Abstract
Patient: Female, 78
Final Diagnosis: Dementia with Lewy body
Symptoms: Dizziness • sycope
Medication: —
Clinical Procedure: —
Specialty: Geriatrics
Objective:
Unusual setting of medical care
Background:
Postprandial hypotension, induced by an absorption of glucose from intestine, could be treated by acarbose; however, it was unclear whether dipeptidyl peptidase-4 inhibitor reduced postprandial hypotension.
Case Report:
A 78-year-old woman who had experienced episodes of dizziness and hypotension after eating was admitted to our hospital. During 24-hour ambulatory blood pressure monitoring, there were repeated episodes of marked postprandial hypotension; i.e., a significant systolic blood pressure reduction within two hours after eating (from ‒58 to ‒64 mm Hg after meals). The patient was diagnosed with dementia with Lewy bodies. The patient exhibited postprandial hyperglycemia and hypotension after a 75 g oral glucose tolerance test. After the administration of 25 mg sitagliptin, the patient’s postprandial and orthostatic hypotension was reduced remarkably. Moreover, her Mini-Mental State Examination score subsequently increased (from 22 to 25 points).
Conclusions:
The dipeptidyl peptidase-4 inhibitor sitagliptin can delay postprandial increases in glucose levels and hypotensive episodes, as well as sympathetic nervous system abnormalities and orthostatic hypotension.
MeSH Keywords: Autonomic Nervous System Diseases; Hypotension; Hypotension, Orthostatic; Lewy Body Disease; Postprandial Period
Background
Postprandial hypotension and orthostatic hypotension can cause syncope and falls in elderly patients, leading to cognitive decline and a reduction in quality of life. One of the causes of postprandial hypotension is carbohydrate ingestion. Carbohydrates in the intestine increase mesenteric arterial blood flow and reduce systemic venous flow, which leads to reductions in cardiac output and systemic blood pressure. In patients with autonomic nervous system dysfunction, delayed systemic blood pressure responses to increased mesenteric arterial flow can cause postprandial hypotension, and α-glucosidase inhibitors such as acarbose have been reported to improve postprandial hypotension [1]. In a recent case report, it was reported that dipeptidyl peptidase-4 (DPP-4) inhibitors can also ameliorate postprandial hypotension [2]. However, there have not been any reports about the relationship between postprandial glucose levels before and after the administration of DPP-4 inhibitors in patients with autonomic nervous system dysfunction due to dementia with Lewy bodies. Moreover, it is unclear whether DPP-4 inhibitors improve orthostatic hypotension and/or postprandial hypotension.
Case Report
A 78-year-old woman, who was suffering from dizziness after meals, was admitted to our hospital. The patient had a previous history of hypertension and ischemic stroke (from the right external capsule to the putamen) six months earlier, and after her stroke, she had dizziness and faintness after meals and also experienced postprandial systolic blood pressure reductions (from 130 mm Hg to 80 mm Hg). Her family felt that she was suffering from cognitive impairment. The patient was referred to our hospital to have her postprandial hypotension evaluated and managed.
No abnormalities were observed during her physical examinations. The findings of her blood examinations are shown in Table 1. Cardiomegaly was not observed on a chest x-ray, and an electrocardiogram showed a normal sinus rhythm. No significant stenosis was detected in the carotid arteries on echosonography.
Table 1.
Characteristics and blood examination of the patient.
| Age, 78, years |
| Gender, Female |
| Body height, 151 cm |
| Body weight, 54 kg |
| Body mass index, 23.7 kg/m2 |
| White blood cells, 5,680 (4,000–7,500)/μL |
| Hemoglobin, 12.7 (11.5–15.5) g/dL |
| Platelet, 14.5×103 (12.0–34.0×103)/μL |
| Fasting blood glucose, 125 (65–110), mg/dL |
| Hemoglobin A1c, 6.0 (4.6–6.2) % |
| Total bilirubin, 0.9 (0.2–1.0), mg/dL |
| Aspartate aminotransferase (AST), 18 (8–38) IU/L |
| Alanine aminotransferase (ALT), 11 (4–44) IU/L |
| Lactate dehydrogenase (LD), 159 (106–211) IU/L |
| Creatinine phosphokinase, 67 (14–180) IU/L |
| Blood urea nitrogen, 15.0 (10–25) mg/dL |
| Creatinine, 1.08 (0.60–1.00) mg/dL |
| Na, 142 (138–146) mEq/L |
| K, 4.5 (3.5–4.7), mEq/L |
| Cl, 105 (97–110) mEq/L |
| C-reactive protein, 0.12 (<0.31) mg/dL |
Data in the parenthesis shows normal values.
