Abstract
The 1858T protein tyrosine phosphatase nonreceptor type 22 (PTPN22 T) allele is one of the main risk factors associated with many autoimmune diseases and correlates with a defective removal of developing autoreactive B cells in humans. To determine whether inhibiting PTPN22 favors the elimination of autoreactive B cells, we first demonstrated that the PTPN22 T allele interfered with the establishment of central B cell tolerance using NOD-scid-common γ chain knockout (NSG) mice engrafted with human hematopoietic stem cells expressing this allele. In contrast, the inhibition of either PTPN22 enzymatic activity or its expression by RNA interference restored defective central B cell tolerance in this model. Thus, PTPN22 blockade may represent a therapeutic strategy for the prevention or treatment of autoimmunity.
INTRODUCTION
Rituximab, an anti-CD20 monoclonal antibody that eliminates B cells, has shown efficacy in type 1 diabetes (T1D), rheumatoid arthritis (RA), and multiple sclerosis (MS) and exposes a role for B cells in promoting autoimmunity (1–3). However, anti–B cell therapy does not reset early B cell tolerance checkpoints defective in T1D likely because these impaired autoreactive B cell counterselection steps may be primary to the development of this autoimmune disease (4). Indeed, asymptomatic individuals carrying the PTPN22 T allele display elevated frequencies of autoreactive B cells in their blood similar to those in patients with T1D, RA, or systemic lupus erythematosus (SLE) (5). This PTPN22 variant harbors a single nucleotide change (cytidine to thymidine) at residue 1858, which results in a single amino acid substitution from arginine to tryptophan at position 620 (620W) of the PTPN22/LYP protein and has been associated with an increased risk for the development of many autoimmune diseases including RA, T1D, and SLE (6). In contrast, the rare loss-of-function 263Q PTPN22 variant was reported to confer protection against SLE and RA, suggesting that decreased PTPN22 phosphatase activity inhibits autoimmunity (7, 8). Here, we aimed to develop an alternative efficient therapy for autoimmune diseases by targeting the intrinsic genetic defects responsible for impaired central B cell tolerance. We therefore assessed whether 620W PTPN22 expression is sufficient to induce defects in central B cell tolerance and whether they could be corrected after inhibiting PTPN22 function.
RESULTS
Central B cell tolerance is defective in humanized mouse engrafted with hematopoietic stem cells carrying PTPN22 T allele(s)
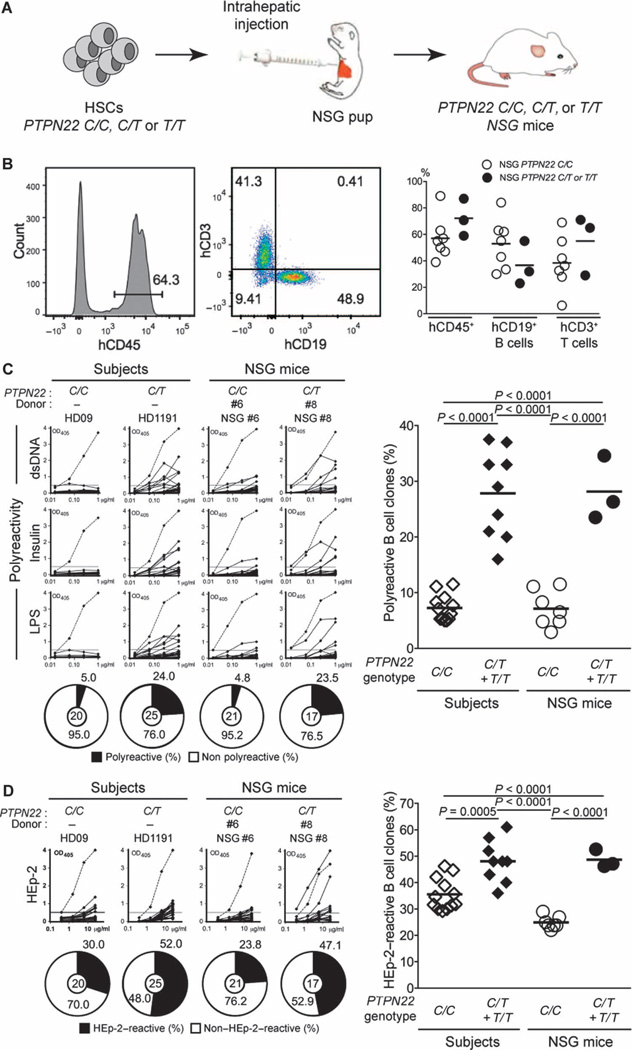
To further study the impact of PTPN22 variants on central B cell tolerance, we engrafted NOD-scid-common γ chain knockout (NSG) immunodeficient mice with CD34+ hematopoietic stem cells (HSCs) isolated from human fetuses that carry (or do not carry) PTPN22 T allele(s) (9–11) (Fig. 1A and table S1). Humanized NSG mice displayed high frequencies of CD45+ human cells detected by flow cytometry about 3 months after engraftment with HSCs, regardless of the presence of PTPN22 T allele(s) (Fig. 1B). Ratios between human CD19+ B andCD3+ T lymphocytes were also similar in NSG mice transplanted with PTPN22 C/C, PTPN22 C/T, or PTPN22 T/T HSCs, demonstrating that the PTPN22 T allele does not affect either B or T cell development (Fig. 