Abstract
Introduction
In the last few years, the use of collagenase clostridium histolyticum for management of Dupuytren's contracture has increased. The procedure of enzymatic fasciectomy has become popular because it is non-invasive, safe and fast to perform.
Sources of data
A systematic search was performed on Medline (PubMed), Web of Science and Scopus databases using the combined keywords ‘Dupuytren collagenase’ and ‘Dupuytren clostridium histolyticum’. Forty-three studies were identified. The quality of the studies was assessed using the Coleman Methodological Score.
Areas of agreement
The use of collagenase clostridium histolyticum provides better outcomes in patients with mild-moderate joint contracture, with lower complications and side effects than open fasciectomy. Manipulation can be performed 2–7 days after the injection. The use of collagenase is cost-effective.
Areas of controversy
Most of the studies did not report patient-related outcomes. The role of dynamic splint has to be investigated with randomized clinical trials.
Growing points
The shorter recovery time and the low incidence of serious or major adverse effects are the main advantages of this new technology.
Areas timely for developing research
There is a need to perform studies with longer follow-up because the recurrence rate seems to increase with time. Further investigations are necessary to assess whether it is safe and effective to inject two or more cords at the same time.
Keywords: Dupuytren, collagenase, non-operative, clostridium histolyticum, contracture
Introduction
Dupuytren's disease (DD) is a common connective disorder of the palmar fascia of the hand, which evolves to progressive contracture in flexion of the fingers, severely impairing function and quality of life.1 The overall prevalence of DD is 0.2%, up to 50% in some subgroups of patients at high risk.2,3 Age and race may be predisposing; it is four to six times more frequent in males than females4; women develop it later. A genetic predisposition has also been recognized.5 High alcohol consumption, smoking, diabetes, epilepsy, hypercholesterolemia and exposure to vibrations are all risk factors.6 Usually, the first appearance is a nodule in the palm of the hand, which usually progresses to form cords.
Open fasciectomy, needle fasciotomy and enzyme fasciectomy have all been successfully performed. The first application of enzymes in patients with DD was in 19657: it was a mixture of trypsin and hyaluronidase; lidocaine8 was introduced later. When the role of immature Type III collagen9 was clarified, enzyme fasciectomy using collagenase became advantageous because, differently from other enzymes, it is collagen specific. It was investigated first in vitro in 1996, using the Clostridial collagenase10; the toxicity was studied later in in vivo studies.11 The first open label study on patients with DD was published in 2000.12 Then, several clinical trials have been undertaken in the USA, Europe and Australia. The Food and Drug Administration approved the collagenase clostridium histolyticum for the management of DD in 2010. This is a comprehensive review of studies published on management of patients with DD using enzyme fasciectomy with collagenase which aims to investigate whether it provides better outcomes compared to other techniques, with lower serious or major side effects and recurrence rates. The costs–benefits of its use have been investigated; the methodological quality of the available studies was also assessed.
Materials and methods
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).13 The keywords ‘Dupuytren collagenase’ and ‘Dupuytren clostridium histolyticum’ were used for the search, with no limits for year of publication. Medline (PubMed), Web of Science and Scopus were accessed on October 12, 2015. Articles in English, Spanish, Italian and French were identified, all published in peer-reviewed journals. Biomechanical studies, studies on animals or cad, avers, technical notes, letter to the editor and instructional courses were excluded. Two authors (A.D.B. and F.S.) independently assessed the abstract of each publication. When the article could not be included or excluded based on the abstract, a full-text version of the article was downloaded. If the abstract was not available, the article was excluded from the study. In addition, the reference list of each selected article was searched by hand to identify additional studies missed at the electronic search.
The two investigators assessed each study according to the Coleman Methodological Score (CMS),14 a score ranging from 0 to 100. A score of 100 was the best study design. Both investigators performed the CMS assessment twice, with an interval of 10 days, and they discussed the scores until consensus was reached when more than a two-point difference was present. Data on demographic features, operative readings, diagnostic methods, follow-up periods, type and rates of complications, return to work activity, recurrence and outcome measures were recorded.
