Abstract
Background:
Ventilator-associated pneumonia (VAP) is a common nosocomial infection, which results in longer hospitalization, increased treatment costs, and higher mortality rates. One major cause of VAP is colonization and microaspiration of oropharyngeal secretions following the formation of dental plaque, which is due to poor oral hygiene and failure to mechanically remove these microorganisms from the teeth. This study was conducted to determine the effect of brushing teeth with distilled water on the incidence of VAP in patients admitted to intensive care unit (ICU).
Materials and Methods:
In this randomized clinical trial, 168 intubated patients, who had at least 20 teeth were randomly assigned to two groups. In the experimental group, the patients’ teeth were brushed twice a day with a children’s toothbrush and distilled water in addition to the routine oral care. The clinical pulmonary infection score (CPIS) was used to diagnose VAP. The data were analyzed using SPSS version 16 software.
Results:
A total of 38.6% of the patients in each group developed VAP. There was a significant difference in incidence of VAP on day five between the two groups (P<0.05). The incidence of VAP had a significant relationship with smoking (P<0.001), underlying diseases (P<0.001), duration of hospitalization (P=0.002), and age (P<0.001). Enterobacter was the most common microorganism identified in both groups.
Conclusion:
According to our results, tooth brushing twice daily with distilled water reduced the incidence of VAP in patients admitted to the ICU. Therefore, it is recommended that nurses caring for ventilator-dependent patients brush the patients’ teeth with distilled water as a part of their routine oral care.
Keywords: Distilled water, Oral hygiene, Ventilator associated pneumonia (VAP), Tooth brushing
INTRODUCTION
Ventilator-associated pneumonia (VAP) is a type of nosocomial infection that develops in mechanically ventilated patients. It is the second most common nosocomial infection and the first most common ICU acquired infection and occurs in patients who are mechanically ventilated for more than 48 hours (1,2). Its prevalence is 10%–65% and it is 5–10 times more common in ICUs than in other wards (3,4). The risk of VAP increases by 1%–3% for every additional day of mechanical ventilation (5). Ventilator associated pneumonia is associated with increased mortality (30%–70%), disability, longer ICU stays (by 7%–9%), longer hospitalization, longer duration of mechanical ventilation, and higher costs of treatment (e.g., $30,000 per patient stay)(6–8). Colonization of the oropharynx and microaspiration of oropharyngeal secretions, which directly introduce bacteria and other pathogens to the lower respiratory tract, are the most important risk factors for development of VAP (9–10). The risk of pneumonia increases in intubated patients by 6%–21% due to easier accessibility of the lower respiratory tract by the colonizing bacteria at the end of the pharynx (11,12). The oral bacterial flora usually changes from gram positive to gram negative bacteria after 48 hours of ICU admission, which leads to bacterial colonization of the pharynx and increased accumulation of dental plaques (13). These dental plaques form at a higher rate in patients admitted to the ICU compared to other patients due to immune dysfunction, underlying diseases, the presence of endotracheal tubes, difficulty with swallowing, side effects of drugs, and decreased oral fluid intake. However, the most important causes of dental plaque formation are poor oral hygiene and the failure to mechanically remove the plaques, which result in growth of bacteria on the plaques. Moreover, there is a strong relationship between the bacteria found in cultures from dental plaques and the bacteria found in tracheal aspirate cultures, which result in VAP (14,15).
The most important strategy for reducing VAP is improving oral hygiene (9). Techniques suggested for removing dental plaques and oral pathogens include mechanical interventions such as brushing and swabbing, and pharmacological interventions such as rinsing with mouthwashes. However, dental plaques are removed only through brushing (16–18). While antibacterial mouthwashes have been shown to improve oral hygiene, they are not routinely used due to development of bacterial resistance, associated complications, and the higher cost associated with them (17). For instance, chlorhexidine is one of the most commonly use solutions in intubated patients, but it is both costly and irritating (17, 19). Therefore, it seems that brushing is more cost-effective and causes less irritation compared to chlorhexidine (20). While water is safe and useful for cleaning the teeth and gums, and preventing xerostomia, it water is a potential source for growth of pseudomonas. Therefore, it is best to use distilled water (19).
