95% CI: 0.0-0.2) at week 4 and month 6, and 0.2% (95% CI: 0.1-0.3) for each subsequent time point up to year 10. Hypersensitivity/paresthesia resolved for 3 of the 7 subjects experiencing changes without treatment or with nonsurgical treatment, such as stimulation exercises or fine needle aspiration. Although such nonsurgical treatments have been used to minimize the negative effects of nipple sensitivity changes from breast augmentation, information on effectiveness is anecdotal and there are no data from systematic, controlled studies to address their effectiveness. The median time to resolution for subjects with resolution was 146 days (range, 6-259 days). In the inframammary cohort, the risk of losing nipple sensation at week 4, month 6, and year 1 was 0.2% (95% CI: 0.1-0.4), 0.3% (95% CI: 0.1-0.4) at years 2 and 3, and 0.3% (95% CI: 0.2-0.5) at all remaining time points. Among the 12 subjects who experienced loss of nipple sensation, half experienced resolution (2 with nonsurgical treatment; 4 without any treatment). The median time to resolution for subjects with resolution was 306 days (range, 182-406 days).
Skin Sensation Changes
Skin sensation changes were separately assessed as hypersensitivity/paresthesia and loss of skin sensation and were also analyzed as overall changes by combining the 2 categories. In the inframammary cohort, the risk of skin changes was 0.0% (95% CI: 0.0-0.2) at week 4, 0.1% (0.0-0.2) at month 6, and 0.1% (0.0-0.3) at all subsequent annual time points. Skin sensation changes occurred in 2 subjects who received FM implants and 3 subjects who received FF implants. The mean implant volume for the 2 subjects with FM implants was 375 cc and, for the 3 subjects with FF implants, was 400 cc. Overall, the mean volumes for all subjects receiving FM and FF implants were 307.6 cc and 365.9 cc, respectively. Of the 5 skin sensation changes reported, 3 (60%) resolved with nonsurgical treatment, including anti-inflammatory drugs, nerve block, or ibuprofen; the 2 unresolved cases were undergoing treatment at the time of analysis. The median (minimum-maximum) time to resolution for skin sensation for subjects with resolution was 223 days (range, 49-754 days). No skin sensation changes were observed in the periareolar cohort.
When analyzed separately for the specific complications of skin hypersensitivity/paresthesia and loss of skin sensation, the risk of skin hypersensitivity/paresthesia was 0.0% (95% CI: 0.0-0.2) for week 4 and month 6, and 0.1% (95% CI: 0.0-0.2) for all remaining time points. The 2 subjects experiencing skin hypersensitivity/paresthesia both had resolution with nonsurgical treatment. The median time to resolution was 489 days (range, 223-754). In the inframammary cohort, there was no risk of loss of skin sensation at week 4 and a low risk (0.1% [95% CI: 0.0-0.2]) at all remaining time points. Of the 3 subjects experiencing loss of skin sensation, 1 (33.3%) achieved resolution with nonsurgical treatment; time to resolution was 49 days. The 2 subjects with unresolved loss of skin sensation continued treatment.
Lactation
In the inframammary cohort, 324 of 2641 subjects with a previous full-term pregnancy, who attempted to breastfeed prior to implantation, experienced lactation issues (Table 4). The most common issues were inadequate milk production (n = 130), mastitis requiring treatment (n = 94), and mastitis not requiring treatment (n = 57). Among the 207 subjects in the periareolar cohort with a previous full-term pregnancy who attempted to breastfeed before implantation, 43 reported lactation issues (Table 4), the most common of which were inadequate milk production (n = 19), mastitis requiring treatment (n = 8), and mastitis not requiring treatment (n = 6).
Table 4.
