Abstract
Background
In a previous study, the authors demonstrated that treatment with expanded adipose-derived stem cells or stromal vascular fraction (SVF)-enriched fat modify the pattern of the dermis in human beings, representing a skin rejuvenation effect. Considering that expanded stem cells require a cell factor, the authors wanted to assess similar results by replacing them with platelet-rich plasma (PRP), which is easier to obtain and for which an empirical regenerative effect has been already described.
Objectives
To determine if PRP injection could replace the cutaneous regenerative effect of adipose-derived stem cells.
Methods
This study was performed in 13 patients who were candidates for facelift. The patients underwent sampling of fat by liposuction from the abdomen and submitted to one of three protocols: injection of SVF-enriched fat or expanded adipose-derived stem cells or fat plus PRP in the preauricular areas. Fragments of skin were removed before and 3 months after treatment and analyzed by optical and electron microscopy.
Results
The use of fat plus PRP led to the presence of more pronounced inflammatory infiltrates and a greater vascular reactivity, increasing in vascular permeability and a certain reactivity of the nervous component. The addition of PRP did not improve the regenerative effect.
Conclusion
The use of PRP did not have significant advantages in skin rejuvenation over the use of expanded adipose-derived stem cells or SVF-enriched fat. The effect of increased vascular reactivity may be useful in pathological situations in which an intense angiogenesis is desirable, such as tissular ischemia.
Level of Evidence: 4
 Therapeutic
Therapeutic
Regenerative medicine is an emerging field that focuses on cell- and tissue-based therapies applicable to plastic surgery procedures. The ability to utilize stem cells to direct the growth of distinct mature cells and the creation of complex 3-dimensional tissue constructs has formed the basis of regenerative medicine. The mechanism that transforms undifferentiated cells into the differentiated cells ultimately forms the specialized tissues that are required for reconstruction, repair, and restoration, including the role of the surrounding environment.1
Stem or progenitor cells are generally considered highly promising candidate cells for regenerative applications not only because they possess a high proliferative capacity, the potential to differentiate into other cell types,2 and important paracrine activity, but also because they can be sourced autologously, eliminating any concerns about rejection or the need for immunosuppressive therapy.3-9
Adult adipose tissue is a multifunctional organ that contains various cellular types, including mature adipocytes, macrophages, endothelial cells, smooth muscle cells, and preadipocytes, supported by connective tissue surrounding fine capillaries. This tissue contains progenitor cells with a perivascular origin,10 called “adipose-derived stem cells,” that are able to differentiate in multiple cell lineages.2 The potential plasticity and therapeutic utility of these cells isolated from adult adipose tissue offer new approaches in regenerative medicine and surgery for tissue and organ function reconstructions.11 There is evidence that stem cells contribute to the restoration of tissue vascularization and organ function. According to the proposed hypothesis of the ischemic nature of radiolesions (the chronic ischemic status of the irradiated tissue),7 treatment with lipoaspirate transplantation is potentially extended to other forms of microangiopathies. The mechanisms of neoangiogenesis appear to be based on the release of angiogenic and antiapoptotic growth factors, which ultimately facilitates the recruitment of endothelial progenitor cells into newly sprouting vessels. The stromal vascular fraction (SVF) of adipose tissue represents a rich reservoir of regenerative precursor cells. The phenomena indicating regeneration were the maturation of stem cells into both adipocytes and vascular cells.12
Adipose tissue has become the autologous filler of choice in plastic, reconstructive, and aesthetic surgery. After fat grafting, there is a progressive improvement of the skin texture, elasticity, and color over a few months.13-15 Therefore, adipose tissue seems to be not only a simple filler but also a dynamic filler with two types of different and supplementary effects, the volumetric effect and the regenerative effect. The physiologic mechanisms of these dynamic phenomena are still not completely elucidated.13 The adipose-derived stem cells have demonstrated their capacity to elicit a regenerative and angiogenic response through autocrine, paracrine, and direct cell-to-cell interactions. Also, there is evidence that the mechanism of repair or regeneration of the overlying skin may be related to stem cells or adipocyte breakdown products within the fat grafts.1
In a previous study, we showed for the first time the analysis of the histologic and ultrastructural changes of the human aged facial and that the aging process of the skin of the human face can be changed by expanded adipose-derived stem cells or fat enriched with its SVF, obtained by centrifugation without an enzymatic process.15 In this sense, we wanted to assess, if possible, similar results without the use of expanded stem cells that require a cell factory and have their use limited by an ethical committee. Therefore, we replaced the stem cells with platelet-rich plasma (PRP), which is easier to obtain and for which a possible regenerative effect has been already described16,17; therefore PRP seems to be an ideal compound.
