Abstract
JAK2 genetic variants are associated with inflammatory bowel disease (IBD) and JAK inhibitors are being evaluated for therapy targeting immune-mediated diseases, including IBD. As JAK pathway-mediated cytokine regulation varies across cell types and stimulation conditions, we examined how JAK signaling and IBD-associated JAK2 variants regulate distinct acute and chronic microbial product exposure outcomes in human myeloid cells, consistent with the conditions of initial entry and ongoing intestinal tissue residence, respectively. Macrophages from controls and ulcerative colitis patients carrying the IBD-risk rs10758669 CC genotype showed increased JAK2 expression and NOD2-induced JAK2 phosphorylation relative to AA carriers. Interestingly, the threshold of JAK2 expression and signaling determined pattern-recognition receptor (PRR)-induced outcomes; while anti-inflammatory cytokines progressively decreased with lower JAK2 expression, pro-inflammatory cytokines switched from decreased to increased secretion below a certain JAK2 expression threshold. Low JAK2-expressing rs10758669 AA macrophages were above this threshold; consequently, both PRR-induced pro- and anti-inflammatory cytokines were decreased. However, relative to rs10758669 CC risk-carriers, AA carrier macrophages switched to increased NOD2-induced pro-inflammatory cytokines at lower therapeutically-used JAK inhibitor doses. Importantly, JAK inhibitors increased pro-inflammatory cytokines secreted by peripheral macrophages following chronic PRR stimulation and by human intestinal myeloid cells following exposure to intestinal pathogens. Mechanistically, the decreased response to and secretion of autocrine/paracrine IL-10, IL-4, IL-22 and thymic stromal lymphopoietin regulated these JAK-dependent outcomes in myeloid cells. Taken together, JAK signaling threshold determines whether PRR-induced pro- and anti-inflammatory cytokines are reciprocally regulated in myeloid cells; consideration of JAK2 genotype and targeting of specific cell types might improve JAK-targeted therapy in immune-mediated diseases.
Keywords: monocytes/macrophages, genetics, signal transduction, cytokines
Introduction
Proper balance between pro- and anti-inflammatory cytokine secretion following host:microbe interactions at mucosal surfaces is essential for intestinal immune homeostasis. Consistently, inflammatory bowel disease (IBD) is characterized by dysregulated cytokines and multiple IBD-risk loci are found in regions encompassing cytokine-associated pathways(1–3).
The recognition and response to microbial products occurs through pattern recognition receptors (PRRs), particularly in myeloid-derived cells. The PRR NOD2 constitutes the highest genetic risk for developing Crohn’s disease(1). Intestinal myeloid cells are derived from peripheral monocytes that migrate into the intestinal lamina propria(4, 5). This initial entry exposes these cells acutely to microbial ligands, including the NOD2 ligand peptidoglycan(6), resulting in both pro- and anti-inflammatory cytokine secretion. However, after ongoing microbial ligand exposure, myeloid-derived cells secrete low levels of cytokines upon additional microbial product exposure(4, 7, 8). Consistently, intestinal myeloid cells similarly secrete very low levels of cytokines to microbial products(4). Therefore, as monocytes enter the intestinal lamina propria, the outcomes following both acute and chronic exposure to microbial ligands are critical in intestinal immune homeostasis. Furthermore, during intestinal injury, peripheral monocyte recruitment to intestinal tissues is increased, which contributes to inflammatory outcomes(5, 9, 10). Moreover, inflamed conditions in the intestinal environment lead to dysregulated conditioning of newly recruited myeloid-derived cells(5, 10). Consistently, pro-inflammatory cytokine secretion by intestinal myeloid cells is increased in IBD(11). NOD2 contributes to both phases of myeloid cell entry into intestinal tissues, to acute recruitment(12, 13) and to optimal cytokine downregulation in resident intestinal myeloid-derived cells(8, 14).
The rs10758669 risk locus associated with both ulcerative colitis and Crohn’s disease is located in the JAK2 region(3). The C risk allele is associated with increased intestinal permeability(15), but it is unclear how it affects other immune outcomes. JAK proteins (JAK1, JAK2, JAK3 and TYK2)(16) are required for signaling through multiple cytokine receptors on various cell types. This, in turn, regulates broad outcomes, including responses to PRR ligand-induced cytokine autocrine/paracrine loops. Gain-of-function mutations in select JAK members in diseases including myelofibrosis have led to therapies inhibiting JAK signaling(16). Consistent with the dysregulation of numerous cytokines in immune-mediated diseases, many of which signal through JAKs, JAK-inhibiting therapies have since proven effective for select immune-mediated diseases, including rheumatoid arthritis and psoriasis(16). Tofacitinib, an inhibitor of multiple JAKs(17), is also being studied in IBD, with phase II(17) and III(18) trials demonstrating efficacy in ulcerative colitis. The phase II tofacitinib trials failed to meet primary endpoints in Crohn’s disease, although the placebo response was rather high in some cases (19, 20). This may also highlight that JAK family members have distinct contributions to ulcerative colitis and Crohn’s disease pathogenesis. For example, given the particularly significant role for innate immunity in Crohn’s disease, one possibility for the distinct outcomes in these trials is that broad JAK inhibition may differentially regulate inflammatory outcomes in myeloid cells (innate immunity) relative to T cells (adaptive immunity). JAK inhibition can decrease T cell-mediated inflammation(21, 22). However, in myeloid cells JAK inhibition decreases proinflammatory outcomes in some studies (21, 23), but increases proinflammatory outcomes in others (24, 25). Increased pro-inflammatory cytokines in myeloid cells with JAK inhibition would be undesirable in intestinal tissues, especially in inflamed conditions. Therefore, distinct JAK inhibition outcomes in different cell subsets might affect the efficacy of therapy targeting IBD and other immune-mediated diseases. As acute and chronic myeloid cell responses to microbial products reflect the initial entry and ongoing residence of macrophages in the intestinal lamina propria, respectively, we questioned how JAK inhibition regulates these two phases of myeloid cell responses. We further questioned how IBD-associated JAK2 variants modulate myeloid cell responses, including during pharmacological JAK inhibition.
In this study, we establish that cooperation between autocrine/paracrine IL-10, IL-4, IL-22 and TSLP is a mechanism through which the JAK pathway contributes to downregulating inflammatory outcomes in human myeloid cells upon microbial exposure. We further determine that inhibiting the JAK pathway in intestinal myeloid-derived cells during live bacterial exposure increases pro-inflammatory cytokines. We define that the JAK2 IBD disease-risk genotype leads to increased JAK2 expression and increased inflammatory responses from myeloid cells. Furthermore, we identify that the JAK signaling threshold is ultimately a critical determinant of the balance between pro-inflammatory and anti-inflammatory cytokines, thereby leading to distinct outcomes when utilizing pharmacological JAK inhibitors in the context of JAK2 IBD risk genotypes. These threshold effects also highlight that the advantage conferred with partial downmodulation in expression/activity of a gene in the context of a protective disease variant may not be reproduced if that same gene is completely inhibited for therapeutic purposes.
Materials and Methods
Patient recruitment and genotyping
Informed consent was obtained per protocol approved by the institutional review board at Yale University. We recruited healthy controls and ulcerative colitis patients (Supplementary Figure 1A). We genotyped JAK2 and NOD2 polymorphisms by TaqMan (Applied Biosystems, Foster City, CA) or Sequenom platform (Sequenom Inc., San Diego, CA). We confirmed that our cohort did not contain any Leu1007insC, R702W, or G908R NOD2 homozygote carriers.
Myeloid cell culture
Monocytes were purified from human peripheral blood mononuclear cells and cultured for 7 days with M-CSF (10ng/ml) (Shenandoah Technology, Warwick, PA) as in(7). Myeloid cells (CD11c purity >75%) were isolated as in(7) from colonic resection specimens from uninvolved intestine in 11 non-IBD patients undergoing surgery for diverticular disease or colon cancer.
