Abstract
Management of relapsed leukemia following allogeneic transplantation is challenging. Intensive chemotherapy, donor lymphocyte infusions (DLI), or second transplantation have some value, but most reported series describe only a limited number of patients surviving beyond 2 or 3 years following relapse. Additionally, understandable selection-bias of reports describing the outcomes of intensive management approaches for relapsed leukemia confound generalizability to a broader population. However numerous reports suggest that second allogeneic transplantation for relapsed leukemia following an initial transplant may produce extended disease control and survival for patients with favorable performance status, remission at the time of second transplant, and most importantly a long interval between initial transplant and relapse. Reduced intensity conditioning for second allografts may be preferable and little data exists to suggest that a new donor will improve disease control by inducing a stronger graft-versus-leukemia effect. Improved measures to prevent the first relapse, however, may protect more patients and produce a greater fraction enjoying extended leukemia-free survival.
Keywords: Allogeneic, Allotransplant, CIBMTR, Center for International Blood and Marrow, Transplant Research, DLI, Donor lymphocyte infusion, EBMT, European Group for Blood and Marrow, Transplant, HCT, Hematopoietic cell transplantation, Leukemia, Second transplant
Introduction
The rigors of allotransplant are survivable, but only control acute leukemia for 40%–70% of allograft recipients depending on the cytogenetic and molecular risk, phenotype, and remission status of the patients. Relapse after allotransplant generally leads to poor survival with only 10%–20% of patients surviving beyond 2 years [1–7]. Notably however, patients receiving intensive chemotherapy supplemented with either donor lymphocyte infusion (DLI) or second transplants can have improved survival over those receiving supportive care alone [6,8–17]. Survival after relapse is inferior in those with circulating blasts at relapse, active infections, or other complications. Second transplant approaches using sibling, unrelated donor (URD), or umbilical cord blood (UCB) transplantation have been reported to have similar outcomes [1,2,9,18–28].
Second transplants are sometimes performed for incomplete donor chimerism with an additional infusion of hematopoietic stem and progenitor cells to boost engraftment. They can also be used as treatment for graft failure, but reconditioning plus a second graft infusion is essential and only successful for a minority. Most second transplants, however, are done for relapse (Table 1).
Table 1.
Second allogeneic transplant: settings and reasons.
| Settings | Rationale |
|---|---|
| Incomplete donor chimerism | Infusion to boost engraftment |
| Graft failure | Reconditioning + infusion for engraftment |
| Relapse | Provides reconditioning and restores GVL |
GVL, graft versus leukemia.
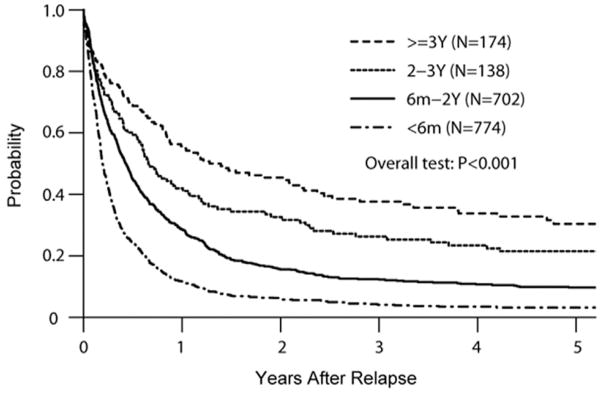
In a large Center for International Blood and Marrow Transplant Research (CIBMTR) analysis of AML relapsing after allografts, only 23% of 1788 relapsed patients survived more than a year post relapse [1]. However, longer survival was associated with later post first transplant relapse (Fig. 1). Five-year survival from relapse was nearly 40% for those relapsing beyond 3 years and 30% for those relapsing 2–3 years after initial allograft. These results were not significantly influenced by patient age, suggesting that disease risk characteristics dominated the outcome. Multivariate analysis confirmed improved survival for those relapsing beyond 2 years, whether treated with DLI or a second allograft. Second transplant success may be similar with matched related donors or unrelated donors, but reported experience with haploidentical donor transplantation for second allografts is scant.
Fig. 1.
Survival following relapse after allogeneic HCT for AML. Longer survival with later relapse (from Bejanyan et al., 2015 [1]).
