Abstract
Background
Adipose tissue transplantation has the benefit of providing both regenerative and aesthetic outcomes in breast cancer treatment. However, the transplanted tissue can stimulate the growth of residual cancer cells.
Objectives
The aim of this study is to identify the interactions between adipose tissue cell subpopulations and human cancer cell lines.
Methods
Intact adipose tissue from lipofilling procedures as well as fibroblasts derived from adipose tissue, were cocultured in the presence of MDA-MB-231, MCF-7 e ZR-75-1 breast cancer cell lines. The influence on cancer cell lines of fibroblasts, induced to differentiate into specific adipocytes, was also assayed.
Results
All cancer cell lines displayed a significant increase in proliferation rate when cocultured in the presence of either intact adipose tissue or induced adipocytes. To a lesser extent, uninduced fibroblasts stimulate breast cancer cell proliferation.
Conclusions
Recent studies have shown that the microenvironment surrounding breast cancer cells may stimulate growth and promote progression of residual cancer cells when surgery is performed on the main tumor mass. Accordingly, the graft of adipose tissue could potentially promote or accelerate the development of a subclinical tumor or support its locoregional recurrence. Our data suggest that adipocytes have a remarkable influence on the proliferation of cancer cell lines. The oncological safety of the lipofilling procedure outcome is still debated; thus, further studies and consistent follow-up examination are needed.
In breast surgery, adipose tissue transplant is applied in aesthetic tissue augmentation, in congenital defects, in the improvement of breast profile defects after conservative surgery, in the treatment of radiotherapy sequelae, in implant coverage after postmastectomy reconstructive surgery, and to reduce the number of more invasive reconstructive procedures such as myocutaneous flaps.1,2 As a result of adipose tissue's well-known regenerative potential and capability to improve tissue quality, adipose tissue transplants are applied in oncologic breast surgery, because stem cells and mesenchymal progenitors (human adipose-derived stem cells) release proangiogenic factors.3 Because adipose tissue essentially consists of adipocytes and stromal fibroblasts, is metabolically active, and secretes several cytokines and growth factors, it does not simply behave as an inert filler and could ultimately influence the microenvironment and recurrence of cancer.4-6 Recently the concept of a potential role of the microenvironment of cancer surrounding the epithelial tumors in the promotion of tumor cell growth has been introduced.7-11 Thus, transplanted adipocytes and/or stromal fibroblasts may contribute to accelerating tumor growth in vivo.
Recent studies have investigated the influence of adipose stem cells and mature adipocytes on the proliferation of residual cancer cells in the tumor bed.12 Any procedure that may transfer these cell types into the lesion site could potentially stimulate residual cells in the tumor bed, thus enhancing the risk of cancer recurrence. However, no clinical studies have currently shown significant results supporting the role of an autologous adipose tissue transplant in cancer relapse, and the biological mechanism remains unclear.
Studies conducted with the murine preadipocytes line 3T3 - L1 have shown that these cells can promote the growth and survival of breast cancer cell lines and that the conditioned medium derived from cultured adipocytes helps the invasiveness of tumor cells.12-14 Additionally, both fibroblasts and adipocytes have proved beneficial to the growth of the tumor, as well as their conditioned media. Furthermore, in vitro differentiated human breast preadipocytes increased the motility of tumor cells, although the mechanism of doing so has not been determined.13,14
Another study utilized murine 3T3 - F442A preadipocytes cocultured in a transwell system with human and murine breast cancer cell lines: in both cases, an increase in tumor invasiveness was observed, leading to the assumption that the factors released by preadipocytes may cause this effect.15 Moreover, studies on the proliferative capability of mesenchymal fat stem cells conclude that these cells do not possess telomerase activity, typical of cancer cells behavior, and that therefore they do not display any aptitude for spontaneous transformation.16-18
In this study, we have isolated two subpopulations of cells from lipoaspirates: adipose-derived mesenchymal stem cell components and progenitors, and mature adipocytes, to provide the basis for to better comprehend the possible supportive role of the stroma in breast cancer relapse during reconstructive surgery.
