Abstract
Background
Swimming-induced pulmonary edema (SIPE) occurs during swimming or scuba diving, often in young individuals with no predisposing conditions, and its pathophysiology is poorly understood. This study tested the hypothesis that pulmonary artery and pulmonary artery wedge pressures are higher in SIPE-susceptible individuals during submerged exercise compared to the general population and are reduced by sildenafil.
Methods and Results
Ten study subjects with a history of SIPE (mean age 41.6 years) and 20 control subjects (mean age 36.2 years) were instrumented with radial artery and pulmonary artery catheters and performed moderate cycle ergometer exercise for 6–7 minutes while submersed in 20°C water. SIPE-susceptible subjects repeated the exercise 150 minutes after oral administration of 50 mg sildenafil. Work rate and mean arterial pressure during exercise were similar in controls and SIPE-susceptibles. Average VO2 and cardiac output (CO) in SIPE-susceptibles and controls were: VO2 2.42 L.min−1 vs. 1.95 L.min−1, P=0.2; CO 17.9 L.min−1 vs. 13.8 L.min−1, P=0.01). Accounting for differences in CO between groups, mean pulmonary artery pressure (MPAP) at CO=13.8 L.min−1 was 22.5 mmHg in controls vs. 34.0 mmHg in SIPE-susceptibles (P=0.004) and the corresponding pulmonary artery wedge pressure (PAWP) 11.0 mmHg vs. 18.8 mmHg (P=0.028). After sildenafil, there were no statistically significant differences in MPAP or PAWP between SIPE-susceptibles and controls.
Conclusions
These observations confirm that SIPE is a form of hemodynamic pulmonary edema. The reduction in pulmonary vascular pressures after sildenafil with no adverse effect on exercise hemodynamics suggests that it may be useful in SIPE prevention.
Clinical Trial Registration Information
ClinicalTrials.gov. Identifier: NCT00815646.
Keywords: Pulmonary edema, Immersion, Hemodynamics, Diving
Journal Subject Terms: Hemodynamics, Pathophysiology
INTRODUCTION
Immersion pulmonary edema (IPE), also known as swimming-induced pulmonary edema (SIPE), is a condition in which cough, dyspnea, hemoptysis and hypoxemia develop after surface swimming or diving, often in young, healthy individuals. Wilmshurst1 first described SIPE in 11 healthy recreational divers. Although first believed to be extremely rare, nearly 300 cases have since been published, including several that describe the syndrome in healthy military recruits during strenuous swimming.2–7 Among military recruits, its prevalence in 2.4–3.6-km open sea swimming trials has been reported between 1.8%–60%, depending upon severity.2,6 In triathletes, 1.4% have reported symptoms consistent with SIPE.8
SIPE usually resolves spontaneously within 24 hours, or with β2 adrenergic agonist or diuretic therapy, but it can be fatal.9,10 Individuals who develop SIPE often have recurrences under the same conditions.2,11,12,6,1 Proposed risk factors for SIPE include cold water,11,12,1 negative static lung load, 5,13 exertion,11,12,4,6,7 fluid loading,7 and low vital capacity.6 Many who experience SIPE have chronic hypertension or develop it later,14–16,8,1 but many cases occur among individuals without hypertension, especially young military recruits, who undergo careful medical screening.2–7
The pathophysiology of SIPE is not fully understood. In one study, an analysis of specimens obtained via bronchoalveolar lavage ruled out an inflammatory process.17 Some instances of SIPE appear to have been precipitated by ventricular dysfunction,9,5 and indeed, transient cardiac abnormalities have been described immediately after an event.16 However, in most cases, cardiac function during recovery is normal.14,16,11,12,17,5,18,10 A hemodynamic cause cannot be reasonably excluded on the basis of post hoc resting measurements on dry land, particularly in view of both plausible rationale and physiological and observational evidence. During immersion in water central redistribution of blood from the extremities occurs,19 and is augmented when the water is cold.20 The resulting engorgement of the central veins, heart and pulmonary vessels causes increased right sided intravascular pressures.21 Wilmshurst and colleagues demonstrated a greater increase in forearm vascular resistance in response to exposure of the head and neck to ice-cold water is greater in SIPE-susceptible individuals compared with control subjects.1 They proposed that hydrostatic pulmonary edema occurs in susceptible individuals due to a combination of immersion-induced central redistribution of blood and idiosyncratic increase in afterload response due to cold. When swimming in the lateral decubitus position, predominantly unilateral edema occurs in the dependent lung, suggesting a hemodynamic mechanism.3,4
This study was performed to advance understanding of SIPE pathogenesis by testing the hypothesis that SIPE-susceptible individuals have higher mean pulmonary artery and pulmonary artery wedge pressures (MPAP and PAWP) during exercise in cold water, compared to the general population. We also tested whether prophylactic sildenafil can attenuate the increase, with the aim of reducing the risk of SIPE.
METHODS
Subjects
After institutional approval and informed consent, ten healthy individuals 18–55 years old, with a history of one or more episodes of SIPE, were recruited from a group of 71 who were screened for the study (ClinicalTrials.gov NCT00815646). Findings were compared to 20 controls who had no history of SIPE and who had participated in other IRB-approved studies, which, in part, have been previously reported.22,21 All subjects had a normal physical exam, chest radiograph, spirometry (FVC, FEV1 and FEF25–75) and 12-lead electrocardiogram. Prior to recruitment, nine SIPE subjects had been evaluated for coronary artery disease, using exercise stress echocardiogram, nuclear imaging, or coronary angiography. For the control subjects, exclusion criteria were cardiovascular disease, abnormal spirometry (FVC, FEV1, FEF25–75), maximum oxygen consumption (VO2max) < 30 mL.kg.min−1, estimated body fat >3% higher than age- and sex-based upper limits, abnormal ECG, age >55 years or pregnancy. The same exclusions, except the body fat criterion, applied to the SIPE subjects. SIPE subjects with a history of mild hypertension were admitted to the study if blood pressure was normal while taking medication.
Instrumentation
Methods have been previously described.22,21 Briefly, on the morning of the study, each subject was instrumented with radial artery and pulmonary artery catheters placed via an antecubital or arm vein. Placement of the catheter tip in the pulmonary artery was confirmed radiographically. Pressure transducers (Hospira, Lake Forest, IL) were calibrated immediately before each run, using an aneroid gauge that had been pre-calibrated against a mercury manometer. All signals were digitized with a data acquisition board (PCI 6014, National Instruments, Austin, TX) and recorded on a personal computer using Labview (version 6.1, National Instruments, Austin, TX).