1B). Pooled immunoglobulin heavy-chain (IgH) sequence analyses from new emigrant B cells of PTPN22 C/T or T/T NSG mice revealed no consistent differences in IgH variable (VH), diversity (D), or joining (J) gene usage, compared to PTPN22 C/C NSG mice (fig. S1, A to C). However, in contrast to new emigrant B cells of PTPN22 C/C NSG mice, the presence of a PTPN22 T allele favored the usage of different D reading frames encoding hydrophobic residues known to favor self-reactivity and which correlated with an abnormal central B cell tolerance checkpoint (12–14) (fig. S1D). The analyses of antibody reactivity revealed that frequencies of polyreactive clones in splenic CD19+CD27−CD10+IgMhiCD21lo new emigrant B cells from NSG mice transplanted with PTPN22 C/C HSCs isolated from seven distinct fetuses were low and similar to those of new emigrant B cells isolated from the blood of PTPN22 C/C healthy donors (Fig. 1C, fig. S2A, and tables S2 to S8). The low frequencies of new emigrant B cells reactive to human epithelial type 2 (HEp-2) cells and the virtual absence of antinuclear clones in this B cell compartment reveal that central B cell tolerance is established normally in humanized mice in the absence of the PTPN22 T allele (Fig. 1D and fig. S2, B and C). In contrast, we found that new emigrant B cells isolated from the spleen of NSG mice engrafted with PTPN22 C/T or T/T HSCs contained many autoreactive clones expressing polyreactive and HEp-2–reactive antibodies with similar frequencies to those observed in healthy donors carrying PTPN22 T allele(s) (5) (Fig. 1, C and D, fig. S2, A and B, and tables S9 to S11). Indirect immunofluorescence assays with HEp-2 cell–coated slides revealed that the proportions of antinuclear new emigrant B cell in NSG mice engrafted with PTPN22 C/T or T/T HSCs were increased but failed to reach significance (fig. S2C). We conclude that the presence of the PTPN22 T allele in HSCs results in defective central B cell tolerance and the release of large numbers of autoreactive B cells from the bone marrow.
Fig. 1. Defective central B cell tolerance in humanized mouse engrafted with HSCs carrying PTPN22 T allele(s).
(A) Schematic diagram depicting the generation of humanized mice. CD34+ HSCs that carry (or do not carry) PTPN22 T allele(s) were injected into the liver of 3-day-old recipient NSG mice. (B) Representative flow cytometry analysis of the frequency of human (h) CD45+, CD3+, and CD19+ cells in the blood of the indicated recipient mice. The summary of blood engraftment from NSG mice transplanted with PTPN22 C/C, C/T, or T/T HSCs is represented. Each dot represents an individual mouse, and the bars indicate mean values. The frequencies of polyreactive (C) and HEp-2–reactive (D) new emigrant B cells from different types of humanized mice transplanted with indicated HSCs were determined and compared with those of healthy donors that carry (or do not carry) PTPN22 T allele(s). Dotted lines show positive control. For each B cell fraction, the frequency of reactive (solid area) and nonreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. In summarized reactivity panels on the right, each diamond represents an individual, and each dot represents a mouse. Averages are shown with a bar, and statistically significant differences are indicated. OD, optical density.