There are inadequate data about improving oral hygiene and reducing VAP using nursing interventions such as tooth brushing. This study was conducted to determine the effect of brushing with distilled water on the incidence of VAP in mechanically ventilated patients admitted to the ICU.
MATERIALS AND METHODS
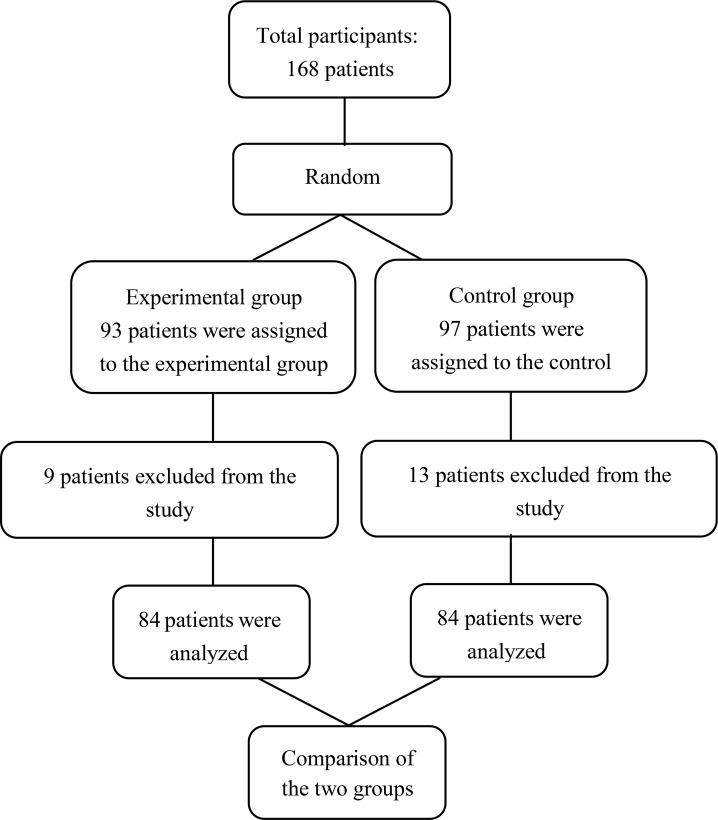
This randomized clinical trial was conducted on 168 ventilator-dependent patients hospitalized in the ICUs. Taking into account the confidence interval of 95% and statistical power of 75%, P1 and P2 were obtained as 0.54 and 0.71, respectively. Eligible patients were randomly assigned to two groups: 84 patients in the intervention group and 84 patients in the control group, using Random Allocation software (Figure 1). Inclusion criteria of this study were as follows: Endotracheal intubation, having at least 20 teeth, and being 20–85 years old. Exclusion criteria were as follows: extubation, signs/symptoms of aspiration, immune compromising conditions, debilitating diseases, coagulation disorders, and jaw fractures. We used a demographic and clinical information questionnaire (age, sex, kind of trauma, history of smoking, underlying diseases, and duration of hospitalization) as well as the standard scale of bacterial pneumonia to collect data.
Figure 1.
Consort flow diagram of study
In the intervention group, in addition to routine oral care, the anterior and posterior surfaces of the teeth and the surface of the tongue were brushed twice a day (in the morning and the afternoon) with a children’s soft toothbrush and distilled water, making sure not to traumatize the teeth. Subsequently, chlorhexidine was applied to a swab and was rubbed on the surface of the tongue, followed by 20 mL of normal saline, and the mouth was deeply suctioned subsequently. The first intervention was performed during the first 24 hours of intubation, and thereafter, oral care was delivered twice daily for the next five days. Oral care was performed by three trained nurses. The patients in the control group received routine oral care per the ICU protocol (control of endotracheal tube cuff, rinsing the mouth with normal saline, applying chlorhexidine to a swab and rubbing it on the surface of the tongue, and oropharyngeal suction) three times a day (in the morning, afternoon, and at night). The treating physician and the data analyst were blinded to patient allocation.