Results
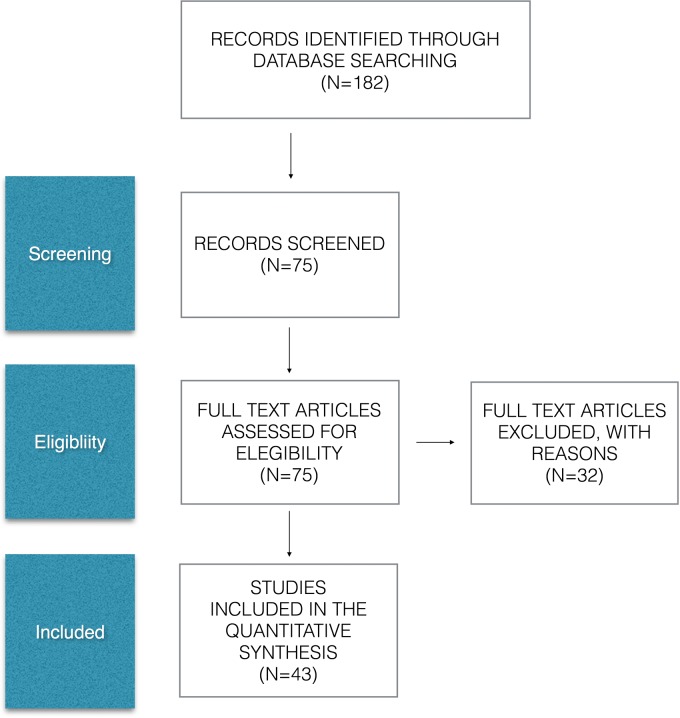
A total of 182 studies were identified at the first search. Of the 75 studies selected on the basis of the abstract, 22 were excluded after the full text had been read; 43 publications relevant to the topic were included (Fig. 1). All the studies were published between 2000 and 2015; some studies include patients who are included in other studies. The total number of patients was 6795: 81% (5195) were male and 19% (1127) female. Gender data were not available in 12 studies.15–26 The average age of the patients at the treatment time was 64 years; the mean follow-up was 15 months, ranging from 1 to 96 months.15
Fig. 1.
PRISMA flow diagram.
Quality assessment
All the Coleman scores are given in Table 1. Articles analyzing the cost analysis without any clinical information were excluded from the assessment with CMS. A score >85 is considered excellent, good from 70 to 84, moderate from 50–69 and poor when <50. The mean CMS was 65.6 (range 39–90). Four studies were graded as excellent, 11 studies as good (Table 1).
Table 1.
General features of the studies
| Authors | Number of patients | Follow-up (m) | Type of study | CMS |
|---|---|---|---|---|
| Badalamente et al. 12 | 35 | 20 | Case series | 69 |
| Badalamente et al. 27 | 80 | 48 | Prospective, randomized, double-blind, placebo-controlled | 90 |
| Badalamente et al. 28 | 35 | 24 | Prospective, randomized, double-blind, placebo-controlled | 79 |
| Hurst et al. 29 | 306 | 3 | Prospective, randomized, double-blind, placebo-controlled | 84 |
| Gilpin et al. 30 | 66 | 12 | Prospective, randomized, double-blind, placebo-controlled | 82 |
| Watt et al. 15 | 8 | 96 | Case series | 62 |
| Chen et al. 31 | 50 | Cost analysis | ||
| Witthaut et al. 32 | 299 | 1 | Prospective, randomized, double-blind, placebo-controlled | 76 |
| Bendon et al. 16 | 4 | Case series | 42 | |
| Rozen et al. 17 | 12 | 12 | Case series | 54 |
| Coleman et al. 33 | 12 | 1 | Case series | 57 |
| Peimer et al. 34 | 463 | Retrospective case control | 55 | |
| Sanjuan-Cerverò et al. 18 | 91 | Retrospective case control | 65 | |
| Hayton et al. 35 | 616 | Retrospective case control | 60 | |
| Martin Ferrero et al. 19 | 35 | Prospective case series | 47 | |
| Nydick et al. 36 | 59 | 6 | Retrospective case control | 67 |
| De Salas-Cansado et al. 20 | 123 | Cost analysis | ||
| Skirven et al. 37 | 21 | 1 | Case series | 59 |
| Witthaut et al. 38 | 587 | 9 | Retrospective analysis | 73 |
| Peimer et al. 39 | 643 | 36 | Retrospective analysis | 63 |
| Baltzer et al. 21 | Cost analysis | |||
| Mickelson et al. 40 | 43 | 1 | Prospective, randomized | 79 |
| Naam41 | 61 | 24 | Retrospective case control | 58 |
| Mc Mahon et al. 42 | 48 | 15 | Retrospective case series | 70 |
| Povisen et al. 22 | 20 | Case control | 42 | |
| Povisen et al. 23 | 20 | Case control | 39 | |
| Sood et al. 43 | 16 | 12 | Retrospective case series | 50 |
| Mehta et al. 44 | 40 | Cost analysis | ||
| Mc Grouther et al. 24 | 58 | 12 | Case series | 87 |
| Coleman et al. 25 | 60 | 2 | Case series | 71 |
| Alberton et al. 45 | 40 | 6 | Case series | 53 |
| Raven et al. 46 | 271 | 1 | Randomized controlled double blind | 86 |
| Manning et al. 47 | 45 | 2,5 | Case series | 61 |
| Atroshi et al. 26 | 32 | Cost analysis | ||
| Kaplan et al. 48 | 37 | 3 | Randomized controlled double blind | 84 |
| Zhou et al. 49 | 132 | 3 | Retrospective matched patients | 70 |
| Atroshi et al. 50 | 164 | 1 | Case series | 57 |
| Gaston et al. 51 | 715 | 2 | Case series | 67 |
| Badalamente et al. 52 | 506 | 1 | Prospective, randomized, double-blind, placebo-controlled | 80 |
| Peimer et al. 53 | 644 | 60 | Retrospective analysis | 85 |
| Verheyden et al.54 | 144 | 1 | Case series | 61 |
