Abstract
Introduction
The number of cases of drug-resistant Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), has risen rapidly in recent years. This has led to the resurgence in repurposing existing drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), for anti-TB treatment.
Sources of data
Evidence from novel drug screening in vitro, in vivo, pharmacokinetic/pharmacodynamics analyses and clinical trials has been used for the preparation of this systematic review of the potential of NSAIDs for use as an adjunct in new TB chemotherapies.
Areas of agreement
Certain NSAIDs have demonstrated inhibitory properties towards actively replicating, dormant and drug-resistant clinical isolates of M. tuberculosis cells.
Areas of controversy
NSAIDs are a diverse class of drugs, which have reported off-target activities, and their endogenous antimicrobial mechanism(s) of action is still unclear.
Growing points
It is essential that clinical trials of NSAIDs continue, in order to assess their suitability for addition to the current TB treatment regimen. Repurposing molecules such as NSAIDs is a vital, low-risk strategy to combat the trend of rapidly increasing antibiotic resistance.
Keywords: tuberculosis, antimicrobial resistance, drug repurposing, NSAIDs, carprofen, Mycobacterium
Introduction
Despite an effective tuberculosis (TB) treatment regimen being in place for several decades, the disease remains responsible for over 1.5 million deaths every year. Nearly one-fifth is due to multidrug-resistant (MDR) strains that do not respond to the front-line drugs available for TB treatment.1 This has provided the much-needed impetus for the discovery and development of novel, more effective and safer anti-tubercular chemotherapeutics.2–4 However, there is a high attrition rate of lead molecules passing from preclinical development to Phase I clinical studies.5 This has led to a shortage of novel molecules available for further development and incorporation into successful anti-tubercular therapy. Whilst pharmaceutical companies are investing upwards of $500 million and 15–20 years’ work in anti-infective research projects, the number of novel molecular entities approved by organizations such as the U.S. Food and Drug Administration (FDA) has been on a declining trend.6 Workers in the field have identified an urgent need for new treatments and are shifting focus to repurpose drugs, either abandoned or still in use to treat other diseases, to combat the shortfall in productivity.7 As a result, the current clinical trials pipeline for anti-TB therapy contains novel and pre-existing drugs that are being tested for their efficacy in combinatorial treatment regimens.8
Repurposing drugs involves discovering novel drug–target interactions of established drug treatments, with an aim to use them to treat different diseases. It is a strategy that has regained the interest of pharmaceutical companies and academic scientists alike for its potential to reduce investments, de-risk clinical activities and shorten the time required to market a drug for its new use. Many drugs have been successfully repurposed to date.7 Amongst these, thalidomide, first used as a morning sickness remedy with disastrous results, was approved for treatment of multiple myeloma and has since been repurposed for leprosy treatment.9 It has also found use in managing severe treatment-associated paradoxical reactions in paediatric TB meningitis.10
The presence of gaps in drug–target interaction profiles is the most common barrier in repurposing molecular entities.11 As discussed by Kinnings et al.,12 integrating the tools presented by computational and systems biology approaches will likely provide new insights, helping to demystify drug-interaction networks by successfully identifying possible off-targets of existing drugs. They report that only 9 out of the 3999 proteins encoded by Mycobacterium tuberculosis are targets for drugs currently in use. Thus, building comprehensive drug–target interaction networks will reveal novel solutions to overcome the various resistance mechanisms developed by the causal pathogen.
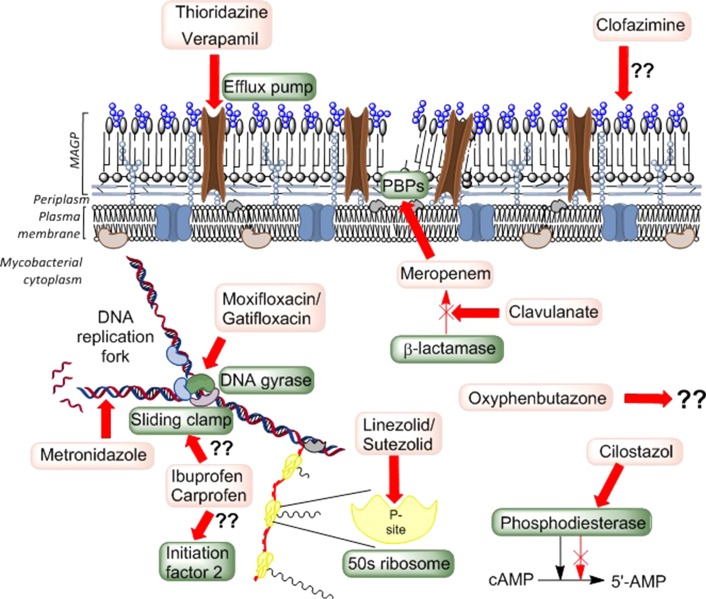
As mentioned earlier, many anti-infectives previously used for other disease indications are being considered for, or are already in various phases of in vitro/in vivo, as well as advanced clinical trial studies (Fig. 1).8,13 Several fluoroquinolone antibiotics and specific drugs such as clofazimine, linezolid and metronidazole are a few examples and have been reviewed elsewhere.14,15 However, therapeutic agents, originally intended to work upon various host-related systems, have shown anti-infective properties specific to M. tuberculosis. Medications used to treat varied, unrelated human conditions such as psychoses and angina, serve to inhibit the multidrug efflux pumps in M. tuberculosis thereby increasing the pathogen's susceptibility to other drugs.16,17 Several phosphodiesterase inhibitors have also shown promise as adjuvants for host-directed therapy.18
Fig. 1.
