Abstract
Background
The earFold™ implantable clip system is a new treatment for prominent ears using an implant made from nickel-titanium alloy, forged into a predetermined shape. The implant is fixed to the cartilage then released, causing the cartilage to fold back.
Objectives
The study aimed to test the safety and behaviour of the implant in vivo.
Methods
This was a Phase 1, prospective, nonrandomised study. Thirty-nine patients were recruited, from 7 to 57 years of age (22 adults and 17 children). Thirty-seven patients were followed up for a minimum of 18 months. A total of 131 implants was used to treat 75 ears. All treatments were performed under local anaesthetic.
Results
Eighteen patients asked for their implants to be left in place permanently. Twenty-one patients agreed to have their implants removed at 6, 12, or 18 months after insertion. Complications affected 8 patients and included extrusion, infection, hypertrophic scarring, and Spock-ear formation. No new complications have arisen in any of the patients since the conclusion of the study, up to a maximum of 47 months. Patients were overwhelmingly satisfied with the outcome of treatment.
Conclusions
earFold can be used as a permanent implant to correct prominence of the human ear. It is best suited for treating prominent ears with a poorly formed or absent antihelical fold. The procedure is quick and predictable with a complication rate comparable to suture-based otoplasty techniques.
This article describes a new technique for correction of prominent ears by subcutaneous placement of a permanent metal implant made of nitinol, a nickel-titanium alloy.1 The earFoldTM (Contract Medical International GmbH, Dresden, Germany) implant is not a suture, although it reproduces many of the same effects, nor is it an external splint. Nevertheless, the implant is able to exert a corrective effect by reshaping the cartilage in a predictable and reproducible manner without the need to sculpt it. Therefore, earFold represents a new approach to the correction of prominent ears.
The authors acknowledge that, in experienced hands, conventional otoplasty surgery is highly successful for prominent ear correction. The intention of earFold is not to replace existing otoplasty techniques but to provide patients with an alternative that is rapid and predictable with low recurrence rates. The rapidity of treatment with earFold also makes it possible to consider treatment under local anaesthetic for all patients, thereby avoiding the risks associated with general anaesthesia. Finally, use of a standardised implant gives patients some measure of control over the outcome of treatment—something not easily achieved with conventional otoplasty surgery.
Herein, we report on the results of a prospective, nonrandomised, first-in-human, pilot study of the earFold implant for treatment of prominent ears. The principal aim of the study was to investigate the safety and behaviour of the implant in the human ear and its ability to correct prominence. The aesthetic outcomes of treatment with earFold were not specifically addressed in this study.
METHODS
The earFold™ Treatment System
The earFold treatment system consists of three components; the earFold implant, the earFold introducer, and the preFold™ positioner. The main function of the implant is to create or enhance the shape of the antihelical fold.
The earFold implant is made of a thin strip of nitinol that is heat-treated to fix it into a predetermined shape. The implant used in the study is metallic gray. Nitinol is a super-elastic alloy of nickel and titanium,1-2 its super-elastic properties ensure that the implant always returns to its pre-programmed shape while exhibiting extreme resistance to metal fatigue. Triangular-shaped tines grip the cartilage when deployed. The implant is thin (approximately 0.15 mm) and lightweight (approximately 0.068 gm). All implants are 5 mm wide and 15 mm long.
The earFold introducer (Figure 1) holds the implant in a flattened position before deployment. The earFold implant is pre-loaded into this introducer and the two components are then sterilised together in ethylene oxide before being packaged and supplied for use. The introducer and implant are intended as single-use items.
Figure 1.

The earFold™ implant in place in earFold introducer.
The preFold positioner (Figures 2 and 3) is identical to the earFold implant in terms of size, shape, and elasticity but does not have triangular-shaped tines. preFold is supplied as a reusable, nonsterile device that is used preoperatively to determine the suitability of a patient for treatment with earFold. preFold must be used before treatment to determine the number, position and orientation of the earFold implants needed for treatment. To achieve this, preFold positioners are placed onto the anterior surface of the ear to create a “new” antihelical fold (Figure 2). The preFold positioners are held in place using double-sided sticky tape (12 mm × 11.4 m, 3M, Scotch™, 3M, Bracknell, UK).
Figure 2.

Images of a 26-year-old male. Preoperative assessment using preFold™ positioners, held in place with double-sided sticky tape (A) Before assessment and (B) with preFold positioners in place.
Figure 3.

(A) Gold-plated, CE-marked version of preFold™ and (B) gold-plated, CE-marked version of earFold™. Images of a 36-year-old male (C) preoperative, (D) with preFold in place, and (E) 5 months postoperative, showing the effect of gold-plating on the visibility of the earFold implant in subcutaneous position.
Although the size, shape, and elasticity of earFold (and preFold) are fixed, the shape and elasticity of the cartilage in the region of the antihelical fold varies from individual to individual and in different positions of the ear. Therefore, treatment with earFold can be individualised by adjusting the number, position, and orientation of the implant(s) along the curve of the new antihelix to achieve more or less folding of the cartilage. During this process of trial and error, the patient is normally involved in the process of choosing how their ears will appear. It is important to note that if the patient and surgeon are unable to agree on the best position for preFold preoperatively, the patient may not be suitable for subsequent treatment with earFold, and an alternative method for correction of prominence should be considered.
Patients
The study commenced in July 2011 and ended in September 2013. Patients were recruited from the clinics of the principle author. Patients were excluded from the study if they were active smokers, had diabetes, suffered from malignancy, took anticoagulants regularly, were undergoing psychiatric or psychological treatment, or were pregnant. Patients were also excluded if they had prominence solely due to a deep conchal bowl, a documented wound healing problem, or a family history of keloid scarring. Smokers were accepted into the study if they had ceased all nicotine intake 3 months before treatment. Patients were considered for inclusion in the study if they were male or female ≥7 years of age (no upper age limit) with a helical-mastoid distance (H-M distance) >20 mm, fully able to understand the requirements of the study, and able to sign a witnessed/informed consent form.
Patients were selected for entry into the study if they met the inclusion criteria and if it proved possible to obtain a satisfactory correction of their prominence using preFold positioners when assessed in the clinic preoperatively. Although the principle purpose of earFold is to improve the definition of the antihelical fold, some patients with a poorly formed/absent antihelical fold and a deep conchal bowl also obtained satisfactory correction of their prominence when the lateral wall of the concha was rolled into the new antihelical fold. Moreover, earFold has no effect on protruding earlobes although the presence of a protruding earlobe was not considered an exclusion criterion for the study.
Ethics
The clinical trial protocol for the study (EF-2011-01) was reviewed and approved by the National Research Ethics Committee (NREC) of the United Kingdom in June 2011. Separate approval for the study was also obtained from the Institutional Review Board of West Hertfordshire NHS Trust, Watford, United Kingdom where the trial was performed. All patients gave their full, written consent to participate in the study in accordance with the Declaration of Helsinki. This study was registered with the Comprehensive Clinical Research Network (CCRN) of the National Institutes of Health Research (NIHR) of the United Kingdom (Study number CCRN 557). This is a voluntary registry of clinical trials.
Outcome Measures
All data were collected prospectively in accordance with the trial protocol and were collated and analysed by an independent clinical-trial review organisation based in Boston, Massachusetts (Health Policy Associates Inc.).