| Muppavarapu et al. 55 | 117 | 15 | Retrospective case control | 60 |
| Tay et al. 56 | 37 | 24 | Retrospective case control | 49 |
Description of subject selection process
The study CORD I,29 a Phase 3 clinical trial based on previous studies by Badalamente et al.,12,27,28 followed strict selection criteria: healthy patients, older than 18 years, with one cord, metacarpophalangeal joint (MP) contracture between 20° and 100°, and proximal interphalangeal joint (PIP) contracture between 20° and 80°. Postmenopausal women or women who had used contraceptive therapy were also included. Exclusion criteria were breast feeding, bleeding disorder, recent stroke, the use of tetracycline, primary arthroplasty, anticoagulant therapy taken within 7 days of the injection, allergy to collagenase and chronic muscular or neuromuscular disorders. Later, 12 studies followed the same entry protocol.17,24,30,32,34,35,38,39,46,49,52,53
Coleman et al. 25,33 and Gaston51 extended the indications to collagenase for patients with at least three joints involved; two injections were administered at the same time.
Skirven et al. 37included only patients with severe PIP contracture >40 S.
Rehabilitation protocol
In the first rehabilitation protocol described by Badalamente et al.,12 the patients were examined the day after the injection. At that stage, passive extension was allowed as patients tolerated up to the rupture of the cord, without any local anesthetic. Later, the protocol was modified by the same group of investigators29 using a static splint in extension over night for 4 months. Many studies followed the same protocol, exception for four studies. Skirven et al. 37 used a custom-made orthosis in extension to extend gradually the contracted PIP joints. Mickleson et al.,40 Manning et al. 47 and Kaplan et al. 48 performed manipulation 1, 4 and 7 days after application of collagenase, showing no differences in terms of efficacy and complications.
Objective outcome
Eighteen studies considered the clinical satisfaction of the patients and a residual contracture <5° as primary end points (Table 2).
Table 2.
Clinical success (residual contracture <5°, up to three injections)
| Authors | MP joint | PIP joint |
|---|---|---|
| Badalamente et al. 12 | 30/34 MP (88%) | 7/9 PIP (77%) |
| Badalamente et al. 27 | 18/18 (100%) | 6/7 (85%) |
| Badalamente et al. 28 | 12/14 (86%) | 9/9 (100%) |
| Hurst et al. 29 | 102/133 (76.7%) | 28/70 (40%) |
| Gilpin et al. 30 | 13/20 (65%) | 7/25 (28%) |
| Witthaut et al. 32 | MP + PIP 126/197 (64%) | |
| Peimer et al. 34 | MP + PIP 310/467 (67%) | |
| Nydick et al. 36 | 14/22 (64%) | 5/12 (42%) |
| Witthaut et al. 38 | 369/531 (70%) | 128/348 (37%) |
| Mickelson et al. 40 | 10/11 (91%) | 4/10 (40%) |
| Mc Grouther et al. 24 | MP + PIP 53/65 (82%) | |
| Coleman et al. 25 | 57/75 (76%) | 15/45 (33%) |
| Alberton et al. 45 | MP + PIP 30/40 (75%) | |
| Raven et al. 46 | 112/167 (67%) | 44/104 (42%) |
| Manning et al. 47 | 35/38 (92%) | |
| Kaplan et al. 48 | 34/37 (91%) | |
| Gaston et al. 51 | 213/325 (66%) | 62/211 (29%) |
| Badalamente et al. 52 | 219/644 (34%) |
Gilpin et al. 30 reported the worst percentage of clinical success for both MP (13/20; 65%) and PIP (7/25; 28%); Badalamente et al. 27 reported the best result for MP (18/18; 100%) and in 2007 for PIP (9/9; 100%). Several studies (Table II and 13,16,19,22,23,33,35,37,41–43,49,50,54–56) reported secondary end points, described as clinical improvement, but these results are difficult to compare because different variables such as range of motion (ROM) changes and decreased contracture were considered. Some studies15,17,39,53 did not report objective outcomes. Cost analysis studies18,20,21,26,31,44 did not report clinical outcomes.
