Abstract
The use of primary mammalian neurons derived from embryonic central nervous system tissue is limited by the fact that once terminally differentiated into mature neurons, the cells can no longer be propagated. Transformed neuronal-like cell lines can be used in vitro to overcome this limitation. However, several caveats exist when utilizing cells derived from malignant tumors. In this context, the popular SH-SY5Y neuroblastoma cell line and its use in in vitro systems is described. Originally derived from a metastatic bone tumor biopsy, SH-SY5Y (ATCC® CRL-2266™) cells are a subline of the parental line SK-N-SH (ATCC® HTB-11™). SK-N-SH were subcloned three times; first to SH-SY, then to SH-SY5, and finally to SH-SY5Y. SH-SY5Y were deposited to the ATCC® in 1970 by June L. Biedler.
Three important characteristics of SH-SY5Y cells should be considered when using these cells in in vitro studies. First, cultures include both adherent and floating cells, both types of which are viable. Few studies address the biological significance of the adherent versus floating phenotypes, but most reported studies utilize adherent populations and discard the floating cells during media changes. Second, early studies by Biedler’s group indicated that the parental differentiated SK-N-SH cells contained two morphologically distinct phenotypes: neuroblast-like cells and epithelial-like cells (Ross et al., J Nat Cancer Inst 71:741–747, 1983). These two phenotypes may correspond to the “N” and “S” types described in later studies in SH-SY5Y by Encinas et al. (J Neurochem 75:991–1003, 2000). Cells with neuroblast-like morphology are positive for tyrosine hydroxylase (TH) and dopamine-β-hydroxylase characteristic of catecholaminergic neurons, whereas the epithelial-like counterpart cells lacked these enzymatic activities (Ross et al., J Nat Cancer Inst 71:741–747, 1983). Third, SH-SY5Y cells can be differentiated to a more mature neuron-like phenotype that is characterized by neuronal markers. There are several methods to differentiate SH-SY5Y cells and are mentioned below. Retinoic acid is the most commonly used means for differentiation and will be addressed in detail.
Keywords: Neuroblastoma, Differentiation, Neuron
1 Introduction
1.1 Preface
A detailed report by Biedler et al. in 1978 describes the original culturing conditions for SH-SY5Y cells [3] and a modified version of this protocol is presented in Subheading 3 of this chapter. In brief, SK-N-SH cells and derived clones including SH-SY5Y are plated at a density of 2 × 105 or 4 × 106 cells/60 mm dish in Eagle’s minimum essential medium supplemented with nonessential amino acids, 15 % fetal bovine serum, penicillin (100 IU/ml) and streptomycin (100 µg/ml). These original plating densities were used to determine the doubling time and saturation densities. Although SH-SY5Y doubling time was not reported specifically, the parental neuroblast-like populations has a doubling time of approximately 27 h and the subclones were reported to have similar doubling times. SH-SY5Y cells are reported to have a growth saturation density of >1 × 106 cells/cm2. In this study, both adherent and floating cells were collected when cells were passed. Floating cells were removed in culture medium, whereas adherent cells were detached with trypsin. The two cell populations were combined, centrifuged, and re-plated at appropriate densities. Combining the suspended and adherent cells may become an important aspect during differentiation protocols and will be discussed in later sections. Cells are grown in a humidified chamber with 5 % CO2 at 37 °C. Little has changed over the past four decades with regard to normal culture conditions for SH-SY5Y cells. These early studies also addressed transmitter properties and indicated that SH-SY5Y consisted of homogeneous neuroblast-like populations. Analyses of specific neuronal enzyme activities in SK-N-SH cells and clones indicated dopamine-β-hydroxylase levels of 3.74 nmol/h/mg in SH-SH5Y cells, although only one set of cell cultures was assayed [3]. Levels of choline acetyl-transferase, acetyl-cholinesterase, and butyryl-cholinesterase were negligible in SK-N-SH and its clones, including SH-SY5Y.
1.2 Undifferentiated Versus Differentiated SH-SY5Y Cells
The ability for researchers to differentiate SH-SY5Y neuroblastoma cells into cells possessing a more mature, neuron-like phenotype through manipulation of the culture medium has afforded numerous benefits in the field of neuroscience research. Advantages include the capacity for large-scale expansion prior to differentiation, with relative ease and low cost to culture compared to primary neurons. Since these cells are considered a cell-line, the ethical concerns associated with primary human neuronal culture are not involved. Additionally, since SH-SY5Y cells are human-derived, they express a number of human-specific proteins and protein isoforms that would not be inherently present in rodent primary cultures. Furthermore, differentiation synchronizes the cell cycle, which can fluctuate dramatically in undifferentiated SH-SY5Y cells and other commonly used cell lines, to produce a homogenous neuronal cell population [1, 2].
Both undifferentiated and differentiated SH-SY5Y cells have been utilized for in vitro experiments requiring neuronal-like cells. Neuronal differentiation entails a number of specific events, including formation and extension of neuritic processes, increased electrical excitability of the plasma membrane, formation of synaptophysin-positive functional synapses, and induction of neuron-specific enzymes, neurotransmitters, and neurotransmitter receptors [4–8]. Thus, when determining whether undifferentiated or differentiated cells should be utilized for a particular experiment, all of these properties should be taken into consideration.