Protocol
On the day before the study, the capacity of each subject to perform dry exercise was tested on a cycle ergometer for 12 minutes to a maximum of 150 W. Subjects were then familiarized with the immersed environment by exercising for 9–12 minutes in the water to a maximum of 125 W external power.
On the day of the study, SIPE subjects were first evaluated during supine dry rest. Dry resting measurements in control subjects were conducted in the upright position (sitting on an exercise bike) with the transducers situated 5 cm inferior to the sternal angle. To measure the hemodynamic effect of rapid submersion, ten of the control subjects and all of the SIPE subjects were placed in the prone position on a rescue litter breathing via a scuba regulator and immersed as quickly as possible in cold water for 2–3 minutes (“dunk”, see Fig. 1 in Wester21). Heart rate (HR), mean arterial pressure (MAP), mean pulmonary artery pressure (MPAP) and pulmonary artery wedge pressure (PAWP) were measured immediately before submersion and at one minute afterward. During this pre-exercise maneuver, pressure transducers were positioned at the level of the subject’s mid-thorax until the subject hit the water, at which point the transducer position was maintained at the water surface level. During underwater exercise, the transducer level was positioned at the level of the water surface. Pressures were averaged over several respiratory cycles. Effective arterial elastance (Ea) was calculated as (2 × Psys+Pdia)/(3× stroke volume),23 where Psys and Pdia represent systolic and diastolic arterial pressures. Pulmonary artery compliance CPA was calculated as stroke volume/PA pulse pressure.24
Exercise on an electronically braked cycle ergometer was then performed for six minutes at 60 rpm while prone and fully submersed to a depth of approximately 50 cm in a pool (volume 4.42 m3) filled with water at 18°C–20°C, as previously described.21 External work rate was set according to the estimated exercise capacity of each subject, which was typically 100–125 W (150–175 W total work rate including the work of moving the legs through the water, previously estimated at 50 W). HR, MAP, MPAP and PAWP were measured immediately before the sixth minute of exercise. In control subjects, resting measurements were also taken, several minutes after the dunk. Resting measurements were not obtained in SIPE subjects in order to minimize the time of exposure to cold water and the risk of SIPE.
Expired gas volume was collected in Douglas bags over one minute during the 5th and 6th minutes of exercise and the volume of each was measured using a calibrated gasometer (model DTM 325–4, American Meter, Nebraska City, NE). Samples of mixed O2 and CO2 expired gas were collected from each bag and measured using mass spectrometry (model 1100 medical gas analyzer, Perkin-Elmer, Pomona, CA), confirmed with gas chromatography (model 3800, Varian, Palo Alto, CA). Arterial and mixed venous blood samples were collected anaerobically in heparinized glass syringes over a 15- to 20-second period during the sixth minute and chilled on ice. Within 15 minutes, the blood samples were analyzed using a blood gas analyzer (Synthesis 15, Instrumentation Laboratory, Lexington, MA) and CO-oximeter (model 682, Instrumentation Laboratory). Concentrations of expired O2 and CO2 were measured using mass spectrometry (model 1100 medical gas analyzer, Perkin-Elmer, Pomona, CA) and confirmed with gas chromatography (model 3800, Varian, Palo Alto, CA). Standard equations were used to calculate oxygen consumption, which was then used to calculate cardiac output in the Fick equation.
Following the first exercise, the SIPE subjects were given 50 mg sildenafil orally (Pfizer, New York, NY). Approximately 150 minutes after sildenafil administration, the protocol was repeated. After each exercise, SIPE subjects were examined for clinical evidence of SIPE and performed spirometry.
Statistical Methods
Unpaired t-tests were used to compare continuous variables between groups obtained under identical circumstances, with correction for multiple comparisons (Tukey-Kramer); paired t-tests were used for comparisons within each group. Categorical variables were compared using Fisher’s exact test. Hemodynamic responses that depended on cardiac output (CO), i.e., systemic and pulmonary vascular pressures and vascular resistances, were compared among the three conditions (controls, SIPE-susceptible before and after sildenafil) using a repeated-measures analysis of covariance, where the covariable was CO (PROC MIXED, SAS 9.3, SAS Institute, Cary, NC, USA). This model allowed pairwise comparisons among the three conditions, adjusted for post-hoc multiple comparisons (Tukey-Kramer), while accounting for the repeated measures within subjects and adjusting for the variable levels of exercise (CO). Given the linear relationship between MPAP and PAWP vs CO within the range of cardiac outputs in this study,25 model estimates between SIPE-susceptible and control subjects were made at the CO of the SIPE-susceptibles (13.8 L.min−1). P<0.05 was considered statistically significant.
Role of the Funding Sources
The funding agencies for this study funded the development of the experimental system and the costs of each study. The funding agencies played no role in study design, data acquisition, or analysis. The investigators and all authors had sole discretion in the data analysis and interpretation, writing of the manuscript and the decision to submit for publication.
RESULTS
Subject Characteristics
A summary of subject recruitment and baseline characteristics is shown in Tables 1 and 2. Table 3 provides details on each SIPE-susceptible subject. The SIPE-susceptible group had a greater proportion of females compared to the control group, but otherwise, there were no statistically significant demographic differences between the two groups. VO2max of the control group was 44.8±8.2 mL.kg.min−1. Two subjects had experienced SIPE while diving, five during a triathlon or in training for a triathlon and two during both. Another subject experienced SIPE when she fell off her windsurfer into a cold river. Echocardiography showed mild left ventricular hypertrophy in subjects 1 and 5. Both ran regularly; one was a triathlete and marathon runner. The echo findings were consistent with “athlete’s heart”. All other subjects had normal echocardiography. Coronary artery disease had previously been excluded by exercise stress echo in six subjects, nuclear stress testing in two subjects and coronary angiography in one subject. Stress testing was not performed in one subject due to her young age (31 years) and regular high-level exercise. One subject was taking candesartan for hypertension. Blood pressure was normal in all subjects during the screening assessment and before the study.
Table 1.