Myeloid cell stimulation
Monocyte-derived macrophages (MDMs) were cultured with muramyl dipeptide (MDP) (Bachem, Torrance, CA), recombinant IL-10, IL-4, IL-22 (R&D Systems) or TSLP (Peprotech). For inhibitor and neutralizing antibody treatments, cells were incubated with JAK inhibitor I (total JAK inhibitor), SD-1029 (JAK2 inhibitor) (Calbiochem, La Jolla, CA), tofacitinib (manufactured by Pfizer, NY, NY), neutralizing anti-IL-10 (5 µg/ml), anti-IL-4 (1 µg/ml; BD Biosciences, San Jose, CA), anti-IL-22 (1.25 µg/ml; Peprotech) or anti-TSLP (0.25 µg/ml; R&D Systems) antibodies 1h prior to treatment. Supernatants were assayed for IL-10 (BD Biosciences), or IL-1β, IL-4, IL-6, IL-8, IL-12, IL-1ra (eBioscience, San Diego, CA), IL-22 (Peprotech) or TSLP (R&D Systems) by ELISA.
Transfection of small interfering RNAs
100nM scrambled or ON-TARGETplus SMARTpool small interfering RNA (siRNA) siRNA against JAK1, JAK2, JAK3, TYK2, IL10RA, IL4RA, IL22RA1 or CRLF2 (GE Dharmacon, Lafayette, CO) were transfected into MDMs for 48h (unless otherwise indicated) using Amaxa nucleofector technology (Amaxa, San Diego, CA).
Protein detection
Intracellular proteins were detected in permeabilized cells by flow cytometry with Alexa Fluor 647-, phycoerythrin- or Alexa Fluor 488-labeled antibodies to phospho-JAK1, phospho-JAK2, phospho-JAK3, phospho-TYK2, phospho-ERK, phospho-p38, phospho-JNK and phospho-IκBα (Cell Signaling Technology, Danvers, MA).
mRNA expression
Following stimulation, RNA was isolated, reverse transcribed and qPCR performed as in(7). Samples were normalized to GAPDH. Primers sequences are available upon request.
Statistical analysis
Mean+SEM was calculated. Significance was assessed using a two-tailed Student’s t-test. p<0.05 was considered significant. To maintain a consistent picogram/milliliter scale for cytokine concentration measures, the higher IL-6 levels (nanogram per milliliter levels) are shown with a multiplication factor. A line across multiple bars indicates same significance for these bars.
Results
JAK pathway signaling regulates NOD2-induced cytokine secretion and tolerance in multiple myeloid cell types
JAK inhibitors are in clinical trials for IBD(17). Although they suppress T cell-mediated inflammation(21, 22), the outcomes in myeloid cells are more controversial. Some studies indicate that JAK inhibition decreases proinflammatory outcomes on exposure to microbial components (21, 23), whereas others show it increases proinflammatory outcomes under these conditions (24, 25). Increased pro-inflammatory cytokines would likely not be favorable when treating IBD patients given the continual exposure of intestinal myeloid cells to microbial products. We therefore examined how JAK protein reduction regulates pro- and anti-inflammatory cytokines during acute and chronic PRR stimulation, as observed with initial entry and prolonged monocyte residence in the intestinal lamina propria, respectively. We effectively knocked down each JAK member (Supplementary Fig. 1B) in human MDMs and ensured cell viability (Supplementary Fig. 1C). JAK knockdown did not decrease PRR levels in MDMs (Supplementary Fig. 1D); such regulation has been reported in human epithelial cells(26). Moreover, we established that NOD2 stimulation activates JAK1, JAK2, JAK3 and TYK2 (Fig. 1A), and that knockdown of each JAK selectively reduced activation of the targeted JAK (Fig. 1A). Knockdown of each JAK, alone or in combination, increased pro-inflammatory (IL-6, IL-12), but decreased anti-inflammatory (IL-10, IL-1ra) cytokine secretion following acute NOD2 stimulation of MDMs and monocytes (Fig. 1B). As JAK inhibition distinctly regulated pro- and anti-inflammatory cytokines, we next examined M1- and M2-polarized macrophages. M1 macrophages secrete increased PRR-induced pro-inflammatory cytokines, and M2 macrophages secrete increased anti-inflammatory cytokines compared to non-polarized MDMs. We ensured proper differentiation of M1 and M2 macrophages (Supplementary Fig. 1E) as per previously published human markers(27). Knockdown of each JAK family member further increased the high levels of NOD2-induced pro-inflammatory cytokines in M1 macrophages, and decreased the anti-inflammatory cytokines in M2 macrophages (Supplementary Fig. 1F).
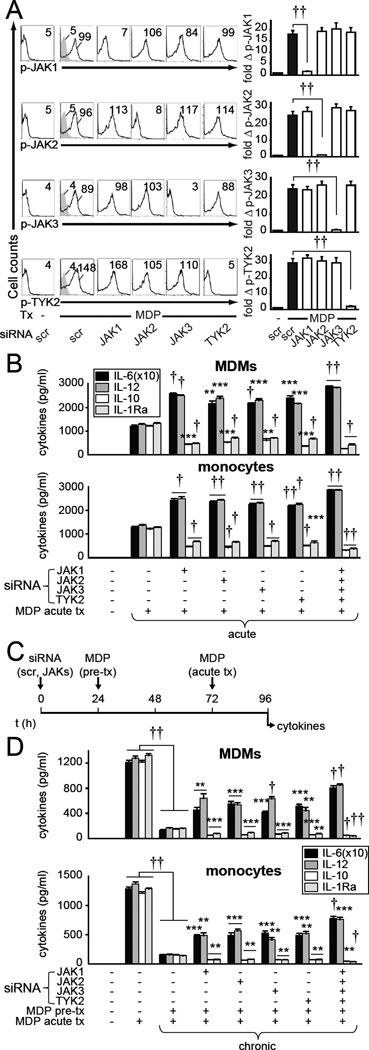
Figure 1. JAK expression knockdown increases pro-inflammatory while decreasing anti-inflammatory cytokines following both acute and chronic NOD2 stimulation.
(A) MDMs (n=4 donors) were transfected with the indicated siRNA and then treated with 100µg/ml MDP (NOD2 ligand) for 15min. Representative flow cytometry with MFI values and summary graphs with fold phospho-protein induction compared with untreated, scrambled siRNA-transfected cells. (B,D) MDMs (n=4 donors, top) or monocytes (n=4 donors, bottom) were transfected with the indicated siRNA, alone or in combination, for 24h and left untreated (acute) or pre-treated with 100µg/ml MDP (chronic) for 48h. Cells were then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to scrambled siRNA-transfected, acute MDP-treated cells (B, acute), or to scrambled siRNA-transfected, MDP-pre-treated and MDP-retreated cells (D, chronic), or as indicated. Similar results were seen for an additional n=8. (C) Timeline schematic for JAK knockdown, MDP pretreatment and subsequent acute MDP treatment. Bonferroni-Holm correction was used for multiple comparisons. **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. scr, scrambled; Tx, treatment.
IL-10, which signals through the JAK pathway(16) and is in turn secreted following JAK signaling(24), contributes to the anti-inflammatory phenotype observed in intestinal macrophages(4, 5). Moreover, early autocrine IL-10 regulates the decreased cytokines observed during NOD2-induced tolerance(28). We therefore hypothesized that JAKs would be required for optimal NOD2-induced tolerance. We found this to be the case for each JAK family member (Fig. 1C & D). Knockdown of all four JAK proteins further reversed the decreased pro-inflammatory cytokine secretion under chronic conditions (Fig. 1D), such that the pro-inflammatory cytokine levels increased from ~10% to ~60% relative to acute NOD2 stimulation. In contrast, and consistent with the requirement we observed for JAK family members in anti-inflammatory cytokine secretion, anti-inflammatory cytokines further decreased in these chronically MDP pre-treated cells (Fig. 1D). TLR2- and TLR4-induced cytokine secretion was regulated by JAKs similarly to that observed with NOD2 (Supplementary Fig. 2). Taken together, dramatically decreasing the expression of JAK family members reciprocally regulates pro-and anti-inflammatory cytokines; pro-inflammatory cytokines are increased and anti-inflammatory cytokines are decreased following both acute and chronic PRR stimulation in multiple primary human myeloid cell types.