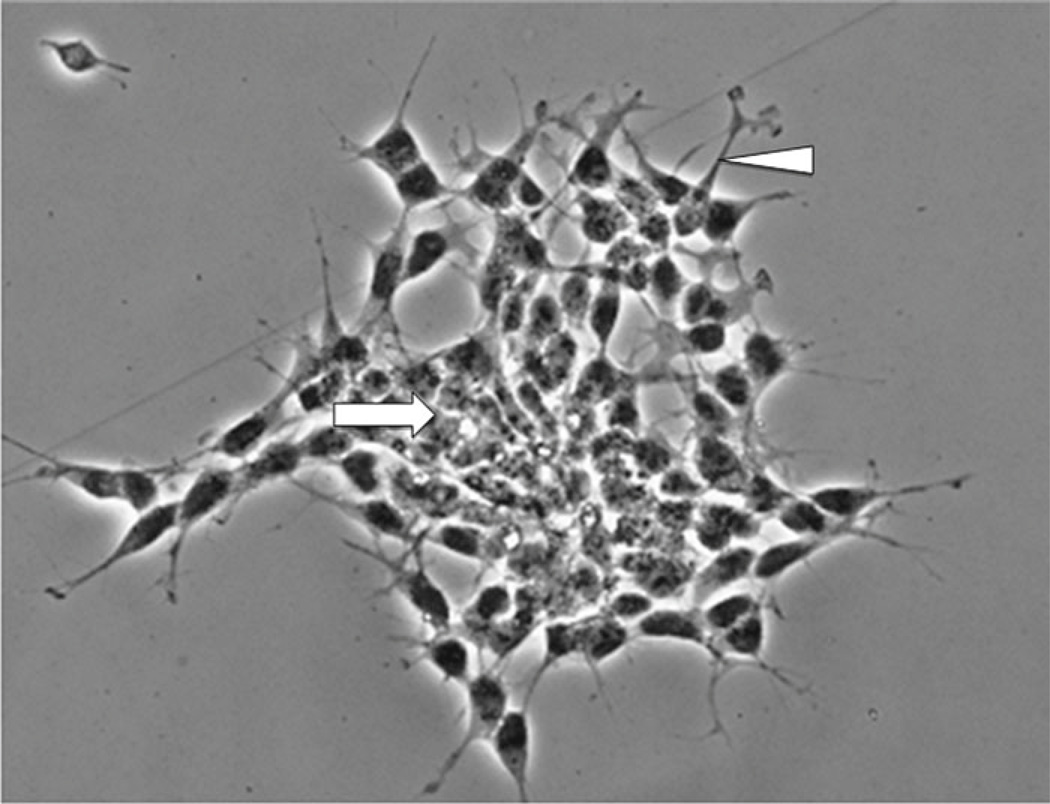
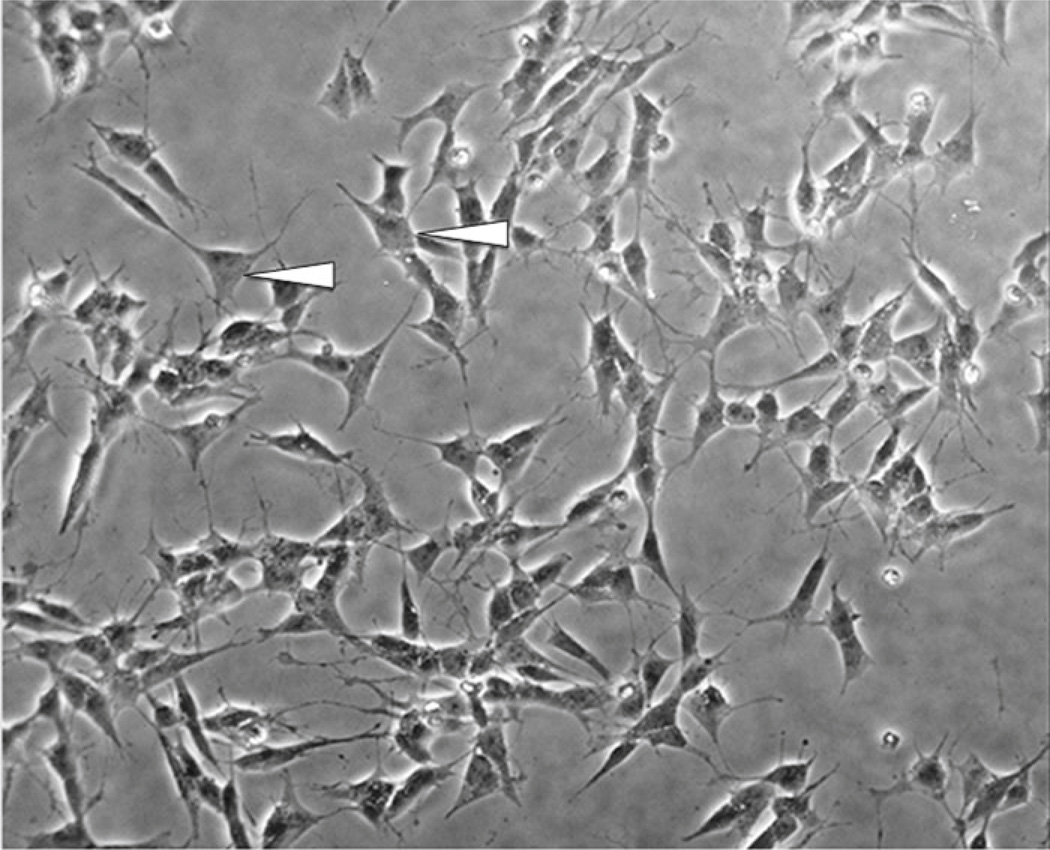
In the undifferentiated form, SH-SY5Y cells are characterized morphologically by neuroblast-like, non-polarized cell bodies with few, truncated processes. The cells tend to grow in clusters and may form clumps as cells appear to grow on top of one another in the central region of a cell mass (Fig. 1). Likewise, the cultures contain both adherent and floating cells, and some studies suggest that the floating cells are more likely to adhere and differentiate into “N” type cells upon RA-differentiation than the adherent cells present in undifferentiated cultures. Undifferentiated SH-SY5Y cells continuously proliferate, express immature neuronal markers, and lack mature neuronal markers [6]. Undifferentiated cells are considered to be most reminiscent of immature catecholaminergic neurons [7, 9]. Following treatment with differentiation-inducing agents, SH-SY5Y cells become morphologically more similar to primary neurons with long, exquisite processes [6] (Fig. 2). Mature cells may exhibit numerous but randomly distributed processes or become distinctly polarized, depending on the differentiation induction method. Differentiation of SH-SY5Y cells also induces a decrease in proliferation rate, as cells are withdrawn from the cell cycle, and an increase in the activity of neuron specific enolase (NSE), the dominant enolase-isoenzyme present in neuronal and neuroendocrine tissues [2, 6]. A number of methods exist for induction of differentiation in SH-SY5Y cells, and are mentioned below. SH-SY5Y cells can be driven toward a variety of adult neuronal phenotypes including cholinergic, adrenergic, or dopaminergic, depending on media conditions [9]. The differentiation method selected for in vitro experiments should ultimately be determined by the desired phenotype following differentiation, as well as for the reduction of non-target effects on experimental pathways in question by particular differentiating agents.
Fig. 1.
Undifferentiated SH-SY5Y cells. Cells tend to grow in clusters and may form clumps of rounded cells on top of one another (arrow). At edges of the cluster, cells begin to extend short neurites (arrowhead)
Fig. 2.
Differentiated SH-SY5Y cells. Cells do not cluster and have a more pyramidal shaped cell body (arrowhead). Neurites begin to extend, reminiscent of dendrites and/or axons
1.3 Retinoic Acid
One of the most commonly implemented and best-characterized methods for induction of differentiation in SH-SY5Y cells is through addition of retinoic acid (RA) to the cell culture medium. Retinoic acid is a vitamin A derivative known to possess powerful growth-inhibiting and cellular differentiation-promoting properties [10, 11]. In fact, vitamin A deficiency is linked to the development of squamous metaplasia in various epithelial tissues, while administration of vitamin A can reverse these effects and restore normal cellular differentiation [10, 11]. Typically, RA is administered at a concentration of 10 µM for a minimum of 3–5 days in serum-free or low serum medium to induce differentiation [6, 12], although slight variations in media are reported.