Subjective outcomes
The patient satisfaction was evaluated in nine studies,15,25,30,33,36,38,41,42,56 all reporting >80% of good satisfaction. In a study,36 there were no differences in terms of satisfaction between patients who had undergone collagenase and needle fasciotomy.
The Disability of Arm, Shoulder and Hand (DASH) score57 was administered in one study.41 After 3 months, patients who had undergone collagenase fared better than those who had undergone open fasciectomy, with no statistical differences at 1 year and 2 years.
The quick DASH was used in one study.42 The Michigan Hand Questionnaire (MHQ)58 was used in two studies. Kaplan et al. 48 did not find any difference between the two groups (patients who received mobilization at Day 1 vs mobilization Day 2); Zhou et al. 49 found larger improvement in MHQ in the collagenase group compared to the fasciectomy group.
Complications
Five studies did not report adverse effects because they were not clinical studies.20,21,26,31,44 In seven studies,15,16,18,22,23,32,39 adverse effects were not reported. In all the remaining studies, adverse effects were widely reported. All the studies reported high rates of minor/mild adverse effects. Hayton et al. 35 reported minor adverse effects in 98% of the patients (604/616), specifically, peripheral edema in 81% (500), pain at the site of injection in 39% (239), hemorrhage at the site of injection in 38% (231), tenderness in 29% (170), swelling at the site of injection in 28% (170), contusion in 65% (402), limb pain in 43% (263), pruritus in 15% (94), ecchymosis in 14% (87), skin lacerations in 13% (79), blood blister in 11% (70), lymphadenopathy in 11% (67). Serious adverse effects occurred in eight studies (Table 3).
Table 3.
Major complications
| Authors | Tendon rupture | Complex regional pain syndrome | Pulley rupture | Sensory abnormality | Deep tissue adhesion | Anapylactic reaction | Hyperestesia | Hemorrhage post-procedural | Deep vein thrombosis |
|---|---|---|---|---|---|---|---|---|---|
| Hurst et al. 29 | 2/306 (0.6%) | 1/306 (0.3%) | |||||||
| Gilpin et al. 30 | 1/66 (1.5%) | 1/66 (1.5%) | |||||||
| Rozen et al. 17 | 2/12 (16%) | ||||||||
| Sanjuan Cervero et al. 18 | 1/43 (2%) | ||||||||
| Mc Mahon et al. 42 | 1/64 (1.5%) | ||||||||
| Coleman et al. 25 | 1/60 (1.6%) | 1/60 (1.6%) | |||||||
| Gaston et al. 51 | 1/715 (0.1%) | 1/715 (0.1%) | 1/715 (0.1%) | 1/715 (0.1%) | |||||
| Badalamente et al. 52 | 2/506 (0.3%) | 1/506 (0.2%) |
Recurrence
Twelve studies reported on recurrences rate (Table 4). varying from 0%29,30,36,41 to 75%.15 In all the studies, the recurrence was considered a decrease in passive extension >20°. Nevertheless, surgery is not recommended when the contracture is <30°.
Table 4.
Recurrences
| Authors | Follow-up (months) | Total number of recurrences |
|---|---|---|
| Badalamene et al. 12 | 20 | 3/35 (8.5%) |
| Badalamene et al. 28 | 24 | 5/35 (14%) |
| Hurst et al. 29 | 3 | 0/306 (0%) |
| Watt et al. 15 | 88 | 6/8 (75%) |
| Gilpin et al. 30 | 12 | 0/66 (0%) |
| Witthaut et al. 38 | 9 | 19/497 (4%) |
| Peimer et al. 39 | 36 | 217/623 (35%) |
| Nydick et al. 36 | 6 | 0/59 (0%) |
| Naam41 | 24 | 0/61 (0%) |
| Mc Mahon et al. 42 | 15 | 13/48 (28%) |
| Alberton et al. 45 | 6 | 2/40 (3.8%) |
| Peimer et al. 53 | 60 | 291/623 (47%) |
Cost analysis
Six studies18,20,21,26,31,44 reported the costs of the application of collagenase. All the authors agreed that the use of collagenase is cost-effective, with savings between 29%18 and 70%44 compared to traditional surgery. According to Chen et al.,31 collagenase is cost-effective only if the drug costs <945 dollars. A Canadian study21 reported that the use of collagenase would be convenient if costs are significantly lower than the current price in USA. All the studies compared only the direct costs without including the costs of low productivity or sick leave. Naam41 highlighted that the return to work was significantly faster after collagenase compared to open fasciectomy (1.9 days vs 37.4 days).
