Abstract
Animals require molecular signals to determine when to divert resources from somatic functions to reproduction. This decision is vital in animals that reproduce in an all-or-nothing mode, such as bristle worms: females committed to reproduction spend roughly half their body mass for yolk and egg production; following mass spawning, the parents die. An enigmatic brain hormone activity suppresses reproduction. We now identify this hormone as the sesquiterpenoid methylfarnesoate. Methylfarnesoate suppresses transcript levels of the yolk precursor Vitellogenin both in cell culture and in vivo, directly inhibiting a central energy–costly step of reproductive maturation. We reveal that contrary to common assumptions, sesquiterpenoids are ancient animal hormones present in marine and terrestrial lophotrochozoans. In turn, insecticides targeting this pathway suppress vitellogenesis in cultured worm cells. These findings challenge current views of animal hormone evolution, and indicate that non-target species and marine ecosystems are susceptible to commonly used insect larvicides.
DOI: http://dx.doi.org/10.7554/eLife.17126.001
Research Organism: P. dumerilii
eLife digest
All organisms need energy to survive and grow. However, sources of energy are limited and so organisms need to decide how to spend the resources they have available. For instance, animals must choose whether they should continue to grow or if they should invest energy into reproduction instead. This decision becomes even more important for animals that reproduce in an “all-or-nothing” manner and invest all their available energy into reproduction and die soon after.
Bristle worms live in coastal areas around world. In mass spawning events, thousands of individuals raise from the sea floor to the surface, to release sperm and eggs. While the fertilized eggs start to develop in the water, the parents invariably die. The female worms spend roughly half their body mass in producing eggs and supplying them with yolk as a source of energy. It has been known for decades that the brains of bristle worms produce a master hormone that promotes growth and suppresses reproduction. Yet the identity of this hormone that controls the life-or-death decision was not clear.
Schenk et al. took advantage of new molecular tools to solve this puzzle. The experiments show that this hormone directly regulates how much yolk the female animals produce. This allowed Schenk et al. to design a new molecular assay that helped to identify the hormone itself. Unexpectedly, the hormone – called methylfarnesoate – belongs to a family of small molecules called sesquiterpenoids, which researchers previously thought were only found in insects and related groups. Hence, many insecticides have been developed to target sesquiterpenoid signaling and they are used in massive amounts to fight pests like the tiger mosquito (which transmits the Zika virus). Schenk et al. also found that these insecticides also cause severe problems in bristle-worms.
These findings challenge current views of how animal hormones have evolved and indicate that common insecticides may be harming bristle worms and other animals in marine environments. The next steps are to find out whether methylfarnesoate is found in other closely related animals, such as snails and mussels, and whether the insecticides are harmful to these animals too. Another future challenge will be to investigate how this hormone actually promotes animal growth.
Introduction
As animals rely on limited energy resources, they require regulatory mechanisms to decide how to best invest available energy and biomaterial. Some of the most extreme changes of energy expenditure are observed in so-called semelparous animals that reproduce only once and typically die afterwards. In both vertebrate and invertebrate representatives of semelparous animals, the switch to the reproductive state is accompanied by a massive breakdown of somatic tissue, in favor of forming and nourishing germ cells: migrating salmon break down about 50% of their white muscle mass while their gonad mass multiplies around six-fold during maturation (Mommsen, 2004). Likewise, female squids exhibit almost complete histolysis of mantle muscle (Jackson and Mladenov, 1994), and in mature female squids up to ~60% of the total body mass at spawning consists of eggs (Rodrigues et al., 2011). Similarly, in the marine annelid worm Nereis virens, oocytes represent up to about 40% of the body mass of the spawning adult (Hoeger et al., 1999).
Insight into the molecules regulating these extreme shifts of energy expenditure has remained limited (Cardon et al., 1981; Mommsen, 2004; Hébert Chatelain et al., 2008). This can largely be attributed to the lack of an appropriate laboratory model system amenable to molecular research. In this study, we explore the marine annelid Platynereis dumerilii, a semelparous animal that can easily be reared in the laboratory (Hauenschild and Fischer, 1969; Fischer and Dorresteijn, 2004) and has been established as a molecular model system over the last decade (reviewed in Zantke et al., 2014). A series of studies show that Platynereis has retained various ancient-type characteristics (Arendt et al., 2004; Raible et al., 2005; Denes et al., 2007; Tessmar-Raible et al., 2007; Keay and Thornton, 2009; Christodoulou et al., 2010), compatible with the notion that the hormones present in this species are also more broadly comparable with other clades.
In Platynereis, the critical switch to the reproductive life stage has been linked to a hormonal activity released by the medial brain. This brain hormonal activity – also termed nereidin – was found to repress sexual maturation and to promote growth of the trunk and posterior regeneration (Figure 1A): Decerebration of Platynereis leads to an accelerated sexual maturation and to the loss of regenerative abilities, whereas re-implantation of a juvenile head with neuroendocrine activity is able to reverse these effects (Hauenschild, 1956a, 1956b, 1960, 1966). In the related species Nereis diversicolor, it could be shown that implantation of ganglia from immature animals into the coelomic cavity of maturing animals, reversed the effects of the decreasing nereidin concentration. In particular, germ cells were resorbed and the ability to regenerate lost segments was re-established (Golding and Yuwono, 1994). These findings highlight the exceptional importance of neuroendocrine signals in semelparous reproduction and suggest that the underlying molecular mechanisms are conserved across different annelids (Hauenschild, 1956b, 1959; Golding, 1967a, 1967b; Durchon and Porchet, 1970; Hofmann, 1976; Cardon et al., 1981; Golding, 1983; Golding and Yuwono, 1994).
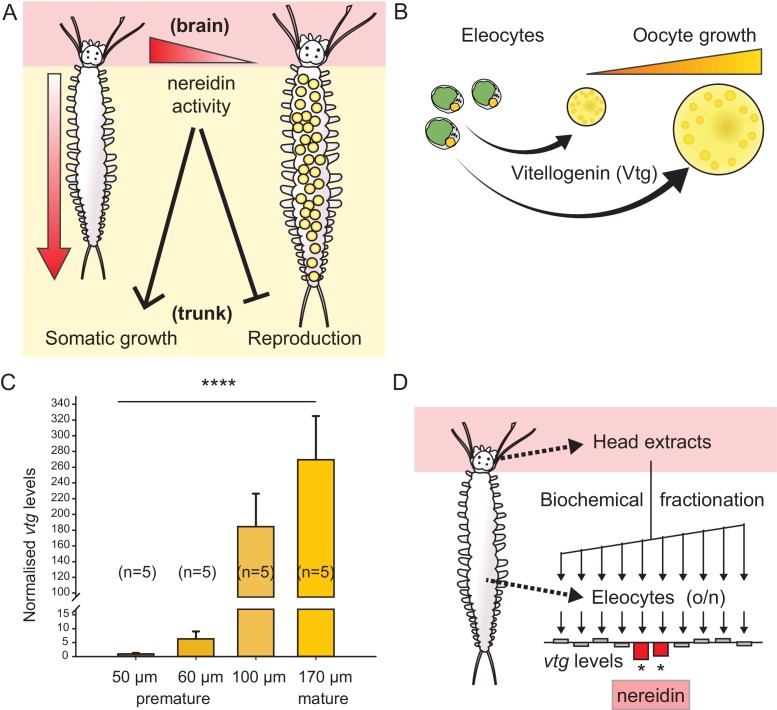
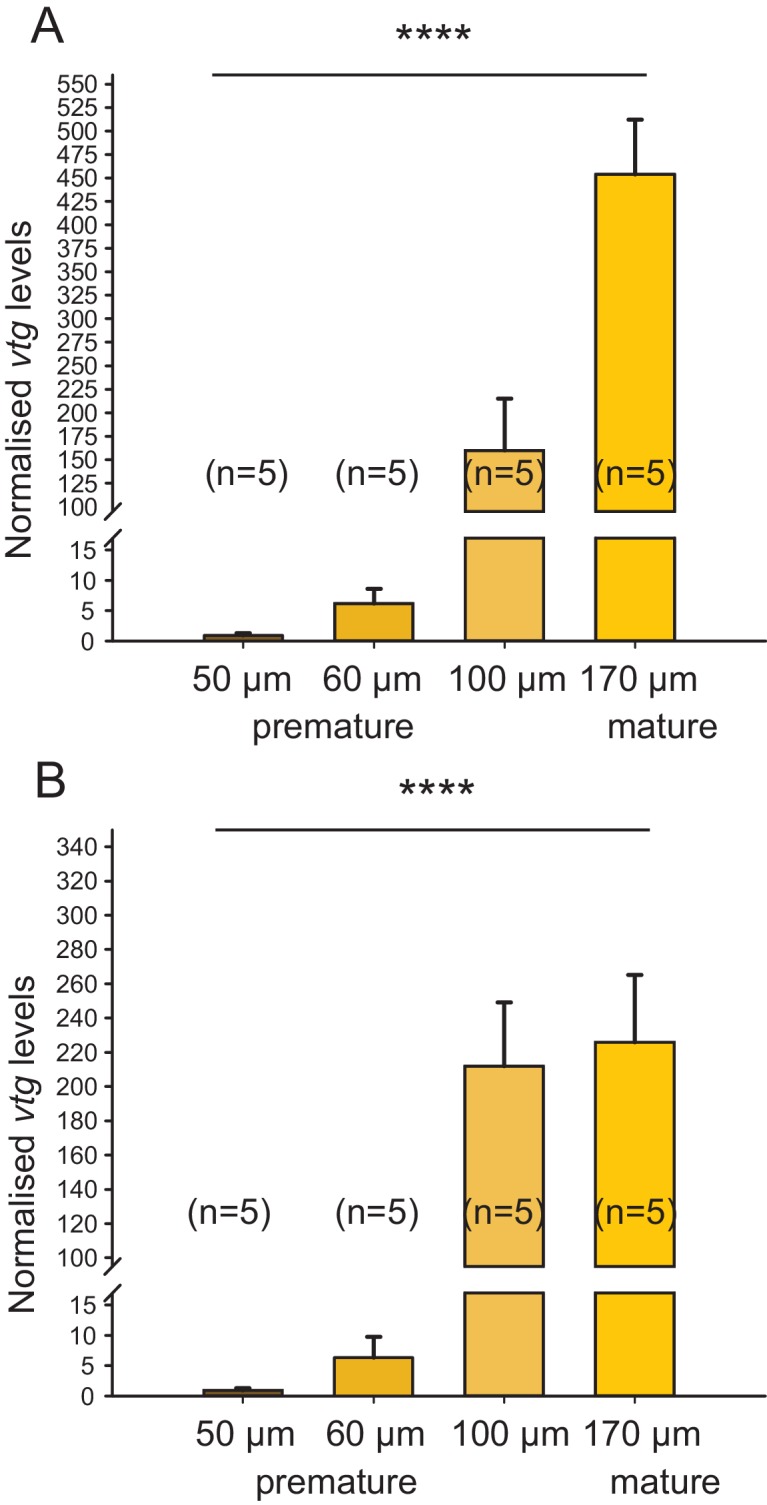
Figure 1. Vitellogenin expression in eleocytes is a quantitative measure for the maturation stage of Platynereis, allowing for the establishment of a bioassay to purify the enigmatic brain hormone, nereidin.
(A) Scheme summarising the critical role of the brain hormone nereidin in energy expenditure, as derived from classical experiments: Before maturation (left), high nereidin levels sustain somatic growth, but repress reproduction; drops in nereidin activity levels initiate the generation of germ cells and sexual maturation (right). (B) Eleocytes in the worm’s coelom have a central role in reproductive commitment, as they synthesise the yolk protein precursor Vitellogenin (Vtg); after release into the coelomic fluid, Vtg is endocytosed by the developing oocytes, leading to oocyte growth. (C) Expression levels of vtg in eleocytes are significantly up-regulated during maturation, confirming vtg as a suitable molecular marker to quantify maturation state. qRT-PCR quantification of vtg levels in eleocytes sampled at different stages of sexual maturation (as assessed by oocyte diameter). The increase in vtg transcripts from premature eleocytes (oocyte diameter: 50 and 60 µm) to those from mature eleocytes (oocytes diameter: 170 µm) is evident; expression levels were calculated against the arithmetic mean of the reference genes cdc5 and rps9, and normalised to the expression of vtg in eleocytes from animals with an oocyte diameter of 50 µm. Statistical significance was tested an one-way ANOVA. ***p<0.001. n: number of biological replicates. (D) Resulting bioassay for the purification of nereidin from head extracts: primary cell cultures were derived from coelomic cells of premature animals; vtg levels were quantified after overnight incubation with different fractions of head extract to determine those fractions containing significant inhibitory activity (nereidin). Data for panel (C) provided in Figure 1—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.17126.003
Figure 1—figure supplement 1. Identification of a Platynereis Vitellogenin orthologue.

Figure 1—figure supplement 2. Irrespective of the chosen reference gene, vitellogenin is regulated over the course of maturation.
Although nereidin activity was recognised as a critical cue more than 60 years ago, the molecular identity of nereidin still remains elusive. However, the results of classical experiments allow for several hypotheses concerning the function and molecular properties of this key brain hormone: (i) When present, nereidin interferes with a critical step required for the animals to enter the cost-intensive process of maturation. (ii) The effective hormone concentration decreases from young animals to mature animals, such that the drop in nereidin concentration allows maturation to proceed (Hauenschild, 1956a, 1956b, 1966; Golding, 1983). (iii) As Nereidin-like activities have been identified in various annelids, this suggests that these substances could be chemically related.