Primary Outcome Measure
The primary outcome measure was the H-M distance measured with a millimetre ruler. The H-M distance was defined as the maximum distance (millimetres) from the mastoid to the most prominent part of the helix when viewing the patient in an anteroposterior direction. This definition was used because the part of the helix that was most prominent sometimes changed after treatment; thus, the position at which the H-M was maximal was sometimes different after treatment. Each measurement was taken at least 3 times and an average was generated and recorded. All treatment encounters were recorded using a standardised Case Report Form.
The H-M distance was used as the primary outcome measure after discussion with the NREC who stipulated that an "objective" measurement should be used to determine the effectiveness of treatment with the implant. Similarly, the minimum H-M distance for inclusion in the study was arbitrarily set at ≥20 mm rather than on the basis of the patient's aesthetic concerns. Finally, after further discussion with the NREC, the protocol set the minimum criterion for a successful outcome of treatment with earFold as a 10% reduction in the H-M distance for each ear that was treated. We acknowledge that use of H-M distance alone is not sufficient to determine the entirety of the outcome for an aesthetic procedure. However, the principle aim of this pilot study was to determine the safety and behaviour of the earFold implant and not the aesthetic success of treatment.
Secondary Outcome Measures
The ease of the procedure was determined by recording all procedures on video. Adverse events were recorded using a standardised Adverse Event Form. Before and after photos were also used to record outcomes. However, these photographs were not taken in a standardised format and were not formally assessed as part of the trial. Patient satisfaction was assessed using a standardised Patient Evaluation Measures (PEM) questionnaire. The PEM was created by the senior author based on a similar (validated) questionnaire used to evaluate Patient Recorded Outcomes after hand surgery.3-4 The PEM was used because the authors were unable to find a suitable questionnaire to evaluate outcomes after prominent ear correction using an implant. Each participant was asked to complete the PEM 3 times: before treatment, 3 months after treatment, and at the conclusion of the study. The patients were identifiable by their initials and patient ID number on each questionnaire. However, analysis of the data from these questionnaires was carried out by an independent party (Health Policy Associates, Boston, Massachusetts) with no knowledge of the patients other than their ID number. A blank copy of the questionnaire is included (Supplementary Appendix 1, available as Supplementary Material at www.aestheticsurgeryjournal.com).
Method of Insertion
Assessment of all ears was performed using preFold positioners (as detailed above). All assessments were performed by the author but 12 patients had their implants inserted by a trainee under close supervision.
Once a decision was made on the number of implants required and their exact position/orientation, this position was marked on the skin. Under local anaesthetic, an incision (8-10 mm long) was made 1-2 mm away from the proximal edge of the skin markings. A subcutaneous tunnel was then developed that was 2 mm wider and longer than the area marked as the footprint for the implant (Figure 4).
Figure 4.

A subcutaneous pocket is created which is 2 mm larger than the area marked as the footprint for the implant.
The introducer, preloaded with an implant, was now inserted in line with the skin markings. The operator pressed the implant firmly onto the anterior surface of the cartilage to fully engage the tines before deployment (Figure 5). The implant was deployed by pushing the top slider of the introducer forwards while maintaining firm downward pressure on the implant to ensure that it remained in close contact with the cartilage until fully deployed.
Figure 5.

Demonstrating the method of deployment of earFold™. The introducer and the implant are inserted and the implant is deployed while maintaining firm downward pressure over the implant to ensure that the tines are fully engaged in the cartilage.
Once deployed, earFold returned immediately to its pre-programmed shape folding the ear cartilage (Figure 6). The skin incision was then closed with interrupted 6-0 Vicryl Rapide™ (Ethicon, Livingstone, UK) sutures and dressed with steristrips™ (3M, Bracknell, UK). No other dressing was employed.
Figure 6.

Mechanism of action of earFold™.
Patients were advised to wash and shower immediately but to avoid sleeping on their ears or sides for 4 weeks. Patients were also advised to avoid all contact sports and swimming for 4 weeks. Thereafter, patients were advised to resume all normal activities. Simple, oral analgesia (ibuprofen or acetaminophen) was prescribed for all patients.
Anterior Scoring
For the 7 patients who had treatment with earFold combined with anterior scoring, the scoring technique used was adapted from Bulstrode et al who used a bent 21G needle (green hub) to score the anterior surface of the cartilage in the area of the antihelical fold was adapted from Bulstrode et al, using a bent 21G needle (green hub) to score the anterior surface of the cartilage in the area of the antihelical fold.5 The needle was introduced through the incision used to insert the earFold implant.
Removal of the Implant
The initial incision was reopened. The proximal edge of the implant was dissected free and grasped firmly with artery forceps. Pulling firmly, the implant would slide out. The skin incision was closed again with absorbable sutures or dressed with steristrips.
All patients were asked to have their implants removed at 6, 12, or 18 months after insertion. The aim was to determine whether earFold had a moulding effect on the underlying cartilage. We tried to ensure that there were at least 3 patients at each time point for adults and for children. The H-M distances were measured at 2 months after removal of the implant.
CE-Mark
Data from this study were submitted for CE-mark approval. Since the conclusion of the study, the design of the earFold implant has been modified to incorporate some of the findings of this pilot study and to simplify large-scale manufacturing. The current version of the earFold implant is now coated with a thin layer of 24-carat gold to reduce its visibility under the skin. Gold-plating has resulted in a small increase in the overall cost of the implant (<5%). Moreover, the middle row of tines has been removed and two small holes have been placed at one end of the implant (Figure 3A,B). These changes have had a major effect in reducing the visibility of the implant under the skin (Figure 3C-E). A full CE-mark for this version of the earFold implant was granted in April 2015.
RESULTS
Demographics and Follow-Up
Thirty-nine patients were recruited (22 female and 17 male). Patients ranged from 7 to 57 years of age (average 24 years). The study included 22 adults (aged ≥16 years) and 17 children (aged 7 to 15 years). None of the patients were active smokers at the time of treatment or at any time after treatment.
Thirty-seven patients returned for their final follow-up assessment at 18 months after treatment. Therefore, mean follow-up time was 17 months. Although the study was formally closed in September 2013 (when the last patient recruited reached their 18 month time-point), follow-up has continued for some of the patients recruited and is now up to a maximum of 47 months. A total of 131 implants were used to treat 75 ears (average 1.7 implants per patient).
Ease of Use
Insertion of earFold is fast. The procedure was defined as having started when the first incision was made and ended when the last suture was inserted. From video recordings, it took approximately 3 minutes to insert each earFold implant. Removal of the implants was also quick, taking an average of 1.7 minutes per implant. The additional step of anterior scoring added another 2 minutes to each implant inserted (ie, 5 minutes per implant). Outcomes from patients treated by the trainee were no different from those treated by the senior author. A video demonstrating the procedure is available online at www.aestheticsurgeryjournal.com.
Typical Outcomes
Typical outcomes from treatment with earFold are shown at 18 months after insertion (Figures 7 and 8 and Supplementary Figures 1-3, available as Supplementary Material at www.aestheticsurgeryjournal.com). The photos show that reasonable correction of prominence and reasonable symmetry were achieved. However, the gray-metal implant was very visible under the skin. In most cases, the scar for insertion of the implant was imperceptible by 3 months. The implant was also used to correct asymmetry (Supplementary Figure 2, available as Supplementary Material at www.aestheticsurgeryjournal.com) and to assist in revision after previous standard otoplasty (Figure 7 and Supplementary Figure 1, available as Supplementary Material at www.aestheticsurgeryjournal.com).