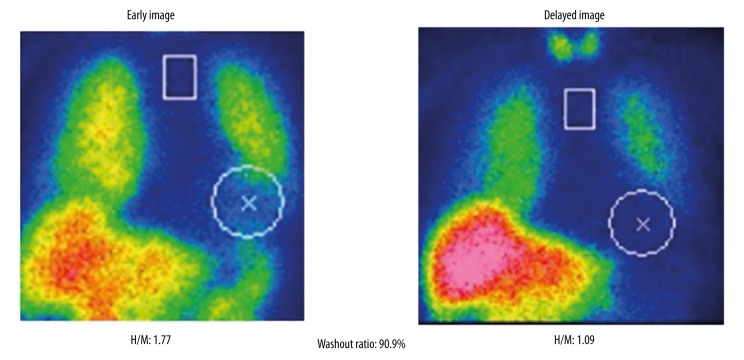
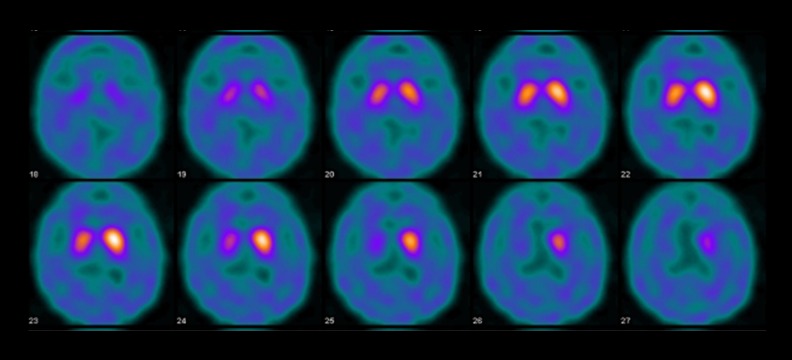
The results of the patient’s 24-hour ambulatory blood pressure monitoring are shown in Figure 1. The patient’s systolic blood pressure dropped from 143 mm Hg to 77 mm Hg at 30 minutes after meals, which was suggestive of marked postprandial hypotension. She also exhibited orthostatic hypotension (a reduction in systolic blood pressure from 160 mm Hg to 120 mm Hg) in the head-up tilt test (Figure 2). In an evaluation of the patient’s heart rate variability during standing up and sitting down (Kiritsu Meijin, Crosswell Inc., Kanagawa, Japan), a reduced sympathetic nervous system activity response, which was measured as the change in the low frequency (LF)/high frequency (HF) power ratio observed after standing up, was detected (Table 2). In metaiodobenzylguanidine (MIBG) scintigraphy, a reduced heart-to-mediastinum uptake ratio (H/M ratio) was observed (1.77 on early images and 1.09 on delayed images), which was indicative of reduced sympathetic nervous function in the heart (Figure 3). In addition, the patient’s Mini-Mental State Examination (MMSE) score was 22 points, and she was considered to be suffering from cognitive impairment without Parkinsonism. The patient was diagnosed with dementia with Lewy bodies based on the following findings: (1) rapid eye movement sleep behavior disorder, (2) autonomic nervous system dysfunction, (3) a reduced H/M ratio on MIBG scintigraphy, and (4) an abnormal pattern on a dopamine transporter (DAT) scan (Figure 4).
Figure 1.