SIPE-Susceptible Subject Recruitment.
| Summary | N |
|---|---|
| Volunteers screened | 71 |
| Excluded | 53 |
| Exceeded maximum age (55 years) | 18 |
| Uncontrolled hypertension | 5 |
| Asthma | 4 |
| Diabetes | 1 |
| Other medical* | 5 |
| Insufficient information to determine eligibility | 23 |
| No past SIPE history (misunderstood study criteria) | 1 |
| Eligible volunteers | 18 |
| Declined to participate | 8 |
| Volunteers studied | 10 |
Pulmonary hypertension (2), sleep apnea and obesity (1)
Table 2.
Subject Characteristics.
| SIPE-Susceptible | Control | P | |
|---|---|---|---|
| N | 10 | 20 | |
| Age (y) | 41.6±7.8 | 36.2±8.3 | 0.09 |
| BMI (kg.m−2) | 25.7±3.9 | 26.0±2.2 | 0.8 |
| M/F | 4/6 | 18/2 | 0.007 |
| Race | 1.0 | ||
| White | 10 | 19 | |
| African | 0 | 1 | |
| American |
Results shown as mean±SD.
BMI, body mass index.
Table 3.
Subjects with Previous SIPE.
| Subj. # | Age (y) | Sex | Ht. (m) | Wt. (kg) | BMI (kg/m2) | History |
|---|---|---|---|---|---|---|
| 1 | 37 | M | 1.85 | 98.8 | 28.7 | Closed circuit rebreather diver, marathon runner; developed shortness of breath and cough during a dive to 43 m. In-hospital SpO2 was 95% on 4 L/min O2. Chest radiograph – pulmonary edema. Echo – no valve disease, mild LVH. Normal systolic and diastolic properties. EKG – intraventricular conduction defect. Stress echo (Bruce stage 5, maximum HR 173 bpm) normal. |
| 2 | 45 | M | 1.78 | 93.0 | 29.4 | Triathlete and recreational diver. Bicycle exercise – up to 145 km at least once a week and then 48–64 km 2–3 other times. Plays soccer 3–4 times a week or swims 1.6 km or runs 8–10 km. Meds – mirtazapine, escitalopram, buproprion. Previous exertional asthma. Recreational diver. Dived to 32 m for 29 minutes breathing 30% O2. During decompression, developed coughing and dyspnea. Chest x-ray – pulmonary edema. EKG – incomplete RBBB. Normal troponin and BNP 83.5. Stress echo normal (17.2 METs), including diastolic function. |
| 3 | 47 | F | 1.63 | 58.9 | 22.3 | Triathlete with 5 episodes of immersion pulmonary edema. Treated for mild hypertension with candesartan 16 mg/day. Stress echo (Bruce stage 7, maximum HR 190 bpm) normal. Took candesartan before study and BP was normal. |
| 4 | 33 | F | 1.70 | 72.6 | 25.1 | Triathlete with 5–6 episodes of immersion pulmonary edema. One occasion, SpO2 92% RA with bilateral pulmonary edema on chest x-ray and CT. EKG normal. Echo normal, no LVH. RV normal. No valve disease. Slight MR. Several months after the study, diagnosed with mild hyperthyroidism. |
| 5 | 37 | M | 1.82 | 86.8 | 26.3 | Two episodes of immersion pulmonary edema during triathlons, 2 episodes while scuba diving. Trivial MR, normal systolic function. Mild LVH. Coronary angiography normal. |
| 6 | 49 | F | 1.60 | 53.2 | 20.8 | Experienced SIPE while windsurfing after falling off her board into cold water, after which she experienced dyspnea and began coughing up pink frothy sputum. Admitted to hospital, with hypoxemia and pulmonary edema. Troponin-I peaked at 0.42 ng/mL (normal 0–0.05). EKG normal except for possible left atrial enlargement. ProBNP reached a high of 351 pg/mL (normal 0–124) the day after admission. Stress echo (Bruce stage 5, peak HR 173 bpm) normal. Transthoracic echocardiography normal with no wall motion abnormalities. LV thickness normal. |
| 7 | 35 | M | 1.78 | 84.5 | 26.7 | Scuba diver with multiple episodes of cough that produced pink sputum during descent or level swimming underwater. Stress echo (15.4 METs) normal. Normal diastolic function. Trivial MR and TR. |
| 8 | 53 | F | 1.68 | 57.3 | 20.4 | Scuba diver and triathlete. Four episodes of SIPE (2 each during scuba and triathlons) with dyspnea, productive cough while swimming or diving. Nuclear stress test to maximum HR 169 bpm negative for ischemia. Normal EF. Echo normal. Mild TR and PR. LVEF >55%. |
| 9 | 49 | F | 1.73 | 73.5 | 24.6 | Triathlete with 5 or more episodes of cough, dyspnea and some pink-tinged sputum during swim portion. LVEF 59%. Exercise EKG to 13.8 METs with nuclear imaging negative for ischemia. |
| 10 | 31 | F | 1.73 | 97.1 | 32.5 | PDA closure at age 5 months. Teaching water aerobics 40 minutes a week. Training for decathlon. Regular kick boxing, biking and running (total running+biking 5–6 hours per week). SIPE during her first swim in cold water in preparation for a triathlon. In-hospital SpO2 was 83%. Chest x-ray and CT scan – pulmonary edema. Echo – normal LV function with no evidence of valve disease. Serum troponin I, BNP and EKG normal. |
BNP brain natriuretic peptide; LV, left ventricle; LVEF, LV ejection fraction; LVH, left ventricular hypertrophy; RV, right ventricle; MR, mitral regurgitation; PDA, patent ductus arteriosus; ProBNP, pro-brain natriuretic peptide; RA, room air; RBBB, right bundle branch block; TR, tricuspid regurgitation.
All subjects completed the study with no adverse effects and with no symptoms, abnormal breath sounds, or changes in spirometry to suggest pulmonary edema.
Supine, Dry Measurements in SIPE-Susceptible Group and Cold Water “Dunk”
Hemodynamic variables of the SIPE-susceptible volunteers in the dry, supine position were normal (Table 4). We have previously observed that sitting at rest on an exercise bicycle, where there is little peripheral muscle tone, often induces low right sided pressures. Thus, dry measurements in the control subjects are not directly comparable to the supine measurements in the SIPE-susceptible group, although they were within normal limits (see Wester21 for 10 of these control measurements). Following sildenafil administration, heart rate and cardiac output were higher (P=0.0141 and 0.0053); systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) were lower (P=0.0007 and 0.017) (Table 4). During the pre-exercise “dunk,” pulmonary artery pressure was greater in the SIPE-susceptible group (P=0.0032, Table 5). Sildenafil significantly attenuated the systemic and pulmonary hypertensive responses to rapid immersion in cold water.