Figure 7.
A 35-year-old female (Patient 26) who underwent standard otoplasty as a child using anterior scoring technique. Prominence recurred (especially at the upper pole), therefore she requested treatment with earFold™ as an adult. The photos show the outcome of treatment with earFold only. (A, C, E) Preoperative H-M distances were 29 mm (left ear) and 30 mm (right ear). (B, D, F) At 18 months postoperative the H-M distances were 14 mm (left ear) and 14 mm (right ear). The implants were left in place permanently at the patient's request.
Figure 8.
A 16-year-old female (Patient 43) with bilateral prominence had undergone no previous treatment. Her request was for her ears to be “pinned back”. Treatment was with earFold™ only. (A, D, G) Preoperative H-M distances were 29 mm (left ear) and 30 mm (right ear). (B, E, H) At 18 months postoperative the H-M distances were 20 mm (left ear) and 20 mm (right ear). All implants were removed at 18 months. (C, F, I) Two months after removal of all implants showing almost complete recurrence of the original prominence. The H-M distances were 26 mm (left ear) and 26 mm (right ear).
H-M Distances
The average H-M distance preoperatively was 29.7 mm per ear (Table 1). Comparison of the H-M distances for all patients before and three months after treatment showed that earFold resulted in a 37% reduction in prominence per ear (ie, a reduction in H-M distance from 29.7 to 18.7 mm). Eighteen patients asked for their implants to be left in place permanently. For these patients, there was a 33.5% reduction in the H-M distance after 3 months in place and a 34.6% reduction after 18 months, suggesting that the correction is stable over this period of observation.
Table 1.
Summary of the H-M distances for patients in the study. Patients shaded in blue were treated with a combination of earFold™ and anterior scoring
| ID | Age (years) | H-M (L) preoperative (mm) | H-M (R) preoperative (mm) | Total implants used | H-M (L) at 3 months (mm) | H-M (R) at 3 months (mm) | H-M (L) at 6 months (mm) | H-M (R) at 6 months (mm) | H-M (L) at 12 months (mm) | H-M (R) at 12 months (mm) | H-M (L) at 18 months | H-M (R) at 18 months (mm) | H-M (L) at 2 months post-removal (mm) | H-M (R) at 2 months post-removal (mm) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 8 | 36 | 35 | 2 | 25 | 25 | 23 | 24 | Implants left in permanently | ||||||
| 5 | 37 | 25 | 26 | 5 | 20 | 20 | 18 | 18 | Implants left in permanently | ||||||
| 7 | 7 | 28 | 30 | 2 | 19 | 19 | 20 | 20 | Implants left in permanently | ||||||
| 10 | 50 | 35 | 37 | 4 | 22 | 21 | 21 | 21 | Implants left in permanently | ||||||
| 16 | 31 | 30 | 25 | 2 | 20 | 20 | 22 | 21 | Implants left in permanently | ||||||
| 22 | 51 | 25 | 25 | 4 | 15 | 14 | 11 | 11 | Implants left in permanently | ||||||
| 23 | 11 | 28 | 31 | 4 | 20 | 20 | 18 | 18 | Implants left in permanently | ||||||
| 26 | 35 | 29 | 30 | 4 | 15 | 16 | 14 | 15 | Implants left in permanently | ||||||
| 31 | 10 | 35 | 28 | 1 | 20 | 20 | 30 | 20 | Implants left in permanently (1 spock ear - on left side - adjusted) | ||||||
| 35 | 8 | 35 | 34 | 2 | 20 | 16 | 20 | 16 | Implants left in permanently (1 implant infected - not removed) | ||||||
| 40 | 11 | 26 | 20 | 1 | 20 | N/A | 19 | N/A | Implants left in permanently | ||||||
| 48 | 54 | 38 | 39 | 4 | 26 | 25 | 26 | 25 | Implants left in permanently | ||||||
| 50 | 11 | 28 | 31 | 4 | 20 | 20 | 20 | 20 | Implants left in permanently | ||||||
| 51 | 38 | 30 | 30 | 2 | 19 | 20 | 20 | 20 | Implants left in permanently | ||||||
| 55 | 11 | 30 | 20 | 2 | 18 | N/A | 18 | N/A | Implants left in permanently | ||||||
| 56 | 11 | 30 | 20 | 2 | 19 | N/A | 18 | N/A | Implants left in permanently (1 implant eroded at 12 months) | ||||||
| 57 | 17 | 29 | 30 | 4 | 18 | 20 | 18 | 20 | Implants left in permanently | ||||||
| 24 | 21 | 25 | 23 | 4 | 17 | 17 | 20 | 15 | 20 | 20 | Implants removed at 6 months | ||||
| 45 | 7 | 30 | 30 | 5 | 18 | 18 | 18 | 18 | 27 | 30 | Implants removed at 6 months (2 implants eroded) | ||||
| 28 | 12 | 32 | 32 | 4 | 18 | 16 | 18 | 17 | 28 | 28 | Implants removed at 12 months | ||||
| 46 | 16 | 26 | 25 | 2 | 16 | 16 | 16 | 16 | 25 | 25 | Implants removed at 12 months (1 implant eroded, 1 hypertrophic scar) | ||||
| 47 | 52 | 37 | 35 | 4 | 24 | 20 | 24 | 20 | 37 | 35 | Implants removed at 12 months | ||||
| 49 | 10 | 28 | 30 | 4 | 21 | 20 | 21 | 20 | 25 | 28 | Implants removed at 12 months (2 implants eroded + 1 infected) | ||||
| 52 | 39 | 25 | 25 | 4 | 13 | 13 | 13 | 13 | 23 | 22 | Implants removed at 12 months | ||||
| 53 | 42 | 30 | 30 | 4 | 18 | 19 | 19 | 19 | 27 | 25 | Implants removed at 12 months (1 implant eroded) | ||||
| 39 | 12 | 34 | 35 | 4 | 18 | 18 | 17 | 17 | 23 | 25 | Implants removed at 18 months | ||||
| 21 | 8 | 34 | 34 | 4 | 21 | 20 | 20 | 20 | 28 | 28 | Implants removed at 18 months | ||||
| 37 | 7 | 28 | 30 | 3 | 20 | 20 | 20 | 18 | 27 | 29 | Implants removed at 18 months | ||||
| 38 | 15 | 30 | 32 | 4 | 19 | 19 | 17 | 16 | 30 | 32 | Implants removed at 18 months | ||||
| 19 | 8 | 35 | 27 | 4 | 20 | 20 | 24 | 18 | 30 | 23 | Implants removed at 18 months | ||||
| 43 | 16 | 30 | 29 | 4 | 21 | 20 | 20 | 20 | 26 | 26 | Implants removed at 18 months | ||||
| 63 | 16 | 35 | 30 | 4 | 20 | 20 | 20 | 20 | Implants left in permanently | ||||||
| 58 | 38 | 30 | 30 | 4 | 16 | 14 | 15 | 14 | 25 | 22 | Implants removed at 6 months | ||||
| 59 | 58 | 26 | 26 | 2 | 15 | 15 | 15 | 15 | 13 | 14 | Implants removed at 6 months | ||||
| 60 | 26 | 26 | 29 | 4 | 13 | 15 | 10 | 12 | 14 | 16 | Implants removed at 6 months | ||||
| 61 | 43 | 30 | 29 | 4 | 14 | 13 | 15 | 13 | 25 | 20 | Implants removed at 6 months | ||||
| 62 | 46 | 38 | 36 | 4 | 20 | 20 | 20 | 20 | 28 | 29 | Implants removed at 6 months | ||||
| 64 | 15 | 22 | 21 | 2 | 20 | 19 | 19 | 19 | 19 | 18 | Implants removed at 6 months (1 hypertrophic scar) | ||||
| 65 | 42 | 30 | 26 | 4 | 20 | 20 | 20 | 20 | 22 | 25 | Implants removed at 6 months |
When the implants were left in place permanently, a comparison of the H-M distances for left and right ears showed that one patient had a difference of 10 mm between the two sides at 18 months after treatment (patient 31, who developed a “Spock-ear” deformity—see below). For the remainder, the H-M distances were either identical on each side or within 1-2 mm of each other. For patients with unilateral prominence (patients 40, 55, and 56), it was possible to achieve a reduction in the H-M distance of the prominent ear that was within 1-2 mm of the “normal” ear (Supplementary Figure 2, available as Supplementary Material at www.aestheticsurgeryjournal.com and Table 1). These data suggest that earFold can be used to achieve a reasonable degree of symmetry and can also be used to correct pre-existing asymmetry. There was greater asymmetry once the implants were removed.