In this study, we confirmed and expanded the results of the previous study15 in a larger study group and analyzed the histological and ultrastructural changes of aged facial skin after the injection of expanded adipose-derived mesenchymal stem cells and fat with its SVF (SVF-enriched fat), comparing the results with those obtained with the use of fat enriched with PRP. Therefore, the purpose of the research was to evaluate if differences exist in the therapeutic results comparing specimens after treatment with three different protocols. We based the first protocol on the injection of expanded MSCs, whereas we utilized decanted fat with the addition of its SVF (obtained by centrifugation without an enzymatic process) in the second protocol and fat enriched with PRP in the third protocol. The last phase of the study was aimed to evaluate the best approach that could be utilized in the regenerative therapy of the face. A secondary aim of this approach was to determine whether subcutaneous connective tissue may constitute a good experimental model to study the effects in the skin of anti-aging mechanisms.
METHODS
Study Design
The study was executed in 13 adult patients (11 females and 2 males) who were candidates for facial rejuvenation surgery (facelift), with a range between 45 and 65 years of age and no history of chronic viral, metabolic, ischemic, or autoimmune diseases or other systemic pathologies. Patients who reported a smoking habit were excluded. The study period was from May 2012 through May 2015.
All patients consented under approved guidelines set forth by the human clinical trial to the Brazilian investigation ethical committee board. This study was carried out according to the ethical principles of the Declaration of Helsinki 2000 and the Declaration of Istanbul 2008. It was submitted and approved by the local ethics research board on May 25th 2012 (no. 28063), and registered to the Brazilian Registry of Clinical Trials (REBEC number: U1111-1145-3081). The study was conducted at the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil) and the University of Verona (Verona, Italy). It did not involve the use of any expensive machinery or devices in which the authors would have a financial interest.
There was a real control group, because before treatment with the three components, we took in each patient a fragment of untreated skin that was compared to similar skin after grafting of the three components.
Sampling of Fat and Skin Biopsies
Under local anesthesia with Xylocaine (lidocaine) 0.5% and epinephrine 1:500,000 U, the patients were subjected to the withdrawal of 30 cc of adipose tissue by liposuction from the infraabdominal region, using a 10 mL syringe (Luer-Lok®; Becton Dickinson) coupled to a liposuction cannula of 3 mm in diameter and 15 cm long containing three distal holes (Tulip®, USA) with a manual vacuum with the first two fingers. The removed fat was processed according to three different protocols.
Part of the fat (10 mL, equivalent to 1286 g), was centrifuged at 3000 rpm for 3 minutes using an IEC Medilite Microcentrifuge, (Thermo Electron Corporation, Byron Medical, Waltham, MA) to obtain two fractions (the adipose fraction and the SVF).18 After centrifugation, the SVF from pellet, rich in regenerative cells, was isolated by mechanical dissociation without an enzymatic process. The pellet was also sent for quantitative analysis of the mesenchymal cell fraction in pellet samples by flow cytometry (CD105+/CD90+/CD73+/CD146+/CD14/CD45−/CD34−), representing the total number of mesenchymal cells, and showed an average of 16,204 ± 5516 mesenchymal stem cells. The pellet was mixed with another layer of intact adipocytes obtained after decantation, creating an enriched fat (SVF-enriched fat); therefore, the centrifugation was utilized only to obtain the pellet. The SVF-enriched fat was injected in the right preauricular area into the subdermal layer, 2 cm forward from the tragus, as part of bedside treatment in the surgical room. It was performed as retrograde injection in a fan-wise manner, in an area of 1 cm2, using a 1.5 mm blunt-tipped cannula connected to a 3-mL syringe.
Another part of the fat (10 mL) was sent to the laboratory to perform expansion of its stem cells. One single passage was necessary to multiply the cells and the immunophenotype analysis before and after the passage was performed. The injection of expanded cells in the left preauricular area, at the level of the tragus, was performed 4 to 5 weeks after in vitro cultivation/expansion. After expansion, the expanded stem cells were diluted in 0.9% saline and packaged in syringes of 1-mL solution containing 2 × 106 mesenchymal cells diluted in a volume of 0.4 mL. We performed the subdermal application of mesenchymal stem cells with a 1-mL syringe (volume, 0.4 mL) coupled with a 30-gauge needle, in the left preauricular area (1-cm2 area), 2 cm distal from the tragus.
The last part of collected fat (10 mL) underwent a decanting process, and the lower layer with blood waste was discarded. Then 1 mL of the middle layer of the adipose fraction was collected and transferred to another 3-mL syringe. This sample was mixed with 1 mL of PRP and injected in the left preauricular area, 2 cm forward from the lobule. The injection was in the subdermal level (1-cm2 area), using a 1.5 mm blunt-tipped cannula connected to a 3-mL syringe.
