Abstract
Capsular contracture is a common sequelae of implant-based breast augmentation. Despite its prevalence, the etiology of capsular contracture remains controversial. Numerous studies have identified microbial biofilms on various implantable materials, including breast implants. Furthermore, biofilms have been implicated in subclinical infections associated with other surgical implants. In this review, we discuss microbial biofilms as a potential etiology of capsular contracture. The review also outlines the key diagnostic modalities available to identify the possible infectious agents found in biofilm, as well as available preventative and treatment measures.
Augmentation and reconstruction mammaplasty are among the most frequently performed cosmetic operations.1 One relatively common sequela of breast augmentation and reconstruction is capsular contracture (CC). CC involves tightening of the collagen capsule that forms around the breast implant, which can be painful and very often distorts the breast. CC remains the most common cause of breast surgery revision. Various studies, including prospective studies that have been done with a considerable degree of follow-up have indicated CC incidences ranging from 5% to 74% of breast reconstructive surgeries.2-12 Surgeons diagnose approximately 45,000 patients with CC annually.9,13 The etiology of CC is not completely understood. Capsule formation itself is known to be a normal response to foreign bodies, however contracture is not. CC formation is likely a multifactorial process and several putative culprits have been proposed. These include placement of incision site, hypertrophic scarring, overactive inflammatory response, and foreign body reaction from powdered gloves, dust, or silicone gel leakage.14,15
Biofilms are microbial communities that are attached to a surface, including living tissue, implants, and medical devices. Infections related to microbial biofilms represent a significant number of all microbial infections in humans. These infections are difficult to treat, and as a result they become persistent and chronic. There is substantial evidence showing a correlation between the presence of microbial biofilms on various medical implants and persistent inflammation of the surrounding tissue.16-19 It appears that microbial biofilms form on breast implants as well and might contribute to a chronic inflammatory response and thus formation of capsular fibrosis and subsequent contracture.20-25 Investigations of biofilms on mammary implants begun by studying CC.26-28 Virden et al20 were among the first to demonstrate a correlation between biofilms on silicone shells and risk of CC.20-24 Several additional studies have attempted to determine the pathophysiology and prognosis of biofilm-related CC, as well as potential prophylactic and therapeutic measures.
Here we review key studies that have investigated the relationship between microbial biofilms and CC of mammary implants. Much of the data presented in this review derive from basic science, preclinical, and small case studies. This review is not a systematic review, but a survey of available and pertinent studies regarding the subject matter. We have included a table summarizing the experimental design and level of evidence for many cited studies (Table 1).
Table 1.
Summary of Capsular Contracture and Biofilm Studies
| Study Topic | Author | Study Design (n) | Notes | Level of Evidence |
|---|---|---|---|---|
| Presence of Biofilms in Capsular contracture | Virden et al,20 | Case-control (40 patients, 55 implants) | Culture and diagnostic SEM 17 of 27 devices developed CC before 12 months; range 2 mo to 5 yr | 3 |
| Dobke et al,21 | Case-control (87 pts, 150 implants) | Culture only | 3 | |
| Pajkos et al, 200322 | Case-control (16 pts, 27 capsules) | Sonication and diagnostic SEM | 3 | |
| Schreml et al,23 | Case-control (45 pts) | Culture only | 3 | |
| Rieger et al,24 | Case series (13 pts, 22 implants) | Culture only (sonication) | 4 | |
| Rieger et al,24 | Case-control (84 pts, 121 implants) | Culture only (sonication) | 3 | |
| Animal Models of biofilm and Capsular contracture | Shah et al,42 | Animal Study (16 rabbits, 20 implants) | Experimental group inoculated with S epidermidis | 5 |
| Kossovsky et al,82 | Animal Study (10 guinea pigs, 20 implants) | Experimental group inoculated with S aureus, diagnostic SEM | 5 | |
| Tamboto et al,83 | Animal Study (6 pigs, 51 implants) | Experimental group inoculated with S epidermidis, diagnostic sonication and SEM | 5 | |
| Role of implant texture, biofilm formation, and CC | Wong et al,6 | Systematic Review (6 RCTs; 235 patients, 470 breasts total) | Smooth implants more likely to undergo CC at 1, 3, and 7 yr | 1 |
| Schreml et al,23 | Case-control (45 pts) | Culture only, no difference in biofilm formation between textures; no follow-up time recorded | 3 | |
| Stevens et al,100 | Sientra's prospective comparative study (2560 patients, 5109 implants) | Smooth implants more likely to undergo CC; 5 year study | 2 | |
| Spear et al,103 | Allergan Core study (715 patients ) | Smooth and textured implants had similar rates of capsular contracture over 10 year follow-up | 2 | |
| Namnoum et al,101 | Allergan Core, 410 and 410 Continued Access prospective comparative study (4412 patients, 8811 implants) | Smooth implants more likely to undergo CC; mean follow-up 37 months | 2 | |
| Jacombs et al,89 | Animal study (16 pigs, 121 implants) | Experimental groups inoculated with S epidermidis, Diagnostic sonication and SEM, no difference in biofilm formation or CC between textures; 16 weeks at explantation | 5 | |
| Liu et al,102 | Meta-Analysis (16 RCTs, 2 case-control studies; 4486 pts, 8867 implants) | Smooth implants more likely to undergo CC, follow-up range 1-5+ yr | 1 | |
| Role of Implant filler and CC | Schaub et al, 2010 | Systematic review (16 studies) | Unable to draw conclusions based on available data | 2 |
| El-Sheikh et al, 2008 | Meta-analysis (4 prospective studies, 8 retrospective) | Pooled odds ratio = 2.25 for silicone implants developing CC; average scientific quality score range 5-9/14 | 2-3 | |
| Prevention | Wixtrom et al,107 | Case series (32 patients, 63 samples) | 6 month follow-up; 22 pts with shield + positive cultures→no CC; 3 pts with shield + neg. cultures → CC (1 was primary augmentation) | 4 |
| Adams et al,110 | Case series (335 patients) | Mean follow-up 14 months; 1.8% overall CC rate | 4 |
MECHANISMS OF BIOFILM FORMATION
Most bacterial species found in nature exist in two different forms, a free-floating form (planktonic), and an attached form (biofilm). The biofilm life cycle begins with planktonic bacteria that can form biofilm upon adhering to a solid surface anywhere in nature including tissue or a foreign material in the host.16,18 The process takes place in three stages: attachment, maturation, and eventually dispersion (Figure 1).29 The initial stage begins with the reversible interactions of the bacterial cells with a surface. These interactions are then reinforced by host and tissue-specific adhesions and ultimately result in the irreversible attachment of the planktonic cells to a surface.29
Figure 1.