The possible endogenous mechanisms of action of repurposed drugs. The drugs and their targets are highlighted in lighter and darker shaded boxes, respectively. The anagram MAGP is used to indicate the ’mycolic acid–arabinogalactan–peptidoglycan’ layer of the mycobacterial cell wall and PBP refers to the penicillin-binding proteins responsible for the maturation of the cell wall peptidoglycan.
Of special interest are common, inexpensive non-steroidal anti-inflammatory drugs (NSAIDs), such as oxyphenbutazone and the 2-arylpropanoic acid family of drugs, which have been found to be anti-tubercular. These drugs act upon replicating, dormant and also drug-resistant clinical isolates of M. tuberculosis. 19,20 Here, we discuss the possibility of these NSAIDs, never previously considered for their anti-infective action, being repurposed as a part of new TB chemotherapy.
NSAIDs offer a promising TB treatment strategy
The great potential for repurposing offered by NSAIDs in the context of anti-TB therapy has been recognized relatively recently.19–21 NSAIDs have captured the attention of the scientific community, both for their biological activities and the ease with which these off-patent drugs can be repurposed. Many members of this class of molecules have shown selective anti-mycobacterial properties, along with possible pleotropic endogenous mechanisms of anti-tubercular action.19
NSAIDs are a group of molecules from chemically diverse families (Fig. 2) that suppress inflammation by inhibiting the formation of prostaglandins, the mediators of inflammatory response. They achieve this by inhibiting cyclooxygenase enzymes, COX-1 and COX-2, involved in the synthesis of prostaglandins and other prostanoids. Classical NSAIDs block both enzymes without differentiation; however, newer COX-2 inhibitors are selective (e.g. celecoxib), and thus exhibit less gastric irritation as an adverse effect—increasing their popularity. There is significant evidence supporting the notion that some of these drugs also modulate immune responses via pathways independent of the cyclooxygenase–prostaglandin route.22,23
Fig. 2.
Chemical classes and the structures of the various NSAIDs under investigation for their antimicrobial properties.
Although originally utilized for anti-inflammatory, analgesic and antipyretic purposes, NSAIDs have been shown to have potential in the treatment of various cancers and neurodegenerative diseases such as Alzheimer's.24 They have subsequently been found to possess inhibitory as well as bactericidal properties against a wide range of pathogens, both Gram-positive and Gram-negative, including Enterococcus faecalis and Helicobacter pylori, respectively.25,26
Diclofenac
Diclofenac sodium was found to be bactericidal against Escherichia coli, Listeria monocytogenes as well as M. tuberculosis. 27 Encouraged by in vitro and in vivo studies using the drug, investigations were extended and synergism was identified with streptomycin in murine TB.28
Diclofenac acid hydrazones and amides have also been shown to reduce lung and spleen bacillary loads by ~3.66 log10 in mouse-infection models at a dose of 25 mg/kg.29 Inhibition of incorporation of thymidine, vital to DNA synthesis, has been reported as one of the likely mechanisms for the bactericidal action of diclofenac in E. coli and Listeria spp.30
Metal complexes
Using metal complexes with active NSAIDs as ligands has proven to be a useful strategy in developing antimicrobials and has also been used to impart anti-tubercular properties to a selected group of NSAIDs that showed no such property prior to modification.31 These organotin complexes of mefenamic, meclofenamic acid, indometacin and tenoxicam showed in vitro minimum inhibitory concentration (MIC) values of <1 µg/mL against M. tuberculosis and can be considered excellent lead molecules for development of a new class of anti-tuberculars.32 However, the report failed to provide information regarding the bacteria-specific selectivity of the aforementioned drug complexes. The toxicity of organotin (IV) towards eukaryotic cells has raised concerns thereby limiting the immediate applications of these complexes in medicinal use.33
Oxyphenbutazone
A high-throughput phenotypic screen revealed that oxyphenbutazone selectively inhibits the non-replicating subset of the M. tuberculosis pathogen whilst having no effect on the replicating bacteria.19 One of the primary reasons for the lengthy duration of TB treatment is the need to eliminate non-replicating bacteria or ‘persisters’, that are difficult to treat due to their physiological status and unique endogenous metabolism.34 In the Gold et al. model, the environment to which the drug was exposed (mildly acidic and high in reactive nitrogen intermediates) resulted in its hydroxylation; and the compound produced was shown to be active against both replicating and non-replicating bacilli in isolation. In addition, it was also found to be synergistic with oxidants and several conventional anti-tubercular drugs such as p-aminosalicylate.19 The modified oxyphenbutazone served to deplete thiols and flavins, thereby potentially affecting a number of enzymatic reactions within the cell. The inability to generate spontaneous mutants further reinforces the argument that the endogenous mechanism of action of this drug may be multifactorial.