Lactation Issues
| Parameter, n (%) | Inframammary Incision | Periareolar Incision |
|---|---|---|
| Pre-implantation | 4621 (100.0) | 306 (100.0) |
| Attempted breastfeeding | 2641 (57.15) | 207 (67.6) |
| Lactation issuesa | 324 (12.3) | 43 (20.8) |
| Inadequate milk production | 130 (40.1) | 19 (44.1) |
| Excess milk production | 15 (4.6) | 2 (4.7) |
| Mastitis not requiring treatment | 57 (17.6) | 6 (14.0) |
| Mastitis requiring treatment | 94 (29.0) | 8 (18.6) |
| Pain | 47 (14.5) | 4 (9.3) |
| Infection/inflammation | - | 1 (2.3) |
| Nipple inversion | 4 (1.2) | 1 (2.3) |
| Other | 18 (5.6) | 3 (7.0) |
| Post-implantation | 4611 (100.0) | 306 (100.0) |
| Attempted breastfeeding | 304 (6.6) | 19 (6.2) |
| Lactation issuesa | 53 (17.4) | 2 (10.5) |
| Inadequate milk production | 34 (64.2) | 2 (100) |
| Excess milk production | 1 (1.9) | - |
| Mastitis not requiring treatment | 3 (5.7) | - |
| Mastitis requiring treatment | 14 (26.4) | - |
| Pain | 7 (13.2) | - |
| Other | 3 (5.7) | - |
aThe sum of lactation issues may exceed the total number of subjects with lactation issues because a subject may have had more than one lactation issue.
Of the 294 subjects in the inframammary augmentation cohort who attempted to breastfeed after 304 pregnancies post-implantation, 53 reported lactation issues. Inadequate milk production (n = 34) and mastitis requiring treatment (n = 14) were the most common issues (Table 4). Of the 19 subjects in the periareolar augmentation cohort who attempted to breastfeed, 2 had lactation issues (inadequate milk production) (Table 4). Seven subjects in the inframammary cohort reported both pre- and post-implantation lactation issues; 2 reported mastitis before implantation and pain following implantation, 3 reported pre- and post-implantation mastitis, 1 reported inadequate milk production before and after implantation, and 1 reported an inability to breastfeed before implantation and inadequate milk production following implantation.
No issues of galactorrhea or inappropriate lactation were reported in either cohort after implantation. Overall, two subjects underwent reoperation after pregnancy and lactation due to ptosis and/or implant size change after lactation.
Implant Assessment at Year 8
For the inframammary augmentation cohort, the majority of physicians reported that the shape of the breast reflected the shape of the implant (96.9%) and that the implant maintained its original position (97.8%) at year 8. Representative photographs are included as Figure 1 and Supplementary material, Figure S1 (available online as Supplementary material, at www.aestheticsurgeryjournal.com). Results were similar among those who attempted to breastfeed vs those who did not (for shape: 95.7% vs 97.0%, respectively; for position: 97.9% vs 97.7%, respectively). For the periareolar augmentation cohort, all physicians reported that the shape of the breast reflected the shape of the implant, and 97.9% reported that the implant maintained its original shape at year 8. Results were similar among those who attempted to breastfeed vs those who did not (for shape: 100.0% for both; for position: 100% vs 97.7%, respectively.
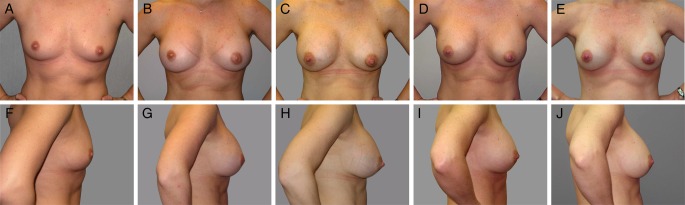
Figure 1.
Photographs of a 29-year-old woman before and at intervals after breast augmentation with silicone implants. This subject had bilateral 375 cc 410 MF implants placed in a partial submuscular position via inframammary incision. The photographs show the subject from the front and side before implantation (A, F), 3 years after implantation before pregnancy (B, G), 4 years after implantation and actively breastfeeding twins (C, H), 5 years after implantation (1 year after giving birth and <3 months after stopping breastfeeding) (D, I), and 7 years after implantation (2 months after giving birth [single birth from second pregnancy] and actively breastfeeding) (E, J).