620W PTPN22 overexpression interferes with central B cell tolerance
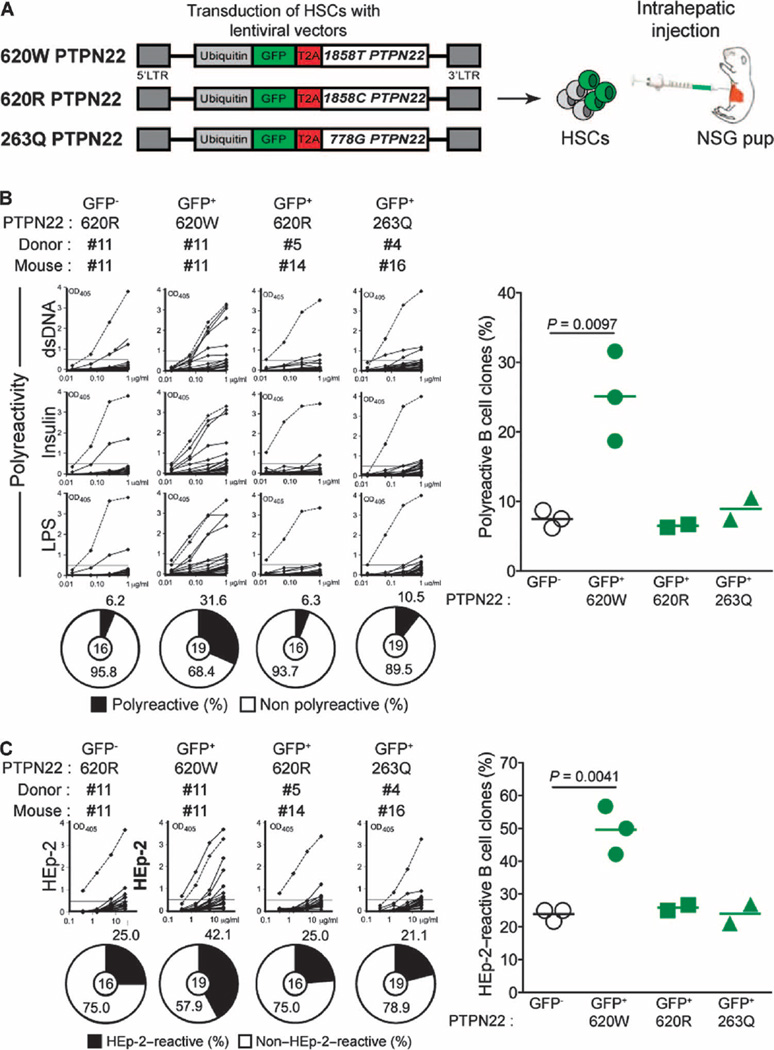
To determine whether B cell–intrinsic expression of 620W PTPN22 phosphatases is sufficient to interfere with the removal of developing autoreactive immature B cells in the bone marrow, we transduced PTPN22 C/C HSCs with lentiviruses expressing green fluorescent protein (GFP) and the 620W PTPN22 autoimmunity-favoring variant, the common 620R or the 263Q loss-of-function PTPN22 enzyme (Fig. 2A). Human CD19+ B cells developed in NSG mice engrafted with HSCs, whether transduced or not with the different lentiviruses, revealed that lentiviral transduction did not alter HSC engraftment or B cell development (fig. S3A). Quantification of lentivirus-encoded PTPN22 expression in GFP-positive sorted B cells revealed that all three PTPN22 variants were at least 20 times more abundant than endogenous PTPN22 proteins in GFP-negative B cell counterparts (fig. S3B). The presence of 620W PTPN22 altered the counterselection of developing autoreactive B cells because GFP-positive new emigrant B cells expressing this variant contained many autoreactive clones that produce polyreactive antibodies (Fig. 2B, fig. S4A, and tables S12 to S17). High proportions of HEp-2–reactive and antinuclear GFP-positive new emigrant B cells corroborated this defective central B cell tolerance checkpoint (Fig. 2C and fig. S4, B to D). In contrast, GFP-negative B cell counterparts that developed in the same NSG mice rarely expressed polyreactive antibodies and displayed low frequencies of HEp-2–reactive and antinuclear clones, revealing that these B cells were properly selected in the absence of 620W PTPN22 expression (Fig. 2, B and C, and fig. S4, A to D). In addition, GFP-positive new emigrant B cells expressing either 620R PTPN22 or the loss-of-function 263Q PTPN22 variant displayed normal proportions of polyreactive, HEp-2–reactive, and antinuclear clones, demonstrating normal central B cell tolerance (Fig. 2, B and C, fig. S4, A to D, and tables S18 to S21). Regardless of how the 620W amino acid replacement alters PTPN22 function, our data demonstrate that B cell–intrinsic 620W PTPN22 expression is sufficient to interfere with the removal of developing autoreactive B cells and the establishment of human central B cell tolerance.
Fig. 2. 620W PTPN22 overexpression interferes with central B cell tolerance.
(A) Humanized mice were generated with CD34+ HSCs transduced with lentiviruses, allowing the expression of different variants of PTPN22 before being injected into the liver of 3-day-old recipient NSG mice. LTR, long terminal repeat. The frequencies of polyreactive (B) and HEp-2–reactive (C) new emigrant B cells from sorted GFP-positive fractions expressing 620W PTPN22, 620R PTPN22, or 263Q PTPN22 were determined and compared with those of GFP-negative new emigrant B cells. Dotted lines show positive control. For each B cell fraction, the frequency of reactive (solid area) and nonreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. In summarized reactivity panels on the right, each symbol represents a mouse overexpressing 620W PTPN22 (green dots), 620R PTPN22 (green squares), or 263Q PTPN22 (green triangles). Averages are shown with a bar, and statistically significant differences are indicated.