NOD2-induced autocrine inhibitory mediators that signal through the JAK pathway regulate NOD2-induced pro-inflammatory cytokines
We next sought to define mechanisms for the JAK-dependence in NOD2-induced anti-inflammatory cytokines and NOD2-mediated tolerance. PRR-stimulated MDMs secrete autocrine/paracrine IL-10 which signals through the JAK pathway(16). Autocrine IL-10 decreases PRR-induced pro-inflammatory cytokine secretion(24) and induces NOD2-mediated tolerance(28). We hypothesized that additional autocrine/paracrine inhibitory mediators that signal through JAKs would be required for these outcomes. We first examined type I IFNs which signal through JAKs and can decrease PRR-induced cytokine secretion(16). JAK signaling was required for NOD2-induced IFN-α in MDMs and pDCs, and IFN-α was also decreased following chronic NOD2 stimulation (Supplementary Fig. 3A & B). IFN-β secretion was similarly regulated in pDCs (Supplementary Fig. 3B), but was not secreted in MDMs (data not shown). However, effective knockdown of IFNAR, required for both IFN-α and IFN-β signaling, neither altered acute nor chronic NOD2-induced cytokine secretion (Supplementary Fig. 3C & D), indicating that autocrine/paracrine type I IFN signaling in and of itself was not required to regulate cytokine secretion during acute or chronic NOD2 stimulation in MDMs.
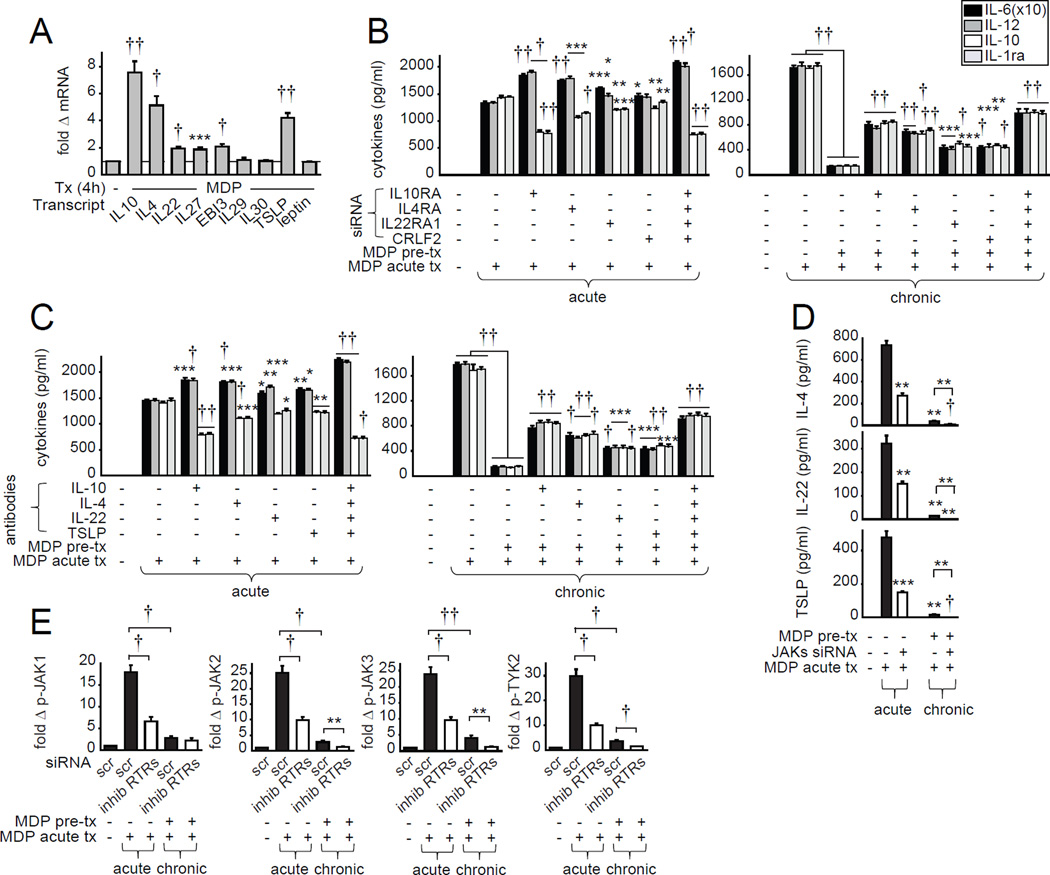
We next examined additional putative inhibitory cytokines that signal through JAKs(29), with a focus on their role in MDMs. We did not detect IL-3, IL-9, IL-11, IL-13, epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) transcripts (data not shown), and did not observe a significant increase in IL-29, IL-30 or leptin mRNA upon NOD2 stimulation (Fig. 2A). However, NOD2 stimulation induced IL-10, IL-4, IL-22, IL-27, EBI3 and TSLP transcripts (Fig. 2A). We therefore knocked down the receptor for each of these regulated cytokines (Supplementary Fig. 3E) and ensured that the cells were viable (data not shown). Autocrine IL-10 and IL-4 signaling most strongly contributed to the reciprocal regulation of acute NOD2-induced pro- and anti-inflammatory cytokine secretion as well as to NOD2-induced tolerance (Fig. 2B). IL-22 and TSLP signaling also regulated these outcomes (Fig. 2B). However, neither IL-27 nor EBI3 signaling contributed to acute or chronic NOD2-induced outcomes (data not shown). Importantly, combined IL-10, IL-4, IL-22 and TSLP receptor knockdown even more dramatically increased acute NOD2-induced pro-inflammatory and decreased anti-inflammatory cytokine secretion and impaired NOD2-mediated tolerance (Fig. 2B). This indicates cooperation between these factors in MDMs (Fig. 2B) and monocytes (data not shown). We confirmed the importance of these autocrine cytokines through an additional independent approach using neutralizing IL-10, IL-4, IL-22 and TSLP Abs (Fig. 2C). We ensured that in addition to IL-10 (Fig 1B), secretion of the inhibitory proteins IL-4, IL-22 and TSLP was observed following acute NOD2 stimulation, but was decreased following chronic stimulation (Fig. 2D). Consistent with the decreased IL-10 and IL-1ra secretion with JAK pathway inhibition, JAK knockdown led to a significant decrease of IL-4, IL-22 and TSLP secretion upon both acute and chronic NOD2 stimulation (Fig. 2D). We next questioned how these autocrine/paracrine cytokines regulate NOD2-induced JAK pathway activation in human MDMs. Combined IL-10, IL-4, IL-22 and TSLP receptor knockdown significantly decreased acute NOD2-induced activation of each JAK member (Fig. 2E). With chronic NOD2 stimulation, activation of each JAK member was decreased relative to that observed with acute NOD2 stimulation (Fig. 2E), although activation was still increased relative to untreated MDMs. Combined IL-10, IL-4, IL-22 and TSLP receptor knockdown further decreased JAK2, JAK3 and TYK2 signaling (Fig. 2E), indicating that these anti-inflammatory cytokines contribute to residual JAK signaling even following chronic NOD2 stimulation. Therefore, upon PRR stimulation, JAKs are both activated in response to the inhibitory autocrine/paracrine cytokines IL-10, IL-4, IL-22 and TSLP (Fig. 2E) and are required for their optimal secretion (Fig. 2D) in a feedforward manner. These cytokines, in turn, cooperate to downregulate proinflammatory and upregulate anti-inflammatory cytokine secretion in myeloid cells following acute NOD2 stimulation, and to the overall downregulation of cytokines observed with NOD2-induced tolerance.
Figure 2. Autocrine IL-10, IL-4, IL-22 and TSLP decrease NOD2-induced pro-inflammatory cytokine secretion and increase JAK pathway signaling.