DISCUSSION
This analysis contributes to the substantial and growing body of evidence affirming the long-term safety of silicone implants in women undergoing primary breast augmentation. As there are limited prospectively and systematically collected data available on the incidence and clinical course of skin and nipple sensation changes after breast augmentation, the current analysis provides such data from follow-up of a large cohort of women with breast augmentation for over 4 years. Our findings demonstrate a low risk (0.1%) of nipple and skin sensation changes and a low incidence of lactation issues, regardless of the surgical approach used for placement of the devices. For the small number of subjects experiencing nipple or skin sensation changes, the proportion achieving resolution of those changes ranged from 33.3% to 100.0%, depending on the type of sensation change experienced. Overall, approximately 50% of nipple or skin sensation changes have been resolved to date, and most of the rest are still being treated. The median time to resolution was between 7 and 8 months.
Women who seek breast augmentation, particularly those with micromastia, are often concerned about postoperative sensory outcomes, perhaps related to studies finding that women with small to normal size breasts are more sensate in the nipple-areolar complex than are women with larger breasts.15,16 A study assessing nipple-areolar complex sensitivity via computerized testing devices found no difference in sensitivity between women with an inframammary incision and those with a periareolar incision.15,16 However, a separate study showed that the periareolar incision may produce less sensory loss in the lower pole of the breast compared with the inframammary incision.17 These findings are similar to ours, which showed a slightly greater risk of nipple and skin sensation changes in the inframammary cohort than in the periareolar cohort (for which there was no risk).
In addition to the incision type, the relative volume of the implant has been shown to impact the nipple-areola complex sensitivity. Larger implants and smaller breasts have shown an increased association of postoperative sensory alterations of the breast.15 In addition, in previous studies, sensory impairments experienced within 3 to 6 months of implantation were not likely to improve with time.15,16 However, in our study, the majority of nipple sensation changes experienced within 6 months of implantation were resolved. A prospective study investigating 37 subjects who underwent augmentation with silicone breast implants assessed sensitivity in 9 regions of the breast before and 6 months after implantation. The relative volume of the implant was found to be associated with sensitivity alterations, although no difference was found between the inframammary and periareolar incision approaches. Other factors, such as breastfeeding before undergoing implantation, were not associated with sensory alterations.18 In our study, the volumes of implants in women experiencing skin sensation changes were slightly higher than the average implant volume of the overall study population. The majority of implant volumes in women with nipple sensation changes was less than the average volume in the overall study population.
We found that the incidence of lactation issues was low in both cohorts, and was slightly less common in the inframammary cohort than in the periareolar cohort (0.7% and 1.1%, respectively). A retrospective study found a greater incidence of lactation insufficiency, defined as inadequate volume of expressed milk and/or infant growth, in women who have undergone breast augmentation than in women who have not (P < .001).19 Among the 42 women who underwent breast augmentation, 27 (64%) had insufficient lactation compared with only 3 (<7%) of the 42 women who had not undergone breast augmentation. In the same study, the periareolar approach was significantly associated with lactation insufficiency (P < .01).19 Lactation and sensitivity appear to be interrelated. For periareolar incisions, intercostal nerves may be damaged and may result in decreased nipple sensitivity, which may affect an infant's suckling reflex.19-21
In our study, there is potential for bias or variability in reporting because end points were self-reported by physicians and were not objectively assessed via computerized or other techniques. However, the prospective monitoring design and the specific experience of surgeons in this study may minimize the impact of this limitation. This was a prospective, observational study that did not include a control group or condition; therefore, only comparisons to previously published data are possible. While the data do not address the role of these breast implants in the incidence and course of nipple and skin changes and lactation problems, it should be noted that this study utilized one specific style of breast implant, the Natrelle 410 silicone gel implant; thus, extrapolation to other styles and types of implants is not warranted. No correlation analyses (periareolar vs inframammary) or significance analyses were performed, preventing definitive assessment of potential differences between the surgical approaches. The low risk of nipple and skin sensation changes for either surgical approach in this study would suggest that the clinical significance of any potential differences between these approaches would not be meaningful. Comparisons would also be limited by the substantial differences in the number of periareolar incisions compared with the number of inframammary incisions.