Discussion
The use of collagenase to manage DD has increased in the last 5 years. Even though the average CMS is moderate, most studies are of good to excellent methodological quality (Table 1). On the contrary, the relatively short follow-up limits the possibility to inform on the recurrence rate in the long term.
In this systematic review, the primary end point was the clinical success considered as a residual contracture <5° at 1 month after the last injection (Table 2). Nevertheless, the various studies report great variability in clinical success rate, especially when referring to the different joints of the hand. Specifically, the mean percentage of ROM of the metacarpophalangeal joint was 79.4% whereas that of the proximal interphalangeal joint was 48.9%. Hurst29 and Gilpin30 reported that joint with contracture <50° for the MCP and <40°for the PIP responded better than more severely contracted joints. The secondary end points were difficult to compare given their heterogeneity.
After manipulation, in almost all the studies, with exception for the three studies by Badalamente et al.,12,27,28 patients were advised to wear a splint overnight for 4 months in order to achieve the maximal extension of the finger. Skirven et al. 37 used for severe PIP contracture (>40°; mean 56°) a custom-made dorsal orthosis allowing gradual progressive extension of the PIP joint to correct the residual flexion contracture. One week after manipulation, a cylinder orthosis in maximal extension was placed on the PIP joint for 4–6 weeks. In this short-term study,37 clinical success (residual contracture <5°) was observed in 55% of the patients after one injection. Three studies40,47,48 showed no statistical differences in patients undergoing collagenase and manipulation after 1, 4 or 7 days, without different occurrence of lesions to the skin and spontaneous ruptures in patients manipulated after 4 days, compared to those manipulated after 1 or 2 days.48 Therefore, manipulation can be performed based on the needs of the patient.
All the studies15,25,30,33,36,38,41,42,56 reported good satisfaction in >80% of the patients. Given that only some studies41,42,48,49 used subjective outcome tools (DASH and MHQ), we now point out that future studies should concentrate on patient-related outcomes.
Major complications have been reported after fasciectomy in ~15% of patients, including injuries to the digital nerve (5.5%) and digital artery (2%), infection (2.4%) and complex regional pain syndrome (5.5%).59 A recent review60 comparing the occurrence of major adverse effects after application of collagenase vs fasciectomy showed lower rates of nerve injury (0% vs 3.8%), neurapraxia (4.4% vs 9.4%), complex regional pain syndrome (0.1% vs 4.5%) and arterial injury (0% vs 5.5%) in patients undergoing collagenase; the occurrence of tendon injury was similar (0.3% vs 0.1%). Our systematic review reported similar findings (Table 3). In addition, an anaphylactic reaction and a case of deep vein thrombosis evolving in pulmonary embolism have also been reported.51 King and Belcher61 reported two cases of cold intolerance after collagenase injection.
In all the studies (Table 4), a recurrence was defined as an increased contracture >20°.29 The wide discrepancy of recurrence rates may be related to the follow-up, with higher recurrences in longer follow-up studies. The CORDLESS39,53 was a long-term study that examined patients enrolled in three previous studies29,30,32 at 3 and 5 years, showing a recurrence rate of 35% at 3 years and 47% at 5 years. Most of the recurrences (219/623; 75%) occurred in the first 3 years after treatment. In a post hoc analysis, Peimer advises to change the criteria of recurrence as a contracture >30° as a contracture of 20° does not need surgery. Using this threshold, the recurrence rate at 5 years was 32% (198/623).53 The study with the longest follow-up (8 years) showed a recurrence rate of 75%,15 with an average contracture of the MP joint of 22°. Van Rijssen et al.,62 using a worsening of 30° as threshold, found a recurrence rate of 85% at 5 years after needle fasciotomy, and 21% after limited fasciectomy. Atroshi et al. 50 and Verheyden et al. 54 used a higher dose than that recommended, injected in different portions of the cord to treat multiple sites of contracture in a single session, showing higher efficacy without increased occurrence of major adverse effects.
Even though Coleman et al. 33 demonstrated good results after management of two cords on the same hand, these findings need to be supported by studies with larger sample size.
All the cost analysis studies18,20,21,26,31,44 agree that collagenase treatment is cost-effective. Furthermore, all the patients treated with collagnease required less medical and physiotherapic cares. All the studies highlight to maintain low the price of the enzyme. According to Naam,41 the time to return to work or daily activity was shorter in the collagenase group compared to the fasciectomy group (average 1.9 days vs 37.4 days).