Table 4.
Resting, Supine Characteristics of SIPE Subjects in the Dry.
| Pre-Sildenafil | Post-Sildenafil | P | |
|---|---|---|---|
| HR (bpm) | 63.4±14.9 | 71.5±16.0 | 0.0141* |
| CO (L.min−1) | 6.1±1.2 | 8.1±2.1 | 0.0053* |
| MAP (mmHg) | 99.2±10.6 | 93.0±9.8 | 0.0233* |
| MPAP (mmHg) | 18.8±4.1 | 17.2±4.2 | 0.2 |
| PAWP (mmHg) | 13.0±3.2 | 12.9±4.6 | 0.7 |
| CVP (mmHg) | 7.8±2.6 | 6.6±5.2 | 0.3 |
| SVR (dyn.s.cm−5) | 1249±226 | 915±258 | 0.0007* |
| PVR (dyn.s.cm−5) | 78±27 | 49±14 | 0.0170* |
| Ea (mmHg.mL−1) | 1.37±0.40 | 1.11±0.29 | 0.0119* |
| CPA (mL.mmHg−1) | 6.25±2.06 | 8.78±3.08 | 0.0121* |
Results shown as mean±SD.
Statistically significant when compared to pre-sildenafil.
CO, cardiac output; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; CVP, central venous pressure; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; Ea, effective arterial elastance, CPA, pulmonary artery compliance (see Methods).
Table 5.
Hemodynamic Effects of Rapid Submersion in 20°C Water (“Dunk”) in All Subjects.
| P values
|
||||||
|---|---|---|---|---|---|---|
| Variable | Controls | SIPE-Susceptible Pre-Sildenafil | SIPE-Susceptible Post-Sildenafil | Pre-S vs. C | Post-S vs. C | Post-S vs. Pre-S |
| HR (bpm) | 93.7±25.8 | 85.2±16.0 | 88.8±16.9 | 0.6 | 0.8 | 0.2 |
| SBP (mmHg) | 195.4±28.9 | 224.3±28.3 | 204.8±28.3 | 0.06 | 0.7 | 0.0008* |
| DBP (mmHg) | 94.0±9.3 | 94.8±13.5 | 86.6±9.3 | 1.0 | 0.3 | 0.0261* |
| MAP (mmHg) | 125.2±13.0 | 133.5±13.9 | 123.9±12.7 | 0.4 | 1.0 | 0.0026* |
| MPAP (mmHg) | 21.7±3.4 | 29.2±6.0 | 24.6±4.1 | 0.0032* | 0.4 | 0.0219* |
| PAWP (mmHg) | 13.5±4.1 | 18.1±3.9 | 15.8±5.1 | 0.07 | 0.5 | 0.1 |
| CVP (mmHg) | 6.9±3.5 | 10.2±3.6 | 7.1±3.2 | 0.1 | 1.0 | 0.0404* |
Results are shown as mean±SD.
Statistically significant.
HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; CVP, central venous pressure.
Exercise Measurements
Hemodynamic and ventilatory parameters during exercise are listed in Table 6. Mean external work rate for the control subjects was 107.8 W (range 50–170 W) and 112.5 W (range 75–200 W) for the SIPE-susceptible subjects. V̇O2 during exercise was lower in the SIPE-susceptible group but the difference was not statistically significant. Tidal volume during exercise was lower in the SIPE-susceptible group (P=0.0036) with no significant difference in respiratory minute volume. Ventilatory frequency was not different between the two groups. CO was lower in the SIPE group (P=0.01). SVR was higher in the SIPE-susceptible group (P=0.0106). Blood gases were not significantly different between groups except after sildenafil, when pH was slightly higher compared to both control (P=0.0087) and pre-sildenafil (P=0.02), and PaO2 was higher in the SIPE-susceptible group compared to pre-sildenafil (P=0.0337).
Table 6.
Hemodynamics and Gas Exchange Measurements in Controls and SIPE Subjects During Exercise, Unadjusted for Cardiac Output.
| P values
|
||||||
|---|---|---|---|---|---|---|
| Controls | SIPE-Susceptible Pre-Sildenafil | SIPE-Susceptible Post-Sildenafil | Pre-S vs. C | Post-S vs. C | Post-S vs. Pre-S | |
| External Work (W)† | 107.8±25.5 | 112.5±37.7 | 112.5±37.7 | 0.9 | 0.9 | 1.0 |
| V̇O2 (L.min−1 STPD) | 2.42±0.46 | 1.95±0.52 | 1.97±0.57 | 0.06 | 0.07 | 0.6 |
| V̇O2 (L.min−1 STPD.kg−1) | 29.1±4.6 | 25.6±5.7 | 25.9±6.2 | 0.2 | 0.3 | 0.5 |
| V̇E (L.min−1 BTPS) | 81.9±20.1 | 59.4±25.0 | 61.0±23.9 | 0.04 | 0.06 | 0.2 |
| Vt (L BTPS) | 2.85±0.50 | 2.12±0.63 | 2.20±0.51 | 0.0036* | 0.0103* | 0.3 |
| Vf (breaths.min−1) | 29.5±8.2 | 28.6±7.8 | 27.8±6.9 | 1.0 | 0.8 | 0.4 |
| HR (bpm) | 143.4±20.4 | 134.6±22.6 | 137.8±19.5 | 0.5 | 0.8 | 0.1 |
| CO (L.min−1) | 17.9±3.4 | 13.8±2.6 | 14.8±4.0 | 0.01* | 0.06 | 0.1 |
| Stroke volume (mL) | 126.4±26.4 | 105.7±31.2 | 109.2±34.9 | 0.2 | 0.3 | 0.3 |
| MAP (mmHg) | 126.2±12.0 | 129.6±14.7 | 128.1±13.3 | 0.8 | 0.9 | 0.7 |
| MPAP (mmHg) | 27.2±6.2 | 34.0±5.7 | 29.4±7.2 | 0.02 | 0.6 | 0.0208* |
| PAWP (mmHg) | 13.1±5.0 | 18.9±5.5 | 16.9±6.2 | 0.03 | 0.2 | 0.1 |
| CVP (mmHg) | 7.1±5.4 | 7.8±5.1 | 5.3±3.3 | 0.9 | 0.6 | 0.3 |
| SVR (dyn.s.cm−5) | 546±107 | 724±138 | 710±211 | 0.0106* | 0.02 | 0.6 |
| PVR (dyn.s.cm−5) | 62.6±15.1 | 84.2±22.4 | 69.3±24.8 | 0.03 | 0.7 | 0.0198* |
| CPA (mL.mmHg−1) | 4.00±0.82 | 3.38±0.75 | 3.74±0.94 | 0.2 | 0.7 | 0.1 |
| Ea (mmHg.mL−1) | 1.48±0.38 | 1.79±0.41 | 1.79±0.50 | 0.06 | 0.07 | 0.9 |
| PaO2 (mmHg) | 107.9±8.9 | 102.1±8.0 | 99.2±5.1 | 0.15 | 0.02 | 0.0337* |
| PaCO2 (mmHg) | 30.7±4.6 | 36.0±5.1 | 35.7±4.7 | 0.02 | 0.03 | 0.7 |
| Arterial pH | 7.31±0.05 | 7.35±0.03 | 7.36±0.03 | 0.07 | 0.0087* | 0.02* |
Results shown as mean±SD.
