Abstract
Methyphenidate (MPH) is the primary drug treatment of choice for ADHD. It is also frequently used off-label as a cognitive enhancer by otherwise healthy individuals from all age groups and walks of life. Military personnel, students, and health professionals use MPH illicitly to increase attention and improve workplace performance over extended periods of work activity. Despite the frequency of its use, the efficacy of MPH to enhance cognitive function across individuals and in a variety of circumstances is not well characterized. We sought to better understand MPH’s cognitive enhancing properties in two different rodent models of attention. We found that MPH could enhance performance in a sustained attention task, but that its effects in this test were subject dependent. More specifically, MPH increased attention in low baseline performing rats but had little to no effect on high performing rats. MPH exerted a similar subject specific effect in a test of flexible attention, i.e. the attention set shifting task. In this test MPH increased behavioral flexibility in animals with poor flexibility but impaired performance in more flexible animals. Overall, our results indicate that the effects of MPH are subject-specific and depend on the baseline level of performance. Furthermore, good performance in in the sustained attention task was correlated with good performance in the flexible attention task; i.e. animals with better vigilance exhibited greater behavioral flexibility. The findings are discussed in terms of potential neurobiological substrates, in particular noradrenergic mechanisms, that might underlie subject specific performance and subject specific responses to MPH.
Keywords: Sustained attention, flexible attention, psychostimulant, behavioral phenotype
Introduction
Methylphenidate (MPH), a catecholamine reuptake inhibitor, is the primary pharmaceutical option for treating Attention Deficit Hyperactivity Disorder (ADHD). Despite the frequency of its use, as many as 30% of ADHD children do not respond to MPH treatment (Barkley 1977). The differential efficacy associated with MPH treatment has led to the conclusion that MPH has many dose dependent and subject specific effects that vary depending on outcomes measures, e.g. cognitive abilities, academic performance, and social behavior (Rapport et al., 1985a; Swanson et al., 1978). For example, MPH in human subjects reduces impulsivity in a linear dose dependent manner as measured by the Matching Familiar Figures test. However, a closer inspection of the changes in each individual found that the dose producing the greatest change in impulsivity differed amongst each subject, suggesting a subject specific effect (Rapport et al., 1985b). MPH also exhibits baseline performance dependent effects on a visual perceptual processing task (Finke et al., 2010). MPH significantly enhanced performance on this test in low baseline individuals, an effect that positively correlated with blood plasma levels of MPH. High baseline individuals showed no improvement with MPH and exhibited a negative correlation with their blood plasma level of MPH. How and why MPH has such variable effects are currently unknown but important for establishing effective dosing regimens in ADHD patients.
In addition to its use as a treatment option for ADHD, MPH is becoming increasingly popular for off-label use as a cognitive enhancer. From 1996 to 2005, the number of prescriptions for MPH increased by approximately 100%. In that same time period, the MPH production increased by 268.9% indicating a growing trend in its illicit use (Swanson and Volkow, 2009). The Substance Abuse and Mental Health Services Administration (SAMHSA) conducts an annual National Survey on Drug Use and Healthy. According to their annual reports, the number of non-medical users of MPH increased from 2.7 million (1.2% of the population) in 2000 to 5.4 million people (2.1% of the population) in 2010. Despite its widespread use both clinically and non-clinically, the mechanism by which MPH enhances cognitive function is poorly understood, much less its actual efficacy as a cognitive enhancer.
The goal of the current study was to establish an animal model that differentiated effects of MPH on specific dimensions of cognitive function. Prior studies in ADHD patients, both children and adults, evaluated the effects of MPH on a battery of tests including alertness, vigilance, divided attention, flexibility, and aspects of selective attention such as focused attention, inhibition, and integration of sensory information (Tucha et al, 2006a; 2006b). In the present study drug effects were assessed in rodent tests of sustained and flexible attention. We adopted this approach to measure the efficacy of the purported pro-cognitive effects of MPH in a normal population.
Methods and Materials
Animals
Twenty-five male Sprague-Dawley rats (150–175 g, Taconic Farms, Germantown, Pennsylvania) were housed in groups of 2 or 3 on a 12 hr light : 12 hr dark cycle (lights on at 07:00 am). Care and testing followed all NIH guidelines for research animal care and all procedures were approved by the Drexel University Institutional Animal Care and Use Committee. All testing took place between 9 am and 5 pm. Following a period of adaptation, rats were reduced to 90% of ad libitum body weight by limiting their water intake to 10 minutes a day.
Drug Administration
Animals were administered MPH or saline orally by ingestion of a drug solution soaked piece of sweetened cereal 15 minutes prior to each test session. MPH was dissolved in saline (2, 8, 16 mg/kg, Sigma-Aldrich, St Louis, MO) and then applied to the piece of cereal. Saline only soaked pieces of cereal served as controls. All animals must have completely consumed the drug and cereal to be included in the data. Drug days were separated by at least one day to washout any drug effect and ensure a return to baseline level of performance.
Sustained Attention Apparatus
The sustained attention apparatus was an operant chamber from Med Associates (St Albans, VT) with a house light, a stimulus light between two retractable levers, and a water dispenser opposite the levers that delivered 40 ul aliquots of water. The levers, lights, and water delivery system were controlled by Med-PC software from Med Associates.
Sustained Attention Testing Procedure
Rats were trained to perform a visual sustained attention task as previously described (Berridge et al., 2012). Briefly, water restricted animals were trained to report the presence or absence of a 15 msec duration stimulus light by pressing one of two levers that were extended on each trial. Correct choices resulted in a water reward while incorrect choices were punished with a timeout. All animals were allowed to complete as many trials as possible within the 45 minute testing period. Performance was measured by vigilance index (VI), a derivative of the sensitivity index used in signal detection theory. Testing began once performance stabilized over three consecutive days with a VI score of 0.35 or greater, which is the threshold representing chance performance. Following completion of the experiments employing the sustained attention paradigm, animals were allowed to freely consume water and food for three days before being food restricted back down to 90% of their ad libitum weight in preparation for testing in the attentional set shifting task.
Sustained Attention Task Data Analysis
There are five possible responses that the rat can emit in the sustained attention task: 1) Hit = correct responding on the signal lever, 2) Correct Rejection = correct response on the non-signal lever, 3) Miss= incorrect response on the signal level, 4) False Alarm= incorrect response on the non-signal lever, 5) Omission = no response (increases correlate with sedative drug effects). For each session the relative number of Hits was calculated h = (#Hits / #Hits + #Misses) as a measure of signaled responding, as well as the relative number of Correct Rejections CR=(#Correct Rejections / #Correct Rejections + #False Alarms) as a measure of non-signaled responding. A measure of relative performance was generated using the relationship of relative hits (h) to false alarms (f, where f=#False Alarms / #False Alarms + #Correct Rejections) using a formula for the index of signal sensitivity: Vigilance Index (VI) = (h-f)/[2*(h+f)-(h+f)2] where: −1 < VI> +1. A value of 0 indicates 50% performance in both signal and non-signal trials and suggests that the subject was performing at chance. A value of +1 signified correct responses in all signal and non-signal trials (excluding omissions). A value of 59% performance in both signal and non-signal trials indicated performance that is significantly greater than chance, which typically results in VI ≥ 0.35.
Attention Set Shifting Apparatus
The set shifting apparatus was a custom built box modeled after the design of Birrell and Brown (Birrell and Brown, 2000). The testing arena (40L × 71W × 20H cm) was constructed from melamine with a plexiglass divider that served as a guillotine door that separated one-third of the arena into a holding area and two-thirds into a testing area. The testing area was further divided in half to provide two pot sampling areas. Terracotta pots were filled with wax to weigh them down with a top layer of scented wax that varied for each pair of pots. The pots were then covered in a fabric and filled with a digging medium that also varied among sets of pots to provide three dimensions in which they could differ.
