Abstract
A wide range of 2-pyridyl and other difficult-to-access heterocyclic N-methyliminodiacetic acid boronates can be readily prepared from the corresponding bromides via a new method involving direct transligation of trialkoxyborate salts with MIDA at elevated temperatures.
Graphical abstract
Most small molecules are highly modular in their constitution.1,2 To maximally harness this inherent modularity, we aim to develop a general platform of building blocks representing the substructural motifs that most commonly appear in a wide range of targeted structures.3 In this regard, a collection of air-stable and environmentally friendly 2-pyridyl building blocks represent a very important objective, as this subunit is found in many pharmaceuticals,4 natural products and/or their derivatives,5 unnatural nucleotides,6 fluorescent probes,7 metal-complexing ligands,8 and materials.9
Although boronic acids are among the most desirable synthetic building blocks with respect to low cost, minimal environmental impact, and lack of toxicity, 2-pyridyl boronic acids are notoriously unstable, which precludes their effective utilization.10 Many different types of surrogates for 2-heterocyclic boronic acids have been developed, including trifluoroborate salts,11 trialkoxy or trihydroxyborate salts,12 diethanolamine adducts,13 sterically bulky boronic esters,14 and boroxines.15 Important advances with 2-heterocyclic silanolates have also recently been reported.16 However, it remains a challenge to develop air-stable and chemically pure 2-pyridyl building blocks.17
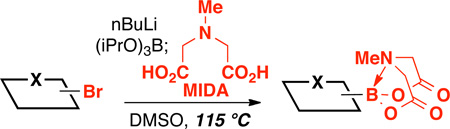
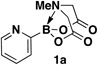
We recently reported that 2-heterocyclic N-methyliminodiacetic acid (MIDA) boronates can serve as stable and highly effective surrogates for a wide range of unstable 2-heterocyclic boronic acids under “slowrelease” cross-coupling conditions.3e We also discovered that 2-pyridyl MIDA boronate 1a is the first air-stable 2-pyridyl borane that can be isolated in chemically pure form and is a very convenient cross-coupling partner under modified slow-release conditions.3e However, our preliminary synthesis of this building block was cumbersome, low yielding, and not scalable.3e Given the broad potential utility of 2-heterocyclic MIDA boronates for many diverse applications,4–9 we pursued a practical, scalable, and general method for their synthesis. Building on the surprising discovery that 2-pyridyl MIDA boronate 1a is stable in anhydrous DMSO even at 130 °C, we herein report that a broad range of 2-pyridyl and other challenging-to-access heterocyclic MIDA boronates can be prepared from the corresponding readily-available bromides via a new method involving direct transligation of trialkoxyborate salts with MIDA at elevated temperatures (Fig. 1).