Retinoic acid treatment has been shown to promote survival of SH-SY5Y cells through activation of the phosphatidylinositol 3-kinase/Akt signaling pathway and upregulation of the antiapoptotic Bcl-2 protein [13, 14]. Furthermore, some studies show that RA-differentiated cells are less vulnerable than undifferentiated cells to toxin-mediated cell death induced by agents including 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), or its metabolite, 1-methyl-4-phenylpyridinium ion (MPP+) than undifferentiated cells [12].
SH-SY5Y cells differentiate primarily to a cholinergic neuron phenotype in response to RA treatment, as evidenced by increased expression of choline acetyl transferase (ChAT) activity and vesicular monamine transporter (VMAT) expression [7, 15]. Cells may also be driven toward a mature dopaminergic phenotype by RA treatment, but this typically requires co-administration of additional agents such as phorbol esters [15]. While controversy exists in the literature over whether dopaminergic markers present in undifferentiated SH-SY5Y cells significantly increase during RA-induced differentiation, studies demonstrate robust increases in tyrosine hydroxylase (TH), dopamine receptor 2 and 3 subtypes (D2R and D3R), and dopamine transporter (DAT) expression when RA administration is followed by treatment with phorbol esters [15]. Interestingly, Encinas et al. reported in 2000 that RA-differentiated SH-SY5Y cells responded to carbachol stimulation by increased release of noradrenaline [2]
1.4 Phorbol Esters
In addition to RA-mediated differentiation, SH-SY5Y cells have been shown to differentiate in the presence of phorbol esters such as 12-O-tetradecanoyl-phorbol-13 acetate (TPA) [5]. In 1981, Påhlman et al. demonstrated that SH-SY5Y cells exposed to 1.6 × 10−8 M TPA for 4 days appeared morphologically differentiated with long, straight processes of uneven appearance and frequent varicosities [5]. TPA treatment also resulted in partial growth inhibition, an approximate twofold increase in NSE activity, and the appearance of cytoplasmic neurosecretory granula, which can be visualized by electron microscopy [5, 6]. One striking difference between TPA- and RA-induced differentiation of SH-SY5Y cells is that TPA treatment increases the cellular noradrenaline content up to 200-fold while RA treatment causes an approximate fourfold induction in noradrenaline [6]. Therefore, use of TPA to induce differentiation of SH-SY5Y cells produces a predominantly adrenergic cellular phenotype [16, 17].