Before injection, a fragment of skin and subcutaneous tissue was removed from the preauricular areas and sent to the Section of Human Anatomy and Histology at the University of Verona (Verona, Italy) and the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil) for morphologic analysis by optical and electron microscopy. Histologic analysis of the skin biopsy specimens was performed by hematoxylin and eosin, picrosirius red (for visualization of collagen), and orcein (for visualization of elastic fibers) staining. The morphologic examination was repeated after 3 months, at which time the action of the lipidic components occurs,7 taking a piece of skin and subcutaneous tissue in the same areas where each kind of treatment was applied. Three months after each treatment, the second biopsy was performed. These biopsies of treated skin were performed at different times, because of the necessity of performing the expansion of mesenchymal stem cells in the laboratory at 4 to 5 weeks. Thus, the right preauricular biopsy (fat and its SVF protocol) and the left preauricular biopsy (fat plus PRP) were performed 4 to 5 weeks before the facelifting operation. The left preauricular biopsy (expanded mesenchymal stem cells) was carried out concurrent with facelifting, to guarantee the same amount of time for the treatment effect of three protocols.
All biopsy specimens, consisting of fragments of skin, were split into two equal parts (0.5 × 0.5 cm). One half was sent in a plastic bottle immersed in 4% formaldehyde to the Institute of Biophysics Carlos Chagas Filho (Laboratory of Immunology, Federal University of Rio de Janeiro) for histologic and immunohistochemical study. The other part (0.5 × 0.5 cm) was sent in a plastic bottle with a solution of buffered 2% glutaraldehyde to the Section of Human Anatomy and Histology of University of Verona, for histomorphometric analysis with electron microscopy.
During the period between the two biopsies, patients were instructed to avoid using any cosmetic products and/or peeling treatments on their face.
Platelet-Rich Plasma Preparation
Peripheral blood was collected using blood collection tubes containing 0.5 mL 3.2% sodium citrate solution. The PRP preparation procedure consisted of two centrifugation steps. All steps were performed in a refrigerated centrifuge (certified Jouan Br4i, Saint-Herblain, Loire-Atlantique, France). Whole blood was centrifuged at 300 g for 5 minutes at 18oC. After the first centrifugation, the whole plasma above the buffy coat was collected, separating platelets from red blood cells and leukocytes (PRP1). PRP1 was centrifuged at 700 g during 17 minutes at 18oC. After the second centrifugation step, the platelet pellet was suspended in 300 μL of platelet-poor plasma (PPP; new fraction named PRP2). Platelet activation was performed adding 20 mm CaCl2 (PRP2-Ca) and 25 IU/mL human plasma thrombin (PRP2-Thr, Ref: T6884; Sigma-Aldrich, St. Louis, MO), incubating at 37°C for 1 hour and at 4°C for 16 hours. To recover the activated PRP2, all of the treated samples were centrifuged at 3000 × g for 20 minutes at 18°C. All samples were stored at –80°C.19
In our current study, the preparation of the PRP was optimized, maximizing platelet recovery and the yield after the two centrifugation steps. We recovered 46.9% to 69.5% of the total initial platelets, and the procedure resulted in a 5.4-fold to 7.3-fold increase in platelet concentration (1.4-1.9 × 106 platelets/μL). We concentrated platelets reaching a baseline of 1 million platelets /μL and finally recovered at least 50% of the initial platelet amount that was higher than what was reported for commercial PRP kits.20,21 Platelets are highly purified; only less than 0.10% and 1.90% from the initial red blood cells and leukocytes, respectively, were present in the final PRP preparation.19
Transmission Electron Microscopy (TEM)
Different samples were fixed with glutaraldehyde 2% in a Sorensen buffer pH 7.4 for 2 h, post-fixed in 1% osmium tetroxide in an aqueous solution for 2 h, dehydrated in graded concentrations of acetone, embedded in Epon-Araldite and cut with an Ultracut E Ultra-microtome (Reichert, Wien, Austria). At the end of dehydrating process, samples were positioned in a multi-well grid for electron microscopy and observed using a TEM Morgagni 268D (FEI Philips).
Scanning Electron Microscopy (SEM)
Specimens were fixed with glutaraldehyde 2% in 0.1 m PB, dehydrated in graded ethanol, critical point dryer (CPD 030, Balzers, Vaduz, Liechtenstein), fixed to stubs with carbon-based adhesive, sputtered with carbon or with gold by a MED 010 coater (Balzers) and examined with an XL30 ESEM (FEI Philips) scanning electron microscope (FEI Company, Eindhoven, Netherlands).
RESULTS
The study was executed in 13 adult patients (11 females and 2 males) who were candidates for facial rejuvenation surgery (facelift), with ages ranging between 45 and 65 years of age (mean, 56.2 years) with no history of chronic viral, metabolic, ischemic, or autoimmune diseases or other systemic pathologies. Patients who reported a smoking habit were excluded.
In a previous study, we described treatment with expanded adipose-derived stem cells and fat with its SVF (SVF-enriched fat obtained by centrifugation) modifying the pattern of collagen and elastic fibers in the dermis with a skin rejuvenation effect.15 In our current study, we have confirmed and expanded the results of our previous study in a larger study group and analyzed the histological and ultrastructural changes of aged facial skin after the injection of expanded adipose-derived stem cells and SVF-enriched fat, comparing the results with those obtained with the use of fat enriched with PRP.