METHODS
Patients, Primary Cells, and Cell Lines
The study protocol was evaluated and approved by the Institutional Ethics Committee of the IRCCS-AOU San Martino-IST in Genoa, Italy. The population of the study (7 cases) was recruited over a 5-month period from October 2014 to February 2015. The data were collected anonymously and included features of the patient (age, gender, medical history), aesthetic defects, characteristics of the tumor in oncologic patients (histology, receptor status). A power analysis was not performed. We utilized a limited set of samples, and the results that we obtained were very consistent. For every sample, we performed three different experiments in duplicate. The selection criteria were gender (only female), had previous oncologic surgery for breast cancer (mastectomy/conservative surgery) or degenerative diseases, and had no comorbidities. All patients provided their written informed consent before their enrollment in the study.
Adipose tissue samples (10 mL) were obtained as by-products of liposuction procedures harvested from elective donor sites such as the internal regions of the knees, the hips, and the gluteus. A 3-mm Coleman cannula connected to a 10-mL Luer Lock syringe was utilized, after we utilized Klein solution infiltration (lidocaine 30 mL, epinephrine 1 mg/mL, 1:1000; lactated Ringer's 1 L) and aspiration was carried out manually. Lipoaspirate samples were processed no later than 12 hours after harvest.
Adipose tissue samples were rinsed twice with phosphate buffer saline solution (PBS, Sigma), centrifuged at 1000 rpm for 1 minute to eliminate residual Klein's solution; the aqueous phase was discarded, and the adipose tissue was digested with Collagenase I (400 U/mL; Biochrom cat. CI-22) for 60 minutes at 37°C with constant agitation. Samples were then centrifuged at 1200 rpm for 5 minutes, supernatants were discarded, and pellets, containing stromal fibroblasts, were seeded in Coon's-F12 medium (Biochrom, cat. F9149) supplemented with 10% fetal bovine serum (FBS, Euroclone, cat ECS0180L) and 1% Glutamine (Gibco cat. 25030-024) in 100 mm Petri dishes. Every other day, nonadherent cells were removed and fresh medium was added to the culture dishes. When adherent cells reached 70% confluence, they were harvested through trypsinization, counted, and seeded in a 24-well plate at a concentration of 25,000 cells/well and subsequently induced to differentiate them into adipocytes by supplementation of the Adipogenic Induction Medium (Lonza, cat. PT-3102B) combined with human mesenchymal stem cell (hMSC) Adipogenic Induction Single Quote (Lonza, cat. PT-4135) for 3 weeks, according to the manufacturer's protocols.
Three breast cancer cell lines—ZR-75-1 (estrogen-responsive: ER+), MCF7 (ER+), and MDA-MB-231 (estrogen unresponsive: ER-)—were obtained from Interlab Cell Line Collection (ICLC, IRCCS-AOU San Martino-IST, Genoa, Italy) and maintained in RPMI 1640 medium (Euroclone, cat. ECB9006L) supplemented with 10% fetal bovine serum (FBS, Euroclone, cat. ECS0180L) and 1% Glutamine (Gibco, cat. 25030-024).
Coculture Experiments
We investigated the effects of unfractionated adipose tissue, and induced and non-induced fibroblasts, on the proliferation of breast cancer cells. Therefore all experiments were performed considering a paracrine effect of adipose-derived cells on breast cancer cell lines. With this purpose in mind, we utilized a Transwell system, which provides a contemporary cultivation but avoids any physical contact between the two cell populations, thus illustrating the effects of secreted soluble factors.
Cocultures of breast cancer cell lines and unfractionated adipose tissues of seven samples were performed. Breast cancer cells were briefly seeded in a 24-well plate at the concentration of 5000 cells/well, and 0.5 mL of fresh adipose tissue were placed in the corresponding transwells. Cancer cell growth was evaluated by the AlamarBlue assay (Invitrogen, cat. DAL1100) after 48 hours.
Either induced or noninduced adipose-derived fibroblasts were cocultured with each of the three breast cancer cell lines. Fibroblasts were briefly seeded in a 24-well plate at the concentration 25,000 cells/well. To induce fibroblasts to differentiate into adipocytes, we utilized the Adipogenic Induction Medium (Lonza, cat. PT-3102B) combined with the hMSC Adipogenic Induction Single Quote (Lonza, cat. PT-4135). At the end of the differentiation protocol, 5000 breast cancer cells were seeded in each transwell and placed over the wells containing induced fibroblasts. Cancer cell proliferation was evaluated through the AlamarBlue assay at 48 hours after seeding. This method permits the evaluation of cell proliferation in the course of time on the same sample without causing cell death, thus decreasing variations among different samples. All samples were tested in duplicate, and each experiment was repeated three times.
