Abstract
Attention deficits and inappropriate regulation of sensory signal processing are hallmarks of many neuropsychiatric conditions, including attention deficit hyperactivity disorder (ADHD), for which methylphenidate (MPH) and atomoxetine (ATX) are commonly prescribed therapeutic treatments. Despite their widespread use and known mechanism of blocking reuptake of catecholamine transmitters in the brain, the resultant actions on individual neuron and neural circuit function that lead to therapeutic efficacy are poorly understood. Given the ability of MPH and ATX to improve cognitive performance in humans and rodent assays of attention, we were interested in their influence on early sensory processing in the dorsal lateral geniculate nucleus (dLGN), the primary thalamic relay for visual information from the retina to the visual cortex. In male rats, dLGN neuronal responses to light stimuli were altered in multiple ways following doses of MPH or ATX observed to enhance performance in visually-guided assays of attention in rats (MPH, 2 mg/kg; ATX, 0.5 mg/kg). Latencies to response onset and to the peak of the primary response were decreased, while the peak intensity and area of the primary response were increased. In addition, some cells that were unresponsive to light stimuli prior to drug treatment displayed a ‘gating effect,’ wherein prominent responses to light stimuli were evident following drug administration. Our results begin to reveal unique effects of MPH and ATX in enhancing sensory signal transmission through visual circuitry, and may yield new insights for understanding the pathophysiology of certain cognitive disorders and inform development of improved therapeutic treatments for these conditions.
Keywords: Atomoxetine, Methylphenidate, Dorsal lateral geniculate nucleus, Norepinephrine, Sensory processing
Introduction
Efficient processing of sensory information is crucial for navigating our surroundings and facilitating adaptive behavioral responses to incoming stimuli within complex and dynamic environments. An essential element in this process is the ability to filter relevant information from an abundance of continuously streaming sensory input. Inappropriate regulation of sensory processing is associated with cognitive deficits common to many neuropsychiatric disorders, including schizophrenia, autism spectrum disorders, and attention deficit hyperactivity disorder (ADHD) (Javitt, 2009; Mangeot et al., 2001; Marco, Hinkley, Hill, & Nagarajan, 2011). ADHD is characterized by inattentiveness and/or hyperactivity and impulsiveness. ADHD patients are easily distracted by environmental stimuli that would otherwise be ignored. Thus, inappropriate sensory signal processing is a core deficit in these individuals. The psychostimulant drug, methylphenidate (MPH; Ritalin ®), and the non-stimulant medication, atomoxetine (ATX; Strattera ®), are commonly prescribed treatments for patients with ADHD and have been reported to enhance attention in otherwise healthy individuals (Bidwell, McClernon, & Kollins, 2011; Greely et al., 2008; Swadlow & Gusev, 2001; Swanson & Volkow, 2002). In addition, both MPH and ATX have been reported to improve elements of cognitive performance in a range of rodent assays that require attention and sensory-guided responses (Berridge et al., 2012; Cain, Wasserman, Waterhouse, & McGaughy, 2011; Jentsch, Aarde, & Seu, 2009; Navarra et al., 2008; Paterson, Ricciardi, Wetzler, & Hanania, 2011; Robinson, 2012). Despite the widespread clinical use and extensive pre-clinical investigation of cognitive function in animal models, the extent to which MPH and ATX alter the physiology of sensory circuits within the central nervous system to contribute to their attention-enhancing effects remain poorly understood.
MPH inhibits reuptake transporters of the catecholamine neurotransmitters, dopamine (DA) and norepinephrine (NE), while ATX is a selective NE transport inhibitor (Berridge et al., 2006; Bymaster et al., 2002). Hence, MPH and ATX share a common mechanism of action by increasing extracellular levels of NE throughout the brain. The ascending locus coeruleus (LC)-NE pathway has been implicated in regulating behavioral states of arousal and modulating state dependent sensory processing (Berridge & Waterhouse, 2003). For example, Aston-Jones and colleagues (1997; 1994) demonstrated that LC neurons respond selectively to target visual stimuli according to the behavioral relevance of such signals, rather than to stimulus attributes. In addition, in this task of sustained attention, enhanced LC responsiveness was positively correlated with improved behavioral performance. At the cellular and circuit level, NE has been shown to potentiate stimulus-evoked discharge and enhance ‘signal to noise’ ratios of responses of sensory processing neurons and networks to afferent stimuli throughout the brain (Berridge & Waterhouse, 2003). MPH was shown to facilitate the processing of incoming stimuli within the sensory cortex, consistent with increased NE in LC terminal fields (Drouin, Page, & Waterhouse, 2006; Drouin, Wang, & Waterhouse, 2007). This result indicates that MPH produces effects characteristic of NE-induced modulation of sensory processing. As such, it is reasonable to predict that blockade of NE reuptake and subsequent elevation of NE concentrations in the LC terminal fields can facilitate processing of behaviorally relevant sensory information, and thereby improve performance in sensory-guided assays of attention.
The rat dorsal lateral geniculate nucleus (dLGN) of the thalamus is the primary sensory relay for visual information from the retina to the cortex and is of interest because it receives substantial input from the LC-NE system (Kromer & Moore, 1980; Latsari, Antonopoulos, Dori, Chiotelli, & Dinopoulos, 2004; Papadopoulos & Parnavelas, 1990). By contrast, DA innervation of the dLGN is minimal (García-Cabezas, Martínez-Sánchez, Sánchez-González, Garzón, & Cavada, 2009). Electrical stimulation of the LC or direct application of NE within the dLGN increases spontaneous discharge of dLGN neurons and facilitates their response to afferent excitatory stimuli (Kayama, 1985; Kromer & Moore, 1980; Rogawski & Aghajanian, 1980a, 1980b) via alpha-1 (α1) post-synaptic adrenoreceptors (Kayama, 1985; Rogawski & Aghajanian, 1982). Given these effects and the mechanisms of action for MPH and ATX, we postulated facilitating effects of these agents on responses of dLGN neurons to visual stimuli. To this end, we investigated the effects of systemic administration of MPH and ATX on response properties of individual dLGN neurons in anesthetized rats.
Methods
Subjects
Nine male Sprague-Dawley rats (250 – 500 g) (Taconic; Germantown, NY) were housed in pairs prior to surgery and individually post-surgery. Rats were allowed to acclimate to the facility, which was temperature and humidity controlled, for at least one week following arrival and maintained under a 12/12 h light/dark cycle (lights on at 7:00 AM) with ad libitum access to food and water. All experimental procedures were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Drexel University Institutional Animal Care and Use Committee.