Attention Set Shifting Procedures
Food restricted animals were tested according to the procedure established by Birrell and Brown 2000. Briefly, each animal was habituated to the testing arena and to terracotta pots. They were then trained to distinguish between each of three pairs of exemplar pots that differed in only one of the three possible features (scent, digging medium, or texture) for a food reward of sweetened cereal. Rats were moved to the next pair of exemplar pots when they correctly made 6 choices in a row. A correct choice was defined as vigorously displacing the digging medium. These pots were never used again.
Animals were then tested on a series of nine different discriminations of increasing difficulty. Fifteen minutes prior to the beginning of testing, animals were administered saline or methylphenidate (8 mg/kg, p.o.). Each rat started with a simple discrimination (SD) in which the pots differed in only one dimension, either odor or medium. After reaching criterion, rats were introduced to the second stage, a compound discrimination (CD), in which pots differed in two dimensions, odor and digging medium, with only one stimulus dimension reinforced. In the third stage, reversal 1 (Rev1), the previously unreinforced stimulus became the relevant choice. The fourth stage consisted of an intra-dimensional shift (IDS) in which a new set of pots with novel stimuli were introduced. The reinforced stimulus remained in the same sensory dimension. For example, a rum scent may be reinforced in the Rev1 stage, which was followed by reinforcement of a pear scent in the ensuing ID stage. In the next stage, the reinforced stimulus was again reversed (Rev2) within the same dimension. In the next stage, the extra-dimensional shift (EDS), novel pots were again introduced, but the reinforced stimulus shifted to a different dimension (odor to digging medium or digging medium to odor). This was followed by another reversal stage (Rev3) and a perseverative stage in which only the irrelevant dimension, texture, was different. The direction of the shift and the order in which stimuli were presented were counterbalanced across all animals.
Attentional Set Shifting Data Analysis
A normal animal that has formed an attentional set typically needs more trials to reach criteria in the ED shift than the ID shift. The number of trials to reach criterion on the ED shift was compared using an ANOVA comparing treatment groups to control groups. A post-hoc Dunnett’s test comparing treatment groups to saline control groups was used if significance was found.
Selection of High vs Low Performers
Animals were divided into a high or low group based on their baseline level of performance in the sustained attention task. Animals with a baseline VI above the group median VI were defined as high performers while animals with a baseline VI below the group median were categorized as low performers. Student’s T-tests were used to compare the various basal characteristics of the low and high performance groups including VI, hit rate, correct rejection rate, omission rate, and motor activity.
Data from the sustained attention task was analyzed using a two way repeated measures ANVOA group (low vs high performers) and drug (Baseline vs MPH) effects. Planned comparisons of drug effect relative to baseline were conducted for each performance group. Spearman’s regression was used to determine correlations between measurements of sustained attention and set shifting performance.
Results
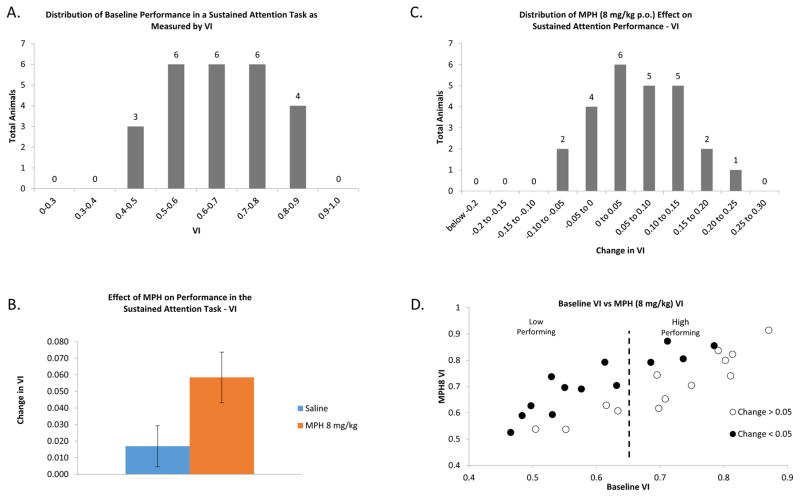
Baseline rodent performance in the sustained attention task varied according to a normal distribution ranging from just above chance to near perfection (Fig. 1A). The distribution of performance measured by VI was not significantly different from normal according to the Shapiro-Wilk test [W(25)= 0.948, p=0.225]. Overall, methylphenidate at a behaviorally relevant dose (8 mg/kg, p.o.) significantly improved performance in the sustained attention task (Fig. 1B) as measured by Vigilance Index (F(1,24)=7.995, p<0.01). The distribution of performance in this population sample following MPH treatment also fit a Gaussian distribution (Fig. 1C). Shapiro- Wilks test for normality showed that the distribution of the VI following MPH treatment was not significantly different from the null hypothesis (W(25)=0.965, p=0.519), as was the change in VI due to MPH (W(25)=0.984, p=0.952). The wide range and normal distribution of sustained attention performance was consistent with that of a heterogeneous population such as the outbred rats used in this study. A scatter plot analysis of the data (Fig. 1D) revealed a correlation between baseline VI and change in VI following MPH administration. MPH improved sustained attention performance in more animals with a lower level of baseline performance than those with a higher baseline level of performance. Only 4 of 13 animals (31%) with a baseline VI above 0.65 showed an increase in VI greater than 0.05 after MPH treatment while 9 of 13 animals (69%) with a baseline VI below 0.65 showed a MPH mediated increase in VI of 0.05 or greater.
Figure 1.
MPH can be used to differentiate between distinct behavioral phenotypes that represent two ends of the spectrum of sustained attention performance. A. Distribution of performance in the sustained attention task (VI). Within the rodent population in our study, performance in the sustained attention task varies along a spectrum, from just above the threshold for chance (VI = 0.35) to near perfect performance (VI = 1.0). Animals were grouped to the next highest tenth arbitrary unit of VI in the histogram seen here to visually demonstrate the distribution of the performance of all the animals used in this study (n=25). The distribution of animals fits a Gaussian curve according to Shapiro Wilks Test for normality (W(25)= 0.948, p=0.225). The median level of performance in this set of animals was 0.634 with a mean of 0.654. B. Overall, methylphenidate at a behaviorally relevant dose (8 mg/kg, p.o.) significantly improved performance in the sustained attention task as measured by Vigilance Index (F(1,24)=7.995, p<0.01). C. Distribution of the effect of MPH (8 mg/kg, p.o.) on individual performance (VI). The change in VI of each individual rat was grouped into the next highest 0.05 bin of the arbitrary VI unit to examine the distribution of the effect of MPH. The distribution of MPH’s effect on performance as measured by change in VI following treatment with MPH (8 mg/kg, p.o.) fits a Gaussian distribution (W(25)=0.984, p=0.952). The mean response was 0.0584 and the median response was 0.0598. D. Correlation between baseline VI and VI with MPH. MPH improved sustained attention in more animals with a lower level of baseline performance than those with a higher baseline level of performance. Only 4 of 13 animals (31%) with a baseline VI above 0.65 (vertical dashed line) showed an increase in VI greater than 0.05 after MPH treatment while 9 of 13 animals (69%) with a baseline VI below 0.65 showed a MPH mediated increase in VI of 0.05 or greater. Filled circles = change in VI > 0.05; open circles = change in VI < 0.05.