Histological analyses of the proposed biosynthetically active brain region (Müller, 1973; Hofmann, 1976) were compatible with a fourth hypothesis, namely that nereidin is a neuropeptide. Based on this hypothesis, large-scale purifications have been performed from head extracts of N. diversicolor and Perinereis cultrifera (Cardon et al., 1981). These finally resulted in the identification of a hexapeptide (PPGPPG). However, this peptide did not display biological activity (Durchon, 1984).
In this study, we take advantage of a newly developed, quantitative cell culture-based bioassay that measures transcript levels of the main Platynereis yolk precursor Vitellogenin (Vtg). Using this assay, we demonstrate that nereidin activity is not sensitive to proteinase treatment, challenging the long-standing hypothesis that nereidin is a peptide. Instead, we correlate nereidin activity with methylfarnesoate (MF), a substance belonging to the hormone class of sesquiterpenoids that has so far been assumed to be restricted to arthropods. We demonstrate that the concentration of MF drops in the course of maturation – as predicted from classical nereidin activity assays – and that eleocytes express the orthologue of a sesquiterpenoid receptor. This, together with our finding that MF exerts its action on vitellogenin production not only in cell culture, but also in vivo, establishes MF as a brain hormone orchestrating a critical metabolic switch in P. dumerilii. We finally show that insecticides targeting the sesquiterpenoid pathway interfere with Platynereis vitellogenesis, revealing a profound impact of insect growth regulators on a presumed non-target organism that serves as a key marine reference species.
Results
A fast and sensitive assay to assess maturation in Platynereis
One of the major challenges faced by previous attempts to identify the brain hormone activity was the absence of a fast and sensitive bioassay to measure the inhibitory effect of nereidin on sexual maturation. Fractionated head extracts could be shown to interfere with the transition of cultured spermatogonia to spermatozoa (Cardon, 1970; Durchon and Porchet, 1970; Cardon et al., 1981). However, the respective assay required more than a week of culturing and substantial input material (thousands of sampled heads). Using this qualitative assay, and systematic fractionating of head extracts, the brain hormone activity could be associated with a small (MR ≤ 2.000), lipophilic molecule (Cardon, 1970; Durchon and Porchet, 1970; Cardon et al., 1981).
In order to establish a sensitive read-out for Platynereis maturation, we decided to focus on the yolk protein precursor Vtg. Yolk production underlies the significant increase in oocyte diameter observed in nereidids during maturation (Fischer, 1984; Fischer and Hoeger, 1993). Yolk protein is derived by endocytotic uptake of Vtg into the oocyte (Fischer et al., 1991), where it accounts for more than 40% of protein (Fischer and Schmitz, 1981), reflecting its central role as reproductive investment. Vtg is synthesised in coelomic cells called eleocytes (Figure 1B) that are present in large numbers (Baert and Slomianny, 1987; Hoeger, 1991). Eleocytes and oocytes are freely floating in the coelomic cavity and can therefore easily be isolated and taken into primary (co)-culture, making them an excellent experimental model system to assess and monitor maturation in Platynereis and other nereidids, and to investigate the concomitant metabolic changes associated with this process.
Based on the central role of eleocytes and Vtg for oocyte growth, we hypothesised that the expression level of vtg in eleocytes might be a suitable molecular read-out for maturation. As a first step, we identified a Platynereis vtg candidate in eleocyte transcriptome data (Sven Schenk, Fritz Sedlazeck, Florian Raible, unpublished). Domain analyses and molecular phylogeny indicate that the protein encoded by this gene is an orthologue of Vtg identified in other animals (insects, molluscs, amphibians and birds; Figure 1—figure supplement 1). Next, we designed qRT-PCR primers to test if vtg transcript levels in P. dumerilii eleocytes increased over female maturation, as suggested by the increase of Vtg protein secretion in P. cultrifera (Baert and Slomianny, 1992). Indeed, we found a strong correlation between vtg-transcript abundance in eleocytes and the maturation state of the animal (as assessed by oocyte diameter, Figure 1C and Figure 1—figure supplement 2), indicating that vtg transcript levels could be used for a quantitative maturation assay. Given the central metabolic role of eleocytes in the reproductive switch, we further hypothesised that nereidin acts directly on eleocytes to regulate vtg production. We therefore designed a bioassay that takes coelomic cells into primary cell culture. After adding fractions of our purification scheme to small aliquots of this culture, and incubating these overnight, we extracted RNA from the cells and analysed them by qRT-PCR to determine the levels of vtg (Figure 1D).
Methylfarnesoate is the active component of the brain hormone and acts at physiological concentrations
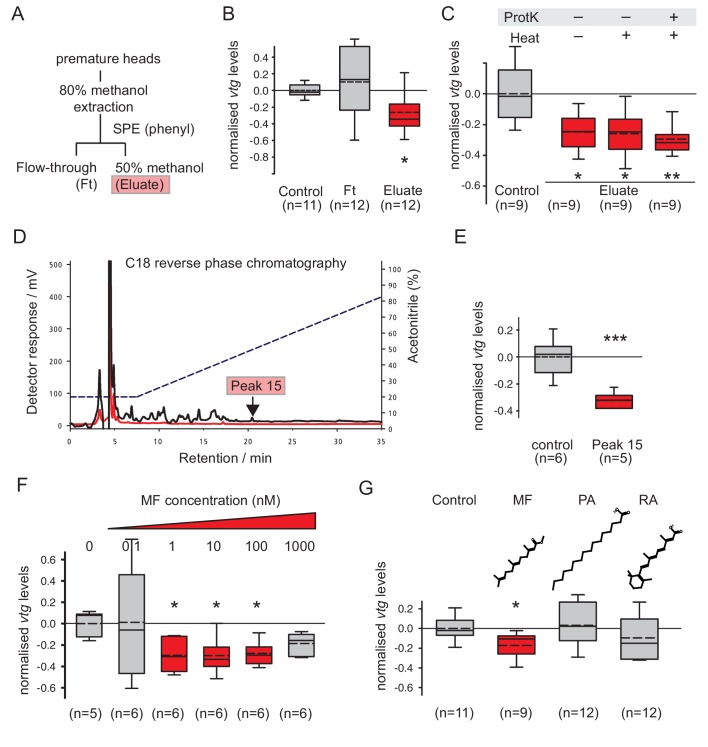
After establishing this bioassay, we used it to chemically isolate the brain hormone-activity of Platynereis. Similar to the method of Cardon (1970), a methanolic extract was prepared from 50 heads of premature animals, i.e. animals becoming sexually distinct, but before the irreversible onset of maturation. After solvent evaporation, the material was reconstituted in an aqueous phase and further fractionated via phenyl-solid phase extraction (SPE, Figure 2A). Consistent with previous characterisations of nereidin, the 50% methanol eluate of this column showed clear activity (Figure 2B) when added to coelomocytes in vitro.
Figure 2. Methylfarnesoate, not protein, constitutes the main nereidin/brain hormone activity.
(A–C) Nereidin activity elutes in lipophilic fractions of head extracts but is not proteinaceous as previously hypothesised. (A) Schematised fractionation of premature Platynereis head extracts, expected to recover the described nereidin activity in the methanolic eluate of SPE. (B) Relative coelomocyte vtg levels after overnight incubation with flowthrough or eluate of (a), normalised against control samples. (C) Insensitivity of nereidin activity against proteinase and heat treatment. Experiment as in (b), with pretreatment of eluate by proteinase K (‘ProtK’) and/or subsequent heat treatment (5’, 95°C; ‘Heat’). Results indicate that the repressive effect of nereidin on eleocyte vtg levels is neither sensitive to proteinase nor heat. (D–G) Identification of methylfarnesoate as a major component of nereidin. (D) Further fractioning of the brain hormone activity (eluate from b) by C18-reverse phase column chromatography. Absorption of eluent at 280 nm (red line) and 215 nm (black line) during application of acetonitrile gradient (dashed line, right ordinate). Peak 15 (marked red), containing the nereidin activity, elutes at a retention time of 20.5 min. (E) Peak 15, containing methylfarnesoate (MF), significantly suppresses vtg levels in the coelomocyte bioassay when compared to the controls. (F) Authentic MF recapitulates the observed effect at physiological concentration ranges; relative vtg levels in coelomocytes treated with 0.1 nM – 1000 nM MF in 0.01% DMSO, normalised to the control group of DMSO-treated cells. (G) Specificity of the repressive effect caused by MF; comparison of coelomocyte vtg levels after incubation in 10 nM MF, palmitic acid (PA) or retinoic acid (RA). Graphs in (B,C,E,F,G) show qRT-PCR quantification of vtg levels after overnight (20 hr) treatment of primary coelomocyte cultures. Expressions values were calculated with respect to the reference gene rps9, and normalised to the levels of controls (untreated cells). Boxplots show the first and third quartile, the median (solid line), and the mean (dashed line). Whiskers denote the 10th and 90th percentile. Statistical significance was tested by a one-sided t-test of the treatment group against the control, or, in case of multiple comparisons, first with an ANOVA, followed by a one-sided t-test with adjustment of the p-values for multiple testing to assess differences between the different groups. *p<0.05. **p<0.01, ***p<0.001. n: number of biological replicates. Raw data for panels B,C,E,F,G provided in Figure 2—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.17126.008
Figure 2—figure supplement 1. Identification of MF as the principal component in peak 15.
Figure 2—figure supplement 2. Stability of the reference gene rps9 in coelomocyte culture.
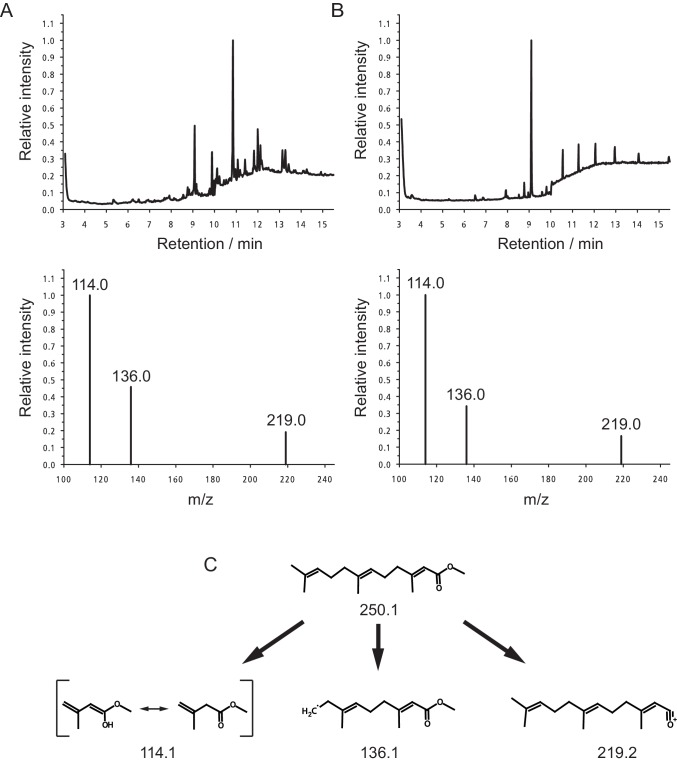
As outlined, it has long been speculated that nereidin is a peptide. This assumption was mainly based on the results of partial characterisation (Durchon, 1984), as well as histological observations, by which the brain regions producing nereidin were shown to contain highly active secretory cells (Müller, 1973; Hofmann, 1976). To assess whether the recovered nereidin activity was of proteinaceous nature, we treated the obtained eluate with proteinase K, and tested if this affected the ability of the eluate to repress vtg levels in cell culture. Our results demonstrate that nereidin is insensitive to proteinase K-treatment, thus strongly arguing against the notion that it is a neuropeptide hormone (Figure 2C).
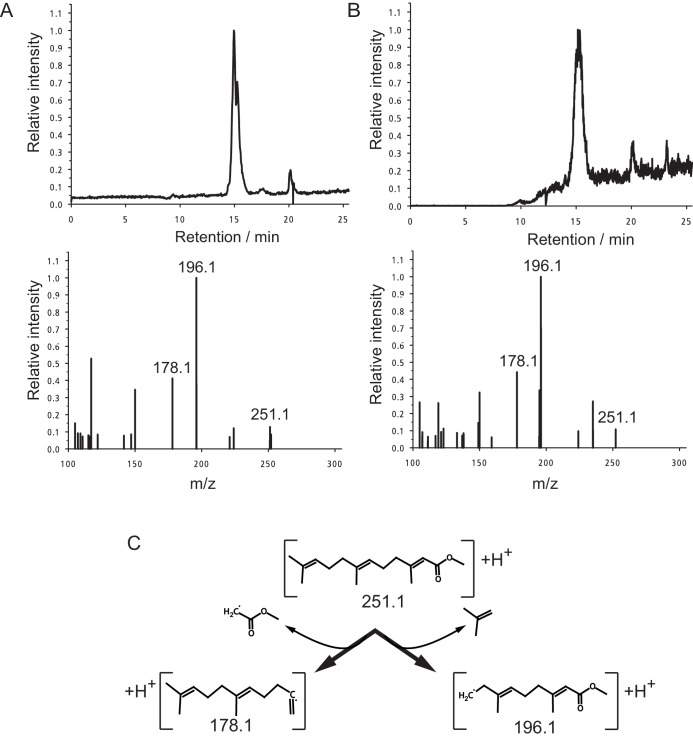
To further characterise the recovered eluate, 250 premature worm heads were extracted and fractionated on a Phenyl-SPE-column. The recovered active fraction was then further fractionated by HPLC on a C18-column (Figure 2D). A total of 16 fractions eluting from the column were collected and tested in the cell culture-based bioassay for their impact on vtg transcript levels. One peak (peak 15) could be identified that robustly and significantly reduced vtg transcripts in two independent experiments and showed a reasonable chromatographic purity (Figure 2D,E). Analysis of peak 15 by LC-MS/MS clearly demonstrated the presence of the sesquiterpenoid methylfarnesoate (MF), when compared to an authentic standard run under the same conditions (Figure 2—figure supplement 1).