This systematic review has several limitations. First, many studies report longer follow-ups of previous studies and some studies utilize the same cohort of patients. Also, although we included studies from several European languages, investigations in non-European languages may have been missed.
Surgical fasciectomy or collagenase injections do not provide a definitive management for patients with DD. Serious adverse events associated with the use of collagenase clostridium histolyticum are uncommon and less frequent compared to the rates of major complications which occur after surgery. In conclusion, the injections of collagenase clostridium histolyticum are satisfying for the patients, and should be encouraged. In cases of recurrence, the injections may be safely repeated.
Conflict of interest statement
The authors have no potential conflicts of interest.
References
- 1.Wilburn J, Mc Kenna SP, Perry-Hinsley D, et al. The impact of Dupuytren disease on patient activity and quality of life. J Hand Surg Am 2013;30:1209–14. [DOI] [PubMed] [Google Scholar]
- 2.Hindocha S, Mc Grouther DA, Bayat A. Epidemiological evaluation of Dupuytren's disease incidence and prevalence rates in relation to etiology. Hand 2009;4:256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanting R, Broekstra DC, Werker PM, et al. A systematic review and meta-analysis on the prevalence of Dupuytren's disease in the general population of We/stern's countries. Plast Reconst Surg 2014;133:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren's contracture in men and women. J Hand Surg Br 1999;24:456–9. [DOI] [PubMed] [Google Scholar]
- 5.Burge P. Genetics of Dupuytren's disease. Hand Clinic 1999;15:63–71. [PubMed] [Google Scholar]
- 6.Burke FD, Proud G, Lawson U, et al. An assessment of the effects of exposure to vibration, smoking, alcohol and diabetes on the prevalence of Dupuytren's disease in 97,537 mines. J Hand Surg Eur Vol 2007;32:400–6. [DOI] [PubMed] [Google Scholar]
- 7.Bassot J. Treatment of Dupuytren's disease by isolated pharmacodynamic ‘exeresis’ or ‘exseresis’ completed by a solely cutaneous plastic step. Lille Chir 1965;20:38–44. [PubMed] [Google Scholar]
- 8.Hueston JT. Enzymatic fasciotomy. Hand 1971;3:38–40. [DOI] [PubMed] [Google Scholar]
- 9.Brickley-Parsons D, Glimcher MJ, Smith RJ, et al. Biochemical changes in the collagen of palmar fascia in patients with Dupuytren's disease. J Bone Joint Surg 1981;63:787–97. [PubMed] [Google Scholar]
- 10.Starkweather KD, Lattuga S, Hurst LC, et al. Collagenase in the treatment of Dupuytren's disease: an in vitro study. J Hand Surg 1996;21:490–5. [DOI] [PubMed] [Google Scholar]
- 11.Badalamente MA, Hurst LC. Enzyme injection as a non-operative treatment for Dupuytren's disease. Drug Deliv 1996;3:35–40. [Google Scholar]
- 12.Badalamente MA, Hurst LC. Enzyme injection as a nonsurgical treatment of Dupuytren's disease. J Hand Surg 2000;25:629–36. [DOI] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DJ, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman BD, Khan KM, Maffulli N et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scan J Med Sci Sports 2000;10:2–11. [DOI] [PubMed] [Google Scholar]
- 15.Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren's disease: 8-year follow-up. J Hand Surg Am 2010;35:534–9. [DOI] [PubMed] [Google Scholar]
- 16.Bendon CL, Giele HP. Collagenase for Dupuytren's disease of the thumb. J Bone Joint Surg Br 2012;94:1390–2. [DOI] [PubMed] [Google Scholar]
- 17.Rozen WM, Edirisinghe Y, Crock J. Late complications of clinical clostridium histolyticum collagenase use in Dupuytren's disease. PLoS One 2012;7:e43406. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sanjuan Cerveró R, Franco Ferrando N, Poquet Jornet J. Use of resources and costs associated with the treatment of Dupuytren's contracture at an orthopedics and traumatology surgery department in Denia (Spain): collagenase clostridium hystolyticum versus subtotal fasciectomy. BMC Musculoskelet Disord 2013;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martín-Ferrero MÁ, Simón-Pérez C, Rodríguez-Mateos JI, et al. [Treatment of Dupuytren's disease using collagenase from clostridium histolyticum]. [Article in Spanish]. Rev Esp Cir Ortop Traumatol 2013;57:398–402. [DOI] [PubMed] [Google Scholar]
- 20.De Salas-Cansado M, Cuadros M, Del Cerro M, et al. Budget impact analysis in Spanish patients with Dupuytren's contracture: fasciectomy vs. collagenase clostridium histolyticum. Chir Main 2013;32:68–73. [DOI] [PubMed] [Google Scholar]