Drugs
MPH and ATX were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were dissolved in sterile saline and administered via intraperitoneal (i.p.) injection in a dosing volume of 1 mg/ml. Doses of MPH (2 mg/kg, i.p.) and ATX (0.5 mg/kg, i.p.) were chosen in accordance with multiple reports of these drugs enhancing measures of cognitive function in normal adult rats (Navarra, Graf et al. 2008; Jentsch, Aarde et al. 2009; Cain, Wasserman et al. 2011; Paterson, Ricciardi et al. 2011; Berridge, Shumsky et al. 2012; Robinson 2012). These doses are also within the range that produce plasma concentrations for exerting therapeutic effects in patients with ADHD and for improving cognitive function in healthy adult humans (Sauer, Ring, & Witcher, 2005; Swanson & Volkow, 2002). Specifically, plasma concentrations following administration of clinically efficacious doses of MPH are within the range of 8 – 40 ng/ml (Swanson & Volkow, 2002) and similar plasma levels are obtained after administration of cognitive-enhancing doses in rats (Berridge et al., 2006).
Surgery
Animals were anesthetized with isoflourane (4% induction, maintained at 1 – 2% in 95% O2 5% CO2) and positioned in flat skull orientation in a stereotaxic frame with atraumatic ear bars. Body temperature was monitored and maintained at 37°C using a rectal probe thermometer and a heating pad. The skull surface was exposed and 4 – 6 jeweler’s screws were fixed to the skull to secure electrode implants. Eight-bundle microelectrodes of formvar-insulated nichrome wires (25μm; A-M Systems, Sequim, WA) were fitted into electrically grounded 26G guide cannulae and implanted bilaterally in the dLGNs of the thalamus (AP –4.8, ML ±3.8, DV –4.0) using stereotaxic coordinates (Paxinos & Watson, 2007). Electrode placement was verified using previously established anatomical and neurophysiological criteria for dLGN thalamic neurons, including activity evoked by light stimulation presented to the contralateral eye with a primary response peak latency prior to 65 msec (Marks, Speciale, & Roffwarg, 1988). Electrode implants were then secured using a connector and dental cement and subjects were allowed to recover for at least one week prior to recording experiments or underwent experimental recording procedures during a terminal surgical session on the same day.
Single-unit recording
For all experimental procedures, the animal was placed in a stereotaxic apparatus with atraumatic ear bars under light isoflourane anesthesia (~2% in 95% O2 5% CO2). A light emitting diode (LED; RadioShack© white, clear lens LED light with luminous intensity: 1100 mcd, chromaticity coordinates: 660, viewing angle: 100 deg) was positioned centrally 1 cm in front of and above the head along the midline, and the room was darkened. Stimuli of three different intensities (5 msec duration) were delivered in pseudorandom order at a rate of 2 stimuli/sec (Hz) using a CED 1401 and a script written using Spike2 software (CED, Cambridge, England). Electrical activity was amplified and discriminated in real time using a combination of acquisition, spike discrimination, and analysis software (Sort Client and NeuroExplorer; Plexon Inc., Dallas, TX) to isolate single-unit waveforms and create cumulative raster plots and peri-stimulus time histograms (PSTHs) of the activity of light-responsive single-units within the dLGN. All recorded activity was stored for off-line analysis. Single-unit activity was re-sorted off-line, according to waveform characteristics and inter-spike interval distribution patterns including peak voltage of the waveform, waveform slopes, scattergram of the waveform’s first two principal components, spike train auto-correlogram, and inter-spike interval histogram (Offline Sorter; Plexon). Following sorting, PSTHs representing single-unit responses were constructed for each stimulus intensity and drug condition, then plotted using bin widths of 1 msec with boxcar smoothing across 3 adjacent bins. Unit responses were examined during vehicle pre-treatment and drug post-treatment conditions.
Analysis of electrophysiological data
Data were analyzed using custom routines written in MATLAB (MathWorks, Inc., Natick, MA). The parameters of neuronal function that were assessed included baseline firing rates and primary light-evoked response properties, including latency to response onset, latency to maximum response, maximum response amplitude, and response area as depicted in Figure 1a. Mean baseline firing rates represented as frequency in impulses/sec were calculated over 100 msec prior to stimulus presentation. Onset and offset of primary response peaks in the PSTHs were determined as the time where two or more consecutive bins crossing a threshold of 3 standard deviations above the average baseline discharge. Onset latencies less than 65 msec were considered to represent primary responses to stimuli, and peaks with longer latencies were excluded from further analysis. Maximum peak amplitude and peak response area (the sum of all bin values from peak onset to peak offset) were corrected for background firing rate by subtracting the mean baseline firing rate from each post-stimulus bin. ‘Gating’ was declared for cells that were initially unresponsive to light stimuli of a given intensity but demonstrated prominent responses to the same intensity stimulus following drug administration. For each cell PSTHs from unit activity were constructed of 15 min intervals under vehicle pre-drug conditions, then again beginning at 15 min following MPH administration (n = 63 units) or 30 min following ATX administration (n = 47 units). Additional control studies included analysis of PSTHs constructed from unit activity collected during a 15 min saline condition time-matched to the vehicle pre-drug condition examined in MPH and ATX studies, then again following a second saline administration, beginning at 15 min to match the MPH administration, and again at 30 min to match ATX administration (n = 26 units). Post-treatment evaluation times were chosen in accordance with time scales reported to demonstrate significant increase of extracellular catecholamine concentrations in the brain (Bymaster et al., 2002) and correspond to clinically relevant plasma levels following administration of MPH in rats (Berridge et al., 2006).
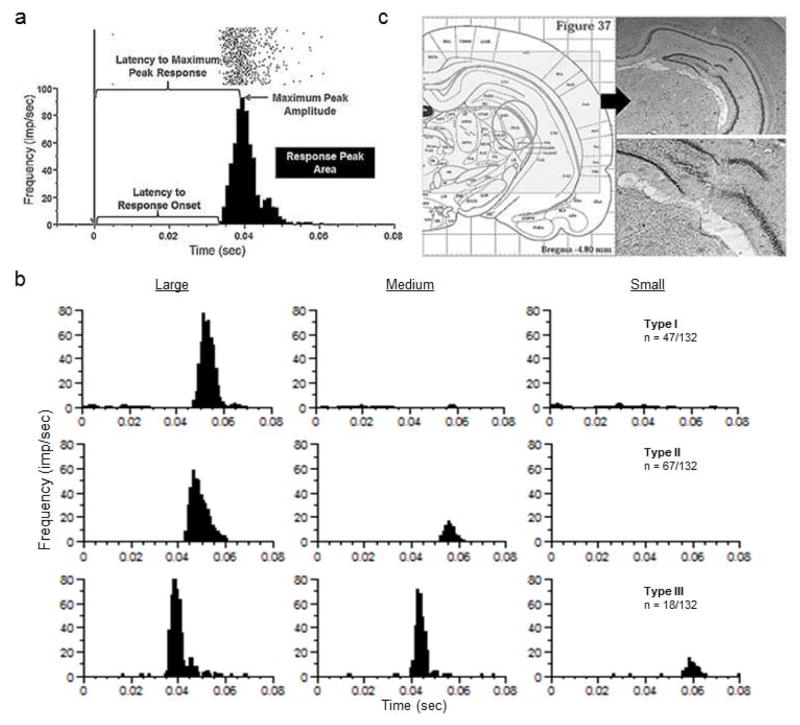
Figure 1.