Inhibition of PTPN22 enzymatic activity resets central B cell tolerance
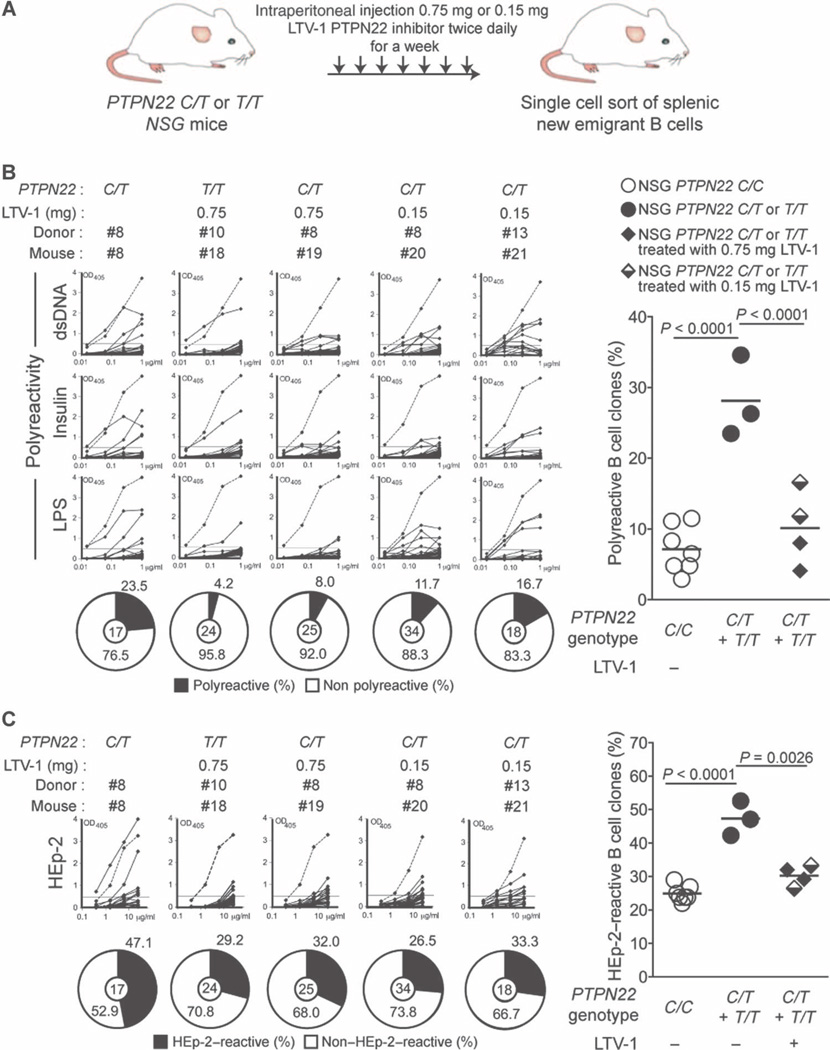
Because the rare loss-of-function 263Q PTPN22 variant was reported to confer protection against SLE and RA, we hypothesize that decreased PTPN22 phosphatase activity may inhibit autoimmunity (7, 8). LTV-1 is a compound that was identified to selectively inhibit human PTPN22 enzymatic activity (15). To assess the impact of the inhibition of 620W PTPN22 enzymatic activity on central B cell tolerance, we injected PTPN22 C/T or T/T engrafted NSG mice about 3 months after transplant with 0.75 mg of the LTV-1 compound twice daily for a week and determined the frequency of autoreactive new emigrant B cells (Fig. 3A). We found that LTV-1 treatment substantially reduced the frequencies of polyreactive new emigrant B cells in PTPN22 C/T or T/T transplanted mice, similar to those in NSG mice engrafted with HSCs that did not carry the PTPN22 T allele (Fig. 3B and tables S22 to S25). In addition, PTPN22 inhibition by LTV-1 also normalized the frequencies of HEp-2–reactive new emigrant B cells in PTPN22 C/T or T/T engrafted mice (Fig. 3C), and antinuclear clone frequencies remained very low (fig. S5). Central B cell tolerance was also restored by injecting five times less LTV-1 (0.15 mg per injection), although polyreactivity frequencies were slightly higher than those in mice treated with 0.75 mg per injection (Fig. 3B). These data therefore suggest a wide range of effective PTPN22 inhibition by LTV-1 (Fig. 3, B and C). Hence, inhibition of 620W PTPN22 enzymatic activity resets central B cell tolerance that is normally impaired by the presence of the PTPN22 T allele.
Fig. 3. Inhibition of PTPN22 enzymatic activity resets central B cell tolerance.
(A) Schematic diagram depicting the PTPN22 inhibitor treatment strategy. NSG mice generated with CD34+ HSCs carrying PTPN22 T allele(s) were injected twice daily with 0.75 or 0.15 mg of the PTPN22 inhibitor for 1 week. The frequencies of polyreactive (B) and HEp-2–reactive (C) new emigrant B cells from NSG mice carrying PTPN22 T allele(s) and treated with the PTPN22 inhibitor were determined and compared with those of nontreated NSG mice. Dotted lines show positive control. For each B cell fraction, the frequency of reactive (solid area) and nonreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. In summarized reactivity panels on the right, each dot represents an untreated mouse and full and half-filled diamonds represent mice treated with either 0.75 or 0.15 mg of the LTV-1 PTPN22 inhibitor, respectively. Averages are shown with a bar, and statistically significant differences are indicated.