1.5 Dibutyryl Cyclic AMP
Hormones and neurotransmitters that upregulate intracellular levels of cyclic AMP (cAMP) promote differentiation and long-term potentiation in neuronal cells [18, 19]. Exposure of SH-SY5Y cells to dibutyryl cyclic AMP (dbcAMP) results in neurite extension, as well as in increased expression of the mature neuronal marker growth-associated protein 43 (GAP43) [19, 20]. Studies demonstrate that treatment with 1 mM dbcAMP for 3 days decreases cell aggregation, reminiscent of the aggregation commonly observed in undifferentiated cultures (Fig. 1), and induces significant neurite elongation and branching [20]. Exposure to dbcAMP also leads to a significant increase in tyrosine hydroxylase (TH) immunoreactivity and in the cellular content of noradrenaline in a protein kinase A (PKA)-dependent manner [14, 20]. In comparison to RA and TPA treatment, which increase expression of Bcl-2, dbcAMP decreases Bcl-2, highlighting one biochemical difference among differentiation methods. These studies indicate that differentiation of SH-SY5Y cells with dbcAMP produces a morphological phenotype similar to that seen in RA and TPA-differentiated cells, and that the differentiated culture is comprised of primarily adrenergic neuron-like cells.
1.6 Additional Methods of Differentiation
A number of less-common alternative methods to induce differentiation in SH-SY5Y cells have been described, as well. Staurosporine, a PKC inhibitor triggers neuritogenesis and cell cycle arrest in SH-SY5Y cells [21, 22]. However, unlike cells differentiated with RA, staurosporine-treated cells display increased vulnerability to toxic insults including cisplatin, 5-fluorouracil, 6-OHDA, and γ-radiation, and express decreased levels of Bcl-2 [22]. Consequently, staurosporine-treated cells undergo apoptosis in a dose-dependent manner. In addition to staurosporine, treatment with growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) has been shown to support differentiation into and maintenance of a mature neuronal phenotype, particularly when used in combination with RA or TPA treatment [2, 23, 24]. Similarly, culturing SH-SY5Y cells in neurobasal medium with B27 supplement, conditions commonly used for primary neuronal culture, has been shown to enhance differentiation [25]. Other methods include treatment with cholesterol, vitamin D, or insulin, or culturing cells on a substrate designed to promote neuronal differentiation and survival [26–29]. Again, selection of a suitable differentiation induction agent should be carefully evaluated depending on the possibility of downstream, unintended effects on output measures caused by treatment alone.
1.7 Markers for Differentiation
Undifferentiated SH-SY5Y cells typically resemble immature catecholaminergic neurons. They are characterized by markers indicative of proliferation, such as proliferating cell nuclear antigen (PCNA), as well as by immature neuronal markers such as nestin [7, 30]. Undifferentiated cells also express differentiation-inhibiting basic helix-loop-helix transcription factors ID1, ID2, and ID3, all of which are significantly decreased following treatment with RA or TPA [13]. Following differentiation, SH-SY5Y cells express a number of mature neuronal markers, including βIII-tubulin, microtubule-associated protein-2 (MAP2), synaptophysin, NeuN, synaptic associated protein-97 (SAP-97), and NSE [7, 12]. Additionally, expression of differentiation-promoting genes NEUROD6 and NEUROD1 increase following RA treatment [13]. Differentiation of SH-SY5Y cells can result in a relatively homogenous population of G0 stage, neuronal-like cells which display an absence of markers for other CNS cell types, such as the astrocytic marker glial fibrillary acidic protein (GFAP) [2, 11], but care should be given to assess proliferation of cells that lack the neuronal-like phenotype as discussed below.