Not including resistive work due to motion of legs through the water, which adds approximately 50 W.
Statistically significant.
V̇O2, oxygen consumption; V̇E, respiratory minute volume; Vt, tidal volume; Vf, ventilatory frequency; HR, heart rate; CO, cardiac output; MAP, mean arterial pressure; CVP, central venous pressure; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; PaO2, PaCO2, arterial PO2 and PCO2, respectively (see Methods).
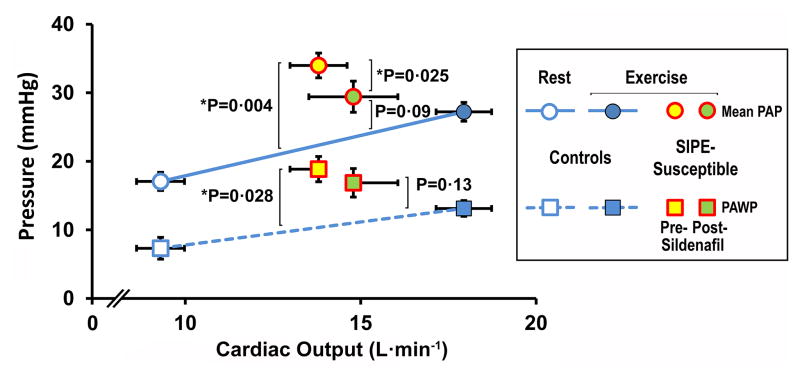
After accounting for differences in CO, both MPAP and PAWP were higher in the SIPE group than in controls during exercise (P=0.004 and P=0.028, respectively), as shown in Fig. 1. After sildenafil, there was a significant decrease in PAP, and neither MPAP nor PAWP in the SIPE group were significantly different from controls. Differences in MAP or CVP among groups were not statistically significant. Similarly, when SVR and PVR in the control group were model-estimated at the cardiac output of the SIPE-susceptible group, there were no differences between SIPE-susceptible individuals and controls, and no effect of sildenafil. During immersed exercise there was no difference between controls and SIPE-susceptible subjects in CPA, either before or after sildenafil.
Figure 1.
Mean PAP and PAWP vs cardiac output. Control subjects were studied at rest and during exercise, while SIPE-susceptible subjects were studied only during exercise. Accounting for differences in cardiac output, mean PAP and PAWP were significantly higher in the SIPE-susceptible group compared to controls (P=0.004 and P=0.028, respectively). After sildenafil, mean PAP was significantly reduced (P=0.025). During the post-sildenafil exercise, neither mean PAP nor PAWP was significantly different from controls. PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure.
DISCUSSION
Our findings indicated an exaggerated increase in MPAP and PAWP during exercise in individuals who have experienced SIPE, supporting the Wilmshurst findings.1 Despite similar external work rates, VO2, VE, HR, CO and arterial pH measures indicated that SIPE-susceptible subjects were not working as hard as the control subjects even though their MPAP and PAWP values were higher. The greater MPAP and PAWP during exercise provide a hemodynamic explanation for SIPE susceptibility. Possible explanations for the lower metabolic rate in the SIPE-susceptible subjects despite similar external work rates include lower baseline oxygen consumption and differences in the rate of pedaling or in leg diameter, either of which would affect the work necessary to move the legs through the water.
The elevation in pulmonary vascular pressures during submersion is primarily due to central redistribution of blood from the extremities,26,19 which engorges the central veins, heart and pulmonary vessels causing higher intracardiac and intravascular pressures,26–28,21 This increase is augmented in cold water.20,21 In a normal lung, PAWP that acutely exceeds a critical value of 18–25 mmHg can cause hydrostatic alveolar edema. 29–31 Pulmonary capillary pressure has a value between MPAP and PAWP;32 thus, acute elevation in either parameter could cause a critical pressure at the alveolar interface due to immersion-related blood redistribution.
Several possible explanations could account for an exaggerated increase in pulmonary vascular pressures in SIPE-susceptible individuals.
Higher blood volume. Increased blood volume and the accompanying increase in cardiac filling pressures are induced by immersion and could be augmented by prior fluid loading, which, importantly, is sometimes encouraged before exercise, particularly in naval recruits before swim training.7 However, SIPE has been reported without fluid loading.6 Moreover, subjects in this experiment did not specifically consume excess fluid before the study.
Higher venous tone. This determines the degree to which capacitance vessels in the arms and splanchnic bed can accommodate blood displaced from the legs. Low venous tone (high venous capacitance) would allow more blood to be accommodated in these veins and thus attenuate immersion-related increases in MPAP and PAWP.28 Conversely, high venous tone, due to increased activity of the sympathetic nervous system 33,34 or mild hypertension,35 would result in higher blood volume in the heart and intrathoracic vessels due to peripheral to central redistribution. Indeed, previous studies in our laboratory have demonstrated that pulmonary artery and pulmonary artery wedge pressures are higher in thermoneutral water compared to the dry and even higher in cold water.21 Among experimental subjects we observed high variability in this response (nearly two-fold), consistent with a variable degree of venous tone. Plausibly, those with a greater increase in pulmonary vascular pressures may represent the subpopulation at greatest risk for SIPE.36 The reduction in MPAP after sildenafil suggests that there may have been active vasoconstriction, perhaps due to excessive sympathetic tone, possibly cold-related. PA compliance was similar between controls and SIPE-susceptibles, and was not affected by sildenafil, thus does not appear to play a role in SIPE-susceptibility.