Relationship between Baseline Performance and the Effect of MPH in the Sustained Attention Task
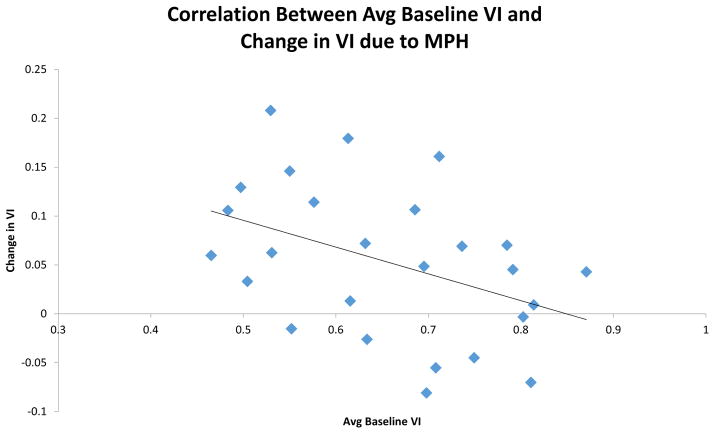
Because of the wide range of baseline VI performance, we ran a post hoc correlation analysis to determine whether baseline performance was associated with the effect of MPH (Fig. 2). Pearson’s correlation found a significant relationship for baseline VI with both VI with MPH treatment (r=0.778, p<0.001) and the change in VI due to MPH treatment (r=−0.423, p<0.05). These results demonstrate a significant relationship between the baseline performance of an individual animal in the sustained attention task and the effect of MPH in that same animal.
Figure 2.
Relationship between baseline performance and performance with MPH (8 mg/kg p.o.). Baseline performance in the sustained attention task negatively correlated with the effect of MPH on performance (rs=−0.423, p<0.05). Animals that demonstrate low performance under basal conditions tend to show a greater response to MPH while animals with a higher baseline level of performance responded minimally to MPH treatment.
Characterization of Animals with High or Low Sustained Attention
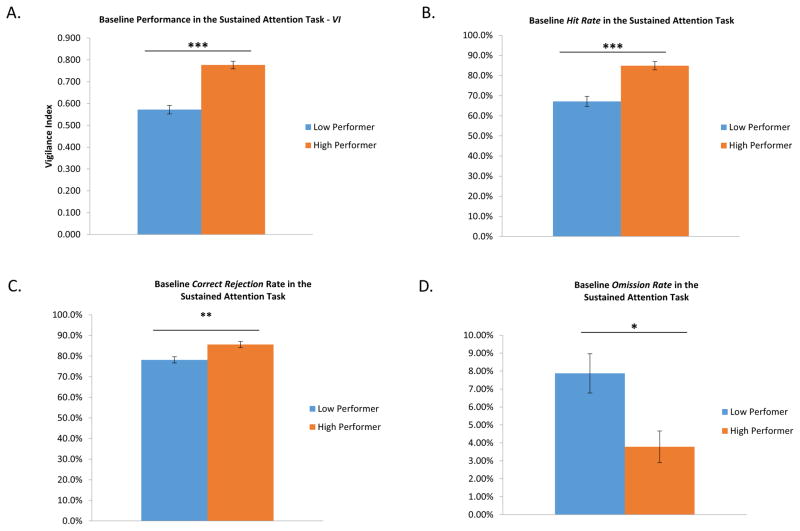
Following the discovery that drug effect was related to baseline performance, rats were grouped for further analysis into one of two phenotypes based on their baseline level of performance in the task (Fig. 3 and Table 1). The median baseline VI of the entire cohort was used to separate the rats into groups of high and low performers. The two groups were of identical age and weight and could not be distinguished based on their physical appearance or general observed behavior. Differences in performance in the sustained attention task could not be attributed to physiological differences such as motor activation (locomotor testing), task acquisition, or visual acuity (Table 1).
Figure 3.
Indices of baseline performance in the sustained attention task in two performance based groups. A. Vigilance Index (VI) - Dividing the animals based on the median VI revealed a significant difference. The low baseline group had a mean VI of 0.572 while the mean VI of high baseline group was 0.776. As expected, the two groups were significantly different (t(23)= −7.970, p<0.001]. B. Hit Rate - The hit rate is a measurement of a subject’s ability to successfully detect the presence of a stimulus. Hit rate is one of the two primary components of performance in the sustained attention task. The mean hit rate for the low baseline group was 67.1%, which was significantly lower than the mean hit rate for the high performance group, 87.4% (t(23)= −5.088, p<0.001). C. Correct Rejection Rate - A correct rejection is a successful detection of the absence of a stimulus. It is the other primary component of rewarded behavior in the sustained attention task. The average correct rejection rate of the low performance group (78.2%) was significantly lower than the average correct rejection rate of the high performance group (85.6%) (t(23)= −3.376, p<0.01). D. Omission Rate - An omission is the lack of a response on a trial. The rate of omissions for the low performance group (7.9%) was more than twice that of the high performance group (3.8%). These rates were significantly different from each other (t(23)= 2.764, p<0.05).
Table 1.
Differences Between Low and High Baseline Performance Groups. The two performance based phenotypes differ across multiple dimensions of cognitive function.
These differences cannot be attributed to differences in sensory perception, visual acuity, or motor function.
| Low Performance | High Performance | Significance | |
|---|---|---|---|
| Baseline Vigilance Index (VI) | 0.572 ± 0.019 | 0.776 ± 0.017 | p<0.001 |
| 1000 ms Vigilance Index (VI) | 0.522 ± 0.015 | 0.592 ± 0.0337 | p<0.05 |
| Sensitivity Index (d′) | 1.62 ± 0.07 | 2.48 ± 0.11 | p<0.001 |
| Rate of Acquisition (1000 ms)(trials to criteria) | 5.27 ± 0.50 | 5.40 ± 0.76 | n.s. |
| Rate of Acquisition (15 ms)(trials to criteria) | 6.36 ± 1.26 | 5.30 ± 1.05 | n.s. |
| Motor Activity (beam breaks) | 1740.94 ± 422.63 | 1807.85 ± 192.12 | n.s. |
| Hit Rate | 67.1% ± 2.5 | 84.9% ± 2.1 | p<0.001 |
| Correct Rejection Rate | 78.2% ± 1.5 | 85.6% ± 1.5 | p<0.01 |
| Omission Rate | 7.9% ± 1.1 | 3.8% ±0.9 | p<0.05 |
The low baseline performance group had a median VI of 0.552 and a mean VI of 0.572 while the high baseline performance group had a median of 0.788 and a mean VI of 0.776 (Fig. 3A). The mean VI of the two groups was significantly different from each other [t(23)=−7.970, p<0.001]. These differences were manifest in two additional measures of performance in the sustained attention task, hit rate (correctly responding on the signal lever) and correct rejection rate (correctly responding on the non-signal lever). The median hit rate of the low and high performance groups was 66.0% and 87.4%, respectively with averages of 67.1% and 84.9% (Fig. 3B),, which were significantly different from each other [t(23)=−5.088, p<0.001]. The mean correct rejection rates of the two groups were significantly different at 78.2% and 85.6% [t(23)=−3.376, p<0.01] with medians of 77.2% and 84.9%, respectively (Fig. 3C). Even the rate of omissions between the two groups was different (Fig. 3D) with the low performance group omitting responses an average of 7.9% of the time compared to an omission rate of 3.8% in the high performance group [t(23)=2.764, p<0.05].
The Effect of MPH on Low and High Baseline Performance Groups in the Sustained Attention Task
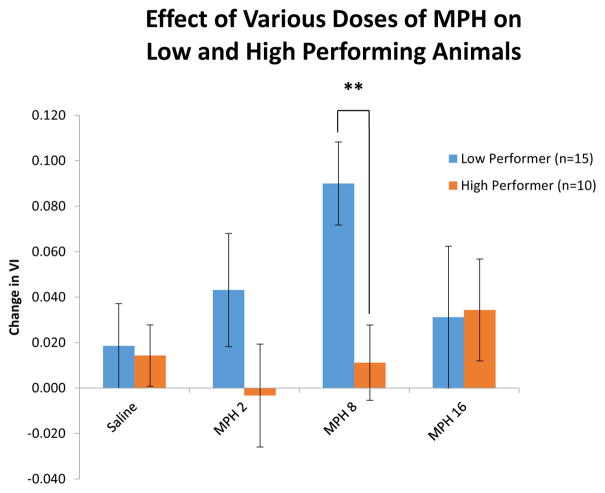
The next experiment examined how various doses of oral methylphenidate (saline, MPH 2 mg/kg, MPH 8 mg/kg, and MPH 16 mg/kg p.o.) affected performance of high and low performing rats in the sustained attention task (Fig. 4). All animals were subjected to each drug condition through repeated testing. Performance was quantified using VI as well as hit rate and correct rejection rate to determine if there were differential effects of dose and baseline performance on these two measures.