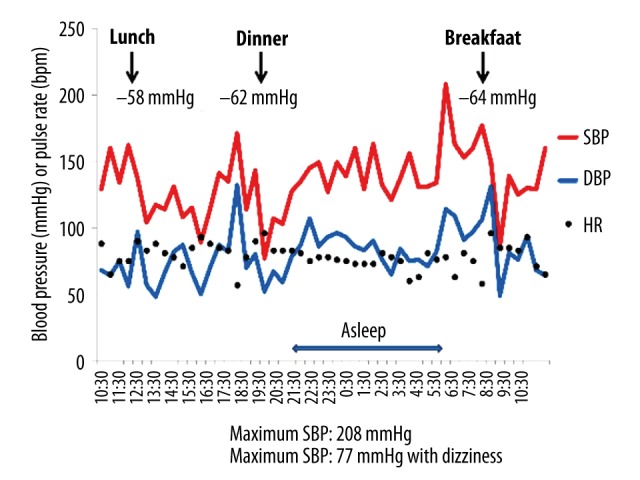
24-hour ambulatory blood pressure monitoring before the administration of sitagliptin. Ambulatory blood pressure monitoring was performed at 15-minute intervals for 24 hours.
Figure 2.
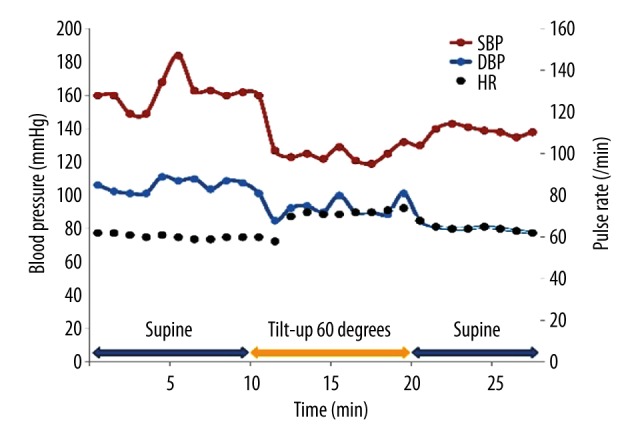
Head-up tilt test results obtained before the administration of sitagliptin. Blood pressure was measured using an automatic cuff-oscillometric device at 5-minute intervals.
Table 2.
Changes in heart rate variability measures after standing-up.
| Rest | Standing-up | Standing | Sitting-down | |
|---|---|---|---|---|
| Baseline | ||||
| Systolic blood pressure, mmHg | 147 | 116 | 106 | 131 |
| Diastolic blood pressure, mmHg | 81 | 67 | 69 | 81 |
| Heart rate, bpm | 62 | 66 | 69 | 69 |
| High frequency (HF), Hz | 134.59 | 137.03 | 55.92 | 46.65 |
| Low frequency (LF), Hz | 124.57 | 111.52 | 57.01 | 63.06 |
| LF/HF ratio | 1.26 | 0.83 | 1.22 | 1.72 |
| After taking sitagliptin 25 mg just after waking up | ||||
| Systolic blood pressure, mmHg | 140 | 127 | 130 | 126 |
| Diastolic blood pressure, mmHg | 79 | 80 | 77 | 77 |
| Heart rate, bpm | 74 | 77 | 76 | 74 |
| High frequency (HF), Hz | 38.73 | 39.20 | 18.35 | 51.40 |
| Low frequency (LF), Hz | 30.28 | 41.11 | 27.53 | 99.77 |
| LF/HF ratio | 0.81 | 1.02 | 1.66 | 2.04 |
Figure 3.
H/M ratio on MIBG scintigraphy before the administration of sitagliptin.
Figure 4.
The patient’s DAT scans.
The results obtained by continuous blood pressure monitoring (Arteriograph24, TensioMed, Hungary) during the 75 g oral glucose ingestion test are shown in Table 3. The patient exhibited a blood pressure reduction at 30 minutes after the ingestion of 75 g oral glucose, which was not affected by her serum insulin levels, and she also displayed postprandial hyperglycemia. On the other hand, the patient did not demonstrate a reduction in blood pressure after the intravenous administration of 20 mL 20% glucose (Table 3), which suggested that the absorption of glucose from the intestine, rather than glucose in the blood, played an important role in the causative mechanism of the patient’s postprandial hypotension.