Impaired left ventricular (LV) systolic function. Transient global myocardial dysfunction with normal coronary arteries has been reported in cases of SIPE.37,9,5 However, in most cases of SIPE, resting echocardiography after the event is normal16–18; and indeed, all SIPE-susceptible subjects in the present study had normal echocardiography, including LV systolic function.
Low diastolic LV compliance. While there was no diastolic dysfunction in their dry echo studies, central blood redistribution in the face of a stiffer left ventricle would lead to a higher LV end-diastolic pressure (LVEDP), PAWP and PA pressures. In normal individuals exercising in the dry, end-diastolic volume increases without a change in end-diastolic pressure.38 However, in individuals who have heart failure with preserved ejection fraction (HFpEF), the greater LV chamber stiffness causes LVEDP during exercise to increase.39 While none of our volunteers had clinical heart failure, the analogy is that augmented preload due to immersion in cold water a slightly greater left ventricular wall stiffness in SIPE-susceptible individuals could be the cause of higher LV filling pressure during exercise in cold water. Small increases in E/A and E/e′ ratios in extremely fit athletes have been attributed to LV remodeling due to prolonged exercise,40 and indeed seven of the 10 SIPE-susceptible subjects in this study were extremely physically fit. It has been proposed that increased arterial stiffness may predispose to diastolic dysfunction, especially among women.41–43 We did not observe a difference in Ea between SIPE-susceptible and control populations during exercise, although sildenafil did induce a statistically significant reduction in Ea in the SIPE-susceptible group during rest.
Compared to controls, the SIPE-susceptible group had a higher SVR during exercise and a greater increase in systolic blood pressure during the cold water “dunk,” which is consistent with an exaggerated peripheral vasoconstrictive response to cold.1 However, since cardiac output during exercise was lower in the SIPE-susceptible subjects, the calculated SVR values in the two groups are not directly comparable. When SVR in the control group was model-estimated at the cardiac output of the SIPE-susceptible group, there was no difference in SVR. Therefore, although cold exposure augmented afterload effects (blood pressure) to a greater degree in SIPE-susceptible subjects during the dunk at rest, during exercise the increased MPAP and PAWP in SIPE-susceptibles could not be attributed to high afterload. It is more likely that higher MPAP and PAWP in the SIPE-susceptible group during exercise are due to enhanced venoconstriction, which elicits increased preload, or lower left ventricular diastolic compliance. These effects, singly or in combination, would cause greater left ventricular filling pressure and hence higher MPAP and PAWP.
Sildenafil has pharmacological effects that probably account for the reduction in MPAP and PAWP in SIPE-susceptible individuals during exercise in cold water. A selective inhibitor of phosphodiesterase-5, sildenafil leads to an increase in intracellular cyclic GMP (cGMP) and relaxation of vascular smooth muscle, and has a small and transient effect on blood pressure and systemic vascular resistance.44 In our subjects, sildenafil administration was associated with a decrease in resting MAP and SVR and an increase in CO. During exercise, sildenafil reduced pulmonary vascular pressures and PVR, but had no effect on other hemodynamic variables. Although we did not assess it in this study, others have demonstrated that sildenafil induces an increase in venous compliance.44 Thus, the sildenafil-induced reduction in pulmonary vascular pressures observed in this study during submersed exercise is likely due to vasodilatation of both pulmonary vessels and peripheral veins. This study demonstrated a hemodynamic effect of sildenafil that may plausibly reduce the likelihood of pulmonary edema in SIPE-susceptible swimmers.
A multicenter randomized trial in patients with a history of HFpEF failed to observe an increase in exercise capacity (peak oxygen uptake during an incremental test) in response to sildenafil treatment.45 However, hemodynamic studies in this population have demonstrated reduced pulmonary artery pressure, PAWP, increased cardiac index, isovolumic relaxation time, increased cardiac output and endothelial function.46–48 Whereas in this study we are focusing on factors that may promote pulmonary edema, there are probably other factors limiting peak oxygen consumption in chronic heart failure such as deconditioning, on which sildenafil is unlikely to have an effect. Although we cannot conclude from this study that sildenafil provides prophylaxis against SIPE, one of our study subjects (subject 3) who had experienced several episodes of SIPE during triathlons, has had no further episodes since using pre-race sildenafil.
There are several shortcomings of our study. While our subjects were not randomly selected from the SIPE-susceptible or general population, we believe that the two groups are similar. In particular, potential subjects with co-morbidities associated with SIPE were excluded. Further, the hypertensive pulmonary vascular response to exercise in cold water that was observed in our subjects may likely be even more exaggerated in the general SIPE-susceptible population, which includes many hypertensives.5 The control group was 90% male, compared to 40% male in the SIPE-susceptible group, raising the possibility that the differences may be due to a fundamental sex-related phenomenon that is not connected with SIPE-susceptibility. We believe this is unlikely, as a previous study showed no gender-related effect on MPAP during rest or exercise in a previous study on 255 males and 101 females.49 The SIPE-susceptible group may also have been fitter than the controls. While none of the controls had experienced SIPE while swimming or diving the possibility that some of them by chance may have been SIPE-susceptible cannot be excluded. However, this is unlikely as in a fit civilian population (triathletes) only 1–2% report SIPE symptoms.8 Although the immersed exercise protocol was identical for both groups, pre-exercise measurements were obtained under different conditions (supine vs. sitting). Thus the congruence of the groups at baseline cannot be established with absolute certainty. Since it was not possible to randomize the order of the sildenafil administration we cannot exclude acute adaptation to the cold as the mechanism for post-sildenafil attenuation of the hemodynamic responses to the dunk and submersed exercise. We believe this is unlikely because the reduction in intravascular pressures during exercise after sildenafil was confined to MPAP and PAWP and others have reported constant norepinephrine response to cold-water (20°C) exposure during repetitive immersions during the same day.50 Furthermore, in our study the change in intravascular pressures during exercise in the second cold water exposure was confined to MPAP and PAWP, with no effect on systemic blood pressure. It could be argued that differences in exercise ventilation might have affected PAP and PAWP, however both PAP and PVR were lower in the control group despite lower pH, which would be expected to increase both parameters. While the observed change in PAWP after sildenafil was not statistically significant, because of the small sample size the possibility that sildenafil can reduce PAWP in this setting cannot be excluded.