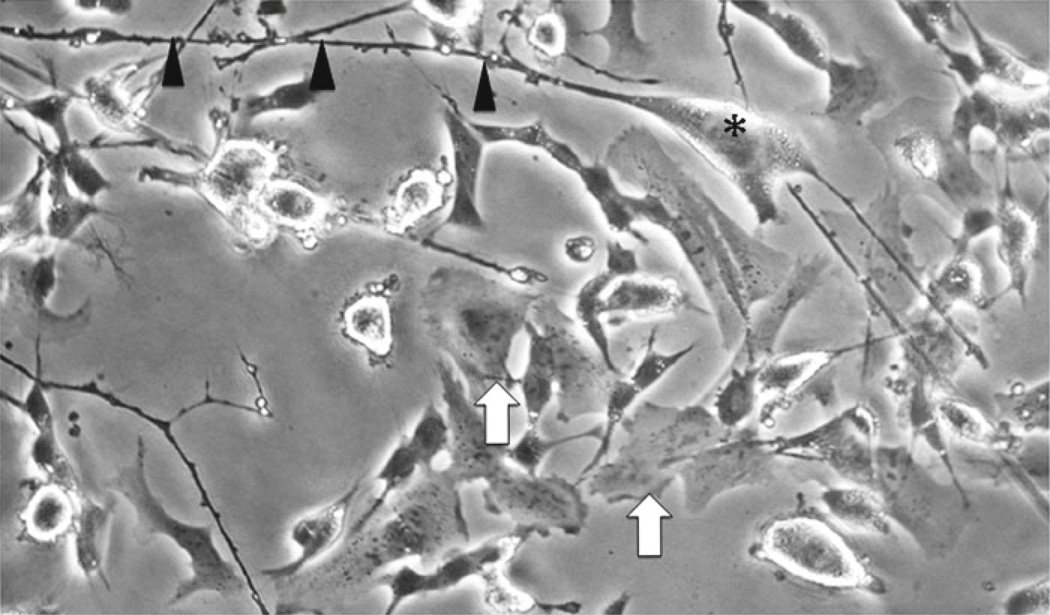
Numerous reports have specifically addressed methods to obtain neuron-like cells from undifferentiated SH-SY5Y. Some reports suggest using only floating cells in differentiation protocols, while others recommend using only adherent cells for differentiation. A detailed description for RA-differentiated, BDNF maintained SH-SY5Y cells was published in 2000 by Encinas et al. [2]. In these studies, the authors classify undifferentiated cells as either S-type (substrate adherent) without neuron-like phenotype or N-type (neuroblastic) characterized by neuritic processes (Fig. 3). In the study by Encinas, cells were plated at 104 cells/cm2 on collagen-coated (0.05 mg/ml) plates in DMEM with 2 mM l-glutamine, penicillin (20 IU/ml), streptomycin (20 mg/ml) and 15 % FBS. Upon differentiation with 10 µm all-trans-RA for 5 days, N-type cells were reported to gain neuron-like characteristics more readily than S-type. However, at day 10 post-RA, the percentage of S-type cells in the culture increased and cultures were overgrown by the S-type population. Thus, short term RA treatment (up to 5 days) appeared to induce differentiation of N-type cells, but longer treatment (>10 days) promoted proliferation of S-type cells thereby promoting unbalanced proportion of S- to N-type cells. Based on results such as these, consideration should be given not only to passage number, but also to cell phenotype in culture when designing in vitro experiments. One potential solution to prevent the imbalance, recommended by Encinas et al. is adding 50 ng/ml BDNF to RA-treated cultures since RA is reported to induce TrkB receptors on SH-SY5Y cells with maximal expression 5 days after BDNF exposure. Removal of serum in combination with BDNF treatment is recommended to prevent replication of S-type cells. Including serum during RA-BDNF treatments can promote S-type proliferation that is observed as a monolayer of S cells under the differentiating N-type cells. Cultures maintained under serum-free, BDNF supplemented conditions were stable for up to 3 weeks without S-type cell over growth with minimal apoptotic cells as determined by TUNEL assay and biochemical analyses. With BDNF cells are arrested, but BDNF withdrawal induces cells to enter the S-phase of the cell cycle [2].
Fig. 3.
Differentiated cells populations consist of two morphologically distinct types: “S” and “N”. The “S” type cell is epithelial-like with no processes (arrows), whereas the “N” type is more neuronal-like with pyramidal shaped bodies (asterisk) and long processes (arrowheads)
Neuronal marker expression of cultures exposed to RA, BDNF and serum-free conditions was assessed by immunocytochemistry and western analyses. In these studies, neither untreated nor RA-BDNF treated SH-SHY5Y cells expressed GFAP, but NSE was detected in all conditions. On the other hand, both medium and high molecular weight neurofilament was detected in untreated and 5-day RA treated neurons, but disappeared after BDNF was added. GAP-43 expression increased after 5 days RA treatment followed by 1 day of BDNF treatment. However, continued BDNF treatment for 3–9 days resulted in return of GAP-43 levels to baseline. The authors suggest that this expression profile coincides with the high levels of neurite extension during which GAP-43 is required. However, the removal of RA from BDNF-containing medium may have contributed, as well.
1.8 Markers for Receptors/Transporters
1.8.1 Dopaminergic Neurons
SH-SY5Y cells in both undifferentiated and differentiated states express a number of dopaminergic neuronal markers. These cells express TH, an enzyme critical for the catalysis of dopamine and, further downstream, noradrenaline (norepinephrine) and adrenaline (epinephrine) [20]. Importantly, SH-SY5Y cells also express the dopamine transporter (DAT), as well as dopamine receptor subtypes 2 and 3 (D2R and D3R), making them an exemplary in vitro system for the study of neurotoxicity in dopaminergic neurons as well as for drugs, which are known to produce primary effects through activation of dopamine receptors [31]. Expression of TH, DAT, D2R, and D3R has been shown to increase following differentiation in a number of studies, particularly when a combination of RA and TPA are utilized to induce differentiation [7, 15, 32]. However, there also exists a body of literature that reports no significant difference in levels of TH or DAT following differentiation, although these studies typically utilized RA alone or agents, which promote a non-dopaminergic phenotype to induce differentiation [12, 33].