The incidence of VAP was determined using the clinical pulmonary infection score (CPIS). This score was developed by Pugin et al. in 1991 and confirmed by the National Center for Infectious Diseases (21). Clinical pulmonary infection score includes body temperature, white cell count, tracheal aspirates in terms of color and smell, oxygenation (PaO2/FiO2), radiographic findings (chest X-ray), and positive tracheal aspirate culture. A score of six or higher shows the presence of pneumonia (CPIS ≥ 6). To determine the incidence of VAP, CPIS was first calculated after two days of intervention, that is, on day three of ICU stay. This was repeated on the fourth and fifth days of the intervention. Test results and chest X-rays were interpreted by the attending physician on daily basis. Data were collected by interviews, observation, inspection, and document analysis by the physician and nurse. In intervention group patients with scores higher than 6, a sterile sample of tracheal aspirate culture was obtained and delivered to the laboratory.
This study was approved by the Research Ethics Committee of Shahid Sadoughi University of Medical Sciences (registered clinical trial IRCR2013102115101N1). Informed consent was obtained from the patients’ guardian after briefing them about the objectives of the study.
Data were analyzed using SPSS v. 16 software. We performed descriptive statistics (mean, standard deviation, and frequency percentage) and inferential statistics (Independent T-test, chi-squared test, and if necessary, Fisher’s exact test). For continuous variables (age and duration of length of hospitalization), independent t-tests were used. For categorical variables (sex, kind of trauma, history of smoking, underlying diseases), chi-squared test was used.
RESULTS
Of the 168 patients, 84 patients were assigned to the control group, and the other 84 patients were assigned to the intervention group. 13 patients in the control group and 9 patients in the intervention group were excluded because of death or extubation. However, new patients were selected again as advised by the statistician to replace them.
At baseline there were no differences in demographic (age, sex) or clinical information (duration of hospitalization, type of trauma, underlying disease, history of smoking) between the patients in the control and the intervention group (P> 0.05) (Table 1).
Table 1.
Comparison of the baseline clinical and demographic data between the two groups
| Variables | Experimental group | Control group | Statistical test | |||
|---|---|---|---|---|---|---|
| Mean | SD a | Mean | SD a | T a | P value | |
| Age (year) | 44.9 | 13.9 | 44.2 | 14 | 0.33 | 0.74 |
| Duration of hospitalization (day) | 12.6 | 4.1 | 12.4 | 4 | 0.4 | 0.69 |
| Sex | N a | % | N a | % | χ2 a | P value |
| Male | 56 | 66.7 | 57 | 67.9 | 0.027 | 0.87 |
| Female | 28 | 33.2 | 27 | 32.1 | ||
| Total | 84 | 100 | 84 | 100 | ||
| Type of trauma | N a | % | N a | % | χ2 a | P value |
| General Organ damage | 21 | 25 | 21 | 25 | 0.033 | 0.98 |
| Neurological damage | 39 | 46.4 | 38 | 45.2 | ||
| General & Neurological damage | 24 | 28.6 | 25 | 29.8 | ||
| Total | 84 | 100 | 84 | 100 | ||
| Underlying disease (yes) | 33 | 39.3 | 37 | 44 | 0.39 | 0.53 |
| Smoking (yes) | 30 | 35.7 | 33 | 39.3 | 0.229 | 0.63 |
Abbreviations: SD, Standard deviation; N: number; χ2, Chi-square test; T: Independent T-test
In each group 38.6% of the patients developed VAP. The incidence VAP in the intervention group and the control group were respectively 4.8% and 6% (P=0.5) on the third day, 14.3% and 20.2% (P=0.31) on the fourth day, and 29.8% and 47.6% on the fifth day (p=0.02) (Table 2).
Table 2.
The frequency distribution of VAP in the experimental and the control group on the fifth day
| Groups | Experimental group | Control group | χ2a | P value | |||
|---|---|---|---|---|---|---|---|
| N a | % | N a | % | ||||
| VAP on the fifth day | Infected | 25 | 29.8 | 40 | 47.6 | 5.65 | 0.02 |
| Uninfected | 59 | 70.2 | 44 | 52.4 | |||
Abbreviations: χ2: Chi-square test, N: number
The following microorganisms were isolated from the endotracheal tube aspirates: enterobacter was found in 14 patients in the experimental group (56%) and 20 patients in the control group (50%). Acinetobacter was found in seven patients in the intervention group (28%) and 12 patients in the control group (30%). Klebsiella was found in four patients in the intervention group (16%) and eight patients in the control group (20%). There were no significant differences between the two groups in this regard (X = 0.69, P = 0.71). Table 3 shows a significant relationship between the incidence of VAP on the fifth day and smoking (P<0.001), underlying diseases (P<0.001), duration of hospitalization (P=0.002), and age (P<0.001) (Table 3).