Figure 1.
A new method that provides access to a wide range of 2-pyridyl and other difficult-to-access MIDA boronates from the corresponding readily-available bromides.
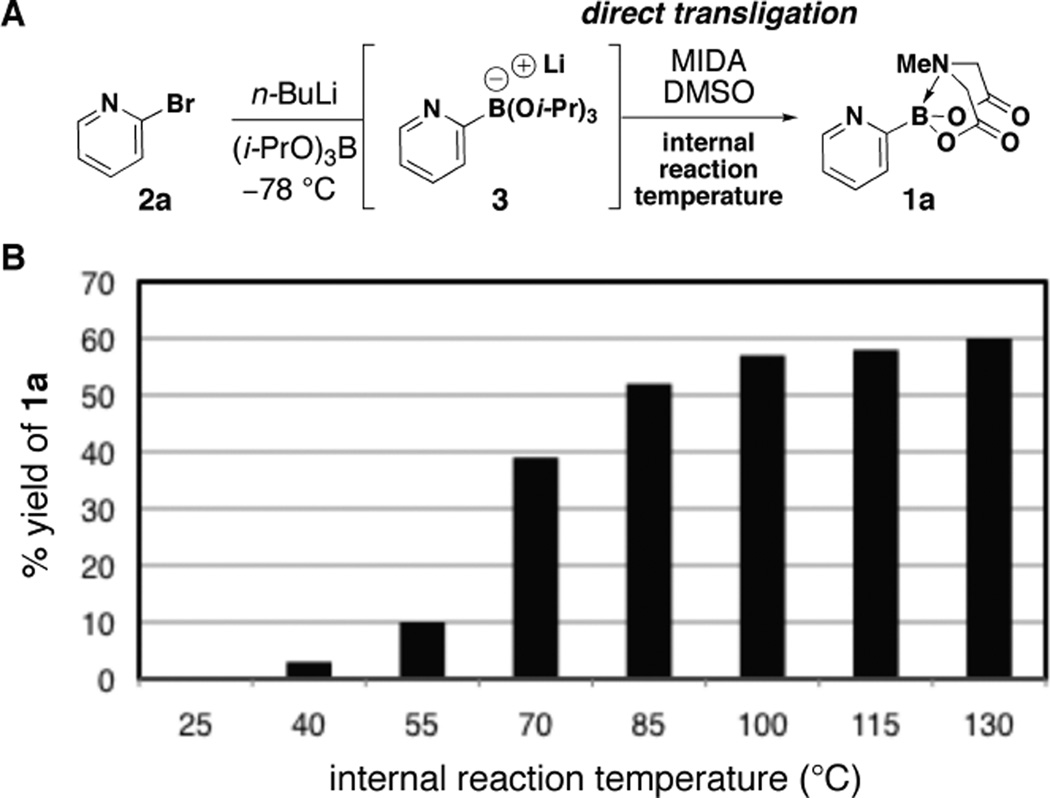
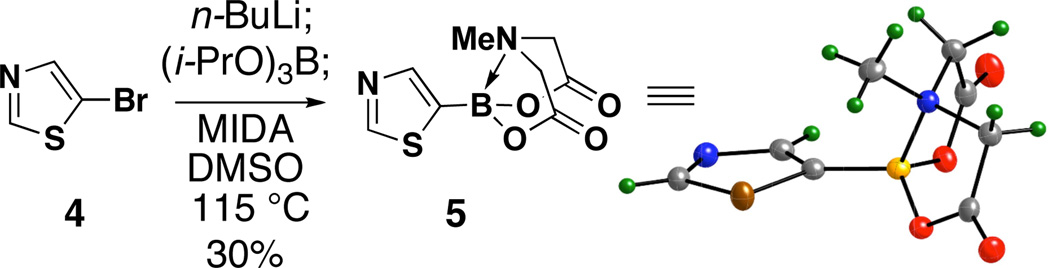
Lithium triisopropyl 2-pyridyl borate18 3 is known to be a useful intermediate for preparing other boronic acid surrogates10,13 and for cross-coupling with a range of aryl halides.12a In preliminary studies, however, we were only able to achieve a low yield of 1a from 3.3e For example, the dropwise addition of a freshly prepared THF solution of 3 to a stirred suspension of MIDA in DMSO at 55 °C over 1 hour (Fig. 2A) resulted in a very low (~10%) and poorly reproducible yield of 1a (Fig. 2B). A major byproduct observed in this reaction was pyridine, suggesting that protodeborylation of the notoriously labile 2-pyridyl–boron bond was a predominant competing pathway.
Figure 2.
A. A method for the preparation of 2-pyridyl MIDA boronate 1a from 2a via the intermediacy of triisopropoxyborate salt 3. B. Yield of 1a (via 1H NMR, average of two runs) as a function of the internal reaction temperature.
With the goal of minimizing this side reaction, we initially explored a wide range of complexation conditions involving milder temperatures. However, as shown in Fig. 2B, reducing the temperature always resulted in even lower yields of 1a. Prompting us to reverse our approach, we discovered in parallel studies that 1a is surprisingly stable in hot DMSO. For example, heating a solution of 1a in d6-DMSO at 130 °C for one hour caused no change in the 1H NMR spectrum (see SI), which suggested that the undesired protodeborylation observed in the transligation reaction was occurring prior to MIDA complexation. This led us to question whether alternatively increasing the reaction temperature might enable a rapid transligation to form the very stable MIDA boronate 1a prior to the decomposition of its precursor 3. Consistent with this hypothesis, we discovered that raising the internal temperature from 55 to ≥ 115 °C resulted in a six-fold increase in the yield of 1a (see Fig. 2B). Moreover, the procedure proved to be highly reproducible at these elevated temperatures.