Representative response properties of visually-sensitive cells and histological verification of electrode placement within the rat dLGN. a) Raster plot of single-unit activity and corresponding PSTH of visually-evoked discharge with respect to light stimuli (arrow at time = 0 msec) recorded from a single-unit in the rat dLGN. Properties of primary light-evoked responses are shown, including latency to response onset, latency to maximum peak response, maximum peak amplitude, and peak response area. b) Three representative types of single-units recorded from the dLGN with different thresholds for stimulus intensity. Top: type I, n = 47/132 cells that responded to high stimuli only, middle: type II, n = 67/106 cells that responded to high and intermediate stimuli, and bottom: type III, n = 18 cells that responded to all three stimuli. Spike rate intensities represented as frequency in impulses/second (imp/sec) in b) and c) are plotted in bin widths of 1msec with boxcar smoothing across 3 adjacent bins. c) Representative coronal section through the dLGN of the rat thalamus collected from one of the subjects upon completion of experimental procedures. Left: corresponding location as depicted in the stereotaxic atlas of the rat brain (Paxinos & Watson, 2007). Right: magnification (top = 1.6x, bottom = 5x) of the electrode guide cannula and electrode tracts terminating in the dLGN.
Light-responsive units were classified according to their pre-drug control responses to the three different light stimulus intensities; type I cells responded to high intensity stimuli only, type II responded to both high and intermediate intensity stimuli, and type III cells responded to all three stimulus intensities – high, intermediate, and low (see Figure 1b). Because distributions of most PSTH parameters were substantially non-normal, and transformations that normalized some group data simultaneously de-normalized others, Kruskal-Wallis tests with post-hoc Dunn’s multiple comparison tests were used to compare responses among stimulus intensities. Single-units that responded to a given stimulus in both pre- and post-treatment epochs were further analyzed with Wilcoxon matched-pairs signed rank tests, comparing pre-treatment to post-treatment PSTHs, both for MPH and ATX. Drug-induced changes in light-evoked discharge were expressed as percentages [(post-treatment – pre-treatment/pre-treatment) x 100]. Mann Whitney tests were used to compare these values to those observed for neurons recorded from rats that received consecutive saline (SAL-SAL) injections. Fisher’s exact tests were used to test whether drug administration exerted differential ‘gating’ effects on neurons that exhibited responses to low versus intermediate intensity stimuli. Statistical analyses and preparation of figures were performed using Prism (GraphPad Software, Inc.) and NeuroExplorer (Plexon) software.
Histological analysis
Upon completion of experiments, animals were deeply anesthetized and perfused with 4% paraformaldehyde. Brains were extracted, frozen, sectioned and mounted in 30 μm slices through the dLGN, and counterstained with neutral red for verification of electrode tracks terminating in the dLGN (Figure 1c).
Results
Prior to drug treatment and time-matched vehicle post-treatment, one hundred and thirty two light-responsive cells were recorded from the dLGN of nine animals. The distribution of cells responding to light stimuli of different intensities was as follows: type I (high only) = 47/132 cells; type II (high and intermediate) = 67/132 cells; type III (high, intermediate, and low) = 18/132 cells (Figure 1b). The effect of decreasing stimulus intensity on the properties of light sensitive neurons was evaluated. There was an overall effect of stimulus intensity on the latency to onset of response (p < 0.0001) with post-hoc analysis revealing that latencies increased in response to low intensity stimuli as compared to intermediate and high intensities (p < 0.05; Figure 2a). Latency to the maximum response was also affected by stimulus intensity (p < 0.0001) with post-hoc tests revealing that latency increased in response to the low intensity stimulus compared to intermediate and high intensities (p < 0.05; Figure 2b). The maximum peak amplitude and the response peak area were significantly decreased as stimulus intensity decreased (p < 0.05 and p = 0.005, respectively) with post-hoc tests revealing significantly smaller magnitudes of response to the low stimulus compared to the high in both conditions (p < 0.05; Figure 2c and d, respectively). Overall, these data indicate that response latencies increase and response magnitudes decrease as stimulus intensity decreases.
Figure 2.
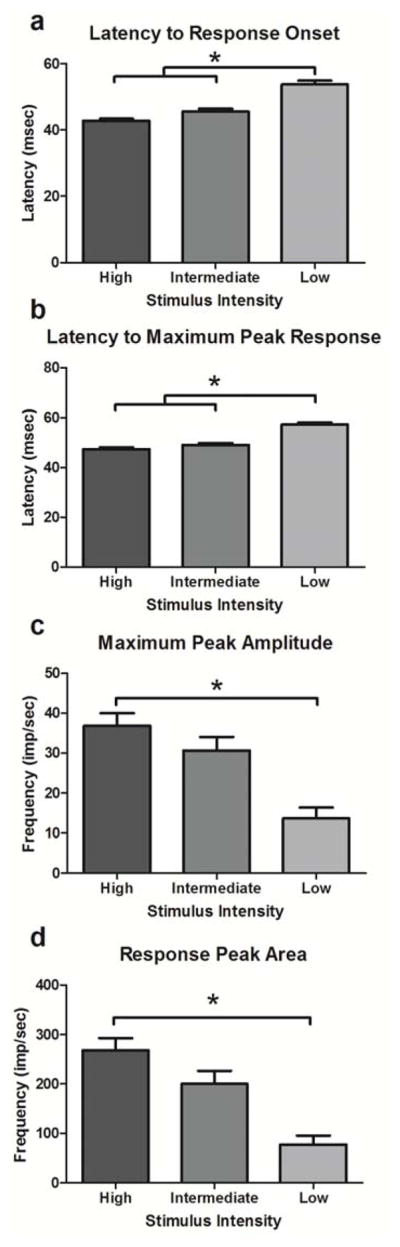
Effects of stimulus intensity on properties of light-evoked responses in the dLGN. Mean and SEM of response properties across decreasing stimulus intensities. N = 132 cells responded to the high intensity stimulus, a subset of these (n = 85/132 cells) responded to the intermediate intensity stimulus, and four cells (n = 18/106) responded to all three stimuli. * denotes p < 0.05 between pairs of stimulus intensity.