Table 3.
The incidence of ventilator associated pneumonia on clinical and demographic characteristics of the fifth day
| Variables | Total samples | Ventilator associated pneumonia | P value | ||
|---|---|---|---|---|---|
| N a | % | ||||
| Sex | Male | 113 | 48 | 42.5 | P=0.149 |
| Female | 55 | 17 | 30.9 | ||
| Type of trauma | General Organ damage | 42 | 21 | 25 | P<0.65 |
| Neurological damage | 77 | 38 | 45.2 | ||
| General & Neurological damage | 49 | 25 | 29.8 | ||
| Underlying disease | Positive | 70 | 48 | 68.6 | P<0.001 |
| Negative | 98 | 17 | 17.3 | ||
| Smoking | Yes | 63 | 42 | 66.7 | P<0.002 |
| No | 105 | 23 | 21.9 | ||
| Variables | Ventilator associated pneumonia | P value | ||
|---|---|---|---|---|
| Infected M±SD a | Uninfected M±SD a | |||
| Age (year) | 48.8±12.5 | 41.9±14.2 | P<0.002 | |
| Duration of hospitalization | 13.9±4 | 11.6±3.8 | P<0.001 | |
Abbreviations: N: number, M: Mean, SD: Standard deviation
DISCUSSION
Our results show that 38.6% of the patients in each group developed VAP. Numerous studies have reported different results on the prevalence of VAP. Pneumonia is the most frequent infection in the ICU with an incidence of 9–68% (7, 22–24). These different results might be attributed to the use of different diagnostic criteria and different study populations.
According to our results, the incidence of VAP in the experimental group on the fifth day was lower than that in the control group, which could be attributed to tooth brushing with distilled water twice day. This is in accordance with results from YAO’s study. YAO et al. showed that tooth brushing twice a day with distilled water reduced the incidence of VAP by 17% (19). In a study by Hutchins et al. a protocol of changing catheters, oral suction every 24 hours, tooth brushing twice a day, mouth washing with 0.12% chlorhexidine and hydrogen peroxide swabs every 4 hours, wetting the mouth and lips, deep oral suction every 12 hours, reduced VAP by 89.7% (1).
Based on our results, there were no differences between the two groups in terms of the type of microorganisms, and the most frequent microorganisms were enterobacter, acinetobacter, and Klebsiella, respectively. While some researchers have reported enterobacter and staphylococcus aureus as the most common bacterial agents causing VAP (25,26), others have reported pseudomonas and acinetobacter, respectively, as the most common bacterial agents which cause VAP (27–29). In studies by Afkhamzadeh et al and Peleg and Hooper, the most frequent microorganisms were Klebsiella and enterobacter (30–31). These different results might be attributed to the impact of different geographical locations, types of prophylactic antibiotics, and different methods of oral care used in those studies.
Moreover, our results showed no significant difference in the incidence of VAP on the fifth day based on sex and kind of trauma. Similarly, studies by Buczko and Nadi et al. did not show any significant relationship between patient sex and VAP (32, 33). Sabery et al. did not find a significant differences between cause of hospitalization and incidence of VAP (P=0.58)(34). However, history of smoking, age, underlying diseases, and duration of hospitalization were significantly related with VAP. The relationship between smoking and pulmonary diseases and VAP has been proven in clinical and epidemiological studies (35, 36). Arvanitis et al, also found significant correlations between the incidence of VAP and age (37). Furthermore, Chao et al. revealed a significant relationship between the incidence of VAP and duration of hospitalization (P=0.000) (38).
Given the physiological complications and psychological, social, and economic effects of VAP, it is necessary to perform interventions to reduce its incidence. According to the hypothesis confirmed in this study, tooth brushing twice a day with distilled water, in addition to performing routine oral hygiene, reduces the incidence of VAP in patients and is recommended as a part of the routine care provided to patients. Moreover, it should be noted that one of the inclusion criteria in this study was the possession of at least 20 teeth, which has not been studied in other studies and is considered the strength of this study. Further studies are also needed to compare the effect of tooth brushing with distilled water with that of tooth brushing with chlorhexidine, on the incidence of VAP in patients admitted to the ICU.