Inhibition of PTPN22 expression during B cell development resets central B cell tolerance
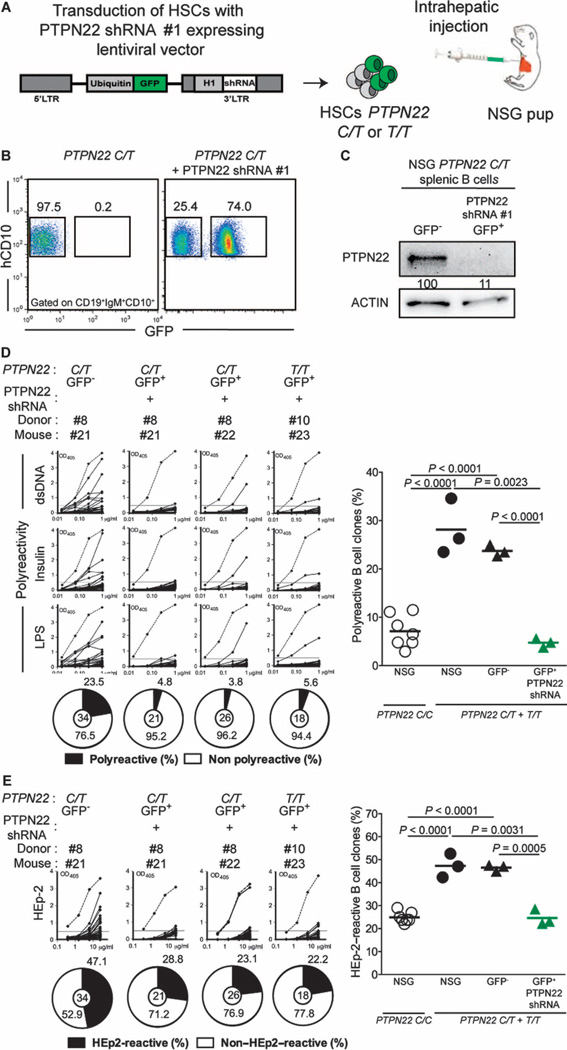
Although central B cell tolerance appears to be mainly regulated by B cell–intrinsic pathways involving B cell receptor (BCR) and, potentially, Toll-like receptor signaling (16), this checkpoint might be restored via B cell–extrinsic pathways normalized by 620W PTPN22 inhibition. In addition, the LTV-1 PTPN22 inhibitor may also nonspecifically alter the function of other phosphatases. To determine whether specific B cell–intrinsic PTPN22 blockade is responsible for the correction of central tolerance, we developed a strategy to inhibit the expression of PTPN22 in developing B cells using RNA interference (17). We engrafted NSG mice with PTPN22 C/T or T/T HSCs transduced with a GFP-tagged lentivirus expressing PTPN22-specific short hairpin RNA (shRNA) (Fig. 4A). We identified two PTPN22-specific shRNA (shRNA #1 and shRNA #3) that could inhibit about 80% of PTPN22 expression detected by Western blot using human RAMOS B cell line and chose shRNA #1 for all further experiments (fig. S6A). A high proportion of GFP-positive human B cells expressing PTPN22 shRNA #1 developed in NSG mice, revealing that transduced HSCs retained engraftment and B cell development capacities (Fig. 4B). In addition, GFP expression correlated with more than 90% decrease of PTPN22 expression in developing B cells (Fig. 4C). Blocking PTPN22 expression in GFP-positive PTPN22 C/T or T/T new emigrant B cells reduced the production of polyreactive and HEp-2–reactive clones compared with GFP-negative counterparts that often expressed autoreactive antibodies (Fig. 4, D and E, fig. S6, B and C, and tables S26 to S31). In addition, we have previously shown using control shRNA lentiviruses that HSC transduction per se does not interfere with the counterselection of autoreactive B cells (17). Together, these data demonstrate that the inhibition of PTPN22 expression in developing B cells can induce efficient removal of autoreactive clones and therefore restore central B cell tolerance that is otherwise impaired when the 620W PTPN22 variant is expressed.
Fig. 4. Inhibition of PTPN22 expression during B cell development resets central B cell tolerance.
(A) CD34+ HSCs carrying PTPN22 T allele(s) were transduced with lentiviruses, allowing the expression of PTPN22 shRNA before injection into the liver of 3-day-old NSG mice. (B) Representative flow cytometry analysis of CD19+ cells isolated from the spleen of NSG mouse engrafted with PTPN22 C/T HSCs transduced with a GFP-tagged lentivirus expressing PTPN22-specific shRNA. CD19+ B cells were stained with anti-hCD19, anti-IgM, and anti-hCD10 antibodies. The frequencies of GFP-negative and GFP-positive shRNA+ new emigrant B cells are shown. (C) PTPN22 protein expression in GFP-negative and GFP-positive shRNA+ hCD19+ cells isolated from the spleen of NSG mice; β-actin is used for normalization of protein expression. Percentage of knockdown is indicated. (D and E) B cell–intrinsic PTPN22 expression is required for central B cell tolerance. The frequencies of polyreactive (D) and HEp-2–reactive (E) new emigrant B cells from sorted GFP-positive fractions expressing PTPN22 shRNA were determined and compared with those of GFP-negative new emigrant B cells. Dotted lines show positive control. For each B cell fraction, the frequency of reactive (solid area) and nonreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. In summarized reactivity panels on the right, each symbol represents a mouse. The average is shown with a bar, and statistically significant differences are indicated.