Moulding Effect of the earFold Implant
Twenty-one patients (53%) agreed to have their implants removed at either 6, 12, or 18 months after insertion (Figure 8 and Supplementary Figure 3, available as Supplementary Material at www.aestheticsurgeryjournal.com). Adults who had treatment with earFold alone experienced a 3.4% reduction in H-M distance after 12 months (patients 52, 47, and 46) and a 12.6% reduction after 18 months in place (patients 43 and 53). The 7 adults who had treatment with earFold and anterior scoring (patients 58-62, 64, and 65) experienced a 29% reduction in prominence after 6 months in place (Supplementary Figure 3, available as Supplementary Material at www.aestheticsurgeryjournal.com). Children who had treatment with earFold alone experienced a 10% reduction in H-M distance after 12 months (patients 28 and 49) and a 13.7% reduction after 18 months in place (patients 19, 21, 37, 38, and 39). These data suggest that the earFold implant does have a small moulding effect on the cartilage in both adults and children. It appears to have a larger moulding effect in adults when combined with a cartilage weakening procedure. However, the implant should be left in place permanently to obtain the maximum effect and to maintain symmetry of any correction. Of the 21 patients who consented to have their implants removed, two patients (both children) subsequently had revision surgery using standard otoplasty to address recurrence of their prominence. A further four patients (3 adults and one child) requested re-insertion of gold-plated implants after the conclusion of the study with a satisfactory outcome from this treatment.
Patient Satisfaction
Comparison of the median PEM responses (Table 2) relating to the patients′ perception of their appearance (Part 2, Q1 to Q10) showed a statistically significant improvement when comparing the baseline and final assessments for all questions (P < 0.001). Part 3 of the survey asked subjects for a final satisfaction assessment. All responses also showed an improvement in median PEM responses with the exception of the responses to Q5, relating to the appearance of the implants under the skin. This suggests a trend for dissatisfaction with the high visibility of the implants under the skin by the end of the study despite overall satisfaction with the correction of the prominence. Although many patients became increasingly concerned about the visibility of the grey-metal implants under the skin, none requested removal of the implants for this reason during the 18 month follow-up specified by the trial. None of the patients complained about palpability of the implants and (by the end of the study) most of the patients said that they were largely unaware of the presence of their implants unless asked.
Table 2.
Results of Patient Evaluation Measures questionnaire. All questions were answered on a Likert scale (1-7)
| Question | Question Details | Which response would be best? |
Difference (Mean) | STD | Range | P-Value* |
|---|---|---|---|---|---|---|
| PEM Part 2 : How are your ears now? – at 18 months after treatment | ||||||
| Part2 Q1 | My ears appear normal (yes, no) | Lower | −2.9 | 2.21 | (−6,1) | <.001 |
| Part2 Q2 | When I look in the mirror, my ears look as though they stick out (yes, no) | Higher | 2.73 | 2.67 | (−2,6) | <.001 |
| Part2 Q3 | Most of the time, I try to cover up my ears with my hair or a hat (all the time, none of the time) | Higher | 2.57 | 2.32 | (−1,6) | <.001 |
| Part2 Q4 | When I go out, I am always aware of people staring at my ears (always, never) | Higher | 2.02 | 2.61 | (−6,6) | <.001 |
| Part2 Q5 | Generally, when other people meet me they look at my ears and then at the rest of my face (always, never) | Higher | 1.73 | 2.51 | (−6,6) | <.001 |
| Part2 Q6 | I avoid meeting other people because of the appearance of my ears (yes, no) | Higher | 0.86 | 1.66 | (−2,6) | <.001 |
| Part2 Q7 | I think about the appearance of my ears (all the time, none of the time) | Higher | 2.35 | 2.11 | (−2,6) | <.001 |
| Part2 Q8 | I hate talking about my ears (yes, no) | Higher | 2.53 | 2.41 | (−3,6) | <.001 |
| Part2 Q9 | When I think about the appearance of my ears I feel (unconcerned, embarrassed) | Lower | −2.35 | 2.25 | (−6,2) | <.001 |
| Part2 Q10 | People comment on the appearance of my ears (all the time, never) | Higher | 1.24 | 2.33 | (−5,6) | <.001 |
| Median | Difference (Mean) | STD | Range | |||
| PEM Part 3 : Overall - at 18 months after treatment | ||||||
| Part3 Q1 | Generally, my treatment at the hospital has been (satisfactory, unsatisfactory) | Lower | 1.00 | 1.17 | 0.43 | (1,3) |
| Part3 Q2 | Generally, my ears are now (satisfactory, unsatisfactory) | Lower | 2.00 | 2.59 | 2.00 | (1,7) |
| Part3 Q3 | Generally, my ears are painful (yes, no) | Higher | 7.00 | 6.25 | 1.36 | (2,7) |
| Part3 Q4 | I have had no problems with the skin on my ears (yes, no) | Lower | 2.50 | 3.61 | 2.57 | (1,7) |
| Part3 Q5 | I am not bothered by the appearance of the implants under the skin (yes, no) | Higher | 3.00 | 3.80 | 2.15 | (1,7) |
| Part3 Q6 | The shape of my ears has improved with treatment (yes, no) | Lower | 1.00 | 2.63 | 2.23 | (1,7) |
Side Effects
The main side effects of treatment were pain, swelling, and bruising of the ears. The pain of surgery subsided after 24-48 hours, requiring only simple analgesia (eg, acetaminophen or ibuprofen). Obvious swelling/bruising occurred in every case and increased within a few hours of treatment but subsided in all cases within 7 days of treatment. No head bandage was used for any patient and most were able to return to work or school within 1 week (or sooner). Temporary sensitivity related to the implant (eg, when lying on their side) was reported by most patients but had disappeared in all patients by 12 weeks.