Acknowledgments
This study was derived from a project approved by the Nursing and Midwifery School of Shahid Sadoughi University of Medical Sciences in Yazd, Iran. The investigators would like to thank the universities of medical sciences of Yazd and Isfahan for their support and cooperation with this project. The investigators would also like to thank the guardians of the patients participating in this study.
REFERENCES
- 1.Hutchins K, Karras G, Erwin J, Sullivan KL. Ventilator-associated pneumonia and oral care: a successful quality improvement project. Am J Infect Control 2009;37(7):590–7. [DOI] [PubMed] [Google Scholar]
- 2.Werarak P, Kiratisin P, Thamlikitkul V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: etiology, clinical outcomes, and impact of antimicrobial resistance. J Med Assoc Thai 2010;93 Suppl 1:S126–38. [PubMed] [Google Scholar]
- 3.Sole ML, Byers JF, Ludy JE, Zhang Y, Banta CM, Brummel K. A multisite survey of suctioning techniques and airway management practices. Am J Crit Care 2003;12(3):220–30; quiz 231–2. [PubMed] [Google Scholar]
- 4.Blot S, Koulenti D, Dimopoulos G, Martin C, Komnos A, Krueger WA, Spina G, Armaganidis A, Rello J, EU-VAP Study Investigators Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med 2014;42(3):601–9. [DOI] [PubMed] [Google Scholar]
- 5.Kalanuria AA, Ziai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care 2014;18(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamishekar H, Shadvar K, Taghizadeh M, Golzari SE, Mojtahedzadeh M, Soleimanpour H, et al. Ventilator-associated pneumonia in patients admitted to intensive care units, using open or closed endotracheal suctioning. Anesth Pain Med 2014;4(5):e21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadani H, Vyas A, Kar AK. A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J Anaesth 2010;54(6):535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird D, Zambuto A, O’Donnell C, Silva J, Korn C, Burke R, et al. Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Arch Surg 2010;145(5):465–70. [DOI] [PubMed] [Google Scholar]
- 9.Keyt H, Faverio P, Restrepo MI. Prevention of ventilator-associated pneumonia in the intensive care unit: a review of the clinically relevant recent advancements. Indian J Med Res 2014;139(6):814–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Dandagi GL. Nosocomial pneumonia in critically ill patients. Lung India 2010;27(3):149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alp E, Voss A. Ventilator associated pneumonia and infection control. Ann Clin Microbiol Antimicrob 2006;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson J. Colonization and infection of the respiratory tract: What do we know? Paediatr Child Health 2004;9(1):21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalifehzadeh A, Parizade A, Hosseini A, Yousefi H. The effects of an oral care practice on incidence of pneumonia among ventilator patients in ICUs of selected hospitals in Isfahan, 2010. Iran J Nurs Midwifery Res 2012;17(3):216–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter study. Crit Care Med 2005;33(8):1728–35. [DOI] [PubMed] [Google Scholar]
- 15.Munro CL, Grap MJ, Jablonski R, Boyle A. Oral health measurement in nursing research: state of the science. Biol Res Nurs 2006;8(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grap MJ, Munro CL, Hamilton VA, Elswick RK, Jr, Sessler CN, Ward KR. Early, single chlorhexidine application reduces ventilator-associated pneumonia in trauma patients. Heart Lung 2011;40(5):e115–22. [DOI] [PubMed] [Google Scholar]
- 17.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care 2009;18(5):428–37; quiz 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patil SP, Patil PB, Kashetty MV. Effectiveness of different tooth brushing techniques on the removal of dental plaque in 6–8 year old children of Gulbarga. J Int Soc Prev Community Dent 2014;4(2):113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao LY, Chang CK, Maa SH, Wang C, Chen CC. Brushing teeth with purified water to reduce ventilator-associated pneumonia. J Nurs Res 2011;19(4):289–97. [DOI] [PubMed] [Google Scholar]