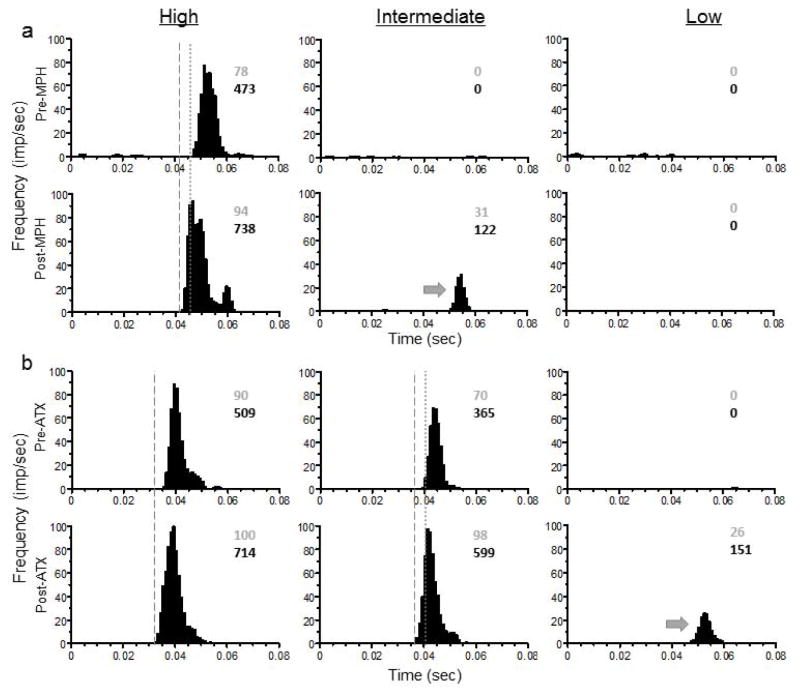
Both MPH and ATX facilitated responses of dLGN neurons to light stimuli. Figure 3 (MPH; 3a, and ATX; 3b) illustrates typical cases where drugs decreased the latency and increased the magnitude of a single-unit response to high and intermediate intensity stimuli. Note also that a response to the intermediate or low intensity stimulus was not evident under control (pre-treatment) conditions but became prominent following drug administration with either MPH or ATX, i.e. ‘gating.’
Figure 3.
Representative PSTHs illustrate the effects of drug treatment (a; methylphenidate; MPH, and b; atomoxetine; ATX) on responses of a single dLGN neuron to high, intermediate, and low intensity visual stimuli. Histograms are aligned to stimulus onset (time = 0) for each stimulus intensity. Stimuli of three intensities were presented in pseudorandom order at 2 Hz across pre-treatment control (top) and drug post-treatment (bottom) conditions. Spike rate intensities represented as frequency in impulses/second (imp/sec) are plotted using bin widths of 1 msec with boxcar smoothing across 3 adjacent bins. Dashed lines: an example of decreased latency to response onset following drug post-treatment. Dotted lines: an example of decreased latency to peak response following drug post-treatment. Numeric values offset from histogram peaks represent maximum peak amplitude (top) and response peak area (bottom), respectively in impulses/second (imp/sec). Arrow denotes an example of a gated response, i.e. one that is not evident under control conditions but becomes apparent following ATX administration.
Effects of MPH on dLGN neuronal responses to visual stimulation
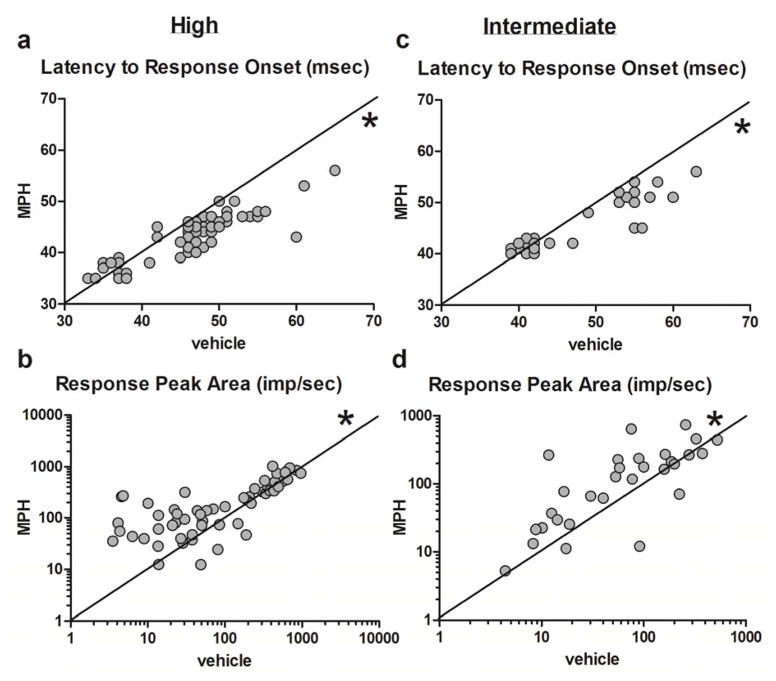
The effects of MPH on responses to high and intermediate intensity stimuli were tested in n = 60 cells recorded from six animals. The bivariate scatter plots in Figures 4 and 5 show the values for latency and magnitude measures for each cell in vehicle pre-treatment condition on the x-axis versus drug post-treatment condition on the y-axis for large and intermediate intensity stimuli (there were only four cells that responded to low intensity stimuli, and they are not represented in Figure 4). Points clustering below the line of identity for latency measures indicate cells whose latencies were greater in vehicle compared to drug condition, signifying a decrease in response latencies or faster responses following drug administration. Additionally, points clustering above the line of identity for measures of magnitude indicate cases where cell responses to light stimuli increased in magnitude following drug treatment. MPH administration significantly decreased the latencies of response to high intensity stimuli (median difference in onset latency = −3 msec, p < 0.0001; Figure 4a, median difference in peak latency = −1 msec, p < 0.001). Following MPH treatment, the maximum amplitude of the response was increased (median difference = 6.9 impulses/sec, p < 0.0001) as was the area of the response peak (median difference = 40.9 impulses/sec, p < 0.001; Figure 4b).
Figure 4.
Effects of methylphenidate (MPH) on properties of visually-evoked responses in the dLGN. Bivariate scatter plots represent vehicle on the x-axis by MPH on the y-axis values for properties of neuronal activity in response to both high (a, b) and intermediate (c, d) stimulus intensities. Points clustering above the 45 degree equivalence line indicate that there was increase in the response measure following MPH treatment whereas those below the line indicate that there was a decrease in the response measure following MPH treatment. * below the line of identity denotes a significant decrease (p < 0.05) in measures post-MPH treatment and above the line of identity denotes a significant increase (p < 0.05) in measures post-MPH treatment.
Figure 5.