To test if MF was indeed the active compound in peak 15, we tested the effect of authentic MF on vtg transcript levels in the established cell culture assay. The results showed a clear down-regulation of vtg transcript levels (Figure 2F). Strongest effects were observed in the range between 1 and 100 nM MF, in agreement with physiological concentrations expected for a hormone. To ascertain the specificity of MF in down regulation of vtg transcripts in vitro, coelomic cells were also challenged with structurally related compounds such as palmitic acid (PA) and all-trans-retinoic acid (RA). As evident from Figure 2G, neither of these molecules showed a significant effect on vitellogenesis, thus establishing the specificity of MF on the observed reduction of vtg transcript levels.
MF concentration drops in the course of maturation
Based on the classical brain transplantation experiments, it was hypothesised that nereidin is present at high levels in young animals, whereas its concentration declines during maturation (Hauenschild, 1956a, 1956b, 1966; Durchon and Porchet, 1970; Golding, 1983). To test whether MF levels decrease over the course of maturation, we next set up a gas chromatography mass spectrometry (GC-MS)-based quantification assay.
Our extraction method followed the method described by Westerlund and Hoffmann (2004), where a methanolic extract is used in combination with a phase partition against an organic solvent to simultaneously denature degrading enzymes and to extract MF. However, as we used heads to extract MF rather than haemolymph, we used in a first step 80% methanol (as in our initial extraction protocol, see above) followed by liquid-liquid extraction into heptane, instead of directly diluting the sample with the methanolic-organic extraction solution (Westerlund and Hoffmann, 2004). The extraction efficiency of our method was determined to be >85%. With an estimated limit of detection of ~2.5 pg MF and a limit of quantification of ~15 pg MF, our assay is comparable to those used by other authors for the identification of MF and related compounds (Westerlund and Hoffmann, 2004; Teal et al., 2014).
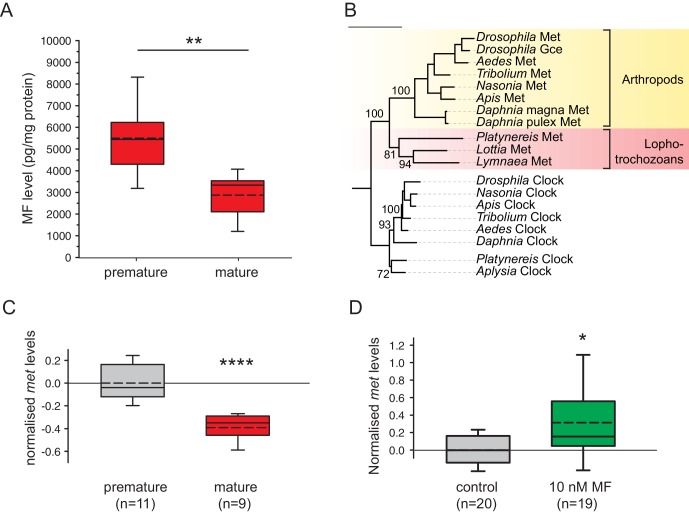
This assay allowed us to unequivocally identify MF in Platynereis heads (Figure 3—figure supplement 1) and to quantify the amount of MF by injecting 2.5 head equivalents into the GC-MS. Measuring MF levels present in heads of different developmental stages showed differences in MF content (Figure 3A). Heads of premature animals were determined to possess ~5510 ± 660 pg MF/mg protein (mean±standard error of the mean), whereas concentrations in the heads of mature animals were found to be significantly lower (~2870 ± 495 pg MF/mg protein). Calculated per head, the MF content is equivalent to ~34.6 ± 8.0 pg/head for premature heads and ~15.4 ± 2.5 pg/head for mature animals (Figure 3—figure supplement 2). Our results are therefore in agreement with the proposed decline of brain hormone concentrations during maturation.
Figure 3. Levels of MF as well as the sesquiterpenoid receptor orthologue Met drop over the course of maturation.
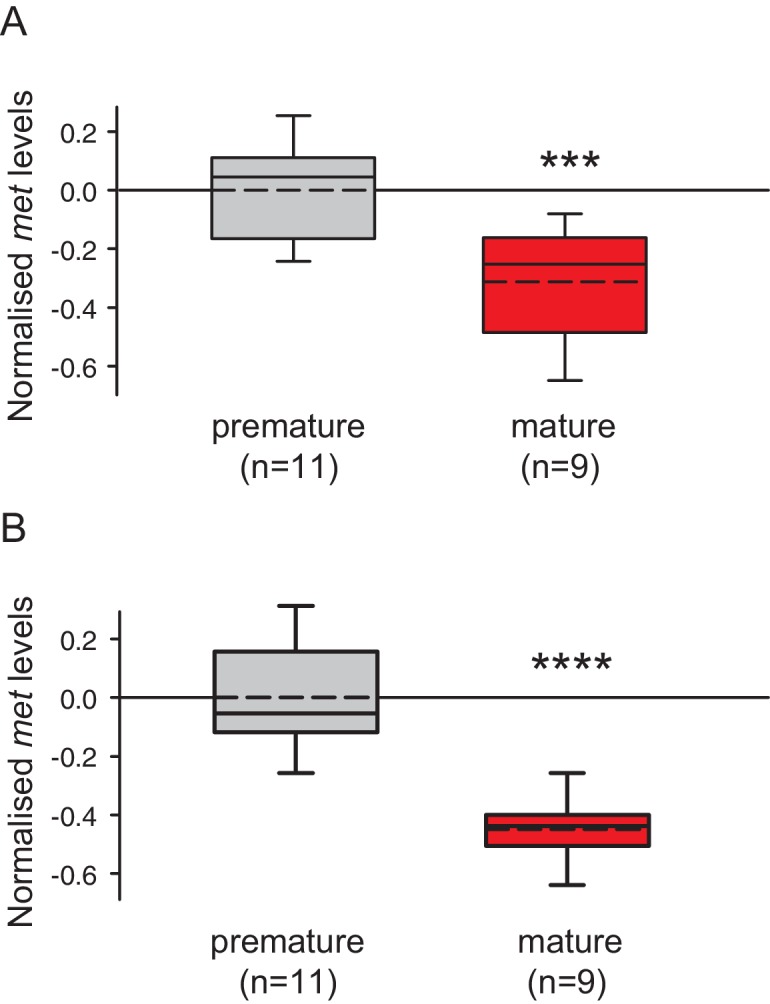
(A) Significant decrease of MF levels between premature and mature heads; boxplots show amounts of MF normalised to the total protein content of the respective head sample. Heads of premature animals contain ~5510 ± 660 (S.E.M) pg MF per mg protein, whereas heads from mature animals contain ~2870 ± 495 pg MF per mg protein, thus roughly 50% of the amount found in heads of premature animals. Amounts are corrected for a MF recovery rate of 85%. The boxes show the first and third quartile, the median (solid line), and the mean (dashed line). Whiskers denote the 10th and 90th percentile. Significance was tested with a one-sided t-test, **p<0.01. n: number of biological replicates. (B–D) Platynereis possesses an orthologue of arthropod sesquiterpenoid receptors that shows co-regulation with MF titres. (B) Maximum Likelihood tree supporting the identity of Platynereis Met as orthologue of the validated arthropod sesquiterpenoid receptors Methoprene-tolerant (Met) and Germ cells expressed (Gce). Phylogeny reconstructed with IQ-TREE 1.3.12 (Minh et al., 2013; Nguyen et al., 2015). By parameter optimisation, the LG+F+I+G4 substitution model was selected. Numbers refer to confidence levels (in %) of major nodes derived from 1000 replicates. Accessions: Drosophila Met: NP_511126.2; Drosophila Gce: NP_511160.2; Aedes Met: AAW82472.1; Nasonia Met: XP_001606775.2; Apis Met: XP_395005.4; Tribolium Met: NP_001092812.1; Daphnia pulex Met: BAM83853.1; Daphnia magna Met: BAM83855.1; Platynereis Met: KU756288 (this study); Lottia Met: XP_009046608.1, Lymnaea Met: FX197139.1. Drosophila Clock: NP_523964.2; Nasonia Clock: XP_008214438.1; Apis Clock: XP_394233.1; Aedes Clock: XP_001662706.1; Tribolium Clock: NP_001106937.1; Daphnia Clock: EFX7997.1; Platynereis Clock: AGX93013.1; Aplysia Clock: XP_005112430.1. (C) Eleocyte met levels drop by ~39% in the course of maturation. qRT-PCR analysis of met transcript levels in eleocytes sampled from premature and mature animals. Relative met levels were calculated with respect to the arithmetical mean of the reference genes sams and cim6pr, and normalised to the level of the premature sample. (D) Eleocyte met levels are increased by the addition of MF. Similar analysis as in (C), indicating that met levels in MF-treated cultured eleocytes (right) are ~30% higher than in untreated controls (left). Relative met levels were calculated with respect to the reference gene rps9, and normalised to the level of the control sample. Boxplots (C,D) show the first and third quartile, the median (solid line), and the mean (dashed line). Whiskers denote the 10th and 90th percentile. Statistical significance in (B,C) was determined by a two-sided t-test.*: p<0.05. ****p<0.0001. n: number of biological replicates. Raw data for panels A,C,D provided in Figure 3—source data 1. Alignment for panel B provided as Figure 3—source data 2.
DOI: http://dx.doi.org/10.7554/eLife.17126.012
Figure 3—figure supplement 1. Quantification of MF in the heads of Platynereis by GC-MS.
Figure 3—figure supplement 2. The levels of MF per head drop over the course of maturation.
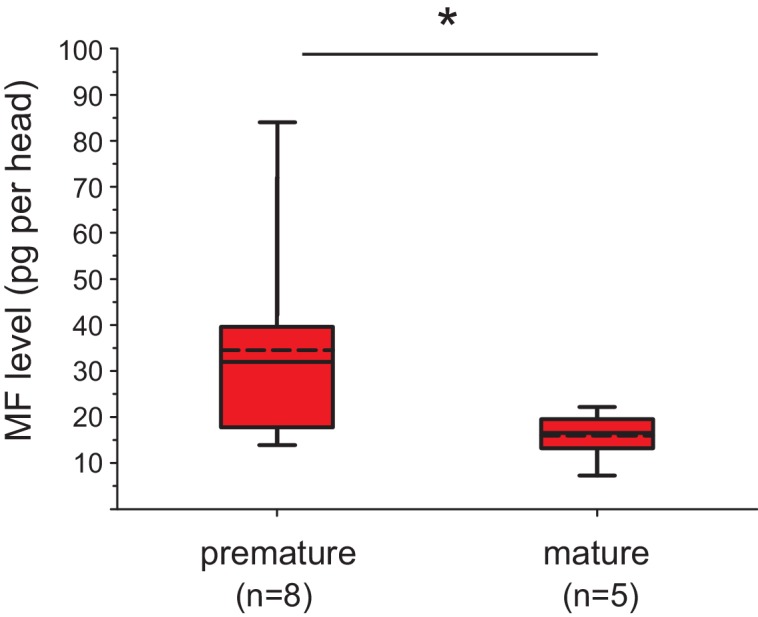
Figure 3—figure supplement 3. Methylfarnesoate is found in the heads of earthworms.
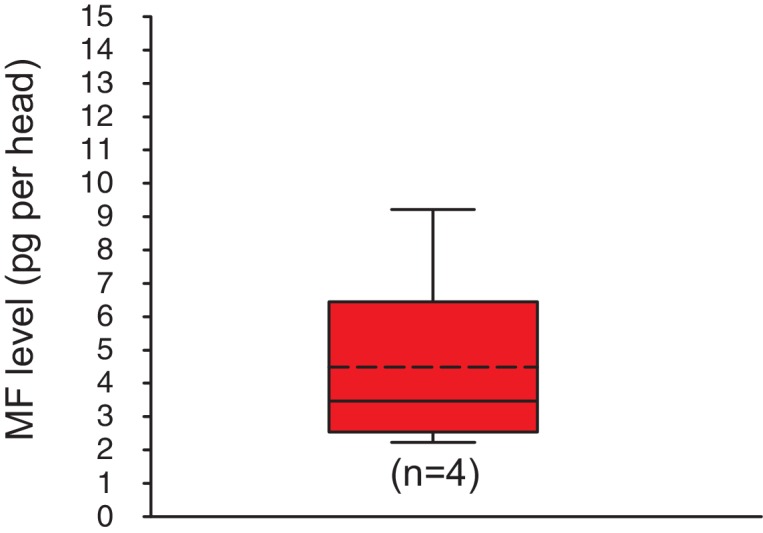
Figure 3—figure supplement 4. The choice of reference genes does not impact on the observed down-regulation of met.
