Nature has designed clever ways for information and material transfer between cells and for intercellular coordination. Mechanisms include direct cell contact, gap junctions, and receptor-ligand signaling. In the last decade, a particular form of exchange has gained increasing attention, and that is intercellular transfer of extracellular vesicles -- natural nanocarriers that deliver biological payloads at long-range1.
Many terms have been coined for vesicles found in the extracellular space, including matrix vesicles, extracellular membrane vesicles, microparticles1, microvesicles1, 2, shedding vesicles3, plasma membrane-derived vesicles4, ectosomes5, exovesicles6 and exosomes7. Sometimes these terms are used interchangeably, however, some have been assigned specific distinguishing features, such as size, context, or protein markers. Bone biologists showed decades ago that extracellular vesicles of a certain type -- “matrix vesicles” -- are central in skeletal mineralization, where they are believed to serve as a nidus for initiation of hydroxyapatite crystal formation8. The general view has been that matrix vesicles are formed by budding off from the plasma membrane. A leader in the field of matrix vesicle biology, H. Clarke Anderson, also identified matrix vesicles in human aortic calcification, one of the first demonstrations that vascular and bone mineralization occur by similar mechanisms9.
Exosomes, which are distinguished by endosomal marker proteins and their origin from a specialized endosomal pathway, arise in a wide variety of cell types, and serve many functions, such as removal of unwanted stress proteins and coordination of membrane biogenesis. They may have a role in disease processes. The payload of exosomes reportedly may include microRNA, proteins, and viral, bacterial, and prion particles10–13.
Matrix vesicles and exosomes are found in other parts of the natural world. As with the hydoxyapatite mineral of bone, the calcium carbonate mineral encasing shellfish was previously believed to arise from matrix vesicles released only from cells of the mantle, a single-cell layer immediately adjacent to the shell’s mineralization front. This process has been studied in the Pacific oyster Crassostrea gigas. The recent sequencing of its genome (which, incidentally, has more genes14 than the human genome) allowed investigators to use proteomic analysis to show that blood cells from other parts of the organism, not just the mantle, deliver exosomes to the mineralization front, where they serve as nucleation sites. In addition to this unexpected mechanism, they found evidence that some exosomes may initiate mineralization even before release from the cells15. Perhaps such mechanisms contribute to mineralization in mammals, and they could explain the frequent association of macrophage-like cells with sites of vascular calcification.
Until now, matrix vesicles, defined as extracellular membrane-invested particles located within the matrix of mineralizing tissue and serving as a nidus for crystal initiation, were believed to form by polarized budding from the outer plasma membrane. At the same time, it has been known that the large extracellular protein, fetuin, which has an important role in inhibiting or limiting mineralization, is internalized and released within some sort of vesicles16. In this issue of Circulation Research, Kapustin and colleagues17 present evidence that both production of matrix vesicles and fetuin “recycling” involve the endosomal/exosomal pathway. The authors also demonstrate, by proteomic analysis, similarities between exosomes from vascular smooth muscle cells and the proteomic profile reported for skeletal osteoblasts. This provides additional support for the growing concept that vascular calcification recapitulates skeletal mineralization. However, it leaves, as an interesting puzzle, the question of how fetuin finds its way to the inside of an ordinary vesicle without having to cross its membrane.
