Abstract
Background
The forehead is a common site for injection of botulinum neurotoxin type A (BoNT-A) to treat hyperactive facial muscles. Unexpected side effects of BoNT-A injection may occur because the anatomy of the forehead musculature is not fully characterized.
Objectives
The authors described the lateral border of the frontalis in terms of facial landmarks and reference lines to determine the safest and most effective forehead injection sites for BoNT-A.
Methods
The hemifaces of 49 embalmed adult Korean cadavers were dissected in a morphometric analysis of the frontalis. L2 was defined in terms of FT (the most protruding point of the frontotemporal region), L0 (the line connecting the infraorbital margin with the tragus), and L1 (the line parallel to L0 and passing through FT) such that L2 was positioned 45° from L1 and passed through FT.
Results
The distance from FT to the superior margin of the orbicularis oculi was 12.3 ± 3.3 mm. The frontalis extended more than 5 cm along L2 in 49 of 49 cases (100%), more than 6 cm in 47 cases (95.9%), more than 7 cm in 34 cases (69.4%), more than 8 cm in 11 cases (22.4%), and more than 9 cm in 3 cases (6.1%). The lateral border of the frontalis ran parallel to and within 1 cm of the medial side of L2.
Conclusions
Surface anatomy mapping can assist with predicting the lateral border of the frontalis to minimize the side effects and maximize the efficiency of BoNT-A injections into the forehead.
Injection of botulinum neurotoxin type A (BoNT-A) is a popular treatment for chronic migraine and to decrease hyperactivity of the facial muscles that produce facial lines over time. BoNT-A relieves pain associated with various headache disorders and may be administered as prophylaxis for episodic migraine, chronic daily headache, tension-type headache, or chronic migraine.1
The safety and efficacy of BoNT-A for the treatment of lines at the forehead and glabella have been demonstrated in several studies.2-4 Steinsapir et al5 described the periocular administration of BoNT-A as microdroplets to treat brow and glabella lines and crow's feet without producing forehead paralysis. However, the anatomy of the forehead musculature remains poorly understood. Physicians performing BoNT-A injections into the forehead typically rely on subjective clinical experience instead of objective anatomic data. Injections may be guided according to landmarks such as the orbital rim and eyebrow. Ahn et al6 suggested targeting 5 equally spaced points along a horizontal line 2 cm above the orbital rim. Cho et al7 proposed 6 injection sites for the frontalis with medial injection sites positioned 1.5 cm above the medial end of the eyebrow and lateral injection sites positioned approximately 1.5 cm laterally and parallel to the medial injection sites. Lorenc et al8 suggested that injection sites should be located 1 to 2 cm above the orbital rim.
The occipitofrontalis comprises an anterior frontal belly and a posterior occipital belly that are joined by a layer of dense fibrous tissue called the galea aponeurotica. The medial, middle, and lateral muscle fibers of the frontal belly (ie, the frontalis) are attached to the procerus, the orbicularis oculi and corrugator supercilii, and the orbicularis oculi, respectively. Contraction of the frontalis raises the eyebrows and the upper eyelid. Over time, these actions produce horizontal forehead lines. The medial border of the frontalis and the depth of the frontalis in the glabella have been described in cadaveric studies.9,10 However, injection sites at the lateral border of the frontalis muscles have not been determined. Injection of BoNT-A into the medial and middle parts of the frontalis without addressing the lateral parts creates an imbalanced state of muscle contraction and relaxation that is visible as drooping of the inner eyebrows and raising of the outer eyebrows (ie, the Mephisto sign or Samurai face).7
We conducted a cadaveric study to better characterize the anatomy of the lateral borders of the frontalis and in turn improve the delivery of BoNT-A into this region. We suggest that precise mapping of reference lines along the forehead will avoid unexpected side effects of BoNT-A injection at this site and will produce safer and more effective outcomes.
METHODS
From March 2013 to June 2014, the hemifaces of 49 embalmed Korean cadavers, donated legally to the Yonsei Medical Center (Seoul, Korea), were dissected in a morphometric analysis of the frontalis. All cadavers had intact faces without signs of trauma or surgery. After removing the skin and subcutaneous tissues covering the forehead, the cadavers were carefully dissected to preserve the lateral fibers of the frontalis. The following landmarks and reference lines then were identified: (1) FT, the most protruding point of the frontotemporal region; (2) L0, the line connecting the infraorbital margin with the tragus; (3) L1, the line parallel to L0 and passing through FT; and (4) L2, the line positioned 45° from L1 and passing through FT (Figure 1).
Figure 1.