Complications
Adverse events affected 8 (20.5%) patients (Table 1). All adverse events occurred at ≤12 months after insertion. Four patients experienced one adverse event related to one implant but 3 patients (2 children and 1 adult) experienced more than one adverse event. Erosion of the skin over the implant affected 7 implants (5 patients or 5.5% of the implants used). Infections affected 2 implants (2 patients or 1.6% of the implants used). Two patients developed hypertrophic scars associated with the incisions to insert the implant. One patient developed a “Spock-ear” deformity, which was noted at 3 months after treatment. There were no recorded episodes of problems with bleeding or haematomas.
Erosion/Extrusion
A total of 7 implants eroded through the skin, affecting 5 patients (Figure 9). All cases of extrusion occurred ≤12 months after insertion of the implants. Two children experienced extrusion of 2 implants each. The rest were adults who experienced extrusion of 1 implant each. Most extrusions occurred in the upper pole, where the skin is thinnest. After reviewing the postoperative pictures, the other common factor was a failure to ensure that the implant was flush with the cartilage at the time of insertion. Leaving a palpable edge appears to increase the risk of extrusion of the implant.
Figure 9.

A 42-year-old male (Patient 53) demonstrating erosion of skin over the upper implant at (A) 12 months postoperative. (B) The same ear 2 months after removal of all implants.
Patients were advised to have the affected implant removed as soon as possible. The skin was then allowed to settle and the patients were offered the option of re-implantation at the same site after 3-6 months. This offer was declined by all the patients who experienced extrusion of their implants. Although not part of the original study protocol, we have continued to follow as many of the patients as possible since the conclusion of the study and are unaware of any new cases of extrusion up to 47 months from the start of the study.
Infection
Infections affected 2 patients (both children), with– 1 implant affected in each case (Figure 10). Only 1 implant needed to be removed (it was extruding). The other case was successfully treated with oral antibiotics, leaving the affected implant in place. There was no recurrence of the infection in this case by the final review at 18 months after insertion. We are not aware of any new episodes of infection since the conclusion of the study.
Figure 10.

(A) An 8-year-old male (Patient 35) with Stahl′s ear deformity in the left ear, treated with earFold™ only. (B) Increased swelling and redness at 18 days postoperative, treated with oral antibiotics only. (C) Eighteen months postoperative. The implant was left in place permanently.
Hypertrophic Scarring
Two hypertrophic scars arose affecting the incision sites of 2 patients (Figure 11), and one was associated with extrusion of an implant. Only 1 patient wished to have treatment of their scar. This was carried out by a combination of excision and a single injection of 2 mg of Adcortyl™ (ER Squibb and Sons, Middlesex, UK). This treatment was successful.
Figure 11.
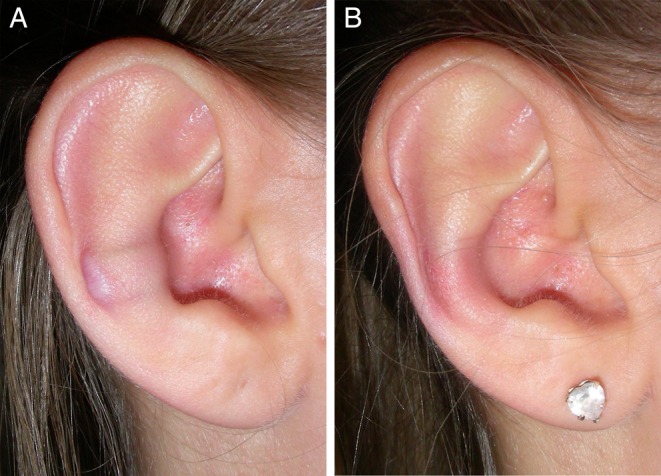
A 15-year-old female (Patient 64) with hypertrophic scarring. (A) Photograph obtained at 6 months postoperative. The implant was removed and the scar was excised. (B) The same ear at 2 months after removal of the implant.
Spock-ear Formation
“Spock-ear” formation affected 1 ear of 1 patient. This was the result of poor orientation of the implant at the time of insertion. As a result, the new antihelical fold was too vertical (Figure 12). The deformity was easily corrected by removal of the implant and replacement of the same implant in a new position.
Figure 12.
A 10-year-old male (Patient 31) with “Spock-ear” deformity. (A) Preoperative, (B) 2 weeks postoperative, and (C) 3 months postoperative, showing "Spock" ear deformity. This was probably the result of creating an antihelical fold which was not sufficiently curved. (D) Implants were removed at 3 months and replaced with single earFold™ implant in a different position. The implant was left in place permanently.
DISCUSSION
earFold is a permanent metal implant which has been designed to facilitate minimally-invasive correction of prominent ears in humans. This pilot study confirmed that earFold can be used to achieve this objective as part of a local anaesthetic procedure which is rapid, simple to perform, and simple to master. Importantly, using preFold positioners for preoperative assessment allows treatment with earFold to have a more predictable outcome compared with standard otoplasty. Moreover, use of preFold gives patients some measure of control over the final outcome.
We have learned that earFold cannot be used to treat every case of prominent ear. The principal effect of the implant is to create or enhance the shape of the antihelical fold. It has no effect on a protruding earlobe. Moreover, cases where the prominence is solely due to a deep conchal bowl may not be suitable for treatment with earFold. Nevertheless, in the present study, we encountered many cases where conchal bowl prominence coexisted with poor development or absence of the antihelical fold. Many of these cases benefited from treatment with earFold because satisfactory reduction in the prominence of the conchal bowl could be achieved by folding of the lateral wall of the concha into the new antihelical fold (Figure 8 and Supplementary Figures 2 and 3, available as Supplementary Material at www.aestheticsurgeryjournal.com). The effectiveness of this approach could be determined with preFold during the preoperative assessment, thereby reducing the risk of disappointment with the outcome in advance. If the patient and the surgeon were unable to achieve a satisfactory improvement using preFold, the patient could then be offered another option for correction of prominence.
It should be noted that the main purpose of the study was to determine the safety and behaviour of the earFold implant in vivo. Concerns about the overall aesthetic outcome were not formally assessed during the study. Similarly, we did not fully understand how the implant would behave before the study, and so did not appreciate that the antihelical fold that the implant creates might be as vertical as those shown in Figures 7 and 8 and in Supplementary Figures 1 and 3, available as Supplementary Material at www.aestheticsurgeryjournal.com. That said, all the patients were shown the correction that would be achieved using preFold in the preoperative assessment. Treatment was only carried out once they were satisfied with the outcome that would be achieved by placing the implants(s) in those specific positions. Therefore, it could be argued that what we (as surgeons) perceive as normal and/or desirable is not necessarily the same as what is perceived as normal and/or desirable by the patients. What is unique with earFold is the ability to show patients before treatment what their ear will look like after treatment. This cannot easily be achieved with standard otoplasty surgery.