The treatment with adipose tissue plus PRP also induced modifications in the papillary dermis, in the reticular dermis, and at the dermoepidermal junction (Figures 1-7).
Figure 2.
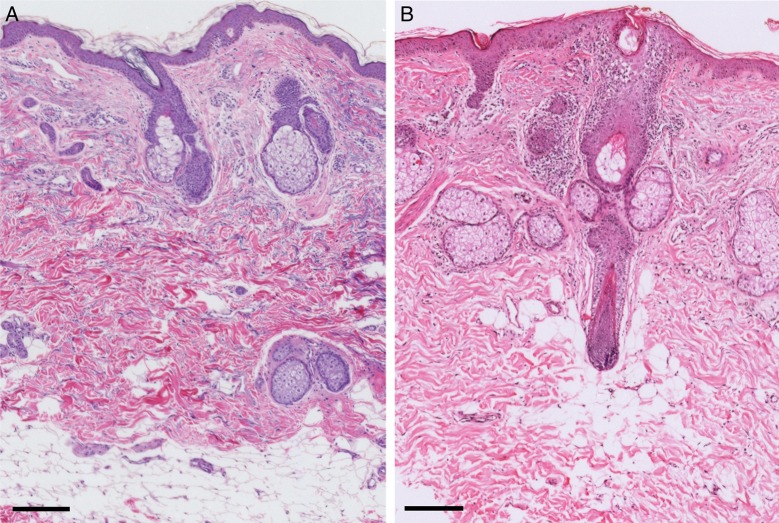
Hematoxylin and eosin stained skin. (A) Control. (B) Treated. Comparing the control (A) and skin treated with fat plus PRP (B), small mononuclear cell infiltrates are visible in the dermis of treated skin. Scale bars: 200 µm.
Figure 3.
Hematoxylin and eosin stained skin. (A) Control. (B) Treated. In the specimen treated with fat plus PRP (B), a mild oedema and disorganized adipose tissue are visible at the dermoepidermal junction. Scale bars: 200 µm.
Figure 4.
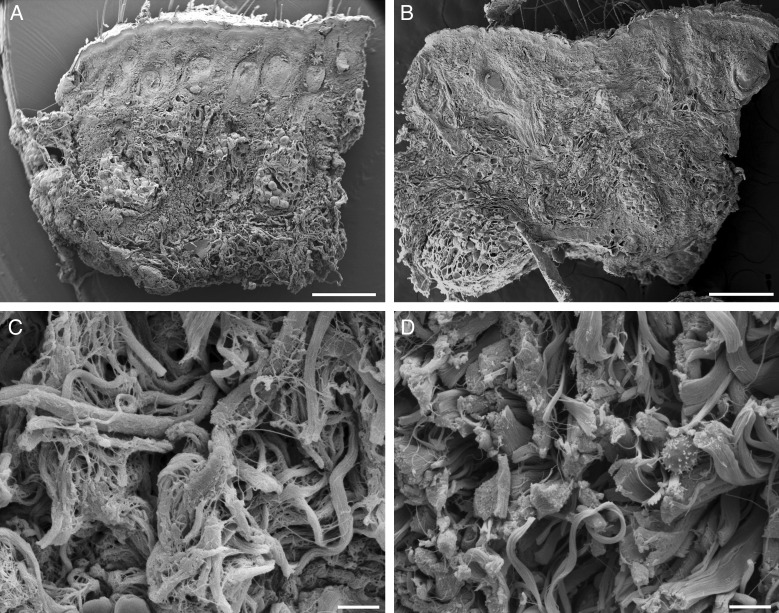
Scanning electron microscopy of the skin. (A,C) Control. (B,D) Treated. At low magnification, no modifications are visible in the skin treated with fat plus PRP. However, at high magnification, in the deep dermis, isolated lymphocytes are visible among the connective bundles that appear separated by wide extracellular spaces. Scale bars: (A,B) 500 µm, (C,D) 50 µm.
Figure 5.