In summary, we have observed that during submerged exercise in cold water, individuals with a history of swimming-induced pulmonary edema have higher MPAP and PAWP than those with no such history. We further demonstrated that these pressures can be reduced with a single 50-mg oral dose of sildenafil.
Clinical Perspectives.
Immersion pulmonary edema (IPE), also known as swimming-induced pulmonary edema (SIPE), occurs during surface swimming or scuba diving in susceptible individuals who are often young and healthy. SIPE usually resolves spontaneously within 24 hours, or with β2 adrenergic agonist or diuretic therapy, but it can be fatal. Some individuals have risk factors for SIPE that include cold water exposure, heavy exertion, fluid loading, hypertension, valve disease and cardiomyopathy. The pathophysiology of SIPE is not fully understood but indirect evidence suggests that it is a form of hemodynamic pulmonary edema caused by an exaggerated increase in pulmonary vascular pressures in response to exercise and immersion in water, especially in the cold. In this study a group of individuals with a history of SIPE and a control group without SIPE were studied during immersed exercise in 20°C water with invasive monitoring of radial and pulmonary artery pressures. Valve disease, cardiomyopathy and ischemic heart disease had been excluded. We confirmed that arterial, pulmonary artery and pulmonary artery wedge pressures were higher in SIPE-susceptible individuals. Mechanisms for the higher pulmonary vascular pressures could include higher blood volume, augmented venous tone and reduced diastolic left ventricular compliance. We further showed that these pressures were reduced by a single oral dose of sildenafil 50 mg, suggesting that sildenafil should be investigated as a possible prophylactic drug.
Acknowledgments
The authors are grateful to the following experts for their technical assistance: Albert Boso, Barry Castle, Owen Doar, Tommy Edwards, Eric Schinazi and Aaron Walker and to the volunteers who were willing to donate their time to participate in the study. The authors appreciate Kathy Gage’s constructive suggestions on the manuscript. Contributors: REM, SDM, DFP, JFP, TEW, ADC and JJF contributed to the study concept, design and conduct, analysis of data and writing of the manuscript. CO, DK and MN participated in the study conduct and data acquisition. WDW performed the statistical analysis.
Funding Sources: This study was funded by the Divers Alert Network and US Naval Sea Systems Command Contracts N61331-03-C-0015 and N0463A-07-C-0002.
Footnotes
Disclosures: None.
References
- 1.Wilmshurst PT, Nuri M, Crowther A, Webb-Peploe MM. Cold-induced pulmonary oedema in scuba divers and swimmers and subsequent development of hypertension. Lancet. 1989;1:62–5. doi: 10.1016/s0140-6736(89)91426-8. [DOI] [PubMed] [Google Scholar]
- 2.Adir Y, Shupak A, Gil A, Peled N, Keynan Y, Domachevsky L, Weiler-Ravell D. Swimming-induced pulmonary edema: clinical presentation and serial lung function. Chest. 2004;126:394–9. doi: 10.1378/chest.126.2.394. [DOI] [PubMed] [Google Scholar]
- 3.Lund KL, Mahon RT, Tanen DA, Bakhda S. Swimming-induced pulmonary edema. Ann Emerg Med. 2003;41:251–6. doi: 10.1067/mem.2003.69. [DOI] [PubMed] [Google Scholar]
- 4.Mahon RT, Kerr S, Amundson D, Parrish JS. Immersion pulmonary edema in special forces combat swimmers. Chest. 2002;122:383–4. doi: 10.1378/chest.122.1.383-a. [DOI] [PubMed] [Google Scholar]
- 5.Peacher DF, Martina SD, Otteni CE, Wester TE, Potter JF, Moon RE. Immersion pulmonary edema and comorbidities: case series and updated review. Med Sci Sports Exerc. 2015;47:1128–34. doi: 10.1249/MSS.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 6.Shupak A, Weiler-Ravell D, Adir Y, Daskalovic YI, Ramon Y, Kerem D. Pulmonary oedema induced by strenuous swimming: a field study. Respir Physiol. 2000;121:25–31. doi: 10.1016/s0034-5687(00)00109-2. [DOI] [PubMed] [Google Scholar]
- 7.Weiler-Ravell D, Shupak A, Goldenberg I, Halpern P, Shoshani O, Hirschhorn G, Margulis A. Pulmonary oedema and haemoptysis induced by strenuous swimming. BMJ. 1995;311:361–2. doi: 10.1136/bmj.311.7001.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller CC, 3rd, Calder-Becker K, Modave F. Swimming-induced pulmonary edema in triathletes. Am J Emerg Med. 2010;28:941–6. doi: 10.1016/j.ajem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Cochard G, Arvieux J, Lacour JM, Madouas G, Mongredien H, Arvieux CC. Pulmonary edema in scuba divers: recurrence and fatal outcome. Undersea Hyperb Med. 2005;32:39–44. [PubMed] [Google Scholar]
- 10.Slade JB, Jr, Hattori T, Ray CS, Bove AA, Cianci P. Pulmonary edema associated with scuba diving: case reports and review. Chest. 2001;120:1686–94. doi: 10.1378/chest.120.5.1686. [DOI] [PubMed] [Google Scholar]
- 11.Hampson NB, Dunford RG. Pulmonary edema of scuba divers. Undersea Hyperb Med. 1997;24:29–33. [PubMed] [Google Scholar]
- 12.Koehle MS, Lepawsky M, McKenzie DC. Pulmonary oedema of immersion. Sports Med. 2005;35:183–90. doi: 10.2165/00007256-200535030-00001. [DOI] [PubMed] [Google Scholar]
- 13.Thorsen E, Skogstad M, Reed JW. Subacute effects of inspiratory resistive loading and head-out water immersion on pulmonary function. Undersea Hyperb Med. 1999;26:137–41. [PubMed] [Google Scholar]
- 14.Casey H, Dastidar AG, MacIver D. Swimming-induced pulmonary oedema in two triathletes: a novel pathophysiological explanation. J R Soc Med. 2014;107:450–2. doi: 10.1177/0141076814543214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gempp E, Demaistre S, Louge P. Hypertension is predictive of recurrent immersion pulmonary edema in scuba divers. Int J Cardiol. 2014;172:528–9. doi: 10.1016/j.ijcard.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Gempp E, Louge P, Henckes A, Demaistre S, Heno P, Blatteau JE. Reversible myocardial dysfunction and clinical outcome in scuba divers with immersion pulmonary edema. Am J Cardiol. 2013;111:1655–9. doi: 10.1016/j.amjcard.2013.01.339. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig BB, Mahon RT, Schwartzman EL. Cardiopulmonary function after recovery from swimming-induced pulmonary edema. Clin J Sport Med. 2006;16:348–51. doi: 10.1097/00042752-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Pons M, Blickenstorfer D, Oechslin E, Hold G, Greminger P, Franzeck UK, Russi EW. Pulmonary oedema in healthy persons during scuba-diving and swimming. Eur Respir J. 1995;8:762–7. [PubMed] [Google Scholar]