In summary, the results of our bioassay and purification scheme demonstrate that the brain hormone-activity present in the heads of P. dumerilii is not a peptide. Instead, we clearly identify MF as the major molecule in Platynereis heads that is able to suppress transcription of vtg in coelomocyte cultures, when added at physiological concentrations. Furthermore, MF concentration drops in the transition from the premature animal to a mature stage, in agreement with classical predictions on the regulation of nereidin.
Platynereis eleocytes possess an orthologue of validated arthropod sesquiterpenoid hormone receptors
The occurrence of MF in the annelid Platynereis is counterintuitive, because sesquiterpenoid hormones have commonly been proposed to be restricted to arthropods, possibly as a secondary consequence of the reduction of a parallel biosynthetic pathway leading to the formation of sterols in this animal group (Bellés et al., 2005; Jindra et al., 2015). To determine whether the Platynereis MF system originated independently, or relies on common descent with arthropod sesquiterpenoid signalling systems, we investigated if Platynereis also possesses an orthologue of arthropod sesquiterpenoid receptors. Based on genetic screens in Drosophila, and subsequent biochemical and bioinformatics studies, homologues of the bHLH-PAS-domain-containing transcription factor Methoprene-tolerant (Met) have been identified as functional arthropod sesquiterpenoid receptors (Miyakawa et al., 2013; Jindra et al., 2015).
In transcriptome data available from Platynereis eleocytes, we identified a transcript encoding a full-length bHLH-PAS-domain-containing transcription factor that showed strong similarity to insect Methoprene-tolerant receptors. Molecular phylogeny revealed this protein to be a clear orthologue of the insect Methoprene-tolerant and germ cell expressed (Gce) receptors, as well as the functionally confirmed Met receptors from the crustacean Daphnia. We therefore named the corresponding Platynereis gene methoprene-tolerant (met). The phylogenetic analysis also revealed Met orthologues in the molluscs Lottia gigantea and Lymnaea stagnalis (Figure 3B).
Using qRT-PCR analysis, we could confirm the expression of Platynereis met in eleocytes. Furthermore, these analyses revealed that the expression level of this gene dropped significantly during maturation, reaching only ~ 61% of the level observed in cells from premature animals (Figure 3C and Figure 3—figure supplement 4). This parallels the observed decline in MF concentration in the head measured by GC-MS (Figure 3A). Furthermore, treatment of cultured premature eleocytes with 10 nM MF induced the expression of Platynereis met, resulting in a 30% up-regulation (Figure 3D). Together, these data confirm the expression of met in eleocytes by an independent method and are also consistent with a positive autoregulation of this putative receptor by MF.
Methylfarnesoate exerts a dominant repressive effect on vitellogenesis in vivo, and worms are susceptible to insecticides targeting the pathway
In vivo, eleocytes are freely floating in the coelomic cavity, compatible with the notion that they are accessible to hormonal factors like MF. However, coelomic fluid also represents a major metabolic compartment, where nutrients and metabolites are exchanged (Fischer, 1979; Baert and Slomianny, 1987; Fischer and Hoeger, 1993; Hoeger and Kunz 1993; Hoeger et al., 1999). This raises the possibility that other factors in the coelomic fluid could modulate or even block the effect of MF that we observe in isolated cell culture and prompted us to investigate the effect of MF on eleocytes in their natural context.
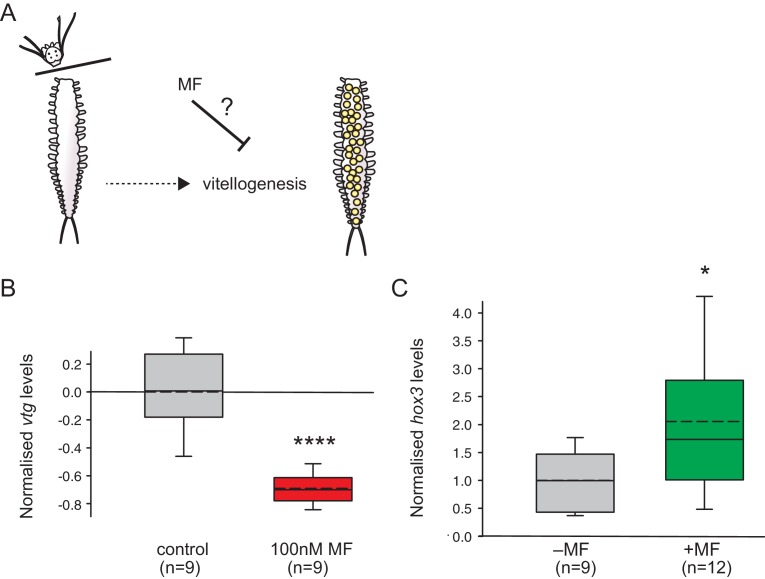
To reliably compare different animals, we used a classical decapitation paradigm. Decapitation leads to accelerated maturation, and, importantly, synchronisation of maturation across different individuals (Hauenschild, 1966, 1974). We then quantified the maturation stage of MF-treated and DMSO-treated animals five days after decapitation, taking advantage of our established vtg qRT-PCR assay (Figure 4A and Figure 4—figure supplement 1).
Figure 4. MF also represses vtg expression in vivo, and sustains expression of a marker for caudal growth upon posterior amputation.
(A) Setup of experiment testing the ability of MF to interfere with vitellogenesis in vivo. Following decapitation, female individuals are known to start vitellogenesis, reflecting loss of nereidin; treatment with MF tests if the repressive effect of nereidin on vtg observed in primary cultures of coelomic cells also occurs in the normal context of these cells. (B) qRT-PCR analysis of vtg expression in decapitated animals 5 days after decapitation. The animals were either treated with 0.1% DMSO (control) or in 100 nM MF in 0.1% DMSO for five days. Expression levels of vtg upon presence of MF are ~70% lower than in treated animals. vtg levels were related to the arithmetical mean of the reference genes rps9 and sams and normalised to the vtg-expression level of the control. (C) MF sustains expression of hox3, a marker diagnostic for caudal regeneration. qRT-PCR analysis of hox3 expression in Platynereis fragments 5 days after posterior amputation. Headless worm fragments treated with 100 nM MF in 0.1% DMSO (right) show twice as high levels of hox3 than headless control fragments (left) only treated with 0.1% DMSO. Hox3-expression is relative to that of the arithmetical mean of the reference genes rps9 and sams and normalised to the hox3-expression level of the untreated samples. (B,C) Boxplots show the first and third quartile, the median (solid line), and the mean (dashed line). Whiskers denote the 10th and 90th percentile. Statistical significance was tested by a one-sided (B) and a two-sided t-test (C), respectively. *p<0.05. ****p<0.0001. n: number of biological replicates. Raw data for panels B,C provided in Figure 4—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.17126.019
Figure 4—figure supplement 1. The choice of reference genes does not impact on the observed down-regulation of vtg.
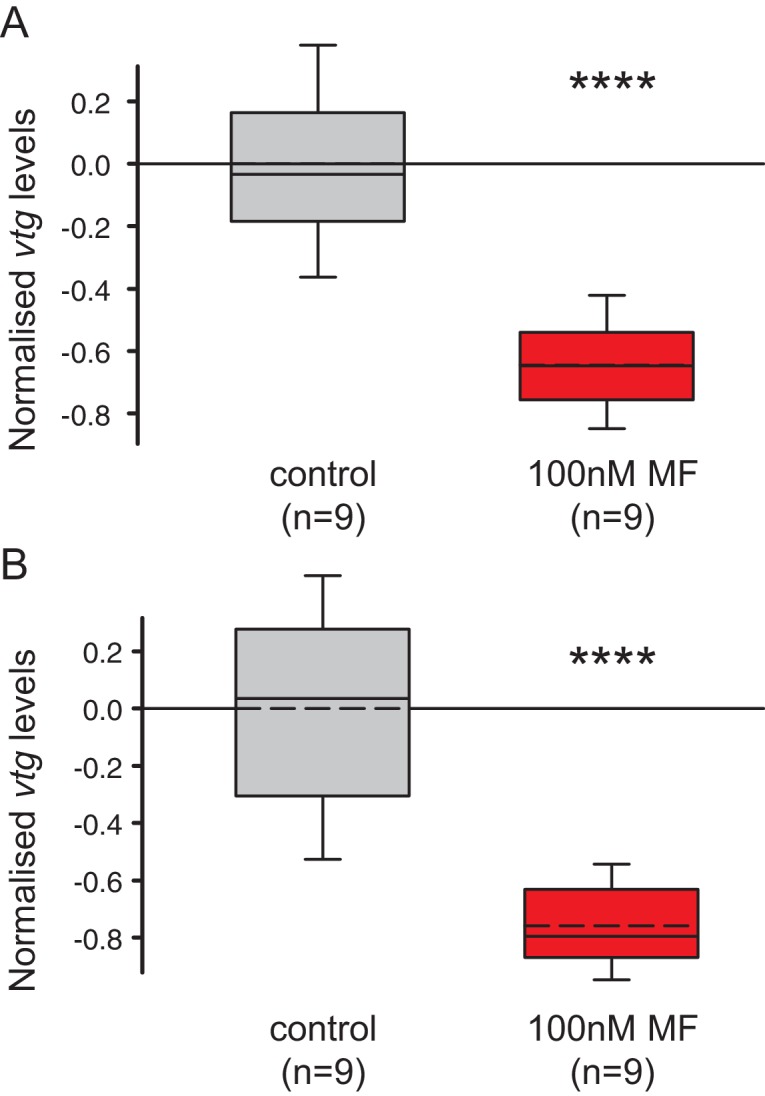
The results of these experiments showed that vtg levels were strongly down-regulated in MF-treated worms when compared to a non-treated control group (Figure 4B). This establishes that MF does not only have an effect on isolated coelomocytes, but also exerts a similar effect in vivo, in spite of other physiological regulators that might be present in the coelomic fluid. Notably, our data are also reminiscent of classical decerebration and re-transplantation experiments that showed that implanted brains could reverse the accelerated maturation observed after decerebration (Hauenschild, 1956a, 1956b, 1966; Golding, 1983). This implicates MF as a major hormonal cue responsible for the anti-maturation effect of the juvenile brain.
The sesquiterpenoid pathway is a prime target for a large variety of insecticides. After observing the effect of MF on annelid reproduction both in vivo and in vitro, we tested whether the juvenile hormone analogue methoprene is also capable of influencing vitellogenesis in Platynereis. We treated decapitated animals as before and assessed changes in vtg levels. In this experimental setup, we did not observe a significant decrease in vtg levels. In contrast, applying methoprene to eleocytes in our cell culture assay clearly led to reduced vtg levels at a concentration of 10 nM (Figure 5). To assess if this effect was specific to methoprene, we also tested the structurally unrelated, yet highly used insecticide pyriproxyfen for its ability to interfere with endogenous vtg transcript levels. Indeed, when applying pyriproxyfen at the same concentration (10 nM), we again observed a significant decrease in the abundance of vtg transcripts when compared to controls. This indicates a profound effect of presumed insect-specific growth regulators on a non-target species.
Figure 5. Vitellogenin expression in worm coelomocytes is suppressed by the hormone agonists methoprene and pyriproxyfen that are considered to act exclusively on insects.
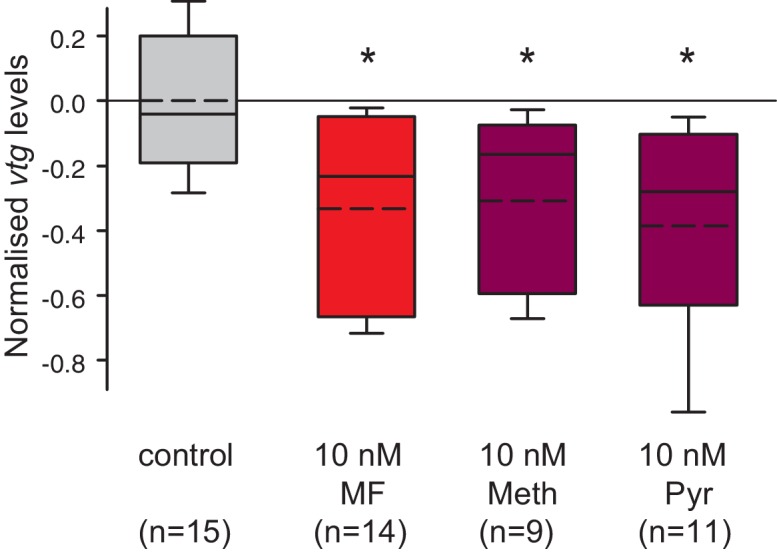
Expression levels of vtg in coelomocytes are significantly down-regulated by treatment with 10 nM methoprene (Meth) and 10 nM pyriproxyfen (Pyr) in 0.01% DMSO. The graph shows qRT-PCR quantification of vtg levels after over night (20 hr) treatment of primary coelomocyte cultures; expression levels are relative to those of the reference gene rps9 and are normalised to the vtg-expression level of the control (DMSO-treated cells). The Boxplots show the first and third quartile, the median (solid line), and the mean (dashed line). Whiskers denote the 10th and 90th percentile. Statistical significance was tested first by an ANOVA, followed by a two-sided t-test with adjustment of the p-values for multiple testing to assess differences between the different groups. *p<0.05. n: number of biological replicates. Raw data provided in Figure 5—source data 1.