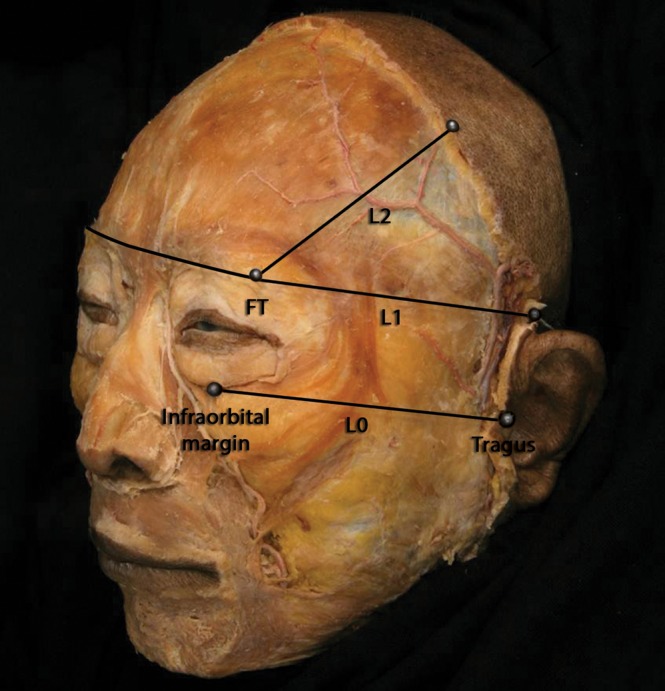
Reference points and lines applied for a morphometric analysis of the frontalis in this 72-year-old male cadaver. FT, the most protruding point of the frontotemporal region; L0, the line connecting the inferior margin of the orbit with the tragus; L1, the line parallel to L0 and passing through FT; L2, the line positioned 45° from L1 and passing through FT.
Landmarks on the dissected specimens were fixed with a pin and thread. L0 was fixed first followed by L1 and then L2. Points were marked on L2 at 1-cm intervals from FT. Intervals from 0 to 1, 1 to 2, 2 to 3, 3 to 4, 4 to 5, 5 to 6, 6 to 7, 7 to 8, and 8 to 9 cm from FT were referred to as sections 1 to 9, respectively (Figure 2). This reference system facilitated several measurements. First, the superior boundary of the orbicularis oculi at the lateral border of the frontalis was characterized given that the orbicularis oculi overlaps the frontalis in the superior orbital region. The minimum distance between FT and this boundary was determined to predict injection sites that would target only the frontalis. Second, the distance along L2 from FT to the musculoaponeurotic junction of the lateral border of the frontalis with the galea aponeurotica was determined. Third, the minimum distance from L2 to the lateral border of the frontalis was ascertained by assigning lengths on the medial side of L2 positive values and assigning lengths on the lateral side of L2 negative values. All measurements were made by means of a digital caliper (CD-15CP, Mitutoyo, Kawasaki, Japan) with a resolution of 0.01 mm. Except where stated otherwise, data were presented as means ± standard deviations (SD). The threshold for statistical significance was set at P < .05.
Figure 2.
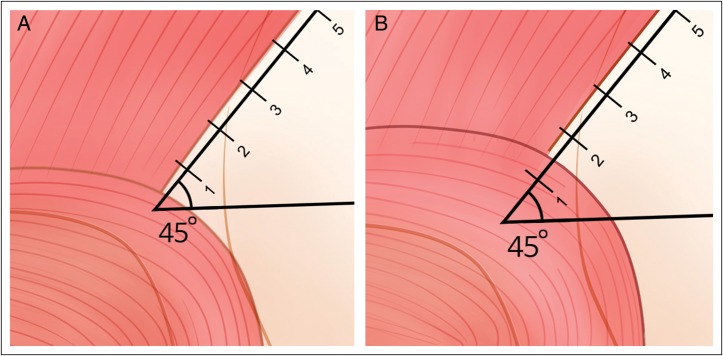
The region in which the orbicularis oculi overlapped the frontalis occurred in (A) section 1 in 15 of 49 cases (30.6%) and in (B) section 2 in 34 cases (69.4%).
The purpose of this study was to provide a guideline for identifying the lateral border of the frontalis on the surface of the patient's face. In other words, this study is not to verify the effectiveness of BoNT-A injection sites during clinical practice but rather to identify accurate injection sites in accordance with cadaveric studies. Therefore, we did not get IRB approval for this study.
RESULTS
The hemifaces of 49 embalmed Korean cadavers (33 males, 16 females; age range, 46-92 years; mean age, 69.71 years) were dissected.