1.8.2 Adrenergic Neurons
SH-SY5Y cells can be driven toward an adrenergic phenotype by RA- or TPA-induced differentiation. Again, the expression of TH is required, as nor-adrenaline and adrenaline are derived from dopamine catalyzed in a TH-dependent manner [20]. Studies demonstrate that both differentiated and high-passage undifferentiated SH-SY5Y cells express sufficient levels of dopamine-β-hydroxylase, the enzyme that catalyzes the formation of nor-adrenaline from dopamine, and are capable of converting intracellular dopamine into nor-adrenaline (nor-epinephrine). Additionally, SH-SY5Y cells express the norepinephrine transporter (NET) and the vesicular monoamine transporter (VMAT), characteristic of adrenergic neurons.
1.8.3 Cholinergic Neurons
The expression of both muscarinic and nicotinic acetylcholine receptors has been reported in SH-SY5Y cells. The G-protein coupled muscarinic receptors are present on membranes of both undifferentiated and differentiated cells, though levels and binding properties are differentially regulated according to method of differentiation. For instance, TPA treatment decreases while RA treatment increases the number of muscarinic binding sites [4]. Both TPA- and RA-treated cells display significantly increased acetylcholinesterase activity compared to undifferentiated cells. However, only RA treatment has been shown to increase choline acetyltransferase activity [4]. The ligand-gated ion channel nicotinic acetylcholine receptors (nAChR) are also present in SH-SY5Y cells and are deemed to be analogous to human ganglia-type nAChR [34, 35]. The nAChR on SH-SY5Y cells have been shown to desensitize in response to nicotine but regain full sensitivity following washout, similarly to what is known to happen in primary neurons upon receptor activation [36].
2 Materials
2.1 Cells
Cells can be purchased from ATCC (ATCC® CRL-2266) http://www.atcc.org/. Cells will arrive on dry ice and should be processed immediately or placed in vapor phase liquid nitrogen until ready for culturing.
2.2 Reagents
Dulbecco’s Minimum Essential Medium (DMEM).
F12 Medium.
Fetal Bovine Serum (FBS), 10 % final concentration.
Penicillin/Streptomycin (Pen/Strep), 1 % final concentration (100 IU/ml, 100 µg/ml, respectively).
Trypsin/EDTA.
Neurobasal medium (NB).
B27 supplement.
GlutaMAX.
All-trans-retinoic acid (ATRA).
DMSO.
1× Phosphate-buffered saline (PBS).
Sterile disposable filter apparatus, with 0.22 µm pore size.
3 Methods
3.1 Initial Culture Conditions
Prepare growth medium: DMEM/F12 (1:1, v:v) medium, (10 % FBS and 1 % pen/strep) and filter through a 0.22 µm pore filter apparatus.
Obtain cells from the ATCC (ATCC® CRL-2266) and thaw quickly at 37 °C.
Gently remove cell suspension from tube and add to a T-75 tissue culture flask containing warm (37 °C) growth medium (see Note 1).
Culture cells at 37 °C, 5 % CO2. Growth medium should be refreshed every 4–7 days (see Note 2). Monitor cells for confluence. When cells reach 80–90 % confluence, subculture as described below.
3.2 Sub-culture Conditions
Aspirate medium under sterile conditions. If a number of floating cells are present, medium can be collected and centrifuged so non-adherent cells can be recovered and re-plated.
Rinse adherent cells once with sterile 1× PBS pre-warmed to 37 °C or room temperature (see Note 3). To prevent detaching cells, add the PBS to the inside surface of the culture flask that does not have cells attached. Do not add PBS directly onto cell monolayer. Gently tip the flask so that the PBS washes over the cell monolayer. Aspirate PBS.
Add trypsin to adherent cells for approximately 2 min or until cells visibly detach from culture flask (see Note 4).
Neutralize trypsin by adding an equal volume of DMEM/F12 medium containing 10 % FBS.
Collect detached cell suspension and centrifuge at 1,500 rpm for 5 min at room temperature to concentrate cell pellet.
Aspirate supernatant carefully without disturbing the cell pellet.
Gently suspend pellet in DMEM/F12 medium containing 10 % FBS.
To separate cells from clumps, pipette up and down gently until the suspension appears homogenous (see Note 1).
Count cells using a hemocytometer and plate at approximately 3 × 103 to 1 × 105 cells/cm2 (see Note 5).
3.3 Differentiation
24–48 h after plating, replace serum-containing medium with Neurobasal medium (containing B27 supplement and GlutaMAX) and 10 µM all-trans-retinoic acid (ATRA) to promote differentiation and neuronal phenotype.
Allow cells to grow in ATRA-containing neurobasal medium for a minimum of 3–5 days, refreshing the medium every 48 h.
Differentiation can be monitored microscopically via morphological assessment of neurite outgrowth (compare Figs. 1 and 2).