However, in spite of being used regularly in veterinary medicine, its use in humans is restricted in the light of sporadic reports of fatal bone marrow depression caused as a side effect of the medication.35
Celecoxib
Amongst the NSAIDs promising fewer adverse effects, the COX-2 selective celecoxib was reported to reverse MDR of methicillin-resistant Staphylococcus aureus by inhibiting the bacterial efflux mechanism.36 A similar effect was noted in Mycobacterium smegmatis and its action is expected to be through an unknown protein that regulates the MDR-1 efflux pumps in bacteria.36 This hypothesis is further substantiated by the fact that the drug exerts effects on COX-2 which in turn regulates the homologous MDR-1 pumps in humans.37 Debilitating the extrusion mechanisms of bacteria is a powerful strategy to reverse resistance and tolerance is seen in planktonic and bacterial biofilms. In the context of combination therapy, this introduces the possibility of reducing the dose or shortening the duration of treatment.
Based on the active pharmacophore of celecoxib, analogues that show potent inhibitory activity against S. aureus and M. tuberculosis have been synthesized and efforts to further optimize these compounds are ongoing.38
Aspirin
Aspirin is a salicylate anti-inflammatory drug which in addition to primary use has shown to potentiate or act synergistically when used in conjunction with the front-line anti-TB drug, pyrazinamide in a mouse-infection model study.39 Gene expression profiling of M. tuberculosis in response to salicylate has shown to down-regulate genes involved in energy production. This could explain the synergy between the salicylate and pyrazinamide which is also known to deplete membrane energy and potential and thereby disrupt transport.
However, aspirin has also demonstrated modest antagonistic activity towards isoniazid raising the importance of evaluating at what time-point in the treatment regimen should NSAIDs be included in the therapy.40,41 A randomised study on the role of aspirin in TB meningitis suggested that aspirin in combination with corticosteroids reduced the incidence of strokes and mortality.42 A similar study on the role of aspirin as an adjunct with steroids for the treatment of HIV-negative adults with TB meningitis in Vietnam is still ongoing (clinical trials identifier: NCT02237365).
Ibuprofen
Our whole-cell phenotypic evaluation studies using Spot culture growth inhibition assay (SPOTi),20,43–45 to screen a library of over-the-counter medicines, revealed commonly used NSAIDs ibuprofen and carprofen, and their analogues selectively inhibited the growth of replicating, non-replicating and even drug-resistant clinical isolates of the tubercle bacilli.20 As some of the previously mentioned NSAIDs, ibuprofen also exhibits antifungal and antibacterial properties.46,47 Moreover, studies have demonstrated that it has a marked effect on reducing bacillary loads in the lung tissue of mouse-infection models—in addition to exhibiting synergism with pyrazinamide.39,48,49 It was shown to be effective at reducing TB burden in necrotizing pulmonary granulomas, thereby conferring a level of protection from the disease. Eisen et al. 49 postulate that this effect is not mediated by a direct anti-inflammatory mechanism, as this would require higher doses of the drug than the 80 mg/kg daily administrations. They propose that ibuprofen acts via inhibiting tumour necrosis factor (TNFα, an inflammatory cytokine) to block granuloma formation.