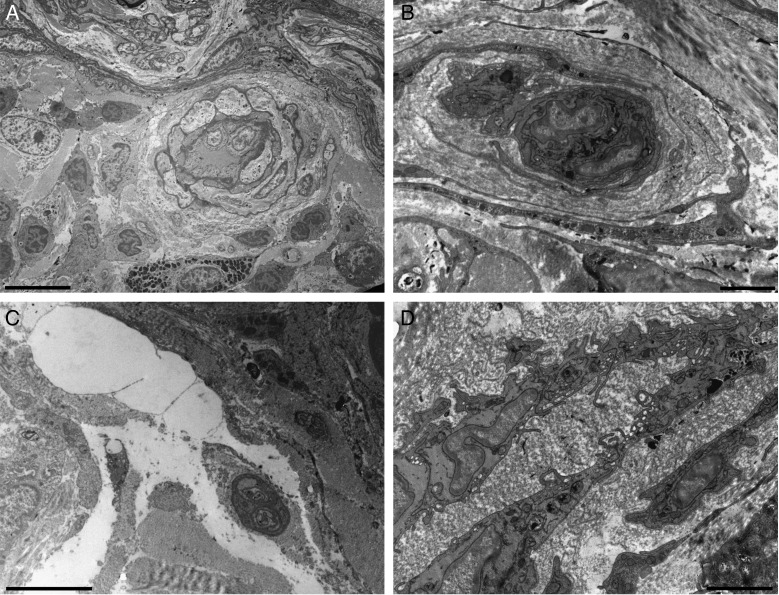
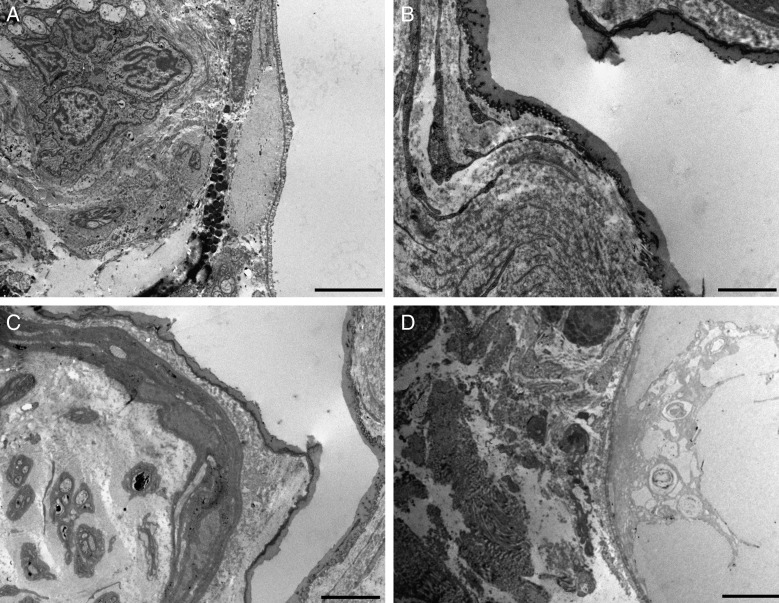
Transmission electron microscopy of the skin after treatment with fat plus PRP. (A) Perivascular infiltrates. (B) Reduplicated vascular basement lamina. (C) Oedema among the connective fibers of the dermis. (D) Activated vascular endothelium is visible. Scale bars: (A) 10 µm, (B) 2 µm, (C) 5 µm, (D) 2 µm.
Figure 6.
Transmission electron microscopy of the skin after treatment with fat plus PRP. (A-D) Ultrastructural aspects of adipocytes at the dermoepidermal junction (the white area of all specimens). The area is rich in vessels (A) and nerves (C). The connective tissue around the adipocytes forms a thick layer (B). The adipocyte can show cytoplasmic alteration and can be surrounded by oedema (D). Scale bars: (A,C,D) 5 µm, (B) 2 µm.
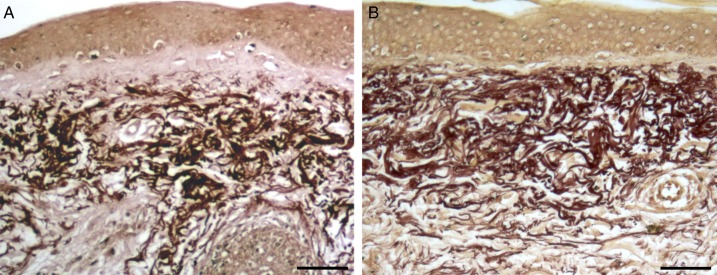
Figure 1.
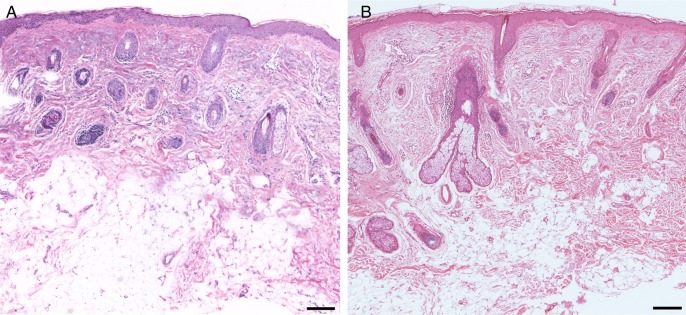
Orcein stained skin. (A) Control. (B) Treated. In the specimen treated with fat plus PRP, the presence of elastosis in reticular dermis and elastic material (thinner fibers) in the papillary dermis are visible. Scale bars: 70 µm.