Same or different donor
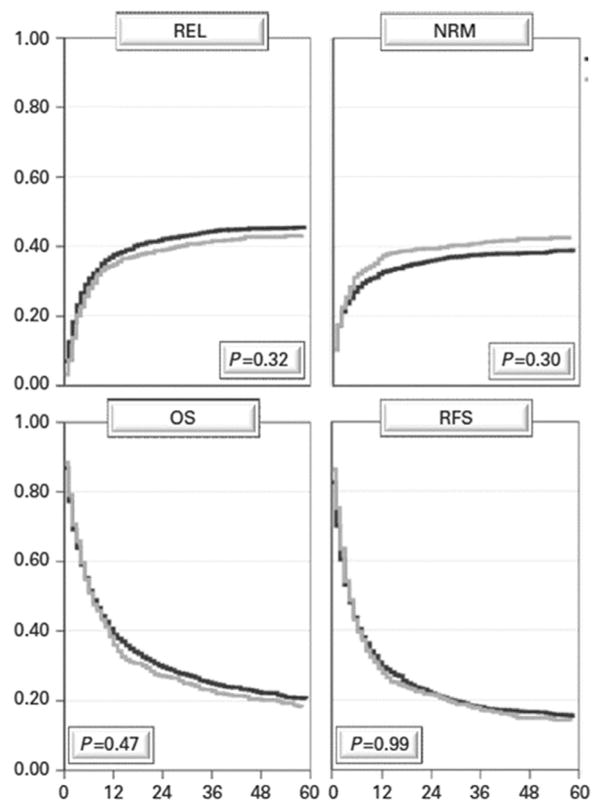
It is often postulated that changing donors for a second allograft may be favorable with hopes of inducing a more potent graft-versus-leukemia (GVL) effect. An earlier CIBMTR and more recent European Group for Blood and Marrow Transplant (EBMT) analyses failed to support this hypothesis [19,20,27]. In the earlier CIBMTR analysis, second transplants were associated with similar risks of acute graft-versus-host disease (GVHD) (perhaps more if acute GVHD had followed the first allograft), but particularly more chronic GVHD if a different donor was used for the second transplant [19]. This did not, however, yield improve survival compared to second transplants performed using the same, original donor. The more recent, large EBMT analysis demonstrated no difference in relapse incidence, non-relapse mortality, overall survival or relapse-free survival when the same donor (N = 1884) or a different donor (N = 712) was chosen for the second transplant [27] (Fig. 2).
Fig. 2.
Survival after 2nd allogeneic HCT for AML: no advantage of changing donors in relapse (REL), non relapse mortality (NRM), overall survival (OS) or relapse-free survival (RFS). Black line same donor (n = 1884); gray line different donor (n = 712) (from Ruutu et al., 2015 [27]. Reprinted by permission from Macmillan Publishers Ltd: Bone Marrow Transplant, copyright 2015.).
Second allograft following earlier autograft
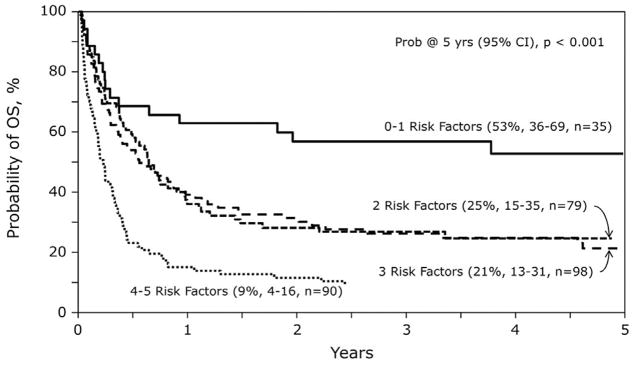
Allografting for treatment of AML relapse after an initial autograft was reported from the CIBMTR in 2013 [29]. In that analysis of 302 URD allograft recipients who had previous autologous transplantation, myeloablative conditioning was associated with excess treatment-related mortality and subsequent inferior survival while reduced intensity conditioning (RIC) was better tolerated (Fig. 3). RIC transplants thus led to improved overall survival at 1 and 5 years. Multivariate analysis identified five risk factors for mortality including: (1) relapse less than 18 months after the autograft; (2) not in complete remission (CR) at URD second transplant; (3) myeloablative conditioning; (4) Karnofsky performance status (KPS) less than 90%; and (5) cytomegalovirus (CMV) seropositive recipient. Patients with only zero to one risk factor had strikingly improved survival at 4 years (53%) vs those with two or three risk factors (21%–25%) or those with four to five risk factors (9%). This again highlights that later relapse, better performance status, and remission at the time of the second transplant offers the best chance for benefit.
Fig. 3.
Survival following URD HCT for relapse after autologous transplantation for AML. Risk factor analysis based upon one or more of the following significant risk factors [relapse < 18 months post autograft: not in CR at URD HCT; myeloablative conditioning; KPS < 90%; CMV seropositive] (from Foran et al., 2013 [29]).