Table 3.
Changes in blood pressure, blood glucose, and insulin level after 75 g oral glucose ingestion or intravenous administration of 20% glucose 20 mL.
| Before | 30 min | 60 min | 90 min | 120 min | |
|---|---|---|---|---|---|
| Baseline | |||||
| 75 g oral glucose ingestion | |||||
| Systolic blood pressure, mm Hg | 162 | 116 | 119 | 129 | 130 |
| Diastolic blood pressure, mm Hg | 90 | 69 | 72 | 80 | 80 |
| Blood glucose, mg/dL | 100 | 208 | 240 | 285 | 274 |
| Insulin, μU/L | 4.4 | 19.2 | 25.8 | 36.3 | 58.5 |
| 20% glucose 20 mL intravenously | |||||
| Systolic blood pressure, mm Hg | 146 | 147 | 157 | 176 | 160 |
| Diastolic blood pressure, mm Hg | 85 | 88 | 91 | 97 | 96 |
| Blood glucose, mg/dl | 100 | 120 | 106 | 102 | 103 |
| Insulin, μU/l | 4.0 | 5.7 | 3.8 | 3.5 | 3.0 |
| After taking sitagliptin 25 mg just after waking | |||||
| 75 g oral glucose ingestion | |||||
| Systolic blood pressure, mm Hg | 190 | 148 | 155 | 137 | 144 |
| Diastolic blood pressure, mm Hg | 103 | 80 | 85 | 81 | 84 |
| Blood glucose, mg/dL | 100 | 157 | 144 | 170 | 186 |
| Insulin, μU/L | 4.5 | 20.7 | 13.7 | 18 | 23.8 |
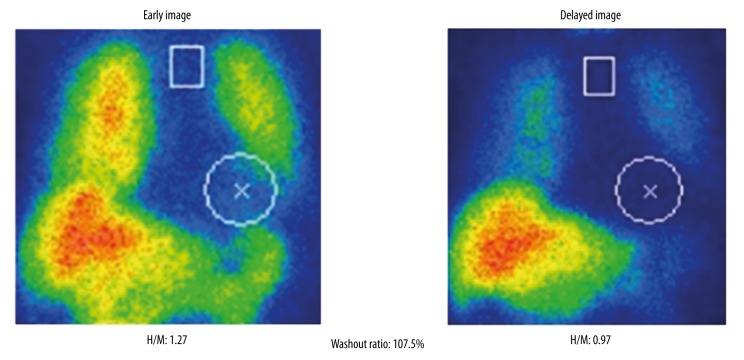
The patient was prescribed 25 mg of the DPP-4 inhibitor sitagliptin to be taken just after she woke. During her subsequent 24-hour ambulatory blood pressure monitoring (Figure 5), it was found that the magnitude of her postprandial hypotension had reduced, especially after breakfast (‒33 mm Hg) and after lunch (‒33 mm Hg). Furthermore, her postprandial blood pressure did not fall below 100 mm Hg, and she did not complain of dizziness. In addition, the patient did not exhibit orthostatic hypotension during the head-up tilt test (Figure 6). In the 75 g oral glucose ingestion test, no blood pressure reduction was seen after the ingestion of glucose (Table 3). In a heart rate variability analysis performed during standing up and sitting down, an improvement in sympathetic nervous system activation; i.e., the change in the LF/HF ratio observed after standing up, was detected (Table 2). However, the H/M ratio detected on MIBG scintigraphy did not change after the patient started taking sitagliptin (Figure 7). After the patient’s postprandial and orthostatic hypotensive episodes ceased, her cognitive impairment; i.e., her MMSE score, improved (22 points to 25 points).
Figure 5.