The FDA-approved maximum daily intake for ibuprofen is 3200 mg, and on prolonged administration of the drug, a peak plasma concentration of up to 90.4 µg/mL can be obtained.50 This is well within the concentrations required to achieve anti-tubercular specific action in vitro. 20 Additionally, ibuprofen has been shown to be safe in various clinical trials,51 with a low risk of irreversible liver damage more commonly associated with paracetamol and aspirin. Additionally, its pharmacokinetic properties include a short plasma half-life (under 3 h), resulting in the drug being eliminated before it can form deleterious metabolites.52 This short time for elimination also reduces the risk of gastrointestinal and renal damage in the long term.53
Carprofen
Amongst the NSAIDs tested, carprofen was found to be the best candidate based on its low MIC of 40 µg/mL and high selectivity index of 25 making it highly specific towards bacterial cells at concentrations non-toxic to eukaryotic cells.20
Carprofen was marketed for systemic human use as an analgesic, for nearly 10 years since the 1980s. The human clinical trials of the drug reported only mild and transient side effects to its use.54 Possessing a higher affinity towards COX-2, it is expected to produce milder side reactions than its non-selective counterparts. In healthy subjects, carprofen is absorbed rapidly and the peak plasma concentration is reached after an hour of its administration.55 Crevosier et al. also reported that the absolute bioavailability of the oral forms reached values of over 90%. Despite limited data implying that it has a good safety profile in humans when used at a range of 150–600 mg/day, carprofen was discontinued from the human market for commercial reasons.56 Carprofen is a photosensitizing drug and reports of phototoxic and photoallergic contact dermatitis may have precipitated the situation further. 57,58 It was reintroduced in several parts of the world under various trade names (Rimadyl, Norocarp, etc.) as pain-relieving medication for veterinary treatment. Pharmacokinetic studies of carprofen in mice and cows showed an extended half-life of the drug; however, in both cases, the concentrations of carprofen administered was lower than the maximum approved dose.59,60
The clinical implications of these findings are yet to be fully realised as there are no conclusive data on the effects of ibuprofen or carprofen on TB treatment outcomes. At present, WHO guidelines recommend incorporating NSAIDs into TB therapies, though this aimed at reducing the joint pain side effects caused by pyrazinamide, rather than as a means to treat the symptoms of TB per se.61 A novel Phase III trial (ClinicalTrials.gov Identifier: NCT02060006) to evaluate the feasibility and efficacy of using meloxicam, a cheap and widely available NSAID, as a preventive intervention for TB-immune reconstituted inflammatory syndrome is currently underway and results from the study are awaited.
Mechanism of action
Developing modified molecules of these drugs with improved anti-mycobacterial efficacy requires knowledge of their mechanisms of action. Based on comparative bioinformatics investigation, initiation factor 2, a key player in protein translation initiation in bacteria,62 appeared to be the likely target of this family of molecules.20 Using transcriptomic analysis, we have also identified significant modulation of a number of key metabolic pathways in M. tuberculosis (unpublished data). Similar analyses also found aspirin to down-regulate the machinery required for transcription and translation in M. tuberculosis. 63 A recent study investigated the binding efficiencies of carprofen, bromfenac and vedaprofen to the DNA polymerase III β subunit (commonly known as the ‘sliding clamp protein’) of E. coli, to verify inhibition of DNA replication and repair as the bactericidal mechanism of action.64 However, the affinity of the drugs to the sliding clamp does not directly equate to their bactericidal property. Vedaprofen has the highest affinity to E. coli sliding clamp yet the MIC of the drug against it is one of the highest (1410 µg/mL). Carprofen exhibits an MIC of 680 µg/mL, one of the best-performing NSAIDs chosen for the study; however, its affinity to the E. coli sliding clamp is moderate at best. This lack of correlation between binding affinities and MIC could be due to differences in the membrane permeability of the drugs. The lower MIC of carprofen also serves as a strong argument in favour of the presence of other endogenous target(s) of the drug in E. coli.
Comparing the detailed crystal structures of the orthologues from E. coli and M. tuberculosis revealed marked differences in the secondary structures forming the key motifs and whether similar binding affinities will be observed with mycobacterial orthologues remains uncertain.65 This warrants for a similar assay with the mycobacterial protein counterparts with the NSAIDs to confirm or disprove the presence of any association between the two.
Unveiling the anti-mycobacterial mechanism of action of the NSAIDs would help in identifying possible interactions with the other front-line drugs used for TB therapy. It is known that aspirin displays alternating effects when used with certain routine anti-TB drugs, potentiating some whereas debilitating others. Antagonism observed in the case of use of aspirin with isoniazid warrants investigation of the effects of adjunctive treatment of aspirin with other hydralazine drugs. Additionally, most NSAIDS have been shown to increase the adverse effects of p-aminosalicylic acid.66 The other challenges in including NSAIDs in therapy would be to overcome the gastrointestinal effects of these drugs; however, these effects are modest compared to the hepatotoxicity exerted by the established anti-TB drugs. To better understand the role of NSAIDs in alleviating infection and its symptoms, the host–pathogen interaction and inflammatory responses of the host require in-depth research.