(A) MDMs (n=12 donors, similar results were seen for an additional n=12) were treated with 100µg/ml MDP for 4h. Fold mRNA expression compared to untreated cells (represented by the dotted line at 1). (B) MDMs were transfected with scrambled or IL-10RA (to block autocrine IL-10), IL-4RA (to block autocrine IL-4), IL-22RA1 (to block autocrine IL-22), or CRLF2 (to block autocrine TSLP) siRNA, alone or in combination, for 24h and left untreated (acute, n=8) or pre-treated with 100µg/ml MDP for 48h (chronic, n=8). Cells were then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to scrambled siRNA-transfected, acute MDP-treated cells (left, acute), or scrambled siRNA-transfected, MDP-pre-treated and acute MDP-treated cells (right, chronic). (C) MDMs were left untreated or treated with neutralizing anti-IL-10, anti-IL-4, anti-IL-22 or anti-TSLP antibodies, alone or in combination, for 1h and left untreated (acute, n=8) or pre-treated with 100µg/ml MDP for 48h (chronic, n=8). Cells were then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to acute MDP-treated cells with no neutralizing antibody treatment (left, acute), or MDP-pre-treated and acute MDP-treated cells with no neutralizing antibody treatment (right, chronic). Similar results were observed in an additional n=16 (B) or n=12 (C). (D) MDMs (n=4 donors) were transfected with JAK1, JAK2, JAK3 and TYK2 siRNA in combination for 24h and left untreated or pre-treated with 100µg/ml MDP. Cells were then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to scrambled siRNA-transfected, acute MDP-treated cells or as indicated. (E) MDMs (n=8 donors, similar results were seen in an additional n=8) were transfected with IL-10RA, IL-4RA, IL-22RA1 and CRLF2 siRNA in combination (inhibitory receptors; ‘inhib RTRs’) for 24h and left untreated (acute) or pre-treated with 100µg/ml MDP for 48h (chronic). Cells were then treated with 100µg/ml MDP for 15min and assessed for phospho-protein induction by flow cytometry. Summarized phospho-JAK induction. (B,E) Bonferroni-Holm correction was used for multiple comparisons. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. scr, scrambled; Tx, treatment.
IL-10, IL-4, IL-22 and TSLP reciprocally regulate acute NOD2-induced pro- and anti-inflammatory cytokines and are sufficient for cytokine downregulation under chronic treatment conditions
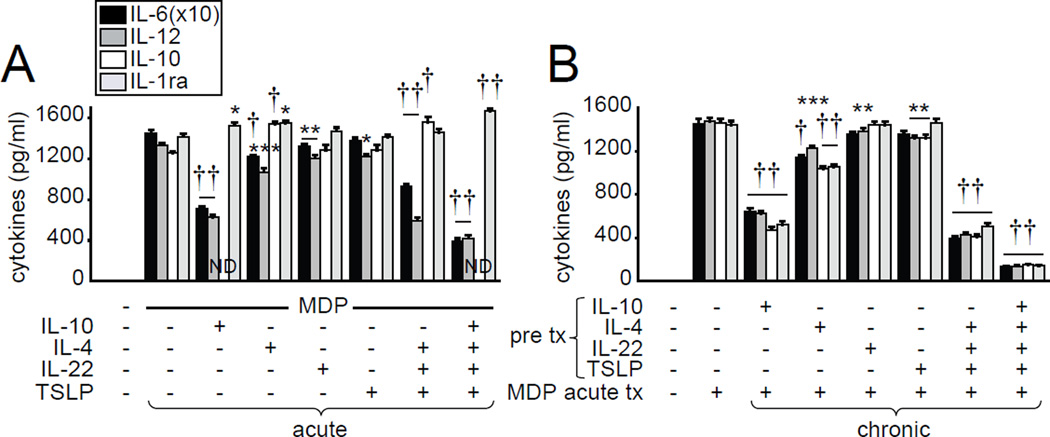
We next questioned if IL-10, IL-4, IL-22 and TSLP treatment is sufficient to reciprocally regulate pro- and anti-inflammatory cytokines during acute NOD2 stimulation, and to induce cytokine downregulation with chronic treatment of MDMs. IL-10 or IL-4 treatment partially decreased pro-inflammatory cytokines during acute NOD2 stimulation, whereas IL-22 or TSLP treatment effects were less pronounced (Fig. 3A). However, combined IL-4, IL-22 and TSLP treatment, and particularly combined IL-10, IL-4, IL-22 and TSLP treatment, cooperated to suppress acute NOD2-mediated pro-inflammatory and enhance anti-inflammatory cytokine secretion (Fig. 3A). We previously found that autocrine IL-10 is required for the optimal decrease in cytokines following chronic NOD2 stimulation(28). In this study, we found that chronic IL-10 treatment was also sufficient to decrease subsequent NOD2-induced cytokine secretion (Fig 3B). Although chronic IL-22 or TSLP treatment was not sufficient to significantly decrease cytokine secretion following NOD2 restimulation, treatment with IL-4, combined IL-4, IL-22 and TSLP, and particularly combined IL-10, IL-4, IL-22 or TSLP (Fig. 3B), decreased cytokines to levels similar to those observed after chronic NOD2 stimulation (Fig. 1). Taken together, combined IL-10, IL-4, IL-22 and TSLP are both necessary and sufficient for the reciprocal pro-inflammatory and anti-inflammatory cytokine regulation during acute NOD2 stimulation and for the cytokine decrease during NOD2-induced tolerance.
Figure 3. IL-10, IL-4, IL-22 and TSLP reciprocally regulate acute NOD2-induced pro- and anti-inflammatory cytokines and decrease cytokine secretion under chronic treatment conditions.
(A) MDMs (n=12 donors, similar results seen in an additional n=8) were treated with 100µg/ml MDP±10ng/ml IL-10, IL-4, IL-22 or TSLP alone or in combination for 24h. (B) MDMs (n=4 donors) were pre-treated with 10ng/ml IL-10, IL-4, IL-22 or TSLP alone or in combination for 48h. Cells were then treated with 100 µg/ml MDP for 24 h. Cytokine secretion+SEM. Significance is compared to acute MDP-treated cells. Bonferroni-Holm correction was used for multiple comparisons. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. ND, not determined due to addition of same recombinant cytokine; Tx, treatment.
JAK signaling is required for decreased pro-inflammatory cytokine secretion in human intestinal myeloid cells
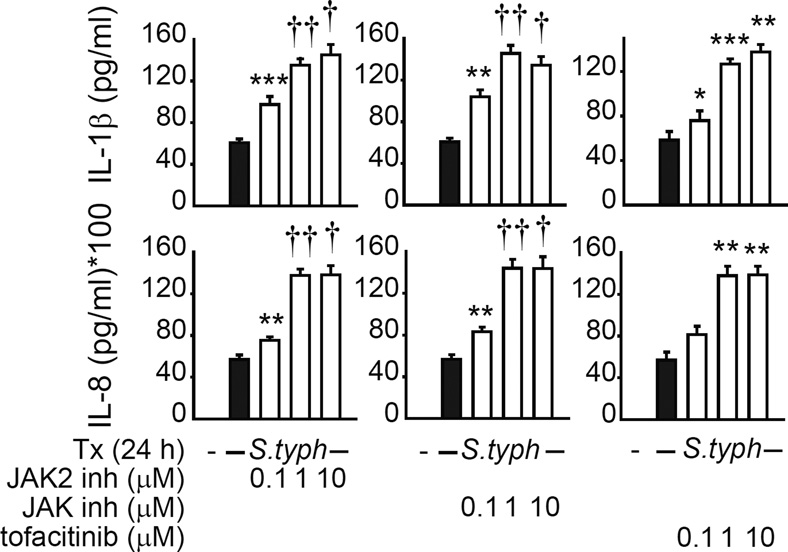
An increase in pro-inflammatory cytokines from myeloid intestinal cells during JAK-inhibitor therapy for IBD may confound the intended beneficial outcomes from other cell subsets. We therefore next questioned if the JAK pathway regulated human intestinal myeloid cell responses to microbes. Compared to peripheral MDMs, PRR ligand-treated intestinal macrophages secrete significantly lower levels of cytokines(4, 7), but can secrete cytokines following S. typhimurium exposure(30, 31). To investigate JAK signaling in intestinal myeloid cells we used three approaches; we targeted JAK2 with SD-1029, inhibited JAK1, JAK2 and JAK3 with JAK inhibitor I, and used tofacitinib, which inhibits multiple JAK members and is in therapeutic trials for IBD. The inhibitors have been commonly used in other studies (32, 33). We used tofacitinib doses that were within range observed in the plasma of subjects treated with this inhibitor(34, 35). Following JAK inhibition, S. typhimurium-infected intestinal myeloid cells secreted increased pro-inflammatory IL-1β and IL-8 levels in a JAK inhibitor dose-dependent manner; a similar increase was observed in peripheral MDMs (Supplementary Fig. 3F). Taken together, JAK inhibitors increase pro-inflammatory cytokine secretion in human peripheral and intestinal myeloid cells following live pathogenic bacterial exposure.