The Boundary of the Orbicularis Oculi at the Frontalis
The minimum distance from FT to the superior boundary of the orbicularis oculi along L2 was 12.3 ± 3.3 mm. This boundary was located in section 1 in 15 of 49 cases (30.6%) and in section 2 in 34 cases (69.4%; Figure 2).
The Musculoaponeurotic Junction of the Frontalis and the Galea Aponeurotica
The distance along L2 from FT to the musculoaponeurotic junction of the lateral frontalis with the galea aponeurotica extended at least to section 5 in 49 of 49 cases (100%). This distance extended to section 6 in 47 cases (95.9%), to section 7 in 34 cases (69.4%), to section 8 in 11 cases (22.4%), and to section 9 in 3 cases (6.1%; Figure 3; Table 1).
Figure 3.
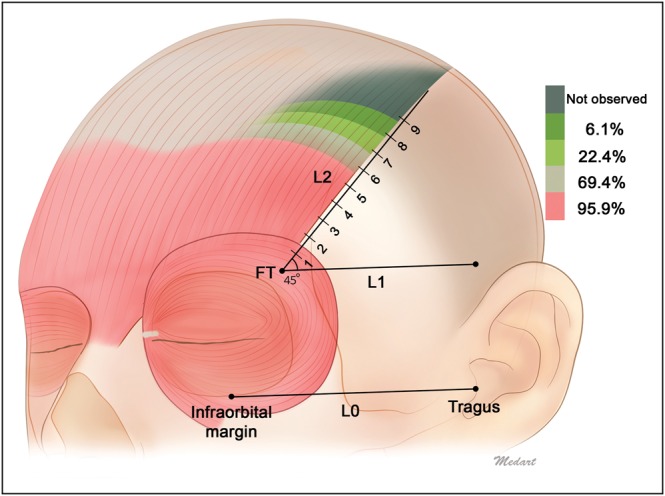
Distance from FT to the musculoaponeurotic junction of the lateral border of the frontalis with the galea aponeurotica. Shading indicates the different running patterns of the lateral border of the frontalis muscle. The lateral border of the frontalis extended at least to section 5 in 49 of 49 cases (100%), to section 6 in 47 cases (95.9%), to section 7 in 34 cases (69.4%), to section 8 in 11 cases (22.4%), and to section 9 in 3 cases (6.1%).
Table 1.
Lateral Extensions of the Frontalis
| Section Intervala | 2-5 | 2-6 | 2-7 | 2-8 | 2-9 | 2-10 |
|---|---|---|---|---|---|---|
| No. (%) of cases | 49 (100%) | 47 (95.9%) | 34 (69.4%) | 11 (22.4%) | 3 (6.1%) | 0 (0%) |
Injections of botulinum neurotoxin type A into sections 2 and beyond avoid the region where the orbicularis oculi overlaps the frontalis. aSections 1 to 9 were defined as distances of 0 to 1, 1 to 2, 2 to 3, 3 to 4, 4 to 5, 5 to 6, 6 to 7, 7 to 8, and 8 to 9 cm from FT along L2, respectively.
The Minimum Distance Between the Lateral Border of the Frontalis and L2
The mean minimum distances from L2 to the lateral border of the frontalis at each section are presented in Table 2. The mean distance for each case was positive, indicating that the lateral border of the frontalis was located on the medial side of L2 in most cases. In addition, the SD associated with each mean was <10 mm. These results indicate that the lateral border of the frontalis runs parallel and within 1 cm of L2. There were no significant differences between the left and right lateral margins of the frontalis regarding the distance from L2 (P > .05; data not shown). No significant difference between genders was observed in this study population, but the mean minimum distance tended to be greater among females.
Table 2.