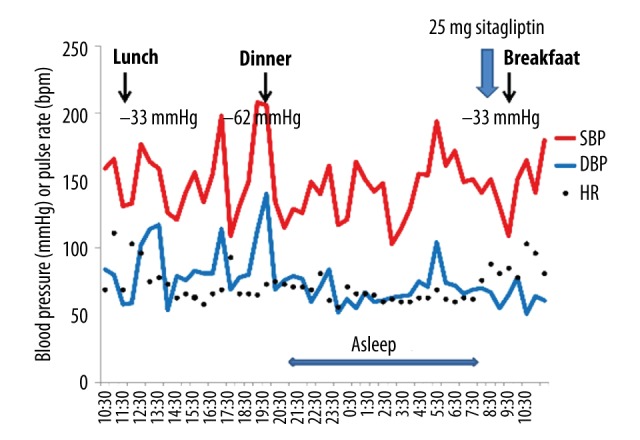
24-hour ambulatory blood pressure monitoring after the administration of sitagliptin. Ambulatory blood pressure monitoring was performed at 15-minute intervals for 24 hours.
Figure 6.
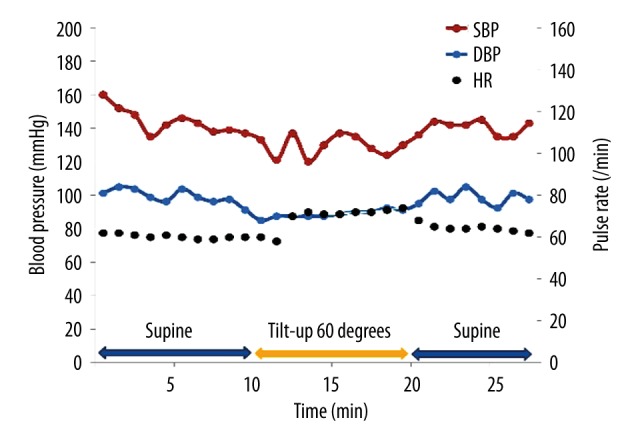
Head-up tilt test results obtained after the administration of sitagliptin. Blood pressure was measured using an automatic cuff-oscillometric device at 5-minute intervals.
Figure 7.
H/M ratio on MIBG scintigraphy after the administration of sitagliptin.
Even after one year of initiation of sitagliptin, the patient did not experience faintness or dizziness after meals. During her 24-hour ambulatory blood pressure monitoring, her postprandial hypotension reduced remarkably (Figure 8). Additionally, the patient did not have any hypoglycemic episodes during the follow-up period.
Figure 8.
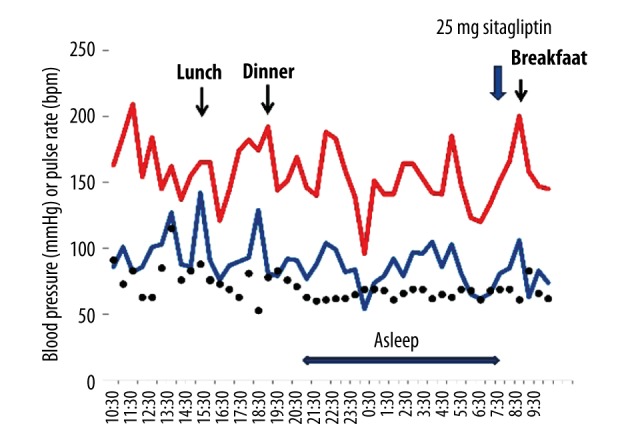
24-hour ambulatory blood pressure monitoring, under the administration of sitagliptin, after one-year follow-up. Ambulatory blood pressure monitoring was performed at 30-minute intervals for 24 hours.
Discussion
We experienced a case in which the patient exhibited postprandial and orthostatic hypotension due to autonomic nervous system dysfunction associated with dementia with Lewy bodies. In this patient, the ingestion of glucose that led to postprandial hypotension was found to play an important role in the mechanism responsible for the postprandial hypotension. The administration of sitagliptin, a DPP-4 inhibitor, reduced the patient’s postprandial hypotension, and moreover, it reduced orthostatic hypotension by increasing sympathetic nervous system activation. The amelioration of the patient’s hypotensive episodes also resulted in an improvement in her cognitive function.