We are unable to disclose the exact costs of the device and the introducer since this information remains commercially sensitive. However, in the clinic where we work, we currently charge a surgeon's fee of approximately £500 ($800 dollars) per patient using the CE-marked (gold-plated) implant. Patients are then charged per implant used giving overall treatment costs (surgeons fee + implant(s) + hospital fee), which vary from £1000 - £2500 ($1500 - $4000). This compares with recent American Society of Aesthetic Plastic Surgeons (ASAPS 2014) statistics which reported an average surgeon's fee for otoplasty of $2,976 per procedure.6 The ASAPS statistics also indicate that each standard otoplasty procedure averaged 2-3 hours and resulted in 5-14 days off work. By comparison, each earFold procedure can be completed in approximately 30 minutes (including assessment, consent, procedure, dressing and discharge paperwork). In the same 2-3 hours used to perform one standard otoplasty, we commonly treat 3 patients and assess another 3 new patients. Therefore, costs to the patient and overall remuneration for the surgeon are currently comparable to standard otoplasty surgery but we anticipate that the cost of the implant will fall in the future. This would allow remuneration for the surgeon to increase while simultaneously reducing overall costs to the patient.
The PEM questionnaire used to assess patient satisfaction for this study was not a validated measure of outcomes after surgery for prominent ears. We acknowledge this as a limitation of this study. It was adapted from a Patient Reported Outcome Measure originally developed (and validated) to assess outcomes after hand surgery.3-4 However, at the time the study protocol was designed in 2010, we were unable to find any properly validated questionnaires specifically directed at determining the effectiveness of an implant for correction of prominent ears. Despite this limitation, our PEM questionnaire appeared to confirm that all the patients were happy with treatment of their prominent ears using earFold implants. However, the survey also confirmed that the visibility of the gray-metal implant under the skin was a concern for many of the patients although they did not complain about palpability of the implants. Failure to ensure that the implant was flush with the cartilage also contributed to increased visibility of the implant. We gradually learnt how to reduce this over the course of the study through improvements in our technique for insertion. Further improvements in the technique for insertion have also been achieved since the conclusion of this study through modification of the design of the earFold introducer. We acknowledge that an implant placed posteriorly would probably be less visible and palpable and this option was certainly investigated during development of the earFold implant. However, the technical difficulties of fixing the implant posteriorly proved insurmountable. Instead, we opted for the simpler alternative of camouflaging the implant with a thin layer of 24-carat gold (Figure 3). This is the version of the implant which has now been granted a CE-Mark.
Our video data show that treatment with earFold was quick, even when combined with additional steps such as anterior scoring. This made it feasible to treat all of our patients (even children as young as 7 years of age) under local anaesthetic since total operating times varied between 5 to 20 minutes, depending on the number of implants inserted. This compares with ASAPS data indicating that each otoplasty procedure takes an average of 2-3 hours.6 Standard otoplasty surgery is a long procedure for a patient, under local anaesthetic. Similarly, removal of earFold implants was quick, although we acknowledge that it is still quicker and easier to remove a suture that is eroding through, than an implant. Finally, training in the use of earFold was simple, without the need for the long learning curve associated with standard otoplasty surgery. It proved easy to show one trainee how to use the implant during the study.
Use of the H-M distance as the primary outcome measure was a potential limitation of this study in a number of different ways. Firstly, we acknowledge that surgery to correct prominent ears is not simply a matter of reducing the H-M distance. That said, measurement of any improvement in aesthetic appearance can be difficult to demonstrate objectively. Therefore, on balance, we feel that the NREC was correct in insisting that we use H-M distance as the primary means for determining whether earFold is able to change the shape of the human ear - especially in the context of a first-in-human pilot study. Secondly, we acknowledge that measurement of the H-M distance can be inaccurate. Parallax errors, positioning of the patient and/or the ruler all play a part. However, in this study, the difference in the average reduction of H-M distance with the implant in place compared with the implant removed was very large (Table 1). Therefore, we do not feel that our conclusion advising that the implant should be left in place permanently (for best results) would be altered by a more accurate measurement of H-M distance.
Another limitation of this study was our failure to include measurements of the conchal-scaphal angle (before and after) as one of our outcome measures. Despite this, we do not believe that measurements of the concha-scaphal angle are superior to measurements of the H-M distance as an objective measure of the effectiveness of prominent ear correction. Moreover, the methods for measuring the concha-scaphal angle are as inaccurate as the methods for measurement of the H-M distance. Therefore, we do not believe that the conclusions we reached (using H-M distance as our primary outcome measure) would change if we had used conchal-scaphal angles instead.
Despite the potential inaccuracies inherent in our use of H-M distance as the primary outcome measure, the data we present (Table 1) and the photos of our patients all suggest that earFold is able to reduce prominence of the ear by the minimum 10% criterion specified by the trial protocol. Moreover, these data suggest that earFold can achieve reasonable symmetry and/or can be used to correct pre-existing asymmetry. Importantly, the data also show that, when the implants were left in place, there was no recurrence of prominence over the 18 months of follow-up specified by the protocol, suggesting that any correction achieved is stable over this period. Although not part of the original study, we have continued to measure the H-M distance in those patients who elected to leave their implants in place beyond 18 months. The data we have shown that the H-M distance in this group remains stable at up to 47 months. Although we recommend that earFold should be left in place as a permanent implant (for both children and adults) the data also suggest that a small moulding effect does occur. The moulding effect is seen for both adults and children, and is probably time-dependent. This moulding effect appears to be enhanced in adults if a cartilage weakening procedure is performed at the same time (eg, anterior scoring). Therefore, we speculated that a permanent moulding effect might occur after much longer periods of implantation (ie, much longer than 18 months).
The postoperative care of patients undergoing treatment with earFold is much simpler compared with standard otoplasty. Following standard otoplasty, many surgeons insist that their patients wear a large head-bandage that gives their patients the appearance of wearing a helmet. This is (typically) left in place for up to two weeks although there is some data suggesting that it is of no benefit in terms of the final outcome.7-8 During this time (and for some weeks afterwards) patients are also asked to avoid contact sports or swimming in case of accidental contact with their healing ears. Although we recommend similar avoidance of contact sports and swimming for earFold, a head-bandage is not necessary and patients are able to shower immediately. The only extra request after earFold is for patients to avoid sleeping on their sides to reduce the risk of dislodging the implants while their ears heal. As a result of these less onerous postoperative demands, patients are often able to return to work within a few days of treatment with earFold.
The overall complication rate for our study (20.5% of patients) was high but comparable to other techniques for prominent ear correction and suture-based methods in particular (Table 3)9-13 In fairness, the complication rates for cartilage only techniques are also relatively high.9,12,14-16 However, given that this was a first-in-human study of an implant using a completely novel approach to prominent ear correction, we were surprised that the complication rate was not higher. For our review of complications (Table 3), the percentages shown relate to the frequency with which a patient experienced an adverse event (eg, infection) rather than the frequency of the event per ear. Comparisons with previous studies shows that our infection rate (5% of patients) and erosion rate (13% of patients) were within the range of those published for conventional, suture-based otoplasty surgery (which is the closest comparison to the earFold treatment system).9-13 Importantly, when left in place permanently, there was no recurrence of the prominence. Furthermore, problems with bleeding, wound dehiscence and haematoma did not occur, possibly because of the minimally invasive nature of the earFold procedure. We acknowledge that the lowest complication rates published so far are associated with surgery using a combination of approaches, especially posterior suturing combined with a fascial flap.12,17,18 However, even with a combined approach, the addition of anterior scoring can result in a large increase in the complication rate.19,20 In contrast, other surgeons have reported on combination approaches (which include anterior scoring or other methods of cartilage shaping) together with posterior sutures, without a similar increase in complications.21-24 However, among this group, it should be noted that the methods described by Marcevich21 and Park23 in particular are relatively complex and might be difficult to reproduce by other surgeons. By comparison, earFold is a technique that should be relatively easy to understand and whose outcomes should be relatively reproducible.