This new method was easily reproduced on the gram scale to provide 2-pyridyl MIDA boronate 1a in 59% isolated yield after silica gel chromatography (Table 1, entry 1). An optimized procedure was also developed for preparing 1a on the decagram scale without the use of chromatography.19 This highly crystalline building block has been stored as a solid on the benchtop under air without decomposition for more than 1 year (see SI for details).
Table 1.
Synthesis 2-pyridyl MIDA boronates.
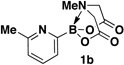
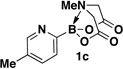
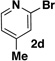
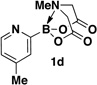
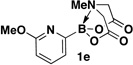
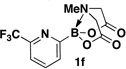
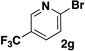
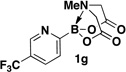
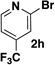
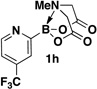
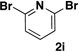
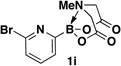
A preliminary survey of scope has further revealed that a wide variety of 2-pyridyl bromides can also be transformed into the corresponding 2-pyridyl MIDA boronates using this same methodology. Specifically, 6-, 5-, and 4-methyl-2-pyridyl subunits appear in a wide variety of pharmaceuticals, materials, and metal ligands, and the corresponding MIDA boronate building blocks 1b–1d can be readily accessed on gram-scale (entries 2–4). It would also be highly advantageous in drug discovery to have access to a collection of air-stable 2-pyridyl building blocks having both electron-releasing and electron-withdrawing groups. Accordingly, 6-methoxy-, and 6-, 5-, and 4-trifluoromethyl-2-pyridyl MIDA boronates 1e–1h were all prepared with this same procedure (entries 5–8). Bromo-substituted 2-pyridyl MIDA boronates 1i and 1j, each having the potential for a range of iterative cross-coupling applications,3 were also conveniently synthesized via monofunctionalization of dibromo pyridines 2i and 2j (entries 9 and 10). Remarkably, all of these 2-pyridyl MIDA boronates 1a–j are air- and chromatographically stable, highly crystalline, monomeric, free-flowing solids (SI).
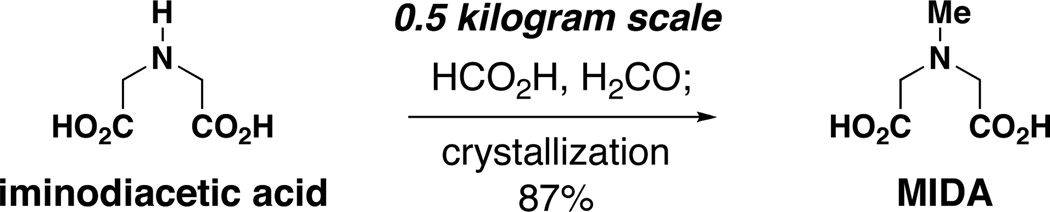
Importantly, all of the reagents used in this novel protocol can be readily accessed for very low cost,20 including the MIDA ligand. Using a new procedure involving reversed order of reagent additions compared to that reported previously,3f,21 MIDA can now be easily prepared on the kilogram scale from the commodity chemicals iminodiacetic acid, formaldehyde, and formic acid (Scheme 1, see SI for details). Tens of thousands of metric tons of iminodiacetic acid are produced each year as a starting material for herbicides, surfactants, and environmental chelating reagents.22 Thus, this starting material is very inexpensive.23 Specifically, mol per mol, iminodiacetic acid is more than five times cheaper than n-BuLi.20,23 MIDA is also completely biodegradable,24 thus making MIDA boronates a very environmentally friendly alternative to the corresponding organostannanes.17 Finally, the highly-crystalline and air-stable nature of MIDA boronates greatly facilitates their isolation, purification, and storage, making them highly attractive intermediates. Given all of these features, this new method stands to provide practical access to a wide range of 2-heterocyclic and other types of very useful MIDA boronate building blocks with an array of important applications. In fact, several of the new building blocks reported herein are already commercially-available.25
Scheme 1.
We have also preliminarily explored the potential of this new protocol to provide access to other types of very challenging-to-access heterocyclic boranes. For example, thiazole subunits appear in many pharmaceuticals, ligands, fluorescent probes, and materials, as well as a wide range of NRPS-derived natural products.1,5c,26,27 However, the corresponding boronic acids again suffer from substantial stability issues.26,27 Using an unoptimized version of this same protocol, 5-thiazolyl MIDA boronate 5 was accessed from the corresponding bromide 4, and this new heterocyclic borane also proved to be indefinitely air-stable (SI, structure confirmed by X-ray, Scheme 2).