3.4 Freezing Down
To freeze down SH-SY5Y cells, begin with undifferentiated cultures.
Harvest 80–90 % confluent monolayer from a T75 flask and pellet cells as described above (see Note 6).
Suspend the cell pellet gently in 1 ml of 90 % FBS, 10 % DMSO in a sterile 1.5 ml screw cap vial appropriate for storage in the vapor phase of liquid nitrogen.
Store cells at −80 °C for approximately 24 h in an insulated cryobox and then transfer tubes to liquid nitrogen for long-term storage.
Footnotes
To improve cell survival, be careful not to introduce air when pipetting/transferring cells between flasks or tubes.
The color of the medium indicates the rate of metabolism of key components by cells. When medium becomes more acidic (color appears more yellow than red), it is likely time to change the medium.
Rinsing with PBS removes the majority of the serum contained in growth medium. Trypsin works more efficiently in the absence of serum as serum inhibits its activity.
Reduce the amount of time cells are exposed to trypsin. Once cells have been exposed to trypsin for about 1 min, you may gently tap the flask to assist in detachment.
You will discover that SHSY5Y cells need to be plated at a density conducive to cell–cell communication to proliferate. If cells are plated too sparsely, growth rate is reduced and cell death is high.
A T75 flask that is about 89–90 % confluent contains an appropriate number of cells to freeze down in approximately 1 ml of freeze medium allowing you to plate into a T75 flask upon thawing and re-culturing. This is important because when cells are thawed and re-plated after having been in liquid nitrogen, inevitably some cells will die. The density of surviving cells should allow the culture to be maintained at least 24 h before sub-culturing is necessary.
References
- 1.Ross RA, Spengler BA, Biedler JL. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983;71(4):741–747. [PubMed] [Google Scholar]
- 2.Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75(3):991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 3.Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- 4.Adem A, Mattsson ME, Nordberg A, Pahlman S. Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: regulation by phorbol ester and retinoic acid-induced differentiation. Brain Res. 1987;430(2):235–242. doi: 10.1016/0165-3806(87)90156-8. [DOI] [PubMed] [Google Scholar]
- 5.Pahlman S, Odelstad L, Larsson E, Grotte G, Nilsson K. Phenotypic changes of human neuroblastoma cells in culture induced by 12-O-tetradecanoyl-phorbol-13-acetate. Int J Cancer. 1981;28(5):583–589. doi: 10.1002/ijc.2910280509. [DOI] [PubMed] [Google Scholar]
- 6.Pahlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14(2):135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 7.Lopes FM, Schroder R, da Frota ML, Jr, Zanotto-Filho A, Muller CB, Pires AS, Meurer RT, Colpo GD, Gelain DP, Kapczinski F, Moreira JC, Fernandes Mda C, Klamt F. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res. 2010;1337:85–94. doi: 10.1016/j.brainres.2010.03.102. [DOI] [PubMed] [Google Scholar]
- 8.Tosetti P, Taglietti V, Toselli M. Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J Neurophysiol. 1998;79(2):648–658. doi: 10.1152/jn.1998.79.2.648. [DOI] [PubMed] [Google Scholar]
- 9.Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J. 2010;123(8):1086–1092. [PubMed] [Google Scholar]
- 10.Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10(9):1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- 11.Melino G, Thiele CJ, Knight RA, Piacentini M. Retinoids and the control of growth/death decisions in human neuroblastoma cell lines. J Neurooncol. 1997;31(1–2):65–83. doi: 10.1023/a:1005733430435. [DOI] [PubMed] [Google Scholar]
- 12.Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30(1):127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Carballo G, Moreno L, Masia S, Perez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277(28):25297–25304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 14.Itano Y, Ito A, Uehara T, Nomura Y. Regulation of Bcl-2 protein expression in human neuroblastoma SH-SY5Y cells: positive and negative effects of protein kinases C and A, respectively. J Neurochem. 1996;67(1):131–137. doi: 10.1046/j.1471-4159.1996.67010131.x. [DOI] [PubMed] [Google Scholar]
- 15.Presgraves SP, Ahmed T, Borwege S, Joyce JN. Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res. 2004;5(8):579–598. doi: 10.1007/BF03033178. [DOI] [PubMed] [Google Scholar]
- 16.Murphy NP, Ball SG, Vaughan PF. Potassium- and carbachol-evoked release of [3H]noradrenaline from human neuroblastoma cells, SH-SY5Y. J Neurochem. 1991;56(5):1810–1815. doi: 10.1111/j.1471-4159.1991.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 17.Scott IG, Akerman KE, Heikkila JE, Kaila K, Andersson LC. Development of a neural phenotype in differentiating ganglion cell- derived human neuroblastoma cells. J Cell Physiol. 1986;128(2):285–292. doi: 10.1002/jcp.1041280221. [DOI] [PubMed] [Google Scholar]