DISCUSSION
Here, we reported that the PTPN22 T allele is responsible for the production of autoreactive B cells that escape central tolerance. These results are in agreement with the presence of PTPN22 T allele(s) correlating with impaired early B cell tolerance checkpoints (5). These observations may also explain why the PTPN22 T allele confers high risk of developing many autoimmune diseases as it induces central B cell tolerance defects observed in patients with T1D, RA, and SLE (4, 18, 19). The impact of the PTPN22 T allele was also investigated by generating knockin mice expressing the analogous mutation R619W in the murine Ptpn22 ortholog (20). The presence of R619W variants altered T and B cell selection and expansion, leading to systemic autoimmunity (20). In addition, B cell–restricted expression of the R619W variant was sufficient to promote autoimmunity, further demonstrating the prevalent tolerogenic role of the 620R PTPN22 variant (20). Together, elevated frequencies of autoreactive B cells resulting from the presence of the 619W or 620W PTPN22 variant in mice and humans, respectively, may increase the probability to present self-antigens and initiate autoimmunity.
Central B cell tolerance could be reset in PTPN22 C/T or T/T subjects by inhibiting PTPN22 enzymatic activity or expression. In line with these observations, decreased PTPN22 phosphatase activity associated with the loss-of-function 263Q PTPN22 variant has already been suggested to protect against autoimmunity, likely by preventing the production of autoreactive B cells in the bone marrow (7, 8). How does PTPN22 inhibition favor the elimination of autoreactive B cells? The presence of the 620W PTPN22 variant decreases both T cell receptor (TCR) and BCR signaling and will therefore affect the selection of the repertoire of these receptors during early T and B cell development, respectively (21–23). Indeed, decreased BCR signaling results in impairment to induce tolerance mechanisms in immature B cells that bind self-antigens and allows some autoreactive clones to escape central B cell tolerance (16, 24). Because PTPN22 inhibition increases TCR signaling (15), it is likely that PTPN22 inhibition will also enhance BCR signaling and will thereby reestablish proper threshold for the removal of developing autoreactive B cells in the bone marrow. PTPN22 inhibition also likely resets TCR signaling thresholds altered by 620W PTPN22 variants (21, 22) and will therefore modify the TCR repertoire of both T effector and regulatory T cells selected in the thymus of PTPN22 T carriers. In conclusion, PTPN22 is a major regulator of human central B cell tolerance; its inhibition can normalize the elimination of developing autoreactive B cells and may thereby thwart the development of autoimmunity.
MATERIALS AND METHODS
Human progenitor cell isolation and injection in NSG mice
Human CD34+ cells were purified from fetal liver samples by density gradient centrifugation followed by positive immunomagnetic selection with anti-human CD34 microbeads (Miltenyi Biotech). Newborn NSG mice (within first 3 days of life) were sublethally irradiated (x-ray irradiation with X-RAD 320 irradiator at 180 cGy), and 100,000 to 150,000 CD34+ cells in 20 µl of phosphate-buffered saline were injected into the liver with a 22-gauge needle (Hamilton Company).Mice were used for experiments 10 to 12 weeks after transplantation. NSG mice treated with the PTPN22 inhibitor were injected with the PTPN22 inhibitor 0.75 or 0.15 mg ip twice daily for a week. All animals were treated, and experiments were conducted in accordance with the Yale institutional reviewed guidelines on treatment of experimental animals.
PTPN22 overexpression and silencing and CD34+ HSC transduction
The pTRIP-Ubi-GFP lentiviral vector was used for overexpression of PTPN22 variants and shRNA delivery. Vector constructions have been previously described (17, 25). The following sequences were used for human PTPN22 targeting: shRNA #1, 5′-CTAGTGCTCTTGGTGTATATT-3′; shRNA #2, 5′-CTGTTGCCAACATCCTCTA-3′; shRNA #3, 5′-AAGAATCCACCTGACTTCC-3′. Lentiviral particles were produced by transient transfection of 293T cells, as previously described (26). Viruses were then used to transduce CD34+ HSCs in the presence of protamine sulfate (Sigma).
Single-cell sorting
B cells were enriched from splenocytes using magnetic separation with CD19 microbeads (Miltenyi Biotech) and stained with CD19–Pacific Blue, CD10-phycoerythrin-Cy7, CD21-allophycocyanin, and IgM-biotin (all from BioLegend) before purification. Single CD19+CD10+CD21loGFP− or CD19+CD10+CD21loGFP+ new emigrant B cells were sorted on a FACSAria (BD Biosciences) into 96-well polymerase chain reaction (PCR) plates and were immediately frozen on dry ice.
cDNA synthesis, Ig gene amplification, antibody production, and antibody purification
RNA from single cells was reverse-transcribed in the original 96-well plate in 12.5-µl reactions containing 100 U of Superscript II RT (Gibco BRL) for 45 min at 42°C. Reverse transcription PCRs, primer sequences, cloning strategy, expression vectors, and antibody expression and purification were as previously described (27).