Scheme 2.
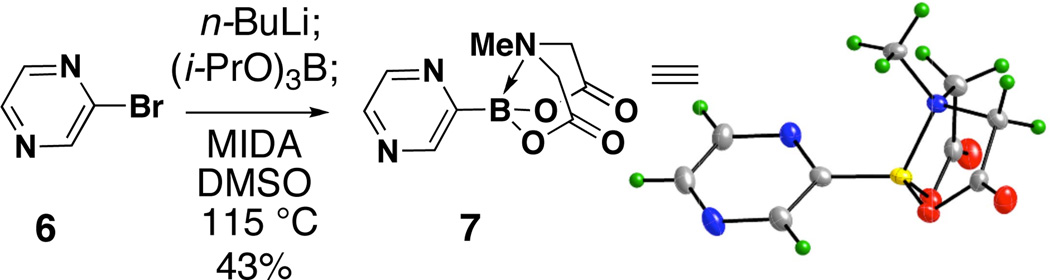
As a final example, 2-pyrazine subunits are very important in medicinal chemistry and many other applications.28 However, to the best of our knowledge, there are no previously reported examples of air-stable 2-pyrazinyl borane building blocks. As shown in Scheme 3, using the standard conditions described above, 2-bromopyrazine 6 was effectively transformed into the corresponding 2-pyrazinyl MIDA boronate 7 (structure confirmed by X-ray), and this new building block has also proven to be indefinitely air-stable (SI).
Scheme 3.
In summary, we have developed a practical, scalable, and cost-effective method for preparing a wide range of previously challenging-to-access heterocyclic MIDA boronates involving direct transligation of the corresponding readily accessible triisopropoxyborate salts. Importantly, this new method completely avoids the intermediacy of boronic acids. In several cases, the building blocks described herein represent the first examples of the corresponding heterocyclic boranes that can be isolated as air-stable materials in chemically pure form. This methodology and the novel MIDA boronates that it can access stand to have a highly enabling impact on the synthesis of a wide range of pharmaceuticals, natural products, and materials.
Supplementary Material
Acknowledgments
We gratefully acknowledge the NSF (CAREER 0747778) and Bristol-Myers Squibb for funding, and Aldrich for reagents. D.M.K. is a NIH CBI Training Grantee, E.P.G. is a BMS Graduate Fellow, and M.D.B. is a HHMI Early Career Scientist, Beckman Young Investigator, Sloan Research Fellow, Dreyfus New Faculty Awardee, Bristol-Myers Squibb Unrestricted Grant in Synthetic Organic Chemistry Awardee, Eli Lilly Grantee, Amgen Young Investigator, and AstraZeneca Excellence in Chemistry Awardee.
Footnotes
Supporting Information Available Procedures and characterization for all new compounds as well as spectral and X-ray crystallographic data.