DOI: http://dx.doi.org/10.7554/eLife.17126.022
Methylfarnesoate sustains expression of a marker diagnostic for caudal re-growth
In addition to its central role in suppressing maturation, nereidin has also been classically defined by the ability of transplanted brain pieces to maintain caudal regenerative capacity (Hauenschild, 1974; Hofmann, 1976). Both functions have been used interchangeably in nereidin research. However, the lack of molecular candidates for nereidin has prevented the assessment of whether both functions rely on identical molecules, or if they can be separated. In a final set of experiments, we therefore tested if MF had any impact on regeneration of caudal body segments. To minimise inter-individual variations in regenerative capacities (Golding, 1967b), we followed a similar approach as Hauenschild (1974) in which hormone-exposed and control fragments were derived from the same animal. In addition, as in our maturation assays, we aimed to develop a quantitative approach to assess the molecular effect of MF on caudal regeneration. For this, we established a qRT-PCR assay quantifying the expression of the homeobox gene hox3. Hox3 expression has earlier been demonstrated to be a highly specific early on marker in caudal regeneration (Pfeifer et al., 2012). More specifically, it has recently been linked to ectodermal cells of likely stem cell character (ectoteleoblasts, Gazave et al., 2013) involved in the regenerative process. Our results show that the expression of hox3 is significantly up-regulated (~2 fold) after 5 days in test fragments treated with 100 nM MF when compared to DMSO-treated control fragments (Figure 4C). This finding is consistent with the idea that besides its role in repressing maturation, MF may also contributes to sustaining putative stem cells involved in regeneration.
Discussion
Together, our results establish methylfarnesoate as a critical neurohormone suppressing maturation in a key lophotrochozoan reference species. Our data are fully consistent with properties classically attributed to nereidin: in light of the massive amounts of Vitellogenin that are produced by animals committed to reproduction, the ability of MF to suppress vtg levels is compatible with a central role of this compound in controlling reproduction-associated energy expenditure, as expected for nereidin. Likewise, our quantifications of MF levels over the course of reproductive development (Figure 3A) are consistent with the decline in nereidin activity present in transplanted heads of different stages. Although our data clearly argue against the long-held assumption that nereidin is a neuropeptide, they are still consistent with the chemical properties (apparent mass, lipophilic nature) that have been derived from previous purification attempts. Likewise, the data are consistent with the notion that related hormones are present in other annelids (see below).
Our findings not only reveal an overall role of this sesquiterpenoid in governing energy expenditure but also highlight critical mechanistic aspects: (i) We show that eleocytes are direct target cells for MF, indicating a direct cross-talk between the brain and a highly relevant metabolic cell type. (ii) Moreover, the observed effect of MF on vitellogenin levels is consistent with a direct function of MF in controlling this key factor for oocyte maturation. (iii) The positive autoregulation of the sesquiterpenoid receptor orthologue Met that we observe is reminiscent of other hormone ligand-receptor systems, such as thyroxin or ecdysone (Thummel, 1995; Bagamasbad and Denver, 2011). In the biological context of semelparous reproduction, such autoregulation provides an attractive model how graded changes in MF levels can be amplified, contributing to sharpening the response of the target cells to above- vs. below-threshold levels.
Future work on the fine-tuning of MF regulation, and its effect on target cells, will likely yield more detailed insight into the role of MF for eleocyte physiology. Such analyses will extend our understanding of molecular changes in the eleocyte, for instance to cover genes involved in nucleotide metabolism (Hoeger et al., 1999). Moreover, they will resolve the question if residual levels of MF signalling are required for proper vitellogenesis, or if vitellogenesis only takes place in complete absence of MF. This addresses classical debates about the requirement of nereidin (Hauenschild, 1966; Durchon and Porchet, 1970; Golding, 1983) but is also interesting for cross-comparisons between the annelid system and insects, where sesquiterpenoids do not only define juvenile stages – similar to Platynereis – but in later life play a – positive – role for vitellogenesis itself.
A second phenomenon where the identification of MF provides important mechanistic insight, and opens up new experimental possibilities concerns the question of how regenerative abilities of Platynereis and other species are controlled. From a global perspective of organismal energy expenditure, it is plausible that semelparous reproduction (investment in growth of germ cells) and regeneration (investment in re-growth of soma) are anti-correlated, although it remains unclear exactly how this is achieved at the molecular level. Our finding that MF sustains expression of a marker (hox3) characteristic for a population of putative stem cells in posterior segment addition (Pfeifer et al., 2012; Gazave et al., 2013) is consistent with the idea that MF also plays a role in regenerative control, but in contrast to vtg regulation in eleocytes, it is less clear if this effect is direct or caused by other mediating factors. Due to the complexity of regeneration, reliable and quantitative short-term assays for regeneration will need to be established to pursue this question.
The identification of MF in Platynereis heads challenges several hypotheses: most directly, it defeats the hypothesis that nereidin is a peptide, a notion that has been propagated in the literature for more than 30 years (Cardon et al., 1981; Durchon, 1984). Furthermore, MF and related sesquiterpenoid hormones have so far only been found in arthropods (Bellés et al., 2005; Jindra et al., 2015), and their evolution has been hypothesised to be a secondary consequence of the loss of the sterol synthesis pathway in this clade (Zandee, 1964; Bellés et al., 2005). As marine nereidid worms were previously shown to possess a functional sterol synthesis pathway (Wootton and Wright, 1962), and Platynereis even has a functional estrogen receptor (Keay and Thornton, 2009), our discovery makes it unlikely that loss of sterol synthesis preceded the evolution of sesquiterpenoid hormones. Rather, it suggests that, like sterols, bioactive sesquiterpenoids, could have been part of the ancient molecular repertoire available in the common ancestor of protostomes, if not even at earlier stages of animal evolution.
Several lines of experimental evidence support this notion: Exogenous MF was found to affect larval development of the annelid Capitella sp. (Laufer and Biggers, 2001), and annelid extracts displayed biological activity in assays for insect juvenile hormones (Schneiderman and Gilbert, 1958). The latter result would suggest that insect juvenile hormones might also be active in the annelid system, but this issue has not yet been addressed experimentally. Moreover, we have detected MF in head extracts of common earthworms (Lumbricus sp., Figure 3—figure supplement 3). Beyond the annelid clade, our identification of Methoprene-tolerant orthologues in the limpet Lottia and the pond snail Lymnaea indicates that the sesquiterpenoid signalling pathway might even exist in molluscs. As annelids and molluscs are large groups within the lophotrochozoan superphylum (Halanych et al., 1995), this implies a potential physiological role for sesquiterpenoids in a broad set of (mostly aquatic) species.
What could such physiological roles be? Semelparity, as it occurs in Platynereis, is considered an extreme form of 'capital breeding', a reproductive strategy where females build up large energy stores in their bodies that are then invested into offspring at particular time points (Drent and Daan, 1980). Capital breeding strategies allow animals to time their reproduction more precisely and have larger clutches of offspring than could be afforded by daily nutrient uptake. It likely represents an ancient reproductive strategy that is very common in poikilothermic animals (Bonnet et al., 1998). Therefore, it is plausible that the role for MF uncovered in the marine annelid Platynereis could reflect a more general role of sesquiterpenoids in regulating reproductive energy expenditure. The inhibitory function for maturation we uncover in Platynereis is reminiscent of the juvenoid functions of insect juvenile hormones: During pre-adult development, these provide a molecular cue for juvenile status, preventing insects from entering into adulthood, and thereby indirectly preventing reproductive energy expenditure as well. Likewise, MF treatment of larval stages of crustaceans can delay metamorphosis or prevent gonad formation (Borst et al., 1987; Tsukimura et al., 2006).
During adulthood, however, insect and crustacean sesquiterpenoids have distinct functions. In contrast to our results in worms, juvenile hormones commonly act as positive regulators of vitellogenesis in the adult insect fat body, whereas their down-regulation is associated with diapause and longevity (Gäde et al., 1997; Wyatt, 1997; Hansen et al., 2014). Similarly, MF promotes adult maturation, vitellogenesis and mating behaviour in crustaceans (Laufer et al., 1987; Homola and Chang, 1997; Subramoniam, 2011), with a separate function in sex determination (Toyota et al., 2015). As Platynereis retains certain MF levels even in the mature stage (see above), it will be a suitable model to address additional roles of MF in the life history of an annelid.
Finally, our discovery of a sesquiterpenoid hormone in an annelid is not only relevant to understand hormone evolution but it likely also has significant ecological implications. Reproductive hormones are attractive targets for pesticides. Juvenile hormone analogues like methoprene and later pyriproxyfen have initially been designed to interfere with insect development and are broadly used to fight insect pests such as Aedes, a vector for dengue, chikungunya, Zika, and yellow fever viruses (reviewed in Henrick, 2007). The discovery the sesquiterpenoid MF in crustaceans by Laufer et al. (1987) already raised concerns if the effect of juvenile hormone analogues is restricted to insect development (Walker et al., 2005; Abe et al., 2015), calling for more specific tests with non-target species. Our results argue that aquatic lophotrochozoans should also be systematically included in specificity tests for compounds targeting sesquiterpenoid signalling pathway, and that such tests should not be restricted to mortality (Henrick, 2007), but include assays on reproductive fitness. For instance, in our studies, pyriproxyfen impacts on vtg levels at concentrations of 10 nM which lies in the range of pyriproxyfen found in surface waters (Sullivan and Goh, 2008). In turn, sesquiterpenoid analogues may also be suitable leads in the search for specific compounds targeting pest species outside the arthropod clade or factors to favourably affect the balance between reproductive and somatic growth in aqua- and mariculture.
Taken together, our data argue for a critical role of sesquiterpenoid hormones outside arthropods and indicate that methylfarnesoate is the major constituent of the long-sought brain hormone nereidin, a key factor in regulating maturation in marine capital breeders. The discovery of this hormone in an annelid provides a new entry point to understand hormonal regulation of energy expenditure and sheds new light on the evolution of animal hormone systems.
Materials and methods
Animals
Platynereis dumerilii were kept in a continuous culture at the Marine Facility of the Max F. Perutz Laboratories in Vienna (for details see Hauenschild and Fischer, 1969).
Cell isolation
After anaesthetisation of the animals in a 1:1 (v/v) mixture of seawater and 7.5% (w/v) MgCl2 in distilled water, the animals were transferred into 500 µL Nereis balanced salt solution (NBSS, see Fischer et al., 1991) and the coelomic cavity was opened with fine scissors. The coelomic cells were gently squeezed out, passed through 136, 70 and 30 µm gauze filters to select for eleocytes (>90% pure as judged by microscopical inspection). Cells were then collected by centrifugation (5 min, 500 ×g, 0°C), and washed once with NBSS. For the isolation of coelomic cells (eleocytes and oocytes), only the first gauze filtration was carried out.
Bioassay
Coelomic cells were isolated as described above and cultured a total of volume of 50 µL of culture medium without hen egg ultra filtrate (Schenk and Hoeger, 2010) overnight at 19°C on a rocking platform, in a BSA-coated 96-well microtitre plate. All animals were in the premature stage, with oocyte diameters between 50 and 65 µm, i.e. before the irreversible onset of maturation assumed to be beginning from oocyte diameters of 85 µm onwards (Hauenschild, 1966). Test substances were diluted in either 100 mM Na2PO4, pH 7.5, or in NBSS, and their final volume never exceeded 10% of the total, DMSO concentration was kept to a maximum of 0.01%. Controls received only the vehicle.
After incubation, cells were harvested by centrifugation (5 min, 500 xg, 0°C) and total RNA was isolated with a commercial kit (RNeasy-Kit, Qiagen, Hilden, Germany) according to the manufacturer’s protocol, stored at −80°C and used for qRT-PCR using primers against Platynereis vitellogenin (sequences see Table 1) to assess changes in vitellogenin transcript abundance. As vitellogenin expression is strongly dependent on the maturation stage (see results) and as individual animals can show considerable variations in their physiology (Fischer and Hoeger, 1993; Hoeger and Kunz 1993) the cells of at least two animals were pooled per treatment group. Each assay consisted of three biological replicates and was at least repeated once.
Table 1.
List of primers used for qRT-PCR, including predicted melting temperature.