MDMs from rs10758669 C disease-risk carriers in the JAK2 region demonstrate increased JAK2 expression and increased NOD2-induced JAK2 phosphorylation and cytokine secretion
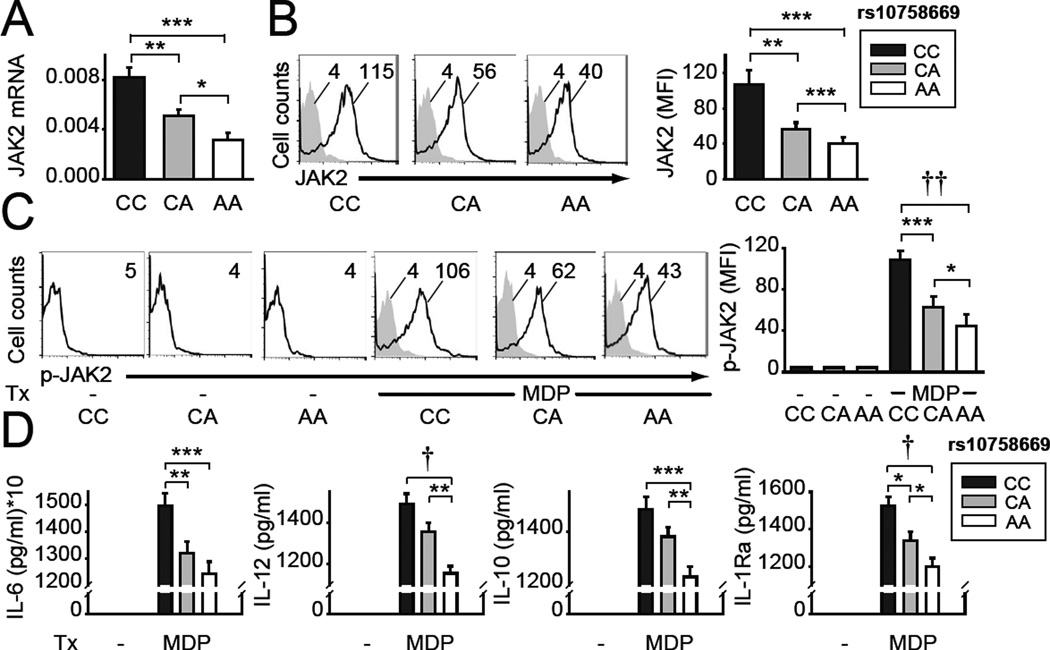
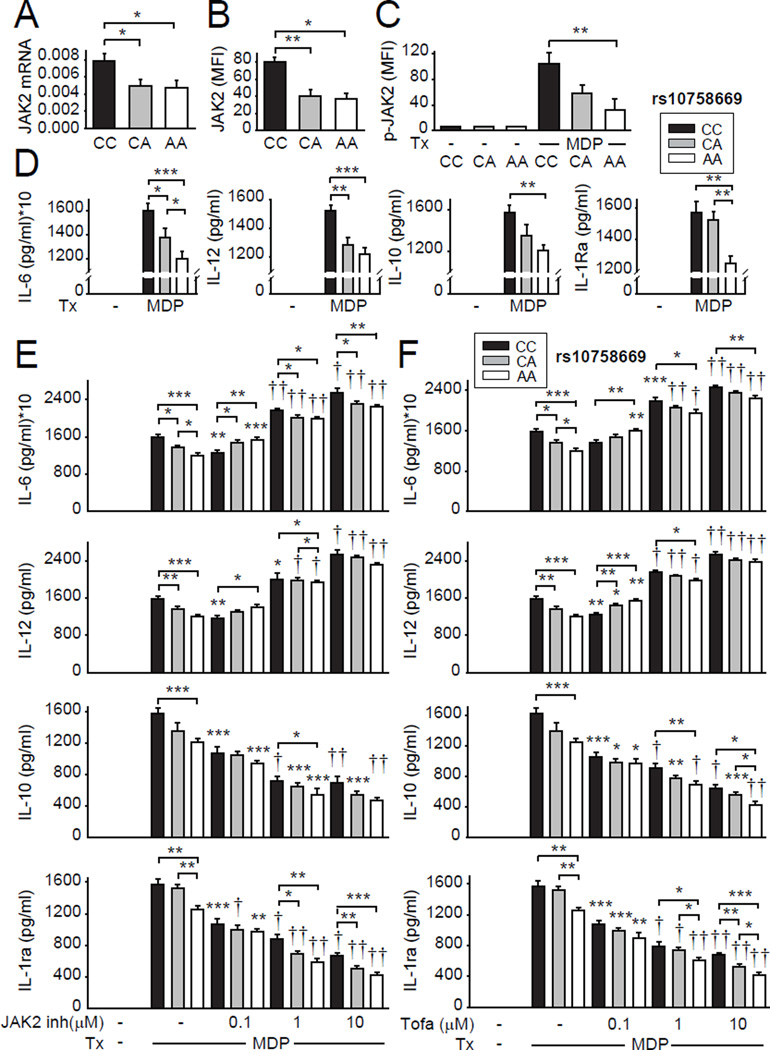
We next sought to define how the IBD-associated rs10758669 polymorphism(3), located in an intergenic region 3kb from JAK2, regulates JAK2 expression and cytokine secretion in human MDMs. Rs10758669 CC risk-carrier MDMs demonstrated increased JAK2 mRNA (Fig. 5A) and protein (Fig. 5B) expression compared to AA carrier MDMs. In contrast, mRNA expression of other genes in the region was not detected (INSL6, INSL4, RLN2, RLN1, PLGRKT) (data not shown) or was not modulated by rs10758669 genotype (CDC37L1, AK3, RCL1, CD274) (Supplementary Fig. 4A). Consistent with the increased JAK2 expression, NOD2-induced JAK2 phosphorylation was increased in rs10758669 CC carrier MDMs (Fig. 5C). Rs10758669 CA carriers showed an intermediate phenotype (Fig. 5A–C).
Figure 5. MDMs from healthy control rs10758669 CC risk carriers express increased JAK2 and show increased NOD2-induced JAK2 activation and cytokines relative to AA carriers.
MDMs from rs10758669 CC, CA or AA healthy control carriers were assessed as follows: (A) JAK2 mRNA expression (change in CT values normalized to GAPDH and represented as a linear scale) (n=15 donors/genotype, similar results were seen in n=10/genotype). (B) Representative and summarized flow cytometry data for JAK2 protein expression (n=10 donors/genotype). (C) Cells were treated with 100µg/ml MDP for 15min. Representative and summarized flow cytometry data for fold phospho-JAK2 induction normalized to untreated cells (n=10 donors/genotype). (D) Cells were treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM (n=15 donors/genotype, similar results were seen in n=8/genotype). Bonferroni-Holm correction was used for multiple comparisons. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. Tx, treatment.
We next questioned how JAK2 genotype regulates cytokines following acute NOD2 stimulation. These acute stimulation conditions simulate myeloid cell entry into the intestinal lamina propria under homeostasis; this entry dramatically increases during acute intestinal injury and inflammation(5, 9, 10). Rs10758669 CC risk allele-carrier MDMs demonstrated increased pro-inflammatory and anti-inflammatory cytokine secretion relative to AA carriers (Fig 5D). Taken together, rs10758669 CC IBD-risk carrier MDMs in the JAK2 region demonstrate increased JAK2 expression and PRR-induced JAK2 activation and cytokine secretion.