Cell-specific Response Test
To evaluate whether the increase in proliferation was specifically caused by adipocytes and adipose tissue–derived cells or it occurred with other cell types, all breast cancer cell lines were cocultured in the presence of two different strains of human bone marrow stromal cells (hBMSC; Lonza) using the same Transwell system. Cancer cell proliferation was measured at 48 hours through the AlamarBlue assay. All samples were tested in duplicate, and each experiment was repeated three times.
Statistical Analysis
In order to evaluate the statistical significance of our results, we performed unpaired t test analysis with 95% CI, using the PRISM software 3.0cx version (Mac version). This test permits the comparison of the mean values of experiments. A P value was considered significant when >.05.
RESULTS
Tissue Samples, Primary Cells, and Adipogenic Induction
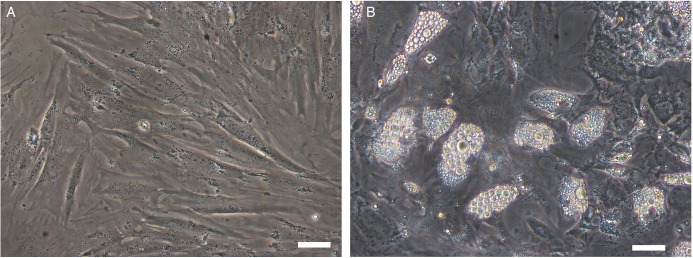
The mean age of the patients recruited, all of whom were women, was 49.2 years (range, 39-54 years). After 7 days in culture, lipoaspirate-derived fibroblasts reached an average value of 0.45 ± 0.19×106 cells per each mL of lipoaspirate utilized. Considering an approximated doubling time of 24 hours, we estimated a frequency of 6×103 fibroblast-like cells in the original samples. The cells displayed the typical fibroblast-like phenotype (Figure 1A), and a pre-adipocyte phenotype with lipid droplets filling the cytoplasm when they were induced to differentiation (Figure 1B).
Figure 1.
(A) Stromal vascular fraction display in culture a rather homogeneous phenotype with most of the cells displaying an elongated, bipolar, fibroblast-like phenotype. (B) After 18 days of stimulation in adipogenic conditions, an important percentage of stromal vascular fraction evidenced a cytoplasmic accumulation of lipid droplets suggesting the shift vs an adipogenic phenotype. Bar = 5 µm.
Cocultures of Adipocytes and Breast Cancer Cells
In the coculture system, an average increase in the proliferation rate of 2.31 folds in the three breast cancer cell lines was observed when the lines were cocultured in the presence of undigested adipose tissue compared with control breast cancer cell lines alone (Figure 2). The increase in the proliferation rate was statistically significant in all breast cancer cell lines (P values: MDA-MB-231 = .0053; MCF7 = .0116; ZR-75-1 = .0017).
Figure 2.
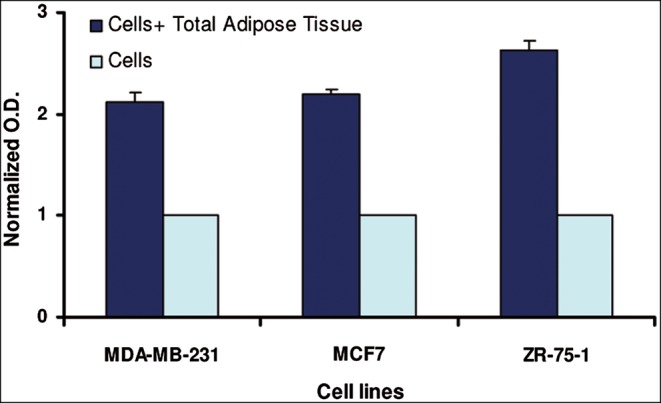
The proliferation of breast cancer cell lines when cocultured for 48 hours in the presence of unprocessed lipoaspirates vs breast cancer cell lines alone (P values: MDA-MB-231 = .0053; MCF7 = .0116; ZR-75-1 = .0017). Each bar represents the average of three separate experiments performed in duplicate on seven different samples.