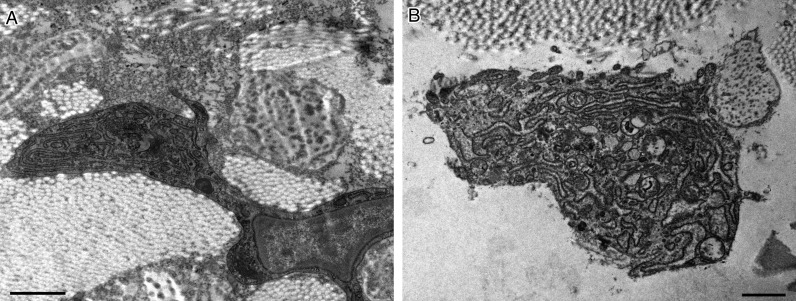
Figure 7.
Transmission electron microscopy of the skin. After treatment with fat plus PRP (A,B), activated fibroblasts are visible in the dermis. Scale bars: 1 µm.
In the papillary dermis, before the treatment, oxytalan and elastic fibers were generally scarce and the treatment resulted in an increase of the oxytalan elastic network that was visible by orcein stain. When observed at TEM, some new elastic fibers were of small diameter and formed an irregular network below the dermoepidermal junction.
In the reticular dermis, before the treatment, a moderate degree of elastosis was found. By orcein stain, a dense and disorganized network of elastic fibers that sometimes formed compact blocks, was visible. At ultrastructural examination, the reticular dermis showed irregularly dispersed elastic fibers generally of large diameter enmeshed in a network of thin collagen fibers. In the deepest portion of the reticular derma, the collagen component was also organized in large-diameter bundles composed of strictly packed fibers. After treatment with fat graft and PRP, the reticular dermis showed a decrease in the elastic fiber network. The ultrastructural examination showed a modified 3-dimensional architecture of the reticular dermis in which the elastic fibers, with respect to the pretreatment specimens of the same patient, appeared with a reduced diameter and with a smoother surface because of a lesser developed fibrillar component at their periphery.
At the dermoepidermal junction, before the treatment, the adipocytes located at the boundary of the dermis and the subcutaneous tissue, were organized in lobules, which were often vertically directed toward the surface of the skin. These lobules formed adiposae papillae, in which a vascular peduncle was often seen. In their apical portion, the papillae often showed a relationship with sebaceous or sweat glands. At this level, the adipocytes were covered by a dense basket of collagen strictly adherent to their plasma membrane.
After treatment with adipose tissue and PRP, in the reticular dermis and dermoepidermal junction, perivascular areas with mononuclear cell infiltration and edema were visible. Accumulation of electrondense material in the form of irregular masses was also detected. These areas showed blood capillaries and arterioles surrounded by edematous areas and mononuclear infiltrates. Often the blood vessels were surrounded by reduplicated and fragmented layers of basal lamina. These vessels were often in relationship with small nerve fibers. In some of the patients before the treatment, infiltrate composed of mononuclear cells was also present around the vessels and glands, but these features appeared more evident after the application of the adipose tissue enriched with PRP.
DISCUSSION
Data about the regeneration of the skin are scarce. More human studies are necessary in this area, because animal models are not useful given the long life of human beings and the peculiarities of their skin with less hair.
In a previous study, we showed for the first time that the aging process of the skin of the human face can be changed by expanded adipose-derived stem cells or fat enriched with its SVF, obtained by centrifugation without an enzymatic process.15 This study showed in particular that, as the largest event of skin aging, elastosis in the reticular dermis can be reduced simultaneously with the increase of new elastic fibers formed in the papillary dermis. To take these results into consideration in the first study, we utilized an advanced protocol that integrated histological, immunohistochemical, and ultrastructural analysis. Although we achieved an excellent result in that study, it is evident that it would be important to optimize the surgical procedures to make them more secure and effective, and to apply them to a high percentage of patients. The use of fat grafts in the face has been reported to show an improvement in skin quality on its macroscopic appearance, such as an improvement in skin hydration.5,13
In this sense, we wished to assess, if possible, similar results without the use of expanded stem cells that require a cell factory and have their use limited by an ethical committee. We therefore replaced the stem cells with PRP, which is easier to obtain and for which a possible regenerative effect has been already described,16,17 even though those results were empirical before now. Furthermore, in the previous study, we observed an important aspect of regeneration: the ability to produce new blood vessels in the deep part of the reticular dermis and the dermal-epidermal junction. PRP was described as having an angiogenic action, increasing fat graft survival by providing nutrient support from its plasma component, enhancing the proliferation of preadipocytes and adipose mesenchymal stem cells22,23; therefore, PRP seems to be an ideal compound for this purpose.