References
- 1.(a) Walsh CT. Science. 2004;303:1805–1810. doi: 10.1126/science.1094318. [DOI] [PubMed] [Google Scholar]; (b) Cane DE, Walsh CT, Khosla C. Science. 1998;282:63–68. doi: 10.1126/science.282.5386.63. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sperry JB, Wright DL. Curr. Opin. Drug Dis. Dev. 2005;8:723–740. [PubMed] [Google Scholar]; (b) Broughton HB, Watson IA. J. Mol. Graph. Mod. 2004;23:51–58. doi: 10.1016/j.jmgm.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 3.(a) Gillis EP, Burke MD. J. Am. Chem. Soc. 2007;129:6716–6717. doi: 10.1021/ja0716204. [DOI] [PubMed] [Google Scholar]; (b) Lee SJ, Gray KC, Paek JS, Burke MD. J. Am. Chem. Soc. 2008;130:466–468. doi: 10.1021/ja078129x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gillis EP, Burke MD. J. Am. Chem. Soc. 2008;130:14084–14085. doi: 10.1021/ja8063759. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Uno BE, Gillis EP, Burke MD. Tetrahedron. 2009;65:3130–3138. [Google Scholar]; (e) Knapp DM, Gillis EP, Burke MD. J. Am. Chem. Soc. 2009;131:6961–6963. doi: 10.1021/ja901416p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ballmer SG, Gillis EP, Burke MD. Org. Synth. 2009;86:344–359. [Google Scholar]; (g) Gillis EP, Burke MD. Aldrichimica Acta. 2009;42:17–27. [PMC free article] [PubMed] [Google Scholar]
- 4.(a) DeGoey DA, Grampovnik DJ, Flentge CA, William WJ, Chen H–J, Yeung CM, Randolph JT, Klein LL, Dekhtyar T, Colletti L, Marsh KC, Stoll V, Mamo M, Morfitt DC, Nguyen B, Schmidt JM, Swanson SJ, Mo H, Kati WM, Molla A, Kempf DJ. J. Med. Chem. 2009;52:2571–2586. doi: 10.1021/jm900044w. [DOI] [PubMed] [Google Scholar]; (b) Heim-Riether A, Taylor SJ, Liang SG, Donghong A, Xiong Z, Michael AE, Collins BK, Farmer BT, Haverty K, Hill-Drzewi M, Junker H-D, Mariana MS, Moss N, Neumann T, Proudfoot JR, Keenan LS, Sekul R, Zhang Q, Li J, Farrow NA. Bioorg. Med. Chem. Lett. 2009;19:5321–5324. doi: 10.1016/j.bmcl.2009.07.151. [DOI] [PubMed] [Google Scholar]
- 5.(a) Aida W, Ohtsuki T, Li X, Ishibashi M. Tetrahedron. 2008;65:369–373. [Google Scholar]; (b) Kubota NK, Ohta E, Ohta S, Koizumi F, Suzuki M, Ichimura M, Ikegami S. Bioorg. Med. Chem. 2003;11:4569–4575. doi: 10.1016/s0968-0896(03)00526-1. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Scarpelli R, Bollbuck B, Werschkun B, Pereira MMA, Wartmann M, Altmann K–H, Zaharevitz D, Gussio R, Giannakakou P. Chem. Bio. 2000;7:593–599. doi: 10.1016/s1074-5521(00)00006-5. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hwang GT, Hari Y, Romesberg FE. Nucleic Acids Res. 2009;37:4757–4763. doi: 10.1093/nar/gkp467. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gutierrez AJ, Terhost TJ, Matteucci MD, Froehler BC. J. Am. Chem. Soc. 1994;116:5540–5554. [Google Scholar]
- 7.Tang B, Yu F, Li P, Tong L, Duan X, Xie T, Wang X. J. Am. Chem. Soc. 2009;131:3016–3023. doi: 10.1021/ja809149g. [DOI] [PubMed] [Google Scholar]
- 8.(a) Havas F, Leygue N, Danel M, Mestre B, Galaup C, Picard C. Tetrahedron. 2009;65:7673–7686. [Google Scholar]; (b) Schubert US, Eschbaumer C. Org. Lett. 1999;1:1027–1029. [Google Scholar]
- 9.(a) Chi CC, Chiang CL, Liu SW, Yueh H, Chen CT, Chen CT. J. Mat. Chem. 2009;19:5561–5571. [Google Scholar]; (b) Whittell GR, Manners I. Adv. Mat. 2007;19:3439–3468. [Google Scholar]; (c) Yamaguchi Y, Kobayashi S, Miyamura S, Okamoto Y, Wakamiya T, Matsubara Y, Yoshida Z. Angew. Chem. Int. Ed. 2004;43:366–369. doi: 10.1002/anie.200352749. [DOI] [PubMed] [Google Scholar]
- 10.Tyrrell E, Brookes P. Synthesis. 2003:469–483. [Google Scholar]