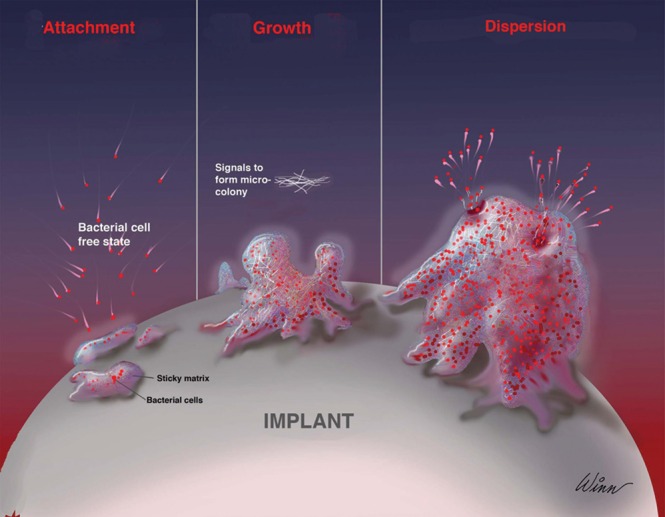
The three stages of the biofilm life cycle; attachment, maturation and dispersion. From Wixtrom et al.107 Reprinted with permission from Oxford University Press.
The next step of biofilm formation is maturation, which is defined by bacterial multiplication and the synthesis of extracellular polymeric substance (EPS).30 EPS consists of proteins, polysaccharides, lipids, and nucleic acids. EPS fulfills several functions. It anchors bacteria to a surface and to each other, provides storage of nutrients, and provides a protective barrier for biofilms.30-33 EPS also plays a role in biofilm-mediated antimicrobial resistance.33 As adherent bacteria divide and secrete EPS, they form a highly structured microcolony, which is anchored to the surface and other microcolonies.32
As the biofilm continues to grow and mature, it becomes highly differentiated and complex. Different bacterial structures form and are intermixed with channels allowing the exchange of nutrients and waste products.34 There are multiple microenvironments within biofilms that vary in pH, oxygen concentration, nutrient availability, and cell density. These microenvironments are characterized by a great deal of heterogeneity in metabolic activity among cells in different locations within the colony, making it difficult to target the entire biofilm with one type of therapy.35-38 For example, metabolically inactive cells within the biofilm colony may be resistant to antimicrobial agents that target actively growing cells, such as penicillin.35,36,39
Detachment of planktonic cells from a biofilm and their subsequent dispersal into the environment comprises the final stage of the biofilm life cycle. As with the other stages of biofilm growth, detachment involves a myriad of environmental signals, bacterial signal transduction pathways, and their effectors. Detachment of planktonic cells facilitates bacterial survival.40 Detachment also facilitates disease transmission.40,41
Biofilms are usually polymicrobial. Under certain conditions a few species may be overrepresented in the biofilm community. Staphylococcus epidermidis is a part of the microflora of the skin and the endogenous flora of the breast. It has been frequently identified on breast implants removed because of CC.20,22,23,42,43 Another common organism found on removed breast implants is Propionibacterium acnes, a commensal species of the skin and gut. These bacteria could gain access to implants at the time of surgery, particularly when surgeons use peri-nipple-areola or trans-nipple-areola approaches.44 Other bacteria implicated in the formation of biofilm on mammary implants include Staphylococcus aureus, and other Staphylococci, Streptococci, Bacillus species, Escherichia coli, Mycobacterium species, Corynebacterium, and Lactobacilli.22,23,45-48
Biofilms are highly resistant to antibiotics and multiple mechanisms contribute to this phenomenon. In addition to the already mentioned mechanisms of resistance, we highlight a few more here. Initiation of the biofilm mode of growth causes differential expression of numerous genes, including those involved in stress response, which allow biofilms to resist harmful conditions or chemicals, including antibiotics.49 Multidrug efflux transporters are upregulated in biofilms and contribute to decreased antibiotic effectiveness.50 The extracellular matrix of a biofilm provides several adaptive traits including accumulation of antibiotic-degrading enzymes such as β-lactamases which results in resistance to antibiotics such as penicillins, cephamycins, and carbapenems.51 Additional information regarding antibiotic resistance in biofilms can be found in several excellent review articles.52-54
INFLAMMATORY RESPONSES TO BIOFILMS
All medical implants, including breast implants, are susceptible to bacterial colonization and biofilm formation.18,55-57 Host response to implants can be divided into several phases: acute or chronic inflammation, foreign body reaction, and fibrous encapsulation.18,58
The immune host response consists of innate (immediate and short-lived) and adaptive immunity (long-lived). During the acute inflammatory phase, host cell damage triggers coagulation and plasminogen cascade which then activate the innate immune system.59 The presence of pathogens also triggers innate immune response through various cell pattern recognition receptors (PRRs). These not only detect molecules released from damaged host cells, but also microbe associated molecules.60 Activation of PRRs triggers production of cytokines and other inflammatory mediators that attract immune cells (such as neutrophils, dendritic cells, macrophages, and myofibroblasts) to the site of infection.18
Immune responses consist of multiple signaling pathways that are orchestrated by cytokines. Pro-inflammatory cytokines, such as interferons and interleukins, are released and activate their respective receptors to promote inflammatory responses. Upon killing and removal of pathogens and dead host cells, macrophages release anti-inflammatory cytokines which suppress inflammation and stimulate tissue remodeling, angiogenesis, and healing. Innate immunity activates adaptive immune response, which is highly specific, long lasting, and adaptable. During these processes, T cells, B cells, and plasma cells are predominant.61,62 The adaptive immunity works in conjunction with the innate immunity to achieve an effective overall immune response. Both are essential to combat infection.
Medical implant biofilms are shown to induce a prolonged inflammatory response as the host attempts to eliminate the biofilms.55,63-66 The host response contributes to the development of tissue destruction through continuous recruitment of pro-inflammatory cells such as macrophages and lymphocytes, release of inflammatory mediators, and proteases.67 While proteases aid in dislodging biofilms, they also damage normal and healing tissue. Finally, microphages may form a fibrous capsule around an implant.18
Different types of fibroblasts, including myofibroblasts, have a very important role in healing. Their number increases in CC.68,69 They are regulated by transforming growth factor beta-1 (TGF-β1) and mechanical stress, and are involved in wound repair.68 However, if present in the wound for too long, they cause excessive fibrosis and scaring through matrix deposition and proliferation of connective tissue.68 A recent study on patients with airway tracheal stenosis showed a correlation between bacterial biofilms and higher expression of TGF-β1 marker, which is consistent with myofibroblast activity and fibrosis.70 Myofibroblasts produce collagen (types I and III) and a specific form of fibronectin71 and may be involved in the formation of a contracted capsule around an implant.72 Bacteria may also exploit this excessive collagen and fibronectin deposition because many bacterial species produce collagen- and fibronectin-binding proteins that mediate bacterial attachment to extracellular matrix components.73-76 Once attached, bacteria multiply and form biofilms. Therefore, we speculate that the increased number and activity of myofibroblasts could contribute to biofilm formation and possible CC.