Table 3.
Comparison of outcomes for different otoplasty techniques. Percentage complications were calculated from number of events per patient rather than per ear
| Author and Year | Technique, used | Number of patients in study | Haematoma and/or bleeding (%) | Infection (%) | Skin necrosis or skin problem (%) | Suture or implant extrusion (%) | Keloid and Hypertrophic scars (%) | Recurrence of prominence (%) | Reoperation to correct a problem (%) | Overall complication rate (all patients who had a problem and/or needed redo surgery) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kang 2014 | earFold™ | 39 | 0.0% | 5.1% | 0.0% | 12.8% | 5.1% | 0.0% | 15.3% | 20% |
| CARTILAGE SCORING ONLY | ||||||||||
| Tan9, 1986 | Anterior scoring | 101 | 8.0% | 0.0% | 0.0% | 0.0% | 0.0% | 10.0% | 7.0% | 20.0% |
| Calder14, 1994 | Anterior scoring | 562 | 2.0% | 5.2% | 1.4% | 0.0% | 2.1% | 8.0% | 8.0% | 28.5% |
| Jeffrey15, 1999 | Anterior scoring | 122 | 3.3% | 3.3% | 1.6% | 0.0% | 0.8% | 3.3% | 3.3% | 27.0% |
| Mandal12, 2006 | Anterior scoring | 68 | 1.4% | 1.4% | 0.6% | 0.0% | 1.5% | 11.0% | 8.8% | 29.0% |
| Bhatti16, 2007 | Anterior scoring | 34 | 2.0% | 1.5% | 1.0% | 0.0% | 0.0% | 0.0% | 0.0% | 14.7% |
| SUTURE ONLY TECHNIQUES | ||||||||||
| Tan9, 1986 | Posterior suture | 45 | 33.0% | 15.5% | 0.0% | 15.5% | 2.0% | 24.4% | 17.0% | 58.0% |
| Adamson10, 1991 | Posterior suture | 119 | 1.6% | 0.0% | 0.0% | 16.1% | 4.8% | 12.7% | 32.0% | 51.0% |
| Messner11, 1996 | Posterior suture | 51 | 0.0% | 2.0% | 3.9% | 9.0% | 2.0% | 30.0% | 3.9% | 50.9% |
| Mandal12, 2006 | Posterior suture | 94 | 5.5% | 1.0% | 0.0% | 2.1% | 2.1% | 8.0% | 6.0% | 28.0% |
| Olivier13, 2009 | Posterior suture | 104 | 0.9% | 0.0% | 0.0% | 4.8% | 3.8% | 7.6% | 2.9% | 27.8% |
| COMBINED TECHNIQUES | ||||||||||
| Horlock17, 2001 | Posterior suture and fascial flap | 51 | 1.9% | 0.0% | 0.0% | 0.0% | 0.0% | 11.8% | 4.0% | 15.6% |
| Bulstrode5, 2003 | Anterior scoring and posterior sutures | 114 | 0.9% | 3.5% | 0.0% | 0.0% | 1.8% | 0.9% | 1.8% | 14.0% |
| Mandal12 2006 | Posterior suture and fascial flap | 41 | 2.4% | 0.0% | 0.0% | 7.3% | 0.0% | 4.9% | 3.6% | 12.0% |
| Scharer19, 2007 | Anterior scoring and posterior suture | 75 | 1.3% | 1.3% | 1.3% | 19.0% | 2.6% | 23.0% | 15.0% | 38.0% |
| Schlegel-Warner20, 2010 | Anterior scoring and posterior sutures | 222 | 5.0% | 6.7% | 7.2% | 6.7% | 3.1% | 14.8% | 1.8% | 35.5% |
| Maricevich21, 2011 | Cartilage sculpting and posterior sutures | 111 | 0.9% | 0.0% | 0.0% | 0.0% | 0.0% | 2.7% | 0.9% | 10.8% |
| De la Fuente22, 2012 | Anterior scoring and posterior suture | 100 | 0.0% | 0.0% | 6.0% | 0.0% | 0.0% | 2.0% | 3.0% | 11% |
| Park23, 2012 | Cartilage grafting and posterior sutures | 39 | 0.0% | 0.0% | 0.0% | 0.0% | 2.5% | 2.5% | 2.5% | 7.6% |
| Ribeiro24, 2012 | Anterior scoring, cartilage excision and posterior sutures | 897 | 0.4% | 0.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.4% | 1.2% |
| Sinha18, 2012 | Posterior suture and fascial flap | 227 | 0.0% | 0.0% | 0.4% | 2.6% | 1.3% | 3.7% | 4.4% | 8.8% |
We acknowledge that extrusion of alloplastic material and infection are all serious complications in the context of otoplasty surgery. Moreover, thinning of the skin over the implant and extrusion rates might increase with increased duration of follow-up beyond the 18 months specified by the protocol for this study. However, we are not aware of any new episodes of infection or extrusion among the 18 patients who elected to leave their implants in place permanently - some of whom have now had their implants in place for >47 months. Furthermore, we have not observed any change in the quality of the skin at 18 months compared with 47 months, in this group. It is our intention to continue monitoring this cohort of patients for as long as possible to determine longer-term outcomes, although this was not specified in the original trial protocol.
When the specific complications of infection or extrusion did occur, remedial action was simple. If the implant eroded through the skin, the implant was removed to reduce the risk of infection. Although removal of an implant is clearly more complicated than removal of an eroding suture, our data show that this took only 1.7 minutes per implant (from opening the skin to applying a dressing) and could be carried out in an office, albeit under local anaesthetic. Similarly, treatment of infection was simple. Only two infections arose. One was associated with an implant eroding through the skin. The other responded to oral antibiotics. Therefore, unusually for an implant, it may also be unnecessary to remove every earFold implant if an infection arises.
Close analysis of the case report forms and photos suggests that most erosions were the result of a failure to ensure that the implant was flush with the cartilage at the time of insertion. Therefore, it should be possible to reduce the erosion rate further with more experience/training in the use of the implant. The aim would be to ensure that there are no palpable edges at the time of insertion. If the edges are palpable, then the implant must be removed and re-deployed. Failure to ensure that the implant was flush with the cartilage also contributed to increased visibility of the implant. Therefore, cosmesis would be improved and complications would be reduced by closer attention to this aspect of deployment of the implant. Since the conclusion of this study, we have addressed these concerns through improvements in our technique for insertion and changes in the design of the earFold introducer. Importantly, although none of the patients who had their implants removed for erosion or infection requested re-implantation, the skin at the site of insertion healed sufficiently well in all of these cases to make this a possibility - if they wished it (Figure 9).
Although two incisions developed hypertrophic scars, this has to be considered against a background of the 131 incisions used to insert each implant for this study. Although not formally assessed as part of this study, all the scars were generally satisfactory with or without the implants in place. Moreover, once any implants were removed, the ears healed very well leaving little evidence that the implants had ever been in place (Figures 8, 9 and 11, and Supplementary Figure 3, available as Supplementary Material at www.aestheticsurgeryjournal.com). At this point, patients could proceed with other forms of otoplasty or re-insertion of the implants, if they wished.