Name |
Sequence 5’→3’ |
Tm / °C |
Efficiency |
|---|---|---|---|
Pdu vtg qPCR1 F |
ACAGGCCATCACATTCACAA |
56.4 |
101% |
Pdu vtg qPCR1 R |
TCTGCTCACGTCTCTTTCCA |
58.4 |
|
Pdu met qPCR1 F |
GGATGATTATGATGTATACCTGCAAC |
62.9 |
102% |
Pdu met qPCR1 R |
AGACCGAACTGGCGTTTG |
56.3 |
|
Pdu hox3 qPCR1 F |
CTACCCCTGGATGAGGGAAT |
60.5 |
95% |
Pdu hox3 qPCR1 R |
ACTTCCGGTTCCTGGTCC |
58.4 |
|
Pdu rps9 F |
CGCCAGAGAGTTGCTGACT |
59.5 |
102% |
Pdu rps9 R |
ACTCCAATACGGACCAGACG |
60.5 |
|
Pdu sams qPCR1 F |
CAGCAACGGTGAAATAACCA |
56.4 |
101% |
Pdu sams qPCR1 R |
CATCACTCACTTGATCGCAAA |
57.5 |
|
Pdu cim6pr qPCR1 F |
ACTTCCCCTGCTGATGAGTG |
60.5 |
99% |
Pdu cim6pr qPCR1 R |
TTCGTAAGTCAGGTTTCCATTG |
58.4 |
|
Pdu cdc5 F |
CCTATTGACATGGACGAAGATG |
60.1 |
100% |
Pdu cdc5 R |
TTCCCTGTGTGTTCGCAAG |
57.5 |
qRT-PCR analyses
Primers for qPCR were designed using the 'Universal Probe Library' web page provided by Hoffman-LaRoche (http://lifescience.roche.com, see Table 1). Available genome information (http://4dx.embl.de/platy/) was used to choose primers across exon/intron junctions. Prior to use, all primers were tested for their dynamic range by serial dilutions (10x, 100x, 1000x) of cDNA. These tests also included included a –RT-control (no reverse transcription). All selected primers showed a linear amplification profile in the tested dilution range, and the primer efficiencies were calculated (E = 101/slope× 100, where "E" is the primer efficiency and "slope" is the calculated slope of the dilution series) to be between 95% and 102% (Table 1), and amplification from –RT controls was not observed. In these primer tests, amplified products were subcloned and the sequence confirmed by Sanger sequencing as described below.
In all assays, the same RNA amount was used for each group to be compared. Generally, this was 5 ng for the assessment of vtg, 150 ng for met, and 300 ng for hox3.
cDNA for qRT-PCR was synthesised using Qiagen’s QuantiTect-Kit and final dilution of the cDNA to 50 µL. Briefly, the desired amount of RNA was diluted to 12 µL with nuclease-free H20, and 2 µL of the Kit`s gDNA-Whipeout buffer (DNase I) were added. The DNase digest was performed for 6 min at 42°C, following cooling to 0°C, cDNA was then synthesised in a total volume of 20 µL for 15 min at 42°C, the reaction was stopped by heat deactivation of the enzymes (5 min, 95°C); finally, the reaction was centrifuged (1 min, 17.000 ×g), diluted to 50 µL with H2O and used for qRT-PCR.
qRT-PCR was performed on a Applied Biosciences StepOne instrument using SybrGreen (Thermo Fisher Scientific, Vienna, Austria) as reporter dye. Five microlitres cDNA were measured in a volume of 20 µL, with two technical replicates per sample. After an initial denaturation for 10 min at 95°C, amplification and reading of the reporter dye were carried out at 60°C for 1 min over a total of 40 cycles with a 3 s denaturation step at 95°C at the beginning of each cycle, this was followed by recording a melting curve of the amplified product (60.0–95.0°C in 0.3°C increments) to ascertain only a single amplified product.
Suitable reference genes were chosen based on (i) the stability of their expression values in the respective tissue and (ii) similar Ct-values for these genes in relation to the target gene. Specifically, the target and the reference gene were aimed to be within a △Ct -range of ±5. Generally, two reference genes were used for normalisation, and the target gene was normalised by the 2–△△Ct –method to the arithmetic mean of these two reference genes. For the coelomocyte cultures, as they were extensively used in the context of the brain hormone fractionation, the limited material available for each assay only allowed us to use a single reference gene, rps9 (ribosomal protein small subunit 9). However, we tested the stability of rps9 in coelomocytes against the reference gene cdc5 (cell-cycle-dependent serine/threonine protein kinase) when we first assessed vtg-transcript levels in eleocytes of different maturation stages (Figure 1C). Separate quantification against either reference gene produced similar results (Figure 1—figure supplement 2). This is in line with the fact that both of these genes were previously shown to be suitable reference genes (Dray et al., 2010; Zantke et al., 2013). Additionally, we also assessed the stability of rps9 in cell culture by comparing the normalised Ct-values within each experimental series. These analyses demonstrated that there is a high stability of these values (SD of 3%) (Figure 2—figure supplement 2). For Platynereis S-adenosylmethionine synthetase (sams; accession: KX907200) and cation-independent-mannose-6-phosphate-receptor (cim6pr; accession: KX907201), independent calculations with the respective second housekeeping gene support their suitability as additional reference genes (Figure 3—figure supplement 4, Figure 4—figure supplement 1).
Data were excluded from the analysis if the △Ct-values were ±1.0x of the mean of the respective treatment group, or if the melt curve analysis indicated ambiguous Tm for the PCR products. In any case, at least five biological replicates were used for analysis.
Brain hormone purification
Platynereis dumerilii were anaesthetised as described above and decapitated in front of the pharynx. The heads were suspended in 80% methanol containing 0.1% formic acid, and disrupted by three ultrasonic bursts for 30 s on ice and extracted twice for 15 min on ice. Insoluble material was pelleted for 15 min and 20,000 ×g at 0°C, after which the pellet was again extracted and centrifuged. To further eliminate insoluble macromolecules, the raw extract was left to precipitate for 30 min at −20°C, and was again centrifuged for 15 min and 20,000 ×g at 0°C. The resulting supernatant was mildly dried down in vacuo and the pellet suspended in 0.1% formic acid, by vigorous vortexing, followed by shaking at 1400 rpm at room temperature on a Thermomixer, and finally centrifuged (2 min, 17,000 ×g). The extract was then applied onto phenyl-extraction columns (Alltech, 100 mg sorbent, ordered from Thermo Fisher Scientific, Vienna, Austria) equilibrated in 0.1% formic acid. The flow-through was discarded, the column washed with 750 µL 0.1% formic acid, and eluted with 500 µL 50% methanol, 0.1% formic acid. The final eluate was dried down in vacuo and finally reconstituted at a concentration of 0.66 heads/µL in 100 mM Na2HPO4, pH 7.4, 10 min by shaking at 1400 rpm in a Thermomixer, and centrifuged (2 min 17,000 ×g). 6.0 head equivalents were used per well in the cell culture assay.
Proteinase treatment
For proteinase treatment, an aliquot of the eluted active fraction was incubated with a final concentration of 1 mg/mL proteinase K (EC 3.4.21.64; Merck Millipore, Vienna, Austria; 30 mU) for 60 min at 56°C. The reaction was stopped by heating the sample to 95°C for 5 min and, after cooling to 0°C by making the sample 0.1 mM in phenylmethylsulphonyl fluoride, finally the samples were clarified by centrifugation. Controls without proteinase K were treated alike. Again, 6.0 head equivalents were used per cell culture assay.
Brain hormone identification
The brain hormone enriched fraction was dried down in vacuo, suspended in 0.1% trifluoroacetic acid and resolved on a Symmetry-ODS-column (Waters) equilibrated in 20% solvent B (0.1% TFA in acetonitrile) and 80% solvent A (0.1% TFA in water), after an isocratic wash for 1.5 min, the column was developed by raising the concentration of B to 100% over 35 min, followed by a plateau of 3.5 min, with a flow rate of 0.5 mL/min. The eluent was monitored at 215 nm and 280 nm and a total of 16 fractions eluting were collected. The fractions were dried down in vacuo and stored at −20°C until use. To determine the biological activity of the collected peaks, each was reconstituted in 100 mM Na2HPO4, pH 7.5 at a concentration of 0.66 heads per µL and used in the cell culture assay as described above. Peaks showing a significant reduction in vitellogenin transcript abundance in two independent experiments were further analysed by LC-MS/MS, and the obtained elution profiles and fragment patterns were compared with authentic standards.
LC-MS/MS was performed on a LTQ Orbitrap (Thermo Fisher Scientific) equipped with a nanospray ion source connected to a Dionex Ultimate 3000 UHPLC (Thermo Fisher Scientific), equipped with a Pepmap100 C18 guard column and a Pepmap100 C18 column (2 cm × 75 μm and 50 cm × 75 µm, respectively, 5 µm particle size, Thermo Fisher Scientific) equilibrated in 1% B (80% acetonitrile, 0.2% formic acid) and 99% A (0.2% formic acid in water). The samples were loaded onto the guard column with a flow rate of 10 μL/min and washed for 10 min using 100% A. The sample was the resolved on the analytical column with a flow of 300 nL/min. The column was developed for 10 min isocratic with 1% B; B was then raised to 20% in one min, before reaching 80% after 49 min. Following a 3-min plateau, the column was washed by raising B to 90% B in 2 min and washed for another 2 min. Mass spectra acquisition started after 10 min; precursor ions were scanned in the range of m/z 100–1000, and the 12 most abundant ions were fragmented in the HCD cell with a normalised fragmentation energy of 40% ± 75%.
Quantification of brain hormone
Heads were collected as described above and stored at −80°C. They were thawed at room temperature, and subjected to 4 freeze-thaw cycle between liquid N2 and 55°C warm water. The heads were then transferred into 1.5 mL auto sampler vials containing a glass bead and covered with 400 µL 80% methanol, and the head capsule was broken with four cycles in an ultra sonic water bath at 55°C for ten minutes. The homogenate was then extracted with 1000 µL heptane for 5 min with vigorous shaking at 1400 rpm at room temperature in a Thermomixer followed by centrifugation at 2500 ×g for 10 min. The organic phase was recovered and the aqueous phase re-extracted with 500 µL as above. The pooled extract was mildly dried down in vacuo, dissolved in 300 µL heptane, transferred into a 300 µL auto sampler vial insert, dried down, and finally dissolved in 10 µL heptane. The remaining aqueous phase was dried down as well, and used for protein determination (see below).
Brain hormone amounts were quantified by GC-MS on an Agilent 6890N GC equipped with an Agilent 5973 mass spectrometer. The GC was operated in splitless mode with constant pressure of 68.6 kPa and an initial flow of 1.0 mL/min with He-gas as carrier. After injection (inlet temperature: 250°C, purge flow: 50 mL/min, purge time: 2.0 min) and an initial plateau phase of 1 min at 90°C, the temperature was raised from 90°C for 280°C at 20 °C/min followed by a plateau for 7.5 min.
The eluting samples were ionised and fragmented by electron impact at an ionisation energy of 2376 eV and monitored in the range from m/z 50.0–400.0 with a sampling rate of 23/s in the total ion channel, and for identification and quantification in selected ion mode (SIM) at m/z 114.0, 136.0 and 219.0 with 3.1 cycles/s and a dwell time of 100 ms per ion. The MS-source was operated at 230°C and the quadrupole at 150°C. One µL of the samples was injected, and the sample was resolved on a DB-5MS column (J&W Scientific, Agilent; 30 m × 0.25 mm, 0.25 µm film thickness).
The amount of methylfarnesoate was determined by comparison to authentic standards of methylfarnesoate (Echelon Biosciences). For quantification, serial dilutions of a 2.5 ng/µL sock solution of MF in heptane were measured. Each data point was measured in triplicate, and a linear regression was fitted. Peak detection and quantification was carried out with the program, MSD ChemStation (Agilent); peaks had to fit into a specified retention time window (± 0.1 min of that of the standard) and meet the qualifier ions. In rare cases, where automatic peak detection failed, peaks were manually integrated and the amount of MF calculated based on the regression generated.
The extraction efficiency of our method was assessed by using the heads of 25 young animals as a matrix and spiking in a total of 12.5 ng MF (out of a 12.5 ng/µL stock solution). The samples were then processed as described, and finally reconstituted in 25 µL heptane, out of which one µl was measured, resulting in an expected MF amount of 500 pg. The stock solution served as reference and was diluted 25-fold immediately before measurement. The extraction efficiency was thus estimated to be >85%. Under our conditions, the limit of detection was 10 fmole MF (2.5 pg MF/µL), and the limit of quantification ~ 60 fmole MF (~15 pg MF/µL).
Earthworm head sampling
Earthworms Lumbricus spec. (Giant Canadian Night Crawlers, Baitmaster) were purchased at a local fishing bait shop (Angelsport Gangl, Wien). The animals were treated with antibiotics (125 mg/L ampicillin, 500 mg/L streptomycin sulphate) for 5 min, before the antibiotics solution was allowed to soak into the soil the earthworms were covered in. After 24 hr in the antibiotics treated soil, the animals were anaesthetised in 15% ethanol at 4°C for 15 min. Two to six heads (prostomium plus the next 4 segments) were collected on ice, snap frozen in liquid N2, and stored at −80°C until further use. For the extraction of MF, the samples were subjected to four freeze-thaw cycles between 55°C and liquid N2. Thereafter, they were covered in 80% MeOH and processed as described for Platynereis heads, with adjustments accounting for the larger tissue size. The finally obtained dried extract was reconstituted in n-heptane to yield ~2 heads per µL. GC-MS measurements were carried out as described for Platynereis heads.
In vivo treatment of worms
For in vivo treatment of worms, premature female animals (max. Oocyte diameter < 65 µm) were selected and kept for 2 days in sterile filtered natural sea water (NSW). They were then decapitated (as described above) to synchronise and to set in motion sexual maturation (Hauenschild, 1966). The animals were transferred in groups of 4–5 into glazed ceramic bowls (treated with 6% PEG 6000 to prevent unspecific hydrophobic binding of the hormones to the glass coating) containing 50 mL sterile filtered NSW supplemented with 0.125 mg/mL ampicillin and 0.500 mg/mL streptomycin sulphate. Worms were treated either with 100 nM methylfarnesoate, 100 nM methoprene (Sigma, both added from a 100 µM stock in DMSO, resulting 0.1% DMSO final concentration), or 0.1% DMSO alone. After 24 hr, water was exchanged, and the antibiotics concentration reduced to 50%, after that, the water exchanged every 24 hr. After 5 days, the animals were snap-frozen in liquid N2 without any residual water and stored at −80°C until RNA extraction.