Long follow-up after second transplants
Long-term outcomes after second allotransplant for acute leukemia are rarely reported [9,19,20,24,25,27]. An EBMT series of 286 patients described 10% 10-year survival and 7% leukemia-free survival [30]. These disappointing outcomes were due to 53% relapse mortality and 35% treatment-related, non-relapse mortality. Favorable factors for long-term survival included: complete remission at second transplant, relapse more than 10 months after the first transplant, and total body irradiation (TBI) in the second transplant conditioning. Three favorable factors yielded encouraging 45% 5-year and 36% 10-year survival while only two favorable factors led to 26% 5-year and 19% 10-year survival. Survival was less than 10% survival at either time point for those with only zero or one favorable factor. Other series corroborate these same general conclusions that later relapse, achievement of complete remission before second transplant, better performance status, and reduced intensity or reduced toxicity conditioning regimens are essential for substantial benefit from a second allograft.
Limited analyses of second transplant costs have been described. Khera reported 55 patients receiving two serial allografts for hematologic malignancies [31]. While the initial hospital stay following each transplant was similar at a median of 25 and 23 days, the 100-day costs were substantially higher (first transplant median $132,000 vs second transplant median $151,000, P = 0.03). Costs were substantially greater if the second transplant involved a mismatched allogeneic donor and the second graft was complicated by GVHD, lung or renal complications, or serious infections.
Conclusions
In sum, second allografts have a place, but the excess toxicity and cost associated with their application should be restricted to circumstances where favorable outcome can be anticipated. Second transplants may only be appropriate if the initial relapse was at least 1 and more favorably greater than 2 years after the first transplant; and if the patient has achieved complete remission prior to the second graft. Supplemental immunologic, small molecule or pharmacologic maintenance therapy may be beneficial to better augment the predilection of relapse following a second transplantation [8,32,33]. Available data also suggests no advantage of changing to a different donor either in augmenting the GVL effect or limiting risks of non-relapse mortality. Safer cellular, vaccine, or drug therapy to prevent initial post-transplant relapse makes much more sense and may in the long run help many more patients. Yet in selected circumstances, second transplants may be of value and even lead to long-term leukemia-free survival.
Footnotes
Conflict of interest
Daniel Weisdorf received consulting fees from Alexion, Kadmon, Enlivex, Amgen, Onyx.
References
- 1.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de LM, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl. 2015;21:454–9. doi: 10.1016/j.bbmt.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejanyan N, Oran B, Shanley R, Warlick E, Ustun C, Vercellotti G, et al. Clinical outcomes of AML patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transpl. 2014;49:1029–35. doi: 10.1038/bmt.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 4.Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Furst S, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma. 2013;54:1228–34. doi: 10.3109/10428194.2012.741230. [DOI] [PubMed] [Google Scholar]
- 5.Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transpl. 2009;44:769–77. doi: 10.1038/bmt.2009.300. [DOI] [PubMed] [Google Scholar]
- 6.Thanarajasingam G, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2013;19:1713–8. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. 1989;7:50–7. doi: 10.1200/JCO.1989.7.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on treatment of relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2010;16:1467–503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan CN, Majhail NS, Brazauskas R, Wang Z, Cahn JY, Frangoul HA, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transpl. 2015;21:151–8. doi: 10.1016/j.bbmt.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loren AW, Porter DL. Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transpl. 2008;41:483–93. doi: 10.1038/sj.bmt.1705898. [DOI] [PubMed] [Google Scholar]
- 11.Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol. 2002;20:405–12. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Lee KH, Kim S, Seol M, Kim SH, Kim WK, et al. Combination chemotherapy of intermediate-dose cytarabine, idarubicin, plus etoposide and subsequent mobilized donor leukocyte infusion for relapsed acute leukemia after allogeneic bone marrow transplantation. Leuk Res. 2001;25:305–12. doi: 10.1016/s0145-2126(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 13.Warlick ED, DeFor T, Blazar BR, Burns L, Verneris MR, Ustun C, et al. Successful remission rates and survival after lymphodepleting chemotherapy and donor lymphocyte infusion for relapsed hematologic malignancies postallogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18:480–6. doi: 10.1016/j.bbmt.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–45. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 15.Choi SJ, Lee JH, Lee JH, Kim S, Seol M, Lee YS, et al. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia. 2004;18:1789–97. doi: 10.1038/sj.leu.2403523. [DOI] [PubMed] [Google Scholar]