The role of immune-modulation in TB
Inflammation, observed due to M. tuberculosis infection, is a host immune-response to the pathogen itself in combination with the action of the cocktail of anti-TB drugs administered. Early inflammatory responses are beneficial to the host as they serve to kill or sequester the organism in highly structured granulomas. However, prolonged inflammatory responses are detrimental, leading to the formation of pathological lesions that play a fundamental role in the transmission of the disease and its exacerbation.67 Therefore, therapy that is tailored to moderate the hypo- as well as hyper-responses could result in improvements in treatment outcome.68
The use of corticosteroids to control inflammation and reduce mortality from TB meningitis is already an established clinical practice and though they effect in very separate ways, this outcome supports the inclusion of NSAIDs in TB treatment regimens. When used as part of anti-TB therapy, NSAIDs are expected to influence both host- and pathogen-directed effects. However, the authors would like to add a caveat that though modulating macrophage response might prove useful to control the disease, the balance between cellular and chemokine responses is an area poorly understood in the context of host response to TB infection and vice versa.
Alternative modes and/or routes of delivery
Recently, there has been an increase in the investigation of delivery routes that optimize anti-TB therapies. In particular, a growing number of researchers support delivery of anti-TB drugs via aerosol formulations. The inhalation of an anti-tubercular drug, using either a nebulizer or a dry powder form, makes it is more likely to penetrate the alveoli and lung parenchyma thereby preventing establishment and progression of TB infection. Furthermore, delivering therapies in this way enables the primary site of the disease to be targeted directly, ensuring that the local concentration of the drug exceeds the MIC. Delivery of second-line drug, capreomycin, in a dry powder form has undergone testing in Phase I clinical trials in a small number of healthy volunteers.69 Promisingly, it was found to be well tolerated. Many other anti-tubercular compounds have been tested with similarly positive results, providing sufficient evidence to support pursing further research in this area. Rifamipicin loaded on chitosan particles is also proving to be an area of interest as these allow for a concentration-independent sustained release which could have significant impact on lowering the side effects of chemotherapy as well as improving patient compliance as the dosing frequency would be reduced.70
A recent study suggests that the NSAID ibuprofen might be administered via the pulmonary route, by encapsulating it within gel micro-particles. However, although the authors found the drug retained 50% activity against Candida albicans, further testing is vital before the potential of this delivery method can be validated.71 In short, the delivery of anti-tubercular drugs via the aerosol route represents an exciting area for improving the efficacy of current TB therapies.69
Conclusion
While it becomes imperative to find new drugs to control TB, it is also important to continually revise, redefine and perhaps, reclassify drugs that are already in use. The advantages offered by repurposing are manifold. It is crucial to understand not only their secondary targets but also the endogenous molecular mechanisms of action and how it would translate in a multidrug combinatorial treatment regimen. Identifying how these drugs work would strengthen their case for inclusion in clinical trials as well as pave the way for designing more targeted drugs. As the search to find novel drugs to tackle antimicrobial resistance deepens, there is a need to evaluate the driving forces of resistance and widen our search to novel concepts as well to find a better cure for TB than what exists today.
NSAIDs have rightly been hailed for their anti-inflammatory properties but their anti-infective property needs to be investigated further as the combined effects promise a significant improvement in treatment outcomes. It is encouraging to note that structural modifications to improve the antimicrobial activities of NSAIDs such as ibuprofen and carprofen are already underway.72
In a TB treatment setting, NSAIDs are primarily used to alleviate the symptoms that arise from the effects of this protracted disease and its therapy. These compounds have proven pharmacokinetic/dynamic and toxicity profiles in basic animal models and there is reasonable evidence to justify their inclusion into early clinical trials. However, the best administration routes and the stage of infection at which treatment is administered (early or late infection) require critical consideration before initiation of any further investigation in a clinical setting.
Author's contributions
SB conceived the project and along with AM prepared the first complete draft of the manuscript. All the other authors critically reviewed, revised and contributed significantly for its further improvement.
Acknowledgements
The authors would like to acknowledge Birkbeck, University of London for the International Merit Scholarship towards AM and MS’ MRes studies, a Birkbeck Anniversary/Wellcome Trust Studentship for AM's PhD study and the Wellcome Trust for a Summer internship to SB.
Conflict of interest statement
The authors have no potential conflicts of interest.
References
- 1.Organization WH. Global tuberculosis report 2015. 2015.