Protein determination
Protein concentration was determined by the bicinchoninic acid assay with BSA as standard (Smith et al., 1985).
Regeneration assay
Fed, young premature (35–50 parapodial segments, pps) worms were kept in sterile-filtered NSW supplemented with 0.125 mg/mL ampicillin and 0.500 mg/mL streptomycin for 24 hr, and further for an additional 24 hr in half concentrated ampicillin/streptomycin. Generally, the regeneration assay was carried out as described by Hauenschild (1974). Briefly, worms were anesthetised as described and cut three times between segments. The first cut was after the 21st pps, the second after the 26th pps, and the third cut was after the 31st pps producing fragments of five segments. The test fragments from each individual animal used for assays consisted of pps 22–26, and 27–31. The fragments were then kept in 1 mL sterile-filtered NSW containing half concentrated ampicillin/streptomycin in GC-auto sampler vials containing either 100 nM methylfarnesoate (added from a 100 µM stock solution in DMSO, fragment ‘+MF’), or vehicle alone (0.1% DMSO, fragment ‘−MF’); during the course of the experiment, the water was exchanged every 24 hr, care was taken to not let the worm fragments dry out. To rule out any effect of the position within the animal on regenerative abilities, +MF and control were alternated between the anterior and posterior fragments. The anterior and posterior +MF- and control fragments of two individual worms were combined, to serve as one biological replicate for +MF, and –MF treatment groups, respectively. They were snap-frozen in liquid nitrogen, stored at −80°C and used for the quantification of hox3 via qRT-PCR.
Gene identification and confirmation
A transcript sequence encoding Platynereis Vitellogenin (accession no. KU756287) was identified from an eleocyte-specific transcriptome dataset (Schenk et al., unpublished), and sequence-validated after PCR cloning using the primers 5’-ATGAAGACTCTCCTGATCTTCG-3’ and 5’-CTAGTAGTAGAATCTTGGTCCTTCAC-3’ (for the list of used sequencing primers see Table 2).
Table 2.
List of primers used for cloning and sequence validation.
Name |
Sequence 5’→3’ |
Tm / °c |
|---|---|---|
|
Pdu vtg 1F |
ATGAAGACTCTCCTGATCTTCG |
60.1 |
|
Pdu vtg 1R |
CTAGTAGTAGAATCTTGGTCCTTCAC |
64.6 |
|
Pdu vtg_seq 1F |
AGCCCTAGAAGCTGCCTCTG |
62.5 |
|
Pdu vtg_seq 2F |
ATTGCTCAATCTGAACTCCCATGC |
63.6 |
|
Pdu vtg_seq 3F |
GCTGTTCCACAGGAAATTGC |
58.4 |
|
Pdu vtg_seq 4F |
GCTTTGGTCAGTGGACTTCC |
60.5 |
|
Pdu vtg_seq 1R |
GGCAATCCTCTGATGTAAACATTCTC |
64.6 |
|
Pdu vtg_seq 2R |
CAAGCGTTTCACGACCAAGAGG |
64.2 |
|
Pdu vtg_seq 3R |
GAAGAGCTTCTTGCTGGAGC |
60.5 |
|
Pdu vtg_seq 4R |
AAGACCAGCTGGCGCGTTATG |
63.2 |
Pdu met F |
ATGGAGCCGAATTCGGAGCAGAATTCGG |
71.8 |
Pdu met R |
TCAACATGTCTCAGTTTCTTTTTGAGCG |
65.6 |
Pdu hox3 F |
CCCCGGGGCTCTTGGTTTT |
61.6 |
Pdu hox3 R |
GCCATCTCTATTCTCCTCGGCCG |
68.3 |
Similarly, we identified a transcript encoding Platynereis Methoprene-tolerant (accession no. KU756288) that was confirmed by sequencing of a fragment sub-cloned with the primers 5’-ATGGAGCCGAATTCGGAGCAGAATTCGG-3’ and 5’-TCAACATGTCTCAGTTTCTTTTTGAGCG-3’.
For cloning, cDNA was prepared with the Transcriptor High Fidelity cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions with oligo dT-priming from eleocyte or whole worm RNA (isolated with the RNeasy Kit, Qiagen).
PCR was performed using Phusion Taq (Thermo), using the following cycle: 1x {98°C, 30”}; 35x {98°C, 7”; x°C, 20”; 72°C, 30”/kb}; 1x {72°C, 7’30”}; 10°C. The annealing temperature ‘x’ was determined by the web page ‘OligoCalc’ (http://www.basic.northwestern.edu/biotools/oligocalc.html), using on the 'salt adjusted' algorithm. If necessary, annealing temperatures were optimized by gradient PCR and adjusted accordingly.
The PCR products were run on agarose gels, stained with SybrSafe (Invitrogen) DNA-dye, the specific products were cut out and purified using the QIAquick gel extraction kit (Qiagen). The purified PCR-products were sub-cloned into a pJET-vector (CloneJet Kit, Thermo), and transformed into NEB-5-alpha (New England Biolabs) chemical competent E. coli. Bacteria plated on Luria-Bertani (LB)-medium agar plates supplemented with ampicillin (amp, 50 µg/mL), after growth over night single colonies were selected and grown over night in LBAmp-medium. The next day, plasmids were purified (Birnboim and Doly, 1979) and submitted for Sanger sequencing using the indicated primers.
Gene orthology and domain analyses
For domain analyses of Platynereis Vtg and lipoproteins representatives for other clades, we used the domain annotation implemented in SMART (http://smart.embl.de; Schultz et al., 1998; Letunic et al., 2015). For determining phylogenetic relationships, we aligned full-length protein sequences representative of distinct clades using MAFFT v7.221 (Katoh and Standley, 2013), and constructed Maximum Likelihood trees with IQ-TREE 1.3.12 (Minh et al., 2013; Nguyen et al., 2015). The consensus tree was visualized using iTOL (http://itol.embl.de/, Letunic and Bork, 2011).
For assessing the phylogenetic relationships of Platynereis Met and other bHLH-PAS domain proteins, BLASTP searches were performed against the nr section of the NCBI sequence repository. Sequences from representatives of selected clades were aligned using MAFFT v7.221 (Katoh and Standley, 2013), and maximum likelihood phylogenetic trees were constructed with IQ-TREE, using the built-in parameter optimisation (Minh et al., 2013; Nguyen et al., 2015).
Data analysis and statistics
All statistical tests were performed with the program R (R Core Team, 2015). First, all data were tested for normal distribution using the Shapiro-Wilk normality test. Statistical significance was then tested with a t-test incorporating Welch`s correction to account for heteroscedasticity. If more than one comparison was to be assessed, statistical significance was first tested by a one-way analysis of variance test (ANOVA), and if significance was given, pair wise comparisons were carried out by a (Welch-corrected) t-test and the obtained p-values were adjusted for multiple testing by the method of Benjamini and Hochberg implemented in R yielding adjusted p-values (padj). A result was considered as statistically significant if p<0.05 in the case of t-tests, and p<0.10 in the case of ANOVA, the following t-test were then again considered to yield a statistically significant result if padj < 0.05. Significance levels of p-values and adjusted p-values are indicated by *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, if not stated otherwise.
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Community`s Seventh Framework Programme (FP7/2007–2013)/ERC Grant Agreement 260304 (to FR). FR and CG acknowledge support by the interdisciplinary Research Platform 'Marine Rhythms of Life' of the University of Vienna; SS was supported by a Lise Meitner-fellowship of the FWF (M1478-B19). The authors are indebted to Agne Valinciute for excellent technical help and for cloning and sequence-validation of the met gene, the Marine Facility staff at MFPL for animal husbandry, all members of the Raible and Tessmar labs for critical input, Dr. Dietrich K. Hofmann (Ruhr-Universität Bochum) for supply with brain hormone literature and continuous discussion, Dr. Ulrich Hoeger (Universität Mainz) for access to HPLC equipment and support, and Dr. Kristin Tessmar-Raible, Dr. Stephanie Bannister, and Dr. Graham Warren (all at the MFPL, Vienna) for helpful comments on the manuscript.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
Austrian Science Fund (FWF) M1478-B19 to Sven Schenk.
Universität Wien Research Platform Marine Rhythms of Life to Christopher Gerner, Florian Raible.
European Research Council Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreement 260304 to Florian Raible.
Additional information
Competing interests
The authors declare that no competing interests exist.
Author contributions
SS, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
CK, Acquisition of data, Drafting or revising the article.
PF, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
CG, Conception and design, Analysis and interpretation of data, Drafting or revising the article.
FR, Conception and design, Acquisition of data, Analysis and interpretation of data, Drafting or revising the article.
Ethics
Animal experimentation: This study only used invertebrate worms from a laboratory culture. These are not covered by the specific ethical regulations applicable to higher invertebrates or vertebrates under Austrian and European legislation.
References
- Abe R, Toyota K, Miyakawa H, Watanabe H, Oka T, Miyagawa S, Nishide H, Uchiyama I, Tollefsen KE, Iguchi T, Tatarazako N. Diofenolan induces male offspring production through binding to the juvenile hormone receptor in Daphnia magna. Aquatic Toxicology. 2015;159:44–51. doi: 10.1016/j.aquatox.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- Baert J-L, Slomtanny M-C. Vitellin accumulation and vitellogenin synthesis in relation to oogenesis in Perinereis cultrifera (Polychaeta Annelida) Invertebrate Reproduction & Development. 1992;21:121–128. doi: 10.1080/07924259.1992.9672228. [DOI] [Google Scholar]
- Baert JL, Slomianny MC. Heterosynthetic origin of the major yolk protein, vitellin, in a nereid, Perinereis cultrifera (polychaete annelid) Comparative Biochemistry and Physiology Part B. 1987;88:1191–1199. doi: 10.1016/0305-0491(87)90023-X. [DOI] [Google Scholar]
- Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. General and Comparative Endocrinology. 2011;170:3–17. doi: 10.1016/j.ygcen.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés X, Martín D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annual Review of Entomology. 2005;50:181–199. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Research. 1979;7:1512–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet X, Bradshaw D, Shine R. Capital versus income breeding: an ectothermic perspective. Oikos. 1998;83:333–342. doi: 10.2307/3546846. [DOI] [Google Scholar]
- Borst DW, Laufer H, Landau M, Chang ES, Hertz WA, Baker FC, Schooley DA. Methyl farnesoate and its role in crustacean reproduction and development. Insect Biochemistry. 1987;17:1123–1127. doi: 10.1016/0020-1790(87)90133-8. [DOI] [Google Scholar]
- Cardon C, Durchon M, Porchet M. Purification par chromatographie liquide de haute pression (HPLC) de l'hormone cérébrale chez Nereis diversicolor et Perinereis cultrifera (Annélides Polychètes) Reproduction Nutrition Développement. 1981;21:383–390. doi: 10.1051/rnd:19810304. [DOI] [PubMed] [Google Scholar]
- Cardon C. Procédés de fractionnement de ganglions cérébroïdes de Nereis diversicolor O. F. Müller en vue de l'isolement de l'hormone inhibitrice de la sexualisation. Bulletin de la Société Zoologique de France. 1970;95:543–549. [Google Scholar]
- Christodoulou F, Raible F, Tomer R, Simakov O, Trachana K, Klaus S, Snyman H, Hannon GJ, Bork P, Arendt D. Ancient animal microRNAs and the evolution of tissue identity. Nature. 2010;463:1084–1088. doi: 10.1038/nature08744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes AS, Jékely G, Steinmetz PR, Raible F, Snyman H, Prud'homme B, Ferrier DE, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Dray N, Tessmar-Raible K, Le Gouar M, Vibert L, Christodoulou F, Schipany K, Guillou A, Zantke J, Snyman H, Béhague J, Vervoort M, Arendt D, Balavoine G. Hedgehog signaling regulates segment formation in the annelid Platynereis. Science. 2010;329:339–342. doi: 10.1126/science.1188913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent RH, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. doi: 10.5253/arde.v68.p225. [DOI] [Google Scholar]
- Durchon M, Porchet M. Dosage de l'activité endocrine cérébrale au cours du cycle génital femelle chez Nereis diversicolor O.F. Müller (Annélide Polychète) Comptes Rendus de l'Académie des Sciences - Series. 1970;270:1689–1691. [PubMed] [Google Scholar]
- Durchon M. Peptidic hormones in annelids. In: Hoffmann J, Durchon M, editors. Biosynthesis, Metabolism and Mode of Action of Invertebrate Hormones. Berlin - Heidelberg - New York - Tokyo: Springer-Verlag; 1984. pp. 10–18. [Google Scholar]
- Fischer A, Dorresteijn A. The polychaete Platynereis dumerilii (Annelida): a laboratory animal with spiralian cleavage, lifelong segment proliferation and a mixed benthic/pelagic life cycle. BioEssays. 2004;26:314–325. doi: 10.1002/bies.10409. [DOI] [PubMed] [Google Scholar]