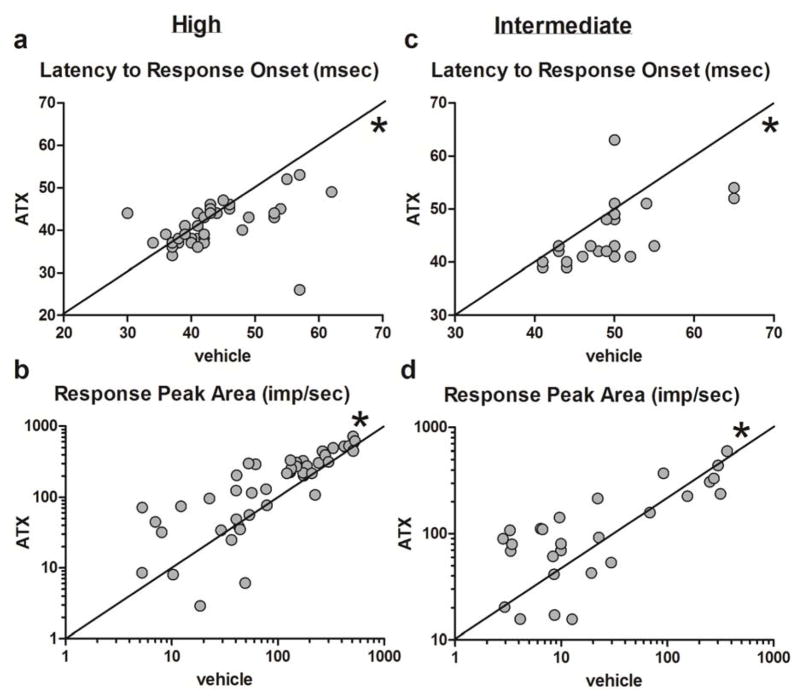
Effects of atomoxetine (ATX) on properties of visually-evoked responses in the dLGN. Bivariate scatter plots represent vehicle on the x-axis by ATX on the y-axis values for properties of neuronal activity in response to both high and intermediate stimulus intensities. Points clustering above the 45 degree equivalence line indicate that there was increase in the response measure following ATX treatment and below indicate that there was a decrease in the response measure following ATX treatment. * below the line of identity denotes a significant decrease (p < 0.05) in measures post-ATX treatment and above the line of identity denotes a significant increase (p < 0.05) in measures post-ATX treatment.
MPH also reduced the latencies to response onset (median difference = −1.5 msec, p < 0.005; Figure 4c) and to the maximum response (median difference = −2 msec, p < 0.005) for intermediate intensity stimuli. Correspondingly, the maximum response amplitude (median difference = 5.6 impulses/sec, p < 0.005) and the area of the response peak (median difference = 22.8 impulses/sec, p < 0.01; Figure 4d) were significantly increased for medium intensity stimuli following MPH treatment. These results indicate that dLGN neurons respond more quickly and more robustly to both high and intermediate intensity light stimuli after systemic administration of MPH.
Visual inspection of Figure 4 suggests that the MPH-induced changes in PSTH peaks are not uniformly distributed across the ranges of response properties in the sample. Latencies to response onset (Figure 4a and c) or to peak response exhibit an overall pattern where the shortest latencies are more or less evenly distributed about the line of identity, while the points with the longest vehicle pre-treatment latencies fall predominantly below the line, indicating that the latencies of the slowest-responding cells are most likely to be impacted by MPH administration. In contrast, the peak magnitude measures (Figure 4b and d) suggest that whereas responses to intermediate intensity stimuli were almost all increased by MPH, irrespective of initial peak size, the responses to high intensity stimuli increased proportionately more if they were relatively small under vehicle treatment conditions. Together these observations suggest a ceiling effect: peaks that are small and/or slow initially are enhanced by MPH, whereas peaks that are initially large and/or fast are less affected.
Effects of ATX on dLGN neuronal responses to visual stimulation
The effects of ATX on responses to high and intermediate intensity stimuli were tested in n = 46 cells recorded from five animals. Results were broadly comparable to those seen with MPH treatment. For high intensity stimuli, the latencies to response onset and to peak response were significantly decreased with ATX treatment (median difference = −1 msec, p < 0.05 (Figure 5a) and p < 0.05, respectively), and the magnitude measures were significantly increased (amplitude; median difference = 7.3 impulses/sec, p < 0.0001; area, median difference = 60.8 impulses/sec, p < 0.001; Figure 5b).
In response to the medium intensity stimulus, there was a significant decrease in latency to the response onset (median difference = −3.5 msec, p < 0.0001; Figure 5c) and a trend toward decreased latency to the maximum response (median difference = −0.5 msec, p = 0.09). Both measures of peak magnitude in response to the intermediate stimulus were significantly increased following ATX (amplitude median difference = 6.3 impulses/sec, p < 0.0001; area median difference = 69.9 impulses/sec, p < 0.0001, Figure 5d). These results indicate that as with MPH, the responses of dLGN neurons to light stimuli occur more quickly and more robustly following ATX treatment. With one exception, ATX facilitated neural responses following the same pattern of enhancing modulatory effects.
The scatterplot patterns in Figure 5 for ATX are not as clear as those of Figure 4 for MPH. Nevertheless, the trends are similar; latency shifts tend to be greater for slower responding cells (Figure 5a and c) and cells with initially smaller response amplitudes are increased to a greater extent than those with initially larger magnitude responses (Figure 5b and d). The net effect of these changes brought about by MPH and ATX is a stronger and faster response of the dLGN visual relay circuit to light stimuli.
Percent change in visually-evoked neuronal activity following post-treatment conditions
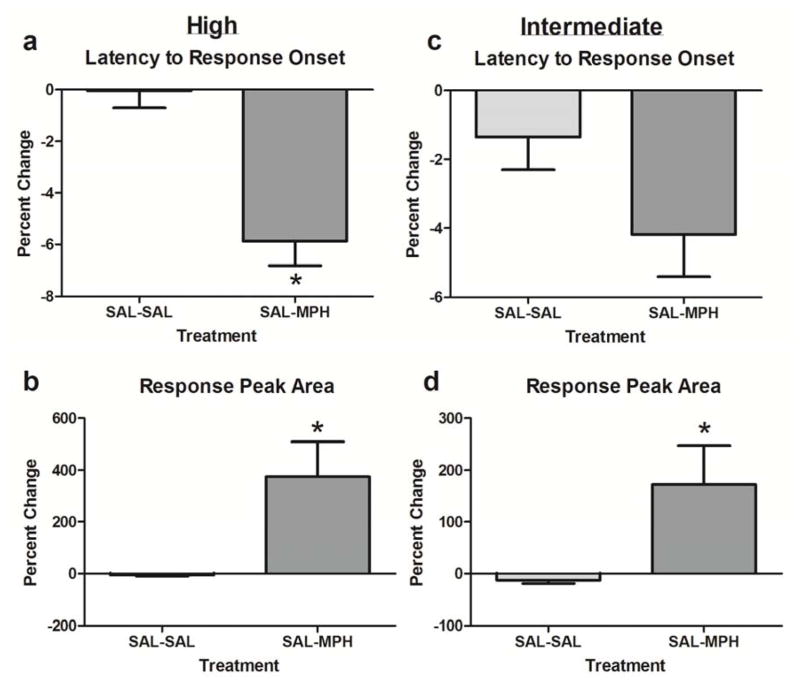
An additional three animals (n = 26 cells) received a second administration of saline to construct PSTHs corresponding with MPH and ATX post-treatment time point analyses and serve as further control conditions. For the high intensity stimulus, the percent change in the latency to response onset (p < 0.0005; Figure 6a) and the latency to the maximum response (p < 0.005) following MPH significantly differed from the saline time-matched control, with MPH decreasing the latencies to a greater degree than its time-matched saline control. Percent changes in response magnitudes to the high intensity stimulus (amplitude; p < 0.0001, and area; p < 0.0005; Figure 6b) were also significantly increased to a greater extent. For the intermediate intensity stimulus, MPH did not significantly alter onset latency compared to time-matched saline (p > 0.05; Figure 6c), but it did reduce peak latency (p < 0.0005). Both measures of magnitude had significantly greater percent changes following MPH in response to the intermediate intensity stimulus (amplitude; p < 0.0001, and area; p < 0.0001; Figure 6d).