- 2.Burman WJ. Rip Van Winkle wakes up: development of tuberculosis treatment in the 21st century. Clin Infect Dis 2010;50:S165–72. [DOI] [PubMed] [Google Scholar]
- 3.Maitra A, Bhakta S.. TB summit 2014: prevention, diagnosis, and treatment of tuberculosis-a meeting report of a euroscicon conference. Virulence 2014;5:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitra A, Danquah CA, Scotti F, et al.. Tackling tuberculosis: insights from an international TB summit in London. Virulence 2015;6:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne DJ, Gwynn MN, Holmes DJ, et al.. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 2007;6:29–40. [DOI] [PubMed] [Google Scholar]
- 6.Adams CP, Brantner VV.. Estimating the cost of new drug development: is it really 802 million dollars. Health Aff (Millwood) 2006;25:420–8. [DOI] [PubMed] [Google Scholar]
- 7.Ashburn TT, Thor KB.. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 2004;3:673–83. [DOI] [PubMed] [Google Scholar]
- 8.Zumla A, Nahid P, Cole ST.. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 2013;12:388–404. [DOI] [PubMed] [Google Scholar]
- 9.Languillon J. The effects ot thalidomide on leprosy reaction. Int J Lepr 1971;39:590–2. [PubMed] [Google Scholar]
- 10.Schoeman JF, Fieggen G, Seller N, et al.. Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J Child Neurol 2006;21:301–8. [DOI] [PubMed] [Google Scholar]
- 11.Oprea TI, Mestres J.. Drug repurposing: far beyond new targets for old drugs. AAPS J 2012;14:759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinnings SL, Xie L, Fung KH, et al.. The mycobacterium tuberculosis drugome and its polypharmacological implications. PLoS Comput Biol 2010;6:e1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palomino JC, Martin A.. Is repositioning of drugs a viable alternative in the treatment of tuberculosis. J Antimicrob Chemother 2013;68:275–83. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie SH, Crook AM, McHugh TD, et al.. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 2014;371:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitra A, Bates S, Kolvekar T, et al.. Repurposing-a ray of hope in tackling extensively drug resistance in tuberculosis. Int J Infect Dis 2015;32:50–5. [DOI] [PubMed] [Google Scholar]
- 16.Amaral L, Viveiros M.. Why thioridazine in combination with antibiotics cures extensively drug-resistant mycobacterium tuberculosis infections. Int J Antimicrob Agents 2012;39:376–80. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Cohen KA, Winglee K, et al.. Efflux inhibition with verapamil potentiates bedaquiline in mycobacterium tuberculosis. Antimicrob Agents Chemother 2014;58:574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiga M, Ammerman NC, Maiga MC, et al.. Adjuvant host-directed therapy with types 3 and 5 but not type 4 phosphodiesterase inhibitors shortens the duration of tuberculosis treatment. J Infect Dis 2013;208:512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold B, Pingle M, Brickner SJ, et al.. Nonsteroidal anti-inflammatory drug sensitizes mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc Natl Acad Sci USA 2012;109:16004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman JD, Evangelopoulos D, Gupta A, et al.. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ Open 2013;3:e002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanyi J, Zumla A.. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. J Infect Dis 2013;208:185–8. [DOI] [PubMed] [Google Scholar]
- 22.Lee YT, Wang Q.. Inhibition of hKv2.1, a major human neuronal voltage-gated K+ channel, by meclofenamic acid. Eur J Pharmacol 1999;378:349–56. [DOI] [PubMed] [Google Scholar]
- 23.Neal TM, Vissers MC, Winterbourn CC.. Inhibition by nonsteroidal anti-inflammatory drugs of superoxide production and granule enzyme release by polymorphonuclear leukocytes stimulated with immune complexes or formyl-methionyl-leucyl-phenylalanine. Biochem Pharmacol 1987;36:2511–7. [DOI] [PubMed] [Google Scholar]
- 24.Imbimbo BP, Solfrizzi V, Panza F.. Are NSAIDs useful to treat Alzheimer's disease or mild cognitive impairment. Front Aging Neurosci 2010;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirin H, Moss SF, Kancherla S, et al.. Non-steroidal anti-inflammatory drugs have bacteriostatic and bactericidal activity against helicobacter pylori. J Gastroenterol Hepatol 2006;21:1388–93. [DOI] [PubMed] [Google Scholar]
- 26.Hersh E, Hammond B, Fleury A.. Antimicrobial activity of flurbiprofen and ibuprofen in vitro against six common periodontal pathogens. J Clin Dent 1990;3:1–5. [PubMed] [Google Scholar]
- 27.Mazumdar K, Dastidar SG, Park JH, et al.. The anti-inflammatory non-antibiotic helper compound diclofenac: an antibacterial drug target. Eur J Clin Microbiol Infect Dis 2009;28:881–891. [DOI] [PubMed] [Google Scholar]