Figure 4.
The effect of various doses of MPH on performance in low and high performance groups. A range of MPH doses (2, 8, 16 mg/kg p.o.) were administered in each animal over multiple testing sessions. The low and high doses of did not affect performance in either group significantly. The moderate dose, which produces a clinically relevant blood plasma concentration comparable to that found in ADHD patients being treated with MPH, significantly improved performance in low baseline animals, but had no effect on high baseline animals [F(1,23)=9.190, p<0.01]. These results demonstrate a dose dependent and subject specific effect of MPH.
A repeated measures ANOVA revealed a significant main effect of the MPH dose on VI [F(5,19)=3.043, p<0.05]. Planned comparisons were performed to determine which doses had a significant effect. These analyses showed that that VI is only affected at a dose of 8 mg/kg p.o. and exclusively in the low performance group [F(1,23)=15.134,p<0.001]. This result was significant for both absolute and relative VI changes from baseline. The lower and higher doses did not significantly affect VI (2 mg/kg [F(1,23)=1.303,p=0.265]; 16 mg/kg [F(1,23)=2.107,p=0.160]). The high performance group was not affected by any dose of MPH. Similarly, hit rate was only enhanced in the low performance group at a dose of MPH 8 mg/kg p.o. The hit rate of the high performance group was not affected at any dose of MPH. In contrast to these results, MPH had no effect on correct rejection rates at any dose in either group. As expected, oral saline administered before or after the MPH dose response curve did not affect performance [F(1,23)=1.749,p=0.199; F(1,23)=1.026,p=0.322].
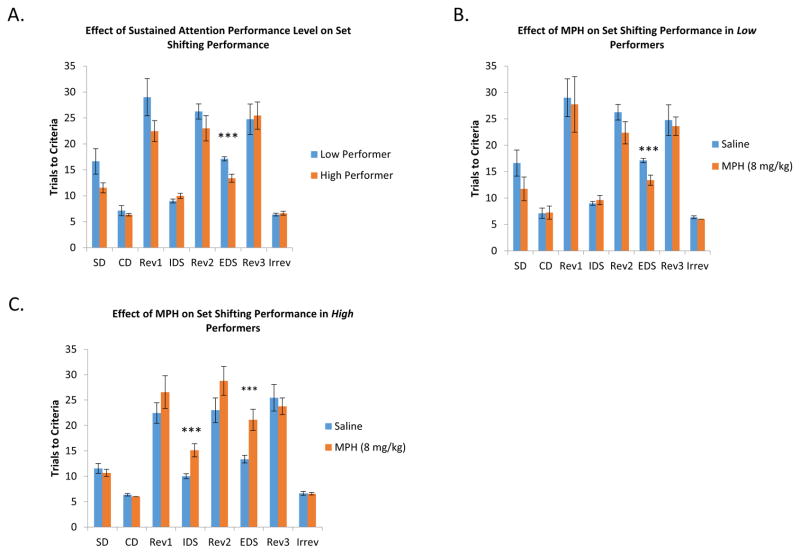
The Effect of MPH and Baseline Performance in the Sustained Attention Task on Performance in the Attention Set Shifting Task
The next set of experiments examined set shifting performance of animals tested (n = 17) in the sustained attention task (Fig. 5). Under control conditions the number of trials to criteria in the intra-dimensional shift were significantly different from the number of trials to criteria in the extra-dimensional shift indicating the animals were forming and shifting their attention sets as expected [F(1,21)=158.270,p<0.001]. Separating animals into the two sustained attention performance based groups yielded a similar significant difference in the number of trials to criteria between the IDS and EDS stages for both the low [t(23)=−8.526, p<0.001]and high groups [t(21)=−7.403, p<0.001]. Further segregation of the data to account for saline (Low, t(11)= −10.482, p<0.001; High, t(10)= −4.086) and MPH (Low, t(21)= −5.025, p<0.001; High, t(10)=−7.416, p<0.01) treatment did not change the significant differences found between the IDS and EDS shifts, demonstrating appropriate formation and shift of attention sets across each group and condition.
Figure 5.
A. The effect of baseline performance in the sustained attention task on set shifting performance. The high performance group required significantly fewer trials to criteria in the extra-dimensional shift compared to the low performance group [F(1,21)=13.758, p<0.001]. These data suggest that animals with better sustained attention may have better behavioral flexibility. B. The effect of MPH on low performance sustained attention animals in the set shifting task. MPH significantly decreased the number of trials to criteria in the extra-dimensional shift in animals with a low baseline level of performance in the sustained attention task [F(1,21)=30.556, p<0.001]. C. The effect of MPH on high baseline sustained attention animals in the set shifting task. MPH significantly impaired performance in the set shifting task by increasing the number of trials to criteria in both the intra-dimensional [F(1,20)=19.563, p<0.001] and extra-dimensional stages [F(1,20)=17.401, p<0.001] in animals with a high baseline level of performance in the sustained attention task
Because of the greater difficulty of the EDS, the number of trials to criteria in the IDS are typically less than the number of trials to criteria for the EDS. A repeated measures ANOVA found an overall difference in behavioral flexibility between the two groups of animals [F(1,21)=13.758, p<0.001]. ANOVA also found a significant main effect of MPH treatment [F(1,21)=8.456, p<0.01] that appeared to increase the number of trials to criteria overall. Drug treatment did not interact with the stage of the set shifting task [F(1,21)=1.394, p=0.251], suggesting that MPH increased trials to criteria in both stages equally. However, separating the performance of the low and high sustained attention animals revealed a significant interaction between the performance group of an animal and the drug treatment [F(1,21)=26.758, p<0.001], which suggested a difference in how the two groups of animals responded to the drug.
An interaction between all three factors [drug x stage x performance group, F(1,21)=23.090, p<0.001] indicated a difference in how the two performance groups responded to drug treatment across both testing stages. Planned comparisons showed that, under normal saline conditions, the high baseline performance group needed significantly fewer trials to criteria in the extra-dimensional shift [F(21)=23.673, p<0.001] compared to the low baseline performance group (Fig. 5A). Treatment with MPH improved the low baseline performance group by reducing the number of trials to criteria in the extra-dimensional shift [F(22)=30.556, p<0.001] (Fig. 5B). In the high performance group, MPH treatment significantly increased the trials to criteria in both the intra-dimensional [F(20)=19.563, p<0.001] and extra-dimensional stages [F(1,20)=17.401, p<0.001], suggesting an impairment in behavioral flexibility due to MPH ( [F(1,21)=9.563, p<0.001]) (Figure 5C).
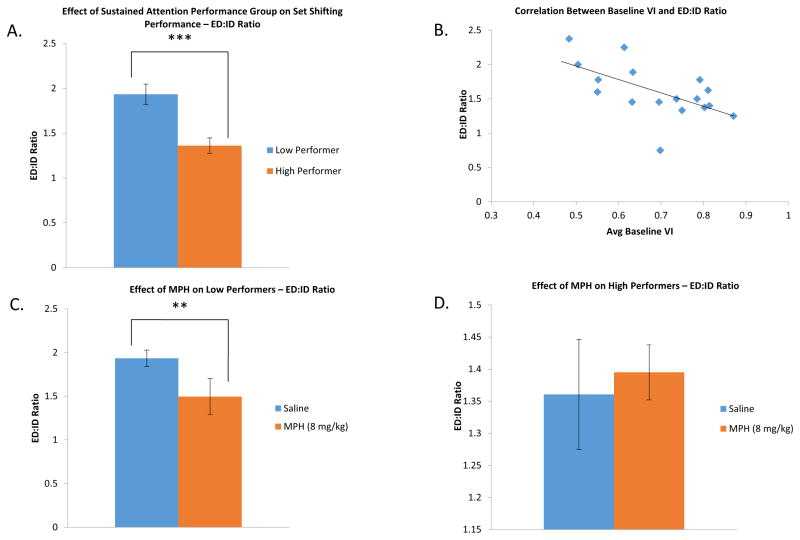
Post hoc tests were used to more specifically characterize the effects of MPH on each group (Fig. 6). To account for the increased number of trials to criteria in the high baseline performance group, we measured behavioral flexibility by comparing the number of trials to criteria in the extra-dimensional shift to the number of trials in the intra-dimensional shift. A repeated measures ANOVA found that MPH treatment had a significant main effect on performance [F(1,21)=5.975, p<0.05]. In addition, the two baseline performance based groups were still significantly different using this different measurement of behavioral flexibility [F(1,21)=14.877, p<0.001] (see Fig. 6A).