Biofilms also serve as one of several mechanisms microorganisms have developed to evade the immune system. It is not completely clear as to why the inflammatory response is not always successful in removing biofilms. However, it appears that biofilms are able to sense and manipulate host immune responses.41,77,78 For example, one study documented that human leukocytes were capable of penetrating S aureus biofilms, but were not capable of phagocytizing these bacteria, suggesting that biofilms have developed mechanisms to prevent normal leukocytes responses.79 Other research studies imply that the biofilms exposed to neutrophils release planktonic bacteria, and this presumably maintains the prolonged inflammatory response.80
The importance of biofilms in the chronic inflammation related to a variety of medical implants has been clearly demonstrated, thus it is reasonable to assume that biofilms may play a role in chronic inflammation and pathogenesis of CC.
ANIMAL STUDIES OF BIOFILMS AND CAPSULAR CONTRACTURE
Multiple animal studies have shown a correlation between biofilms and CC. These studies utilized the Baker Grading Scale in their evaluations.81 Baker grade III or IV is usually defined as “capsular contracture.”
The first animal model study that examined the role of biofilms in CC was a rabbit model.42 All rabbits underwent bilateral silicone implant placement. Experimental implant pockets were inoculated with S epidermidis in varying concentrations. Baker grade III-IV CC was identified with the inoculated pockets, while control pockets were grade I-II, suggesting that S epidermidis biofilms may contribute to CC. Another study investigated S aureus biofilms and CC in a guinea pig model.82 Animals underwent bilateral silicone implant placement. Experimental group implants were inoculated with S aureus culture overnight prior to placement. All surviving experimental animals had grade III CC, while none of the control animals did. Although these two studies used rodent animal models and a limited number of animals, their results suggest involvement of bacterial biofilms in CC.
Using a porcine model and an excellent study design, Tamboto et al83 were able to successfully establish a causal relationship between biofilms and the development of CC following augmentation mammaplasty. Submammary pockets were inoculated with S epidermidis or control (phosphate-buffered saline) prior to implantation of silicone prosthesis. Implants and intact surrounding capsule were removed after 13 weeks. Bacteria were then cultured from biofilms that formed on both capsules and implants. Presence of biofilms was confirmed by scanning-electron microcopy (SEM), which is currently the only direct method for biofilm conformation. Biofilms were detected on 72.2% of inoculated pockets. Of the inoculated implants, 77.8% had CC (Baker grade III/IV). Five of 15 control pockets developed biofilms from endogenous bacterial species, and four of these developed CC. Biofilm formation was associated with a 4-fold increased risk of developing contracted capsules.83 Although microbial biofilms certainly are not the only cause of CC, these studies suggest a strong correlation between implant/pocket biofilms and development of CC.
CLINICAL EVIDENCE OF BIOFILMS IN CAPSULAR CONTRACTURE
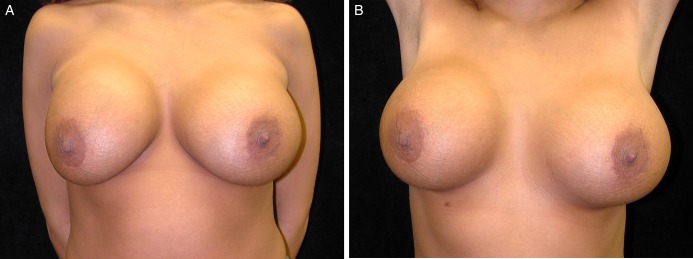
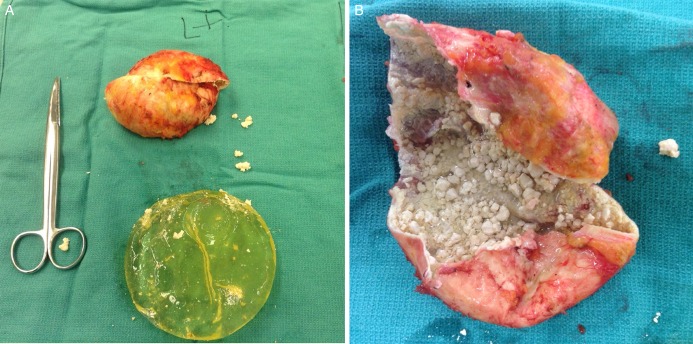
Multiple clinical studies have demonstrated significant correlation between presence of biofilms/bacterial colonization and CC of breast implants.20-22,24,25 All of these studies used an objective measure for CC, known as the Baker Grading Scale.81 Typical appearance of CC grade III can be seen in Figures 2 and 3. A summary of these studies can be found in Table 1. The Wilflingseder histological classification is a rarely used but objective measure. The reader is referred to a following article for further reading regarding this classification.84
Figure 2.
(A, B) A 24-year-old woman with capsular contracture in the right breast (Baker grade III). Patient had 350 cc saline implants placed in the subglandular plane 1.5 years prior to presentation. The implant is displaced laterally, and the implant margin is clearly demarcated. Palpation demonstrated firmness of the breast. Photographs courtesy of Christopher Salgado, MD.
Figure 3.
(A) Breast implant with Baker grade IV capsular contracture after explantation and capsulectomy. The patient was 54-years-old at the time of removal; augmentation had been performed 30 years prior. Manufacturer information was unavailable. (B) Capsule has been incised, exposing the interior surface. Photographs courtesy Zubin Panthaki, MD.
Virden et al performed one of the first studies to examine the link between CC and biofilms of breast implants.20 Fifty-five silicone implants and tissue expanders were explanted due to CC after a follow-up ranging from 2 months to 5 years.20 All implants were explanted with their capsules and examined. Biofilms were detected by SEM on approximately 56% of all implants.20 In another study, Dobke et al examined 150 silicone wall mammary implants.21 In this study, 76% of contracted capsules harbored bacteria. Unfortunately, SEM was not performed in this study. Although bacteria were detected on a large number of contracted implants, the presence of biofilm structures was not confirmed. In the third study, Pajkos et al evaluated 19 contracted and 8 non-contracted breast implants and capsules for bacterial presence.22 Bacteria were detected in 89.5% of breast implants with CC out of which 57.9% had biofilms. Presence of biofilms was confirmed by SEM.22 In contrast, bacteria were present in only two (10.5%) of the non-contracted implants.
These studies, despite some shortcomings, consistently show a significant incidence of bacterial colonization and/or biofilms in CC, suggesting correlation of CC and biofilms. Given the vast amount of clinical data supporting development of biofilm-related complications in other types of medical implants,16-19 these pioneering findings warrant further research.
DETECTION OF BIOFILMS ON CONTRACTED IMPLANTS
There is no clinical standard for detection of biofilms on medical implants, including breast implants. Diagnostic modalities used investigationally include bacterial culturing with or without sonication of specimens, polymerase chain reaction (PCR) and/or 16S RNA sequencing for bacterial DNA identification, and SEM for direct visualization and conformation of the biofilms on samples. Future utilization of some or all of these methods in clinical practice may help identify a cohort of patients who would otherwise undergo multiple revisions for recurrent CC secondary to lingering biofilm.