- 19.Lange L, Lange S, Echt M, Gauer OH. Heart volume in relation to body posture and immersion in a thermo-neutral bath: a roentgenometric study. Pflugers Arch. 1974;352:219–26. doi: 10.1007/BF00590487. [DOI] [PubMed] [Google Scholar]
- 20.Kurss DI, Lundgren CEG, Pasche AJ. Effect of water temperature on vital capacity in head-out immersion. In: Bachrach AJ, Matzen MM, editors. Underwater Physiology VII Proceedings of the 7th Symposium on Underwater Physiology. Bethesda, MD: Undersea Medical Society; 1981. pp. 297–301. [Google Scholar]
- 21.Wester TE, Cherry AD, Pollock NW, Freiberger JJ, Natoli MJ, Schinazi EA, Doar PO, Boso AE, Alford EL, Walker AJ, Uguccioni DM, Kernagis D, Moon RE. Effects of head and body cooling on hemodynamics during immersed prone exercise at 1 ATA. J Appl Physiol (1985) 2009;106:691–700. doi: 10.1152/japplphysiol.91237.2008. [DOI] [PubMed] [Google Scholar]
- 22.Cherry AD, Forkner IF, Frederick HJ, Natoli MJ, Schinazi EA, Longphre JP, Conard JL, White WD, Freiberger JJ, Stolp BW, Pollock NW, Doar PO, Boso AE, Alford EL, Walker AJ, Ma AC, Rhodes MA, Moon RE. Predictors of increased PaCO2 during immersed prone exercise at 4.7 ATA. J Appl Physiol (1985) 2009;106:316–25. doi: 10.1152/japplphysiol.00885.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 24.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Naeije R, Chesler N. Pulmonary circulation at exercise. Compr Physiol. 2012;2:711–41. doi: 10.1002/cphy.c100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arborelius M, Jr, Balldin UI, Lilja B, Lundgren CEG. Hemodynamic changes in man during immersion with the head above water. Aerosp Med. 1972;43:592–8. [PubMed] [Google Scholar]
- 27.Christie JL, Sheldahl LM, Tristani FE, Wann LS, Sagar KB, Levandoski SG, Ptacin MJ, Sobocinski KA, Morris RD. Cardiovascular regulation during head-out water immersion exercise. J Appl Physiol (1985) 1990;69:657–64. doi: 10.1152/jappl.1990.69.2.657. [DOI] [PubMed] [Google Scholar]
- 28.Echt M, Lange L, Gauer OH. Changes of peripheral venous tone and central transmural venous pressure during immersion in a thermo-neutral bath. Pflugers Archiv. 1974;352:211–7. doi: 10.1007/BF00590486. [DOI] [PubMed] [Google Scholar]
- 29.Grainger RG. II. Interstitial pulmonary oedema and its radiological diagnosis: a sign of pulmonary venous and capillary hypertension. Br J Radiol. 1958;31:201–17. doi: 10.1259/0007-1285-31-364-201. [DOI] [PubMed] [Google Scholar]
- 30.Staub NC. Pulmonary edema. Physiol Rev. 1974;54:678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- 31.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353:2788–96. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 32.Ganter CC, Jakob SM, Takala J. Pulmonary capillary pressure. A review. Minerva Anestesiol. 2006;72:21–36. [PubMed] [Google Scholar]
- 33.Merritt FL, Weissler AM. Reflex venomotor alterations during exercise and hyperventilation. Am Heart J. 1959;58:382–7. [Google Scholar]
- 34.Sharpey-Schafer EP. Venous tone: effects of reflex changes, humoral agents and exercise. Br Med Bull. 1963;19:145–8. doi: 10.1093/oxfordjournals.bmb.a070034. [DOI] [PubMed] [Google Scholar]
- 35.Delaney EP, Young CN, DiSabatino A, Stillabower ME, Farquhar WB. Limb venous tone and responsiveness in hypertensive humans. J Appl Physiol (1985) 2008;105:894–201. doi: 10.1152/japplphysiol.90574.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peacher DF, Pecorella SR, Freiberger JJ, Natoli MJ, Schinazi EA, Doar PO, Boso AE, Walker AJ, Gill M, Kernagis D, Uguccioni D, Moon RE. Effects of hyperoxia on ventilation and pulmonary hemodynamics during immersed prone exercise at 4.7 ATA: possible implications for immersion pulmonary edema. J Appl Physiol (1985) 2010;109:68–78. doi: 10.1152/japplphysiol.01431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beinart R, Matetzky S, Arad T, Hod H. Cold water-induced pulmonary edema. Am J Med. 2007;120:e3. doi: 10.1016/j.amjmed.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 38.Nonogi H, Hess OM, Ritter M, Krayenbuehl HP. Diastolic properties of the normal left ventricle during supine exercise. Br Heart J. 1988;60:30–8. doi: 10.1136/hrt.60.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28:236–44. doi: 10.1016/j.echo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 43.Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, Chung N, Ha JW. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–33. doi: 10.1016/j.jacc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 44.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 45.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borlaug BA, Lewis GD, McNulty SE, Semigran MJ, LeWinter M, Chen H, Lin G, Deswal A, Margulies KB, Redfield MM. Effects of sildenafil on ventricular and vascular function in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:533–41. doi: 10.1161/CIRCHEARTFAILURE.114.001915. Epub 2015 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 48.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–74. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–94. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 50.Castellani JW, Young AJ, Sawka MN, Pandolf KB. Human thermoregulatory responses during serial cold-water immersions. J Appl Physiol (1985) 1998;85:204–9. doi: 10.1152/jappl.1998.85.1.204. [DOI] [PubMed] [Google Scholar]