Fat has also been utilized in combination with platelet-enriched autologous plasma (PRP), considering that PRP is rich in growth factors and cytokines. In recent years, topical and injectable growth factors and cytokines have emerged as intriguing therapeutic modalities, with burgeoning interest in their potential to serve as actives for skin rejuvenation.16 PRP is developed from autologous blood and consists of a volume of autologous plasma with a platelet concentration above the baseline; therefore, an increased concentration of platelet growth factors is also present. Platelets are enucleated circulating blood particles that derive from the fragmentation of megakaryocytes. They circulate in an inactivate state until they come into contact with endothelia damage areas. Platelets work through the degranulation of the α-granules in platelets, which contain the synthesized and prepacked growth factors. The most potent platelets to restore damaged tissues are platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-beta), insulin growth factor (IGF), vascular endothelial growth factor (VEGF), and endothelial growth factor (EGF). The synthesized growth factors are directly bound to the surface of the cell membrane to stimulate haemostasis and normal healing. They induce internal cellular signaling that activates angiogenesis, cell proliferation, cell differentiation, and new matrix formation for tissue repair. Platelets are also a natural reservoir of growth factors that could be utilized to regenerate tissues. For this reason, PRP is assumed to have a high potential of healing; however, there is a paucity of data to support or oppose the use of PRP in fat graft surgery.17
Cervelli et al24 studied the effect of PRP on adipose-derived stem cells and found that PRP alone did not increase the adipogenesis of adipose-derived mesenchymal stem cells. Craft et al25 showed increased fat graft survival in nude mice by the effect of long-term delivery of PDGF by microspheres. Gentile et al16 demonstrated that PRP could yield similar volume maintenance of fat graft as SVF, by 69% and 63%, respectively. In addition, the PDGF exhibits controversial roles on adipogenesis. Although most growth factors do not favor adipogenesis, most of them indeed stimulate angiogenesis.22
The results of this study seem to largely confirm those of our previous analysis,15 in optical and electron microscopy, showing the same pattern on the development of collagen and elastic fibers in the skin after the injection of adipose tissue enriched with PRP, compared with controls treated with only expanded adipose-derived stem cells or SVF-enriched fat. Considering the three kinds of treatments, the presence of fat's components, as the adipose-derived stem cells, is a common factor in all of these. This fact could justify the similarity in the pattern of results; however, the use of PRP has not proved able to change the structure of the elastic component of the skin better than the previous experimental paradigm.
The use of PRP led to the presence of more pronounced inflammatory infiltrates and a greater vascular reactivity shown by the presence of vascular reduplication of the basal membrane, which indicated an increase in vascular permeability through its wall. It seems that there was also a certain reactivity of the nervous component. The vessels were often in relationship with small nerve fibers. This effect of increased vascular reactivity and the nervous component may be useful in some pathological situations in which an intense angiogenesis is desirable, such as in situations of tissue ischemia. Overall it does not seem that the use of PRP may have significant advantages in terms of rejuvenation over the use of fat enriched with its SVF, obtained by centrifugation without an enzymatic process, or expanded adipose-derived stem cells.
As a tool for tissue engineering, PRP could utilize the full potential of the platelet concentrate as a stimulating culture medium, as a biological binder, and as a supporting scaffold for cells. The concept is to build in vitro an engineered tissue using mesenchymal stems cells growing in a platelet gel, with the fibrin scaffold serving as the physical support of the graft, without any additional solid material. However, several authors have also noticed that the best results were always obtained when the platelet gel was reinforced with fibrin glue. As tissue engineering applications, the platelets and growth factors alone are not sufficient.26
A major limitation of this study is the absence of a precise quantification of the changes induced by the various treatments. This is primarily because of the inherent limitations of the ultrastructural histological investigations. Particularly for the latter, this refers to the lack of extension of the examined areas that make examinations necessarily more qualitative than quantitative. However, we must also consider the interindividual variability of the patients examined that is linked to different modifying factors, such as exposure to the sun. However, the use of investigative techniques and multiple comparisons to previous studies conducted with similar methods and samples helps to analyze the results in detail and allows identifying with sufficient certainty what is the target of this kind of tissue treatment. Other limitations of this study are the use of a unique ratio between the volume of fat and PRP (1:1) on the skin to observe the regenerative effect and the absence of randomized, double-blinded control studies to provide powerful evidence-based support. Future proposals in combining therapies for skin regeneration should be conducted to determine the effective ratio between the biomaterials utilized to specifically aim for different cutaneous conditions.