Bacterial Culturing
“Conventional/traditional” bacterial identification is defined as bacterial growth on a selective media (on agar plates or in the liquid media). Additionally, biochemical tests can be used for bacterial identification. However, these methods have poor sensitivity for detection of biofilms. In the study by Virden et al, standard plating culture techniques detected bacteria in only 3 out of 27 implants with CC. However, using an experimental culturing protocol with prolonged broth incubation, detection increased to 56% (15/27). Presence of biofilm was confirmed with SEM in all 15 positive specimens. This demonstrated the importance of exploring alternate culturing methods in identifying biofilms in CC.
Sonication
Sonication has been used experimentally to improve the sensitivity and specificity in detecting biofilms on implanted devices and prostheses.24,85-87 Biofilm matrix encases bacteria present on the surface of implanted devices, making conventional culturing on selective media difficult.85 In sonication, each implant is exposed to high frequency sonic energy, which releases the bacteria from the biofilm matrix.85 Bacteria are then grown aerobically or anaerobically on blood agar plates and enumerated. Currently, sonication is not routinely performed clinically. However, given the ease of the technique, sonication followed by culturing may become standard in detection of subclinical infection in prosthetics such as breast implant capsules.
PCR-Identification of Bacterial DNA
Rapid detection and identification of the surgical implant biofilms can be done using molecular biology methods such as PCR. PCR is a very sensitive method that involves amplification of a few copies of bacterial DNA using bacteria-specific primers or universal broad-range primers that can recognize any bacteria present in the sample.88 Tissue specimens and/or sonication treatment of explanted prosthesis is usually required to obtain adequate DNA for PCR. PCR can detect bacterial DNA in the samples that failed to show positive results using conventional bacterial identification.88 Furthermore, PCR is a rapid process, taking 2 to 4 hours to complete.
Sonication followed by culturing and PCR detection of microorganisms in biofilms is currently utilized in numerous research laboratories, but only in selected clinical/hospital microbiology laboratories. Future utilization of some or all of these methods in clinical practice may help identify a cohort of patients who would otherwise undergo multiple revisions for recurrent CC secondary to lingering biofilm.
IMPLANT TEXTURE, BIOFILMS, AND CAPSULAR CONTRACTURE
The role that breast-implant texture plays in biofilm formation and CC is not completely clear. Scherml et al found no quantitative difference in the bacterial colonization on smooth and textured implants.23 In contrast, Jacombs et al reported a 72-fold biofilm increase in textured implants compared to smooth implants in vitro after 24 hours incubation with bacteria.89 This outcome was similar to the several other in vitro implant studies.90-93 However, the in vivo (porcine-model) portion of Jacomb's study demonstrated very little difference in development of CC on smooth (82.6%) and textured (83.7%) implants after approximately 19 weeks following inoculation of implant pockets with S epidermidis. Interestingly, initial bacterial attachment was 20-fold higher on textured implants, which is not surprising since numerous in vitro studies have shown enhanced bacterial adhesion and biofilms development on rough surfaces.94-99 Therefore, it is reasonable to conclude from a number of studies that implant texture would affect initial biofilm growth.90-93 Once mature biofilms are formed, difference in implant texture may be negated. This could explain why both smooth and textured implants had no statistical difference in biofilm formation in Jacomb's study.89
The findings explained above are somewhat contradictory to several clinical studies. Stevens et al studied risk factors of CC in smooth and textured implants in Sientra's 5-year prospective study.100 After a follow-up of 5 years, incidence of CC was significantly higher in smooth implants vs textured implants (odds ratio 2.3, P < 0.0001). No attempts were made to detect biofilms on these implants. Other studies have shown similar results.6,101,102 Spear et al reviewed CC rates for patients enrolled in Allergan's 10-year Core study.103 Risk of CC was not significantly different between surface texture types.
“Smooth” and “textured” are somewhat arbitrary designations, as all breast implant surfaces show irregularity on microscopic scales. Barr et al examined surfaces of 5 implant types.104 The “smooth” surface shell, Allergan Smooth surface (Allergan Medical Corporation, Santa Barbara, CA), contained parallel surface ripples measuring 5 µm. Studies have shown that parallel grooves measuring 5 µm or less facilitate fibroblast migration and organized collagen deposition.105,106 Thus, this may be one of the reasons why smooth surface implants are correlated with higher incidence of CC. The four “textured” implants' grooves ranged from 200 to 500 µm. These grooves are much larger than the approximate diameter of a fibroblast (25 µm), and they presumably interfere with fibroblast migration and the orientation of collagen deposition, which may result in lower incidence of CC.
Biofilm formation is favored when the average roughness of a surface is greater than 0.2 µm.94 This suggests that both “smooth” and “rough” implant surfaces provide enough roughness for biofilm formation. These data further suggest that, while topographic features of all types of breast implants most likely allow formation of biofilms, “smooth” implant surfaces may further contribute to CC by promoting enhanced collagen deposition. The degree to which these factors contribute to CC remains a topic of investigation. Biological advantages of textured implants, including better tissue ingrowth and a potential reduction in long-term incidence of CC, need to be balanced by the increased risk of bacterial attachment and initial biofilm development.
PREVENTION AND TREATMENT OF BIOFILM-RELATED CAPSULAR CONTRACTURE
Multiple preventative techniques that may contribute to lower CC incidence have been described. We have summarized these in in Table 2. Wixtrom et al used Tegaderm (3 M, Two Harbors, MN) nipple shield to reduce implant contamination from endogenous breast flora.107 After a 6 month follow-up, three of the 32 patients developed CC despite nipple shielding. Wixtrom et al did not correlate the incidence of CC with the presence of a positive nipple culture. However, of those three, two had undergone multiple revisions for CC. This suggests that one possible cause of CC may be incomplete biofilm removal from previous operations. Breast pocket irrigation with antibiotics and/or antibacterial agents has been practiced and recommended for many years. Due to the implication of polymicrobial infections associated with CC, finding the optimal broad-spectrum irrigation remains unsettled. In 2000, Adams et al conducted a study comparing the most commonly used breast pocket irrigations in vitro.108 At a lower concentration compared to other solutions tested, betadine, gentamicin, and cefazolin solution was 100% effective against bacteria. Due to concerns that betadine-caused implant deflation, the US Food and Drug Administration banned immersion of breast implants in betadine solution in 2000. This prompted testing for alternative broad-spectrum solutions. Adams et al reported use of bacitracin, cefazolin, and gentamicin solution.109 After a mean follow-up of 14 months (range 6 to 75 months), incidence of CC was 4- to 5-fold less for breast augmentation compared to manufacturer pre-market approval data.109 Breast pocket irrigation alternatives for patients allergic to antibiotics are presented in Table 3.110
Table 2.