Mean Minimum Distances from L2 to the Lateral Border of the Frontalis
| Sectiona | Mean (± SD) Distance, All Cases, mm | Mean (± SD) Distance, Male Cases, mm | Mean (± SD) Distance, Female Cases, mm |
|---|---|---|---|
| 1 | 5.6 (± 3.6) | 2.7 (± 3.1) | 7.6 (± 2.6) |
| 2 | 3.7 (± 3.6) | 2.7 (± 3.3) | 5.7 (± 3.6) |
| 3 | 3.9 (± 3.9) | 2.8 (± 3.8) | 6.3 (± 2.9) |
| 4 | 3.3 (± 3.7) | 2.3 (± 3.6) | 5.2 (± 3.2) |
| 5 | 3.5 (± 4.0) | 2.7 (± 4.1) | 5.2 (± 3.3) |
| 6 | 4.1 (± 5.8) | 2.9 (± 5.3) | 6.6 (± 6.2) |
| 7 | 4.3 (± 5.3) | 3.6 (± 5.7) | 6.3 (± 3.4) |
| 8 | 7.9 (± 4.0) | 7.6 (± 4.3) | 8.5 (± 3.7) |
| 9 | 6.6 (± 7.9) | 2.2 (± 3.1) | 15.4b |
Positive and negative values were assigned to measurements made where the lateral border of the frontalis was located medially and laterally to L2, respectively. L2 is defined in terms of FT (the most protruding point of the frontotemporal region), L0 (the line connecting the infraorbital margin with the tragus), and L1 (the line parallel to L0 and passing through FT) such that L2 is positioned 45° from L1 and passes through FT. SD, standard deviation. aSections 1 to 9 were defined as distances of 0 to 1, 1 to 2, 2 to 3, 3 to 4, 4 to 5, 5 to 6, 6 to 7, 7 to 8, and 8 to 9 cm from FT along L2, respectively. bIt was not possible to calculate an SD for this measurement because it corresponded to only 1 sample.
DISCUSSION
Most skeletal muscles originate from and insert into bony structures and can be visualized in terms of location and gross morphology on the outer surface of the body. In contrast, the borders and locations of facial muscles are difficult to demarcate from the surface anatomy because their insertions and origins are close to the subcutaneous layer and skin. For these reasons, designating the location and length of the frontalis is challenging.
Before BoNT-A injection, the borders of target muscles must be identified because injection of BoNT-A into unintended muscles or incomplete injection into the target muscle could produce unwanted side effects, such as asymmetric or otherwise altered contractility. Incorrectly placed injection also reduces the effectiveness of BoNT-A on the target muscle. Moreover, the biological effectiveness of BoNT-A is greatest when the preparation is distributed in small aliquots throughout the muscle rather than as a single dose.11 Thus, BoNT-A injection into the forehead should span the frontalis rather than being focused at the points described in previous studies.6-8
We conducted a morphometric analysis of fixed cadavers to map the precise position of the frontalis. We considered a study of chemically fixed hemifaces to be optimal for high-resolution structural assessments because fixed muscles are not subject to the laws of physics in the same way as fresh specimens. However, a comparison of our results with those obtained with fresh cadavers is warranted. The reference lines we described enable treatment over a defined injection area based on surface anatomy. Reference L0 passes through the infraorbital margin and tragus, which are easily identifiable and palpable structures on the surface of the face. Similarly, FT is a palpable landmark located superolaterally to the orbit where it separates into temporal and frontal components. From FT and L0, L1 and L2 can readily be determined, and the margins of the frontalis can subsequently be predicted.
We found that the superior border of the orbicularis oculi where it overlaps with the frontalis was located 12.3 ± 3.3 mm from FT. Therefore, the orbicularis oculi is positioned 0 to 2 cm from FT along L2. Accordingly, we advocate that clinicians performing BoNT-A treatment of the frontalis should position the injection at least 2 cm from FT along L2. Second, we measured the distance from FT to the musculoaponeurotic junction of the lateral border of the frontalis with the galea aponeurotica. Our findings indicate that injections targeting the frontalis should be performed in sections 2 to 5 or sections 2 to 6 along L2 (Figure 3; Table 1). In the majority of cases, the lateral border of the frontalis occurred parallel to and within 1 cm of the medial side of L2. In 9 of 49 cases (18.4%), the lateral border of the frontalis occurred on the lateral side of L2. However, the greatest distance between L2 and the frontalis was 0.9 cm for these cases (ie, within 1 cm).
Based on the findings of the present study, we suggest several steps for BoNT-A injection into the forehead area (Figure 4). First, locate the infraorbital margin, tragus, and FT by palpation. Second, draw a line connecting the infraorbital margin and tragus (ie, L0) and a parallel line passing through FT (ie, L1). Third, draw an oblique line from FT at an angle of 45° from L1 (ie, L2). With this facial map, the frontalis can be targeted with injections at 1-cm intervals within sections 2 to 6 along L2. This approach avoids sections 0 to 2, which span the overlap of the orbicularis oculi and frontalis. The lateral border of the frontalis occurs predominantly in sections 0 to 6, and most of the lateral border of the frontalis runs within 1 cm medial to L2.
Figure 4.