- 21.Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren's contracture. A Canadian cost- utility analysis of current and future management strategies. Bone Joint J 2013;95-B:1094–100. [DOI] [PubMed] [Google Scholar]
- 22.Povlsen B, Povlsen SD. What is the better treatment for single digit Dupuytren's contracture: surgical release or collagenase clostridium histolyticum (Xiapex) injection. Hand Surg 2014;19:389–92. [DOI] [PubMed] [Google Scholar]
- 23.Povlsen B, Shields AM, Bhabra GS. Resource utilisation associated with single digit Dupuytren's contracture treated with either surgery or injection of collagenase clostridium histolyticum. Hand Surg 2014;19:205–9. [DOI] [PubMed] [Google Scholar]
- 24.McGrouther DA, Jenkins A, Brown S, et al. The efficacy and safety of collagenase clostridium histolyticum in the treatment of patients with moderate Dupuytren's contracture. Curr Med Res Opin 2014;30:733–9. [DOI] [PubMed] [Google Scholar]
- 25.Coleman S, Gilpin D, Kaplan FT, et al. Efficacy and safety of concurrent collagenase clostridium histolyticum injections for multiple Dupuytren contractures. J Hand Surg Am 2014;39:57–64. [DOI] [PubMed] [Google Scholar]
- 26.Atroshi I, Strandberg E, Lauritzson A, et al. Costs for collagenase injections compared with fasciectomy in the treatment of Dupuytren's contracture: a retrospective cohort study. BMJ Open 2014;4:e004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren's disease. J Hand Surg Am 2002;27:788–98. [DOI] [PubMed] [Google Scholar]
- 28.Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren's contracture. J Hand Surg Am 2007;32:767–74. [DOI] [PubMed] [Google Scholar]
- 29.Hurst LC, Badalamente MA, CORD I Study Group et al. Injectable collagenase clostridium histolyticum for Dupuytren's contracture. N Engl J Med 2009;361:968–79. [DOI] [PubMed] [Google Scholar]
- 30.Gilpin D, Coleman S, Hall S, et al. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren's disease. J Hand Surg Am 2010;35:2027–38. [DOI] [PubMed] [Google Scholar]
- 31.Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am 2011;36:1826–34. [DOI] [PubMed] [Google Scholar]
- 32.Witthaut J, Bushmakin AG, Gerber RA, et al. Determining clinically important changes in range of motion in patients with Dupuytren's Contracture: secondary analysis of the randomized, double-blind, placebo-controlled CORD I study. Clin Drug Investig 2011;31:791–8. [DOI] [PubMed] [Google Scholar]
- 33.Coleman S, Gilpin D, Tursi J, et al. Multiple concurrent collagenase clostridium histolyticum injections to Dupuytren's cords: an exploratory study. BMC Musculoskelet Disord 2012;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for Dupuytren contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am 2013;38:2370–6. [DOI] [PubMed] [Google Scholar]
- 35.Hayton MJ, Bayat A, Chapman DS, et al. Isolated and spontaneous correction of proximal interphalangeal joint contractures in Dupuytren's disease: an exploratory analysis of the efficacy and safety of collagenase clostridium histolyticum. Clin Drug Investig 2013;33:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nydick JA, Olliff BW, Garcia MJ, et al. A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. J Hand Surg Am 2013;38:2377–80. [DOI] [PubMed] [Google Scholar]
- 37.Skirven TM, Bachoura A, Jacoby SM, et al. The effect of a therapy protocol for increasing correction of severely contracted proximal interphalangeal joints caused by Dupuytren disease and treated with collagenase injection. J Hand Surg Am 2013;38:684–9. [DOI] [PubMed] [Google Scholar]
- 38.Witthaut J, Jones G, Skrepnik N, et al. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am 2013;38:2–11. [DOI] [PubMed] [Google Scholar]
- 39.Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am 2013;38:12–22. [DOI] [PubMed] [Google Scholar]
- 40.Mickelson DT, Noland SS, Watt AJ, et al. Prospective randomized controlled trial comparing 1- versus 7-day manipulation following collagenase injection for Dupuytren contracture. J Hand Surg Am 2014;39:1933–41. [DOI] [PubMed] [Google Scholar]