- 20.Berry AM, Davidson PM, Masters J, Rolls K. Systematic literature review of oral hygiene practices for intensive care patients receiving mechanical ventilation. Am J Crit Care 2007;16(6):552–62; quiz 563. [PubMed] [Google Scholar]
- 21.Johnston BL, Conly JM. Diagnosis of ventilator-acquired Pneumonia: Where Do We Go From Here? Can J Infect Dis 2003;14(2):77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev 2006;19(4):637–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalifehzadeh A, Parizade A, Hosseini A, Yousefi H. The effects of an oral care practice on incidence of pneumonia among ventilator patients in ICUs of selected hospitals in Isfahan, 2010. Iran J Nurs Midwifery Res 2012;17(3):216–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Hajibagheri A, Fini IA. Mouth Care in Patients Receiving Mechanical Ventilation: A Systematic Review. Nursing and Midwifery Studies 2012;1(2):51–61. [Google Scholar]
- 25.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA 1995;274(8):639–44. [PubMed] [Google Scholar]
- 26.Resende MM, Monteiro SG, Callegari B, Figueiredo PM, Monteiro CR, Monteiro-Neto V. Epidemiology and outcomes of ventilator-associated pneumonia in northern Brazil: an analytical descriptive prospective cohort study. BMC Infect Dis 2013;13:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diouf E, Beye MD, Diop Ndoye M, Kane O, Seydi AA, Ndiaye PI, et al. Nosocomial ventilator-associated pneumonia in a tropical intensive care unit. Dakar Med 2006;51(2):81–8. [PubMed] [Google Scholar]
- 28.Chi SY, Kim TO, Park CW, Yu JY, Lee B, Lee HS, et al. Bacterial pathogens of ventilator associated pneumonia in a tertiary referral hospital. Tuberc Respir Dis (Seoul) 2012;73(1):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nhu NT, Lan NP, Campbell JI, Parry CM, Thompson C, Tuyen HT, et al. Emergence of carbapenem-resistant Acinetobacter baumannii as the major cause of ventilator-associated pneumonia in intensive care unit patients at an infectious disease hospital in southern Vietnam. J Med Microbiol 2014;63(Pt 10):1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afkhamzadeh AR, Lahoorpour F, Delpisheh A, Janmardi R. Incidence of ventilator-associated pneumonia (VAP) and bacterial resistance pattern in adult patients hospitalised at the intensive care unit of Besat Hospital in Sanandaj. Scientific Journal of Kurdistan University of Medical Sciences 2011;16(1):Pe20–6. [Google Scholar]
- 31.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 2010;362(19):1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buczko W. Ventilator-associated pneumonia among elderly Medicare beneficiaries in long-term care hospitals. Health Care Financ Rev 2010;31(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Nadi E, Nekouii B, Mobin A, Nekouii A, Moghim Beigi A. Frequency of Nosocomial Pneumonia in ICUs of Hospitals of Hamadan University of Medical Sciences. Journal of Isfahan Medical School 2011;29(153). [Google Scholar]
- 34.Sabery M, Shiri H, Taghadosi M, Gilasi HR, Khamechian M. The frequency and risk factors for early-onset ventilator-associated pneumonia in intensive care units of Kashan Shahid-Beheshti hospital during 2009–2010. Feyz Journals of Kashan University of Medical Sciences 2013;16(6). [Google Scholar]
- 35.Rao RN, Goodman LR, Tomashefski JF., Jr. Smoking-related interstitial lung disease. Ann Diagn Pathol 2008;12(6):445–57. [DOI] [PubMed] [Google Scholar]
- 36.Margaritopoulos GA, Harari S, Caminati A, Antoniou KM. Smoking-related idiopathic interstitial pneumonia: A review. Respirology 2016;21(1):57–64. [DOI] [PubMed] [Google Scholar]
- 37.Arvanitis M, Anagnostou T, Kourkoumpetis TK, Ziakas PD, Desalermos A, Mylonakis E. The impact of antimicrobial resistance and aging in VAP outcomes: experience from a large tertiary care center. PLoS One 2014;9(2):e89984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao YF, Chen YY, Wang KW, Lee RP, Tsai H. Removal of oral secretion prior to position change can reduce the incidence of ventilator-associated pneumonia for adult ICU patients: a clinical controlled trial study. J Clin Nurs 2009;18(1):22–8. [DOI] [PubMed] [Google Scholar]