CONCLUSION
The treatment with adipose tissue plus PRP (1:1) induced modifications in the papillary dermis, in the reticular dermis, and at the dermoepidermal junction, probably because of the presence of the fat component. The addition of PRP did not have significant advantages in terms of regeneration over the use of expanded adipose-derived stem cells or SVF-enriched fat in skin rejuvenation. The use of PRP led to the presence of more pronounced inflammatory infiltrates and increased vascular and nervous component reactivity. These may be useful in pathological situations in which an intense angiogenesis is desirable, such as tissular ischemia, or for nervous repair. However, the inflammation is an unfavorable event in normal tissular physiology. Based on the results of this study, an agent that causes inflammation could be not indicated in a procedure utilized to obtain skin rejuvenation.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1.D'Amico RA, Rubin JP, Neumeister MW et al. American Society of Plastic Surgeons/Plastic Surgery Foundation Regenerative Medicine Task Force. A Report of the ASPS Task Force on regenerative medicine: opportunities for plastic surgery. Plast Reconstr Surg. 2013;1312:393-399. [DOI] [PubMed] [Google Scholar]
- 2.Rigotti G, Marchi A, Sbarbati A. Adipose-derived mesenchymal stem cells: past, present, and future. Aesthet Plast Surg. 2009;333:271-273. [DOI] [PubMed] [Google Scholar]
- 3.Del Vecchio D, Rohrich RJ. A classification of clinical fat graft: different problems, different solutions. Plast Reconstr Surg. 2012;1303:511-522. [DOI] [PubMed] [Google Scholar]
- 4.Bosset S, Barré P, Chalon A et al. Skin ageing: clinical and histopathologic study of permanent and reducible wrinkles. Eur J Dermatol. 2002;123:247-252. [PubMed] [Google Scholar]
- 5.Kim JH, Jung M, Kim HS et al. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol. 2011;205:383-387. [DOI] [PubMed] [Google Scholar]
- 6.Meruane MA, Rojas M, Marcelain K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2012;1301:53-63. [DOI] [PubMed] [Google Scholar]
- 7.Rigotti G, Marchi A, Galiè M et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;1195:1409-1422; discussion 1423-1424. [DOI] [PubMed] [Google Scholar]
- 8.Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;693:249-256. [DOI] [PubMed] [Google Scholar]
- 9.Boyette LB, Tuan RS. Adult stem cells and diseases of aging. J Clin Med. 2014;31:88-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;1312:4279-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scioli MG, Bielli A, Gentile P et al. The biomolecular basis of adipogenic differentiation of adipose-derived stem cells. Int J Mol Sci. 2014;154:6517-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gir P, Oni G, Brown SA et al. Human adipose stem cell: current clinical applications. Plast Reconstr Surg. 2012;1296:1277-1290. [DOI] [PubMed] [Google Scholar]
- 13.Mojallal A, Lequeux C, Shipkov C et al. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009;1243:765-774. [DOI] [PubMed] [Google Scholar]
- 14.Mailey B, Saba S, Baker J et al. A comparison of cell-enriched fat transfer to conventional fat grafting after aesthetic procedures using a patient satisfaction survey. Ann Plast Surg. 2013;704:410-415. [DOI] [PubMed] [Google Scholar]
- 15.Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. 2015;1354:999-1009. [DOI] [PubMed] [Google Scholar]
- 16.Gentile P, Orlandi A, Scioli MG et al. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cell Transl Med. 2012;13:230-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pires Fraga MF, Nishio RT, Ishikawa RS. Increased survival of free fat grafts with platelet-rich plasma in rabbits. J Plast Reconstr Aesthet Surg. 2010;6312:e818-e822. [DOI] [PubMed] [Google Scholar]
- 18.Condé-Green A, de Amorim NFG, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;638:1375-1381. [DOI] [PubMed] [Google Scholar]
- 19.Amable PR, Carias RB, Teixeira MV et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013;43:67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzucco L, Balbo V, Cattana E et al. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Platelnex and one manual procedure. Vox Sang. 2009;972:110-118. [DOI] [PubMed] [Google Scholar]
- 21.Kaux JF, Le Goff C, Seidel L et al. Comparative study of the five techniques of preparation of platelet-rich plasma. [Article in French] Pathol Biol (Paris). 2011;593:157-160. [DOI] [PubMed] [Google Scholar]
- 22.Blanton MW, Hadad I, Johnstone BH et al. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg. 2009;123(2 Suppl.):56S-64S. [DOI] [PubMed] [Google Scholar]
- 23.Fukaya Y, Kuroda M, Aoyagi Y et al. Platelet-rich plasma inhibits the apoptosis of highly adipogenic homogeneous preadipocytes in an in vitro culture system. Exp Mol Med. 2012;445:330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervelli V, Scioli MG, Gentile P et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med. 2012;13:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craft RO, Rophael J, Morrison WA et al. Effect of local, long-term delivery of platelet-derived growth factor (PDGF) on injected fat graft survival in severe combined immunodeficient (SCID) mice. J Plast Reconstr Aesthet Surg. 2009;622:235-243. [DOI] [PubMed] [Google Scholar]
- 26.Zhu SJ, Choi BH, Jung JH et al. A comparative histologic analysis of tissue-engineered bone using platelet-rich plasma and platelet-enriched fibrin glue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;1022:175-179. [DOI] [PubMed] [Google Scholar]