Figure 6.
Effect of performance in the sustained attention task and MPH on the ratio of ED to ID trials to criteria. Comparing the trials to criteria in the ED shift compared to the number of trials in the ID shift is one way to compare performance in the set shifting task between groups. A lower value in this metric indicates greater behavioral flexibility. Overall, there was a significant decrease in the ED:ID ratio following treatment with MPH [F(1,21)=14.877, p<0.001]. A. The effect of performance group on ED to ID ratio. The high performance group showed greater behavioral flexibility compared to the low performance group [F(1,21)=14.877, p<0.001]. B. Baseline performance in the sustained attention task negatively correlated with performance in the set shifting task (rs=−0.751, p<0.001 indicating animals with better vigilance have better behavioral flexibility. C. The effect of MPH on ED to ID ratio in the low performance group. There was a significant decrease in the ratio of ED to ID trials to criteria in the animals with a low baseline level of performance in the sustained attention task, indicating a significant drug-mediated increase in behavioral flexibility [t(11)=3.286, p<0.01]. D. The effect of MPH on the ED to ID ratio of the high performance group. MPH did not significantly change the ratio of trials to criteria [t(10)=−0.344, p=0.738]. Even though there was an increase in the number of trials to criteria with MPH, there was a parallel increase in the trials to criteria in the intra-dimensional stage that mitigated the relative effect of the increase in the trials to criteria in the extra-dimensional stage.
An interaction between drug treatment and the baseline performance groups [F(1,21)=8.033, p<0.01] indicated a difference in how the two groups of animals responded to drug treatment. The high baseline performance group had a ED:ID ratio of 1.36 under saline control conditions (Fig. 6D) while the low baseline performance group had a significantly higher ratio of 1.93 (Fig. 6C) [F(1,21)=31.307, p<0.001], indicating diminished flexibility. The ED:ID ratio in the high performance baseline group was 1.40 following MPH treatment (Fig. 6D), which was not significantly different from the low baseline performance group’s ED:ID ratio of 1.49 (Fig. 6C) [F(1,21)=0.483, p=0.495]. MPH treatment did not significantly alter the ED:ID ratio of the high baseline performance group [t(10)= −0.344, p=0.738] (Fig. 6D), but it did significantly alter the ratio in the low baseline performance group [t(11)=3.286, p<0.01] (Fig. 6C).
Relationship between Performance on the Sustained Attention Task and Performance on the Attention Set Shifting Task
A post-hoc correlational analysis of the various performance measures on the two tasks in all animals that were tested was conducted to determine whether performance in one task was predictive of performance in the other task. Spearman’s correlation analysis was used to compare the average baseline vigilance index from the sustained attention task to the IDS:EDS ratio from the attention set shifting task. Using this approach we determined that baseline performance (VI) in the sustained attention task was negatively correlated with baseline performance (ED:ID ratio) in the set shifting task (rs=−0.751, p<0.001 – Fig. 6B), i.e. more vigilant animals tended to have greater behavioral flexibility.
Discussion
We have shown that the subject specific effects of MPH observed in the clinical population are represented in a rodent model of cognition. The distribution of performance in a rodent test of sustained attention varied according to a normal distribution. An individual animal’s baseline performance in the sustained attention task correlated with the effect of MPH at 8 mg/kg p.o. in that same animal, ie an animal with high baseline performance showed little effect from MPH while an animal with low baseline performance showed a significant improvement in performance following MPH treatment.
Animals at the two ends of the performance spectrum in the sustained attention task differed in their cognitive function only. This difference was not due to alternative factors such as visual acuity, motor activity, or rate of learning. The only difference was in their ability to sustain attention to the task-related stimulus and respond appropriately. In addition, the effect of MPH on performance in the sustained attention task varied between the high and low performing groups.
The differences between animals that were revealed in the sustained attention task were also evident in a different a test of cognitive function, the attention set shifting task. Animals with poor performance in the sustained attention task showed poor ability to efficiently shift attention from one reinforcing stimulus to another in the attention set shifting task.
Technical considerations
Route of administration
We chose to administer the drug orally to mimic the clinical route of administration as closely as possible. To do so, we fed rats sweetened cereal treats infused with a MPH solution. This method required close monitoring to ensure that each rat consumed the proper amount of drug. However, this approach minimizes the stress experienced by the animal compared to oral gavage or intra-peritoneal injections and thus is a better route of administration for a cognitive task that has been shown to be sensitive to the effects of stress.
Dosing
MPH’s effect on cognition has previously been shown to be dose dependent, task dependent, and context dependent (Barkley, 1977; Dodds et al., 2008; Finke et al., 2010; Hale et al., 2011; Konrad et al., 2004; Marquand et al., 2011; Swanson et al., 1978). We selected only a primary dose based on pilot data from our laboratory that found 8 mg/kg p.o. to be the optimal dose for enhancing sustained attention performance. In ADHD children, the optimal dose of MPH for improving behavior is not the same as the optimal dose for improving cognitive function in these children, demonstrating variable behavioral results for different doses of MPH in the same subjects (Hale et al., 2011). In addition, MPH has been shown to have subject specific effects that are baseline performance dependent ( Finke et al., 2010; Rapport et al., 1985a).
Doses used to treat ADHD typically produce blood plasma levels of 8–40 ng/ml (Swanson et al., 1999; Swanson and Volkow, 2002). Oral doses of MPH at 0.5, 2.0, and 3.5 mg/kg in adult male Wistar rats produced peak blood plasma level s of 2.1, 36, and 62 ng/ml (Aoyama et al., 1990). Subsequent efforts in adolescent male Sprague Dawley rats closely replicated these results with 1.0 mg/kg p.o. producing a 9.3 ng/ml peak concentration (Kuczenski and Segal, 2002). The primary dose used in our study was 8.0 mg/kg p.o. which likely produces a blood plasma level above the range typically seen in clinical populations. However, pilot testing in our laboratory has shown that such a dose produces the most improvement in rodent performance of the sustained attention task. The discrepancy between the optimal dose to treat ADHD symptoms in humans and improve rodent performance on behavioral tasks could be due to differences in the norepinephrine transporter (NET), which, while functionally similar, can be genetically distinct with different capacities for monoaminergic uptake (Gu et al., 2001). While there is a less than four-fold difference in Ki values between the rodent and human NET, a small difference in binding between the two transporters could be enough to account for the higher doses needed to produce optimal behavioral improvements in the rat (Han and Gu, 2006). It is also important to note the differential sensitivity of rodent tests of executive function to different doses of MPH. In animals MPH improves performance on spatial working memory tasks at lower doses and across a narrower dose range than MPH at higher doses and across a broader range that improve performance on sustained attention tasks (Berridge et al., 2012). Thus, drug dosing for MPH must be considered in the context of the behavioral task being examined.