Enzyme-linked immunosorbent assays and immunofluorescence assays
Antibody reactivity analysis was performed as previously described with the highly polyreactive ED38 antibody as positive control for HEp-2 reactivity and polyreactivity (28). Antibodies were considered polyreactive when they recognized all three distinct antigens: double-stranded DNA (dsDNA), insulin, and lipopolysaccharide (LPS). For indirect immunofluorescence assays, HEp-2 cell–coated slides (Bion Enterprises Ltd.) were incubated in a moist chamber at room temperature with purified recombinant antibodies at 50 to 100 µg/ml, according to the manufacturer’s instructions. Fluorescein isothiocyanate–conjugated goat anti-human IgG was used as detection reagent.
Flow cytometry
The following monoclonal antibodies against human antigens were used: anti-CD10 (HI10a), anti-CD19 (HIB19), anti-CD27 (O323), anti-CD45 (HI30), anti-CD21 (B-ly4), and anti-IgM (G20-127) (the first four antibodies were from BioLegend and the latter two were from BD Biosciences). Cells were acquired with an LSR II (BD Biosciences) and analyzed with FlowJo software. Representative gating strategies are shown in fig. S7.
Immunoblot
Total cell lysates were separated by SDS–polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, probed with mouse anti-PTPN22 (Invitrogen), and detected by chemiluminescence (Amersham ECL Prime Western Blotting Detection Reagent) using a GBox documentation system (Syngene). For quantification, blots were stripped with stripping buffer (Pierce) and reprobed with a mouse anti–β-actin antibody (Sigma-Aldrich). Unprocessed immunoblot images are shown in fig. S8.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5.0; GraphPad). Data are reported as means ± SD. Differences between groups of research subjects were analyzed for statistical significance with unpaired two-tailed Student’s t tests. A P value of ≤0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank M. Kalp for advice on the PTPN22 inhibitor and L. Devine and C. Wang for cell sorting. Funding: This work was supported by a grant provided by AbbVie.
J.-N.S. and E.M. are authors on U. S. Patent #47162-5220-P1-US.605388 “Compositions and Methods for Inhibiting PTPN22.”
Footnotes
SUPPLEMENTARY MATERIALS
immunology.sciencemag.org/cgi/content/full/1/1/aaf7153/DC1
Fig. S1. New emigrant B cells isolated from NSG mice engrafted with PTPN22 C/T or T/T HSCs display normal IgH repertoire.
Fig. S2. Defective central B cell tolerance in humanized mouse engrafted with HSCs carrying PTPN22 T allele(s).
Fig. S3. Overexpression of PTPN22 variants in NSG mice.
Fig. S4. 620W PTPN22 overexpression interferes with the central B cell tolerance checkpoint.
Fig. S5. Frequencies of antinuclear new emigrant B cells in PTPN22 C/T or T/T NSG mice treated with the LTV-1 PTPN22 inhibitor.
Fig. S6. Inhibition of PTPN22 expression during B cell development resets central B cell tolerance.
Fig. S7. Representative gating strategies.
Fig. S8. Unprocessed immunoblot images.
Table S1. Fetal donor characteristics.
Table S2. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #1.
Table S3. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #2.
Table S4. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #3.
Table S5. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #4.
Table S6. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #5.
Table S7. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #6.
Table S8. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #7.
Table S9. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #8.
Table S10. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #9.
Table S11. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #10.
Table S12. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #11.
Table S13. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing 620W PTPN22 from mouse #11.
Table S14. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #12.
Table S15. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing 620W PTPN22 from mouse #12.
Table S16. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #13.
Table S17. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing 620W PTPN22 from mouse #13.
Table S18. Repertoire and reactivity of antibodies GFP-positive new emigrant B cells expressing R620 PTPN22 from mouse #14.
Table S19. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing R620 PTPN22 from mouse #15.
Table S20. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing 263Q PTPN22 from mouse #16.
Table S21. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing 263Q PTPN22 from mouse #17.
Table S22. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #18 treated with 0.75 mg of the LTV-1 PTPN22 inhibitor.
Table S23. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #19 treated with 0.75 mg of the LTV-1 PTPN22 inhibitor.
Table S24. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #20 treated with 0.15 mg of the LTV-1 PTPN22 inhibitor.
Table S25. Repertoire and reactivity of antibodies from new emigrant B cells of mouse #21 treated with 0.15 mg of the LTV-1 PTPN22 inhibitor.
Table S26. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #21.
Table S27. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing PTPN22 shRNA from mouse #21.
Table S28. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #22.
Table S29. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing PTPN22 shRNA from mouse #22.
Table S30. Repertoire and reactivity of antibodies from GFP-negative new emigrant B cells of mouse #23.
Table S31. Repertoire and reactivity of antibodies from GFP-positive new emigrant B cells expressing PTPN22 shRNA from mouse #23.