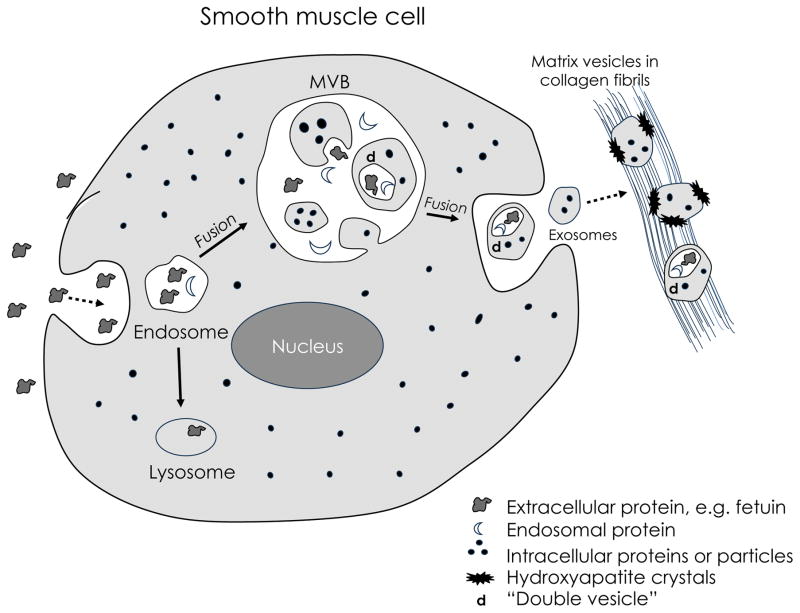
From a brief perusal of the literature, it appears that the biogenesis of exosomes occurs as shown in the schematic (Figure). During fluid-phase endocytosis (which the authors may have termed “liquid flow”), the plasma membrane invaginates to take in extracellular fluid, which may contain particles or proteins, such as fetuin. The resulting endosomes contain extracellular-derived fluid and fetuin (white with dark-gray objects). Some of these endosomes subsequently fuse with large “multivesicular bodies” (MVB), releasing their contents into the MVB. In the next step, the MVB membrane undergoes a secondary invagination, producing simple microvesicles (~100 nm), which contain intracellular fluid contents (gray with black dots).
Figure. Possible schematic of exosomal biogenesis.
It appears that exosomes arise through a multistep process. Invagination of the plasma membrane during pinocytosis, or fluid-phase endocytosis, produces endosomes, which may contain, in addition to extracelluar-derived fluid (white), both extracellular-derived particles and proteins, such as fetuin (dark-gray objects). Some endosomes then fuse with large multivesicular bodies (MVB; ~700–1000 nm), which contain extracellular-derived fluid, fetuin, endosomal marker proteins (crescents), and microvesicles (~100 nm). Simple microvesicles are formed by a “secondary” invagination involving the MVB membrane, and their contents include cytoplasmic fluid and proteins (gray with black dots). To account for the experimental observation of fetuin within extracellular vesicles, we speculate that some of the microvesicles may undergo a “secondary” invagination to engulf fetuin and/or endosomal marker proteins, to create a “double vesicle”, i.e. a microvesicle-within-a-microvesicle (d). In the last stage of the endosomal-exosomal pathway, the MVB fuses with the plasma membrane, releasing extracellular-derived fluid, fetuin, endosomal marker proteins, and microvesicles into the extracellular space. Once in the extracellular milieu, the secreted microvesicles are known as “exosomes.” Based on the topology of this scheme, unless the hypothetical tertiary invagination took place and produced double vesicles, the exosomes should contain only intracellular-derived contents, and any fetuin would be released only in free form (without a surrounding membrane). Ultimately, the exosomes may be deposited onto collagen fibrils, where they are believed to serve as a nidus for mineralization. The presence of fetuin within the exosomes would delimit mineralization. If this hypothetical tertiary invagination concept is correct, barring dissolution of a membrane, one may predict that fetuin (and other large proteins and extracellular particles such as viruses) would not be found in simple exosomes, but only in complex exosomes, having 2 - or a larger even number - of layers between the fetuin and the extracellular space.
At this stage, the fetuin is excluded from the simple microvesicles. It would be difficult to reconcile how Kapustin et al. found fetuin inside vesicles in the extracellular matrix17, unless we invoke an additional process. In one such hypothetical process, the MVB microvesicles would undergo a tertiary invagination: Fetuin (or other extracellular protein or particle) would be engulfed once again, this time by a microvesicle within the MVB. This would result in what we might term a “double vesicle”, i.e. a microvesicle-within-a-microvesicle (d in the Figure).
Returning to the known exosomal biogenesis pathway, the final step is fusion of the MVB with the plasma membrane, releasing microvesicles that are, in this extracellular context, termed “exosomes” together with fluid and free proteins or particles that were originally derived from the extracellular space. The exosomes would contain only intracellular-derived contents, and any fetuin would be released only in free form (without a surrounding membrane), unless the hypothetical tertiary invagination took place and produced double vesicles. If this hypothetical step is correct, barring dissolution of a membrane, one may predict that simple exosomes would not contain fetuin. It would be found only in complex exosomes, having 2 - or a larger even number - of layers between the fetuin and the extracellular space.