- Fischer A, Hoeger U. Metabolic links between somatic sexual maturation and oogenesis in nereid annelids—a brief review. Invertebrate Reproduction & Development. 1993;23:131–138. doi: 10.1080/07924259.1993.9672304. [DOI] [Google Scholar]
- Fischer A, Rabien H, Heacox AE. Specific, concentration-dependent uptake of vitellin by the oocytes of Nereis virens (Annelida, Polychaeta) in vitro. Journal of Experimental Zoology. 1991;260:106–115. doi: 10.1002/jez.1402600114. [DOI] [Google Scholar]
- Fischer A, Schmitz K. Preparation, properties, and composition of Nereis Vitellin, the yolk protein of the annelid, Nereis virens. Differentiation. 1981;19:103–108. doi: 10.1111/j.1432-0436.1981.tb01136.x. [DOI] [Google Scholar]
- Fischer A. A vitellin-like antigen in the coelomic fluid of maturing Nereis virens females. Die Naturwissenschaften. 1979;66:316–317. doi: 10.1007/BF00441280. [DOI] [PubMed] [Google Scholar]
- Fischer A. Control of oocyte differentiation in nereids (Annelida, Polychaeta) - facts and ideas. In: Fischer A, Pfannenstiel H-D, editors. Fortschr. Zool, Vol. 29. Stuttgart, New York: Gustav Fischer Verlag; 1984. pp. 227–245. [Google Scholar]
- Gazave E, Béhague J, Laplane L, Guillou A, Préau L, Demilly A, Balavoine G, Vervoort M. Posterior elongation in the annelid Platynereis dumerilii involves stem cells molecularly related to primordial germ cells. Developmental Biology. 2013;382:246–267. doi: 10.1016/j.ydbio.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Golding DW, Yuwono E. Latent capacities for gametogenic cycling in the semelparous invertebrate Nereis. PNAS. 1994;91:11777–11781. doi: 10.1073/pnas.91.25.11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding DW. Neurosecretion and regeneration in Nereis. I. Regeneration and the role of the supraesophageal ganglion. General and Comparative Endocrinology. 1967a;8:348–355. doi: 10.1016/0016-6480(67)90084-6. [DOI] [PubMed] [Google Scholar]
- Golding DW. Regeneration and growth control in Nereis. I. Growth and regeneration. Journal of Embryology and Experimental Morphology. 1967b;18:67–77. [PubMed] [Google Scholar]
- Golding DW. Endocrine programmed development and reproduction in Nereis. General and Comparative Endocrinology. 1983;52:456–466. doi: 10.1016/0016-6480(83)90186-7. [DOI] [PubMed] [Google Scholar]
- Gäde G, Hoffmann KH, Spring JH. Hormonal regulation in insects: facts, gaps, and future directions. Physiological Reviews. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- Halanych KM, Bacheller JD, Aguinaldo AM, Liva SM, Hillis DM, Lake JA. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science. 1995;267:1641–1643. doi: 10.1126/science.7886451. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Frontiers in Physiology. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauenschild C, Fischer A. Großes Zoologisches Praktikum, Vol. 10b. Stuttgart: Gustav Fischer Verlag; 1969. Platynereis dumerilii. [Google Scholar]
- Hauenschild C. Hormonale Hemmung der Geschlechtsreife und Metamorphose bei dem Polychaeten Platynereis dumerilii. Zeitschrift Für Naturforschung B. 1956a;11:125–132. doi: 10.1515/znb-1956-0302. [DOI] [Google Scholar]
- Hauenschild C. Weitere Versuche zur Frage Des Juvenilhormons bei Platynereis. Zeitschrift Für Naturforschung B. 1956b;11:610–612. doi: 10.1515/znb-1956-9-1026. [DOI] [Google Scholar]
- Hauenschild C. Hemmender Einfluß der Proventrikelregion auf Stolonisation und Oocyten-Entwicklung bei dem Polychaeten Autolytus prolifer. Zeitschrift Für Naturforschung B. 1959;14:87–89. doi: 10.1515/znb-1959-0205. [DOI] [Google Scholar]
- Hauenschild C. Abhängigkeit der Regenerationsleistung Von der inneren Sekretion im Prostomium bei Platynereis dumerilii. Zeitschrift Für Naturforschung B. 1960;15:52–55. doi: 10.1515/znb-1960-0111. [DOI] [Google Scholar]
- Hauenschild C. Der hormonale Einfluss des Gehirns auf die sexuelle Entwicklung bei dem Polychaeten Platynereis dumerilii. General and Comparative Endocrinology. 1966;6:26–73. doi: 10.1016/0016-6480(66)90108-0. [DOI] [Google Scholar]
- Hauenschild C. Normalisierung der geschlechtlichen Entwicklung kopfloser Fragmente junger ♀♀ von Platynereis dumerilii (Polychaeta) durch Behandlung mit konservierten Prostomien juveniler Individuen. Helgoländer Wissenschaftliche Meeresuntersuchungen. 1974;26:63–81. doi: 10.1007/BF01613305. [DOI] [Google Scholar]
- Henrick CA. Methoprene. Journal of the American Mosquito Control Association. 2007;23:225–239. doi: 10.2987/8756-971X(2007)23[225:M]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hoeger U, Kunz I. Metabolic enzymes in coelomic cells (eleocytes) of the polychaete Nereis virens: sex specific changes during sexual maturation. Marine Biology. 1993;115:653–660. doi: 10.1007/BF00349373. [DOI] [Google Scholar]
- Hoeger U, Rebscher N, Geier G. Metabolite supply in growing oocytes of Nereis virens: role of nucleosides. Hydrobiologia. 1999;402:163–174. doi: 10.1023/A:1003792525942. [DOI] [Google Scholar]
- Hoeger U. Hydrolytic enzymes in the coelomic cells of the polychaete Nereis virens during sexual maturation. Marine Biology. 1991;110:7–12. doi: 10.1007/BF01313086. [DOI] [Google Scholar]
- Hofmann DK. Regeneration and endocrinology in the polychaete Platynereis dumerilii. Wilhelm Roux's Archives of Developmental Biology. 1976;180:47–71. doi: 10.1007/BF00848884. [DOI] [PubMed] [Google Scholar]
- Homola E, Chang ES. Methyl farnesoate: crustacean juvenile hormone in search of functions. Comparative Biochemistry and Physiology Part B. 1997;117:347–356. doi: 10.1016/S0305-0491(96)00337-9. [DOI] [Google Scholar]
- Hébert Chatelain É, Breton S, Lemieux H, Blier PU. Epitoky in Nereis (Neanthes) virens (Polychaeta: Nereididae): a story about sex and death. Comparative Biochemistry and Physiology Part B. 2008;149:202–208. doi: 10.1016/j.cbpb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Mladenov PV. Terminal spawning in the deepwater squid Moroteuthis ingens (Cephalopoda: Onychoteuthidae) Journal of Zoology. 1994;234:189–201. doi: 10.1111/j.1469-7998.1994.tb06067.x. [DOI] [Google Scholar]
- Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genetics. 2015;11:e1005394. doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay J, Thornton JW. Hormone-activated estrogen receptors in annelid invertebrates: implications for evolution and endocrine disruption. Endocrinology. 2009;150:1731–1738. doi: 10.1210/en.2008-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer H, Biggers WJ. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. American Zoologist. 2001;41:442–457. doi: 10.1093/icb/41.3.442. [DOI] [Google Scholar]
- Laufer H, Borst D, Baker FC, Reuter CC, Tsai LW, Schooley DA, Carrasco C, Sinkus M. Identification of a juvenile hormone-like compound in a crustacean. Science. 1987;235:202–205. doi: 10.1126/science.235.4785.202. [DOI] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Research. 2011;39:W475–W478. doi: 10.1093/nar/gkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART: recent updates, new developments and status in 2015. Nucleic Acids Research. 2015;43:D257–D260. doi: 10.1093/nar/gku949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minh BQ, Nguyen MA, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution. 2013;30:1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, Oda S, Tatarazako N, Miura T, Colbourne JK, Iguchi T. A mutation in the receptor Methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nature Communications. 2013;4:1856. doi: 10.1038/ncomms2868. [DOI] [PubMed] [Google Scholar]
- Mommsen TP. Salmon spawning migration and muscle protein metabolism: the August Krogh principle at work. Comparative Biochemistry and Physiology Part B. 2004;139:383–400. doi: 10.1016/j.cbpc.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Müller WA. Autoradiographische Untersuchungen über die synthetische Aktivität neurosekretorischer Zellen im Gehirn von Platynereis dumerilii während der sexuellen Entwicklung und Regeneration. Zeitschrift Für Zellforschung Und Mikroskopische Anatomie. 1973;139:487–510. doi: 10.1007/BF02028390. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer K, Dorresteijn AW, Fröbius AC. Activation of Hox genes during caudal regeneration of the polychaete annelid Platynereis dumerilii. Development Genes and Evolution. 2012;222:165–179. doi: 10.1007/s00427-012-0402-z. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria: 2015. [Google Scholar]
- Raible F, Tessmar-Raible K, Osoegawa K, Wincker P, Jubin C, Balavoine G, Ferrier D, Benes V, de Jong P, Weissenbach J, Bork P, Arendt D. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science. 2005;310:1325–1326. doi: 10.1126/science.1119089. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Garcí ME, Troncoso JS, Guerra Ángel. Spawning strategy in Atlantic bobtail squid Sepiola atlantica (Cephalopoda: Sepiolidae) Helgoland Marine Research. 2011;65:43–49. doi: 10.1007/s10152-010-0199-y. [DOI] [Google Scholar]
- Schenk S, Hoeger U. Lipid accumulation and metabolism in polychaete spermatogenesis: Role of the large discoidal lipoprotein. Molecular Reproduction and Development. 2010;77:710–719. doi: 10.1002/mrd.21208. [DOI] [PubMed] [Google Scholar]
- Schneiderman HA, Gilbert LI. Substances with juvenile hormone activity in crustacea and other invertebrates. The Biological Bulletin. 1958;115:530–535. doi: 10.2307/1539115. [DOI] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. PNAS. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Analytical Biochemistry. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Subramoniam T. Mechanisms and control of vitellogenesis in crustaceans. Fisheries Science. 2011;77:1–21. doi: 10.1007/s12562-010-0301-z. [DOI] [Google Scholar]
- Sullivan JJ, Goh KS. Environmental fate and properties of pyriproxyfen. Journal of Pesticide Science. 2008;33:339–350. doi: 10.1584/jpestics.R08-02. [DOI] [Google Scholar]
- Teal PE, Jones D, Jones G, Torto B, Nyasembe V, Borgemeister C, Alborn HT, Kaplan F, Boucias D, Lietze VU. Identification of methyl farnesoate from the hemolymph of insects. Journal of Natural Products. 2014;77:402–405. doi: 10.1021/np400807v. [DOI] [PubMed] [Google Scholar]
- Tessmar-Raible K, Raible F, Christodoulou F, Guy K, Rembold M, Hausen H, Arendt D. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Thummel CS. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell. 1995;83:871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- Toyota K, Miyakawa H, Hiruta C, Furuta K, Ogino Y, Shinoda T, Tatarazako N, Miyagawa S, Shaw JR, Iguchi T. Methyl farnesoate synthesis is necessary for the environmental sex determination in the water flea Daphnia pulex. Journal of Insect Physiology. 2015;80:22–30. doi: 10.1016/j.jinsphys.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Tsukimura B, Nelson WK, Linder CJ. Inhibition of ovarian development by methyl farnesoate in the tadpole shrimp, Triops longicaudatus. Comparative Biochemistry and Physiology Part A. 2006;144:135–144. doi: 10.1016/j.cbpa.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Walker AN, Bush P, Puritz J, Wilson T, Chang ES, Miller T, Holloway K, Horst MN. Bioaccumulation and metabolic effects of the endocrine disruptor methoprene in the lobster, Homarus americanus. Integrative and Comparative Biology. 2005;45:118–126. doi: 10.1093/icb/45.1.118. [DOI] [PubMed] [Google Scholar]
- Westerlund SA, Hoffmann KH. Rapid quantification of juvenile hormones and their metabolites in insect haemolymph by liquid chromatography-mass spectrometry (LC-MS) Analytical and Bioanalytical Chemistry. 2004;379:540–543. doi: 10.1007/s00216-004-2598-x. [DOI] [PubMed] [Google Scholar]
- Wootton JA, Wright LD. A comparative study of sterol biosynthesis in Annelida. Comparative Biochemistry and Physiology. 1962;5:253–264. doi: 10.1016/0010-406X(62)90055-5. [DOI] [PubMed] [Google Scholar]
- Wyatt GR. Juvenile hormone in insect reproduction - a paradox? European Journal of Entomology. 1997;94:323–333. [Google Scholar]
- Zandee DI. Absence of sterol synthesis in some arthropods. Nature. 1964;202:1335–1336. doi: 10.1038/2021335a0. [DOI] [PubMed] [Google Scholar]
- Zantke J, Bannister S, Rajan VB, Raible F, Tessmar-Raible K. Genetic and genomic tools for the marine annelid Platynereis dumerilii. Genetics. 2014;197:19–31. doi: 10.1534/genetics.112.148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zantke J, Ishikawa-Fujiwara T, Arboleda E, Lohs C, Schipany K, Hallay N, Straw AD, Todo T, Tessmar-Raible K. Circadian and circalunar clock interactions in a marine annelid. Cell Reports. 2013;5:99–113. doi: 10.1016/j.celrep.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]