Summary of Preventive Strategies Suggested by Authors for Minimizing Risk of Biofilm
| Phase of Procedure | Recommendation |
|---|---|
| Implant adjuncts | Antibiotic mesh |
| Aseptic preparation | Nipple shield |
| Irrigation of breast pocket with antibiotic solution | |
| Preoperative IV antibiotics | Standard prophylaxis for surgical site infections (cefazolin, ampicillin-sulbactam, clindamycin); no benefit in CC vs placebo in 12 months112 |
| Surgical Technique | Avoidance of peri-nipple-areola incision, especially with subglandular implant placement |
| Atraumatic technique | |
| Meticulous hemostasis | |
| “no touch” technique with Keller Funnel |
Table 3.
Recommended Alternative Solutions for Breast Irrigation for Substitution of the Bacitracin-Cefazolin-Gentamicin Triple Antibiotic Solution Components
| Allergen | Recommended Alternative Irrigation Solution |
|---|---|
| Cephalosporin or penicillin | Gentamicin (80 mg), providone-iodine solution (250 mL), normal saline (250 mL) |
| Bacitracin | Cefazolin (1 g), gentamicin (80 mg), providone-iodine solution (50 mL), normal saline (50 mL) |
| Gentamicin/aminoglycoside | Providone-iodine (250 mL) and normal saline (250 mL) |
| Iodine | Bacitracin (50,000 units), cefazolin (1 g), gentamicin (80 mg), normal saline (500 mL) |
Prophylactic intravenous (IV) antibiotics have also been studied. Arad et al conducted an animal study using rats and IV vancomycin.111 This treatment was more efficacious against immature biofilms and soft-tissue infection. It had limited efficacy against mature biofilm. Preoperative antibiotics in prevention of CC have also been evaluated clinically.112 After a 12-month follow-up, there were no statistical differences between control and antibiotic groups regarding prevalence of CC (47% and 53%, respectively). Presence of biofilm was not assessed. These data suggest that local treatment with antibiotic irrigation is more effective in prevention of bacterial colonization and initial biofilm formation compared to systemic perioperative antibiotics. Additionally, irrigation may decrease selection of antibiotic-resistant bacteria compared to IV prophylaxis.
Jacombs et al used a porcine model to examine the effectiveness of antibiotic impregnated mesh in the prevention of biofilm formation and CC. Researchers implanted a total of 28 prostheses into 5 pigs. All 28 implants and their pockets were inoculated with S epidermidis isolated from a human patient with CC. Fourteen implants were inserted with antibiotic mesh (treatment) and the other 14 were untreated (control). All untreated implants developed Baker grade III/IV CC. In contrast, all treated implants were Grade I/II after 16 weeks, (P < 0.001).113 Specimens with CC had at least 10-fold higher bacterial counts. Bacterial colonization of mesh-covered implants was typically single-layered, if present. In contrast, multilayered biofilms were detected by SEM in all untreated implants.113 This study highlights that prevention of biofilm formation in its early stage using antibiotic coating of implants, rather than treating biofilm related infections, would be more desirable in clinical settings. However, due to the rise of antibiotic resistance, additional approaches are also needed. Alternative antimicrobial or anti-adhesion coating agents currently used for other medical implants should be studied as novel preventative solutions.114,115
Moyer et al conducted a cadaver study to assess the amount of skin contact and skin and breast parenchyma contamination with standard implantation compared to delivery via the Keller Funnel (Keller Medical Inc., Stuart, FL).116 The funnel is composed of rip-stop nylon and a hydrophilic inner coating and is designed to facilitate implant placement without skin contact. Bacterial transfer from the breast parenchyma to implant surface with the funnel was 37.5%, while with the standard implantation technique it was 62.5%. Since this was a cadaver study, no long-term data regarding CC could be determined.
Surgical technique may also affect implant contamination with microorganisms.15 A retrospective study by Wiener demonstrated the effect of the incision on the development of CC in over 400 patients. Patients who had an inframammary incision had a 0.59% incidence of CC compared with 9.5% in patients who had a peri-nipple-areolar incision. Peri-nipple-areola approach transects ducts near the nipple, which harbor the greatest amount of bacteria. These ducts can continue to release bacteria until they have scarred, healed, and sealed. Thus this approach increases the potential for bacterial contamination and biofilm formation. On the other hand, the intramammary incision is in a plane deep to most of the ducts, hence there is less risk of exposure to endogenous bacteria.117 Pocket location also appears to have an impact on development of CC. Incidence of CC is higher in implants placed in the subglandular vs subpectoral plane.100,101 This is also likely due to proximity to bacteria harbored by mammary ducts.
Gold standard treatment of CC is total capsulectomy with implant removal and replacement. Using a new implant when treating the CC is imperative, due to possible presence of biofilm and their notorious antibiotic resistance. Change in pocket location could also be considered at the time of revision.15
Other non-surgical modalities have been considered for patients with established contracture. These include vitamin E, steroids,118 nonsteroidal anti-inflammatory drugs (NSAIDs), and leukotriene inhibitors.15 Findings from studies by Scuderi et al suggest that zafirlukast, a leukotriene receptor antagonist (LTRA), may reduce pain and breast capsule distortion.119,120 More recently, Mazzocchi et al also studied the effects of zafirlukast on CC. They found a significant reduction in mammary compliance values and severity of CC. However, mammary compliance values gradually increased after drug withdrawal.121 The efficacy of anti-inflammatory drugs (such as steroids or leukotriene inhibitors) in treatment of CC supports the hypothesis that inflammatory processes, including biofilm-induced inflammatory processes, are involved in the genesis of CC.
CONCLUSIONS
All medical devices, including breast implants, are susceptible to microbial attachment and formation of biofilms. Development of CC is most likely multifactorial. However, many experimental studies demonstrated a significant link between biofilm infections and increased incidence of CC. In addition, several clinical studies suggest a clinically relevant causal relationship.
Detection of biofilms remains one of the greatest challenges of biofilm infections. New molecular methods should be introduced in the practice. These innovative methods are expected to provide a more sensitive bacterial enumeration and detection that would contribute to more complete picture of microbial biofilm infections encountered in plastic surgery, especially CC.