Interestingly, if it were to happen that a microvesicle in the MVB underwent multiple tertiary invaginations, the result would be a microvesicle containing multiple microvesicles. Once exocytosed, it would be an extracellular multivesicular body. Such structures have been described, as in the report by Yang et al.18 of studies of zebrafish skeletal formation which showed, not only typical single-membrane “matrix vesicles,” but also multivesicular bodies in the extracellular matrix milieu.
In addition to clinical relevance with respect to identifying therapeutic targets that may control cell processing of infectious agents, research on calcifying exosomes has inspired surface engineering of nanocomposites for targeted delivery of pharmaceuticals and other therapeutic agents. It turns out that exosomes coated with inorganic hydroxyapatite mineral have special properties that stabilize the nanoparticles and enhance targeting function. Biomimetic synthesis of organic-inorganic hybrid nanocarriers for targeted drug delivery has been carried out using electrostatically absorbed hyaluronic acid as a reaction site for deposition of calcium phosphate mineral19. Addition of such a hydroxyapatite jacket was found to confer the ability to target CD44 overexpressed cancer cells20.
The findings of Kapustin et al.17 suggest a more intricate mechanism of intracellular control and compartmentalization of biomineralization in smooth muscle cells than previously thought. In addition, a topological analysis of their finding – that exosomal matrix vesicles contain fetuin and endosomal marker proteins – suggests an additional mechanism - tertiary invagination to form “double vesicles” -- as a necessary step for exosomal packaging of proteins or particles from the extracellular space, such as viruses, bacteria, or, even prions.
References
- 1.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90:1549–57. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 2.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–11. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer research. 2003;63:4331–7. [PubMed] [Google Scholar]
- 4.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Fed Proc. 1976;35:156–62. [PubMed] [Google Scholar]
- 5.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer seminars in immunopathology. 2005;27:375–87. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 6.Obregon C, Rothen-Rutishauser B, Gitahi SK, Gehr P, Nicod LP. Exovesicles from human activated dendritic cells fuse with resting dendritic cells, allowing them to present alloantigens. Am J Pathol. 2006;169:2127–36. doi: 10.2353/ajpath.2006.060453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood JL, Pan H, Lanza GM, Wickline SA Consortium for Translational Research in Advanced I, Nanomedicine. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–28. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson HC. Mineralization by matrix vesicles. Scan Electron Microsc. 1984:953–64. [PubMed] [Google Scholar]
- 10.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. Altered MicroRNA Expression Profile in Exosomes during Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLoS One. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–90. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 12.Porto-Carreiro I, Fevrier B, Paquet S, Vilette D, Raposo G. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood cells, molecules & diseases. 2005;35:143–8. doi: 10.1016/j.bcmd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–89. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Li L, Zhu Y, Du Y, Song X, Chen Y, Huang R, Que H, Fang X, Zhang G. Oyster Shell Proteins Originate from Multiple Organs and Their Probable Transport Pathway to the Shell Formation Front. PLoS One. 2013;8:e66522. doi: 10.1371/journal.pone.0066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mount AS, Wheeler AP, Paradkar RP, Snider D. Hemocyte-mediated shell mineralization in the eastern oyster. Science. 2004;304:297–300. doi: 10.1126/science.1090506. [DOI] [PubMed] [Google Scholar]
- 16.Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circulation research. 2011;108:1494–509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 17.Kapustin ANN, Chatrou MLL, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RTM, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular Smooth Muscle Cell Calcification is Mediated by Regulated Exosome Secretion. CIRC RES. doi: 10.1161/CIRCRESAHA.116.305012. IN PRESS. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Zhang Y, Cui FZ. Two types of mineral-related matrix vesicles in the bone mineralization of zebrafish. Biomedical materials. 2007;2:21–5. doi: 10.1088/1748-6041/2/1/004. [DOI] [PubMed] [Google Scholar]
- 19.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS nano. 2013;7:7698–710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Li Z, Lin Y, Yin M, Ren J, Qu X. Biomineralization inspired surface engineering of nanocarriers for pH-responsive, targeted drug delivery. Biomaterials. 2013;34:1364–71. doi: 10.1016/j.biomaterials.2012.10.060. [DOI] [PubMed] [Google Scholar]