The mechanism responsible for postprandial hypotension is poorly understood. The intake of carbohydrates induces the secretion of insulin and neurotensin, which in turn increase mesenteric arterial flow and portal venous flow, leading to peripheral vascular dilatation. Patients with autonomic nervous system dysfunction display delay compensatory blood pressure rises and are likely to suffer hypotensive episodes. Recently, Vanis et al. [3,4] reported that the administration of saline to the stomach did not induce postprandial hypotension, but the administration of glucose did. These findings showed that the postprandial hypotension induced by glucose was not caused by dilation of the gastroenterological system, which is considered to cause hypotension and dumping syndrome. Glucagon-like peptide-1 (GLP-1) secretion might affect the causative mechanism of postprandial hypotension, as GLP-1 was reported to enhance glucose-dependent insulin secretion and to delay gastric emptying.
In this present case report, the patient’s orthostatic hypotension was reduced, but the mechanism responsible for this was unclear. The reactivity of the patient’s sympathetic nervous system, which was measured as the change in the LF/HF ratio observed after standing up, was improved after the administration of sitagliptin. No previous studies have found that DPP-4 inhibitors attenuate autonomic function, and therefore, we need to clarify the mechanism responsible for this by further study of patients.
In patients with dementia with Lewy bodies, postprandial and orthostatic hypotension contributes to cognitive decline. However, these aspects of the condition might be treatable. It has been reported that excessively low blood pressure in medicated hypertensive patients was associated with cognitive impairment [5]. The administration of α-glucosidase inhibitors or DPP-4 inhibitors could be used to prevent excessive blood pressure reductions in patients with autonomic dysfunction. Additionally, some experimental studies have demonstrated that GLP-1 agonists and DPP-4 inhibitors had direct neuronal effects, leading to improvement of cognitive function [6].
Conclusions
We report on a case of postprandial hypotension and orthostatic hypotension due to autonomic dysfunction in a patient with dementia with Lewy bodies. The administration of the DPP-4 inhibitor sitagliptin improved the patient’s postprandial hypotensive episodes, as well as her episodes of orthostatic hypotension, by modifying the reactivity of her sympathetic nervous system. The patient did not suffer any adverse effects of sitagliptin treatment. The amelioration of the patient’s hypotensive episodes led to an improvement in her cognitive function.
References:
- 1.Shibao C, Gamboa A, Diedrich A, et al. Acarbose, an α-glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension. 2007;50:54–61. doi: 10.1161/HYPERTENSIONAHA.107.091355. [DOI] [PubMed] [Google Scholar]
- 2.Yonenaga A, Ota H, Honda M, et al. Marked improvement of elderly post-prandial hypotension by dipeptidyl peptidase IV inhibitor. Geriatr Gerontol Int. 2013;13:227–29. doi: 10.1111/j.1447-0594.2012.00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanis L, Gentilcore D, Rayner CK, et al. Effects of small intestinal glucose load on blood pressure, splanchnic blood flow, glycemia, and GLP-1 release in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1524–31. doi: 10.1152/ajpregu.00378.2010. [DOI] [PubMed] [Google Scholar]
- 4.Vanis L, Gentilcore D, Lange K, et al. Effects of variations in intragastric volume on blood pressure and splanchnic blood flow during intraduodenal glucose infusion in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2012;302:R391–99. doi: 10.1152/ajpregu.00464.2011. [DOI] [PubMed] [Google Scholar]
- 5.Mossello E, Pieraccioli M, Nesti N, et al. Effects of lowblood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175:578–85. doi: 10.1001/jamainternmed.2014.8164. [DOI] [PubMed] [Google Scholar]
- 6.Groeneveld ON, Kappelle LJ, Biessels GJ. Potentials of incretin-based therapies in dementia and stroke in type 2 diabetes mellitus. J Diabetes Investig. 2016;7:5–16. doi: 10.1111/jdi.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]