- 11.(a) Molander GA, Ellis N. Acc. Chem. Res. 2007;40:275–286. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]; (b) Darses S, Genet JP. Chem. Rev. 2008;108:288–325. doi: 10.1021/cr0509758. [DOI] [PubMed] [Google Scholar]; (c) Stefani HA, Cella R, Vieira AS. Tetrahedron. 2007;63:3623–3658. [Google Scholar]; (d) Molander GA, Biolatto B. J. Org. Chem. 2003;68:4302–4314. doi: 10.1021/jo0342368. [DOI] [PubMed] [Google Scholar]; (e) Molander GA, Canturk B, Kennedy LE. J. Org. Chem. 2009;74:973–980. doi: 10.1021/jo802590b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Billingsley KL, Buchwald SL. Angew. Chem. Int. Ed. 2008;47:4695–4698. doi: 10.1002/anie.200801465. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yamamoto Y, Takizawa M, Yu X-Q, Miyaura N. Angew. Chem. Int. Ed. 2008;47:928–931. doi: 10.1002/anie.200704162. [DOI] [PubMed] [Google Scholar]; (c) Cammidge AN, Goddard VHM, Gopee H, Harrison NL, Hughes DL, Schubert CJ, Sutton BM, Watts GL, Whitehead AJ. Org. Lett. 2006;8:4071–4074. doi: 10.1021/ol061564w. [DOI] [PubMed] [Google Scholar]; (d) O’Neill BT, Yohannes D, Bundesmann MW, Arnold EP. Org. Lett. 2000;2:4201–4204. doi: 10.1021/ol0067538. [DOI] [PubMed] [Google Scholar]; (e) Sindkhedkar MD, Mulla HR, Wurth MA, Cammers-Goodwin A. Tetrahedron. 2001;57:2991–2996. [Google Scholar]; (f) Fernando SRL, Maharoof USM, Deshayes KD, Kinstle TH, Ogawa MY. J. Am. Chem. Soc. 1996;118:5783–5790. [Google Scholar]
- 13.(a) N-phenyldiethanolamine 2-pyridylboronate is prepared as a structurally undefined complex containing variable quantities of isopropyl and N-phenyldiethanolamine groups and a stoichiometric quantity of lithium: Hodgson PB, Salingue FH. Tetrahedron Lett. 2004;45:685–687. Jones NA, Antoon JW, Bowie AL, Borak JB, Stevens EP. J. Heterocyclic Chem. 2007;44:363–367. (c) Solid-supported diethanolamine adducts: Gravel M, Thompson KA, Zak M, Bérubé C, Hall DG. J. Org. Chem. 2002;67:3–15. doi: 10.1021/jo0106501. (d) A solid-supported diethanolamine-bound 2-pyridyl reagent has also been reported: Gros P, Doudouh A, Fort Y. Tetrahedron Lett. 2004;45:6239–6241.
- 14.(a) Yang DX, Colletti SL, Wu K, Song M, Li GY, Shen HC. Org. Lett. 2009;11:381–384. doi: 10.1021/ol802642g. [DOI] [PubMed] [Google Scholar]; (c) Deng JZ, Paone DV, Ginnetti AT, Kurihara H, Dreher SD, Weissman SA, Stauffer SR, Burgey CS. Org. Lett. 2009;11:345–347. doi: 10.1021/ol802556f. [DOI] [PubMed] [Google Scholar]
- 15.(a) Perkins JR, Carter RG. J. Am. Chem. Soc. 2008;130:3290–3291. doi: 10.1021/ja7113486. [DOI] [PubMed] [Google Scholar]; (b) Kerins F, O’Shea DF. J. Org. Chem. 2002;67:4968–4971. doi: 10.1021/jo020074o. [DOI] [PubMed] [Google Scholar]; (c) Cioffi CL, Spencer WT, Richards JJ, Herr RJ. J. Org. Chem. 2004;69:2210–2212. doi: 10.1021/jo034664d. [DOI] [PubMed] [Google Scholar]
- 16.a) Denmark SE, Smith RC, Chang WT, Muhuhi JM. J. Am. Chem. Soc. 2009;131:3104–3118. doi: 10.1021/ja8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Denmark SE, Baird JD, Regens CS. J. Org. Chem. 2008;73:1440–1455. doi: 10.1021/jo7023784. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Denmark SE, Baird JD. Org. Lett. 2006;8:793–795. doi: 10.1021/ol053165r. [DOI] [PubMed] [Google Scholar]
- 17.2-pyridyl stannanes represent stable and effective alternatives, but these reagents suffer from substantial toxicity: Bailey TR. Tetrahedron Lett. 1986;27:4407–4410. Cragg ST. In: Patty’s Toxicology. Bingham E, Cohrssen B, Powell CH, editors. Hoboken, NJ: Wiley; 2001.