Figure 6.
Percent change in latency and magnitude of visually-evoked responses following methylphenidate (MPH) as compared to saline (SAL) time-matched control. Percent changes following MPH (n = 60 cells) or SAL (n = 26) were calculated as [(post-treatment – pre-treatment/pre-treatment) x 100]. * denotes significance (p < 0.05) of post-treatment effect between groups.
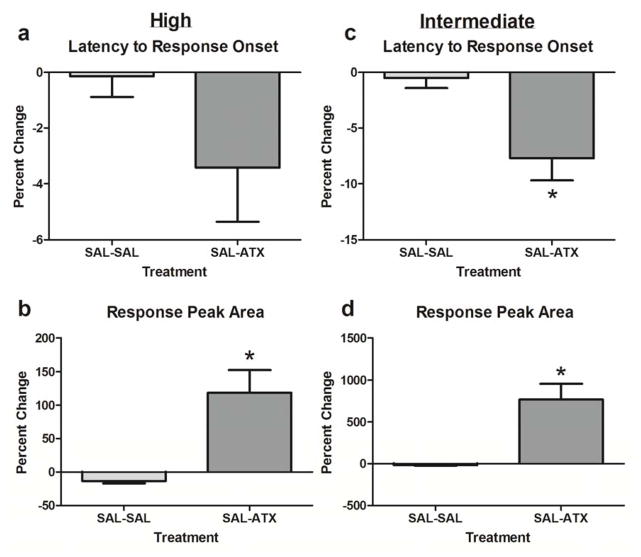
Compared to time-matched controls, ATX also significantly shortened latencies (p < 0.01 – p < 0.0005), except for the onset latency of the response to the high intensity stimulus (p > 0.05; Figure 7). As observed with MPH, both measures of magnitude (peak amplitude and area) exhibited significantly greater percent changes following ATX in response to both high and intermediate intensity stimuli (p < 0.0001 for each comparison; Figure 7).
Figure 7.
Percent change in latency and magnitude of visually-evoked responses following atomoxetine (ATX) as compared to saline (SAL) time-matched control. Percent changes following ATX (n = 46 cells) or SAL (n = 26) were calculated as [(post-treatment – pre-treatment/pre-treatment) x 100]. * denotes significance (p < 0.05) of post-treatment effect between groups.
Gating effects of MPH and ATX across stimulus intensities
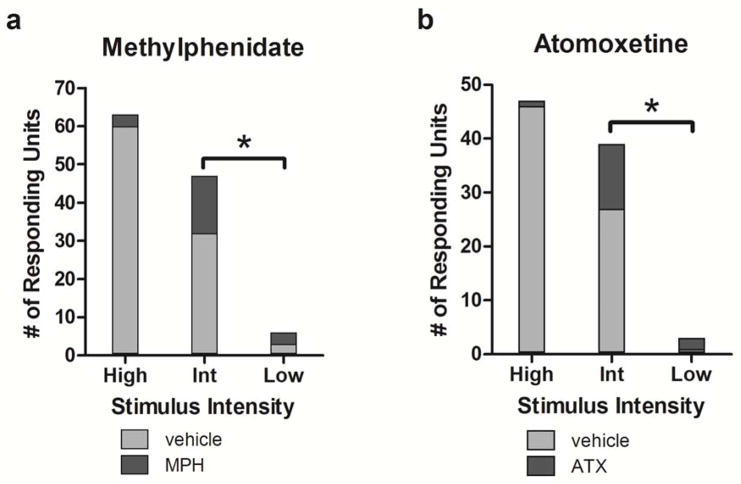
Neurons were also examined for gating effects following MPH and ATX administration. Gating of sensory-evoked discharges in noradrenergic terminal fields has been observed previously following LC stimulation or local application of NE in both cortical and thalamic sensory circuits (Devilbiss & Waterhouse, 2011; Waterhouse et al., 1988). In these previous experiments, neurons initially unresponsive to afferent sensory inputs become responsive during periods of elevated noradrenergic neurotransmission. In the current study, this analysis was limited to cells that were initially light-responsive to at least the high intensity stimuli. As a result, the available pool of cells in which gating could be observed was limited; n = 60 cells following MPH administration and n = 46 cells following ATX administration. After MPH, 15 of 28 neurons previously unresponsive to the intermediate intensity stimuli exhibited a response and 3/57 became responsive to low intensity stimuli. Similarly, after ATX, 12 of 19 previously unresponsive neurons exhibited a response to intermediate intensity stimuli and 2/45 responded to low intensity stimuli. Thus, this analysis showed that following treatment with either MPH or ATX, gating effects were greater in response to the intermediate intensity stimulus as compared to the low intensity stimulus (p < 0.0001 against the null hypothesis of equal proportions of neurons gated from the available pool of neurons for each intensity tested; Figure 6a and b), indicating that pharmacological manipulation is more likely to induce responsiveness to an intermediate intensity stimulus than to a small intensity stimulus. In addition to the above sample, cells with no prior response to light stimuli at any intensity exhibited responses to high intensity stimuli for three neurons following MPH administration and one neuron following ATX administration. Overall, the results indicate that MPH and ATX can recruit neurons into the sensory response pool.
Discussion
The results of the current study show that MPH and ATX, two agents used in the treatment of ADHD, popular for enhancing cognitive function in otherwise healthy people, and effective in enhancing rodent performance in sensory-guided attention tasks, exert prominent facilitating effects on early sensory signal processing in the rat visual thalamus. Both MPH and ATX enhanced the responses of dLGN neurons, reducing the latency and increasing the magnitude of responses to light stimuli, resembling the changes associated with increasing stimulus intensity. Neurons with weaker and/or longer latency responses realized the greatest gains following drug administration as observed by Drouin et al. (2007) in the sensory cortex following systemic MPH treatment. In addition, many cells that were previously unresponsive to light stimuli exhibited well defined responses to visual inputs following MPH or ATX treatment, indicating that both agents were capable of gating responses of dLGN neurons to otherwise sub-threshold stimuli and recruiting these cells into the sensory response pool. As a result of these actions, light-evoked responses in the dLGN circuitry had shorter latencies and were more robust following drug administration, indicating that attention-enhancing drugs can affect early sensory processing. Previous studies have provided evidence that more efficient processing of visual information in the dLGN strengthens the dynamics of synaptic transmission to the visual cortex, thus increasing information transfer to cortical targets and facilitating higher order processing of visual signals (Alonso, Usrey, & Reid, 1996; Saalmann & Kastner, 2009; Swadlow & Gusev, 2001). This early facilitation of visual processing may lead to improvements in cognitive performance under conditions where attention to visual cues is necessary for favorable behavioral outcomes.