No conclusions can be drawn on the impact of implant position on the risk of nipple or skin sensation changes and lactation issues. Similarly, no conclusions can be drawn regarding the potential impact of the implant style, relative volume, and length of time following surgery on the lactation issues. Indeed, some women who have not undergone any breast augmentation procedures are unable to breastfeed because of lactation issues.19,21 In our study, lactation issues following implantation ranged from 10% to 17%, which is similar to the incidence in the general population of postpartum women who elect to breastfeed. In an analysis of the Infant Feeding Practices Study II, a longitudinal study of US women, 12.1% of 2335 women who attempted breastfeeding reported disrupted lactation (defined as at least 2 of the following problems: breast pain, low milk supply, and difficulty with infant latch) during the first year of life.22 A higher incidence of lactation issues in the general population (44.3%) was reported in another study of 431 women, in which delayed onset of lactation was defined as lactation not occurring within 72 hours.23
Primiparity (no prior pregnancies) has been shown to be associated with delayed lactation onset.22,24-26 In our study, parity did not seem to be a factor in lactation. Both subjects who experienced lactation issues after periareolar implantation were multiparous (had prior pregnancies) and, of the 53 subjects with lactation issues after inframammary implantation, the number of primiparous women (n = 28) and multiparous women (n = 25) was similar.
CONCLUSIONS
This study provides ongoing long-term safety data on subjects receiving form-stable implants for primary breast augmentation. The results indicate that the risk of complications from nipple or skin sensation changes and lactation issues is low. Over 10 years, subjects with periareolar incisions experienced no risk of nipple or skin changes, and subjects with inframammary incisions had minimal nipple and skin changes. Many of the changes that occurred were resolved. The incidence of post-implantation lactation issues was similar to the incidence of lactation issues reported in the general population of postpartum women who do not have implants and breastfed their babies.
Acknowledgments
The authors thank Ramkumar Krish, MS, of Allergan plc for providing statistical analyses. Writing and editorial support for this article was provided by Emily H. Seidman, MSc, CMPP, of Peloton Advantage (Parsippany, NJ) and was funded by Allergan plc. The authors would like to acknowledge the principal and coinvestigators who also participated in the 410 Continued Access Study (the full list of investigators is available online as Supplementary Material).
Supplementary Material
This article contains supplementary material located online at www.aestheticsurgeryjournal.com.
Disclosures
Dr Lund is a consultant for Allergan plc (Dublin, Ireland). Dr Turkle is a speaker for Allergan plc. Dr Jewell has served as a consultant for Allergan plc, Solta (Hayward, CA), Keller Medical (Stuart, FL), and New Beauty Magazine (New York, NY); has received research funding through grants or contracts from Pfizer-Excaliard (New York, NY), Allergan plc, Mentor (Santa Barbara, CA), and Solta; and holds patents (or has patents pending) with Pfizer-Excaliard and AorTech (Weybridge, UK). Ms Murphy is an employee of Allergan plc and is a stockholder in that company. The opinions expressed in this article are those of the authors. The authors received no honoraria or other form of financial support related to the development of this article.
Funding
Allergan plc, the manufacturer of the device discussed in this study, designed and funded the study and performed statistical analysis of the data. Editorial support for this article was provided by Peloton Advantage (Parsippany, NJ) and was funded by Allergan plc.