- 28.Dutta NK, Mazumdar K, Dastidar SG, et al.. Activity of diclofenac used alone and in combination with streptomycin against mycobacterium tuberculosis in mice. Int J Antimicrob Agents 2007;30:336–40. [DOI] [PubMed] [Google Scholar]
- 29.Sriram D, Yogeeswari P, Devakaram RV.. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg Med Chem 2006;14:3113–8. [DOI] [PubMed] [Google Scholar]
- 30.Dutta NK, Mazumdar K, Seok SH, et al.. The anti-inflammatory drug diclofenac retains anti-listerial activity in vivo. Lett Appl Microbiol 2008;47:106–11. [DOI] [PubMed] [Google Scholar]
- 31.Chiniforoshan H, Tabrizi L, Hadizade M, et al.. Anti-inflammatory drugs interacting with Zn (II) metal ion based on thiocyanate and azide ligands: synthesis, spectroscopic studies, DFT calculations and antibacterial assays. Spectrochim Acta A Mol Biomol Spectrosc 2014;128:183–90. [DOI] [PubMed] [Google Scholar]
- 32.Kovala-Demertzi D, Dokorou V, Primikiri A, et al.. Organotin meclofenamic complexes: synthesis, crystal structures and antiproliferative activity of the first complexes of meclofenamic acid–novel anti-tuberculosis agents. J Inorg Biochem 2009;103:738–44. [DOI] [PubMed] [Google Scholar]
- 33.Niu L, Li Y, Li Q.. Medicinal properties of organotin compounds and their limitations caused by toxicity. Inorganica Chim Acta 2014;423:2–13. [Google Scholar]
- 34.Jindani A, Aber VR, Edwards EA, et al.. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 1980;121:939–49. [DOI] [PubMed] [Google Scholar]
- 35.Inman WH. Study of fatal bone marrow depression with special reference to phenylbutazone and oxyphenbutazone. Br Med J 1977;1:1500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalle AM, Rizvi A.. Inhibition of bacterial multidrug resistance by celecoxib, a cyclooxygenase-2 inhibitor. Antimicrob Agents Chemother 2011;55:439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel VA, Dunn MJ, Sorokin A.. Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol Chem 2002;277:38915–20. [DOI] [PubMed] [Google Scholar]
- 38.Salunke SB, Azad AK, Kapuriya NP, et al.. Design and synthesis of novel anti-tuberculosis agents from the celecoxib pharmacophore. Bioorg Med Chem 2015;23:1935–43. [DOI] [PubMed] [Google Scholar]
- 39.Byrne ST, Denkin SM, Zhang Y.. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J Antimicrob Chemother 2007;59:313–6. [DOI] [PubMed] [Google Scholar]
- 40.Schaller A, Sun Z, Yang Y, et al.. Salicylate reduces susceptibility of mycobacterium tuberculosis to multiple antituberculosis drugs. Antimicrob Agents Chemother 2002;46:2636–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne ST, Denkin SM, Zhang Y.. Aspirin antagonism in isoniazid treatment of tuberculosis in mice. Antimicrob Agents Chemother 2007;51:794–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misra UK, Kalita J, Nair PP.. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 2010;293:12–7. [DOI] [PubMed] [Google Scholar]
- 43.Evangelopoulos D, Bhakta S.. Rapid methods for testing inhibitors of mycobacterial growth, 2nd edn, Springer: Antibiotic Resistance Protocols, 2010;193–201. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A, Bhakta S.. An integrated surrogate model for screening of drugs against mycobacterium tuberculosis. J Antimicrob Chemother 2012;67:1380–91. [DOI] [PubMed] [Google Scholar]
- 45.Danquah CA, AM, Gibbons S, et al. HT-SPOTi: a rapid, drug susceptibility test (DST), to evaluate antibiotic resistance profiles and novel chemicals for anti-infective drug discovery. Curr Protoc Microbiol 2016;8:17–8. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal A, Roy D, Chowdhury B, et al.. Ibuprofen, a unique anti‐inflammatory compound with antifungal activity against dermatophytes. Lett Appl Microbiol 1993;17:109–11. [Google Scholar]
- 47.Elvers K, Wright S.. Antibacterial activity of the anti‐inflammatory compound ibuprofen. Lett Appl Microbiol 1995;20:82–4. [DOI] [PubMed] [Google Scholar]
- 48.Vilaplana C, Marzo E, Tapia G, et al.. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J Infect Dis 2013;208:199–202. [DOI] [PubMed] [Google Scholar]
- 49.Eisen DP, McBryde ES, Walduck A.. Low-dose aspirin and ibuprofen's sterilizing effects on mycobacterium tuberculosis suggest safe new adjuvant therapies for tuberculosis. J Infect Dis 2013;208:1925–7. [DOI] [PubMed] [Google Scholar]
- 50.Konstan MW, Krenicky JE, Finney MR, et al.. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther 2003;306:1086–91. [DOI] [PubMed] [Google Scholar]
- 51.Pierce CA, Voss B.. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother 2010;44:489–506. [DOI] [PubMed] [Google Scholar]
- 52.Davies NM. Clinical pharmacokinetics of ibuprofen. Clin Pharmacokinet 1998;34:101–54. [DOI] [PubMed] [Google Scholar]
- 53.Rainsford K. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 2009;17:275–342. [DOI] [PubMed] [Google Scholar]
- 54.Carprofen Summary Report (1). In: EMEA (ed). Committee for Veterinary Medicinal Products London, UK: 1999.