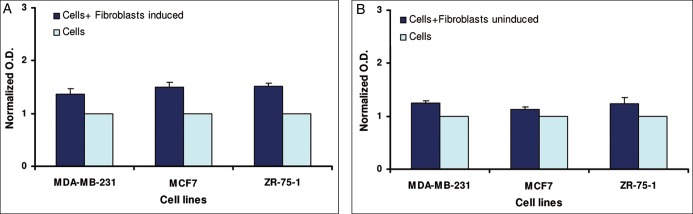
Induced fibroblasts significantly stimulated the proliferation of breast cancer cells, but to a lesser extent regarding total adipose tissue, with an average increase of approximately 1.46 folds. (Figure 3A). The increase in the proliferation rate in the presence of induced fibroblasts was statistically significant in all breast cancer cell lines (P values: MDA-MB-231 = .0062; MCF7 = .0109; ZR-75-1 = .0035). Cocultures with noninduced fibroblasts displayed an average increase in proliferation rate of 1.21 (P values: MDA-MB-231 = .0115; MCF7 = .0449; ZR-75-1 = .0194) (Figure 3B).
Figure 3.
The proliferation of breast cancer cell lines when cocultured for 48 hours in the presence of: (A) induced adipocytes vs breast cancer cell lines alone; (B) noninduced adipocytes vs breast cancer cell lines alone. Each bar represents the average of three separate experiments performed in duplicate on seven different samples. (P values: (A) MDA-MB-231 = .0062; MCF7 = .0109; ZR-75-1 = .0035; (B) MDA-MB-231 = .0115; MCF7 = .0449; ZR-75-1 = .0194).
Furthermore, to evaluate the specificity of fat tissue–derived cell stimulation, we utilized hBMSC as a source of fibroblasts unrelated to tissue. In this case, we did not observe any significant variation in the proliferation of breast cancer cell lines (Figure 4).
Figure 4.
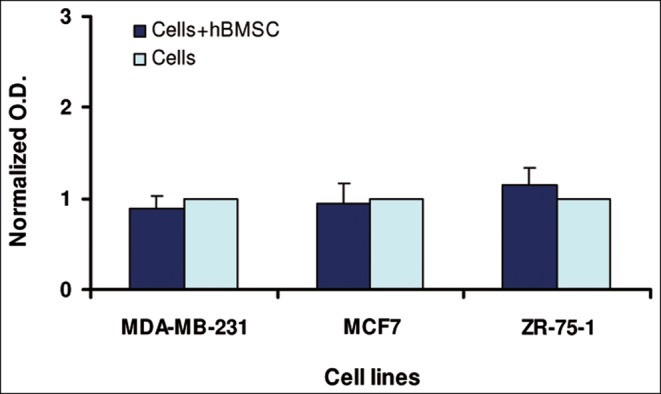
The proliferation rate of breast cancer cell lines cocultured in the presence of BMSC or alone at 48 hours. Each bar represents the average of two separate experiments performed in duplicate on seven different samples.
DISCUSSION
Adipocytes constitute most of the fat tissue, but there are also other cell types including fibroblasts and preadipocytes at a different maturation stage. In this study, considering that the same pattern of cell populations is also represented in lipoaspirate samples, we wanted to identify which cell component would hold a significant effect in influencing the proliferation of breast cancer cells.
It is well known that the different components of fat tissue secrete a significant variety of signaling molecules.19-21 our study was aimed at identifying interactions between adipose tissue cell subpopulations and human cancer cell lines. The study was conducted only on human samples in all of our experiments, trying to reproduce the interactions operating on humans, whereas most previous studies reported in the literature analyzed adipocytes on animal models.22-24 Moreover, a subpopulation analysis was performed to identify which cellular component in lipoaspirates could be responsible for the eventual adverse effects.