REFERENCES
- 1.Cosmetic surgery national data bank statistics. Aesthet Surg J. 2015;35(Suppl 2):1-24. [DOI] [PubMed] [Google Scholar]
- 2.Jewell ML. Silicone gel breast implants at 50: the state of the science. Aesthet Surg J. 2012;328:1031-1034. [DOI] [PubMed] [Google Scholar]
- 3.Hedén P. Breast augmentation with anatomic, high-cohesiveness silicone gel implants (European experience). In: Spear SL, Willey SC, Robb GL, Hammond DC, Nahabedian MY, eds. Surgery of the Breast: Principles and Art. 3rd ed Philadelphia, PA: Lippincott, Williams and Wilkins; 2011:1322-1345. [Google Scholar]
- 4.Tebbetts JB, Adams WP. Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process. Plast Reconstr Surg. 2005;1167:2005-2016. [PubMed] [Google Scholar]
- 5.Spear SL, Murphy DK. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;1336:1354-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barone FE, Perry L, Keller T, Maxwell GP. The biomechanical and histopathologic effects of surface texturing with silicone and polyurethane in tissue implantation and expansion. Plast Reconstr Surg. 1992;901:77-86. [DOI] [PubMed] [Google Scholar]
- 7.Heden P. Cohesive gel breast implants. In: Peters W, Brandon H, Jerina KL, Wolf C, Young VL, eds. Biomaterials in Plastic Surgery. Cambridge, United Kingdom: Woodhead Publishing Limited; 2012:68-95. [Google Scholar]
- 8.Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;326:709-717. [DOI] [PubMed] [Google Scholar]
- 9.Natrelle® 410 highly cohesive anatomically shaped silicone-filled breast implants [directions for use]. Dublin, Ireland; Allergan plc; 2014. http://www.allergan.com/miscellaneous-pages/allergan-pdf-files/l3717_410_dfu. [Google Scholar]
- 10.Natrelle® Breast Implants. Dublin, Ireland: Allergan plc; Available at: http://www.natrelle.com/professional/pdf/natrelle_product_catalog.pdf Accessed April 20, 2015. [Google Scholar]
- 11.Maxwell GP, Van Natta BW, Bengtson BP, Murphy DK. Ten-year results from the Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Surg J. 2015;352:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidance for Industry and FDA Staff: Saline, Silicone Gel, and Alternative Breast Implants. U.S. Department of Health and Human Services, Food and Drug Administration Center for Devices and Radiological Health. Available at: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm071233.pdf Accessed April 15, 2015.
- 13.Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):40S-48S. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;1334:567e-583e. [DOI] [PubMed] [Google Scholar]
- 15.Mofid MM, Klatsky SA, Singh NK, Nahabedian MY. Nipple-areola complex sensitivity after primary breast augmentation: a comparison of periareolar and inframammary incision approaches. Plast Reconstr Surg. 2006;1176:1694-1698. [DOI] [PubMed] [Google Scholar]
- 16.Slezak S, Dellon AL. Quantitation of sensibility in gigantomastia and alteration following reduction mammaplasty. Plast Reconstr Surg. 1993;917:1265-1269. [DOI] [PubMed] [Google Scholar]
- 17.Okwueze MI, Spear ME, Zwyghuizen AM et al. Effect of augmentation mammaplasty on breast sensation. Plast Reconstr Surg. 2006;1171:73-83. [DOI] [PubMed] [Google Scholar]
- 18.Pitanguy I, Vaena M, Radwanski HN, Nunes D, Vargas AF. Relative implant volume and sensibility alterations after breast augmentation. Aesthetic Plast Surg. 2007;313:238-243. [DOI] [PubMed] [Google Scholar]
- 19.Hurst NM. Lactation after augmentation mammoplasty. Obstet Gynecol. 1996;871:30-34. [DOI] [PubMed] [Google Scholar]
- 20.Brody GS. Lactation after augmentation mammoplasty [letter]. Obstet Gynecol. 1996;876:1062-1063. [DOI] [PubMed] [Google Scholar]
- 21.Michalopoulos K. The effects of breast augmentation surgery on future ability to lactate. Breast J. 2007;131:62-67. [DOI] [PubMed] [Google Scholar]
- 22.Stuebe AM, Horton BJ, Chetwynd E et al. Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. J Womens Health (Larchmt). 2014;235:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nommsen-Rivers LA, Chantry CJ, Peerson JM, Cohen RJ, Dewey KG. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am J Clin Nutr. 2010;923:574-584. [DOI] [PubMed] [Google Scholar]
- 24.Dewey KG, Nommsen-Rivers LA, Heinig MJ, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3 Pt 1):607-619. [DOI] [PubMed] [Google Scholar]
- 25.Scott JA, Binns CW, Oddy WH. Predictors of delayed onset of lactation. Matern Child Nutr. 2007;33:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman DJ, Perez-Escamilla R. Identification of risk factors for delayed onset of lactation. J Am Diet Assoc. 1999;994:450-454. [DOI] [PubMed] [Google Scholar]