Vendor and Genetic background
Different genetic backgrounds could lead to different baseline levels of performance or drug sensitivities. Studies comparing behavioral performance of inbred strains to their outbred stocks have found significant differences in cognitive function. For example, the spontaneous hypertensive rat is an inbred strain of the Wistar Kyoto stock, but the two strains show differences in sustained attention and set shifting performance (Kantak et al., 2008; Thanos et al., 2010). The subjects used in this study were of the Sprague-Dawley outbred stock which is purportedly representative of the genetic diversity found in a heterogeneous population of wild type animals. Despite the best efforts of the vendor that supplied our subjects, the animals included in our study may not actually be representative of the genetic diversity of a heterogeneous population. For example, improper cross breeding procedures by the vendor for just a few generations would result in a shift in the genetic background of the subsequent generations of rats. Those rats, then, would not have the genetic diversity of the overall outbred stock, which could alter the behavioral performance of that sub-population of rats.
Behavioral Outcomes
Subject-specific effects of MPH – Sustained Attention
Because MPH reportedly produces subject specific effects in the clinical population (Barkley, 1977; Hale et al., 2011; Rapport et al., 1985a; Swanson et al., 1978), we examined the data for subject specific effects of MPH within an animal population. The effects of MPH in normal healthy human subjects have been consistently inconsistent in that the drug improves cognitive performance in some individuals in some tasks but does not have a universal effect across all individuals (Finke et al., 2010). Our results confirmed that the subject specific effects reported in clinical populations carried over into an animal population. The animals that improved the most from MPH treatment tended to have the lowest baseline levels of performance in the sustained attention task. Conversely, animals that showed no improvement from MPH treatment had higher baseline levels of performance. Our statistical analysis confirmed a strong correlation between baseline performance in the sustained attention task and the effect of MPH (8 mg/kg) on performance in the task. We subsequently split the population into low and high groups for further analysis based on their baseline performance and the effect of MPH on their performance.
The overall difference between low and high performing animals also carried over into the two primary components of performance in the sustained attention task, hit rate and correct rejection rate, both of which were higher in the high performance group. We examined other aspects of these two groups of animals to determine whether some factor other than differences in cognitive function could account for the variation in baseline performance in the two groups. We found no differences in motor activity, learning, and signal detection between the two groups of animals. We even found that the difference in performance carried over from an earlier training version of the sustained attention task that is easier due to the longer stimulus duration. The two groups of animals are, thus, two distinct behavioral phenotypes that differ only in tested dimensions of their cognitive function.
We repeated sustained attention testing in these same animals with different doses of MPH, i.e. ones that produced blood plasma levels above or below the behaviorally relevant range. We found that a dose below the optimal dose for improving task performance did not have a significant effect overall, nor did it have an effect on either performance group individually. Treating animals with a dose above our optimal dose did not improve performance in the sustained attention task, but it did reduce sustained attention in the low performance group.
The effects of MPH on behavioral flexibility
The set shifting task was designed to be analogous to the Wisconsin card sorting task, a test of behavioral flexibility in humans (Birrell and Brown, 2000). The results from our experiments are consistent with the dependence on the PFC and monoamine system reported in the human and nonhuman primate equivalents (Advokat, 2010; Dias et al., 1996; Koelega, 1993; Konrad et al., 2004; Mehta et al., 2004; Pietrzak et al., 2006; Robbins, 2007; Rogers et al., 1999; Tannock and Schachar, 1992; ). In our experiments MPH 8 mg/kg p.o. improved behavioral flexibility, most likely mediated by the α1 receptor (Lapiz and Morilak, 2006). The correlation between baseline set shifting performance and the effect of MPH on that performance indicates that it primarily enhances flexibility in animals that demonstrate initial poor behavioral flexibility. Quantifying behavioral flexibility using the ratio of ED to ID trials to criteria to compare performance across conditions suggested that the high baseline performance group was not affected by MPH treatment in the set shifting task. However, the total number of trials needed to reach criteria increased across both the intra-dimensional and the extra-dimensional stages, demonstrating a worsening of performance overall, despite the lack of relative change in behavioral flexibility according to the ED:ID ratio. These results suggest that poor behavioral flexibility can be improved by MPH administration. However, subjects with better baseline behavioral flexibility may be impaired by MPH treatment.
Correlations between MPH and performance in two different cognitive tasks
Our correlational analysis uncovered a number of relationships between performance in the two cognitive tasks. Our first finding was a correlation between baseline performance in the sustained attention task and the effect of MPH in that same animal’s performance in the sustained attention task. We also found a similar correlation between baseline performance in the set shifting task and the effect of MPH on an animal’s performance in the set shifting task. Prior studies in non-medicated ADHD patients, both children and adults, have demonstrated deficits in performance across a spectrum of tasks that evaluate multiple forms of attention including sustained and flexible capabilities (Tucha et al, 2006a; Tucha et al, 2006b). Finally, Animals with higher baseline sustained attention performance correlated with lower EDS:IDS ratios, i.e. more vigilant animals tended to have greater behavioral flexibility. Interestingly, MPH’s effect on one cognitive task did not correlate with its effect on the other task, but it is possible that the sample size was not large enough to reach such a conclusion.
Potential Biological Differences between Low and High Performing Animals
The different performance outcomes and differential MPH sensitivity observed in the two phenotypes characterized are suggestive of potential differences in the NE and/or DA systems. In ongoing and previous studies using an array of pharmacologic blocking agents we and others have determined that MPH effects on performance of sustained and flexible attention tasks are dependent, at least in part, upon interactions with noradrenergic neurotransmission (Berridge et al, 2012; Cain et al, 2011; Lapiz and Morilak, 2006; McGaughy et al, 2008; Navarra et al. 2008; 2013). The factors that would influence noradrenergic system function and subsequent behavioral phenotype include but are not limited to transporter function, presynaptic regulation of transmitter release, postsynaptic receptor availability, and neuroanatomical variations in locus coeruleus projections to signal processing and decision making circuits.
One testable hypothesis is that the behavioral differences between the two groups of animals is due to a variance in the norepinephrine transporter (NET) capacity to clear NE from the extracellular space owing to differences in uptake capacity or plasma membrane expression. In vitro models, for example, have shown the NET to be relatively plastic in its plasmalemmal surface expression and function (Annamalai et al., 2010; Binda et al., 2006; Dipace et al., 2007; Hahn et al., 2003; 2005; Mandela and Ordway, 2006; Matthies et al., 2010; Sung et al., 2003;). The plasmalemmal expression of NET varies between control and chronically stressed animals demonstrating plasticity of NET trafficking in vivo (Miner et al., 2006). NET expression has even been shown to vary from region to region within the same animal (Park et al., 2011).
Alternatively, there may be differences in transporter capacity that account for the differential response to MPH treatment. Peripheral system NET has been shown to be somewhat plastic in its uptake function as shown by its sensitivity to cold exposure (King et al., 1999). Human studies of NET polymorphisms have found an association between different alleles and different physiological responses demonstrating a possible link between transporter function and behavioral phenotype (Kohli et al., 2011). Differences in uptake capacity could alter the basal concentration of NE in the extracellular space, similar to the differences in baseline NE observed in human patients. In addition Wistar and Wistar-Kyoto rats chronically treated with desipramine show differences in forced swim test times that may be due to differences in transporter function measured in synaptosomes (Jeannotte et al., 2009). The low and high performance groups may exhibit a similar differential transporter uptake capacity which could account for the differential response to MPH. The same extracellular concentration of NE could produce varying influences on neural circuit functions and behavioral outcomes if presynaptic α2a autoceptors between groups of animals differ in their sensitivity or efficiency. A disparity in autoceptor function would result in an alteration in NE release across groups of animals, indirectly modulating function of the NET, which is sensitive to the extracellular concentration of NE (Zhu and Ordway, 1997). A variance in autoceptor function would thus lead to greater swings in NE concentration due to its indirect modulation of NET uptake capacity.