The threshold of JAK2 signaling determines if NOD2-induced pro-inflammatory cytokines are increased or decreased
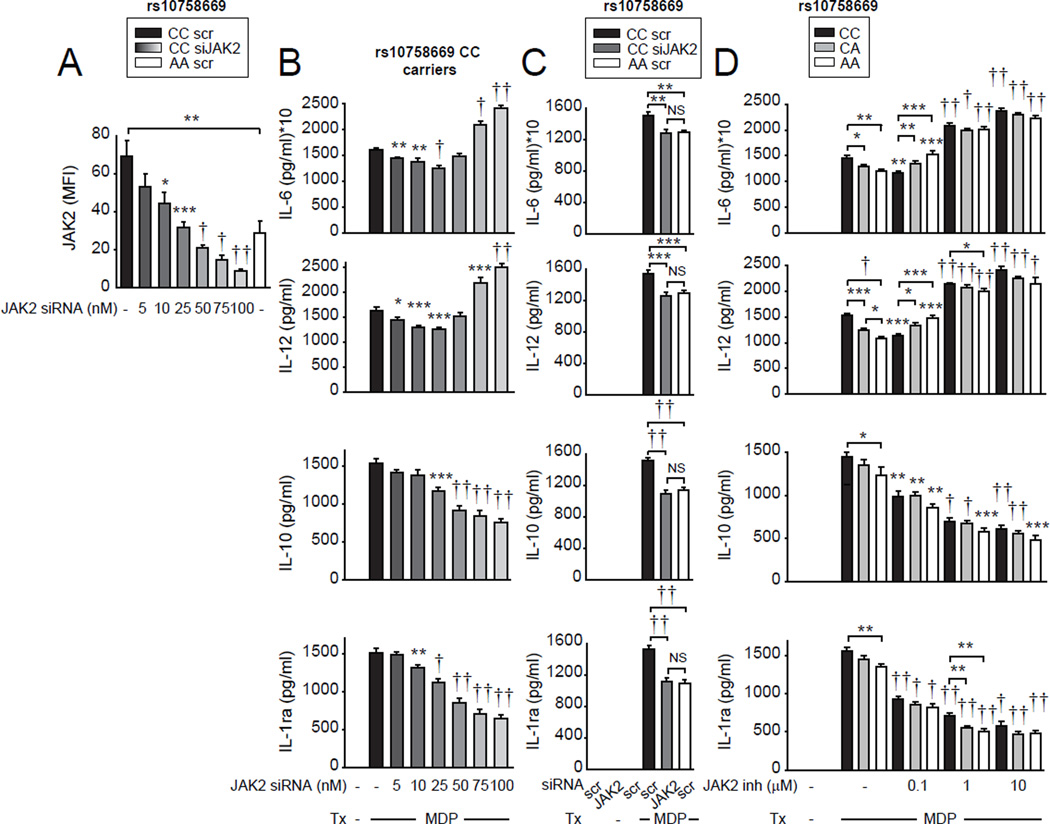
The decreased secretion of both pro- and anti-inflammatory cytokines following acute NOD2 stimulation by the lower JAK2-expressing rs10758669 AA carrier MDMs (Fig. 5D) was surprising given that JAK2 knockdown resulted in increased pro-inflammatory cytokines (Fig. 1). We hypothesized that these differences may reflect distinct outcomes based on the threshold of JAK2 expression and signaling. As such, if JAK2 expression or signaling falls below a particular threshold, NOD2-induced pro-inflammatory cytokines would now increase due to greatly reduced anti-inflammatory cytokines. A partial JAK2 expression decrease as is observed in rs10758669 AA carriers could then modulate cytokines differently relative to the more dramatic reduction in JAK2 expression under the knockdown conditions assessed above. To address this hypothesis, we progressively decreased JAK2 expression in high JAK2-expressing rs10758669 CC MDMs through dose-dependent siRNA targeting (Fig. 6A). With mild JAK2 expression reduction, levels of NOD2-induced pro-inflammatory cytokines progressively decreased (Fig. 6B). However, once JAK2 expression decreased to less than ~30% of the levels observed in CC carrier MDMs, NOD2-induced pro-inflammatory cytokines stopped decreasing, and began to increase (Fig. 6B). In contrast, anti-inflammatory cytokine secretion progressively decreased through the full range of JAK2 expression reduction (Fig. 6B). Furthermore, side-by-side JAK2 expression knock-down in rs10758669 CC carrier MDMs to the levels observed in AA carrier cells (Fig. 6A) resulted in comparable cytokine secretion between the two genotypes (Fig. 6C). This highlights that at the levels of reduced JAK2 expression in rs10758669 AA non-risk carrier MDMs, JAK2 expression is above the threshold of reciprocal regulation of pro- and anti-inflammatory cytokines, such that PRR-induced pro-inflammatory and anti-inflammatory cytokines are both decreased. Taken together, with acute NOD2 stimulation, once JAK2 expression or signaling decreases below a threshold, proinflammatory cytokine secretion progressively increases.
Figure 6. As the strength of JAK expression and signaling decreases below a threshold, pro-inflammatory cytokine secretion increases rather than decreases upon acute NOD2 stimulation.
(A–B) MDMs from healthy control rs10758669 carriers were transfected with JAK2 siRNA at the indicated concentrations. (A) Summarized flow cytometry data for JAK2 protein expression (n=8 donors/genotype). (B) Transfected cells were treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM (n=8 donors, similar results were seen in an additional n=8). (C) MDMs from healthy control rs10758669 CC carriers or AA carriers (n=15/genotype, similar results were seen in n=6/genotype) were transfected with 25nM scrambled or JAK2 siRNA. Cells were then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Bonferroni-Holm correction was used for multiple comparisons. (D) MDMs from healthy control rs10758669 carriers (n=8 donors/genotype) were pre-incubated with SD-1029 (JAK2 specific inhibitor) at the indicated doses and then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to scrambled siRNA-transfected, untreated (A) or MDP-treated (B) CC carrier MDMs, or MDP-treated cells of the same genotype in the absence of inhibitor (D) or as indicated. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. inh, inhibitor; scr, scrambled; Tx, treatment.
Given that the threshold of JAK2 expression determines how pro-inflammatory cytokine secretion is regulated, we questioned how JAK inhibition affects cytokine regulation in the context of rs10758669 genotype. Such genotype:inhibitor dose interactions may have implications for JAK inhibitor therapy. At low levels of JAK2 (Fig. 6D) and JAK (tofacitinib, Supplementary Fig. 4B) inhibition, rs10758669 CC carrier MDMs demonstrated decreased NOD2-induced pro-inflammatory cytokines. However, with more potent JAK2 and JAK inhibition NOD2-induced pro-inflammatory cytokines increased. In contrast, anti-inflammatory cytokine secretion decreased progressively with increasing JAK inhibition (Fig. 6D and Supplementary Fig. 4B). Interestingly, at the lower 0.1 µM JAK2 inhibitor (SD-1029) or tofacitinib dose, NOD2-induced cytokine trends in rs10758669 CC and AA carrier MDMs were reversed. AA carriers expressed higher pro-inflammatory cytokines relative to CC carrier MDMs at that same inhibitor dose (Fig. 6D and Supplementary Fig. 4B), and relative to non-JAK2 inhibitor treated AA MDMs, whereas pro-inflammatory cytokines from CC MDMs were still decreased relative to non-JAK2 inhibitor treated cells. Therefore, the lower JAK2-expressing rs10758669 AA MDMs switch from a decrease to an increase in PRR-induced pro-inflammatory cytokines at a lower dose of JAK pharmacological inhibitor treatment than do rs10758669 CC MDMs. Taken together, pro-inflammatory cytokine regulation depends on the level of JAK2 expression and JAK signaling during acute NOD2 stimulation, and JAK inhibition leads to a ‘switch’ from decreased to increased inflammatory cytokines at lower inhibitor doses in the lower JAK2-expressing rs10758669 AA MDMs.