The one case of “Spock-ear” formation was easily remedied by removal of the implant and replacement in a new position to avoid creating an excessively vertical antihelical fold. Our ability to do so at 3 months post-insertion underlines the reversibility and adjustability of the earFold treatment system in the event of an unsatisfactory outcome. This is particularly true if treatment is carried out with earFold alone. It may be less true if a cartilage weakening procedure is performed simultaneously since this might enhance the speed with which any moulding effect occurs.
CONCLUSION
In conclusion, we present a new technique for correction of prominent ears. As for all surgical techniques, earFold has its own risks and drawbacks. Moreover, it cannot be used to treat every case of prominent ear. To address some of the concerns about visibility under the skin, the design of the implant has been changed since the conclusion of this study. Although complications did occur, they were easily corrected and most appeared to be the result of technical errors at the time of implantation. It is hoped that with increasing experience, these can be minimised. Further studies are planned.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the team at Northwood Medical Innovations Ltd, the engineers at Contract Medical Innovations GMBH, the team in the dissection room at Guy's Hospital in London, the team at Health Enterprise East, the team at Health Policy Associates, Inc., the engineers at Mediconcepts, and the trainees and nursing staff who have helped along the way, especially Mrs Johannah Adegoke-Dickson.
Supplementary Material
This article contains supplementary material located online at www.aestheticsurgeryjournal.com.
Disclosures
Dr Kang is the inventor of the earFold™ implant used for this investigation. He is also the chairman and chief technical officer for the company (Northwood Medical Innovations [NMI] Ltd) that distributes the earFold implant. He is a shareholder of NMI Ltd. Dr Kerstein has nothing to disclose.
Funding
Funding support for the study was provided by the distributor (NMI Ltd) of the earFold™ implant. The hospital where the study was performed and NMI Ltd acted as co-sponsors for the study since the hospital is a part-owner of NMI Ltd. All implants and materials were provided free and gratis by NMI Ltd. None of the patients received any compensation to participate in the study, either direct or in kind. The principal author received no compensation of any kind to perform the study or to prepare this report.
REFERENCES
- 1.Pelton AR, Stockel D, Duerig TW. Medical uses of nitinol. Materials Science Forum. 2000;327-328:63-70. (Proceedings of the International Symposium on Shape Memory Materials). [Google Scholar]
- 2.Thompson S. An overview of nickel-titanium alloys used in dentistry. International Endodontic Journal. 2000;33:297-310. [DOI] [PubMed] [Google Scholar]
- 3.Macey AC, Burke FD, Abbott K et al. Outcomes of hand surgery. British Society for Surgery of the Hand. J Hand Surg Br. 1995;206:841-855. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R, Dias JJ. Validity and reliability of three generic outcome measures for hand disorders. J Hand Surg Br. 2000;256:593-600. [DOI] [PubMed] [Google Scholar]
- 5.Bulstrode NW, Huang S, Martin DL. Otoplasty by percutaneous anterior scoring. Another twist to the story: a long-term study of 114 patients. Br J Plast Surg. 2003;562:145-149. [DOI] [PubMed] [Google Scholar]
- 6.Cosmetic Surgery National Data Bank Statistics. Aesthet Surg J. 2015;35(Suppl 2):1-24. [DOI] [PubMed] [Google Scholar]
- 7.Powell BW. The value of head dressings in the postoperative management of the prominent ear. Br J Plast Surg. 1989;426:692-694. [DOI] [PubMed] [Google Scholar]
- 8.Ramkumar S, Narayanan V, Laing JH. Twenty-four hours or 10 days? A prospective randomised controlled trial in children comparing head bandages following pinnaplasty. J Plast Reconstr Aesthet Surg. 2006;599:969-974. [DOI] [PubMed] [Google Scholar]
- 9.Tan KH. Long-term survey of prominent ear surgery: a comparison of two methods. Br J Plast Surg. 1986;392:270-273. [DOI] [PubMed] [Google Scholar]
- 10.Adamson PA, McGraw BL, Tropper GJ. Otoplasty: critical review of clinical results. Laryngoscope. 1991;1018:883-888. [DOI] [PubMed] [Google Scholar]
- 11.Messner AH, Crysdale WS. Otoplasty. Clinical protocol and long-term results. Arch Otolaryngol Head Neck Surg. 1996;1227:773-777. [DOI] [PubMed] [Google Scholar]
- 12.Mandal A, Bahia H, Ahmad T, Stewart KJ. Comparison of cartilage scoring and cartilage sparing otoplasty--A study of 203 cases. J Plast Reconstr Aesthet Surg. 2006;5911:1170-1176. [DOI] [PubMed] [Google Scholar]
- 13.Olivier B, Mohammad H, Christian A, Akram R. Retrospective study of the long-term results of otoplasty using a modified Mustarde (cartilage-sparing) technique. J Otolaryngol Head Neck Surg. 2009;383:340-347. [PubMed] [Google Scholar]
- 14.Calder JC, Naasan A. Morbidity of otoplasty: a review of 562 consecutive cases. Br J Plast Surg. 1994;473:170-174. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery SL. Complications following correction of prominent ears: an audit review of 122 cases. Br J Plast Surg. 1999;527:588-590. [DOI] [PubMed] [Google Scholar]
- 16.Bhatti AZ, Donovan DO. Sutureless otoplasty by scoring of the cartilage: a study in 34 patients. Br J Oral Maxillofac Surg. 2007;453:217-220. [DOI] [PubMed] [Google Scholar]
- 17.Horlock N, Misra A, Gault DT. The postauricular fascial flap as an adjunct to Mustarde and Furnas type otoplasty. Plast Reconstr Surg. 2001;1086:1487-1490. discussion 91. [DOI] [PubMed] [Google Scholar]
- 18.Sinha M, Richard B. Postauricular fascial flap and suture otoplasty: a prospective outcome study of 227 patients. J Plast Reconstr Aesthet Surg. 2012;653:367-371. [DOI] [PubMed] [Google Scholar]
- 19.Scharer SA, Farrior EH, Farrior RT. Retrospective analysis of the Farrior technique for otoplasty. Arch Facial Plast Surg. 2007;93:167-173. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel-Wagner C, Pabst G, Muller W, Linder T. Otoplasty using a modified anterior scoring technique: standardized measurements of long-term results. Arch Facial Plast Surg. 2010;123:143-148. [DOI] [PubMed] [Google Scholar]
- 21.Maricevich P, Gontijo de Amorim NF, Duprat R, Freitas F, Pitanguy I. Island technique for prominent ears: an update of the Ivo Pitanguy clinic experience. Aesthet Surg J. 2011;316:623-633. [DOI] [PubMed] [Google Scholar]
- 22.de la Fuente A, Sordo G. Minimally invasive otoplasty: technical details and long-term results. Aesthetic Plast Surg. 2012;361:77-82. [DOI] [PubMed] [Google Scholar]
- 23.Park C, Jeong TW. Antihelical shaping of prominent ears using conchal cartilage-grafting adhesion. Laryngoscope. 2012;1226:1238-1245. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro JA, da Silva GS. Finesse in otoplasty in four steps. Aesthetic Plast Surg. 2012;364:846-852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