- 18.Mikhailov BM, Kozminskaya TK. Izv. Akad. Nauk. SSSR Ser. Khim. 1959;76:1866–1867. [Google Scholar]
- 19.(See SI for full details) To a stirred solution of 2-bromopyridine (9.5 mL, 97 mmol) and triisopropyl borate (19.5 mL, 84.8 mmol) in THF (175 mL) at −78 °C was added dropwise n-BuLi (2.5 M in hexanes, 36 mL, 90 mmol). The resulting solution was warmed to 23 °C and added over one hour to a stirred solution of MIDA (25.4 g, 173 mmol) in DMSO (175 mL) at 115 °C. The resulting mixture was cooled to 23 °C and filtered. The filtrate was deacidified with solid K3PO4 (72.6 g, 342 mmol) and concentrated in vacuo. The resulting residue was precipitated from CH3CN:CH2Cl2:Et2O to afford 1a (11 g, 55%).
- 20.2-Bromopyridine, n-BuLi, and (i-PrO)3B can be purchased on the kg scale for <$20/mol (Spectrum Chemicals, Gardena, CA, USA), <$31/mol (Beta Pharma, New Haven, CT, USA) and <$28/mol (Matrix Scientific, Columbia, SC, USA), respectively.
- 21.(a) Childs AF, Goldsworthy LJ, Harding GF, King FE, Nineham AW, Norris WL, Plant SGP, Selton B, Tompsett ALL. J. Chem. Soc. 1948:2174–2177. [Google Scholar]; (b) Chase BH, Downes AM. J. Chem. Soc. 1953:3874–3877. [Google Scholar]
- 22.Yangong Z. China Chemical Reporter. 2005;16(8):16. [Google Scholar]
- 23.Iminodiacetic acid can be purchased on the kg scale for <$5.50/mol (City Chemical, West Haven, CT, USA).
- 24.Warren CB, Malec EJ. Science. 1972;176:277–279. doi: 10.1126/science.176.4032.277. [DOI] [PubMed] [Google Scholar]
- 25.Sigma-Aldrich: 1a 719390, 1b 723959, 1e 723053.
- 26.(a) For the recent synthesis and reactivity of 5-thiazol pinacol boronic ester, see: Primas N, Bouillon A, Lancelot J-C, El-Kashef H, Rault S. Tetrahedron. 2009;65:5739–5746. Stanetty P, Schnürch M, Mihovilovic MD. J. Org. Chem. 2006;71:3754–3761. doi: 10.1021/jo0601009.
- 27.(a) Beguin C, Duncan KK, Munro TA, Ho DM, Xu W, Liu-Chen L–Y, Carlezon WA, Cohen BM. Bioorg. Med. Chem. 2009;17:1370–1380. doi: 10.1016/j.bmc.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rewinkel J, Enthoven M, Golstein I, van der Rijst M, Scholten A, van Tilborg M, de Weys D, Wisse J, Hamersma H. Bioorg. Med. Chem. 2008;16:2753–2763. doi: 10.1016/j.bmc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 28.(a) Lu R–J, Tucker JA, Zinevitch T, Kirichenko O, Konoplev V, Kuznetsova S, Sviridov S, Pickens J, Tandel S, Brahmachary E, Yang Y, Wang J, Freel S, Fisher S, Sullivan A, Zhou J, Stanfield-Oakley S, Greenberg M, Bolognesi D, Bray B, Koszalka B, Jeffs P, Khasanov A, Ma Y–A, Jeffries C, Liu C, Proskurina T, Zhu T, Chucholowski A, Li R, Sexton C. J. Med. Chem. 2007;50:6535–6544. doi: 10.1021/jm070650e. [DOI] [PubMed] [Google Scholar]; (b) Gillespie RJ, Bamford SJ, Botting R, Comer M, Denny S, Gaur S, Griffin M, Jordan AM, Knight AR, Lerpiniere J, Leonardi S, Lightowler S, McAteer S, Merett A, Mira A, Padfield A, Reece M, Saadi M, Selwood DL, Stratton GC, Surry D, Todd R, Tong X, Ruston V, Upton R, Weiss SM. J. Med. Chem. 2009;52:33–47. doi: 10.1021/jm800961g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.