Biofilm infections are difficult to treat with conventional antibiotics, and this treatment is further hindered due to the increase of antibiotic resistance. Therefore prevention, rather than treatment, of possible biofilm-related CC might be a better strategy. Future implants may be manufactured with antimicrobial or anti-adhesion coating, which could limit risk of CC. At this moment associated risk factors can be minimized through meticulous surgical technique, proper aseptic preparation, and minimal skin contact. Delivery systems such as the Keller funnel hold promise, but studies reporting long-term outcomes and cost effectiveness are lacking. With regards to implant characteristics, there is no clear evidence regarding shell texture or implant type and development of biofilm-related CC.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.Cosmetic Surgery National Data Bank: Statistics 2013. Aesthet Surg J. 2014;34(1 suppl):1S-22S. [DOI] [PubMed] [Google Scholar]
- 2.Asplund O. Capsular contracture in silicone gel and saline-filled breast implants after reconstruction. Plast Reconstr Surg. 1984;732:270-275. [DOI] [PubMed] [Google Scholar]
- 3.Asplund O, Gylbert L, Jurell G, Ward C. Textured or smooth implants for submuscular breast augmentation: a controlled study. Plast Reconstr Surg. 1996;976:1200-1206. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel SE, Woods JE, O'Fallon WM, Beard CM, Kurland LT, Melton LJ III. Complications leading to surgery after breast implantation. N Engl J Med. 1997;33610:677-682. [DOI] [PubMed] [Google Scholar]
- 5.Wyatt LE, Sinow JD, Wollman JS, Sami DA, Miller TA. The influence of time on human breast capsule histology: smooth and textured silicone-surfaced implants. Plast Reconstr Surg. 1998;1026:1922-1931. [DOI] [PubMed] [Google Scholar]
- 6.Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg. 2006;1185:1224-1236. [DOI] [PubMed] [Google Scholar]
- 7.Handel N, Cordray T, Gutierrez J, Jensen JA. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg.. 2006;1173:757-767. discussion 768-772. [DOI] [PubMed] [Google Scholar]
- 8.Benediktsson K, Perbeck L. Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg. 2006;591:27-34. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham B. The Mentor Core Study on Silicone MemoryGel Breast Implants. Plast Reconstr Surg. 2007;120(7 Suppl 1):19S-29S. discussion 30S-32S. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;333:440-444. [DOI] [PubMed] [Google Scholar]
- 11.Hvilsom GB, Holmich LR, Henriksen TF, Lipworth L, McLaughlin JK, Friis S. Local complications after cosmetic breast augmentation: results from the Danish Registry for Plastic Surgery of the breast. Plast Reconstr Surg. 2009;1243:919-925. [DOI] [PubMed] [Google Scholar]
- 12.Walker PS, Walls B, Murphy DK. Natrelle saline-filled breast implants: a prospective 10-year study. Aesthet Surg J. 2009;291:19-25. [DOI] [PubMed] [Google Scholar]
- 13.Spear SL, Murphy DK, Slicton A, Walker PS, Inamed Silicone Breast Implant USSG. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7 Suppl 1):8S-16S. discussion 17S-18S. [DOI] [PubMed] [Google Scholar]
- 14.Breast Augmentation, Principles and Practice. In: Shiffman MA (ed.) Berlin Heidelberg: Springer-Verlag; 2009. [Google Scholar]
- 15.Adams WP., Jr Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;361:119-126, vii. [DOI] [PubMed] [Google Scholar]
- 16.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews Microbiology. 2004;22:95-108. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Montanaro L, Arciola CR. Biofilm in implant infections: its production and regulation. Int J Artif Organs. 2005;2811:1062-1068. [DOI] [PubMed] [Google Scholar]
- 18.Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;1001:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;3326:5967-5982. [DOI] [PubMed] [Google Scholar]
- 20.Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg. Spring 1992;162:173-179. [DOI] [PubMed] [Google Scholar]
- 21.Dobke MK, Svahn JK, Vastine VL, Landon BN, Stein PC, Parsons CL. Characterization of microbial presence at the surface of silicone mammary implants. Ann Plast Surg. 1995;346:563-569. disscusion 570-561. [DOI] [PubMed] [Google Scholar]
- 22.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;1115:1605-1611. [DOI] [PubMed] [Google Scholar]
- 23.Schreml S, Heine N, Eisenmann-Klein M, Prantl L. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Ann Plast Surg. 2007;592:126-130. [DOI] [PubMed] [Google Scholar]
- 24.Rieger UM, Pierer G, Luscher NJ, Trampuz A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plast Surg. 2009;333:404-408. [DOI] [PubMed] [Google Scholar]
- 25.Rieger UM, Mesina J, Kalbermatten DF et al. . Bacterial biofilms and capsular contracture in patients with breast implants. Br J Surg. 2013;1006:768-774. [DOI] [PubMed] [Google Scholar]
- 26.Blount AL, Martin MD, Lineberry KD, Kettaneh N, Alfonso DR. Capsular contracture rate in a low-risk population after primary augmentation mammaplasty. Aesthet Surg J. 2013;334:516-521. [DOI] [PubMed] [Google Scholar]
- 27.Silverman BG, Brown SL, Bright RA, Kaczmarek RG, Arrowsmith-Lowe JB, Kessler DA. Reported complications of silicone gel breast implants: an epidemiologic review. Ann Intern Med. 1996;1248:744-756. [DOI] [PubMed] [Google Scholar]
- 28.Berry MG, Cucchiara V, Davies DM. Breast augmentation: Part II--Adverse capsular contracture. J Plast Reconstr Aesthet Surg. 2010;6312:2098-2107. [DOI] [PubMed] [Google Scholar]
- 29.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;213:1599-1607. [DOI] [PubMed] [Google Scholar]
- 30.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;91:34-39. [DOI] [PubMed] [Google Scholar]
- 31.Ajdic D, Chen Z. A novel phosphotransferase system of Streptococcus mutans is responsible for transport of carbohydrates with alpha-1,3 linkage. Molecular oral microbiology. 2013;282:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;27:a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;89:623-633. [DOI] [PubMed] [Google Scholar]
- 34.Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;131:7-10. [DOI] [PubMed] [Google Scholar]
- 35.Fux CA, Wilson S, Stoodley P. Detachment characteristics and oxacillin resistance of Staphyloccocus aureus biofilm emboli in an in vitro catheter infection model. J Bacteriol. 2004;18614:4486-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chambless JD, Hunt SM, Stewart PS. A three-dimensional computer model of four hypothetical mechanisms protecting biofilms from antimicrobials. Appl Environ Microbiol. 2006;723:2005-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu KD, Stewart PS, Xia F, Huang CT, McFeters GA. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998;6410:4035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol. 2008;681:223-240. [DOI] [PubMed] [Google Scholar]
- 39.O'Connell HA, Kottkamp GS, Eppelbaum JL, Stubblefield BA, Gilbert SE, Gilbert ES. Influences of biofilm structure and antibiotic resistance mechanisms on indirect pathogenicity in a model polymicrobial biofilm. Appl Environ Microbiol. 2006;727:5013-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;732:310-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;61:56-60. [DOI] [PubMed] [Google Scholar]