- 18.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez S, Jimenez C, Carrera AC, Diaz-Nido J, Avila J, Wandosell F. A cAMP-activated pathway, including PKA and PI3K, regulates neuronal differentiation. Neurochem Int. 2004;44(4):231–242. doi: 10.1016/s0197-0186(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 20.Kume T, Kawato Y, Osakada F, Izumi Y, Katsuki H, Nakagawa T, Kaneko S, Niidome T, Takada-Takatori Y, Akaike A. Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neurosci Lett. 2008;443(3):199–203. doi: 10.1016/j.neulet.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 21.Leli U, Shea TB, Cataldo A, Hauser G, Grynspan F, Beermann ML, Liepkalns VA, Nixon RA, Parker PJ. Differential expression and subcellular localization of protein kinase C alpha, beta, gamma, delta, and epsilon isoforms in SH-SY5Y neuroblastoma cells: modifications during differentiation. J Neurochem. 1993;60(1):289–298. doi: 10.1111/j.1471-4159.1993.tb05850.x. [DOI] [PubMed] [Google Scholar]
- 22.Tieu K, Zuo DM, Yu PH. Differential effects of staurosporine and retinoic acid on the vulnerability of the SH-SY5Y neuroblastoma cells: involvement of bcl-2 and p53 proteins. J Neurosci Res. 1999;58(3):426–435. [PubMed] [Google Scholar]
- 23.Chen J, Chattopadhyay B, Venkatakrishnan G, Ross AH. Nerve growth factor-induced differentiation of human neuroblastoma and neuroepithelioma cell lines. Cell Growth Differ. 1990;1(2):79–85. [PubMed] [Google Scholar]
- 24.Simpson PB, Bacha JI, Palfreyman EL, Woollacott AJ, McKernan RM, Kerby J. Retinoic acid evoked-differentiation of neuroblastoma cells predominates over growth factor stimulation: an automated image capture and quantitation approach to neuritogenesis. Anal Biochem. 2001;298(2):163–169. doi: 10.1006/abio.2001.5346. [DOI] [PubMed] [Google Scholar]
- 25.Sayas CL, Moreno-Flores MT, Avila J, Wandosell F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J Biol Chem. 1999;274(52):37046–37052. doi: 10.1074/jbc.274.52.37046. [DOI] [PubMed] [Google Scholar]
- 26.Sarkanen JR, Nykky J, Siikanen J, Selinummi J, Ylikomi T, Jalonen TO. Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J Neurochem. 2007;102(6):1941–1952. doi: 10.1111/j.1471-4159.2007.04676.x. [DOI] [PubMed] [Google Scholar]
- 27.Recio-Pinto E, Ishii DN. Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984;302(2):323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- 28.Reddy CD, Patti R, Guttapalli A, Maris JM, Yanamandra N, Rachamallu A, Sutton LN, Phillips PC, Posner GH. Anticancer effects of the novel 1alpha,25-dihydroxyvitamin D3 hybrid analog QW1624F2-2 in human neuroblastoma. J Cell Biochem. 2006;97(1):198–206. doi: 10.1002/jcb.20629. [DOI] [PubMed] [Google Scholar]
- 29.Agholme L, Lindstrom T, Kagedal K, Marcusson J, Hallbeck M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis. 2010;20(4):1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- 30.Cuende J, Moreno S, Bolanos JP, Almeida A. Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene. 2008;27(23):3339–3344. doi: 10.1038/sj.onc.1210987. [DOI] [PubMed] [Google Scholar]
- 31.Arun P, Madhavarao CN, Moffett JR, Namboodiri AM. Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J Neurochem. 2008;106(4):1669–1680. doi: 10.1111/j.1471-4159.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- 32.Constantinescu R, Constantinescu AT, Reichmann H, Janetzky B. Neuronal differentiation and long-term culture of the human neuroblastoma line SH-SY5Y. J Neural Transm Suppl. 2007;72:17–28. doi: 10.1007/978-3-211-73574-9_3. [DOI] [PubMed] [Google Scholar]
- 33.Guarnieri S, Pilla R, Morabito C, Sacchetti S, Mancinelli R, Fano G, Mariggio MA. Extracellular guanosine and GTP promote expression of differentiation markers and induce S-phase cell-cycle arrest in human SH-SY5Y neuroblastoma cells. Int J Dev Neurosci. 2009;27(2):135–147. doi: 10.1016/j.ijdevneu.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Gould J, Reeve HL, Vaughan PF, Peers C. Nicotinic acetylcholine receptors in human neuroblastoma (SH-SY5Y) cells. Neurosci Lett. 1992;145(2):201–204. doi: 10.1016/0304-3940(92)90022-y. [DOI] [PubMed] [Google Scholar]
- 35.Lukas RJ, Norman SA, Lucero L. Characterization of nicotinic acetylcholine receptors expressed by cells of the SH-SY5Y human neuroblastoma clonal line. Mol Cell Neurosci. 1993;4(1):1–12. doi: 10.1006/mcne.1993.1001. [DOI] [PubMed] [Google Scholar]
- 36.Sokolova E, Matteoni C, Nistri A. Desensitization of neuronal nicotinic receptors of human neuroblastoma SH-SY5Y cells during short or long exposure to nicotine. Br J Pharmacol. 2005;146(8):1087–1095. doi: 10.1038/sj.bjp.0706426. [DOI] [PMC free article] [PubMed] [Google Scholar]