We initially verified the influence of total unfractionated lipoaspirate on the proliferation of cancer cells. To this end, we cocultured unprocessed lipoaspirates with three different breast cancer cell lines, and we observed that the proliferation rate was enhanced, with an average value of 2.31 folds for all cell lines tested. This evidence is in agreement with the previous results of Dirat and coworkers25 reporting that cancer-associated adipocytes (CAA), as well as cancer associated fibroblasts (CAF), can promote tumor growth.26
To identify the influence of the different components of adipose tissue, we cocultured either uninduced fibroblasts or fibroblasts induced to adipocytes with breast cancer cells. Fibroblasts induced to adipocytes were able to enhance breast cancer cell proliferation on a significant 46% average on all cell lines utilized. Uninduced fibroblasts displayed a lower stimulation on cancer cell proliferation (21%). Unrelated fibroblasts, derived from bone marrow, did not significantly influence cancer cell proliferation, suggesting a specificity of the paracrine effect of adipose tissue cells. It is otherwise true that a potential limitation of our study is that the effects were observed in a controlled system in vitro, which might not completely reflect the microenvironment present in human organisms, even though it is simultaneously close to the real scenario. Moreover, the lack of power analysis on population recruitment could be a further limitation of our study.
Some authors demonstrated that not only breast cancer is influenced by adipocytes, but also skin cancer such as melanoma is affected, and it showed an increase in the proliferation of cells.27
However, from a clinical point of view, the oncological role of the transplanted adipose tissue in promoting local recurrence has not yet been clarified. Many authors have investigated the eventual increase in breast cancer occurrence and/or recurrence,28-31 but many studies have limitations in patients enrollment, follow-up time, and statistical significance.32,33 Petit et al demonstrated an increased risk of recurrence in intraepithelial neoplasia, this increased risk was not observed in other types of breast cancer.34 Riggio et al concluded that in stage 1 of the tumor, node, metastases (TNM) classification, the risk is low, and in stage 2, local failure is not significantly higher.6
International guidelines for the lipofilling procedure propose that when the risk of recurrence is higher (as in partial breast removal), fat grafting should be performed not sooner than 3-5 years after the surgery. Furthermore in patients with positive familial history or genetic alteration in BRCA1/2 genes, it should be mandatory that fat grafting be avoided.35-37
Future perspectives of our study could be represented by grafting procedures in animal models that take advantage of harvested fat tissue that is depleted of specific cell subpopulations, potentially promoting cancer proliferation.38
CONCLUSION
An increased proliferation of breast cancer cell lines has been observed in all types of cocultures, demonstrating that there is a significant effect related to adipose tissue presence. It is evident that clinicians should consider this evidence when programming an intervention for patients subjected to oncological treatments. Therefore, it is necessary to make a careful selection of patients who are suitable for the intervention and to wait an adequate amount of time between surgery and the first fat grafting treatment, especially after a conservative surgery. Finally, it is necessary to perform a careful follow-up to monitor the oncological safety.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Lab costs and salaries for Drs Massa and Repaci were partially supported by funds from the local government (Regione Liguria), Grant #PAR-FAS 2007-2013.
REFERENCES
- 1.Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthetic Plast Surg. 2009;335:706-715. [DOI] [PubMed] [Google Scholar]
- 2.Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;295:360-376. [DOI] [PubMed] [Google Scholar]
- 3.Rigotti G, Marchi A, Galiè M et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;1195:1409-1422; discussion 1423-1424. [DOI] [PubMed] [Google Scholar]
- 4.Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology . J Plast Reconstr Aesthet Surg. 2012;653:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YY, Lehuédé C, Laurent V et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;3242:142-151. [DOI] [PubMed] [Google Scholar]
- 6.Riggio E, Bordoni D, Nava MB. Oncologic surveillance of breast cancer patients after lipofilling. Aesthetic Plast Surg. 2013;374:728-735. [DOI] [PubMed] [Google Scholar]
- 7.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression . Nature. 2004;4327015:332-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmick NA, Chytil A, Plieth D et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;3035659:848-851. [DOI] [PubMed] [Google Scholar]