- 16.Arellano ML, Langston A, Winton E, Flowers CR, Waller EK. Treatment of relapsed acute leukemia after allogeneic transplantation: a single center experience. Biol Blood Marrow Transpl. 2007;13:116–23. doi: 10.1016/j.bbmt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bishop MR, Alyea EP, III, Cairo MS, Falkenburg JH, June CH, Kroger N, et al. National Cancer Institute's first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transpl. 2011;17:443–54. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kedmi M, Resnick IB, Dray L, Aker M, Samuel S, Gesundheit B, et al. A retrospective review of the outcome after second or subsequent allogeneic transplantation. Biol Blood Marrow Transpl. 2009;15:483–9. doi: 10.1016/j.bbmt.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transpl. 2004;34:721–7. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 20.Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhauser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31:3259–71. doi: 10.1200/JCO.2012.44.7961. [DOI] [PubMed] [Google Scholar]
- 21.Shaw BE, Mufti GJ, Mackinnon S, Cavenagh JD, Pearce RM, Towlson KE, et al. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transpl. 2008;42:783–9. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 22.Chiang KY, Weisdorf DJ, Davies SM, Enright H, Kersey JH, McGlave PB, et al. Outcome of second bone marrow transplantation following a uniform conditioning regimen as therapy for malignant relapse. Bone Marrow Transpl. 1996;17:39–42. [PubMed] [Google Scholar]
- 23.Martino R, Badell I, Brunet S, Sureda A, Nomdedeu J, Altes A, et al. Second bone marrow transplantation for leukemia in untreated relapse. Bone Marrow Transpl. 1994;14:589–93. [PubMed] [Google Scholar]
- 24.Christopeit M. When is second allogeneic HSCT for relapse of acute leukaemia an option? Bone Marrow Transpl. 2016;51:184–5. doi: 10.1038/bmt.2015.285. [DOI] [PubMed] [Google Scholar]
- 25.Vrhovac R, Labopin M, Ciceri F, Finke J, Holler E, Tischer J, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. Bone Marrow Transpl. 2016;51:186–93. doi: 10.1038/bmt.2015.221. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani K, Kanfer EJ, Marin D, Gabriel I, Rahemtulla A, Taylor A, et al. EBMT risk score predicts outcome of allogeneic hematopoietic stem cell transplantation in patients who have failed a previous transplantation procedure. Biol Blood Marrow Transpl. 2012;18:235–40. doi: 10.1016/j.bbmt.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Ruutu T, de Wreede LC, van BA, Brand R, Mohty M, Dreger P, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transpl. 2015;50:1542–50. doi: 10.1038/bmt.2015.186. [DOI] [PubMed] [Google Scholar]
- 28.Arfons LM, Tomblyn M, Rocha V, Lazarus HM. Second hematopoietic stem cell transplantation in myeloid malignancies. Curr Opin Hematol. 2009;16:112–23. doi: 10.1097/MOH.0b013e3283257a87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foran JM, Pavletic SZ, Logan BR, govi-Johnson MA, Perez WS, Bolwell BJ, et al. Unrelated donor allogeneic transplantation after failure of autologous transplantation for acute myelogenous leukemia: a study from the center for international blood and marrow transplantation research. Biol Blood Marrow Transpl. 2013;19:1102–8. doi: 10.1016/j.bbmt.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreola G, Labopin M, Beelen D, Chevallier P, Tabrizi R, Bosi A, et al. Long-term outcome and prognostic factors of second allogeneic hematopoietic stem cell transplant for acute leukemia in patients with a median follow-up of 10 years. Bone Marrow Transpl. 2015;50:1508–12. doi: 10.1038/bmt.2015.193. [DOI] [PubMed] [Google Scholar]
- 31.Khera N, Emmert A, Storer BE, Sandmaier BM, Alyea EP, Lee SJ. Costs of allogeneic hematopoietic cell transplantation using reduced intensity conditioning regimens. Oncologist. 2014;19:639–44. doi: 10.1634/theoncologist.2013-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lima M, Bonamino M, Vasconcelos Z, Colares M, Diamond H, Zalcberg I, et al. Prophylactic donor lymphocyte infusions after moderately ablative chemotherapy and stem cell transplantation for hematological malignancies: high remission rate among poor prognosis patients at the expense of graft-versus-host disease. Bone Marrow Transpl. 2001;27:73–8. doi: 10.1038/sj.bmt.1702726. [DOI] [PubMed] [Google Scholar]
- 33.de Lima M, Giralt S, Thall PF, de Padua SL, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–31. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]