The local mechanisms underlying drug-induced modulation of light-evoked dLGN responses require further investigation. The dLGN receives direct input from the retina, but retino-geniculate projections account for less than 10% of the input to this nucleus (Van Horn, Erişir, & Sherman, 2000). Ninety percent of the afferents to the dLGN are derived from areas other than the retina and thus could serve as sources of modulatory influences to this thalamic relay nucleus (Kayama, 1985; Kromer & Moore, 1980; Papadopoulos & Parnavelas, 1990; Van Horn et al., 2000). MPH blocks reuptake of both NE and DA, thereby increasing the extracellular concentrations of these transmitters in catecholamine terminal fields (Bymaster et al., 2002). However, the high selectivity of ATX for blocking NE, but not DA, transporters suggests that modulation of sensory-evoked responses as observed here is dependent upon increased noradrenergic transmission. This interpretation is further supported by reports that DA innervation of the dLGN is sparse if not non-existent (García-Cabezas et al., 2009).
Facilitation of signal processing in sensory circuits by the LC-NE system is well documented, as reviewed by Berridge and Waterhouse (2003). Likewise, noradrenergic ‘gating’ actions have also been demonstrated in both thalamic and cortical regions of the brain (Devilbiss & Waterhouse, 2011; Waterhouse et al., 1988). More specifically, enhancement of dLGN neuronal responses to excitatory synaptic inputs by LC stimulation or local iontophoretic application of NE has been linked to activation of α1 receptors (Kayama, 1985; Kromer & Moore, 1980; Rogawski & Aghajanian, 1980a, 1980b, 1982). Together with these previous results, the present findings suggest that the facilitating effects of MPH and ATX on dLGN signal processing are the result of blockade of NE transporter function leading to elevated extracellular levels of NE within the dLGN and local α1 mediated modulatory influences on stimulus-driven excitation.
In addition to local modulation within the dLGN, the remote actions of MPH and ATX on neural circuitry afferent to the dLGN could be responsible for alterations in responses to light stimuli. A likely target in such a scenario would be the visual cortex, which sends feedback to dLGN, directly and indirectly via the thalamic reticular nucleus, and is a prominent recipient of noradrenergic projections from the LC. Considering possibilities relevant to the relationship between attention and sensory processing, MPH and ATX are both capable of increasing both NE and DA in the prefrontal cortex (PFC). The PFC is a cortical region associated with abnormal catecholaminergic neurotransmission in ADHD (Arnsten & Pliszka, 2011) and may influence the dLGN via projections to the LC (Jodo, Chiang, & Aston-Jones, 1998). DA elevation in the PFC depends on the NE reuptake transporter (NET), given the relative abundance of NET over dopamine transporters (DAT) within the PFC (Carboni, Tanda, Frau, & Chiara, 1990; Sesack, Hawrylak, Matus, Guido, & Levey, 1998). Thus, both drugs could be impacting dLGN function via NE and DA mechanisms in the PFC. Local drug infusion studies are currently underway to address the consequences of remote effects as well as the contribution of additional neuromodulatory systems, such as DA, that may influence dLGN neuronal responses.
It is important to acknowledge seemingly paradoxical reports that both MPH and ATX dose-dependently decrease, not increase, tonic output from the LC (Bari & Aston-Jones, 2013; Devilbiss & Berridge, 2006). However, the results of microdialysis studies (Berridge et al., 2006; Bymaster et al., 2002; Drouin et al., 2006) indicate that the net impact of blocking NE transporter uptake following systemic drug administration is increased extracellular levels of NE in noradrenergic terminal fields, resulting in the facilitation of stimulus-driven responses (Devilbiss & Berridge, 2008; Drouin et al., 2007).
Our results demonstrate the potential for cognitive-enhancing agents to facilitate early sensory processing within the dLGN by increasing the speed of transmission and magnitude of response to visual stimuli. As modulation of sensory processing is deficient in disorders of cognitive function, these findings suggest that improvement of sensory signal processing may be a prominent dimension of the therapeutic action of MPH and ATX in attention disorders. Likewise, the effects demonstrated here could translate to improved sensory function in normal individuals, thus providing at least one physiological explanation for off-label use of these agents as cognitive enhancers (Greely et al., 2008). In addition, our findings provide insight regarding interpretation of the results of recent reports that demonstrated facilitating effects of attention on early visual signal processing in the LGN of both monkeys (McAlonan, Cavanaugh, & Wurtz, 2008) and humans (O'Connor, Fukui, Pinsk, & Kastner, 2002; Schneider & Kastner, 2009). For example, McAlonan et al. (2008) showed that LGN activity was greater in response to a peripheral stimulus when monkeys covertly directed attention to that stimulus during a fixation task of selective attention. Taken together, the ability of MPH and ATX to enhance performance in tasks of attention, the impact of these agents on early sensory signal processing as shown here, and the evidence that states of attention modulate LGN circuit function collectively point to early sensory signal processing as a prominent site of action for cognitive enhancing drugs. Overall, the results of the current work indicate that further efforts to elucidate the mechanisms by which attention-enhancing medications influence early sensory processing may yield new insights regarding the pathophysiology of attention disorders and lead to development of improved therapeutic treatments.
Figure 8.
Treatment-induced responsiveness of visually-sensitive dLGN neurons. Illustrated by the bar graphs are the number of cells that discharged in response to light stimuli under pre-treatment control conditions (light gray) and the number of additional cells where stimulus-evoked discharge became evident following drug treatment; i.e. “gated” responses (dark gray) for each stimulus intensity; high, intermediate (Int), and low. * denotes p < 0.0001 against the null hypothesis of equal proportions of neurons recruited from available pools of neurons between stimulus intensities.
Acknowledgments
This research was supported by National Institute on Drug Abuse/National Institutes of Health (NIDA/NIH) Grant DA017960-06. This finding source had no other role other than financial support. All authors contributed in a significant way to the manuscript.
We thank David P. Kowalski for assistance.
Footnotes
Author’s note: This manuscript is original, not previously published, and not under concurrent consideration elsewhere, although the results presented here were the subject of a preliminary report presented on a poster at the Society for Neuroscience meeting in 2012.
Disclousres
All authors have read and approved the final manuscript.
The authors declare no real or potential conflicts of interest.
Contributor Information
Rachel L. Navarra, Drexel University College of Medicine, Pharmacology and Physiology
Brian D. Clark, Drexel University College of Medicine, Neurobiology and Anatomy.
Gerard A. Zitnik, Drexel University College of Medicine, Neurobiology and Anatomy
Barry D. Waterhouse, Email: Barry.Waterhouse@drexelmed.edu, Drexel University College of Medicine, Neurobiology and Anatomy, 2900 Queen Lane, Philadelphia, PA 19129, Phone: 215-991-8411, Fax: 215-843-5810.