- 42.Shah Z, Lehman JA Jr., Tan J. Does infection play a role in breast capsular contracture? Plast Reconstr Surg. 1981;681:34-42. [DOI] [PubMed] [Google Scholar]
- 43.Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;987:1225-1229. [DOI] [PubMed] [Google Scholar]
- 44.Courtiss EH, Goldwyn RM, Anastasi GW. The fate of breast implants with infections around them. Plast Reconstr Surg. 1979;636:812-816. [PubMed] [Google Scholar]
- 45.Washer LL, Gutowski K. Breast implant infections. Infect Dis Clin North Am. 2012;261:111-125. [DOI] [PubMed] [Google Scholar]
- 46.Macadam SA, Mehling BM, Fanning A et al. . Nontuberculous mycobacterial breast implant infections. Plast Reconstr Surg. 2007;1191:337-344. [DOI] [PubMed] [Google Scholar]
- 47.Vinh DC, Rendina A, Turner R, Embil JM. Breast implant infection with Mycobacterium fortuitum group: report of case and review. J Infect. 2006;523:e63-e67. [DOI] [PubMed] [Google Scholar]
- 48.Del Pozo JL, Tran NV, Petty PM et al. . Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol. 2009;475:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez L, Breidenstein EB, Hancock RE. Creeping baselines and adaptive resistance to antibiotics. Drug Resist Updat. 2011;141:1-21. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol. 2008;19013:4447-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;447:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Fuente-Nunez C, Reffuveille F, Fernandez L, Hancock RE. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opin Microbiol. 2013;165:580-589. [DOI] [PubMed] [Google Scholar]
- 53.Jolivet-Gougeon A, Bonnaure-Mallet M. Biofilms as a mechanism of bacterial resistance. Drug discovery today Technologies. 2014;11:49-56. [DOI] [PubMed] [Google Scholar]
- 54.Abdallah M, Benoliel C, Drider D, Dhulster P, Chihib NE. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch Microbiol. 2014;1967:453-472. [DOI] [PubMed] [Google Scholar]
- 55.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;2845418:1318-1322. [DOI] [PubMed] [Google Scholar]
- 56.Castelli P, Caronno R, Ferrarese S et al. . New trends in prosthesis infection in cardiovascular surgery. Surg Infect (Larchmt). 2006;7 Suppl 2:S45-S47. [DOI] [PubMed] [Google Scholar]
- 57.Veerachamy S, Yarlagadda T, Manivasagam G, Yarlagadda PK. Bacterial adherence and biofilm formation on medical implants: a review. Proc Inst Mech Eng H. 2014;22810:1083-1099. [DOI] [PubMed] [Google Scholar]
- 58.Anderson JM. Biological Responses to Materials. Annual review of materials research. 2001;31:30. [Google Scholar]
- 59.Rhoads DD, Wolcott RW, Cutting KF, Percival SL. Evidence of Biofilms in Wounds and the Potential Ramifications. In: MCBain A, Allison D, Pratte J, Spratt D, Upton M, Verran J, eds. Biofilms: Coming of Age. Cardiff: Bioline; 2007:131-143. [Google Scholar]
- 60.Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol. 2011;38 Suppl 11:28-35. [DOI] [PubMed] [Google Scholar]
- 61.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216-248. [DOI] [PubMed] [Google Scholar]
- 62.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;343:235-249. [PubMed] [Google Scholar]
- 63.Zhao G, Usui ML, Lippman SI et al. . Biofilms and Inflammation in Chronic Wounds. Advances in wound care. 2013;27:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tankersley A, Frank MB, Bebak M, Brennan R. Early effects of Staphylococcus aureus biofilm secreted products on inflammatory responses of human epithelial keratinocytes. J Inflamm. 2014;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolcott RD, Rhoads DD, Dowd SE. Biofilms and chronic wound inflammation. J Wound Care. 2008;178:333-341. [DOI] [PubMed] [Google Scholar]
- 66.Panagakos F, Scannapieco F. Periodontal Inflammation: Fron Gingivitis to Systemic Disease? In: Panagakos F, ed. Gingival Diseases- Their Aetiology, Prevention, and Treatment: InTech; 2011. [Google Scholar]
- 67.Shankar R, H. G. Biomaterials Science: An introduction to materials in medicine. New York: Academic Press; 1996. [Google Scholar]
- 68.Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Advances in wound care. 2013;24:122-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rinkevich Y, Walmsley GG, Hu MS et al. . Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;3486232:aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazhar K, Gunawardana M, Webster P et al. . Bacterial biofilms and increased bacterial counts are associated with airway stenosis. Otolaryngol Head Neck Surg. 2014;1505:834-840. [DOI] [PubMed] [Google Scholar]
- 71.Vedrenne N, Coulomb B, Danigo A, Bonte F, Desmouliere A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol Biol.. 2012;601:20-27. [DOI] [PubMed] [Google Scholar]
- 72.Hwang K, Sim HB, Huan F, Kim DJ. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic Plast Surg. 2010;346:716-721. [DOI] [PubMed] [Google Scholar]
- 73.Switalski LM, Patti JM, Butcher W, Gristina AG, Speziale P, Hook M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993;71:99-107. [DOI] [PubMed] [Google Scholar]
- 74.Kreikemeyer B, Nakata M, Oehmcke S, Gschwendtner C, Normann J, Podbielski A. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J Biol Chem. 2005;28039:33228-33239. [DOI] [PubMed] [Google Scholar]
- 75.Menzies BE. The role of fibronectin binding proteins in the pathogenesis of Staphylococcus aureus infections. Curr Opin Infect Dis. 2003;163:225-229. [DOI] [PubMed] [Google Scholar]
- 76.Talay SR, Valentin-Weigand P, Jerlstrom PG, Timmis KN, Chhatwal GS. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;609:3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu L, Estrada O, Zaborina O et al. . Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;3095735:774-777. [DOI] [PubMed] [Google Scholar]
- 78.Tateda K, Ishii Y, Horikawa M et al. . The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;7110:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. Journal of immunology. 2005;17511:7512-7518. [DOI] [PubMed] [Google Scholar]
- 80.Jesaitis AJ, Franklin MJ, Berglund D et al. . Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. Journal of immunology. 2003;1718:4329-4339. [DOI] [PubMed] [Google Scholar]
- 81.Baker JL. Symposium on Aesthetic Surgery of the Breast: proceedings of the symposium of the Educational Foundation of the American Society of Plastic and Reconstructive Surgeons, inc., and the American Society for Aesthetic Plastic Surgery, inc., held at Scottsdale, Arizona, November 23-26, 1975. Saint Louis: Mosby; 1978. [Google Scholar]
- 82.Kossovsky N, Heggers JP, Parsons RW, Robson MC. Acceleration of capsule formation around silicone implants by infection in a guinea pig model. Plast Reconstr Surg. 1984;731:91-98. [DOI] [PubMed] [Google Scholar]
- 83.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;1263:835-842. [DOI] [PubMed] [Google Scholar]
- 84.Wilflingseder P, Porbst A, Mikuz G, Hoinkes G.. Constrictive fibrosis post augmentation mammaplasty. Paper presented at: Transactions of the Sixth International Congress of Plastic and Reconstructive Surgery 1976; Paris. [Google Scholar]