Differential expression or sensitivity of post-synaptic receptors between animals could result in different responses to the same concentration of NE in the extracellular space. The increased sensitivity to MPH shown in the low performance group in our experiments could indicate that these animals may be more sensitive to changes in NE efflux either through greater expression or increased sensitivity of post-synaptic receptors similar to the effect of polymorphisms of the α1 receptor in humans (Lei et al., 2005). Rodents have also been shown to exhibit changes in post-synaptic receptor sensitivity and α2 receptor binding affinity in rodent models such as the NET-KO mice and reserpine mediated monoamine depleted rats (Ugedo et al., 1993; Xu et al., 2000). The postsynaptic α2 receptor may be even more adaptive as shown by the change in receptor affinity (Kd) following both acute and chronic reserpine treatment of neural membrane extracted from various areas of the brain including the hypothalamus, parieto-occipital cortex, brainstem, and striatum (Giralt and Garcia-Sevilla, 1989).
Finally, differences in behavioral outcomes and MPH sensitivity could be due to neuroanatomical variations in LC projections to circuits engaged in attention tasks. Studies in our laboratory have shown that neurons in the LC are segregated with respect to their connections to the frontal cortex (Chandler and Waterhouse, 2012). Each neuron that projects to the cortex almost always projects to only a single cortical area and is distinct from other LC neurons that project to alternate cortical areas, although there are some LC cells that project to multiple distal targets. Variations in the relative proportion of LC neurons projecting to each sub-region of the frontal cortex would result in differences in the strength of the connectivity between the LC and frontal cortical targets between subjects that could be manifested as differential cognitive function. Alternatively, a change in the number of LC cells that project to multiple distal targets would result in a loss of fine control over PFC function that could also result in behavioral differences.
Implications
Implications of low performance group as a potential model of impaired cognitive function
Even though the animals used were purportedly of a normal wild type background, the low performance group exhibit a similar behavioral phenotype compared to clinical populations with impaired cognitive function such as ADHD. ADHD patients have been shown to be reliably impaired in tests of sustained attention and other executive functions (Barkley et al., 1992; Douglas, 1972; Seidman et al., 1998; 2004; Sergeant et al., 2002). The low performance group characterized in our experiments shows a similar phenotype to ADHD children, who, like the low performing rats here, show impaired sustained attention, poor cognitive flexibility, and good behavioral response to MPH treatment. The low performance rats may share a common mechanism that could provide insight into attention disorders for which the molecular and genetic etiology is poorly understood.
Implications for treating ADHD patients
Our results are consistent with growing evidence of subject specific effects of MPH (Barkley, 1976; Hale et al., 2011; Husain and Mehta, 2011). Each individual subject may need a slightly different dose to produce the best behavioral and/or cognitive improvement. Our experiment broadly split the population into two groups that responded differently to the same dose of MPH. Yet, within each group subjects responded similarly to the same dose of MPH, suggesting that MPH’s effects in a specific subject can be predicted based on a subject’s behavioral phenotype. The human clinical population also demonstrates subject specific responses to MPH (Barkley, 1976). The current methodology of slowly titrating the dose of MPH for a patient takes a number of weeks if not months to find the optimal dose for a patient, frequently delaying relief from symptoms for the patient. There is both preclinical (Blondeau and Dellu-Hagedorn, 2007) and clinical (Hale et al., 2011) evidence that a subject’s response to MPH can be predicted based on the behavioral phenotype of the subject. Our results support the possibility of developing behavioral phenotypes, based on neuropsychological screens such as a sustained attention test or set shifting test, which can be used to closely predict the most optimal dose for a patient based on his or her phenotypic profile.
Implications for off-label users of MPH
Many off-label users anecdotally claim improved cognition function following self-administration of low dose MPH. However, results from clinical studies of MPH’s effects on cognition in normal adult populations depict a more mixed picture ( Husain and Mehta, 2011; Repantis et al., 2010). Reaction time in the continuous performance task (CPT), the human analog of the sustained attention task used in our experiments, was consistently reduced in normal subjects, but improvements in cognition overall were inconsistent. One study found an improvement in a visual processing speed and short term memory, but only in subjects with a low baseline level of performance (Finke et al., 2010). Our experiments produced similar results with MPH enhancing low baseline performance but not high baseline performance in a rodent model of sustained attention. The lack of improvement in the high baseline performance group in our experiment suggests that high performing humans who self-administer MPH as a cognitive enhancer may not be benefiting as much as they might believe.
Conclusion
We have shown that the subject specific effects of MPH observed in the clinical population carry over to a rodent model of cognition. The distribution of performance in a rodent test of sustained attention varied according to a normal distribution. An individual animal’s baseline performance in the sustained attention task correlated with the effect of MPH at 8 mg/kg p.o. in that same animal, ie an animal with high baseline performance showed little effect from MPH while an animal with low baseline performance showed a significant improvement following MPH treatment. The only difference was in their ability to sustain attention to a stimulus and respond appropriately. The differences in sustained attention performance carried over to a test of a different cognitive function, flexible attention. Animals with poor sustained attention showed poor ability to efficiently shift attention from one reinforcing stimulus to another. As was the case with sustained attention, the performance enhancing effects of MPH on flexible attention were most evident in animals with initially weak performance in the attention set shifting task. Although the NE-LC system is not solely responsible for executive function, the results of the present study identify behavioral phenotypes that could result from variations in noradrenergic function.
Acknowledgments
This work was supported by NIH NIDA R01 017960 to BDW, NIH NIMH 1R21MH087921 to Jill McGaughy – University of New Hampshire, CURE grant from the Pennsylvania Tobacco Settlement, and a fellowship award to RC from the Drexel University Human Cognition Enhancement Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LIST OF REFERENCES
- Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Annamalai B, Mannangatti P, Arapulisamy O, Ramamoorthy S, Jayanthi LD. Involvement of threonine 258 and serine 259 motif in amphetamine-induced norepinephrine transporter endocytosis. J Neurochem. 2010;115:23–35. doi: 10.1111/j.1471-4159.2010.06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Kotaki H, Iga T. Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. J Pharmacobiodyn. 1990;13:647–652. doi: 10.1248/bpb1978.13.647. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Predicting the response of hyperkinetic children to stimulant drugs: a review. J Abnorm Child Psychol. 1976;4:327–348. doi: 10.1007/BF00922531. [DOI] [PubMed] [Google Scholar]
- Barkley RA. A review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry. 1977;18:137–165. doi: 10.1111/j.1469-7610.1977.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Grodzinsky G, DuPaul GJ. Frontal lobe functions in attention deficit disorder with and without hyperactivity: a review and research report. J Abnorm Child Psychol. 1992;20:163–188. doi: 10.1007/BF00916547. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, Waterhouse BD. Differential sensitivity to psychostimulants across prefrontal cognitive tasks: differential involvement of noradrenergic alpha - and alpha-receptors. Biol Psychiatry. 2012;71:467–473. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda F, Lute BJ, Dipace C, Blakely RD, Galli A. The N-terminus of the norepinephrine transporter regulates the magnitude and selectivity of the transporter-associated leak current. Neuropharmacology. 2006;50:354–361. doi: 10.1016/j.neuropharm.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau C, Dellu-Hagedorn F. Dimensional analysis of ADHD subtypes in rats. Biol Psychiatry. 2007;61:1340–1350. doi: 10.1016/j.biopsych.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates Attentional Set Shifting in adolescent rats. Dev Cogn Neurosci. 2011;1:552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Waterhouse BD. Evidence for broad versus segregated projections from cholinergic and noradrenergic nuclei to functionally and anatomically discrete subregions of prefrontal cortex. Frontiers in Behav Neurosci. 2012;6:20. doi: 10.3389/fnbeh.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dipace C, Sung U, Binda F, Blakely RD, Galli A. Amphetamine induces a calcium/calmodulin-dependent protein kinase II-dependent reduction in norepinephrine transporter surface expression linked to changes in syntaxin 1A/transporter complexes. Mol Pharmacol. 2007;71:230–239. doi: 10.1124/mol.106.026690. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas VI. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Canadian Journal of Behavioural Science. 1972;4:259–282. [Google Scholar]
- Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, Muller U. Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 2010;210:317–329. doi: 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- Giralt MT, Garcia-Sevilla JA. Acute and long-term regulation of brain alpha 2-adrenoceptors after manipulation of noradrenergic transmission in the rat. Eur J Pharmacol. 1989;164:455–466. doi: 10.1016/0014-2999(89)90253-7. [DOI] [PubMed] [Google Scholar]
- Gu HH, Wu X, Giros B, Caron MG, Caplan MJ, Rudnick G. The NH(2)-terminus of norepinephrine transporter contains a basolateral localization signal for epithelial cells. Mol Biol Cell. 2001;12:3797–3807. doi: 10.1091/mbc.12.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Robertson D, Blakely RD. A mutation in the human norepinephrine transporter gene (SLC6A2) associated with orthostatic intolerance disrupts surface expression of mutant and wild-type transporters. J Neurosci. 2003;23:4470–4478. doi: 10.1523/JNEUROSCI.23-11-04470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Mazei-Robison MS, Blakely RD. Single nucleotide polymorphisms in the human norepinephrine transporter gene affect expression, trafficking, antidepressant interaction, and protein kinase C regulation. Mol Pharmacol. 2005;68:457–466. doi: 10.1124/mol.105.011270. [DOI] [PubMed] [Google Scholar]
- Hale JB, Reddy LA, Semrud-Clikeman M, Hain LA, Whitaker J, Morley J, Lawrence K, Smith A, Jones N. Executive impairment determines ADHD medication response: implications for academic achievement. J Learn Disabil. 2011;44:196–212. doi: 10.1177/0022219410391191. [DOI] [PubMed] [Google Scholar]
- Husain M, Mehta MA. Cognitive enhancement by drugs in health and disease. Trends Cogn Sci. 2011;15:28–36. doi: 10.1016/j.tics.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannotte AM, McCarthy JG, Redei EE, Sidhu A. Desipramine modulation of alpha-, gamma-synuclein, and the norepinephrine transporter in an animal model of depression. Neuropsychopharmacology. 2009;34:987–998. doi: 10.1038/npp.2008.146. [DOI] [PubMed] [Google Scholar]
- Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A. Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 2002;59:662–663. doi: 10.1001/archpsyc.59.7.662. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Singh T, Kerstetter KA, Dembro KA, Mutebi MM, Harvey RC, Deschepper CF, Dwoskin LP. Advancing the spontaneous hypertensive rat model of attention deficit/hyperactivity disorder. Behav Neurosci. 2008;122:340–357. doi: 10.1037/0735-7044.122.2.340. [DOI] [PubMed] [Google Scholar]
- King VL, Dwoskin LP, Cassis LA. Cold exposure regulates the norepinephrine uptake transporter in rat brown adipose tissue. Am J Physiol. 1999;276:R143–151. doi: 10.1152/ajpregu.1999.276.1.R143. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology (Berl) 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Kohli U, Hahn MK, English BA, Sofowora GG, Muszkat M, Li C, Blakely RD, Stein CM, Kurnik D. Genetic variation in the presynaptic norepinephrine transporter is associated with blood pressure responses to exercise in healthy humans. Pharmacogenet Genomics. 2011;21:171–178. doi: 10.1097/FPC.0b013e328344f63e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Gunther T, Hanisch C, Herpertz-Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:191–198. doi: 10.1097/00004583-200402000-00015. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lei B, Morris DP, Smith MP, Svetkey LP, Newman MF, Rotter JI, Buchanan TA, Beckstrom-Sternberg SM, Green ED, Schwinn DA. Novel human alpha1a-adrenoceptor single nucleotide polymorphisms alter receptor pharmacology and biological function. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:229–239. doi: 10.1007/s00210-005-1019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandela P, Ordway GA. The norepinephrine transporter and its regulation. J Neurochem. 2006;97:310–333. doi: 10.1111/j.1471-4159.2006.03717.x. [DOI] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, O’Daly OG, Williams SC, Mourao-Miranda J, Mehta MA. Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology. 2011;36:1237–1247. doi: 10.1038/npp.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies HJ, Moore JL, Saunders C, Matthies DS, Lapierre LA, Goldenring JR, Blakely RD, Galli A. Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking. J Neurosci. 2010;30:7863–7877. doi: 10.1523/JNEUROSCI.4574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Navarra RL, Clark BD, Zitnik GA, Waterhouse BD. Methylphenidate and atomoxetine enhance sensory-evoked neuronal activity in the rat visual thalamus. Experimental and Clinical Psychopharmacology. 2013;21:363–74. doi: 10.1037/a0033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30:1225–1245. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S. Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. J Abnorm Child Psychol. 1985a;13:227–243. doi: 10.1007/BF00910644. [DOI] [PubMed] [Google Scholar]
- Rapport MD, DuPaul GJ, Stoner G, Birmingham BK, Masse G. Attention deficit disorder with hyperactivity: differential effects of methylphenidate on impulsivity. Pediatrics. 1985b;76:938–943. [PubMed] [Google Scholar]
- Repantis D, Schlattmann P, Laisney O, Heuser I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol Res. 2010;62:187–206. doi: 10.1016/j.phrs.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Blackshaw AJ, Middleton HC, Matthews K, Hawtin K, Crowley C, Hopwood A, Wallace C, Deakin JF, Sahakian BJ, Robbins TW. Tryptophan depletion impairs stimulus-reward learning while methylphenidate disrupts attentional control in healthy young adults: implications for the monoaminergic basis of impulsive behaviour. Psychopharmacology (Berl) 1999;146:482–491. doi: 10.1007/pl00005494. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Weber W, Hatch M, Faraone SV. Neuropsychological function in adults with attention-deficit hyperactivity disorder. Biol Psychiatry. 1998;44:260–268. doi: 10.1016/s0006-3223(97)00392-2. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Doyle A, Fried R, Valera E, Crum K, Matthews L. Neuropsychological function in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:261–282. doi: 10.1016/j.psc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Kinsbourne M, Roberts W, Zucker K. Time-response analysis of the effect of stimulant medication on the learning ability of children referred for hyperactivity. Pediatrics. 1978;61:21–29. [PubMed] [Google Scholar]
- Swanson J, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Psychopharmacology: concepts and opinions about the use of stimulant medications. J Child Psychol Psychiatry. 2009;50:180–193. doi: 10.1111/j.1469-7610.2008.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry. 1992;33:1217–1228. doi: 10.1111/j.1469-7610.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang GJ, Swanson JM, Newcorn JH, Volkow ND. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol Biochem Behav. 2010;94:374–379. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucha O, Mecklinger L, Laufkötter R, Klein HE, Walitza S, Lange KW. Methylphenidate-induced improvements of various measures of attention in adults with attention deficit hyperactivity disorder. J Neural Transm. 2006a;113:1575–92. doi: 10.1007/s00702-005-0437-7. [DOI] [PubMed] [Google Scholar]
- Tucha O, Prell S, Mecklinger L, Bormann-Kischkel C, Kübber S, Linder M, Walitza S, Lange KW. Effects of methylphenidate on multiple components of attention in children with attention deficit hyperactivity disorder. Psychopharmacology. 2006b;185:315–26. doi: 10.1007/s00213-006-0318-2. [DOI] [PubMed] [Google Scholar]
- Ugedo L, Garro MA, Pineda J, Giralt MT, Miralles A, Olmos G, Garcia-Sevilla JA, Menargues A, Obach R. Acute and chronic effects of reserpine on biochemical and functional parameters of central and peripheral alpha 2-adrenoceptors. Eur J Pharmacol. 1993;239:149–157. doi: 10.1016/0014-2999(93)90988-t. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Ordway GA. Down-regulation of norepinephrine transporters on PC12 cells by transporter inhibitors. J Neurochem. 1997;68:134–141. doi: 10.1046/j.1471-4159.1997.68010134.x. [DOI] [PubMed] [Google Scholar]