- 41.Naam NH. Functional outcome of collagenase injections compared with fasciectomy in treatment of Dupuytren's contracture. Hand (N Y) 2013;8:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon HA, Bachoura A, Jacoby SM, et al. Examining the efficacy and maintenance of contracture correction after collagenase clostridium hisolyticum treatment for Dupuytren's disease. Hand (N Y) 2013;8:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sood A, Therattil PJ, Paik AM, et al. Treatment of Dupuytren disease with injectable collagenase in a veteran population: a case series at the department of veterans affairs new jersey health care system. Eplasty 2014;14:e13. [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta S, Belcher HJ. A single-centre cost comparison analysis of collagenase injection versus surgical fasciectomy for Dupuytren's contracture of the hand. J Plast Reconstr Aesthet Surg 2014;67:368–72. [DOI] [PubMed] [Google Scholar]
- 45.Alberton F, Corain M, Garofano A, et al. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: report of 40 cases. Musculoskelet Surg 2014;98:225–32. [DOI] [PubMed] [Google Scholar]
- 46.Raven RB III, Kushner H, Nguyen D, et al. Analysis of efficacy and safety of treatment with collagenase clostridium histolyticum among subgroups of patients with Dupuytren contracture. Ann Plast Surg 2014;73:286–90. [DOI] [PubMed] [Google Scholar]
- 47.Manning CJ, Delaney R, Hayton MJ. Efficacy and tolerability of day 2 manipulation and local anesthesia after collagenase injection in patients with Dupuytren's contracture. J Hand Surg Eur Vol 2014;39:466–71. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan FT, Badalamente MA, Hurst LC, et al. Delayed manipulation after collagenase clostridium histolyticum injection for Dupuytren contracture. Hand (N Y) 2015;10:578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou C, Hovius SE, Slijper HP, et al. Collagenase clostridium histolyticum versus limited fasciectomy for Dupuytren's contracture: outcomes from a multicenter propensity score matched study. Plast Reconstr Surg 2015;136:87–97. [DOI] [PubMed] [Google Scholar]
- 50.Atroshi I, Nordenskjöld J, Lauritzson A, et al. Collagenase treatment of Dupuytren's contracture using a modified injection method: a prospective cohort study of skin tears in 164 hands, including short-term outcome. Acta Orthop 2015;86:310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaston RG, Larsen SE, Pess GM, et al. The efficacy and safety of concurrent collagenase clostridium histolyticum injections for 2 Dupuytren contractures in the same hand: a prospective, multicenter study. J Hand Surg Am 2015;40:1963–71. [DOI] [PubMed] [Google Scholar]
- 52.Badalamente MA, Hurst LC, Benhaim P, et al. Efficacy and safety of collagenase clostridium histolyticum in the treatment of proximal interphalangeal joints in Dupuytren contracture: combined analysis of 4 phase 3 clinical trials. J Hand Surg Am 2015;40:975–83. [DOI] [PubMed] [Google Scholar]
- 53.Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 5-year data. J Hand Surg Am 2015;40:1597–605. [DOI] [PubMed] [Google Scholar]
- 54.Verheyden JR. Early outcomes of a sequential series of 144 patients with Dupuytren's contracture treated by collagenase injection using an increased dose, multicore technique. J Hand Surg Eur Vol 2015;40:133–40. [DOI] [PubMed] [Google Scholar]
- 55.Muppavarapu RC, Waters MJ, Leibman MI, et al. Clinical outcomes following collagenase injections compared to fasciectomy in the treatment of Dupuytren's contracture. Hand (N Y) 2015;10:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tay TK, Tien H, Lim EY. Comparison between collagenase injection and partial fasciectomy in the treatment of Dupuytren's contracture. Hand Surg 2015;20:386–90. [DOI] [PubMed] [Google Scholar]
- 57.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand). The Upper Extemity Collaborative Group (UECG). Am J Ind Med 1996;29:602–8. [DOI] [PubMed] [Google Scholar]
- 58.Chung KC, Pillsbury MS, Walters MR, et al. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg 1998;23A:575–87. [DOI] [PubMed] [Google Scholar]
- 59.Denkler K. Surgical complications associated with fasciectomy for Dupuytren's disease: a 20 year review of the english literature. Eplasty 2010;10:e15. [PMC free article] [PubMed] [Google Scholar]
- 60.Peimer CA, Wilbrand S, Gerber RA, et al. Safety and tolerability of collagenase clostridium histolyticum and fascietcomy for Dupuytren's contracture. J Hand Surg Eur Vol 2015;40:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King I, Belcher H. Cold intolerance following collagenase clostridium histolyticum treatment for Dupuytren contracture. J Hand Surg Am 2014;39:808–9. [DOI] [PubMed] [Google Scholar]
- 62.Van Rijssen A, ter Linden H, Werker PMN. Five-year results of randomized clinical trial on treatment in Dupuytren's disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg 2012;129:469–77. [DOI] [PubMed] [Google Scholar]