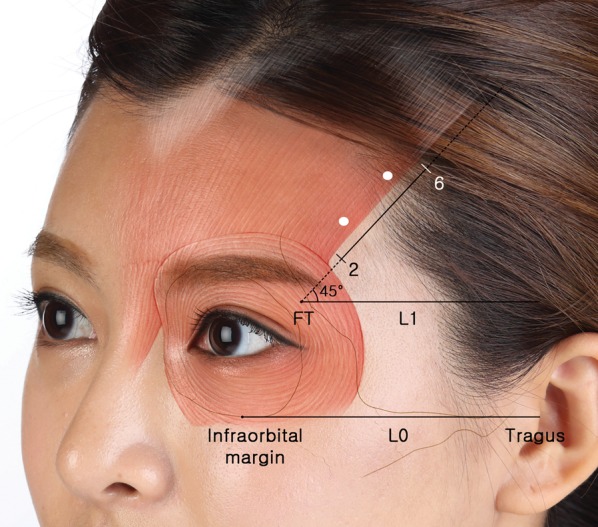
The forehead musculature and reference map described in this study are superimposed on a photograph of this 24-year-old woman. It is difficult to definitively locate the lateral border of the frontalis in a clinical setting. However, the reference points (infraorbital margin, tragus, and FT) and lines (L0, L1, and L2) described in this study may be helpful for identifying the lateral border of the frontalis on the skin prior to injection of botulinum neurotoxin type A into the forehead.
There were no significant differences in minimum distances from L2 to the lateral frontalis between the left and right sides of the head. However, we observed a nonsignificant tendency of female cases to exhibit a greater minimum distance. In a cadaveric study, Spiegel et al9 reported gender differences in the location and running aspect of the medial border of the frontalis. These authors noted that the angle created by the bilateral medial borders of the frontalis was 62° in males and 38° in females; that is, the frontalis ran more vertically in females than in males.9 The present findings suggest that this may also be the case for the lateral border. The study by Spiegel et al9 was conducted with Caucasian cadavers and addressed the medial borders of the frontalis. The present study was conducted with Asian cadavers and addressed the lateral borders of the frontalis. Therefore, direct comparisons of these studies are limited. A subsequent study of the medial borders of the frontalis in Asian cadavers is planned. We expect that the results of this study will enable a comparison of this facial muscle in Asians and Caucasians.
CONCLUSIONS
The infraorbital margin, tragus, and FT can be readily observed in a clinical setting before injection of BoNT-A. L2 follows from these reference points and facilitates the identification of the lateral border of the frontalis on the skin. These findings may be helpful for minimizing the side effects and maximizing the efficiency of BoNT-A injection into the forehead.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Acknowledgments
The authors thank Sang-Hoon Kwon from the College of Arts and Sciences, New York University, and Hwi Eun Hur from Davidson College for their assistance with our discussion of anatomic procedures and with revision of this manuscript.
REFERENCES
- 1.Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406-1418. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel- group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003;29:461-467. [DOI] [PubMed] [Google Scholar]
- 3.Carruthers JA, Lowe NJ, Menter MA et al. . A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46:840-849. [DOI] [PubMed] [Google Scholar]
- 4.Carruthers JD, Glogau RG, Blitzer A, Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies--consensus recommendations. Plast Reconstr Surg. 2008;121:5S-30S. [DOI] [PubMed] [Google Scholar]
- 5.Steinsapir KD, Rootman D, Wulc A, Hwang C. Cosmetic microdroplet botulinum toxin A forehead lift: a new treatment paradigm. Ophthal Plast Reconstr Surg. 2015;31:263-268. [DOI] [PubMed] [Google Scholar]
- 6.Ahn BK, Kim YS, Kim HJ, Rho NK, Kim HS. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39:1843-1860. [DOI] [PubMed] [Google Scholar]
- 7.Cho ES, Hwang JY, Kim ST. A proposal to prevent the “mephisto sign” side effect of botulinum toxin type a injection in chronic migraine. Yonsei Med J. 2013;54:1542-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal aesthetic botulinum toxin type A therapy. Aesthetic Plast Surg. 2013;37:975-983. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel JH, Goerig RC, Lufler RS, Hoagland TM. Frontalis midline dehiscence: an anatomical study and discussion of clinical relevance. J Plast Reconstr Aesthet Surg. 2009;62:950-954. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald MR, Spiegel JH, Raven RB, Kabaker SS, Maas CS. An anatomical approach to glabellar rhytids. Arch Otolaryngol Head Neck Surg. 1998;124:1315-1320. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z. Diffusion, spread, and migration of botulinum toxin. Mov Disord. 2013;28:1775-1783. [DOI] [PubMed] [Google Scholar]