MDMS from rs10758669 CC disease risk carrier ulcerative colitis risk patients demonstrate increased JAK2 expression, and increased NOD2-induced JAK2 phosphorylation and cytokine secretion relative to A allele carriers
Paralleling outcomes in healthy control MDMs, rs10758669 CC disease risk carrier cells from ulcerative colitis patients (Supplementary Figure 1A) showed increased JAK2 RNA and protein expression, and increased acute NOD2-induced JAK2 phosphorylation and secretion of both pro- and anti-inflammatory cytokines relative to A carrier MDMs (Fig. 7A–D). MDMs from rs10758669 AA disease carriers similarly required a lower JAK2 inhibitor and tofacitinib dose to switch from decreased to increased NOD2-induced pro-inflammatory cytokines than did C carrier MDMs (Fig. 7E & F). Therefore, PRR-induced pro-inflammatory cytokine secretion in MDMs is regulated in a JAK2 genotype-dependent manner in both healthy controls and ulcerative colitis patients.
Figure 7. MDMs from ulcerative colitis rs10758669 CC risk carriers express increased JAK2, show increased NOD2-induced JAK2 activation and cytokines, and required higher JAK2 inhibitor and tofacitinib doses to increase PRR-induced pro-inflammatory cytokines relative to AA carriers.
MDMs from rs10758669 CC, CA or AA ulcerative colitis carriers were assessed as follows: (A) JAK2 mRNA expression (change in CT values normalized to GAPDH and represented as a linear scale) (n=8 donors/genotype). (B) Summarized flow cytometry data for JAK2 protein expression (n=7 donors/genotype). (C) Cells were treated with 100µg/ml MDP for 15min. Summarized flow cytometry data for fold phospho-JAK2 induction normalized to untreated cells (n=7 donors/genotype). (D) Cells were treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM (n=8 donors/genotype). (E-F) Cells were pre-incubated with SD-1029 (JAK2 specific inhibitor) (E) or tofacitinib (F) at the indicated doses and then treated with 100µg/ml MDP for 24h. Cytokine secretion+SEM. Significance is compared to MDP-treated cells of the same genotype (in the absence of inhibitors) or as indicated. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. inh, inhibitor; Tofa, tofacitinib; Tx, treatment.
Discussion
In this study we define that the threshold of JAK pathway signaling determines whether PRR-induced pro- and anti-inflammatory cytokines are similarly or reciprocally regulated in human myeloid-derived cells. We also elucidate mechanisms of JAK-mediated cytokine secretion, and determine consequences of the JAK2 region rs10758669 IBD-risk variant (Supplementary Fig. 4C). Near complete JAK pathway inhibition increases pro-inflammatory and decreases anti-inflammatory cytokines in myeloid cells upon both acute and chronic microbial product exposure (both phases relevant in intestinal tissues), and in intestinal myeloid cells on live pathogenic bacterial exposure. These effects on innate cells may be counteracting other beneficial outcomes when administering JAK inhibitors for IBD therapy. As such, in myelofibrosis patients treated with JAK inhibitors, both diarrhea and systemic inflammatory responses are observed(36). Therefore, whereas JAK inhibition may downregulate T cell-mediated inflammation(16), it can upregulate myeloid cell-mediated inflammatory outcomes under intestinally-relevant conditions per our studies, and impair epithelial cell restitution in experimental colitis models(37). Taken together, JAK inhibition effects in non-T cell subsets may ultimately prevent optimal responses during IBD therapy. Minimizing potentially unfavorable effects of JAK inhibitors on myeloid cells through cell-specific targeting of JAK inhibitors to T cells may improve IBD therapy outcomes.
Our findings may also provide insight into the contrasting results between ulcerative colitis and Crohn’s disease patients in the JAK inhibitor trials. The lack of efficacy in Crohn’s disease patients in Phase II trials with the broad JAK inhibitor tofacitinib may be due to the significant role that innate immune pathways play in Crohn’s disease pathogenesis. Interestingly, a recent Phase II trial with a selective JAK1 inhibitor has yielded more promising results in Crohn’s disease patients(38). In fact, we observe considerable redundancy in PRR-induced cytokine regulation in macrophages with knockdown of each of the individual JAK family members. One possibility for this redundancy is that the PRR-induced autocrine inhibitory cytokines signal through multiple JAK family members. For example, IL-10 signals through JAK1, JAK2 and TYK2, and IL-4 through JAK1 and JAK3(29). Therefore, autocrine inhibitory cytokine outcomes are affected by knock down of each JAK protein. However, we observe the most dramatic effects on PRR-induced cytokine secretion when JAKs are knocked down in combination. Therefore, it is possible that inhibition of multiple JAKs with tofacitinib might more easily reach the threshold of JAK signaling required to switch the direction of pro-inflammatory cytokine secretion from decreased to increased relative to the inhibition of a single JAK family member, such as JAK1. As such, in addition, to minimizing the effects of JAK inhibitors on myeloid cells through cell-specific targeting of JAK inhibitors to T cells, selective JAK family member targeting and/or modulating JAK inhibitor doses may improve IBD therapy outcomes, in particular in Crohn’s disease patients.
In contrast to anti-inflammatory cytokines, the direction of pro-inflammatory cytokine secretion depends on the threshold of JAK signaling. Mildly decreased JAK2 expression and/or signaling decreases both pro- and anti-inflammatory cytokines in PRR-stimulated MDMs (Fig. 6D, 7D), whereas a more pronounced JAK signaling reduction leads to a progressive decrease in the anti-inflammatory cytokine feedforward loop and an increase in pro-inflammatory cytokines. To our knowledge, this study is the first to define that the threshold of JAK signaling determines whether pro- and anti-inflammatory cytokines are uniformly or reciprocally regulated, and likely clarifies previous conflicting reports in myeloid cells(21, 23–25). With identification of disease-associated alleles that modulate gene expression, it will be critical to define how the threshold of expression/activity of the implicated genetic pathway regulates downstream outcomes. Whereas partial expression/activity down-modulation might decrease disease susceptibility, complete inhibition of the same pathway for therapeutic purposes may result in adverse outcomes. As the IBD-risk rs10758669 genotype that regulates JAK2 expression modulates the threshold of sensitivity to inhibition of JAKs by tofacitinib (Fig. 6&7 and Supplementary Fig. 4B), JAK inhibitor dose-mediated regulation of in vivo outcomes during IBD therapy in the context of JAK2 genotype will be of interest in future studies. Examples of personalized drug dosing include thiopurine in IBD patients per TMPT genotype, methotrexate in bone marrow transplant patients per MTHFR genotype (39), and warfarin in patients requiring anti-coagulation per CYP2C9 genotype(40).
We find that JAK signaling is required for both the responses to and the autocrine/paracrine secretion of IL-10, IL-4, IL-22 and TSLP(16), thereby invoking a feedforward loop. These autocrine cytokines cooperate for optimal outcomes (Figs. 2,3). IL-22 has been associated with epithelial cell proliferation and anti-microbial protein induction(41), but has not been well-defined in mediating myeloid cell outcomes. TSLP mediates dendritic cell maturation and T cell outcomes, but its effects on monocytes and macrophages are not well-established(42). We now confirm that IL-22 and TSLP mediate JAK activation and define that they downregulate pro-inflammatory and upregulate anti-inflammatory cytokine secretion in myeloid cells. The JAK inhibition- and/or knockdown-mediated pro-inflammatory cytokine increase is likely a combination of defective suppression by anti-inflammatory mediators and of JAK-independent signaling mechanisms that induce pro-inflammatory cytokines. Consistently, JAK inhibition less effectively decreased PRR-induced pro-inflammatory cytokines in IL-10−/− compared to wild-type mouse BMDMs(24).