- 85.Oliva A, Nguyen BL, Mascellino MT et al. . Sonication of explanted cardiac implants improves microbial detection in cardiac device infections. J Clin Microbiol. 2013;512:496-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trampuz A, Piper KE, Jacobson MJ et al. . Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;3577:654-663. [DOI] [PubMed] [Google Scholar]
- 87.Mason PK, Dimarco JP, Ferguson JD et al. . Sonication of explanted cardiac rhythm management devices for the diagnosis of pocket infections and asymptomatic bacterial colonization. Pacing Clin Electrophysiol. 2011;342:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tunney MM, Patrick S, Curran MD et al. . Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;3710:3281-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacombs A, Tahir S, Hu H et al. . In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;1334:471e-480e. [DOI] [PubMed] [Google Scholar]
- 90.Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013;24 Suppl A100:49-56. [DOI] [PubMed] [Google Scholar]
- 91.Burgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010;212:156-164. [DOI] [PubMed] [Google Scholar]
- 92.O'Mahony A, MacNeill SR, Cobb CM. Design features that may influence bacterial plaque retention: a retrospective analysis of failed implants. Quintessence Int. 2000;314:249-256. [PubMed] [Google Scholar]
- 93.Yeo IS, Kim HY, Lim KS, Han JS. Implant surface factors and bacterial adhesion: a review of the literature. Int J Artif Organs. 2012;3510:762-772. [DOI] [PubMed] [Google Scholar]
- 94.Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17 Suppl 2:68-81. [DOI] [PubMed] [Google Scholar]
- 95.Mitik-Dineva N, Wang J, Truong VK et al. . Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol. 2009;583:268-273. [DOI] [PubMed] [Google Scholar]
- 96.Mei L, Busscher HJ, van der Mei HC, Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent Mater. 2011;278:770-778. [DOI] [PubMed] [Google Scholar]
- 97.Wu Y, Zitelli JP, TenHuisen KS, Yu X, Libera MR. Differential response of Staphylococci and osteoblasts to varying titanium surface roughness. Biomaterials. 2011;324:951-960. [DOI] [PubMed] [Google Scholar]
- 98.Arnold JW, Bailey GW. Surface finishes on stainless steel reduce bacterial attachment and early biofilm formation: scanning electron and atomic force microscopy study. Poult Sci. 2000;7912:1839-1845. [DOI] [PubMed] [Google Scholar]
- 99.Myint AA, Lee W, Mun S, Ahn CH, Lee S, Yoon J. Influence of membrane surface properties on the behavior of initial bacterial adhesion and biofilm development onto nanofiltration membranes. Biofouling. 2010;263:313-321. [DOI] [PubMed] [Google Scholar]
- 100.Stevens WG, Nahabedian MY, Calobrace MB et al. . Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2013;1325:1115-1123. [DOI] [PubMed] [Google Scholar]
- 101.Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;669:1165-1172. [DOI] [PubMed] [Google Scholar]
- 102.Liu X, Zhou L, Pan F, Gao Y, Yuan X, Fan D. Comparison of the postoperative incidence rate of capsular contracture among different breast implants: a cumulative meta-analysis. PloS one. 2015;102:e0116071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Spear SL, Murphy DK, Allergan Silicone Breast Implant USCCSG. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg. 2014;1336:1354-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barr S, Hill E, Bayat A. Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility. Eplasty. 2009;9:e22. [PMC free article] [PubMed] [Google Scholar]
- 105.den Braber ET, de Ruijter JE, Ginsel LA, von Recum AF, Jansen JA. Orientation of ECM protein deposition, fibroblast cytoskeleton, and attachment complex components on silicone microgrooved surfaces. J Biomed Mater Res. 1998;402:291-300. [DOI] [PubMed] [Google Scholar]
- 106.den Braber ET, de Ruijter JE, Smits HT, Ginsel LA, von Recum AF, Jansen JA. Effect of parallel surface microgrooves and surface energy on cell growth. J Biomed Mater Res. 1995;294:511-518. [DOI] [PubMed] [Google Scholar]
- 107.Wixtrom RN, Stutman RL, Burke RM, Mahoney AK, Codner MA. Risk of breast implant bacterial contamination from endogenous breast flora, prevention with nipple shields, and implications for biofilm formation. Aesthet Surg J. 2012;328:956-963. [DOI] [PubMed] [Google Scholar]
- 108.Adams WP Jr., Conner WC, Barton FE Jr., Rohrich RJ. Optimizing breast pocket irrigation: an in vitro study and clinical implications. Plast Reconstr Surg. 2000;1051:334-338. discussion 339-343. [DOI] [PubMed] [Google Scholar]
- 109.Adams WP Jr., Conner WC, Barton FE Jr., Rohrich RJ. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;1076:1596-1601. [DOI] [PubMed] [Google Scholar]
- 110.Adams WP Jr., Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;1171:30-36. [PubMed] [Google Scholar]
- 111.Arad E, Navon-Venezia S, Gur E et al. . Novel rat model of methicillin-resistant Staphylococcus aureus-infected silicone breast implants: a study of biofilm pathogenesis. Plast Reconstr Surg. 2013;1312:205-214. [DOI] [PubMed] [Google Scholar]
- 112.Gylbert L, Asplund O, Berggren A, Jurell G, Ransjo U, Ostrup L. Preoperative antibiotics and capsular contracture in augmentation mammaplasty. Plast Reconstr Surg. 1990;862:260-267. discussion 268-269. [PubMed] [Google Scholar]
- 113.Jacombs A, Allan J, Hu H et al. . Prevention of biofilm-induced capsular contracture with antibiotic-impregnated mesh in a porcine model. Aesthet Surg J. 2012;327:886-891. [DOI] [PubMed] [Google Scholar]
- 114.Chen M, Yu Q, Sun H. Novel strategies for the prevention and treatment of biofilm related infections. Int J Mol Sci 2013;149:18488-18501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Heerden J, Turner M, Hoffmann D, Moolman J. Antimicrobial coating agents: can biofilm formation on a breast implant be prevented? J Plast Reconstr Aesthet Surg. 2009;625:610-617. [DOI] [PubMed] [Google Scholar]
- 116.Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J. 2012;322:194-199. [DOI] [PubMed] [Google Scholar]
- 117.Wiener TC. Relationship of incision choice to capsular contracture. Aesthetic Plast Surg. 2008;322:303-306. [DOI] [PubMed] [Google Scholar]
- 118.Costagliola M, Atiyeh BS, Rampillon F. An innovative procedure for the treatment of primary and recurrent capsular contracture (CC) following breast augmentation. Aesthet Surg J. 2013;337:1008-1017. [DOI] [PubMed] [Google Scholar]
- 119.Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G. The effects of zafirlukast on capsular contracture: preliminary report. Aesthetic Plast Surg. 2006;305:513-520. [DOI] [PubMed] [Google Scholar]
- 120.Scuderi N, Mazzocchi M, Rubino C. Effects of zafirlukast on capsular contracture: controlled study measuring the mammary compliance. Int J Immunopathol Pharmacol. 2007;203:577-584. [DOI] [PubMed] [Google Scholar]
- 121.Mazzocchi M, Dessy LA, Alfano C, Scuderi N. Effects of zafirlukast on capsular contracture: long-term results. Int J Immunopathol Pharmacol. 2012;254:935-944. [DOI] [PubMed] [Google Scholar]
- 122.Feldman EM, Kontoyiannis DP, Sharabi SE, Lee E, Kaufman Y, Heller L. Breast implant infections: is cefazolin enough? Plast Reconstr Surg. 2010;1263:779-785. [DOI] [PubMed] [Google Scholar]