- 9.Orimo A, Gupta PB, Sgroi DC et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;1213:335-348. [DOI] [PubMed] [Google Scholar]
- 10.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;605:1254-1260. [PubMed] [Google Scholar]
- 11.Olumi AF, Grossfeld GD, Hayward SW et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;5919:5002-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;183110:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adib T, Henderson S, Perrett C et al. Predicting biomarkers for ovarian cancer using gene-expression microarrays. Br J Cancer. 2004;903:686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Exp Mol Pathol. 2012;923:312-317. [DOI] [PubMed] [Google Scholar]
- 15.Dirat B, Bochet L, Dabek M et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;717:2455-2465. [DOI] [PubMed] [Google Scholar]
- 16.Nava MB, Catanuto G, Pennati AE et al. Lack of activation of telomere maintenance mechanisms in human adipose stromal cells derived from fatty portion of lipoaspirates. Plast Reconstr Surg. 2015;1351:114e-123e. [DOI] [PubMed] [Google Scholar]
- 17.Ogura F, Wakao S, Kuroda Y et al. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;237:717-728. [DOI] [PubMed] [Google Scholar]
- 18.Zaman WS, Makpol S, Sathapan S, Chua KH. Long-term in vitro expansion of human adipose-derived stem cells showed low risk of tumourigenicity. J Tissue Eng Regen Med. 2014;81:67-76. [DOI] [PubMed] [Google Scholar]
- 19.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;2012:221-228. [DOI] [PubMed] [Google Scholar]
- 20.Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;52:103-110. [DOI] [PubMed] [Google Scholar]
- 21.Kinnaird T, Stabile E, Burnett MS et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;945:678-685. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Daquinag A, Traktuev DO et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;6912:5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Daquinag A, Amaya-Manzanares F et al. Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res. 2012;7220:5198-5208. [DOI] [PubMed] [Google Scholar]
- 24.Gale K, Rakha E, Ball G et al. A case controlled study of the oncological safety of fat grafting. Plast Reconstr Surg. 2015;135:1263-1275. [DOI] [PubMed] [Google Scholar]
- 25.Dirat B, Bochet L, Escourrou G et al. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45-52. [DOI] [PubMed] [Google Scholar]
- 26.Jotzu C, Alt E, Welte G et al. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Oncol (Dordr). 2011;341:55-67. [DOI] [PubMed] [Google Scholar]
- 27.Pallua N, Paul NE, Burghardt B et al. Adipose tissue increases the proliferation of melanoma cell lines in vitro . J Craniofac Surg. 2015;264:1403-1407. [DOI] [PubMed] [Google Scholar]
- 28.Agha RA, Fowler AJ, Herlin C et al. Use of autologous fat grafting for breast reconstruction: a systematic review with meta-analysis of oncological outcomes. J Plast Reconstr Aesthet Surg. 2015;682:143-161. [DOI] [PubMed] [Google Scholar]
- 29.Simorre M, Chaput B, Voglimacci Stephanopoli M et al. [Lipofilling in breast reconstruction: is there any population with higher risk of local recurrence? Literature systematic review]. Gynecol Obstet Fertil. 2015;434:309-318. [DOI] [PubMed] [Google Scholar]
- 30.Bielli A, Scioli MG, Gentile P et al. Adult adipose-derived stem cells and breast cancer: a controversial relationship. Springerplus. 2014;3:345-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweizer R, Tsuji W, Gorantla VS et al. The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem Cells Int. 2015;2015:120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigotti G, Marchi A, Stringhini P et al. Determining the oncological risk of autologous lipoaspirate grafting for post-mastectomy breast reconstruction. Aesthetic Plast Surg. 2010;344:475-480. [DOI] [PubMed] [Google Scholar]
- 33.Rietjens M, De Lorenzi F, Rossetto F et al. Safety of fat grafting in secondary breast reconstruction after cancer . J Plast Reconstr Aesthet Surg. 2011;644:477-483. [DOI] [PubMed] [Google Scholar]
- 34.Petit JY, Rietjens M, Botteri E et al. Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;246:1479-1484. [DOI] [PubMed] [Google Scholar]
- 35.Krumboeck A, Giovanoli P, Plock JA. Fat grafting and stem cell enhanced fat grafting to the breast under oncological aspects--recommendations for patient selection. Breast. 2013;225:579-584. [DOI] [PubMed] [Google Scholar]
- 36.SOFCPRE. Recommandations concernant les transferts de graisse dans le sein. 2012.
- 37.GutowskiKA; ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg. 2009;1241:272-280. [DOI] [PubMed] [Google Scholar]
- 38.Bertolini F, Petit JY, Kolonin MG. Stem cells from adipose tissue and breast cancer: hype, risks and hope. Br J Cancer. 2015;1123:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]