Author contributions: E.M. started the collaboration with R.F. and T.S. that shared protocols for the generation of engrafted NSG mice. P.S.-S. built and provided the pTRIP lentiviral vector. J.-N.S. and E.M. designed the experiments. J.-N.S., M.K., A.B., J.M.B., C.M., H.W., N.K., T.O., and L.M. performed the experiments and were responsible for the statistical analysis. J.-N.S. and E.M. wrote the manuscript. All authors reviewed the manuscript and provided scientific input.
Competing interests: The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N. Engl. J. Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JCW, Szczepański L, Szechiński J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell–targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH HERMES Trial Group. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain N, Massad C, Oe T, Cantaert T, Herold KC, Meffre E Type 1 Diabetes TrialNet Pathway to Prevention Study Group. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 2016;126:282–287. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menard L, Saadoun D, Isnardi I, Ng Y-S, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: Multifunctional regulator of immune signaling, development, and disease. Annu. Rev. Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orrú V, Tsai SJ, Rueda B, Fiorillo E, Stanford SM, Dasgupta J, Hartiala J, Zhao L, Ortego-Centeno N, D’Alfonso S, Italian Collaborative Group. Arnett FC, Wu H, Gonzalez-Gay MA, Tsao BP, Pons-Estel B, Alarcon-Riquelme ME, He Y, Zhang Z-Y, Allayee H, Chen XS, Martin J, Bottini N. A loss-of-function variant of PTPN22 is associated with reduced risk of systemic lupus erythematosus. Hum. Mol. Genet. 2009;18:569–579. doi: 10.1093/hmg/ddn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Rodríguez L, Taib WRW, Topless R, Steer S, González-Escribano MF, Balsa A, Pascual-Salcedo D, González-Gay MA, Raya E, Fernandez-Gutierrez B, González-Álvaro I, Bottini N, Witte T, Viken MK, Coenen MJH, van Riel PLCM, Franke B, den Heijer M, Radstake TRDJ, Wordsworth P, Lie BA, Merriman TR, Martín J. The PTPN22 R263Q polymorphism is a risk factor for rheumatoid arthritis in Caucasian case–control samples. Arthritis Rheum. 2011;63:365–372. doi: 10.1002/art.30145. [DOI] [PubMed] [Google Scholar]
- 9.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hematopoietic stem cells. J. Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 10.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, Greenberg E, Spitzer TR, Savage DG, Tahara H, Choi G, Yang Y-G, Sykes M. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl. Med. 2012;4:125ra30. doi: 10.1126/scitranslmed.3003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett SJ, Tomlinson IM, Sonnhammer ELL, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: A systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J. Mol. Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 13.Ng Y-S, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J. Exp. Med. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers G, Ng Y-S, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debré M, Rieux-Laucat F, Conley ME, Cunningham-Rundles C, Durandy A, Meffre E. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J, Tasken K, Tremblay ML, Lie BA, Page R, Mustelin T, Rahmouni S, Rickert RC, Tautz L. LYP inhibits T-cell activation when dissociated from CSK. Nat. Chem. Biol. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meffre E. The establishment of early B cell tolerance in humans: Lessons from primary immunodeficiency diseases. Ann. N. Y. Acad. Sci. 2011;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantaert T, Schickel J-N, Bannock JM, Ng Y-S, Massad C, Oe T, Wu R, Lavoie A, Walter JE, Notarangelo LD, Al-Herz W, Kilic SS, Ochs HD, Nonoyama S, Durandy A, Meffre E. Activation-induced cytidine deaminase expression in human B cell precursors is essential for central B cell tolerance. Immunity. 2015;43:884–895. doi: 10.1016/j.immuni.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuels J, Ng Y-S, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J. Exp. Med. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, Moon RT, Liggitt D, Wolf-Yadlin A, Buckner JH, Rawlings DJ. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest. 2013;123:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J. Immunol. 2007;179:4704–4710. doi: 10.4049/jimmunol.179.7.4704. [DOI] [PubMed] [Google Scholar]
- 22.Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat. Immunol. 2014;15:875–883. doi: 10.1038/ni.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arechiga AF, Habib T, He Y, Zhang X, Zhang Z-Y, Funk A, Buckner JH. Cutting edge: The PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J. Immunol. 2009;182:3343–3347. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J. Immunol. 2005;174:1775–1781. doi: 10.4049/jimmunol.174.4.1775. [DOI] [PubMed] [Google Scholar]
- 25.Ruer-Laventie J, Simoni L, Schickel J-N, Soley A, Duval M, Knapp A-M, Marcellin L, Lamon D, Korganow A-S, Martin T, Pasquali J-L, Soulas-Sprauel P. Overexpression of Fkbp11, a feature of lupus B cells, leads to B cell tolerance breakdown and initiates plasma cell differentiation. Immun. Inflamm. Dis. 2015;3:265–279. doi: 10.1002/iid3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schickel J-N, Pasquali J-L, Soley A, Knapp A-M, Decossas M, Kern A, Fauny J-D, Marcellin L, Korganow A-S, Martin T, Soulas-Sprauel P. Carabin deficiency in B cells increases BCR-TLR9 costimulation-induced autoimmunity. EMBO Mol. Med. 2012;4:1261–1275. doi: 10.1002/emmm.201201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.