- 55.Crevoisier C. Pharmacokinetic properties of carprofen in humans. Eur J Rheumatol Inflamm 1982;5:492–502. [PubMed] [Google Scholar]
- 56.Kerr A, Muller F, Ferguson J, et al.. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol 2008;159:1303–8. [DOI] [PubMed] [Google Scholar]
- 57.Roelandts R. Photosensitivity associated with carprofen. Dermatologica 1986;172:64–5. [DOI] [PubMed] [Google Scholar]
- 58.Bosca F, Miranda MA.. Photosensitizing drugs containing the benzophenone chromophore. J Photochem Photobiol B 1998;43:1–26. [DOI] [PubMed] [Google Scholar]
- 59.Ingrao JC, Johnson R, Tor E, et al.. Aqueous stability and oral pharmacokinetics of meloxicam and carprofen in male C57BL/6 mice. J Am Assoc Lab Anim Sci 2013;52:553–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Brentnall C, Cheng Z, McKellar QA, et al.. Influence of oxytetracycline on carprofen pharmacodynamics and pharmacokinetics in calves. J Vet Pharmacol Ther 2013;36:320–8. [DOI] [PubMed] [Google Scholar]
- 61.Organization WH. Treatment of tuberculosis: guidelines. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 62.Simonetti A, Marzi S, Billas IM, et al.. Involvement of protein IF2 N domain in ribosomal subunit joining revealed from architecture and function of the full-length initiation factor. Proc Natl Acad Sci USA 2013; 110: 15656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denkin S, Byrne S, Jie C, et al.. Gene expression profiling analysis of mycobacterium tuberculosis genes in response to salicylate. Arch Microbiol 2005;184:152–7. [DOI] [PubMed] [Google Scholar]
- 64.Yin Z, Wang Y, Whittell LR, et al.. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem Biol 2014;21:481–7. [DOI] [PubMed] [Google Scholar]
- 65.Gui W-J, Lin S-Q, Chen Y-Y, et al.. Crystal structure of DNA polymerase III β sliding clamp from mycobacterium tuberculosis. Biochem Biophys Res Commun 2011;405:272–7. [DOI] [PubMed] [Google Scholar]
- 66.Arbex MA, Varella MdCL, Siqueira HRd, et al. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations-part 2: second line drugs. J Bras Pneumol 2010;36:641–56. [DOI] [PubMed] [Google Scholar]
- 67.Kaufmann SH, Dorhoi A.. Inflammation in tuberculosis: interactions, imbalances and interventions. Curr Opin Immunol 2013;25:441–9. [DOI] [PubMed] [Google Scholar]
- 68.Flynn JL, Chan J, Lin PL.. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol 2011;4:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hickey A, Durham P, Dharmadhikari A, et al.. Inhaled drug treatment for tuberculosis: past progress and future prospects. J Control Release 2015. (in Press corrected proof). [DOI] [PubMed]
- 70.Gelperina S, Kisich K, Iseman MD, et al.. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med 2005;172:1487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hariyadi DM, Lin SC-Y, Wang Y, et al. Diffusion loading and drug delivery characteristics of alginate gel microparticles produced by a novel impinging aerosols method. J Drug Target 2010;18:831–41. [DOI] [PubMed] [Google Scholar]
- 72.Bartzatt R, Cirillo SL, Cirillo JD.. Determination of molecular properties effectuating the growth inhibition of mycobacterium tuberculosis by various small molecule hydrazides. Lett Drug Des Discov 2008;5:162–8. [Google Scholar]