We identify mechanisms wherein JAK pathway signaling distinctly regulates pro- and anti-inflammatory cytokine secretion in multiple myeloid cell types upon intestinal-pertinent acute and chronic PRR stimulation conditions, establish that the JAK signaling threshold and IBD-risk JAK2 variant regulate these outcomes, and integrate JAK2 genotype with therapeutically utilized doses of JAK inhibitors (in ongoing IBD clinical trials) for effects on these outcomes. The JAK inhibitor dose effects on intestinal-relevant myeloid cell responses highlights considerations with respect to dosing, including in the context of JAK2 genotype, to JAK member-selective targeting, and to cell subset-specific targeting when using JAK inhibitors for therapy of immune-mediated diseases.
Supplementary Material
Figure 4. JAK inhibition induces pro-inflammatory cytokine secretion in S. typhimurium treated human intestinal myeloid cells.
Human intestinal myeloid cells were preincubated with the indicated doses of SD-1029 (JAK2 inhibitor) (n=6 donors), JAK inhibitor I (JAK1, JAK2 and JAK3 inhibitor) (n=6 donors), or tofacitinib (n=5 donors) and then co-cultured with S. typhimurium (S. typh) at MOI 10:1 for 24h. Cytokine secretion+SEM. *, p<0.05; **, p<0.01; ***, p<0.001; †, p<1x10−4; ††, p<1x10−5. inh, inhibitor; Tx, treatment.
Acknowledgments
We gratefully acknowledge the contribution of blood donors and thank Maria Ciarleglio for helpful advice.
Abbreviations
- CD
Crohn’s disease
- IBD
inflammatory bowel disease
- MDM
monocyte-derived macrophage
- MDP
muramyl dipeptide
- NOD
nucleotide-binding oligomerization domain
- PRR
pattern-recognition receptor
Footnotes
This work was supported by the NIH: R01DK099097, U01DK062422, and P30-DK34989, and by the American Gastroenterological Association-Pfizer IBD grant (the grant was judged/selected by the American Gastroenterological Association, and Pfizer was not involved in the design, conduct or reporting of the current study, or in the provision of tofacitinib).
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Consortium NIG, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBDC, C. Wellcome Trust Case Control. Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 6.Zheng S, Abraham C. NF-kappaB1 inhibits NOD2-induced cytokine secretion through ATF3-dependent mechanisms. Mol Cell Biol. 2013;33:4857–4871. doi: 10.1128/MCB.00797-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, Kitani A, Strober W. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Lahiri A, Haines GK, 3rd, Flavell RA, Abraham C. NOD2 regulates CXCR3-dependent CD8+ T cell accumulation in intestinal tissues with acute injury. J Immunol. 2014;192:3409–3418. doi: 10.4049/jimmunol.1302436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 Sensor Promotes Intestinal Pathogen Eradication via the Chemokine CCL2-Dependent Recruitment of Inflammatory Monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahiri A, Abraham C. Activation of pattern recognition receptors up-regulates metallothioneins, thereby increasing intracellular accumulation of zinc, autophagy, and bacterial clearance by macrophages. Gastroenterology. 2014;147:835–846. doi: 10.1053/j.gastro.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prager M, Buttner J, Haas V, Baumgart DC, Sturm A, Zeitz M, Buning C. The JAK2 variant rs10758669 in Crohn’s disease: altering the intestinal barrier as one mechanism of action. Int J Colorectal Dis. 2012;27:565–573. doi: 10.1007/s00384-011-1345-y. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 18.Sandborn W, Sands BE, D’Haens GR, Vermeire S, Schreiber S, Danese S, Panés J, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth DA, Mukherjee A, Healey PJ, Zhang H, Su C. Efficacy and Safety of Oral Tofacitinib As Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis: Results From Two Phase 3 Randomized Controlled Trials. Gastroenterology. 2016;150:S157. [Google Scholar]
- 19.Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W Study A3921043 Investigators. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:1485–1493. doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Panés J, Sandborn W, Schreiber S, Sands BE, Vermeire S, Chan G, Moscariello M, Wang W, Niezychowski W, Marren A, Healey PJ, Maller E. Efficacy and Safety of Tofacitinib for Oral Induction Therapy in Patients With Moderate to Severe Crohn’s Disease: Results of a Phase 2B Randomized Placebo-Controlled Trial. Gastroenterology. 2016;150:S182–183. [Google Scholar]
- 21.Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, Warner JD, Tanaka M, Steward-Tharp SM, Gadina M, Thomas CJ, Minnerly JC, Storer CE, LaBranche TP, Radi ZA, Dowty ME, Head RD, Meyer DM, Kishore N, O’Shea JJ. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186:4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derenzini E, Lemoine M, Buglio D, Katayama H, Ji Y, Davis RE, Sen S, Younes A. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J. 2011;1:e46. doi: 10.1038/bcj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarilina A, Xu K, Chan CH, Ivashkiv LB. Regulation of Inflammatory Responses in Tumor Necrosis Factor-Activated and Rheumatoid Arthritis Synovial Macrophages by JAK Inhibitors. Arthritis and Rheumatism. 2012;64:3856–3866. doi: 10.1002/art.37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattison MJ, Mackenzie KF, Arthur JS. Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10-mediated feedback. J Immunol. 2012;189:2784–2792. doi: 10.4049/jimmunol.1200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Brown J, Gao S, Liang S, Jotwani R, Zhou H, Suttles J, Scott DA, Lamont RJ. The role of JAK-3 in regulating TLR-mediated inflammatory cytokine production in innate immune cells. J Immunol. 2013;191:1164–1174. doi: 10.4049/jimmunol.1203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Role of Janus Kinase 3 in Predisposition to Obesity associated Metabolic Syndrome. J Biol Chem. 2015;290:29301–29312. doi: 10.1074/jbc.M115.670331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 28.Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology. 2011;140:231–241. doi: 10.1053/j.gastro.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):i111–i115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franchi L, Kamada N, Nakamura Y, Burberry A, Kuffa P, Suzuki S, Shaw MH, Kim YG, Nunez G. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13:449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedl M, Abraham C. Nod2-induced autocrine interleukin-1 alters signaling by ERK and p38 to differentially regulate secretion of inflammatory cytokines. Gastroenterology. 2012;143:1530–1543. doi: 10.1053/j.gastro.2012.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai L, Lidie KB, Chen Q, Adelsberger JW, Zheng X, Huang D, Yang J, Lempicki RA, Rehman T, Dewar RL, Wang Y, Hornung RL, Canizales KA, Lockett SJ, Lane HC, Imamichi T. IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation. J Exp Med. 2013;210:517–534. doi: 10.1084/jem.20120572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guzzo C, Ayer A, Basta S, Banfield BW, Gee K. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J Immunol. 2012;188:864–873. doi: 10.4049/jimmunol.1101912. [DOI] [PubMed] [Google Scholar]
- 34.Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, Chan GL, Krishnaswami S, Prakash C. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos. 2014;42:759–773. doi: 10.1124/dmd.113.054940. [DOI] [PubMed] [Google Scholar]
- 35.Krishnaswami S, Chow V, Boy M, Wang C, Chan G. Pharmacokinetics of tofacitinib, a janus kinase inhibitor, in patients with impaired renal function and end-stage renal disease. J Clin Pharmacol. 2014;54:46–52. doi: 10.1002/jcph.178. [DOI] [PubMed] [Google Scholar]
- 36.Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S, Vaddi K, Levy R, Tefferi A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis. J Biol Chem. 2013;288:31795–31806. doi: 10.1074/jbc.M113.504126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis E, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Tasset A, Van der Aa A, Harrison P. Filgotinib (GLPG0634), an Oral JAK1 Selective Inhibitor, Induces Clinical Remission in Patients With Moderate-to-Severe Crohn’s Disease: Results From the Phase 2 FITZROY Study Interim Analysis. Gastroenterology. 2016;150:S1267. [Google Scholar]
- 39.Ulrich CM, Yasui Y, Storb R, Schubert MM, Wagner JL, Bigler J, Ariail KS, Keener CL, Li S, Liu H, Farin FM, Potter JD. Pharmacogenetics of methotrexate: toxicity among marrow transplantation patients varies with the methylenetetrahydrofolate reductase C677T polymorphism. Blood. 2001;98:231–234. doi: 10.1182/blood.v98.1.231. [DOI] [PubMed] [Google Scholar]
- 40.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 41.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–795. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.