References
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. [10.1038/38 3815a 0] Nature. 1996;383(6603):815–819. doi: 10.1038/383815a0. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Pliszka SR. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology Biochemistry and Behavior. 2011;99(2):211–216. doi: 10.1016/j.pbb.2011.01.020. http://dx.doi.org/10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80(3):697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. The Journal of Neuroscience. 1994;14(7):4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Aston-Jones G. Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology. 2013;64(0):53–64. doi: 10.1016/j.neuropharm.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, Spencer RC. Methylphenidate Preferentially Increases Catecholamine Neurotransmission within the Prefrontal Cortex at Low Doses that Enhance Cognitive Function. Biological Psychiatry. 2006;60(10):1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Shumsky JS, Andrzejewski ME, McGaughy JA, Spencer RC, Devilbiss DM, Waterhouse BD. Differential Sensitivity to Psychostimulants Across Prefrontal Cognitive Tasks: Differential Involvement of Noradrenergic α1- and α2-Receptors. Biological Psychiatry. 2012;71(5):467–473. doi: 10.1016/j.biopsych.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacology Biochemistry and Behavior. 2011;99(2):262–274. doi: 10.1016/j.pbb.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Perry KW. Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat: A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology. 2002;27(5):699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Cain RE, Wasserman MC, Waterhouse BD, McGaughy JA. Atomoxetine facilitates attentional set shifting in adolescent rats. Developmental Cognitive Neuroscience. 2011;1(4):552–559. doi: 10.1016/j.dcn.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Tanda G, Frau R, Chiara GD. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. Journal of neurochemistry. 1990;55(3):1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Low-Dose Methylphenidate Actions on Tonic and Phasic Locus Coeruleus Discharge. Journal of Pharmacology and Experimental Therapeutics. 2006;319(3):1327–1335. doi: 10.1124/jpet.106.110015. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Berridge CW. Cognition-Enhancing Doses of Methylphenidate Preferentially Increase Prefrontal Cortex Neuronal Responsiveness. Biological Psychiatry. 2008;64(7):626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. Phasic and Tonic Patterns of Locus Coeruleus Output Differentially Modulate Sensory Network Function in the Awake Rat. Journal of Neurophysiology. 2011;105(1):69–87. doi: 10.1152/jn.00445.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin C, Page M, Waterhouse B. Methylphenidate Enhances Noradrenergic Transmission and Suppresses Mid- and Long-Latency Sensory Responses in the Primary Somatosensory Cortex of Awake Rats. Journal of Neurophysiology. 2006;96(2):622–632. doi: 10.1152/jn.01310.2005. [DOI] [PubMed] [Google Scholar]
- Drouin C, Wang D, Waterhouse BD. Neurophysiological actions of methylphenidate in the primary somatosensory cortex. Synapse. 2007;61(12):985–990. doi: 10.1002/syn.20454. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MÁ, Martínez-Sánchez P, Sánchez-González MÁ, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cerebral Cortex. 2009;19(2):424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. [10.1038/456702a] Nature. 2008;456(7223):702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Sensory Processing in Schizophrenia: Neither Simple nor Intact. Schizophrenia Bulletin. 2009;35(6):1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology. 2009;202(1):497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83(1):63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- Kayama Y. Ascending, descending and local control of neuronal activity in the rat lateral geniculate nucleus. Vision Research. 1985;25(3):339–347. doi: 10.1016/0042-6989(85)90058-6. [DOI] [PubMed] [Google Scholar]
- Kromer LF, Moore RY. A study of the organization of the locus coeruleus projections to the lateral geniculate nuclei in the albino rat. Neuroscience. 1980;5(2):255–271. doi: 10.1016/0306-4522(80)90102-5. [DOI] [PubMed] [Google Scholar]
- Latsari M, Antonopoulos J, Dori I, Chiotelli M, Dinopoulos A. Postnatal development of the noradrenergic system in the dorsal lateral geniculate nucleus of the rat. Developmental Brain Research. 2004;149(1):79–83. doi: 10.1016/j.devbrainres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Mangeot SD, Miller LJ, McIntosh DN, McGrath-Clarke J, Simon J, Hagerman RJ, Goldson E. Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Developmental Medicine & Child Neurology. 2001;43(6):399–406. doi: 10.1017/s0012162201000743. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr Res. 2011;69(5 Part 2 of 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GA, Speciale SG, Roffwarg HP. A method for concurrent local intracranial drug infusion and electrophysiological recording. Physiology & Behavior. 1988;43(2):249–252. doi: 10.1016/0031-9384(88)90248-x. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. [10.1038/nature07382] Nature. 2008;456(7220):391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(1):34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. [10.1038/nn957] Nat Neurosci. 2002;5(11):1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Papadopoulos GC, Parnavelas JG. Distribution and synaptic organization of serotoninergic and noradrenergic axons in the lateral geniculate nucleus of the rat. The Journal of Comparative Neurology. 1990;294(3):345–355. doi: 10.1002/cne.902940304. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neuroscience Research. 2011;69(1):41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic press; 2007. [DOI] [PubMed] [Google Scholar]
- Robinson E. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology. 2012;219(2):303–312. doi: 10.1007/s00213-011-2420-3. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. [10.1038/287731a0] Nature. 1980a;287(5784):731–734. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Norepinephrine and serotonin: Opposite effects on the activity of lateral geniculate neurons evoked by optic pathway stimulation. Experimental Neurology. 1980b;69(3):678–694. doi: 10.1016/0014-4886(80)90060-6. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK. Activation of lateral geniculate neurons by locus coeruleus or dorsal noradrenergic bundle stimulation: Selective blockade by the alpha1-adrenoceptor antagonist prazosin. Brain Research. 1982;250(1):31–39. doi: 10.1016/0006-8993(82)90950-7. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Gain control in the visual thalamus during perception and cognition. Current Opinion in Neurobiology. 2009;19(4):408–414. doi: 10.1016/j.conb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JM, Ring BJ, Witcher JW. Clinical pharmacokinetics of atomoxetine. Clinical pharmacokinetics. 2005;44(6):571–590. doi: 10.2165/00003088-200544060-00002. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Kastner S. Effects of Sustained Spatial Attention in the Human Lateral Geniculate Nucleus and Superior Colliculus. The Journal of Neuroscience. 2009;29(6):1784–1795. doi: 10.1523/JNEUROSCI.4452-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine Axon Varicosities in the Prelimbic Division of the Rat Prefrontal Cortex Exhibit Sparse Immunoreactivity for the Dopamine Transporter. The Journal of Neuroscience. 1998;18(7):2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. The impact of 'bursting' thalamic impulses at a neocortical synapse. [10.1038/86054] Nat Neurosci. 2001;4(4):402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behavioural Brain Research. 2002;130(1–2):73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Van Horn SC, Erişir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. The Journal of Comparative Neurology. 2000;416(4):509–520. [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Jung-Tung C, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Research Bulletin. 1988;21(3):425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]